Synthesis method of chiral monophosphorus ligand
A synthesis method and ligand technology, which are applied in chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of many wastes, synthesis methods not in line with atom economy, long routes, etc.
Inactive Publication Date: 2010-11-17
CHENGDU UNIVERSITY OF TECHNOLOGY
View PDF0 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The synthesis method in the literature does not conform to the principle of atom economy, and the route is longer, there are more three wastes, it does not meet the requirements of green chemistry, and the cost is much higher
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
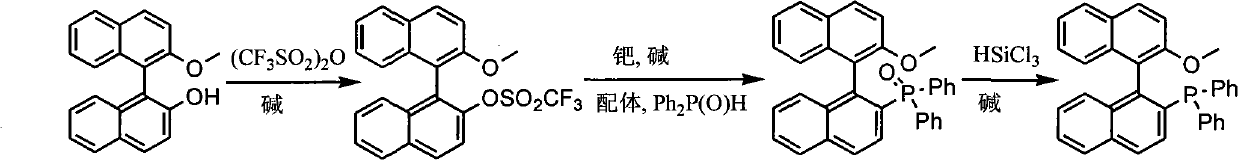
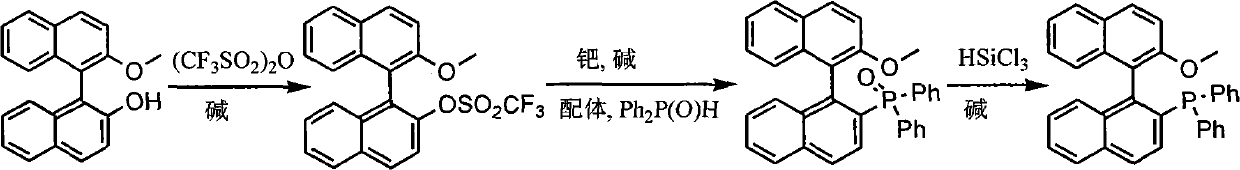
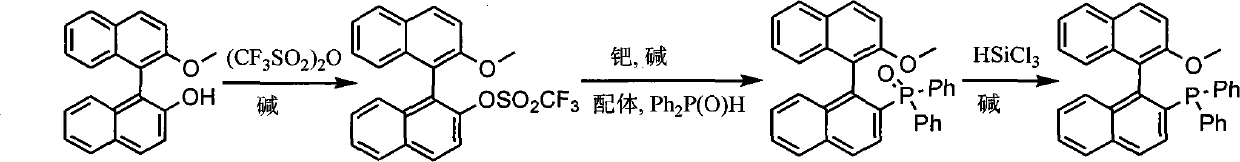
The invention discloses a synthesis method of chiral mono-phosphorus ligand 2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl. The chiral mono-phosphorus ligand is widely applied to the asymmetric catalysis field. The synthesis method comprises the following steps: allowing optically pure binaphthalene monomethyl ether to react with trifluoromethanesulfonic anhydride to obtain 2-trifluoromethyl sulfonyloxy-2'-methoxy-1,1'-binaphthyl; and allowing the obtained compound to react with diphenylphosphine oxide under the catalytic action of palladium to obtain 2-diphenylphosphine acyl-2'-methoxy-1,1'-binaphthyl, and then reducing with trichlorosilane to finally obtain the optically pure mono-phosphorus ligand 2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl. The synthesis method has short synthetic route and high atom economy, thus greatly lowering cost; and the synthetic route is shown in the following reaction equation.
Description
technical field The invention belongs to organic chemistry, asymmetric catalysis, and synthesis of chiral ligands. More specifically, it uses binaphthyl diol monomethyl ether to synthesize 2-diphenylphosphine-2'-methoxyl-1,1'- binaphthyl. Background technique (S)- or (R)-2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl is an important chiral ligand, which can be used in palladium, rhodium, nickel and other metal catalysis Asymmetric allylation, hydrosilation of alkenes, hydroboration of 1,3-enynes and other asymmetric reactions (T.Hayashi.Acc.Chem.Res.2000, 33:354-362). It is reported in the literature that the method for synthesizing 2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl is to generate binaphthyl diol bistrifluoromethane from binaphthyl diol and two moles of trifluoromethanesulfonic anhydride Sulfonate, then under palladium catalysis, diphenylphosphine oxide reacts with one of the trifluoromethanesulfonate groups, and the remaining trifluoromethanesulfonate group ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07F9/50
Inventor 曾庆乐曾辉
Owner CHENGDU UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com