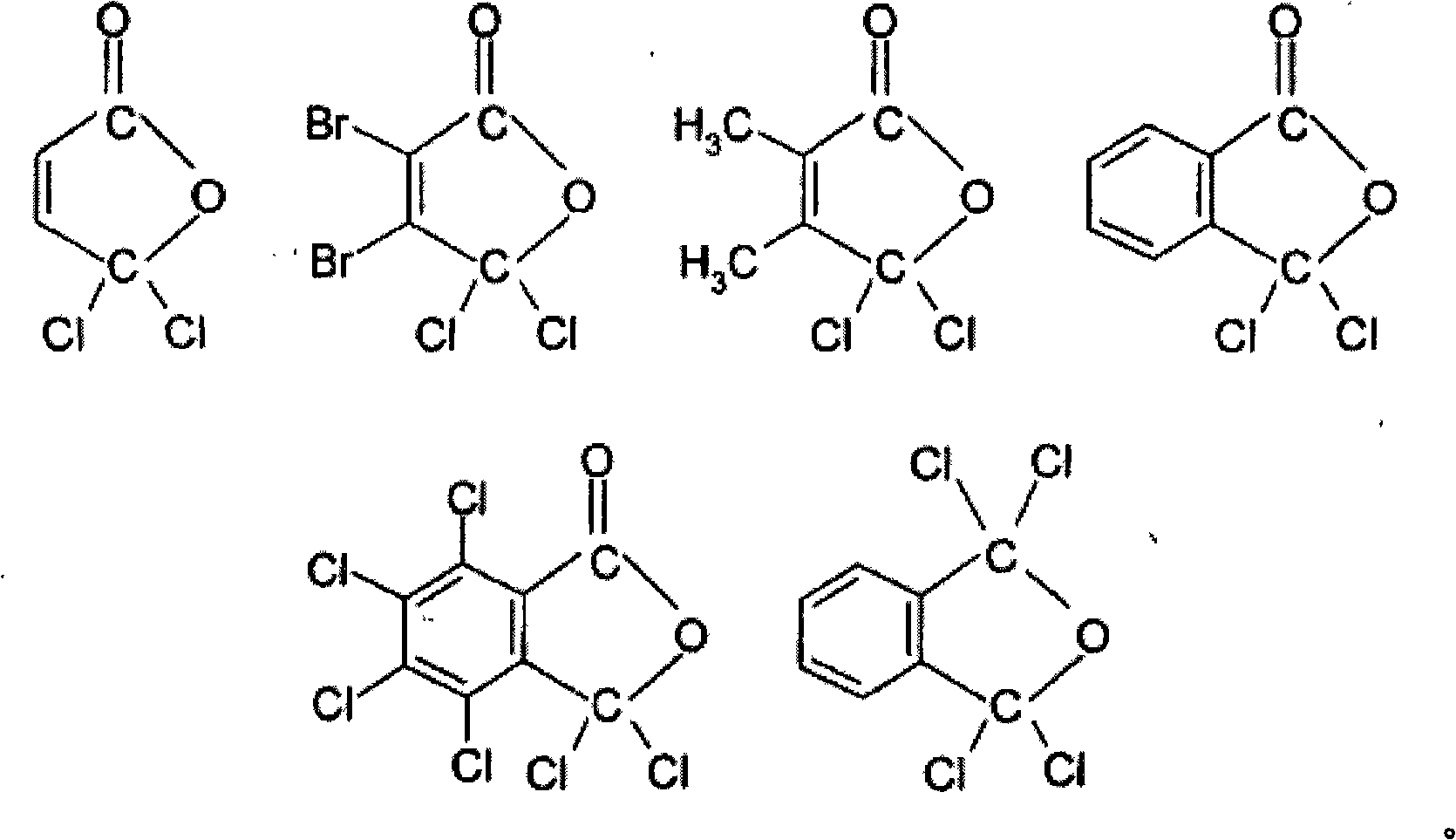
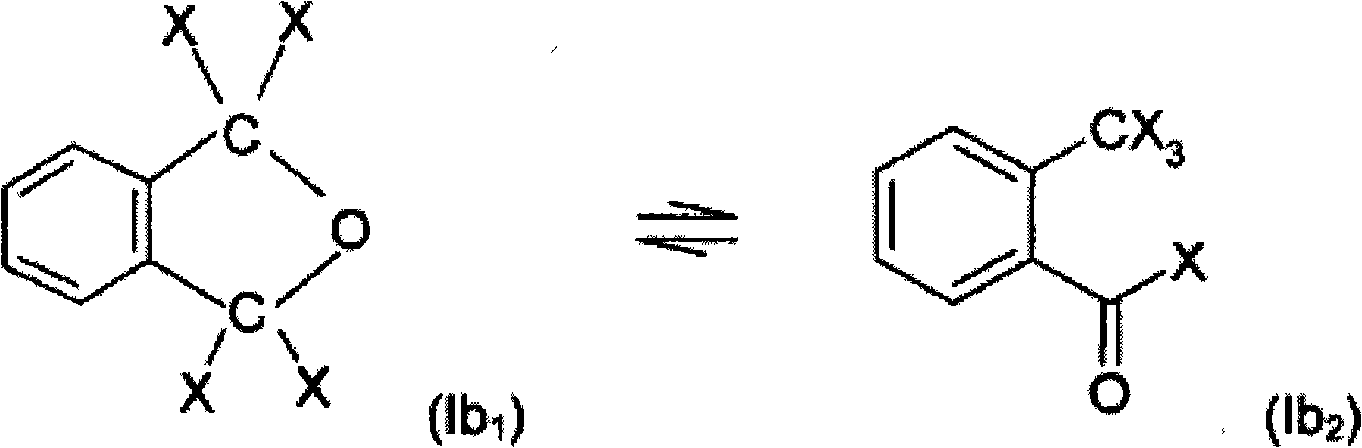
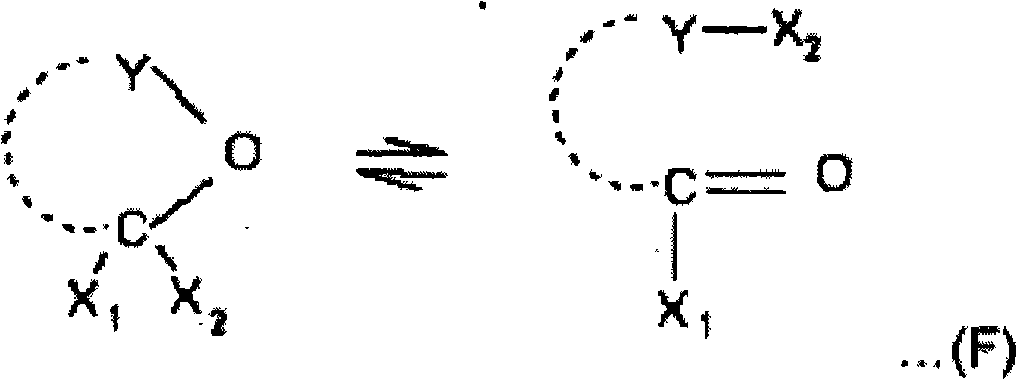Process for preparing a sulphonic acid anhydride
A technology of sulfonic acid anhydride and sulfonic acid, applied in the preparation of acid anhydride, the preparation of symmetrical or mixed anhydride, the preparation of "anhydridetriflique", and the field of trifluoromethanesulfonic anhydride, which can solve the problems of difficulty and cost of phosphoric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Preparation of trifluoromethanesulfonic anhydride
[0124] A 250 ml reactor was used which was heated with a double jacket and equipped with a central mechanical stirrer, dropping funnel and elbow which was cooled to 15°C via a) a dry ice trap b) a wash bottle containing sodium hydroxide solution and Feed to collector in atmosphere.
[0125] 73 g of RO constituting the "pied" of the reaction mixture were charged; trichloromethylbenzoyl chloride used was equilibrated in a molar ratio of 20 / 80 with its pseudochloride tautomeric form.
[0126] The primer was heated to 120°C.
[0127] Using a dropping funnel, 157 g of trifluoromethanesulfonic acid was gradually added over 3 hours, keeping the temperature of the reaction mass at 115°C to 135°C.
[0128] Evolution of HCl was observed from the beginning of the addition and crude TAA condensed after about 15 minutes.
[0129] The HCl stream was converted to organic vapor by condensation in a dry ice trap.
[0130] After the...
Embodiment 2 and 3
[0138] Comparative test A-O
[0139] A series of qualitative experiments were performed to demonstrate the effect of the type of reagent used.
[0140] Before detailing the examples and experiments, a description of the apparatus used and the protocols followed will be provided.
[0141] device used
[0142] The device used is Temperature gradient oven (Glass Oven B 580).
[0143] It is in the form of a tubular glass furnace into which a single-necked reaction flask is introduced, which is connected to two receiving flasks.
[0144] The above-mentioned whole is linked together and can be rotated by a motor.
[0145] The light products of the reaction were trapped at the outlet by dry ice into two receiving flasks.
[0146] Visually monitor for possible gas emissions with a bubble meter.
[0147] operating procedures
[0148] Reagents were weighed (max weight 10 g) in reaction vials in a fume hood.
[0149] The molar ratio between sulfonic acid and reagent is sho...
Embodiment 2
[0175] In this example, the sulfonic acid used is trifluoromethanesulfonic acid, and the reagent used is a compound having at least one gem-dichloro carbon atom involved in the tautomerization of the pseudoacyl chloride.
[0176] The results are shown in Table (IV).
[0177] Table (IV)
[0178] Test label
[0179] The recovered distillate was analyzed by fluorine NMR spectroscopy. It consists essentially of trifluoromethanesulfonic anhydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com