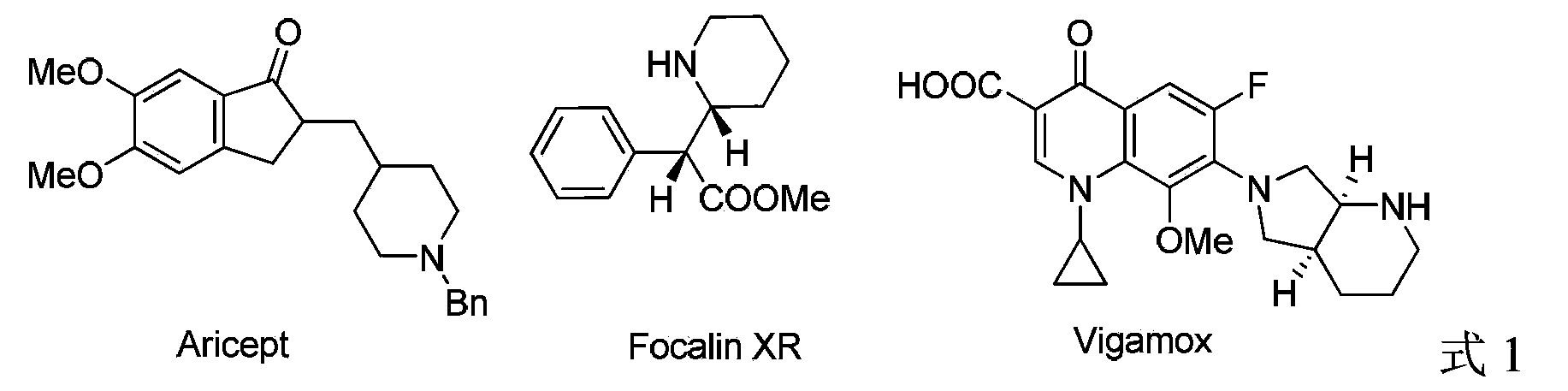
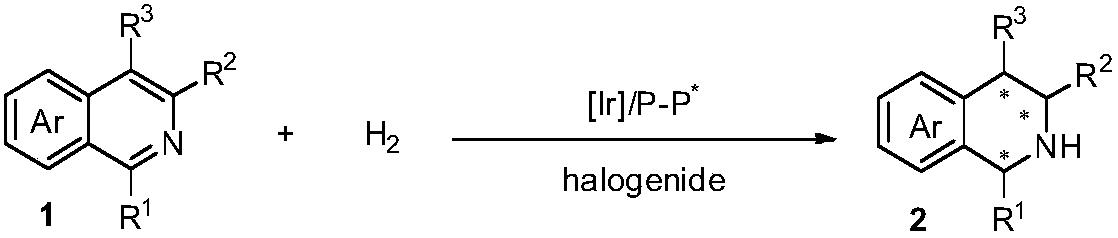Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Iridium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is useful for preparing other iridium compounds. The anhydrous salt is a dark green crystalline solid.
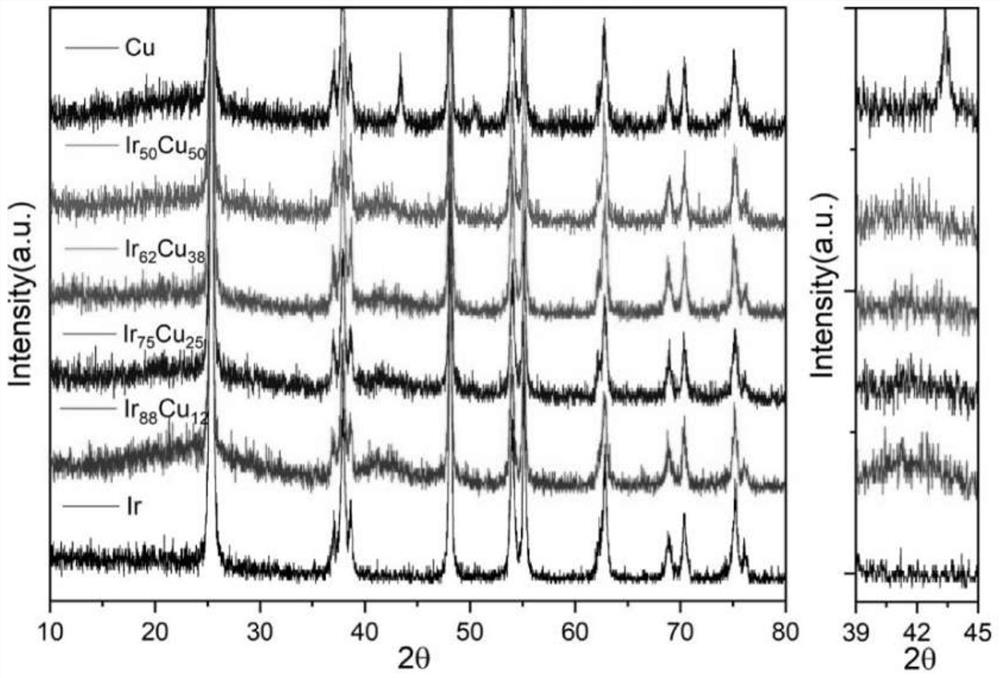
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
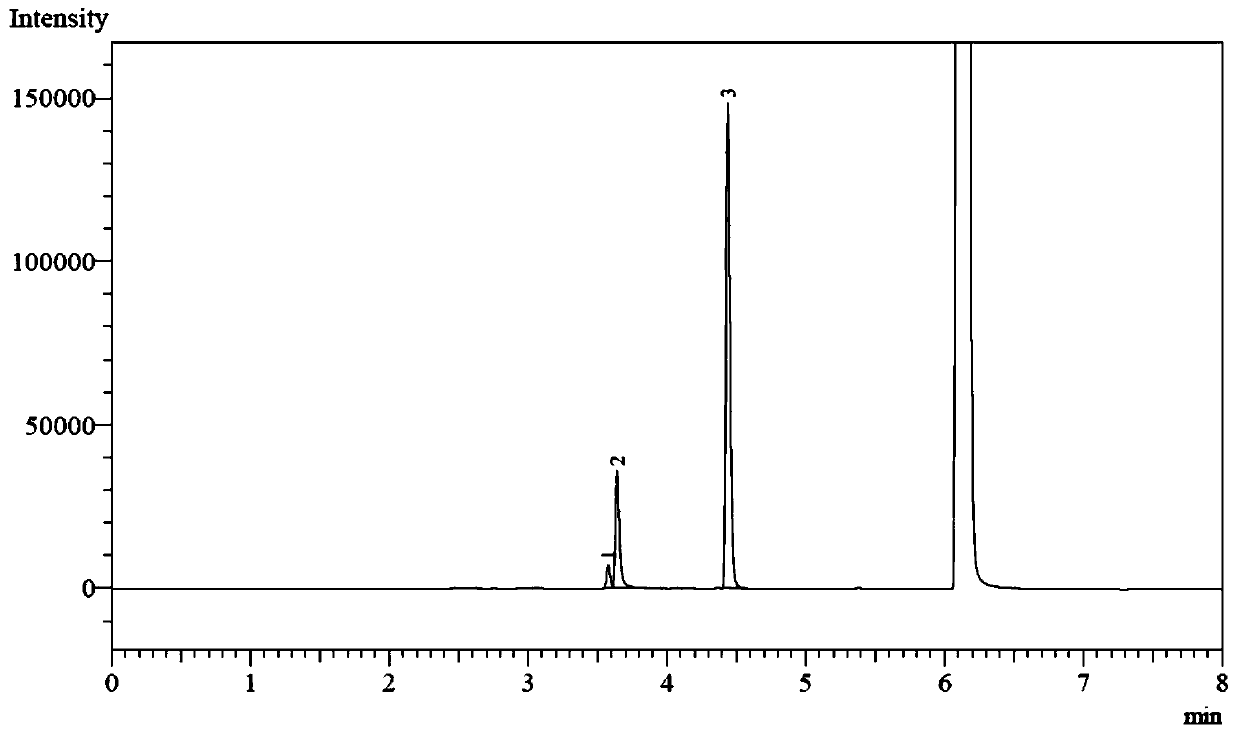
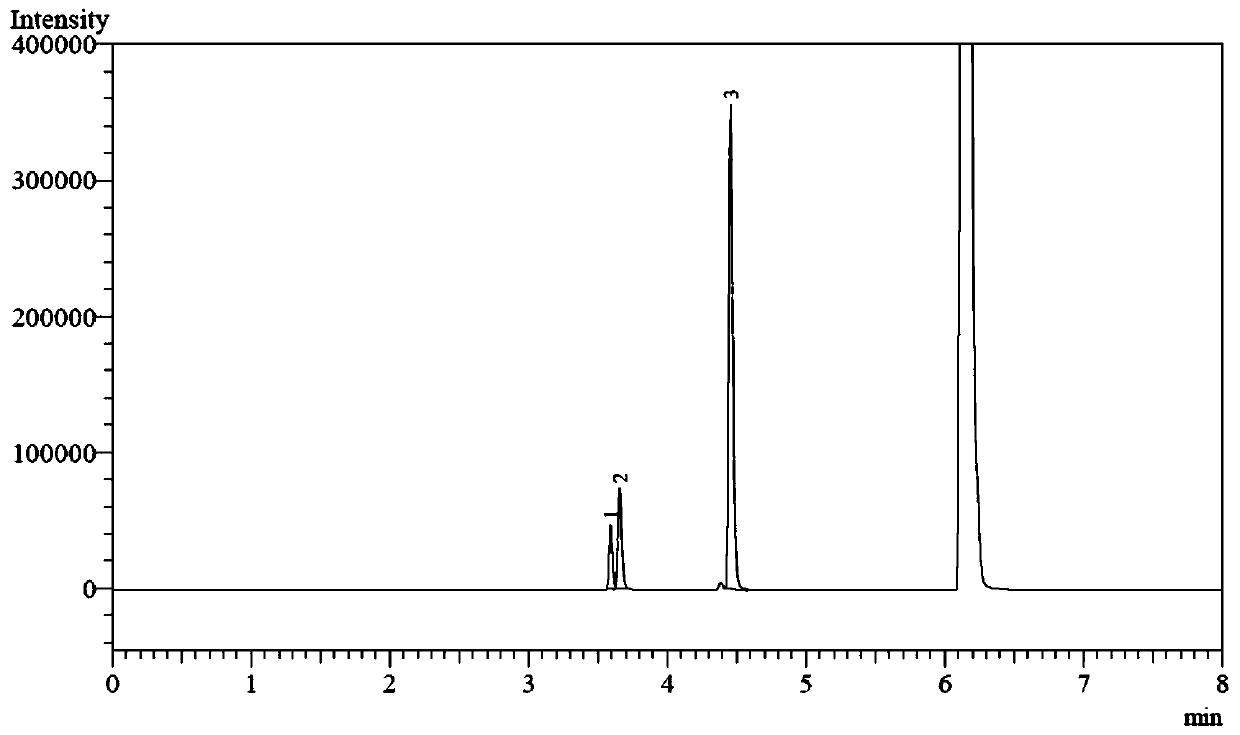
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
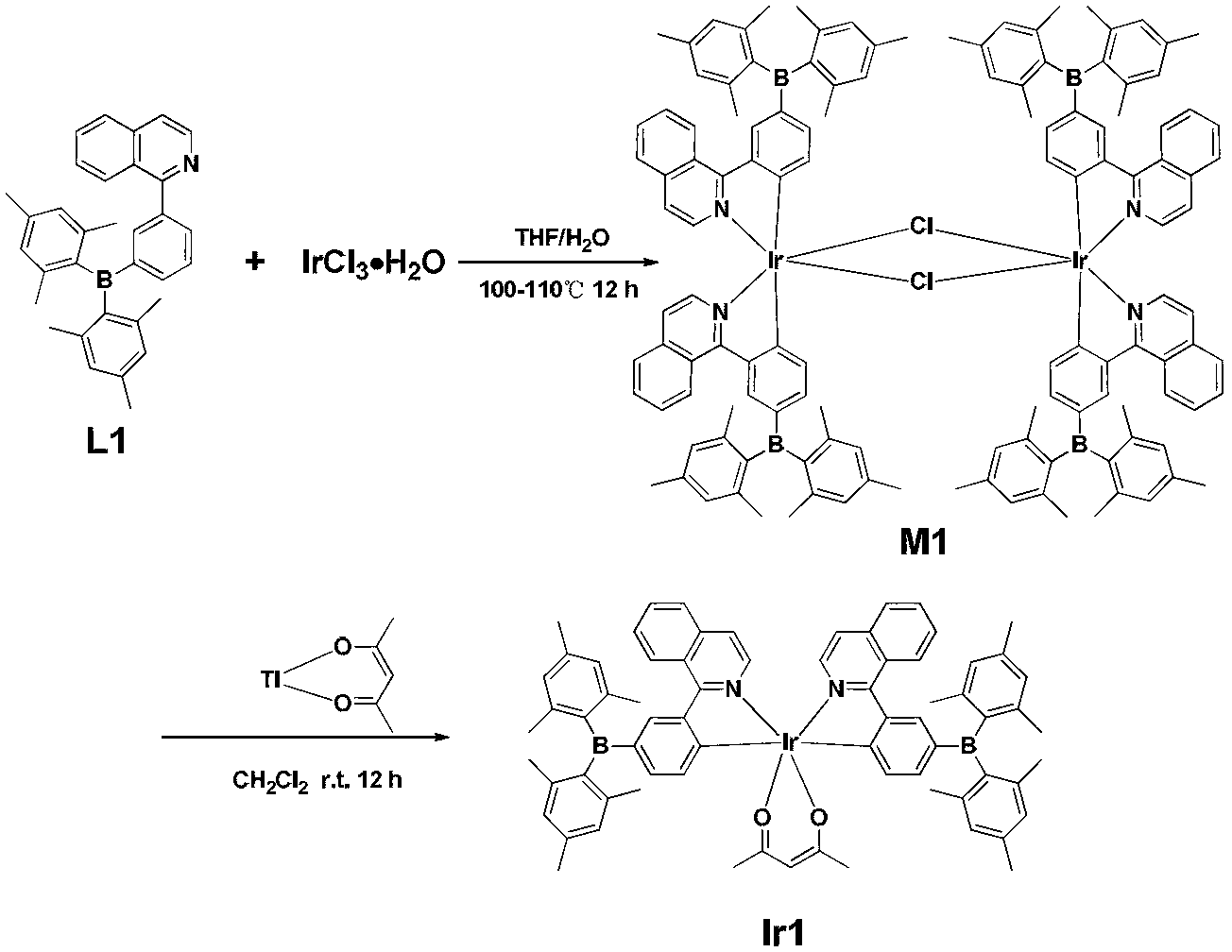
Organic electroluminescent iridium coordination compound and preparation method and application thereof
InactiveCN101759685AImprove thermal stabilityStrong electrical propertiesOrganic chemistrySolid-state devicesBenzaldehydeGlycerol
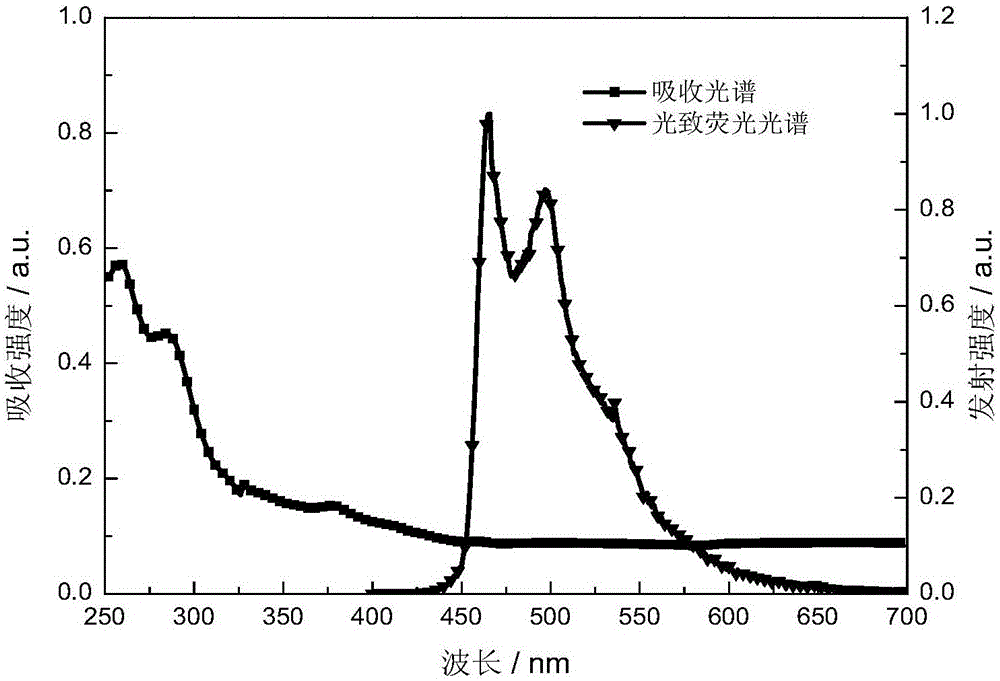
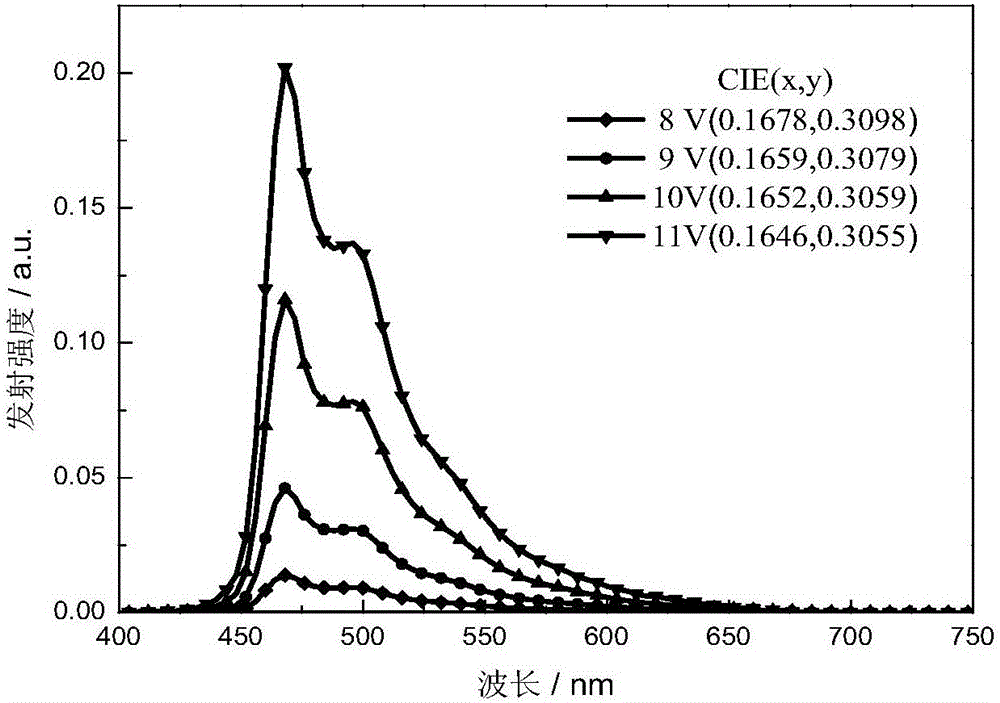
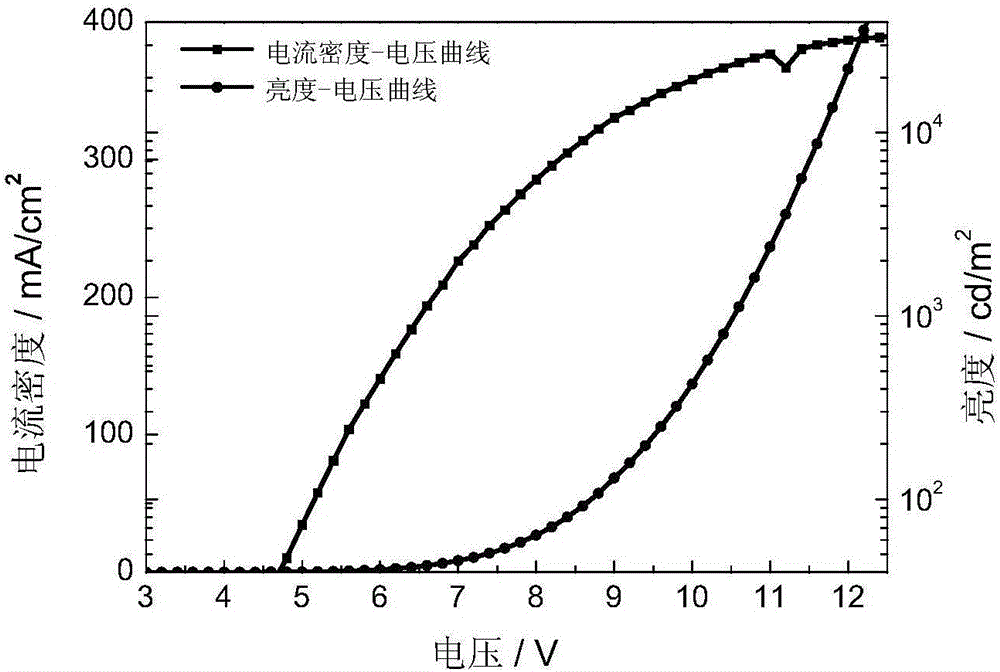
The invention provides an organic electroluminescent iridium coordination compound and a preparation method and application thereof, relating to an iridium-containing organic electroluminescent material and a preparation method thereof and application thereof. The invention solves the problems of the electroluminescent iridium coordination compound with a host-guest intergraded structure such as difficult synthesis, bad thermo-stabilization performance, bad solution processing performance and low electroluminescent efficiency. The coordination compound has the following formula. The method comprises the steps of: reacting benzaldehyde or ramification of the benzaldehyde with o-phenylenediamine; reacting a product after the reaction with hole transport group-substituted bromine to obtain ligand; reacting the ligand with iridium chloride; and reacting a product with glycerol and potassium carbonate to obtain the coordination compound. The organic electroluminescent iridium coordination compound is used in an organic electroluminescent device. The thermal decomposition temperature of the coordination compound is 402 DEG C, the synthesis is easy, the current density of the electroluminescent device is 200-450 mA / square centimeter, the luminosity is 2800-11000 cd / square meter, and the maximum external quatum efficiency is 6%.
Owner:HEILONGJIANG UNIV
Norbornene, styrene and nikasol ternary polymerization catalyst and ternary polymerization method
The invention relates to a norbornene, styrene and nikasol ternary polymerization catalyst and a ternary polymerization method. The preparation method of the catalyst comprises the steps that (pentamethyl cyclopentadiene) iridium chloride, aluminum alkyl and a ligand are dissolved into a first solvent in a dry three-mouth flask in the inertia atmosphere, stirring is performed at the constant temperature of 20-50 DEG C at the speed of 110-130 rpm for 20-50 min, and an iridium-aluminum complex catalyst is obtained. The polymerization method comprises the steps that a norbornene monomer, a styrene monomer and a nikasol monomer are taken and added into a reaction kettle, and a second solvent is added for dissolution; then, the iridium-aluminum complex catalyst is added, a reaction is performed under the certain stable pressure, and the product is washed to obtain the terpolymer. Catalytic activity can be generated at low temperature, norbornene, styrene and nikasol ternary polymerization is catalyzed, the reaction temperature is low, the catalysis efficiency is high, the copolymer yield is high, and the terpolymer is good in photoetching performance.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
Method for synthesis of chiral piperidine derivative through iridium-catalyzed asymmetric hydrogenation of pyridine
ActiveCN103387533AHigh reactivityHigh enantioselectivityAsymmetric synthesesDiphosphinesIridium chloride
A method for synthesis of chiral piperidine derivatives through iridium-catalyzed asymmetric hydrogenation of pyridine employs a catalytic system of chiral diphosphine complex of iridium. The reaction can be carried out under the following conditions: temperature of 25-60 DEG C; a mixed solvent of toluene / dichloromethane (V / V=1:1); pressure of 13-50 barometric pressure; a ratio of substrate and catalyst of 50 / 1; and a catalyst of a complex of (1,5-cyclo-octadiene) iridium chloride dimer and diphosphine ligand. Hydrogenation of pyridine salt can obtain a corresponding chiral 2-substituted piperidine derivative, whose enantiomeric excess can reach 93%. The invention has advantages of simple and practical operation, easily available raw materials, high enantioselectivity, and good yield; in addition, the reaction has green atom economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
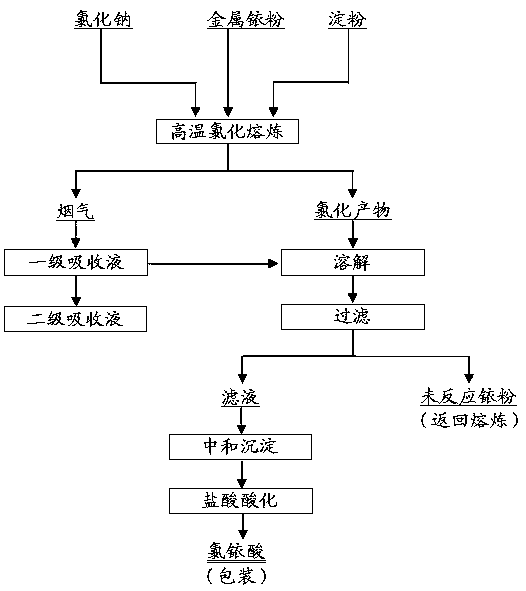
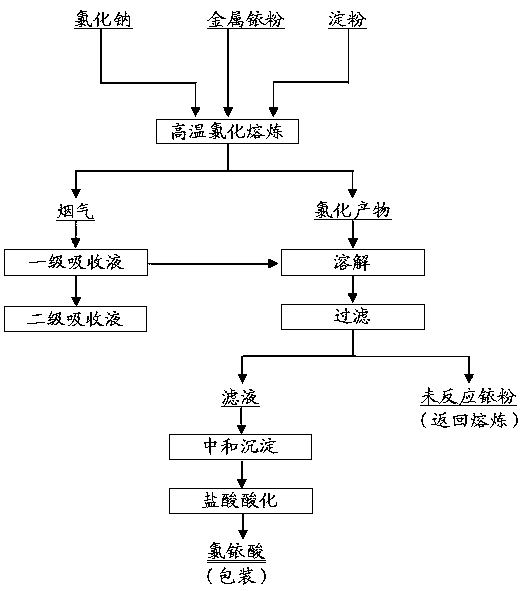
Method for preparing chloroiridic acid
ActiveCN107758752AFully convertedPromote conversionRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsPhysical chemistryHearth
The invention relates to a method for preparing chloroiridic acid. According to the method disclosed by the invention, firstly, metal iridium powder and sodium chloride are fused at a high temperature, meanwhile, a little amount of starch is added, chlorine is introduced into the fused melt, and most iridium powder is transformed into iridium sodium chloride by a measure of chlorine strengthened stirring; a little part of produced iridium chloride gas is absorbed by using a diluted hydrochloric acid solution; iridium sodium chloride obtained by high-temperature chlorination smelting reaction is merged with a diluted hydrochloric acid absorbing solution, and then sodium hydroxide is added for neutralization to produce iridium oxide, a product is filtered and washed for multiple times to remove sodion, hydrochloric acid is added and dissolved and then concentration is performed to prepare the chloroiridic acid solution. According to the invention, chlorine is introduced into a melt and stirred adequately, and by a measure of smelting in a furnace hearth, transformation of iridium is efficiently promoted, and the leaching efficiency of iridium powder reaches 80% or above; by adding starch, passivation of the surface of the iridium powder in the heating process can be avoided, sufficient transformation of iridium powder is promoted, and by performing secondary absorption on tail gas, comprehensive utilization of a hydrochloric acid absorbing solution is realized, and the comprehensive recovery rate of iridium is increased.
Owner:CENT SOUTH UNIV
Tantalum ruthenium mixing type electrolytic capacitor and preparation method thereof
The invention discloses a tantalum ruthenium blending electrolytic capacitor applied for high power and a process for preparation belonging to the electrolytic capacitor preparing technical field. The structure of the blending capacitor comprises a barred, slice and slice series capacitor structure and sintering tantalum anode. The electrolytic solution and the ruthenium oxide cathode are sealed in the tantalum housing to form the tantalum ruthenium blending electrolytic capacitor which are both equipped with the characteristics of tantalum electrolytic capacitor and ruthenium oxide super capacitor. The sintering anode utilizes high specific volume tantalum powder as raw material and prepares for slice anode after process flows of weighing, forming, sintering, energizing and the like. The ruthenium oxide cathode utilizes titanium metal as electrode basal body, ruthenium chloride, and iridium chloride and tetrabutyl titanate as reacting precursors, employs oxygenizing and sintering treatment with 380 DEG C to obtain metal oxide-coated cathode, the ruthenium oxide cathode surface prepares resin micro-projecting dot matrix to replace traditional membrane. The invention is expected to have wide application in various fields of electronics, cars, astronavigation, military and the like.
Owner:TSINGHUA UNIV
Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one
InactiveCN103288829AHigh reactivityHigh enantioselectivityAsymmetric synthesesMorpholineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 20-50 DEG C; the solvent is a dichloromethane-toluene mixed solvent (V / V=1:2); the pressure is 13-50 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; the catalyst is a complex of (1,5-cyclooctadiene)iridium chloride dimer and diphosphine ligand; and the additive is morpholine trifluoroacetate or piperidine hydrochloride. Seven-element cyclic pyrrolo[2,1-c][1,4]-benzodiazepino-5-one is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 96%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine
InactiveCN103288828AHigh reactivityHigh enantioselectivityOrganic chemistryBenzodiazepineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 0-50 DEG C; the solvent is benzene or toluene; the pressure is 13-60 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; and the catalyst is a complex of (1,6-cyclooctadiene)iridium chloride dimer and diphosphine ligand. Seven-element cyclic indolo[2,1-c][1,4]-benzodiazepine is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 89%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Iridium rhodium nanosheet assembly electrocatalyst for catalyzing hydrogen evolution reaction and preparation method thereof
InactiveCN111001405AHigh yieldSimplify the experimental stepsMaterial nanotechnologyCatalyst activation/preparationPtru catalystIridium chloride
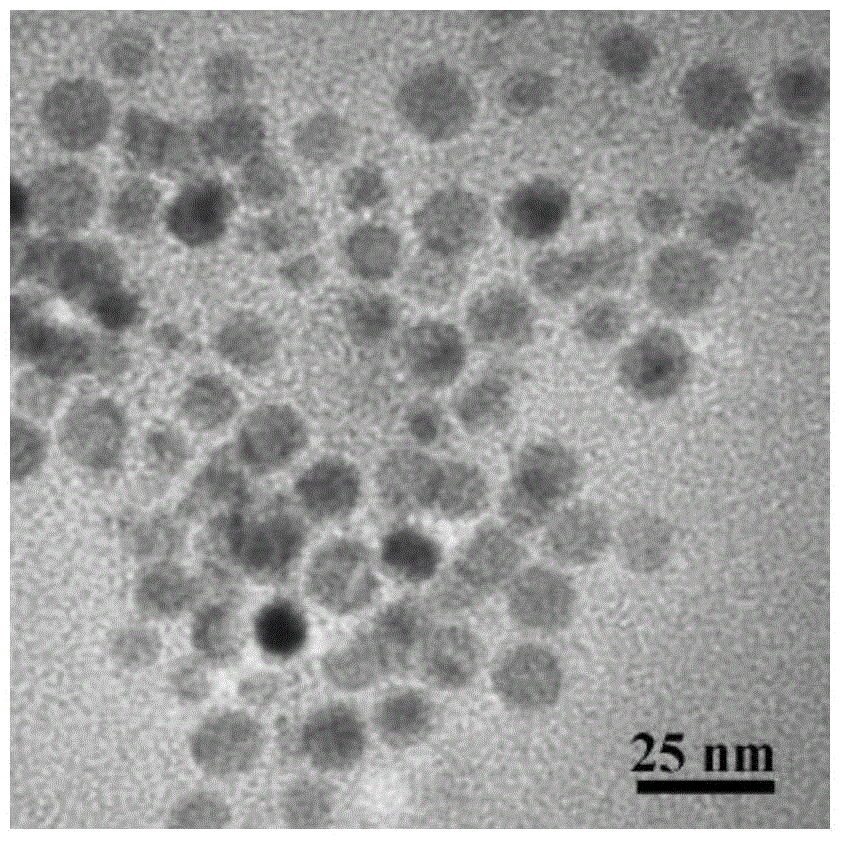
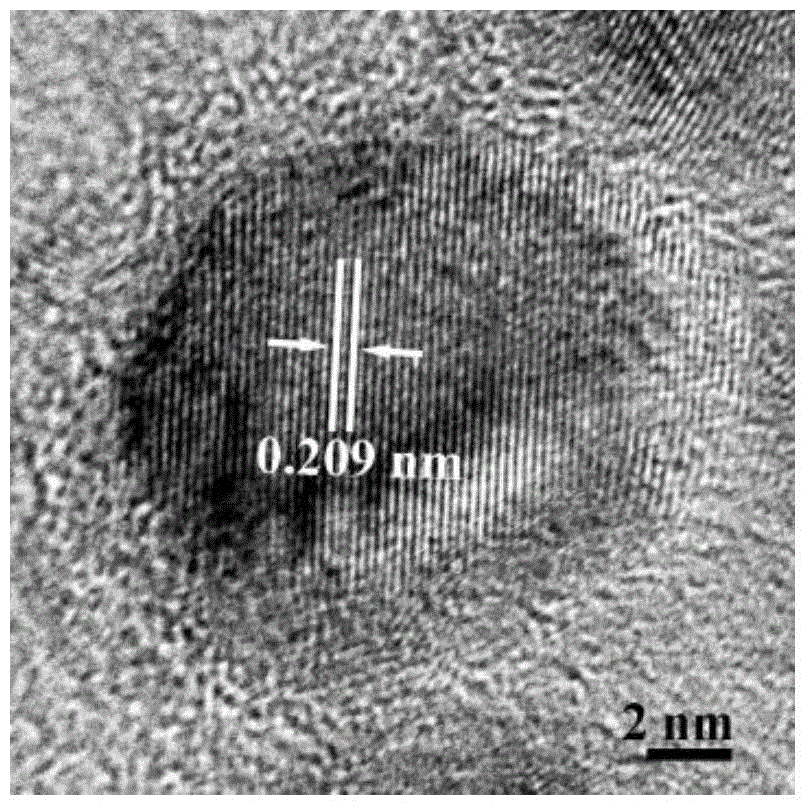
The invention relates to an iridium rhodium nanosheet assembly electrocatalyst for catalyzing hydrogen evolution reaction and a preparation method thereof. The preparation method comprises the following steps: respectively preparing a iridium chloride solution and a rhodium chloride solution with the concentrations of 5-100mM; taking 0.5-3 mL of N, N-dimethylformamide , and dissolving 2-20 mg of PS-b-PEO in N, N-dimethylformamide; then adding the iridium chloride solution and the rhodium chloride solution with the total volume of 2mL; finally, adding 0.2-5mL of a formic acid solution, and uniformly mixing; after solution fully mixing, putting an obtained mixed solution into a water bath kettle, heating to 40-95 DEG C, reacting for 2-10 hours, carrying out centrifugal separation, collectinga precipitate, and washing to obtain the iridium rhodium nanosheet assembly electrocatalyst. The operation steps are simple, the reaction conditions are mild, and the iridium rhodium nanosheet assembly prepared through the one-step method has excellent electro-catalytic hydrogen evolution reaction performance.
Owner:ZHEJIANG UNIV OF TECH
Diadema setosum-type PdCuIr nitrogen reduction electrocatalyst and preparation method thereof
ActiveCN109174122AHigh yieldEasy to synthesizeCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsCopper chlorideCentrifugation
The invention discloses a diadema setosum-type PdCuIr nitrogen reduction electrocatalyst and a preparation method thereof. The preparation method comprises: dissolving sodium chloropalladite, copper chloride and iridium chloride in deionized water, controlling concentrations in a range of 1 and 40 mM, mixing the solution, adding 10 to 500 mg of KBr, 10 to 100 mg of a surfactant F127, 0.5 to 5 mL of ascorbic acid having a concentration of 0.01 to 0.1 mol / L and 0.1 to 1 mL of a HCl solution with a concentration of 1 to 10 mol / L into the mixture, carrying out ultrasound treatment for 5 to 15 minto obtain a clear solution, putting the clear solution in an oil bath pot, carrying out a reaction process at 80 to 120 DEG C for 5 to 90 min, and carrying out washing, centrifugation and drying to obtain the diadema setosum-type PdCuIr nitrogen reduction electrocatalyst. The preparation method has simple processes and very short reaction time. The diadema setosum-type PdCuIr nitrogen reduction electrocatalyst has excellent electrochemical synthetic ammonia performances.
Owner:ZHEJIANG UNIV OF TECH
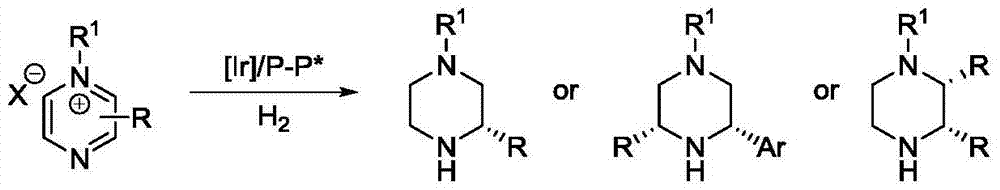
Iridium catalytic method for asymmetric hydrogenation to synthesize piperazine derivatives
InactiveCN106995413AHigh reactivityHigh enantioselectivityOrganic chemistry methodsCatalytic methodPyrazine
In an iridium catalytic method for asymmetric hydrogenation to synthesize piperazine derivatives, an iridium chiral diphosphine complex is used as a catalyst. The reaction is carried out in the following condition that the temperature is in a range of -20 to 70 DEG C, the solvent is toluene, tetrahydrofuran and ethyl acetate, the pressure is 10-80 times of atmospheric pressure, the ratio of a substrate to the catalyst is 50 / 1, and the catalyst is a complex of (1,5-cyclo-octadiene) iridium chloride dimer and chiral diphosphine. 3-substitued, 3,5-disubstitued, and 2,3-disubstitued pyrazine salt can all be well hydrogenated to obtain corresponding chiral piperazine derivatives, with the highest enantiomeric excess reaching 96%. The method is simple to operate, available in raw materials, high in enantioselectivity, and good in yield, and an atom-economic eco-friendly route is provided for synthesizing 3-substitued, 3,5-disubstitued, and 2,3-disubstitued piperazine derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
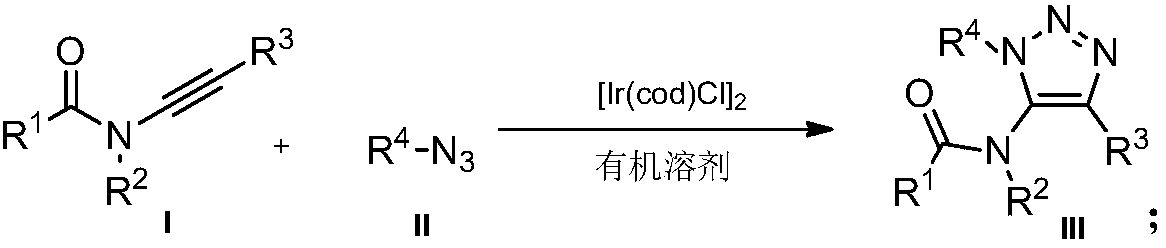
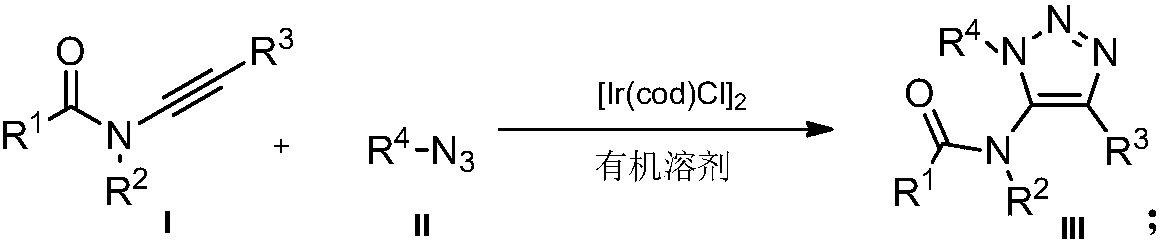
Preparation method of novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole
InactiveCN107721984AMild reaction conditionsReaction conditions greenOrganic chemistryOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis, and relates to a preparation method of novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole. The novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole is prepared by catalyzing a ynamine compound and nitrine in an organic solvent and under the action of a catalyst of 1,5-cyclooctadiene iridium chloride dipolymer ([Ir(COD)Cl]2). The preparation method of a 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole product is mild in reaction conditions, and the yield of the product is not lower than 70 percent; the preparation method is mild in reaction conditions, is green and is high in reaction efficiency and is more suitable for large-scale production requirements, and a prepared novel 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole compound has potential physiological activity.
Owner:DALIAN UNIV OF TECH
Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine
InactiveCN103288830AHigh reactivityHigh enantioselectivityAsymmetric synthesesBenzodiazepineDiphosphines
The invention relates to a method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine by iridium-catalyzed asymmetric hydrogenation. The reaction conditions are as follows: the temperature is 20-50 DEG C; the solvent is benzene or toluene; the pressure is 13-50 atmospheric pressures; the ratio of the substrate to the catalyst is 50 / 1; and the catalyst is a complex of (1,5-cyclooctadiene)iridium chloride dimer and diphosphine ligand. Seven-element cyclic pyrrolo[2,1-c][1,4]-benzodiazepine is hydrogenated to obtain the corresponding chiral dihydro product, and the enantiomeric excess can reach 96%. The invention is simple and practical to operate, and has the advantages of accessible raw materials, favorable selectivity for antipode, high yield, atomic economical efficiency for reaction, and environment friendliness.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of Cu-Ir alloy polyhedral nano cage
InactiveCN105664965AHigh oxygen generation reaction electrocatalytic activityMaterial nanotechnologyElectrolysis componentsElectrolysisCopper acetylacetonate
The invention belongs to the technical field of noble metal nano particle synthesis and especially relates to a preparation method of a Cu-Ir alloy polyhedral nano cage having high catalytic activity in water electrolysis for producing oxygen. The method includes the steps of adding copper acetylacetonate in oleamine, increasing the temperature gradually to 270 DEG C under magnetic stirring with protection by nitrogen gas, maintaining the temperature for 15 min, reducing the temperature to an injection temperature, injecting an oleamine solution of iridium chloride, and maintaining the injection temperature for 2 h to obtain the Cu-Ir alloy polyhedral nano cage. The method is simple and convenient. The Cu-Ir alloy polyhedral nano cage has high electrically catalytic activity in an oxygen production reaction.
Owner:JILIN UNIV
Preparation method of platinum-precious metal-copper ternary alloy nano hollow cube
InactiveCN108520965AGood size controlShape is easy to controlMaterial nanotechnologyCell electrodesPotassiumIridium chloride
The invention discloses a preparation method of a platinum-precious metal-copper ternary alloy nano hollow cube. The method takes sodium polyacrylate as a stabilizer and ascorbic acid as a reducing agent, a cuprous oxide nano cube is synthesized firstly, then the cuprous oxide nano cube serves as a template, potassium chloropalladite, precious metal salt (such as chloroauric acid, rhodium chlorideand iridium chloride) are subjected to galvanic displacement with cuprous oxide, and the platinum-precious metal-copper ternary alloy nano hollow cube with the regular shape, uniform size and good dispersibility and stability is obtained. The preparation method is simple and economic, the prepared platinum-precious metal-copper ternary alloy nano hollow cube has the excellent electrocatalytic activity and stability on the ethanol catalytic reaction, has the potential application prospect in the field of direct ethanol fuel cells, and is suitable for industrialized large-scale production.
Owner:SHAANXI NORMAL UNIV
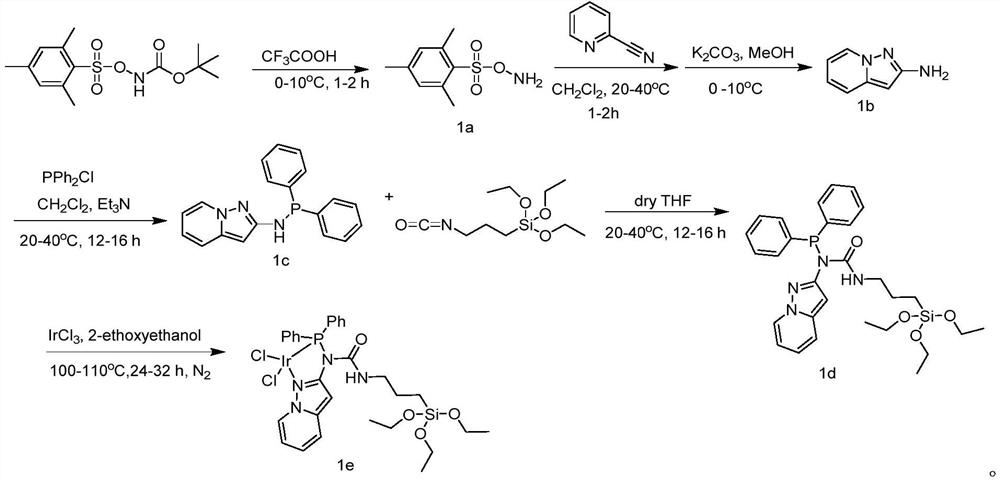
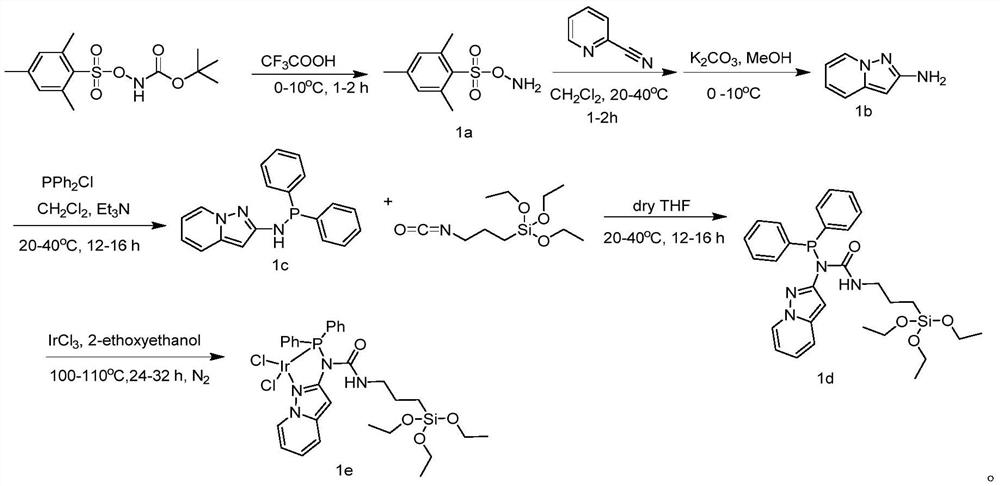
Carbene iridium catalyst and preparation method and application thereof
ActiveCN111068785AReduced stabilityImprove stabilityIndium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEthylic acid
The invention provides a carbene iridium catalyst and a preparation method and application thereof, the carbene iridium catalyst has good application in preparation of methyl acetate through methanolcarbonylation, and the carbene iridium catalyst is mainly prepared from a CAAC ligand, 1, 5-cyclooctadiene iridium chloride and bis(trimethylsilane) amino potassium as raw materials. The preparation method comprises the following steps: drying raw materials for preparing the carbene iridium catalyst, replacing with nitrogen, sequentially adding acetone and liquid nitrogen, performing reacting at-78 DEG C, and extracting a reaction product with a solvent. According to the invention, the CAAC-IrCl (COD) catalyst of the CAAC ligand with relatively strong electron donating capability is applied tothe aspect of preparing methyl acetate through methanol carbonylation, so that the stability of the catalyst is improved, the methyl acetate is directly prepared under relatively mild and anhydrous conditions, the preparation process is simplified, and the operation cost is reduced.
Owner:NANJING YANCHANG REACTION TECH RES INST CO LTD +1
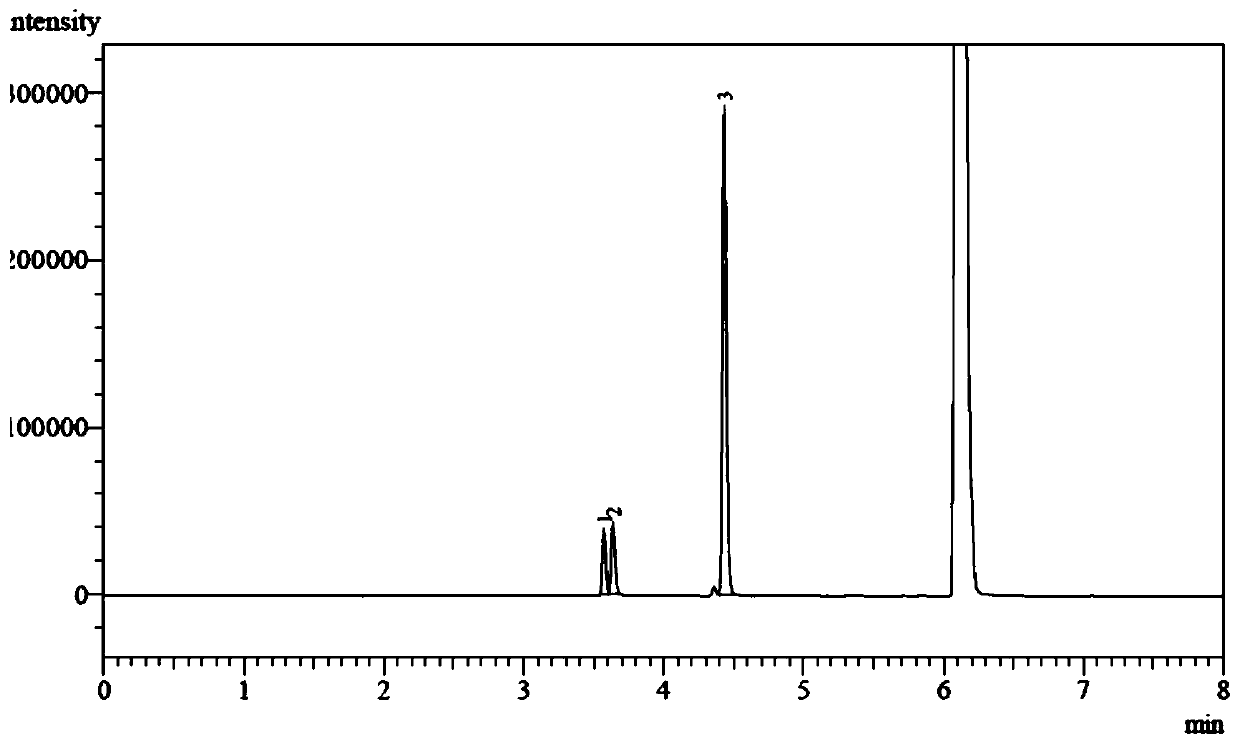
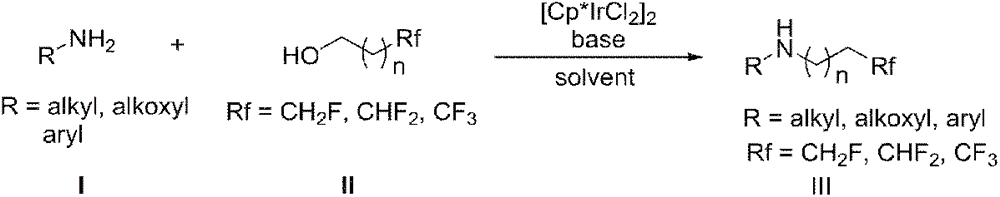
Method for preparing fluorine-containing secondary amine
InactiveCN106748802AHigh yieldEasy to getOrganic compound preparationAmino compound preparationPentamethylcyclopentadieneAlcohol
The invention discloses a synthetic method of a fluorine-containing secondary amine compound. Pentamethylcyclopentadienyl iridium chloride dimer is used as a metal catalyst, fluoroalkyl comes from fluorinated alcohol, alkali is used as a co-catalyst, and the fluorine-containing secondary amine is prepared via the following step: primary amine reacts with the fluorinated alcohol under the action of the transition metal iridium catalyst and the alkali in an organic solvent to obtain the fluorine-containing secondary amine compound. The method overcomes the defects in the prior art that the fluorine-containing alkylating agent is high in toxicity and instable and needs to be prepared in advance, the reaction operation is complex, the steps are fussy and the like, and can obtain considerable yield. The adopted fluorinated alcohol is readily available, stable in chemical property and low in toxicity. The method is simple in reaction, has very high atom economy and step economy, and is energy-saving, environment-friendly and simple in operation. By adopting [Cp*IrCl2]2 as the catalyst, ligands are not needed, and the system is easy to realize.
Owner:NANJING UNIV OF SCI & TECH
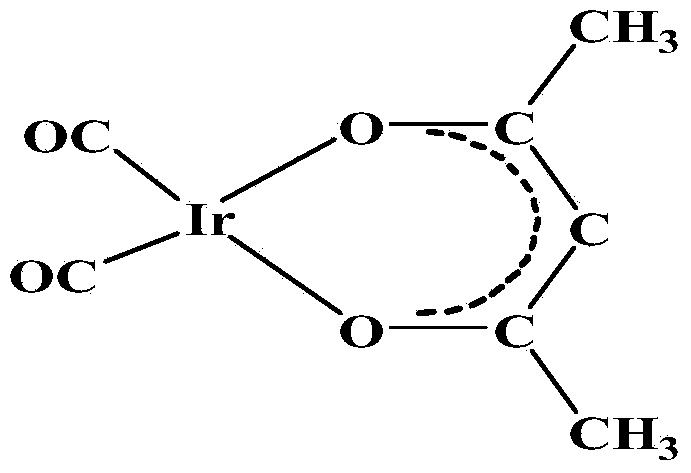
Method for preparing dicarbonyl iridium acetylacetonate (I)
InactiveCN104230999AShort reaction timeHigh yieldGroup 8/9/10/18 element organic compoundsIridium chlorideHomogeneous catalysis
The invention discloses a method for preparing dicarbonyl iridium acetylacetonate (I). Dicarbonyl iridium acetylacetonate (I) is a precursor compound for preparing an iridium coating by MOCVD (metal-organic chemical vapor deposition), and is also a common carbonyl addition homogeneous catalyst. The method mainly comprises the following steps: (1) reacting iridous chloride hydrate and 1,5-cyclooctadiene to generate an intermediate (1,5-cyclooctadiene) iridium chloride (I) dimer; and (2) reacting the intermediate and carbon monoxide to introduce carbonyl group, reacting with acetylacetone to obtain the dicarbonyl iridium acetylacetonate (I). The preparation method has the advantages of simple technical process, high yield and high product purity, and is suitable for mass preparation of [Ir(CO)2(acac)].
Owner:KUNMING INST OF PRECIOUS METALS
Catalyst for synthesizing replacement ketone compounds and preparation method
ActiveCN111974457AStrong catalytic efficiencyImprove catalytic abilityIndium organic compoundsOrganic compound preparationPtru catalystOrganic synthesis
The invention discloses a catalyst for synthesizing replacement ketone compounds and a preparation method, and belongs to the field of chemical materials and medicines. A nitrogen-phosphorus-containing ligand is obtained through organic synthesis, the ligand is combined with iridium chloride and then loaded on a carrier of MCM-41 to prepare an iridium catalyst containing the MCM-41 loaded with thenitrogen-phosphorus-containing ligand. The catalyst prepared by the preparation method can be used to catalyzed synthesis of the replacement ketone compounds and synthesis of bisphenol F. The novel iridium heterogeneous catalyst containing the nitrogen-phosphorus-containing ligand is an environmentally friendly catalyst, compared with previous experimental schemes for synthesizing ketones, the catalyst has the advantages that the catalytic efficiency is high, the reaction conditions are mild, and the catalyst can be reused, and the catalyst is in line with the development concept of environmental protection and atomic economy; and when the catalyst is used in the synthesis reaction of bisphenol F, the catalytic activity is high, the application range is wide, and the application prospectsare good.
Owner:JIANGNAN UNIV
Controlled reduction preparation method of iridium trichloride
ActiveCN106854001AAvoid introducingHigh purityRuthenium/rhodium/palladium/osmium/iridium/platinum halidesRefluxIridium chloride
Belonging to the technical field of reduction, the invention discloses a controlled reduction preparation method of iridium trichloride. The method includes: adding a quadrivalent iridium chloride and a reducing agent in a three-mouth flask according to certain proportion, under a magnetic stirring condition, and conducting constant temperature reduction until quadrivalent iridium in the solution is completely converted to trivalence. A condensation reflux device is used in the reaction process to reduce the volatilization of the reducing agent and control the reaction system stable. The method has the advantages of simple operation, short process, fully controllable reduction process and stable content, can guarantee that quadrivalent iridium can be completely reduced to trivalence, and the prepared iridium trichloride product has high purity.
Owner:GRIKIN ADVANCED MATERIALS
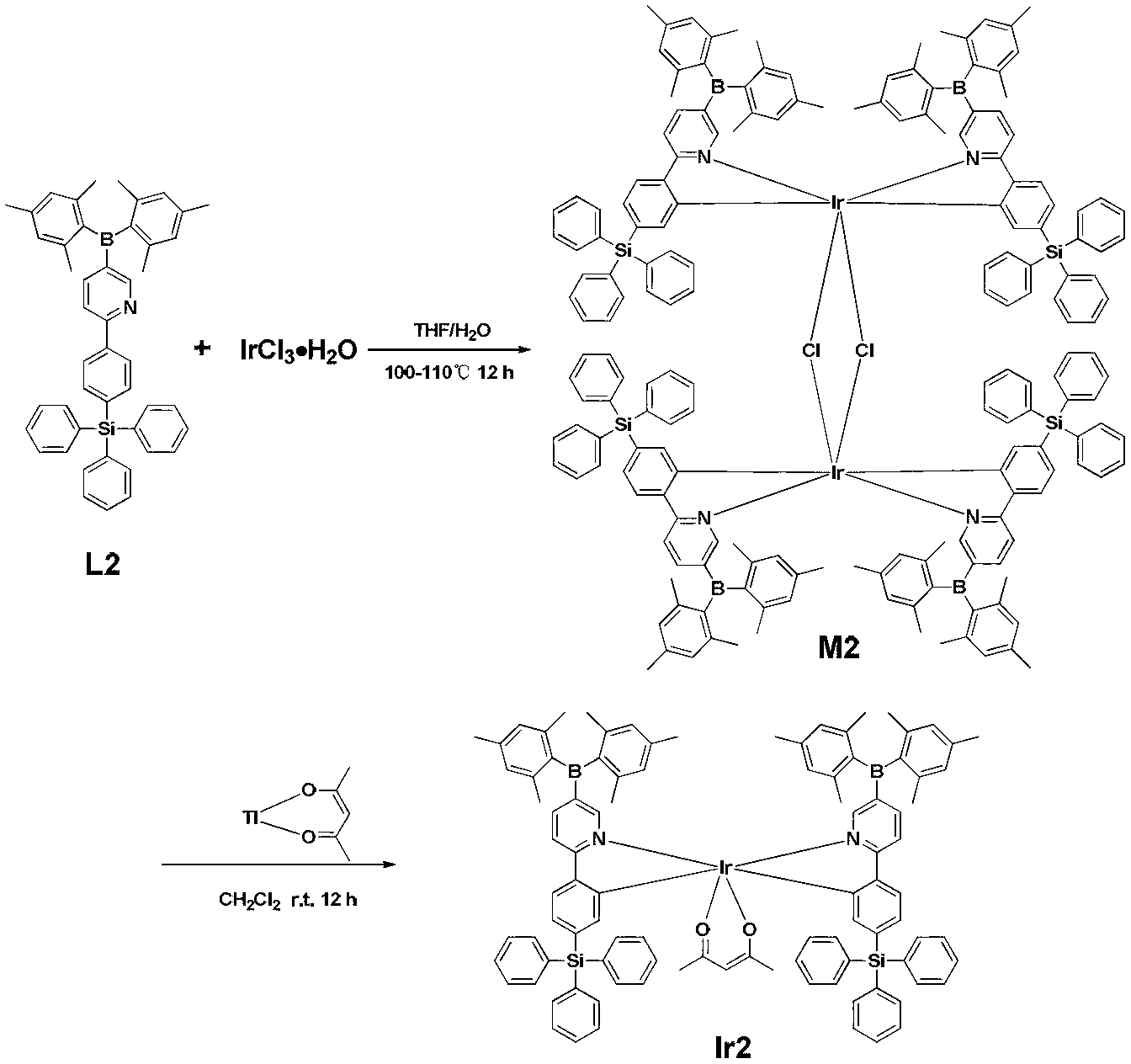
Green-emission iridium phosphors complex and preparation method thereof
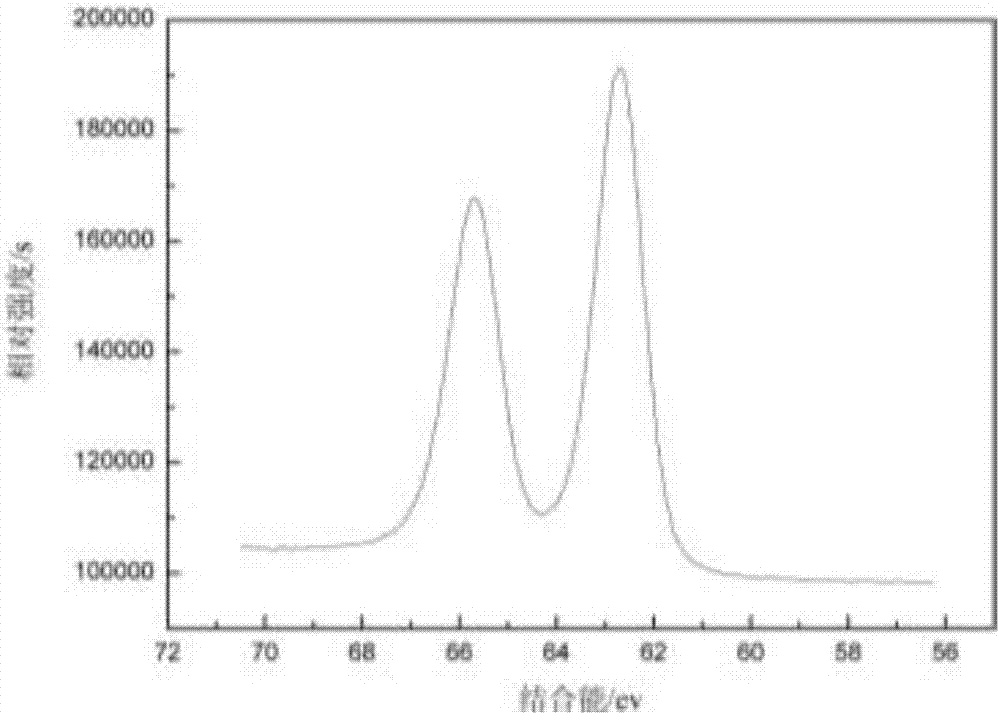
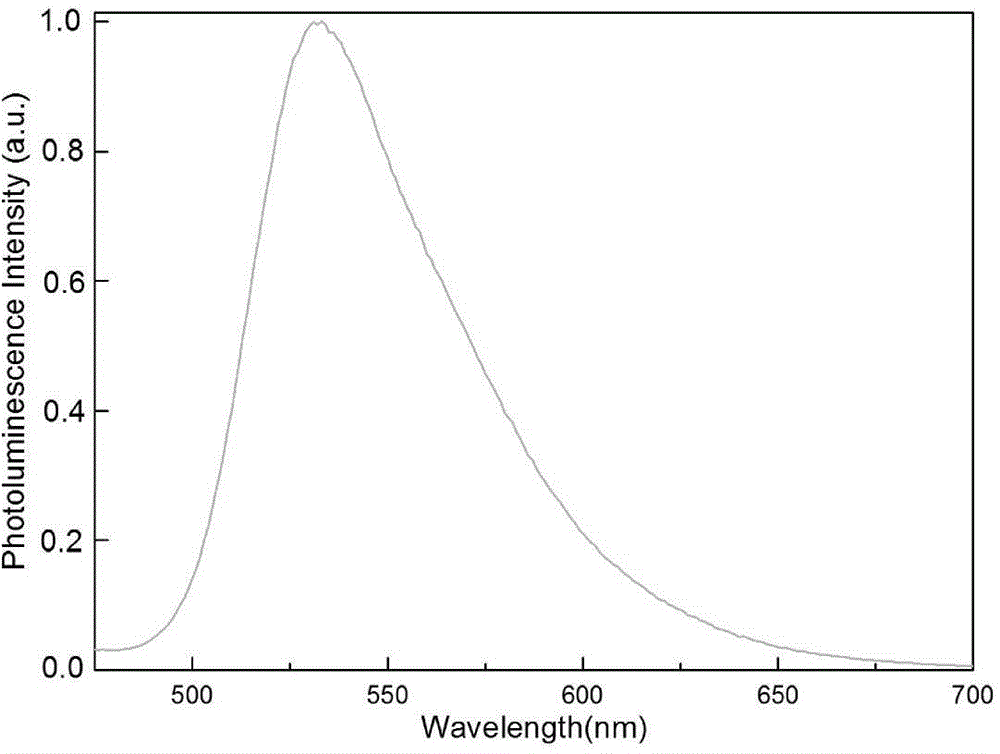
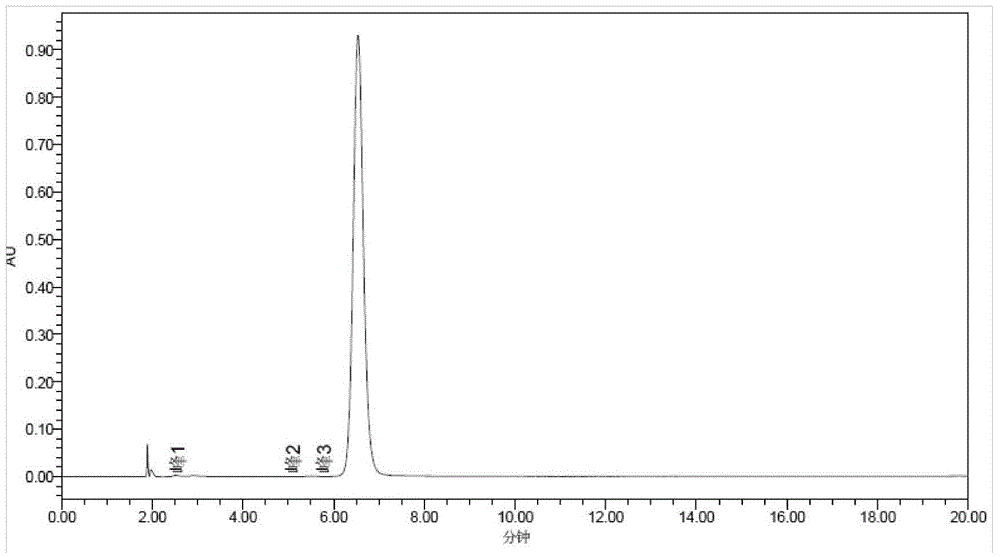
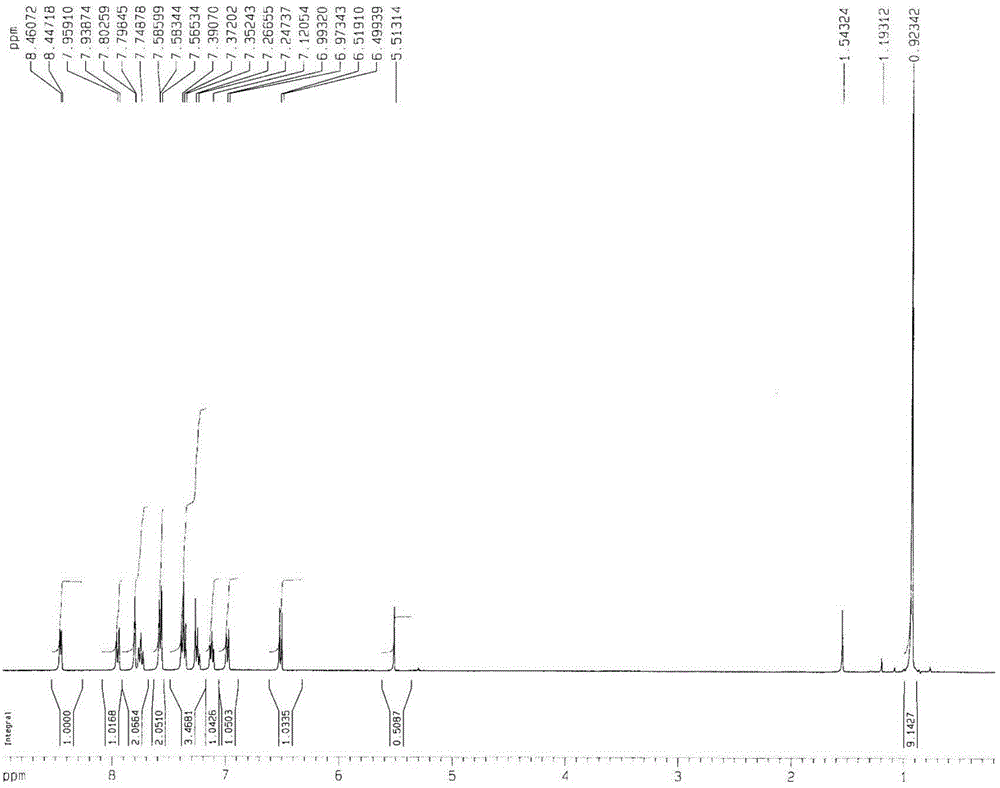
The invention discloses a green-emission iridium phosphors complex and a preparation method thereof. The iridium phosphors complex is that 2-(xenyl) pyridine is used as a cyclometalated ligand, and 2, 2, 6, 6-tetramethyl-3, 5-heptadione (tmd) is used as an auxiliary ligand; the maximum emission wavelength of (Ir(bppy)2(tmd)) is 533nm, which is the typical green-light emission wavelength; iridium chloride hydrate is used as a raw material to synthesize iridium-chloride bridged dimer (dppy)2Ir2Cl2(dppy)2 which reacts with tmd under an alkaline condition to obtain (Ir(bppy)2(tmd)); and the yield is up to 85%, and the purity is up to 99.5%.
Owner:KUNMING INST OF PRECIOUS METALS
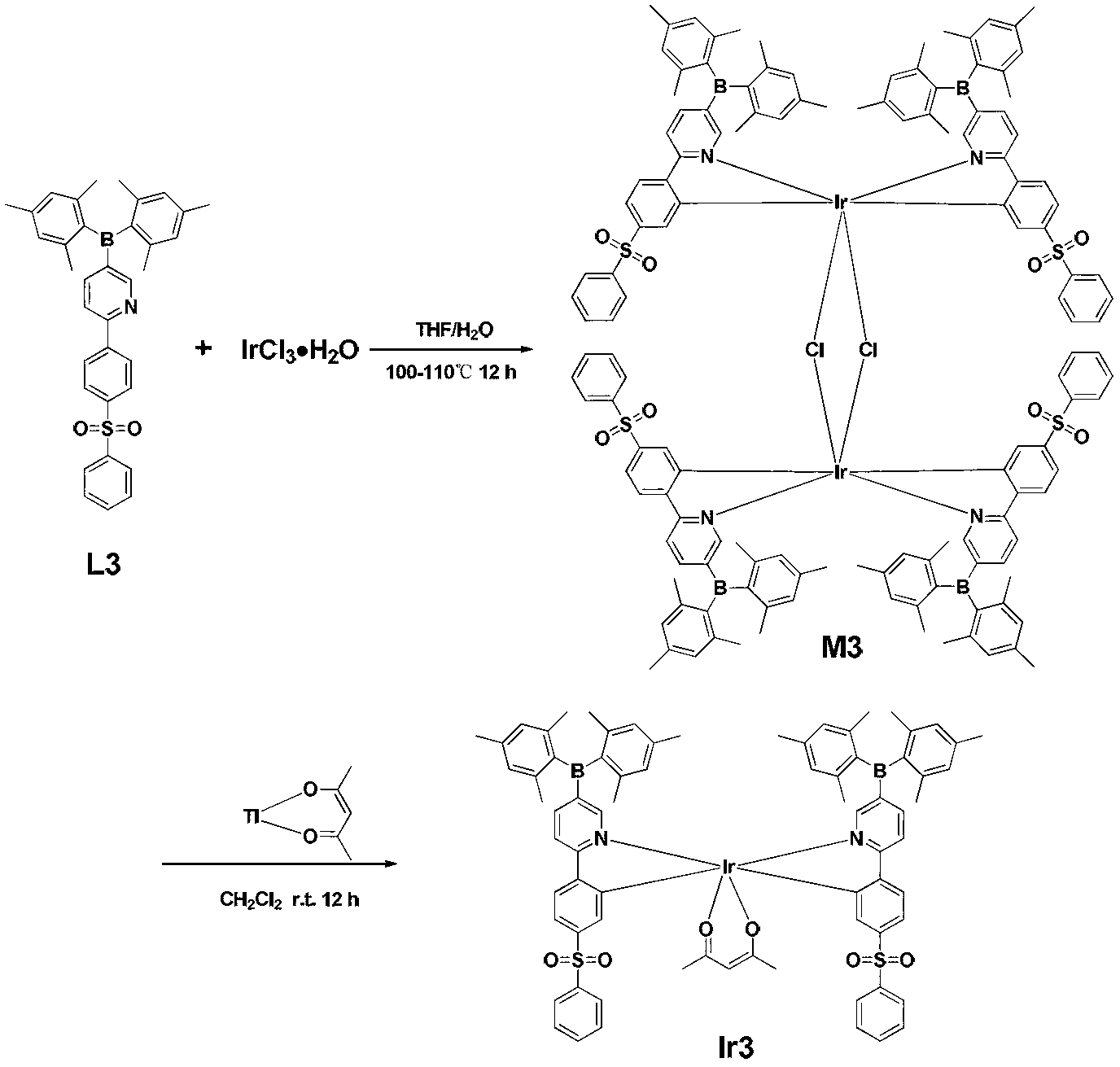
Multi-substituted phenylpyridine iridium (III) complex and preparation method and application thereof
InactiveCN106317123AImprove quantum efficiencyDual emission with blue lightIndium organic compoundsLuminescent compositionsStructural formulaIridium chloride
The invention provides a multi-substituted phenylpyridine iridium (III) complex and a preparation method and application thereof. The structural formula of the complex is as shown in the formula I or II. Please see the formula in the description. R1, R2, R3, R4 and R5 are independently hydrogen, fluorine, methyl or trifluoromethyl. The preparation method includes the steps that multi-substituted phenylpyridine reacts with iridium chloride trihydrate to obtain iridium (III) chloro-bridged dimer; then the iridium (III) chloro-bridged dimmer reacts with 2-pyridinecarboxylic acid or 3-trifluoromethyl-5-pyridyl triazole, and the multi-substituted phenylpyridine iridium (III) complex can be obtained. The multi-substituted phenylpyridine iridium (III) complex has the advantages of blue light dual emission, extremely high quantum efficiency and the like, can serve as an electroluminescent phosphorescent material and a phosphorescent doping material to be used in an organic electroluminescent device, can achieve blue light emission, and can be doped with yellow-orange light to achieve white light emission.
Owner:NANJING UNIV OF POSTS & TELECOMM
A kind of method preparing chloroiridic acid
ActiveCN107758752BFully convertedPromote conversionRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsPhysical chemistryHearth
The invention relates to a method for preparing chloroiridic acid. According to the method disclosed by the invention, firstly, metal iridium powder and sodium chloride are fused at a high temperature, meanwhile, a little amount of starch is added, chlorine is introduced into the fused melt, and most iridium powder is transformed into iridium sodium chloride by a measure of chlorine strengthened stirring; a little part of produced iridium chloride gas is absorbed by using a diluted hydrochloric acid solution; iridium sodium chloride obtained by high-temperature chlorination smelting reaction is merged with a diluted hydrochloric acid absorbing solution, and then sodium hydroxide is added for neutralization to produce iridium oxide, a product is filtered and washed for multiple times to remove sodion, hydrochloric acid is added and dissolved and then concentration is performed to prepare the chloroiridic acid solution. According to the invention, chlorine is introduced into a melt and stirred adequately, and by a measure of smelting in a furnace hearth, transformation of iridium is efficiently promoted, and the leaching efficiency of iridium powder reaches 80% or above; by adding starch, passivation of the surface of the iridium powder in the heating process can be avoided, sufficient transformation of iridium powder is promoted, and by performing secondary absorption on tail gas, comprehensive utilization of a hydrochloric acid absorbing solution is realized, and the comprehensive recovery rate of iridium is increased.
Owner:CENT SOUTH UNIV
Iridium-catalyzed production method for organosilicon compounds
InactiveUS7956210B2Solution to short lifeImprove production yieldSilicon organic compoundsMetal/metal-oxides/metal-hydroxide catalystsUnsaturated hydrocarbonIridium chloride
Alkyl silanes are prepared by silylating an unsaturated hydrocarbon with an Si—H functional silane employing an iridium chloride coordination compound as a catalyst and a polymeric polyene as a cocatalyst. Reaction bottoms can be worked up to provide an iridium-containing composition which remains catalytically active.
Owner:WACKER CHEM GMBH
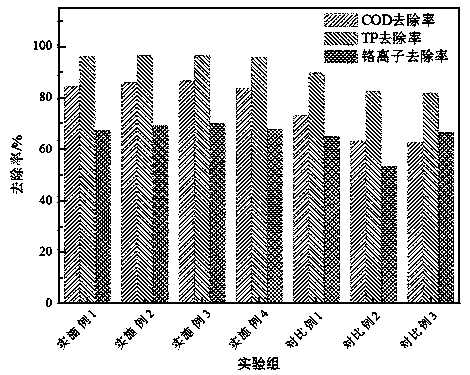
Sewage treatment flocculant and preparation method thereof
ActiveCN111115782AGood removal effectImprove use valueSustainable biological treatmentWater/sewage treatment by flocculation/precipitationPolyethylene glycolWater chlorination
The invention discloses a preparation method of a sewage treatment flocculant. The preparation method comprises the following steps: (1) preparing an aqueous solution of chitosan acetic acid and iridium chloride, stirring, adding an ethanol solution of span-80 and polyethylene glycol into the solution, carrying out water bath treatment at constant temperature, and adding glutaraldehyde; (2) adjusting the pH value to 9-10, carrying out solid-liquid separation, and washing and drying the obtained solid phase; (3) preparing a black brin-kiwi fruit extract, adjusting the pH value to 13-14, addingthe solid phase prepared in step (2), heating in a nitrogen atmosphere, cooling, filtering, washing and drying to obtain a solid phase B; (4) adding the solid phase B and aluminum phosphate into 2,3-dihydropyran, keeping the temperature constant, carrying out solid-liquid separation, and washing and drying to obtain a solid phase C; (5) sequentially soaking the solid phase C in an aqueous solutionof hydrogen peroxide and sodium acrylate, and adding epoxy chloropropane to obtain a solid phase D; and (6) performing acid soaking, filtering, washing and drying on the solid phase D to obtain the sewage treatment flocculant. The flocculant prepared in the invention has an obvious sewage treatment effect and stable performance.
Owner:江西省地质环境监测总站
Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone
InactiveCN103288831AHigh reactivityHigh enantioselectivityAsymmetric synthesesBenzodiazepineEnantiomer
The invention discloses a method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone by asymmetric hydrogenation under catalysis of iridium. The reaction conditions are that the temperature is 0-50 DEG C; a solvent adopts a dichloromethane and methylbenzene mixed solvent in which the volume ratio of dichloromethane to methylbenzene is 1 to 2; the pressure is 13-50 atm; the proportion of a primer to a catalyst is 50 to 1; the catalyst is a complex consisting of (1,5-cyclooctadiene) iridium chloride dimer and diphosphine; an additive is morpholine trifluoroacetate or piperidine hydrochloride; a corresponding chiral dihydro product is obtained by hydrogenating seven-membered ring-shaped benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone; enantiomer is excessive and can reach 96 percent. The method is easy to operate and practicable; raw materials are readily available; the enantioselectivity is high; the yield is high.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Organic metal iridium complex luminescent material and synthetic method of material
InactiveCN103172677AImprove solubilityImprove transfer rateGroup 8/9/10/18 element organic compoundsLuminescent compositionsOrganic layerNitrogen gas
The invention discloses an organic metal iridium complex luminescent material and a synthetic method of the material. The synthetic method disclosed by the invention comprises the following steps of: mixing an organic complex with iridium chloride hydrate in nitrogen atmosphere, subsequently adding a mixed solvent of tetrahydrofuran and water, heating in the nitrogen atmosphere and cooling to the room temperature; separating the reaction mixture liquid to acquire a tetrahydrofuran organic layer, and drying to remove the tetrahydrofuran solvent so as to acquire a metal iridium dimer complex; completely dissolving the metal iridium dimer complex and acetyl acetone thallium in dichloromethane and stirring at the room temperature in the nitrogen atmosphere; then purifying the metal iridium dimer complex on a chromatography silica gel column or a self-manufactured thin layer silica gel chromatography plate to acquire the final product which is the organic metal iridium complex luminescent material. According to the invention, the mixed solvent of the tetrahydrofuran and the water is taken as a reaction solvent for synthesizing the organic metal iridium complex, so that the synthetic yield of the organic metal iridium complex can be significantly improved; and the reaction after-treatment is simplified, so that the after-treatment time is shortened and the energy resources are saved.
Owner:XI AN JIAOTONG UNIV
Method for asymmetric hydrogenation of isoquinoline by activation of halogen bond
InactiveCN109422682AHigh reactivityHigh enantioselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesBiological activation
Disclosed is a method for asymmetric hydrogenation of isoquinoline by activation of a halogen bond. A catalytic system used by the method is a chiral diphosphine complex of iridium, and an activator is halides. An reaction can be carried out under the following conditions: the temperature is 25-100 DEG C, a solvent is tetrahydrofuran, the pressure is 13-100 atmospheres, a ratio of a substrate to acatalyst is 50 / 1, and the catalyst is a complex of (1,5-cyclooctadiene) iridium chloride dimer and a bisphosphine ligand. The isoquinoline is hydrogenated to obtain corresponding chiral tetrahydroisoquinoline derivatives, and an enantiometric excess can reach 99%. The method has the advantages of simple and practical operation, easy availability of raw materials, high enantioselectivity and goodyield, and the reaction has the advantages of green atom economy, environmental friendliness and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing iridium chloride solid-phase mercury-free catalyst
InactiveCN108031494ANot easy to losePreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsActivated carbonRed mud
The invention provides a method for preparing an iridium chloride solid-phase mercury-free catalyst. The iridium chloride solid-phase mercury-free catalyst contains, by weight, 3.69% of iridium chloride and is 1651m<2> / g in specific surface area and 4.3-4.6nm in average pore size. The preparation method includes activated carbon preparation, adsorbent preparation and adsorption treatment. Activated carbon preparation includes steps: mixing red mud, pyroligneous liquor and coal tar according to a mass ratio of 1:1.8:1.5, heating to 89-92 DEG C, adding MCM-41, sodium thiocyanate, cassava meal and graphene, and well stirring to obtain a mixture. By adoption of the method, the acetylene conversion rate of the iridium chloride solid-phase mercury-free catalyst in vinyl chloride synthesis reaction is greatly increased.
Owner:NINGXIA JINHAI CHUANGKE CHEM TECH
IrCu/TiO2 nanosheet catalyst as well as preparation method and application thereof
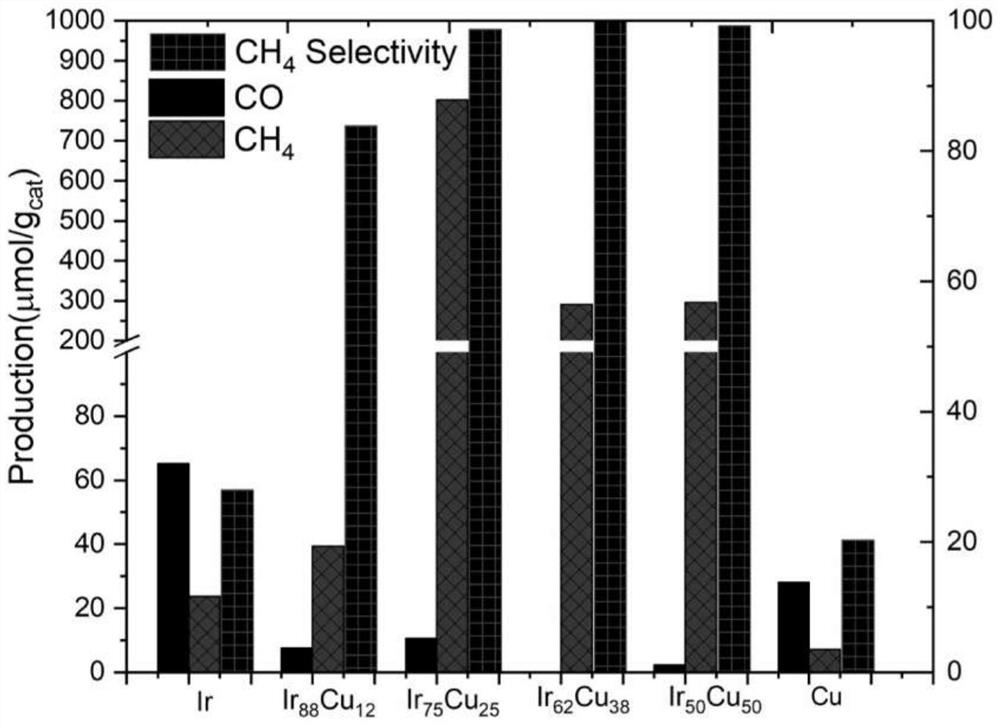
ActiveCN113262802AHigh activityExcellent CH <sub>4<</sub> Hydrocarbon from carbon oxidesCatalystsPtru catalystPhoto catalytic
The invention discloses an IrCu / TiO2 nanosheet catalyst as well as a preparation method and application thereof, and belongs to the technical field of CO2 photocatalytic reduction. The method comprises the following steps: dissolving iridium chloride and copper nitrate into ethylene glycol, and preparing IrCu alloy by a microwave-assisted method; and dissolving the IrCu alloy in ethanol, dropwise adding the IrCu alloy into a TiO2 nanosheet ethanol solution in a dipping manner, drying by distillation in a water bath, drying and grinding to obtain the IrCu / TiO2 nanosheet catalyst. The catalyst shows extremely excellent activity and CH4 selectivity in a CO2 photocatalytic reduction reaction.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

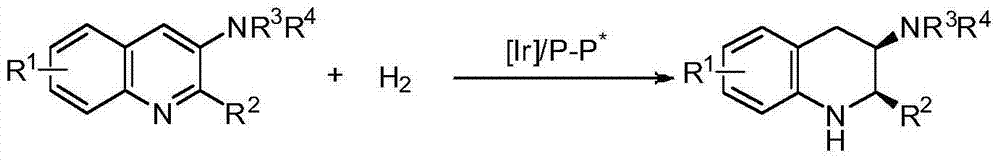


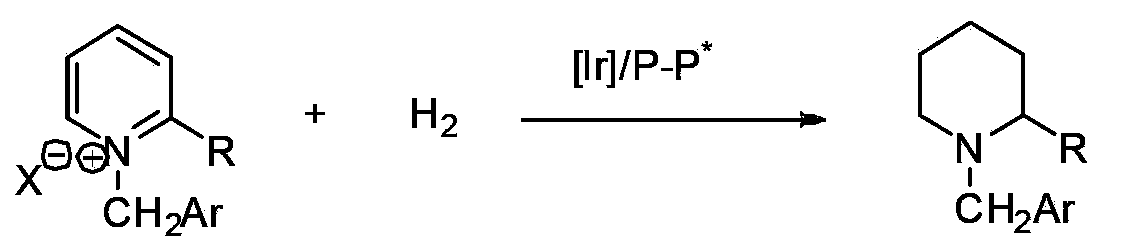

![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/FDA0000138253650000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000021.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepino-5-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8721003a-0f8e-4840-b620-3c6dcd1a65ad/BDA0000138253660000051.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/FDA0000138251660000011.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/BDA0000138251670000021.png)
![Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-6H-indolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/25bec905-6e0d-4049-a28e-94b0f58c6f5c/BDA0000138251670000051.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/FDA0000138247160000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/BDA0000138247170000011.png)
![Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine Method for synthesizing chiral dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ea8510d-18df-48d9-bf5f-addc10a7ec9b/BDA0000138247170000021.png)



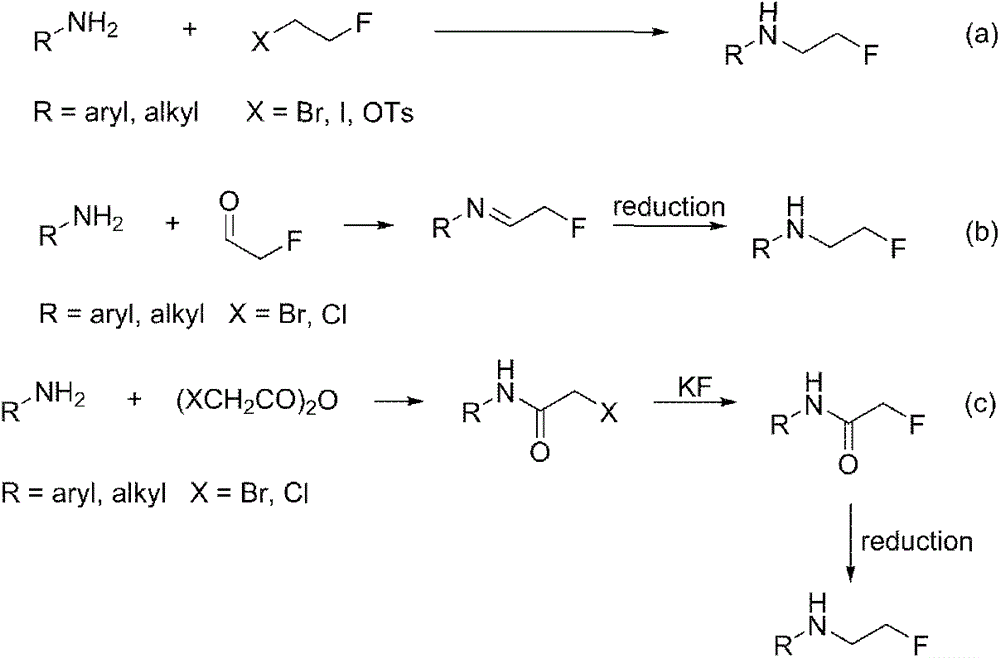

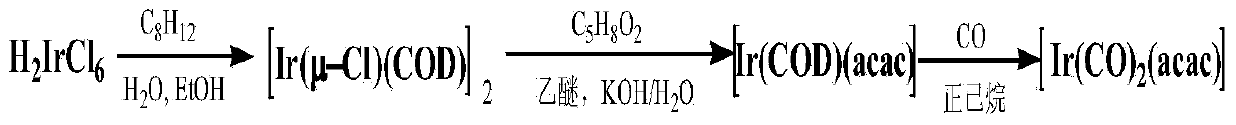


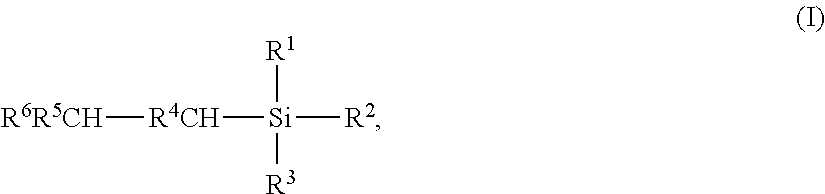


![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/FDA0000138255680000011.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000021.png)
![Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone Method for synthesizing chiral dihydro-6H-benzpyrole-[2,1-c][1,4]-benzodiazepine-6-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e6d181c0-4f2b-4590-96d9-77acc6e90e6f/BDA0000138255690000051.png)