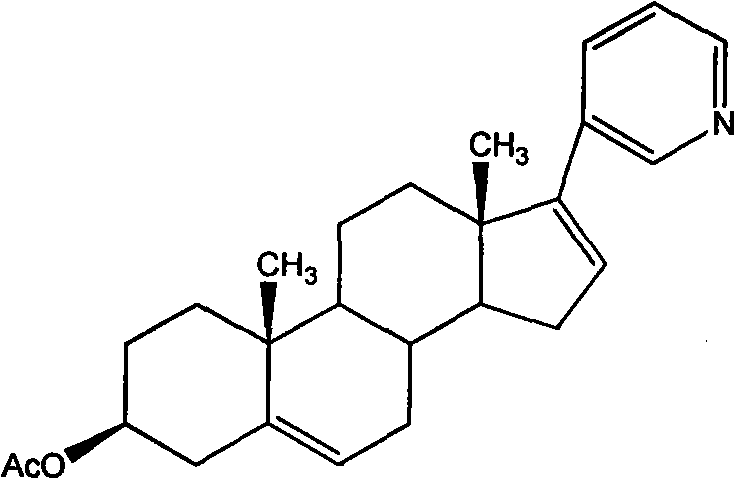
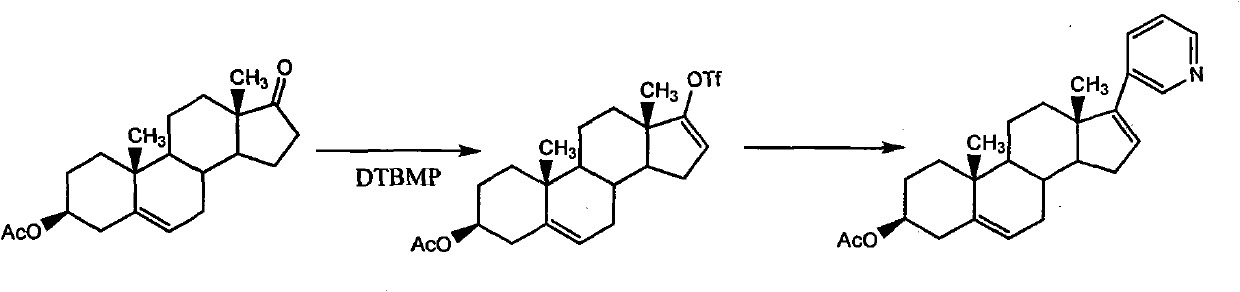
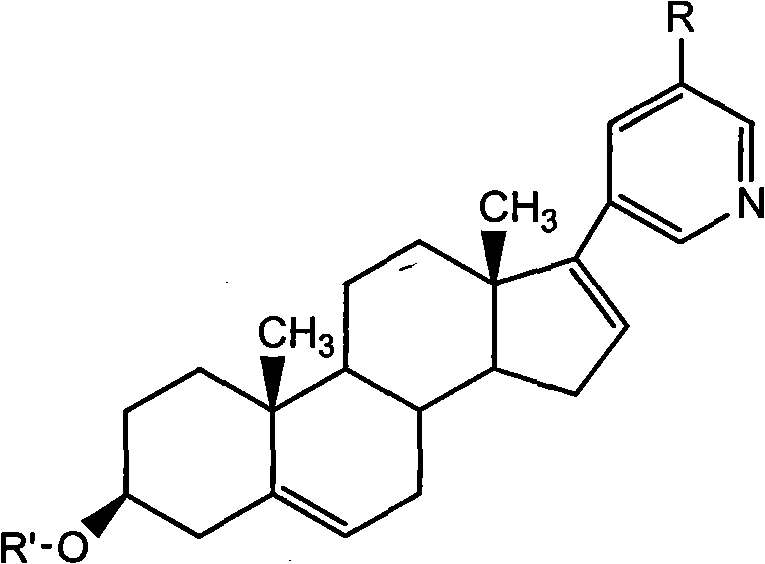
Synthetic method applicable to industrial production of Abiraterone acetate
A technology of abiraterone acetate and a synthetic method, applied in the synthetic field of industrialized mass production of abiraterone acetate, can solve the problem that the interval time between trifluoromethanesulfonic anhydride and alkali is short, cannot meet needs, and by-products 1 and 2 are removed, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1 Preparation of 3β-acetoxyandrost-5,16-dien-17-yl triflate
[0031] Add dehydroepiandrosterone acetate (40 g, 121 mmol) to the reactor, add CH 2 Cl 2 (800ml) was stirred and dissolved, and cooled to -5-5°C under positive pressure of argon. Quickly join Tf 2 O (22.4ml, 133mmol, 1.1eq), stirred and reacted at -5~5°C for 40 minutes, then quickly added 2,6-lutidine (14.4ml, 121mmol, 1eq) at -5~5°C , The resulting reaction solution was stirred and reacted at room temperature for 3 hours. The reaction was quenched by adding water (400ml), the layers were separated and washed with CH 2 Cl 2 (200ml) to extract the aqueous layer, and combine the organic layers. The organic solution was washed with 2N HCl (200ml). After separation, use 120g anhydrous MgSO 4 Dry for 7 min, filter through a filter lined with acid alumina, and wash with CH 2 Cl 2 The filter cake was washed, and the filtrate and washings were concentrated to obtain a reddish-brown oil. HPLC showed tha...
Embodiment 2
[0037] Step 1 Preparation of 3β-acetoxyandrost-5,16-dien-17-yl triflate
[0038] Add dehydroepiandrosterone acetate (200g, 0.606mol) in the reactor, replace with argon three times, add CH 2 Cl 2 (4000ml) was stirred and dissolved, and cooled to -20~-15°C under the positive pressure of argon. Quickly join Tf 2O (112ml, 0.666mol, 1.1 equivalent), stirred and reacted at -20~-15°C for 40 minutes, then at -20~-15°C, quickly added 2,6-lutidine (70ml, 0.6mol, 1 equivalent), the resulting reaction solution was stirred and reacted at room temperature for 2 hours. The reaction was quenched by adding water (2000ml), the layers were separated and washed with CH 2 Cl 2 (1000ml) to extract the aqueous layer, and combine the organic layers. The organic solution was washed with 2N HCl (1000ml). After separation, anhydrous MgSO 4 (600g) was dried for 7 minutes, filtered through a filter lined with acid alumina, and washed with CH 2 Cl 2 The filter cake was washed, and the filtrate an...
Embodiment 3
[0044] Step 1 Preparation of 3β-acetoxyandrost-5,16-dien-17-yl triflate
[0045] Add dehydroepiandrosterone acetate (100g, 0.303mol) in the reactor, replace with argon three times, add CH 2 Cl 2 (2000ml) was stirred and dissolved, and cooled to -10~-5°C under the positive pressure of argon. Quickly join Tf 2 O (56ml, 0.333mol, 1.1 equivalent), stirred and reacted at -10~-5°C for 40 minutes, then quickly added N,N-diisopropylethylamine (DIPEA) (54ml , 0.6mol, 1 equivalent), the resulting reaction solution was stirred and reacted at room temperature for 5 hours. The reaction was quenched by adding water (1000ml), the layers were separated and washed with CH 2 Cl 2 (500ml) extracted the aqueous layer, and combined the organic layers. The organic solution was washed with 2N HCl (500ml). After separation, anhydrous MgSO 4 (300g) was dried for 7 minutes, filtered through a filter covered with diatomaceous earth, and washed with CH 2 Cl 2 The filter cake was washed and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com