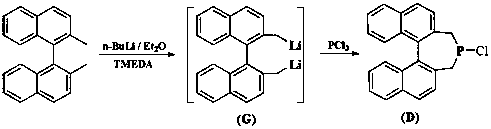
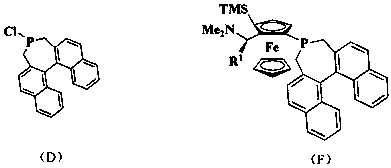
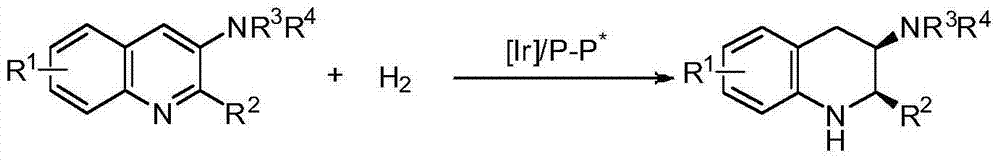Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
284 results about "Diphosphines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH₂(PPh₂)₂. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.
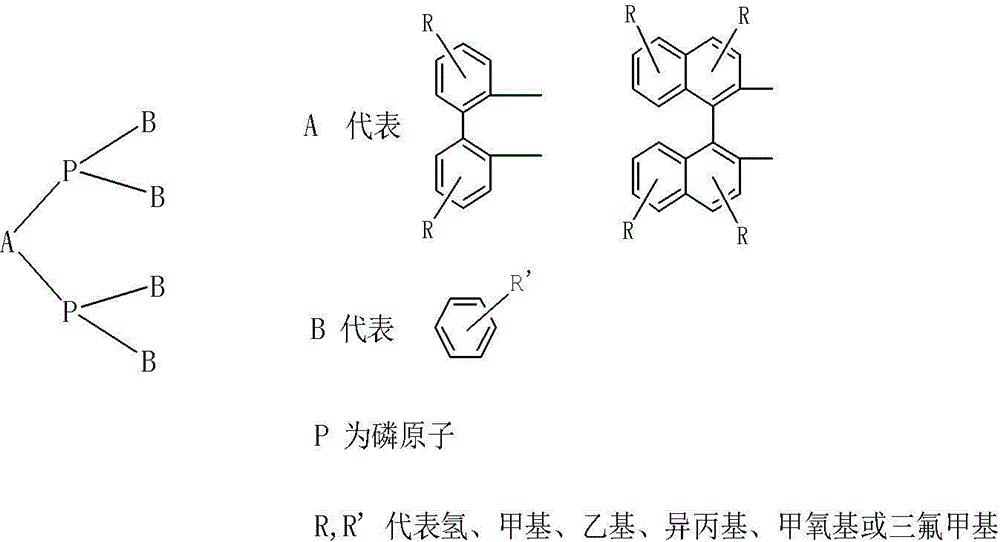
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
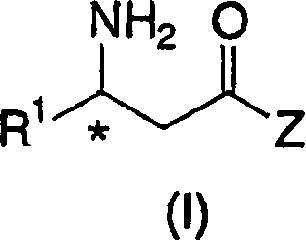
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
ActiveUS7468459B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleEnantiomer
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME LLC
Method for producing diphosphines and the use thereof
InactiveUS6924389B2Carry-out safelyHigh purityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAliphatic hydrocarbonDiphosphines
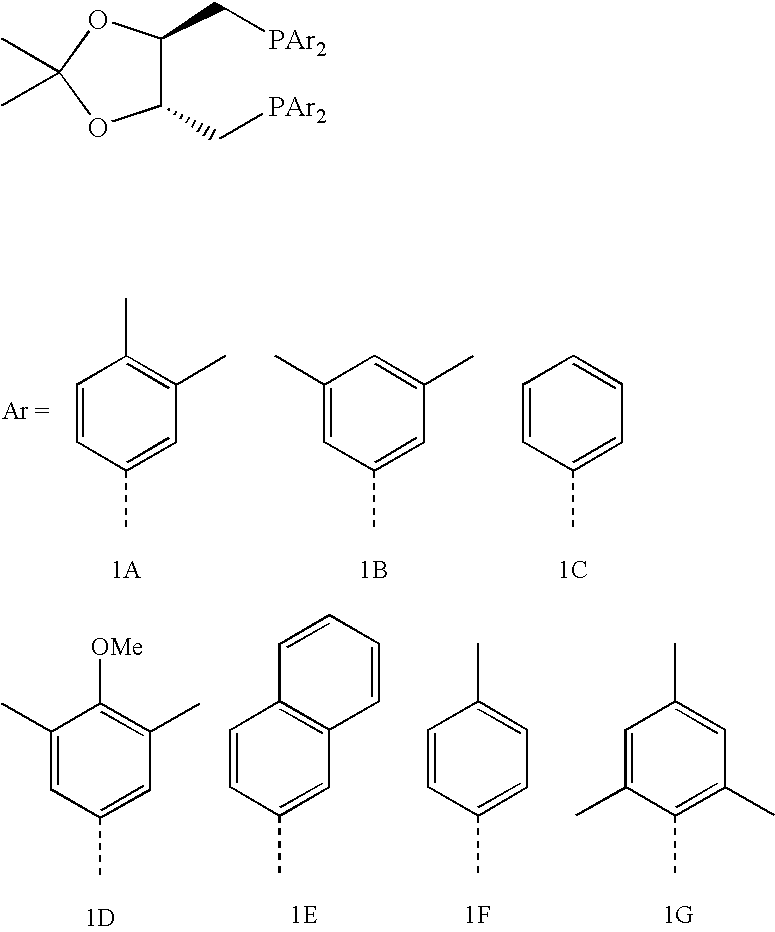
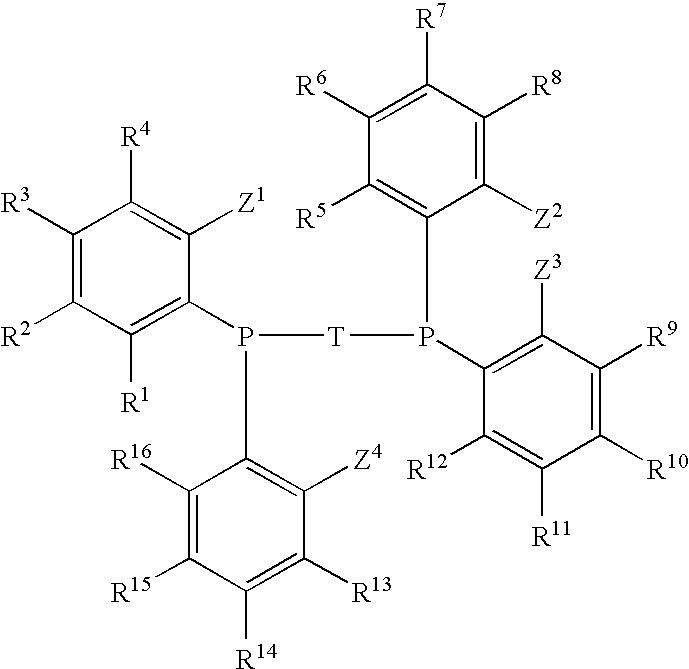
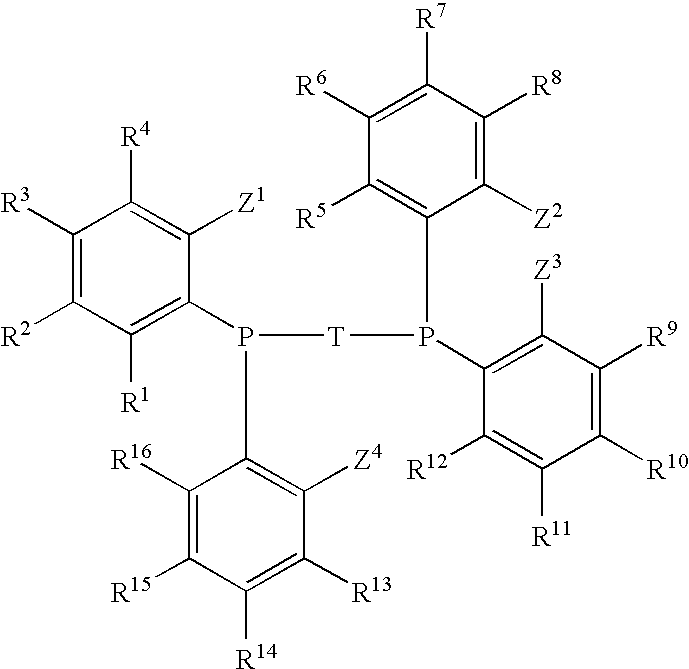
Diphosphine of formula IR1R2P—H2C—Ar—CH2PR1R2 (I)are prepared by a process that comprises a) reacting a dimethyl compound of formula IIH3C—Ar—CH3 (II)with a base and an N-substituted aminophosphorous halide to form bis(aminophosphinomethyl) compound of the formula IIIR3R4P—H2C—Ar—CH2—PR3R4 (III)b) reacting the compound of formula III with HCl to form bis(dichlorophosphinomethyl) compound of formula IVCl2P—H2C—Ar—CH2—PCl2 (IV)and c) reacting the compound of formula IV with an organometallic reagent to give the product compound of formula I, wherein groups R1 and R2 are each, independently of one another, a substituted or unsubstituted aromatic, heteroaromatic or aliphatic hydrocarbon group and may have a covalent bond connecting them and Ar is a substituted or unsubstituted aromatic or heteroaromatic hydrocarbon group an R3 and R4 are each, independently of one another, an N-substituted alkylamino or arylamino group.
Owner:EVONIK DEGUSSA GMBH
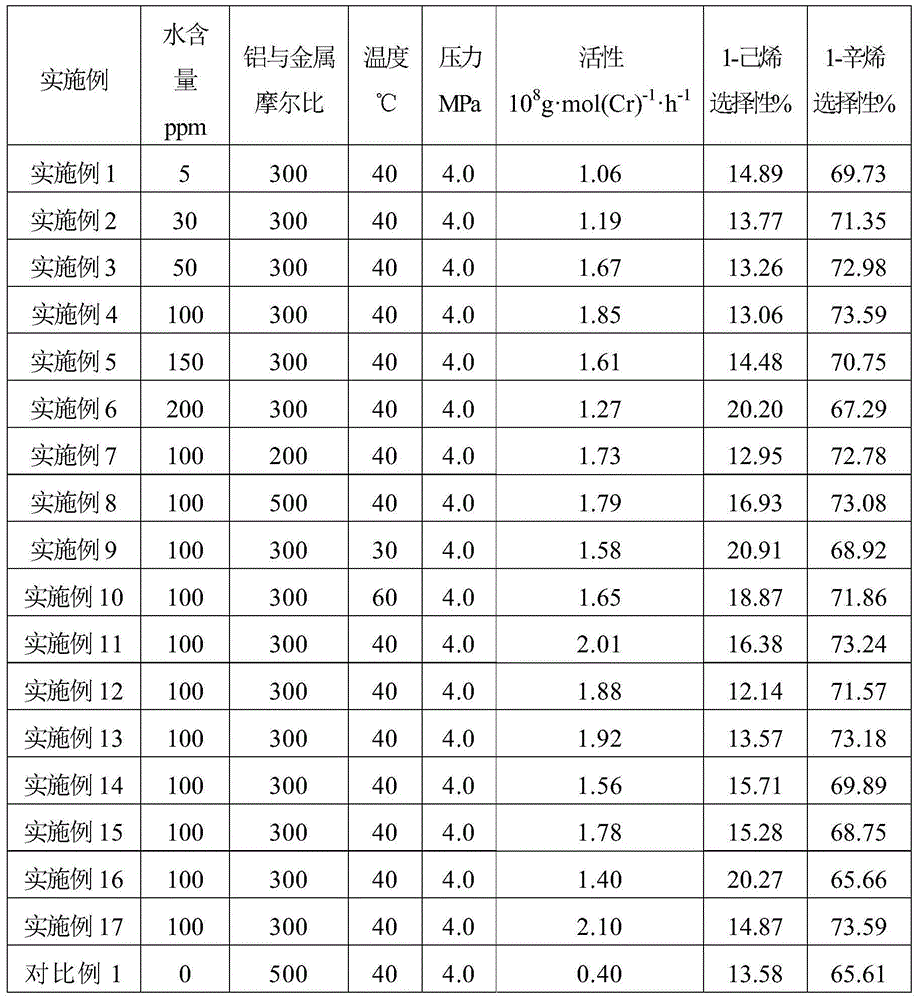
Phosphine Ligand-Metal Compositions, Complexes, and Catalysts For Ethylene Trimerizations
This invention relates to a method to selectively oligomerize olefins comprising contacting olefins with: 1) at least one diaryl-substituted diphosphine ligand; 2) a chromium metal precursor; and 3) optionally, one or more activators. In a particular embodiment, the method for selectively oligomerizing olefins includes trimerizing ethylene to selectively form 1-hexene.
Owner:EXXONMOBIL CHEM PAT INC
Method for preparing aldehyde through linear chain olefin hydroformylation
InactiveCN102911021AReduce dosageGuaranteed uptimeOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionFormylation reactionDiphosphines
The invention relates to a method for preparing aldehyde through linear chain olefin hydroformylation. According to the method, a continuous reaction mode is used for performing olefin hydroformylation reaction in a homogeneous catalyst system; a catalyst is a composite catalysis system composed of a rhodium complex, a biphenyl backbone or biphenyl backbone diphosphine ligands and triphenylphosphine or triphenyl phosphate monophosphorous ligands; a reaction solvent comprises butyraldehyde, valeraldehyde, toluene or isodecanol; when the catalyst system uses propylene or butene-1 as raw materials under the condition of a low molar ratio of the diphosphine ligands to the rhodium complex, contents of the aldehyde generated by hydroformylation of the propylene and the butene-1 is larger than 97% and 95% respectively; and when the catalyst system uses a mixture of the butene-1 and butene-2 as raw materials, and the content of n-valeraldehyde in hydroformylation reaction products can reach above 85%.
Owner:QINGDAO SANLI BENNUO CHEM IND +1
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
InactiveCN1761642AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleAsymmetric hydrogenation
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME BV
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
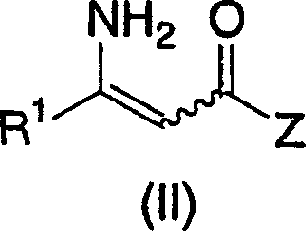
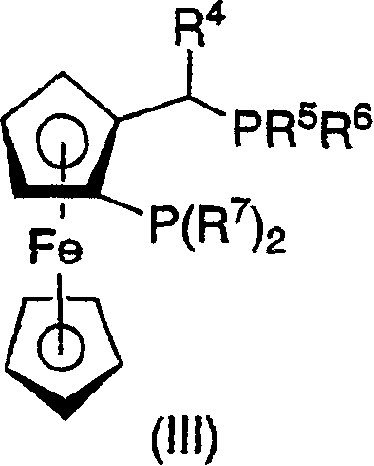
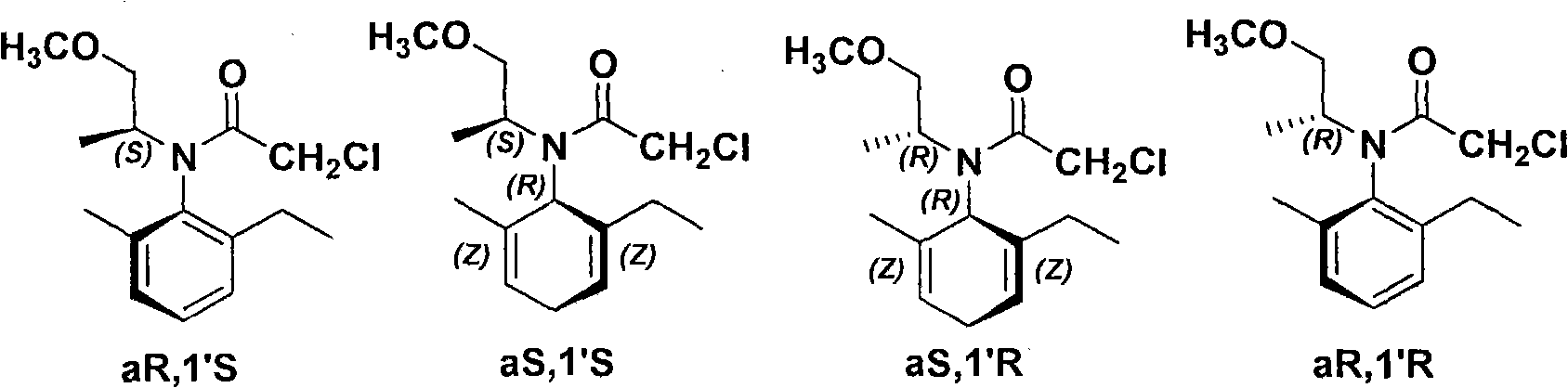
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
Ethylene oligomerization
InactiveUS20120101321A1Organic-compounds/hydrides/coordination-complexes catalystsHydrocarbons from unsaturated hydrocarbon additionAluminoxaneAromatic solvent
The oligomerization of ethylene using a chromium catalyst having a bridged diphosphine ligand can produce a selective product distribution (to predominantly hexene or predominantly octene / hexene) when activated with an aluminoxane. The oligomerization reaction also produces polymer by product—particularly when the aluminoxane is provided in a non-aromatic solvent. The present invention mitigates this problem.
Owner:NOVA CHEM (INT) SA
Process for the carbonylation of a conuugated diene
InactiveUS20060235241A1Improve rigidityPromote resultsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen atomDiphosphines
A process for the carbonylation of a conjugated diene, which involves reacting the conjugated diene with carbon monoxide and a co-reactant having a mobile hydrogen atom in the presence of a catalyst system including: (a) a source of palladium; and (b) a bidentate diphosphine ligand of formula II, R1R2>P1—R3m—R—R4n—P2<R5R6 (II) wherein P1 and P2 represent phosphorus atoms; R1, R2, R5 and R6 independently represent the same or different optionally substituted organic radical containing a tertiary carbon atom through which each radical is linked to the phosphorus atom; R3 and R4 independently represent the same or different optionally substituted methylene groups; R represents an organic group having the bivalent bridging group C1—C2 through which R is connected to R3 and R4; m and n independently represent a natural number in the range of from 0 to 4, wherein the rotation about the bond between the carbon atoms C1 and C2 of the bridging group is restricted a temperature in the range of from 0° C. to 250° C., and wherein the dihedral angle between the plane occupied by the three atom sequence composed of C1, C2 and the atom directly bonded to C1 in the direction of P1, and the plane occupied by the three atom sequence C1, C2 and the atom directly bonded to C2 in the direction of P2, is in the range of from 0 to 120°; and (c) a source of an anion.
Owner:SHELL OIL CO
Bulk ethylene oligomerization using a low concentration of chromium catalyst and three-part activator
InactiveUS20140142360A1Organic-compounds/hydrides/coordination-complexes catalystsCatalystsOligomerAluminoxane
This invention enables the “bulk” oligomerization of ethylene (i.e. the oligomerization of ethylene in the presence of the oligomer product) using a catalyst system comprising 1) a very low concentration of a chromium catalyst and 2) a three part activator. The chromium catalyst contains a diphosphine ligand, preferably a so called P—N—P ligand. The activator includes an aluminoxane, trimethyl aluminum, and triethyl aluminum.
Owner:NOVA CHEM (INT) SA
Ethylene tetrapolymerization catalyst composition and ethylene tetrapolymerization method
ActiveCN105562099AHigh polymerization reactivityRapid responseOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsArylOrganic solvent
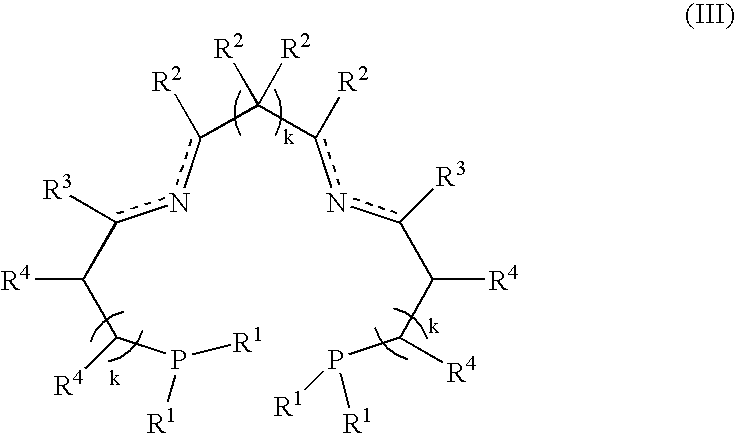
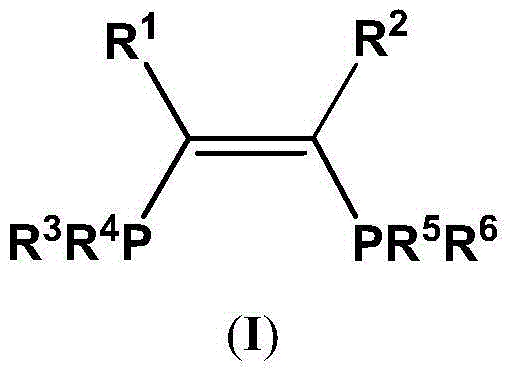
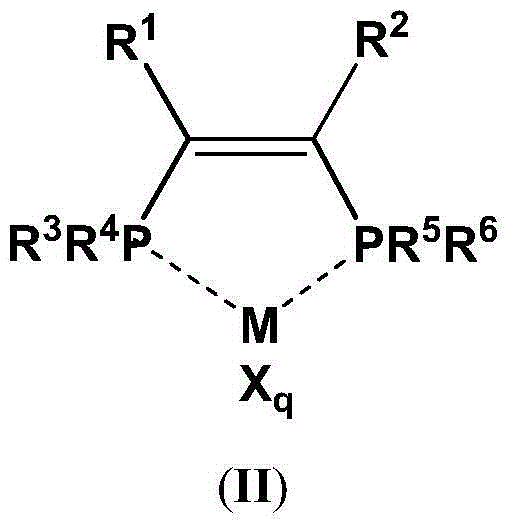
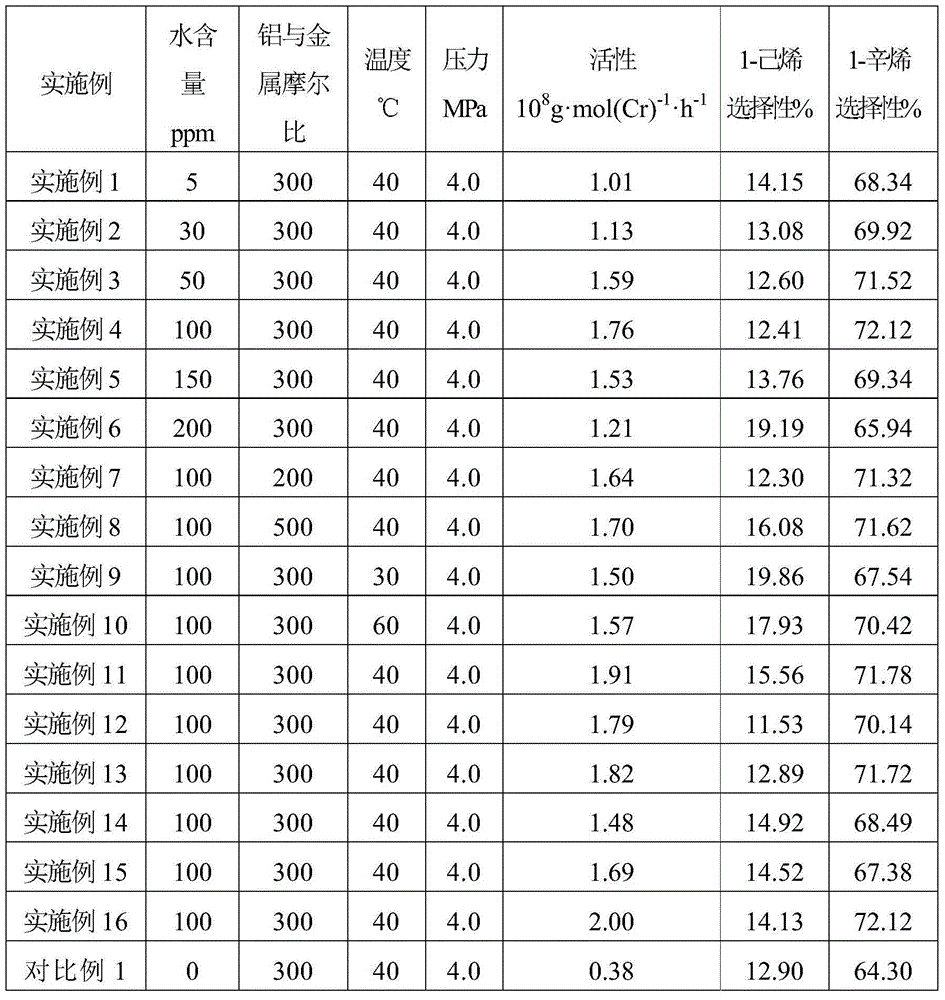
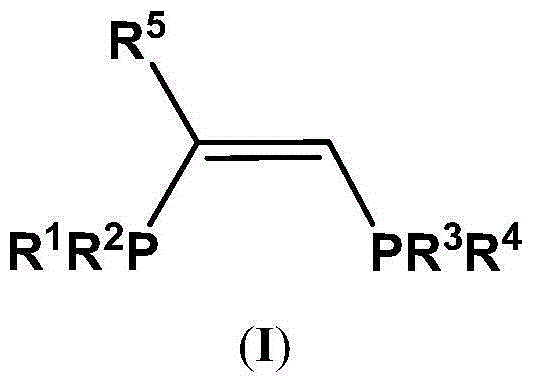
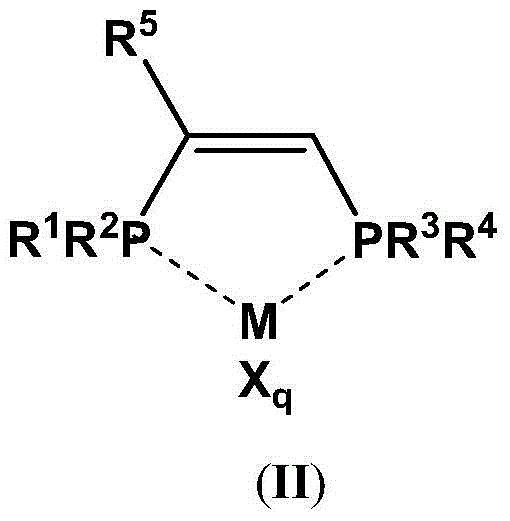
The present invention discloses an ethylene tetrapolymerization catalyst composition, which comprises a diphosphine ligand represented by a formula I, a transition metal compound, an aluminum-containing co-catalyst, water and an organic solvent, wherein R1, R2, R3 and R4 are respectively and independently selected from alkyl, cycloalkyl and aryl, and R5 is selected from alkyl, cycloalkyl and aryl. The invention further discloses a method for carrying out an ethylene tetrapolymerization reaction by using the catalyst composition. The formula I is defined in the specification.
Owner:CHINA PETROLEUM & CHEM CORP +1
Rare earth complexes chelated by aromatic base substituted diphosphine dioxide and its synthesizing process
InactiveCN1687080AElectrical apparatusGroup 5/15 element organic compoundsHigh current densityRare earth
The present invention relates to a kind of high-performance photoelectric function rare earth complex material and its preparation method. Said invention uses substituted diphosphine dioxide containing aromatic group as ligand, and utilizes complexation reaction to synthesize a series of high-effective rare earth photoelectric function complexes, and prepares doped red electroluminescent device containing above-mentioned rare earth photoelectric function material, said electroluminescent device can obtan satisfactory result in the luminance, current density and efficiency under the high current density.
Owner:FUDAN UNIV
Direct hydrocarbonylation process
A direct hydrocarbonylation process for the production of 1,4-butanediol is described. The process comprises reacting allyl alcohol with carbon monoxide and hydrogen in an alcohol solvent in the presence of a catalyst system comprising a rhodium complex, a trialkyl phosphine, and a diphosphine. The process gives a high yield of 1,4-butanediol in a one-step reaction.
Owner:LYONDELL CHEM TECH LP
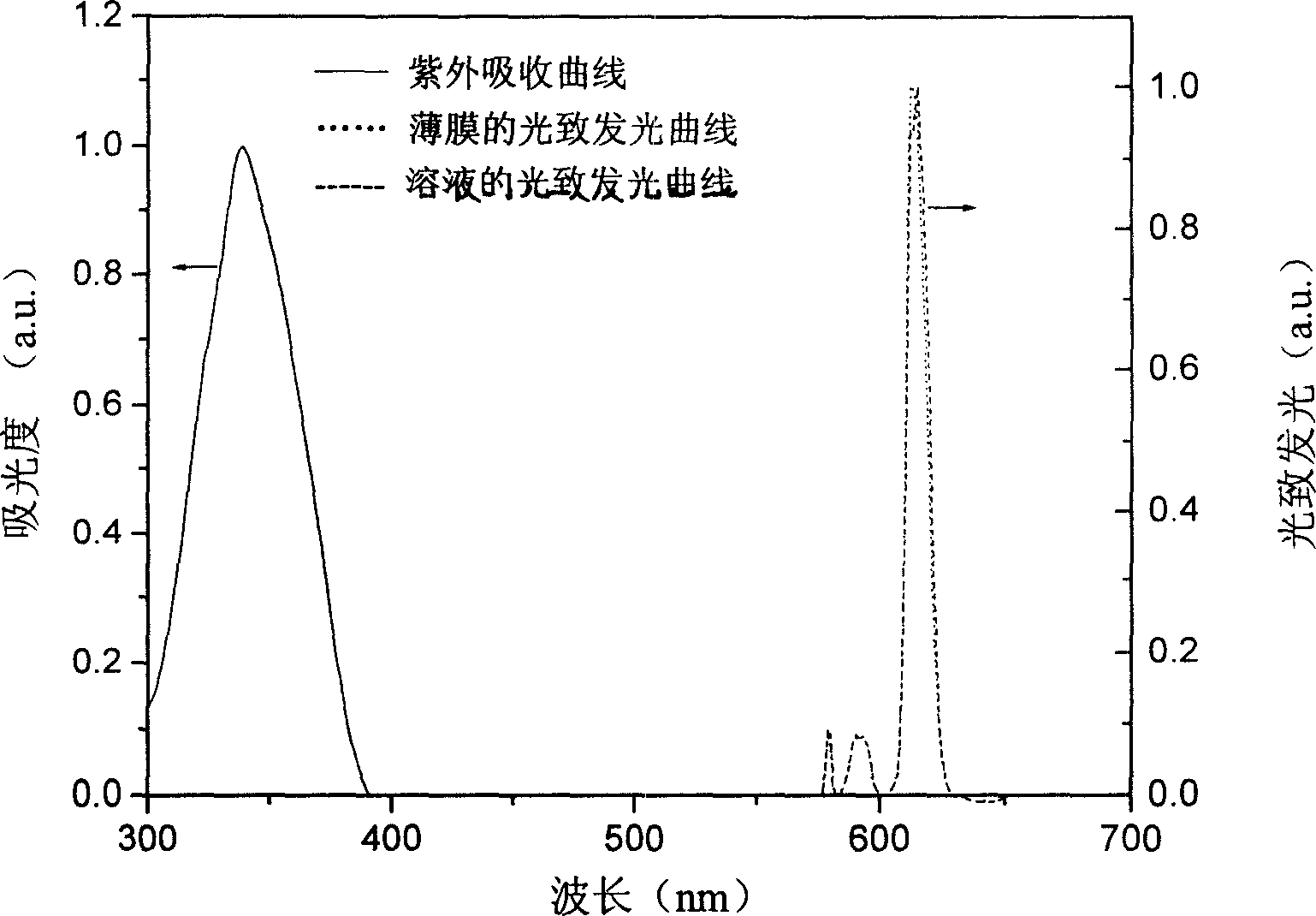
Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof
InactiveCN103951683AGroup 5/15 element organic compoundsCopper organic compoundsPhotoluminescenceDiphosphines
The invention relates to luminescent novel material synthesis technologies and particularly relates to blue-green materials with novel tri / tetra-coordination dual-core copper [I] coordination compounds and a preparation method thereof. According to the preparation method, the novel dual-core copper [I] coordination compound blue-green materials, namely [Cu2[bmptzH][[mu]-dppm]2][ClO4]2 and [Cu2[fptz][[mu]-dppm]2][ClO4], which simultaneously have two coordination bonding manners, namely tri-coordination and tetra-coordination, and have special structures, are synthesized from functionalized pyridine triazole bidentate chelating ligands (5-tert-butyl-3-[6-methyl-pyridine-2-yl]-1,2,4-triazole and 5-trifluoromethyl-3-[pyridine-2-yl]-1,2,4-triazole) and an organic diphosphine auxiliary ligand (bis[diphenylphosphino]methane). The blue-green materials show very good photoluminescence properties in a solid state, and the photoinduced quantum efficiency is 66% and 77% respectively.
Owner:JIANGXI UNIV OF SCI & TECH
Radioactive transition metal nitride heterocomplex
InactiveUS6270745B1Impairing activityHigh yieldGroup 5/15 element organic compoundsRadioactive preparation carriersRheniumTechnetium
The present invention provides a single radioactive transition metal nitride heterocomplex which permits labeling of a physiologically active substance such as a peptide, hormone or the like without impairing the activity of the substance. The radioactive transition metal nitride heterocomplex of the present invention is represented by the following formula (I):wherein a radioactive transition metal M is radioactive technetium or radioactive rhenium, N is a nitrogen atom, X is a diphosphine compound or a diarsine compound, and Y is a bindentate ligand having a combination of electron-donating atoms.
Owner:NIHON MEDI PHYSICS CO LTD
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
A process for the hydrogenation, using molecular hydrogen (H2), of a catalytic system, wherein the catalytic system comprises a base and a complex of formula; (II) wherein Y represents simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and N2P2 is a tetradentate diimine-diphosphino ligand.
Owner:FIRMENICH SA
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
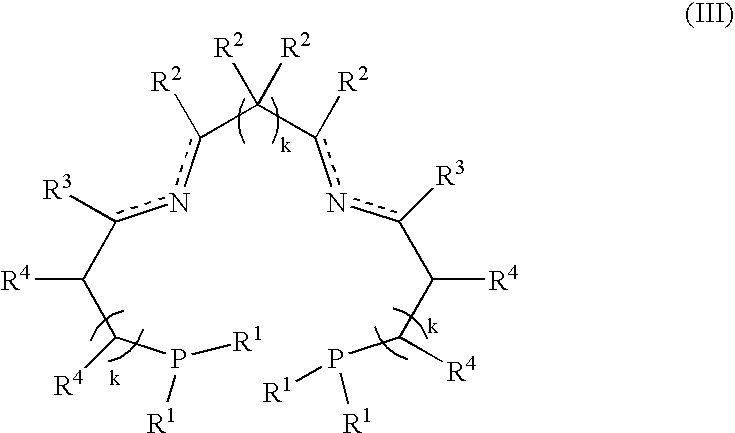
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Iridium catalyzed enantiotropic hydrosubstituting process of aromatic pyridine ring and pyrazine ring
InactiveCN1468852AHigh enantiomeric excessMild reaction conditionsOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoxaline
The iridium catalyzed enantiotropic hydrosubstituting process of aromatic pyridine ring and pyrazine ring uses catalyst system comprising chiral coordination compound of iridium and additive compound. The reaction conditions includes temperature of 0-80 deg.c; solvent of dichloromethane, toluene, tetrahydrofuaran, 1, 2-dichloro ethane, isopropanol, etc.; additive compound of tetrabutyl ammonium iodide, iodine, amine, etc.; pressure of 1-100 atm; substrate / catalyst ratio up to 5000; and chiral coordination compound of diphosphine, N-P compound, S-P compound, etc. The catalyst system is prepared through reaction of iridium precursor and chiral compound in the said solvent and the addition of the additive compound while stirring; and can produce asymmetrical induction up to 96 %. The process of the present invention is environment friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chiral diphosphine ligand and chiral catalyst, and preparation and application method thereof
ActiveCN103059064AHigh affinityReduced activityOrganic compound preparationGroup 5/15 element organic compoundsCyclic processDiphosphines
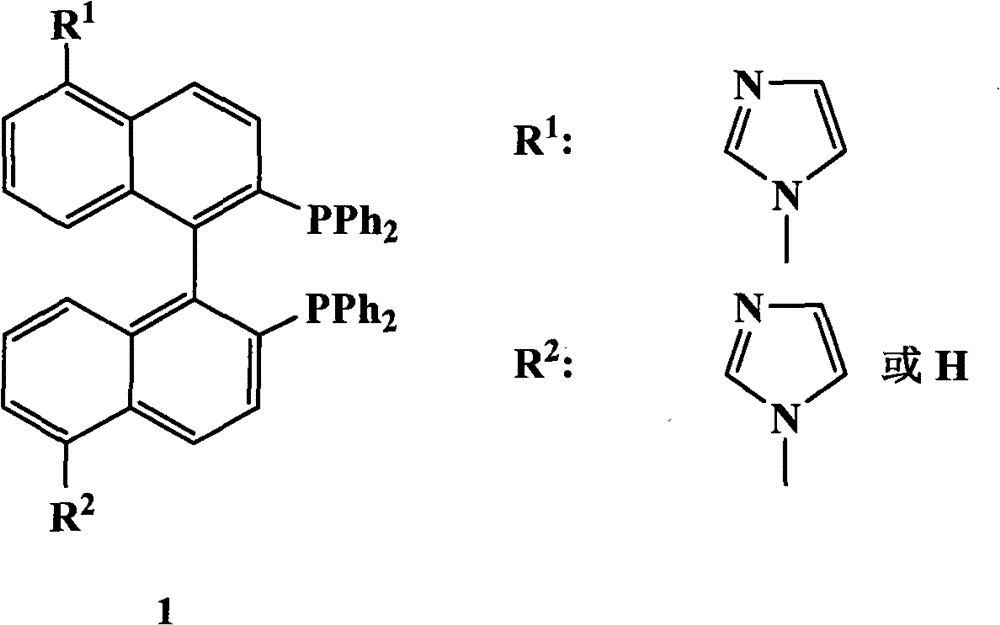
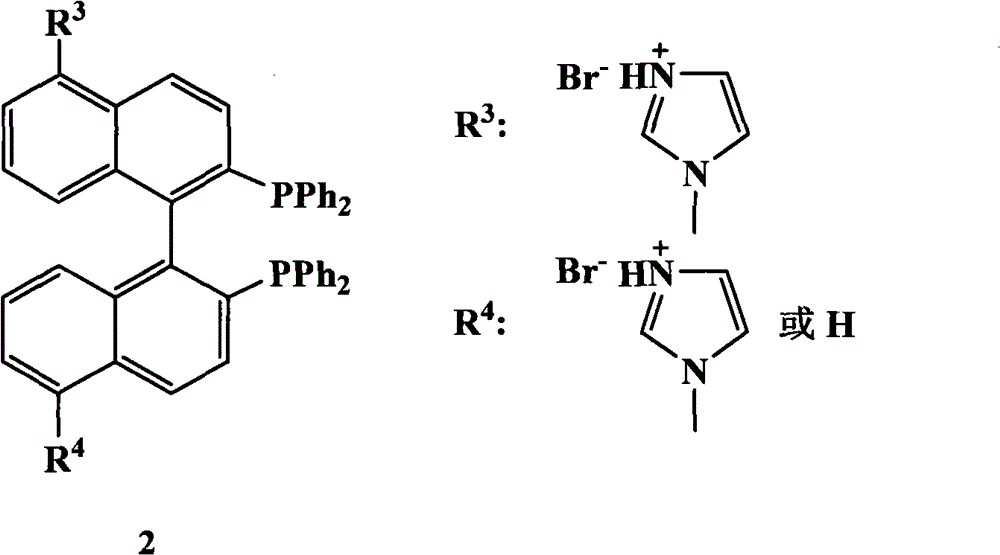
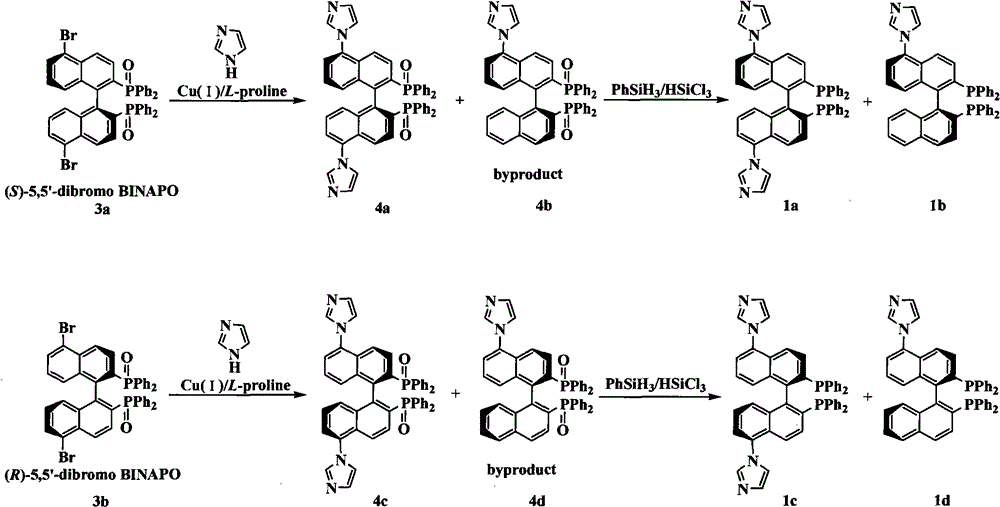
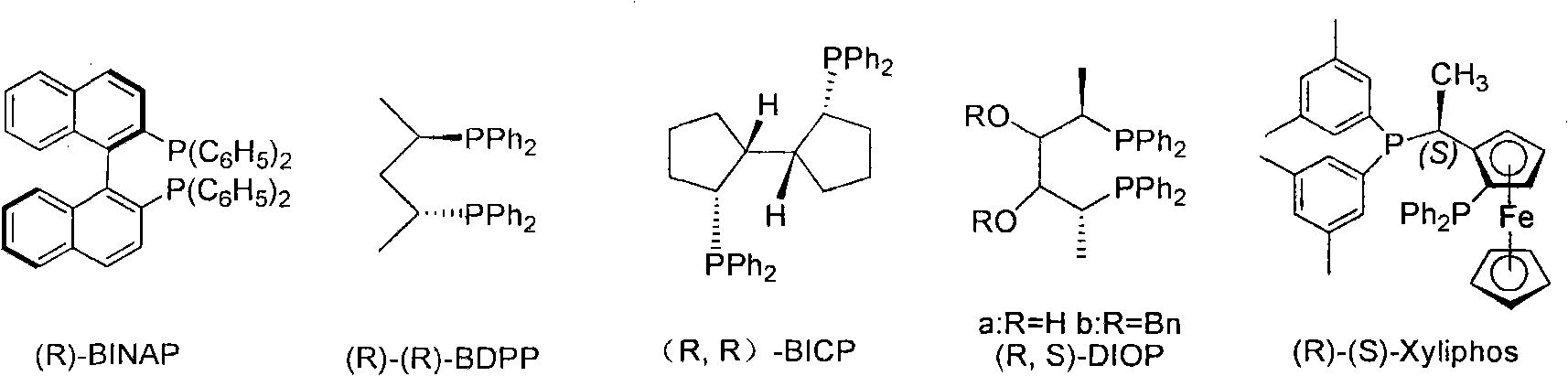
The invention relates to a chiral diphosphine ligand and a chiral catalyst, and a preparation and application method thereof. The preparation method of a BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) chiral diphosphine ligand based on novel imidazole and imidazole cation modification. Imidazole and imidazole cation are introduced to the 5,5'- position of the BINAP molecule framework to synthesize the chiral diphosphine ligand. The assembly of the imidazole and imidazole cation into the chiral diphosphine ligand molecule can effectively enhance the stability of the chiral catalyst in an ionic liquid and the affinity with the imidazole ionic liquid, and avoids loss of the catalyst in the cyclic process. The imidazole-modified chiral diphosphine ligand has the following chemical structural formula, wherein the spatial configuration of the imidazole-modified chiral diphosphine ligand is S type or R type.
Owner:山东聚强绿洲生物科技有限公司
Preparation method of ezetimibe and its intermediate
InactiveCN102952055AHigh catalytic efficiencyD.e. high valueOrganic chemistryBulk chemical productionDiphosphinesOxygen
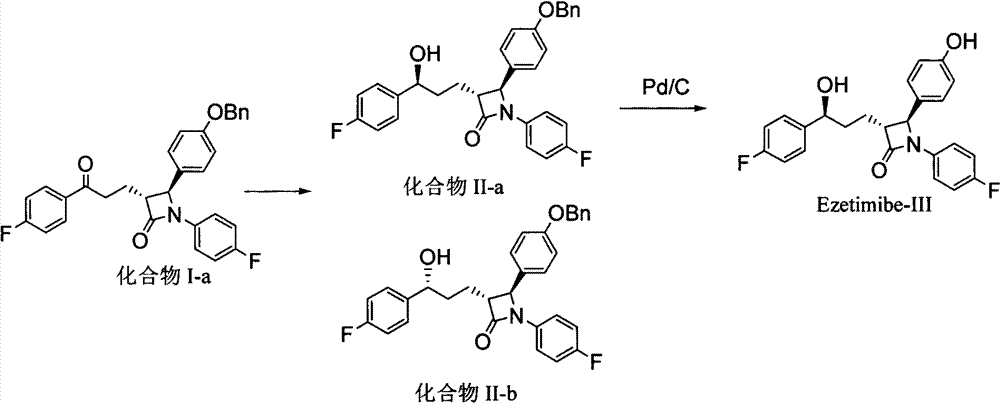
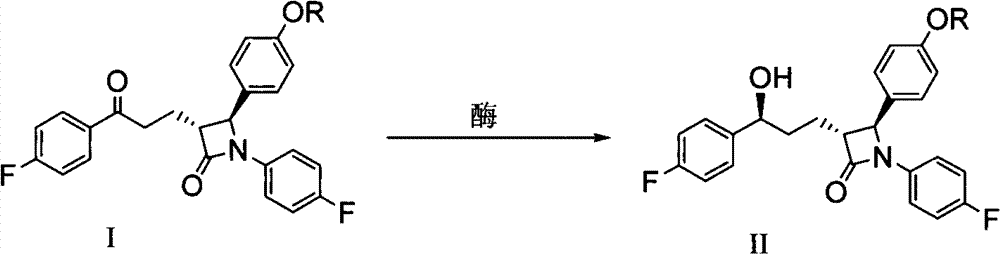
The invention discloses a preparation method of an ezetimibe intermediate compound as shown in formula II. The method includes the step of: in an organic solvent, in the absence of water and oxygen, and under the effects of an asymmetric hydrogenation catalyst and an organic alkali, subjecting compound I and hydrogen to an asymmetric catalytic hydrogenation reaction as shown in the following. Specifically, R is a conventional protecting group in the field, can protect a phenolic hydroxyl group and is stable under alkaline conditions. The asymmetric hydrogenation catalyst is a ruthenium-diphosphine-diamine catalyst invented by Ryoji Noyori, a Japanese scientist and a Nobel Prize winner. The preparation method provided in the invention has mild reaction conditions, can prepare products with high optical purity, yield and purity, and has after-treatment that is convenient to operate, thus being easy to realize industrialized production.
Owner:CHIRAL QUEST (SUZHOU) CO LTD
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
A process for the hydrogenation, using molecular hydrogen (H2) of a catalytic system, wherein the catalytic system includes a base and a complex of formula (II):Ru(P2N2)Y2 (II)wherein Y represent simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and P2N2 is a tetradentate diimino-diphosphine ligand.
Owner:FIRMENICH SA
Novel ruthenium complex and method for preparing methanol and diol
ActiveCN103772142AFacilitate large-scale industrial productionImprove efficiencyRuthenium organic compoundsOrganic compound preparationOrganic solventHydrogen atmosphere
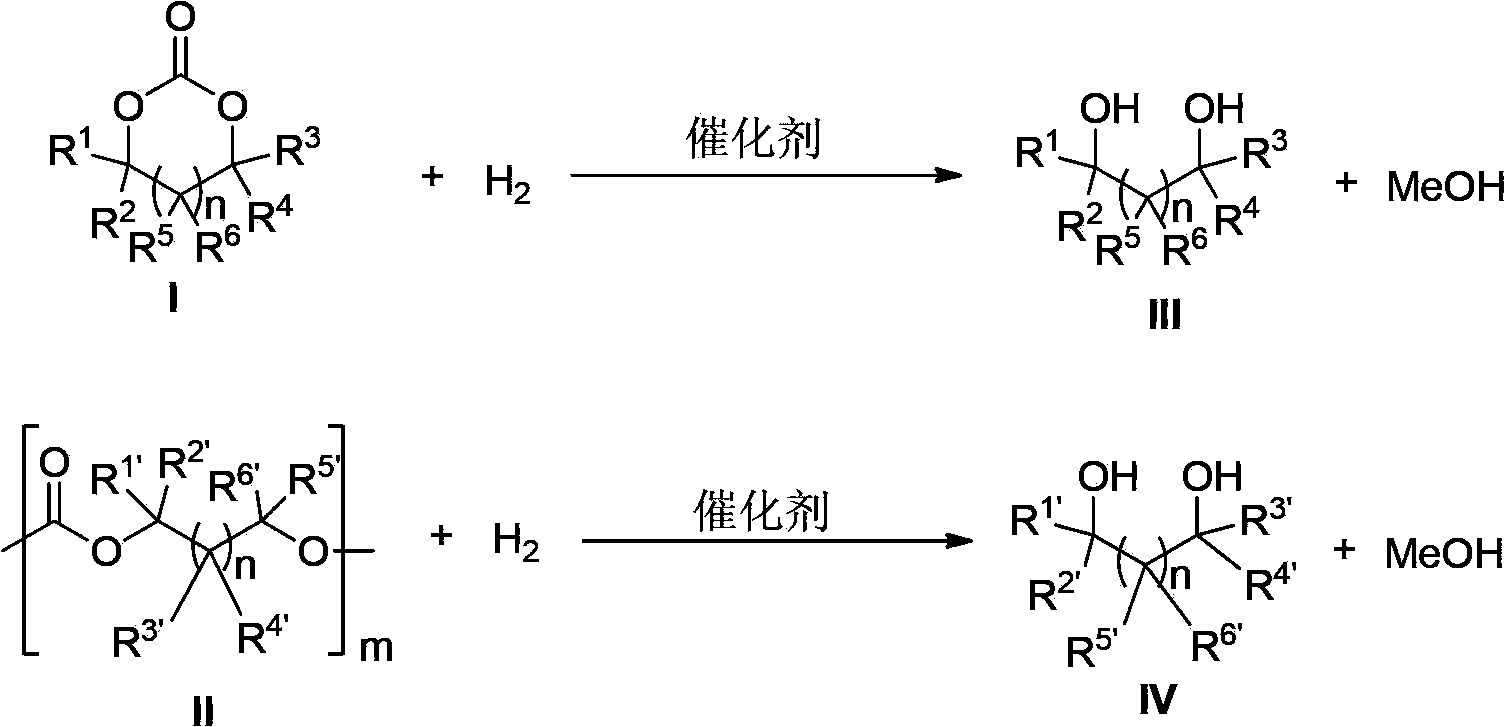
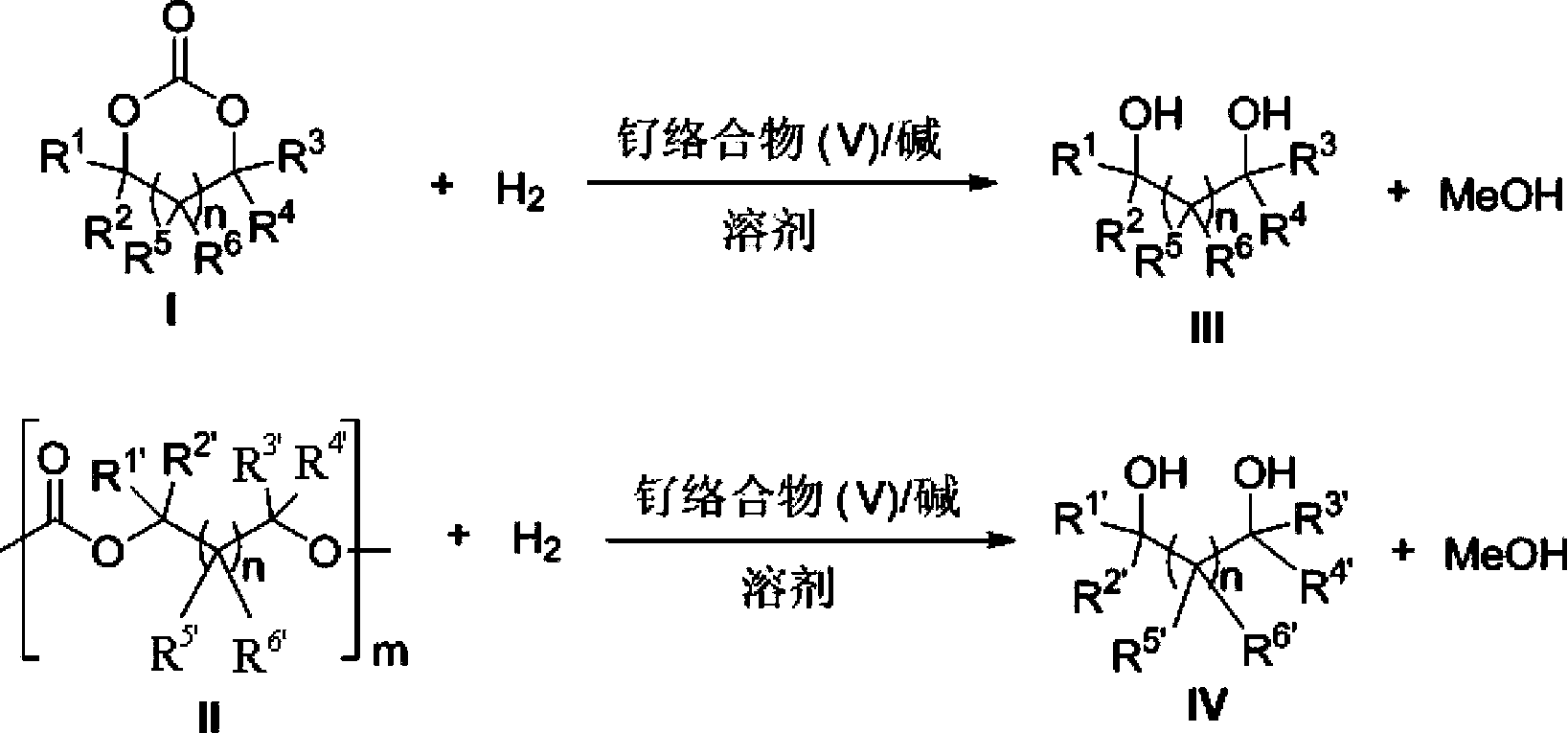
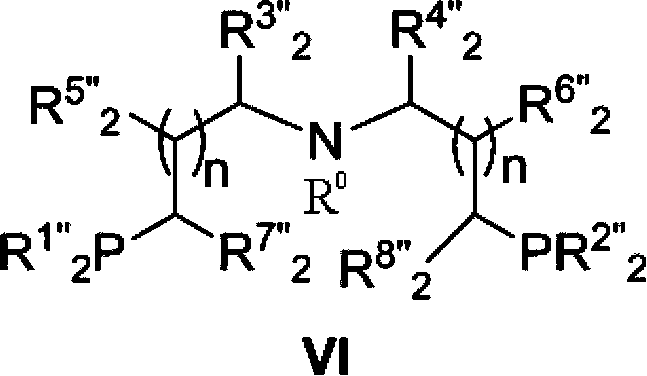
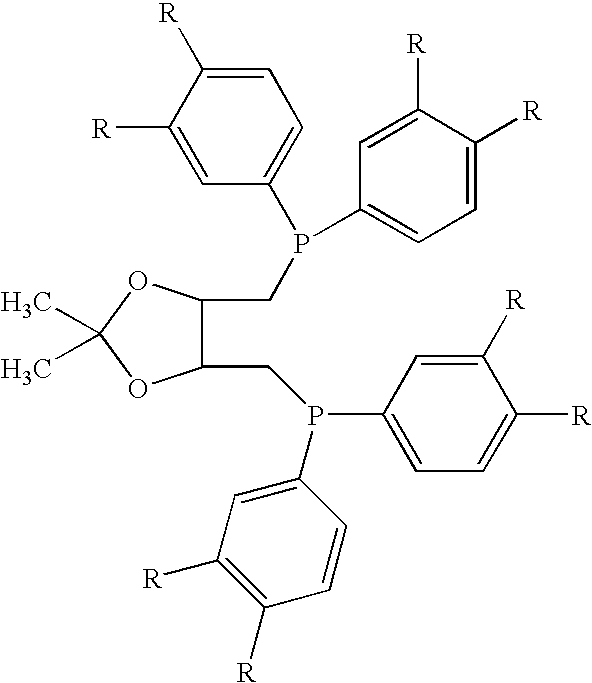
The invention provides a method for preparing methanol and diol from cyclic carbonates and polycarbonates. The method comprises: in hydrogen atmosphere, in an organic solvent, and in the presence of a ruthenium complex (Ru(L)XYY') and an alkali, performing hydrogenation reduction reaction on a cyclic carbonate or a polycarbonate, so as to obtain methanol and diol, wherein all groups in the formula are defined in the specification. The invention also provides the ruthenium complex formed by ruthenium and a tridentate amino diphosphine ligand. The invention also provides a method for preparing deuterated methanol and deuterated diol by employing the above preparation method. The method provided by the invention is high in efficiency, high in selectivity, economic, environment-friendly, and simple for operation, can be performed under mild conditions, and has complete atom economy.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Hydroformylation process
InactiveUS20100292514A1Raise the ratioOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenCyclobutane
A process for the production of 4-hydroxybutyraldehyde is described. The process comprises reacting allyl alcohol with a mixture of carbon monoxide and hydrogen in the presence of a solvent and a catalyst comprising a rhodium complex and a diphosphine. The diphoshine is a trans-1,2 -bis(bis(3,4-di-n-alkylphenyl)phosphinomethyl)cyclobutane and / or a 2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis[bis(3,4-di-n-alkylphenyl)phosphino]butane. The process gives high yield of 4-hydroxybutyraldehyde compared to 3-hydroxy-2-methylpropionaldehyde.
Owner:LYONDELL CHEM TECH LP
Nitrogen heterocycle ligand transition metal complex, and preparation and catalytic application thereof
ActiveCN102417523AThe synthesis method is simpleRuthenium organic compoundsOrganic compound preparationDiphosphinesKetone
The invention relates to a novel nitrogen heterocycle ligand / phosphine ligand transition metal complex, and preparation and application thereof in asymmetric catalytic hydrogenation and hydrogen transfer. The complex has the following structural formula: [MLnL' XY], wherein the transition metal M is Ru, Rh, Ir, Pd, Pt, Co, Ni or Os. The complex also contains a nitrogen heterocycle ligand, two monophosphine ligands or one diphosphine ligand, and the like. The transition metal compound, dinitrogen or mononitrogen ligand and diphosphine or monophosphine ligand react at 0-120 DEG C in an organic solvent for 0.5-20 hours to obtain the complex. The complex is used for asymmetric catalytic transfer hydrogenation or asymmetric hydrogenation reaction, and especially for asymmetric catalytic hydrogenation reaction of ketones, esters, hypnones and derivatives thereof, diphenyl ketones and derivatives thereof, beta-N,N-dimethylamino-alpha hypnones and derivatives thereof and other ketone compounds of which the alpha site is a large steric hindrance alkyl group.
Owner:ENANTIOTECH CORP
Ethylene tetrapolymerization catalyst composition and ethylene tetrapolymerization method
ActiveCN105562095AHigh polymerization reactivityRapid responseOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsArylOrganic solvent
The present invention discloses an ethylene tetrapolymerization catalyst composition, which comprises a diphosphine ligand represented by a formula I, a transition metal compound, an aluminum-containing co-catalyst, water and an organic solvent, wherein R1 and R2 are respectively and independently selected from alkyl, cycloalkyl and aryl, and R3, R4, R5 and R6 are respectively and independently selected from aryl and cycloalkyl. The invention further discloses a method for carrying out an ethylene tetrapolymerization reaction by using the catalyst composition. The formula I is defined in the specification.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalytic hydrogenation processes
ActiveUS20040063966A1High hydrogenation yieldHigh yieldRuthenium organic compoundsOrganic compound preparationHalogenHydrogen
The catalysts of formula (II): [Ru(L)m(L')wXY], wherein X and Y represent simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, m is 1 or 2, w is 1 when m is 1 and w is 0 when m is 2, L is a phosphino-amine or phosphino-imine bidentate ligand and L' a diphosphine, are useful for the hydrogenation of substrates having a carbon-hetero atom double bond.
Owner:FIRMENICH SA
Oligomerisation of ethylene to mixtures of 1-hexene and 1-octene
A process for the otigomerisation of ethylene to predominantly 1-hexene or 1-octene or mixtures of 1-hexene and 1-octene includes contacting ethylene with a catalyst under ethylene oligomerisation conditions. The catalyst comprises a source of chromium, a diphosphine ligating compound, and optionally an activator. The diphosphine ligating compound includes at least one optionally substituted fused cyclic structure including at least two rings, the optionally substituted fused cyclic structure including a 5- to 7- membered aromatic first ring bonded to a phosphorus atom, the aromatic first ring being fused to a 4- to 8-membered heterocyclic second ring, the heterocyclic second ring including a heteroatom which is separated by two ring atoms along the shortest connecting path from the phosphorous atom that is bonded to the first aromatic ring.
Owner:SASOL TECHNOLOGY (PTY) LTD
Method for preparing biphenyl diphosphine ligand
ActiveCN102010442ASimple and fast operationSuitable for industrial productionGroup 5/15 element organic compoundsButyl chlorideDiphosphines
The invention discloses a new method for preparing a biphenyl diphosphine ligand 2,2'-diphenylphosphinomethyl-1,1'-biphenyl (BISBI) and derivatives thereof. The method is characterized by comprising the following steps: (1) under the protection of inert gases, adding 40-60 parts of triphenylphosphine and 250-360 parts of ether solvents to a reaction flask, stirring the mixture to dissolve the mixture and adding 3-20 parts of alkali metals and 0-5 parts of electron-rich aromatics to the reaction flask to react for 2-24 hours at 20-120 DEG C to prepare a diphenulphosphine metal compound and a phenyl metal compound; (2) dropwise adding 15-20 parts of tertiary butyl chloride to the reaction solution to remove the generated phenyl metal compound; and (3) dropwise adding the solution formed by 15-40 parts of 2,2'-disubstituted methyl-1,1'-biaryl compound and 20-40 parts of ether solvent to the reaction solution with phenyl metal compound removed to react for 6-24 hours at 0-40 DEG C and carrying out extraction, drying, concentration and recrystallization to obtain the diphosphine ligand 2,2'-diphenylphosphinomethyl-1,1'-biphenyl (BISBI) and the derivatives thereof. The method has the characteristics of cheap and easily obtained raw materials and mild reaction conditions, is simple and convenient for operation, and is easy to realize industrial production.
Owner:成都欣华源科技有限责任公司
Chiral ferrocene diphosphine ligands, preparation method and application thereof
ActiveCN108929345AEasy to synthesizeStabilized oxygenOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFerroceneAsymmetric hydrogenation
The invention discloses chiral ferrocene diphosphine ligands. The ligands take chiral ferrocene amine as the raw material, after ortho-lithiation, the lithiated raw material reacts with a phosphine chloride to obtain a monophosphine intermediate, and the intermediate reacts with different secondary phosphines, thus obtaining chiral ferrocene diphosphine ligands with different chiral centers. The diphosphine ligands provided by the invention have the characteristics of novel structure, stability in air and simple synthesis, the complex catalysts formed by the diphosphine ligands and metals havestrong substrate universality, and show high catalytic activity and stereoselectivity in asymmetric hydrogenation reaction of latent chiral olefins, latent chiral ketones, latent chiral imines and the like.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000011.PNG)
![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000012.PNG)
![Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof Novel tri/tetra-coordination dual-core copper [I] coordination compound blue-green materials and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a12936d2-d316-4ce2-8f0f-eb88c0a93e3b/HDA0000504082740000021.PNG)