Bithienofluorene, its derivative and preparation method
A technology for bis-thieno-fluorene derivatives, applied in the field of bis-thieno-fluorene derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
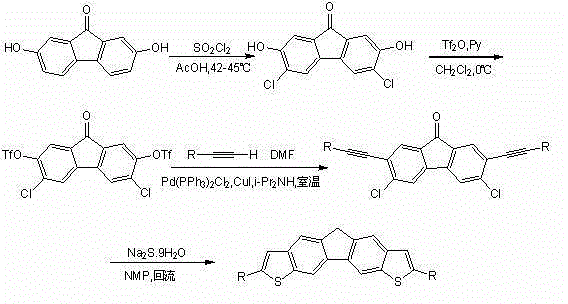
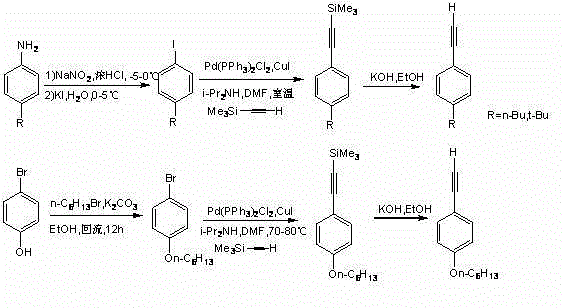
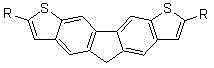
[0046] See attached figure 1 , the invention discloses a bisthienofluorene and its derivatives, which are characterized in that fluorene is connected to two thiophene groups in parallel rings, and there is a substituent at the 2-position of thiophene, and sodium sulfide is not only used as the sulfur element of the thiophene ring Source and as a reducing agent for fluorenone carbonyl reduction; its preparation is carried out in the following steps:
[0047] Preparation of 3,6-dichloro-2,7-dihydroxy-9-fluorenone (a)
[0048] Under nitrogen protection, 2,7-dihydroxyfluorenone (10.490 g, 49.5 mmol) was dissolved in 250 mL of glacial acetic acid to form a suspension, stirred, and slowly heated to 42 °C. Sulfonyl chloride (20 mL, 247.4 mmol) was slowly added dropwise, keeping the temperature below 45 °C. The suspension gradually changed from dark red to orange. React for 40 minutes after dropping. Cool, filter with suction, and wash with water and acetone. An orange sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com