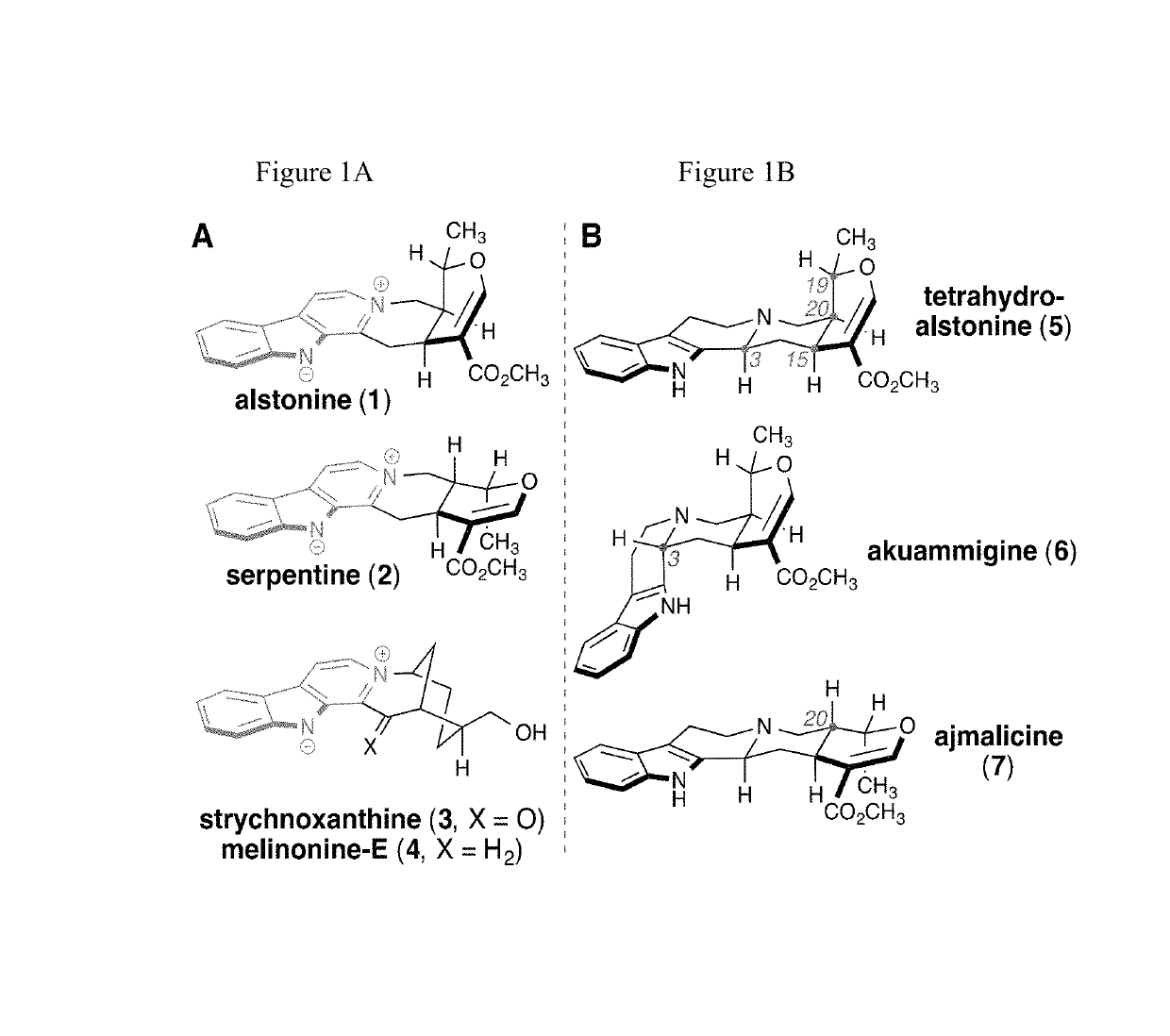
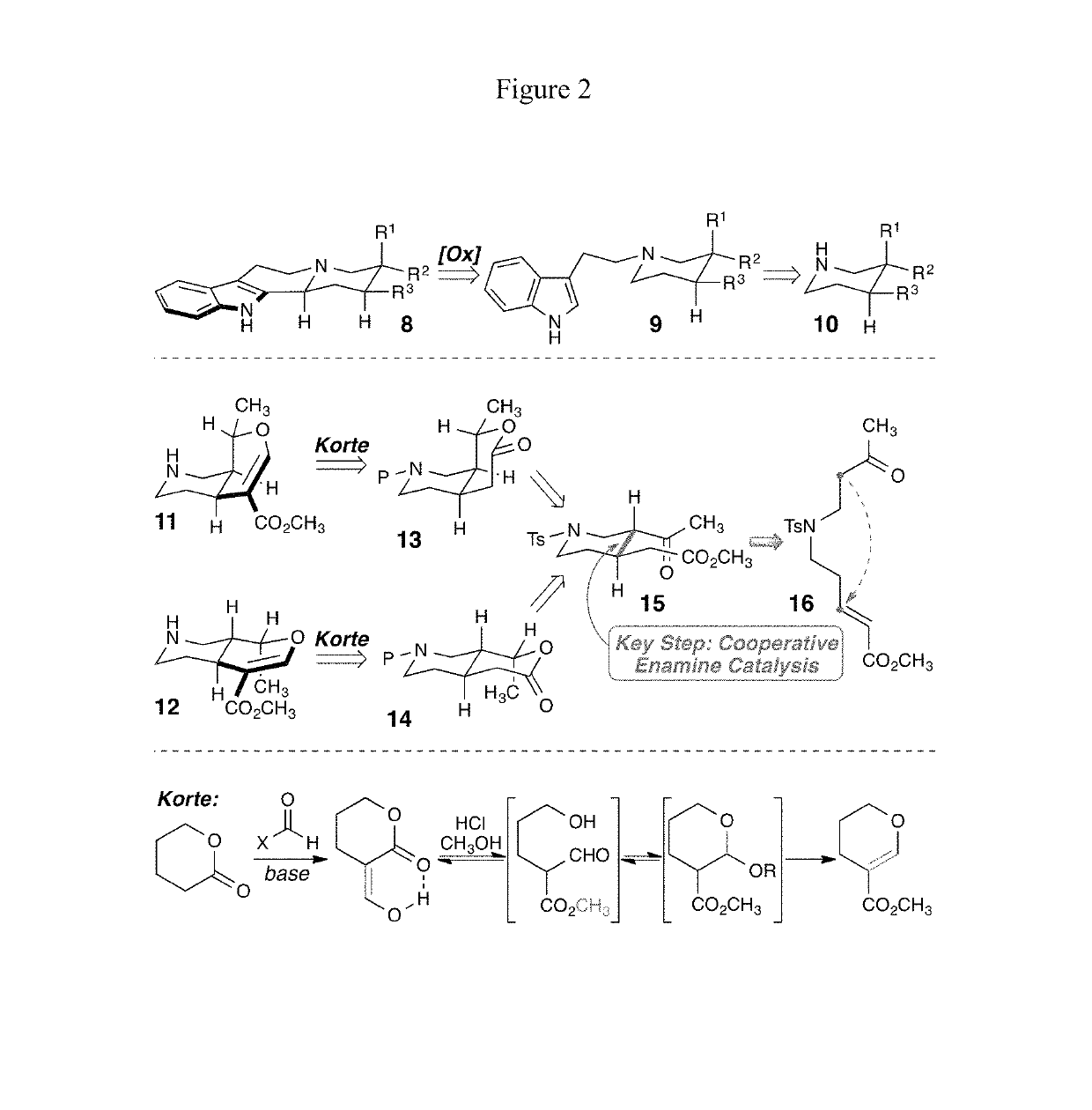
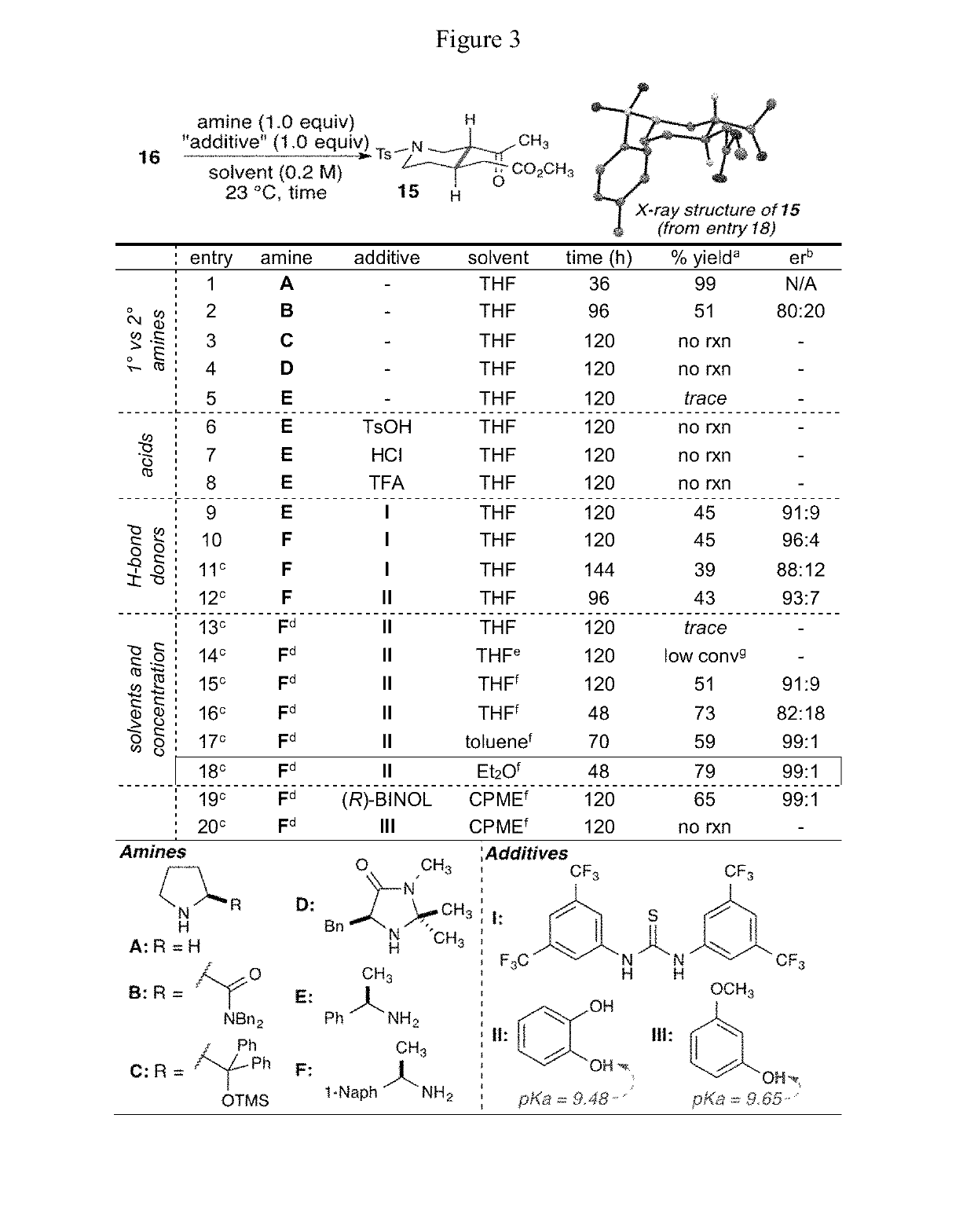
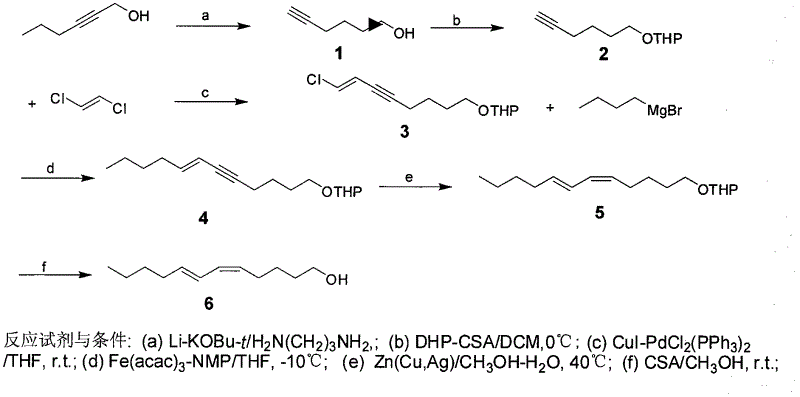
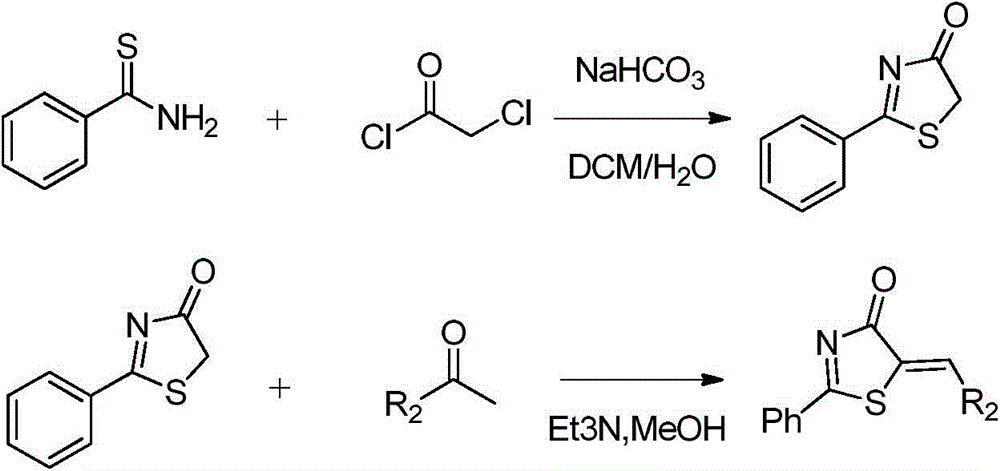
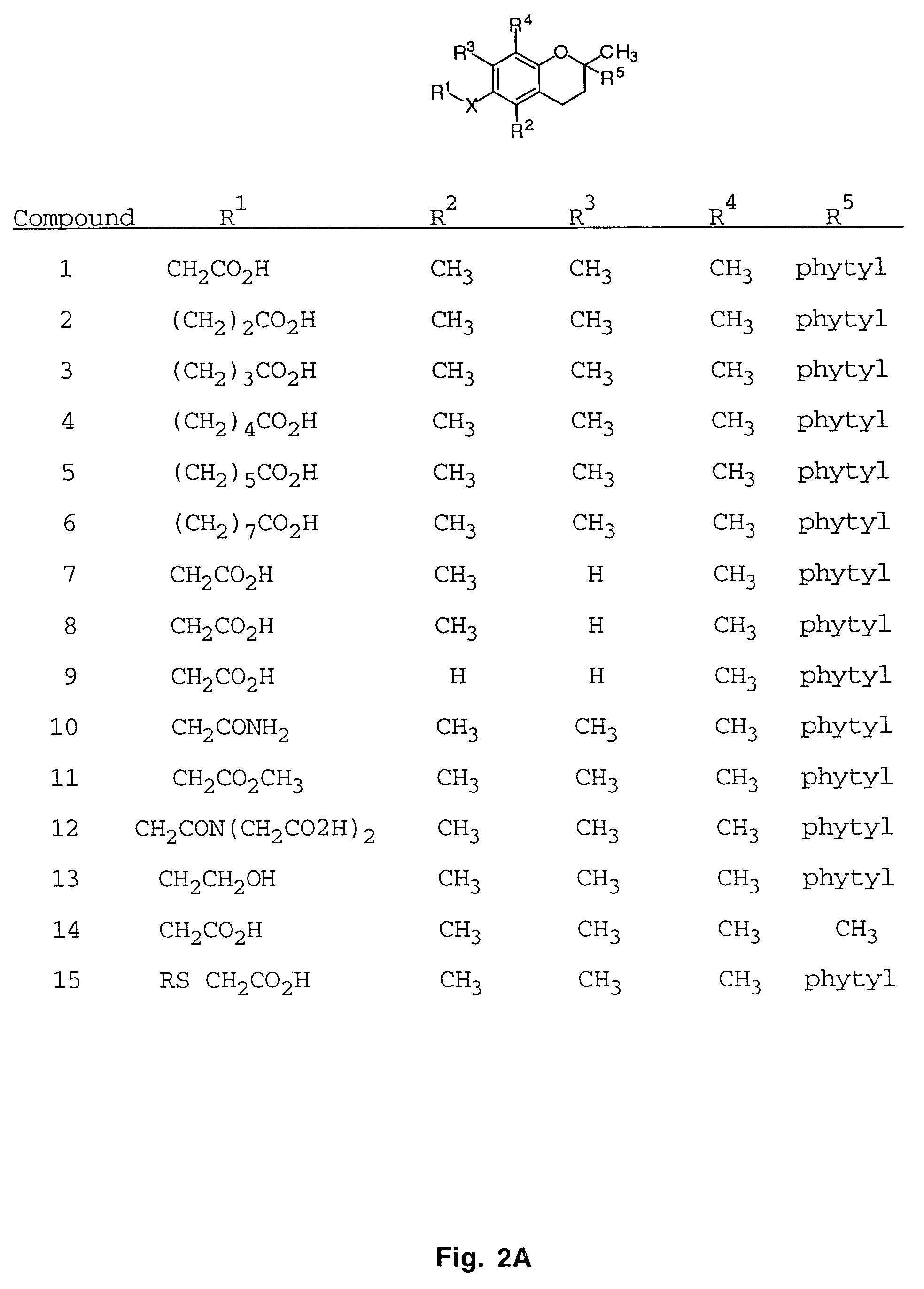
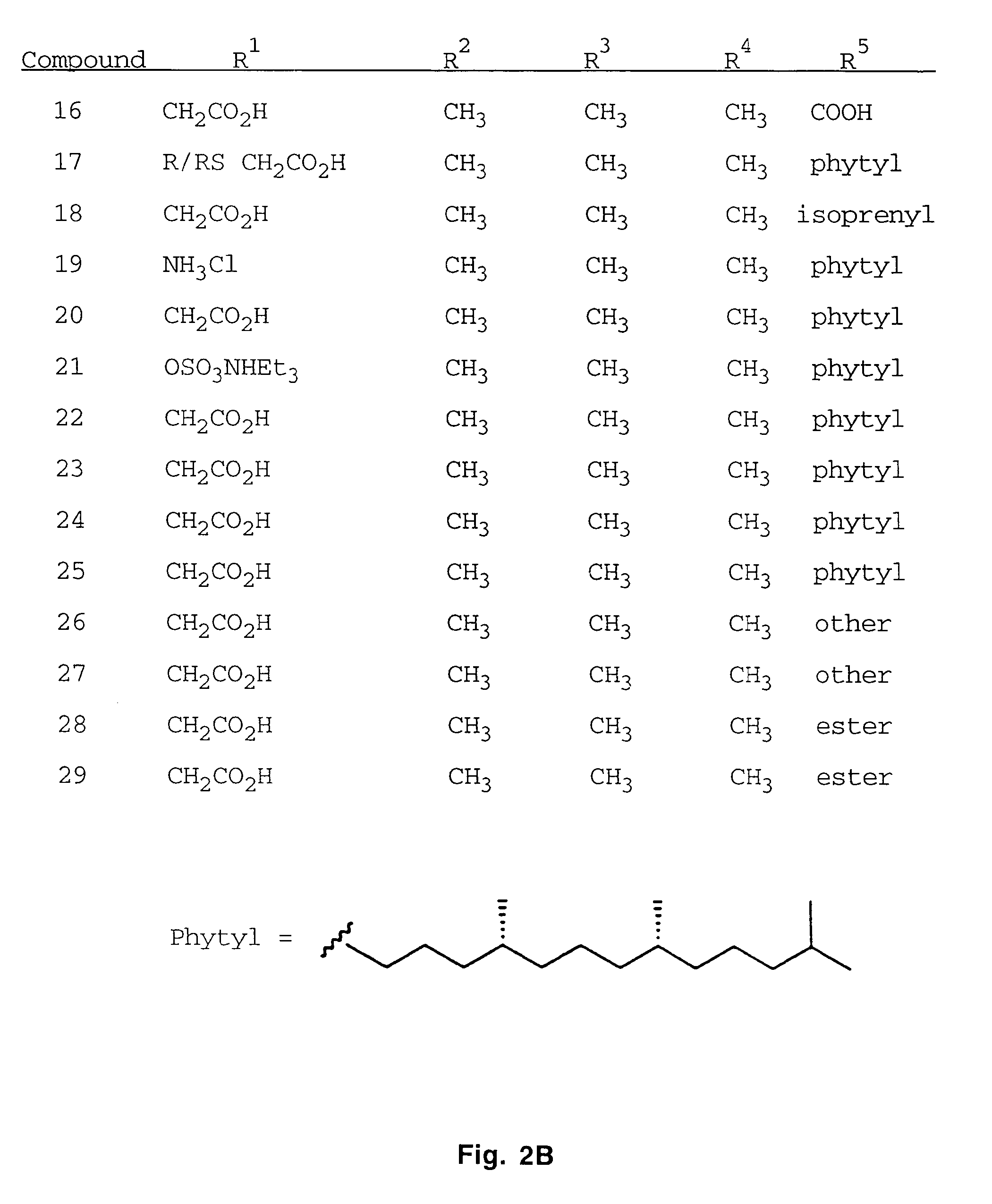
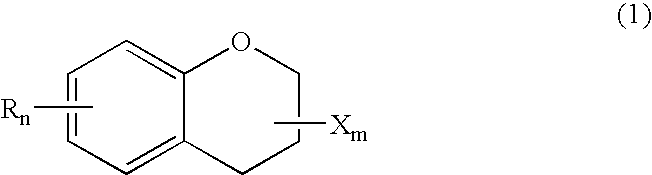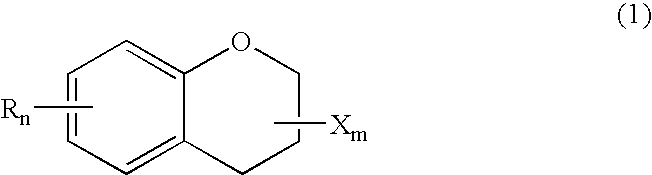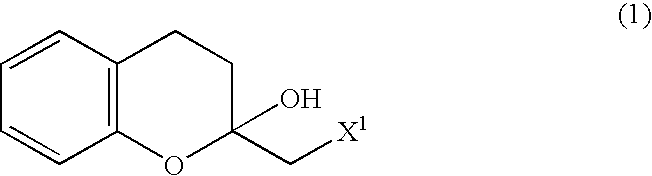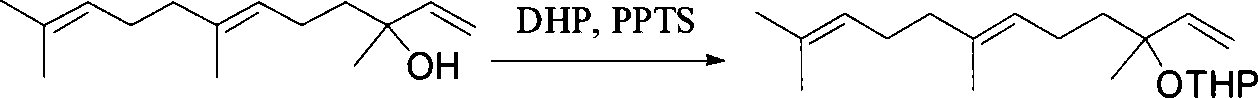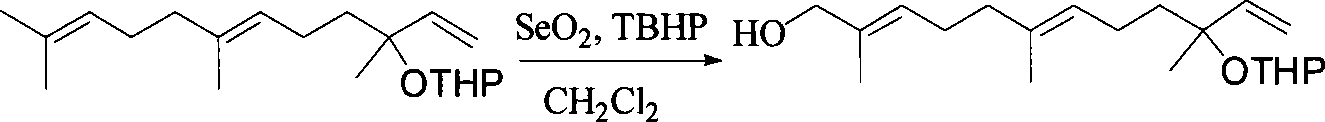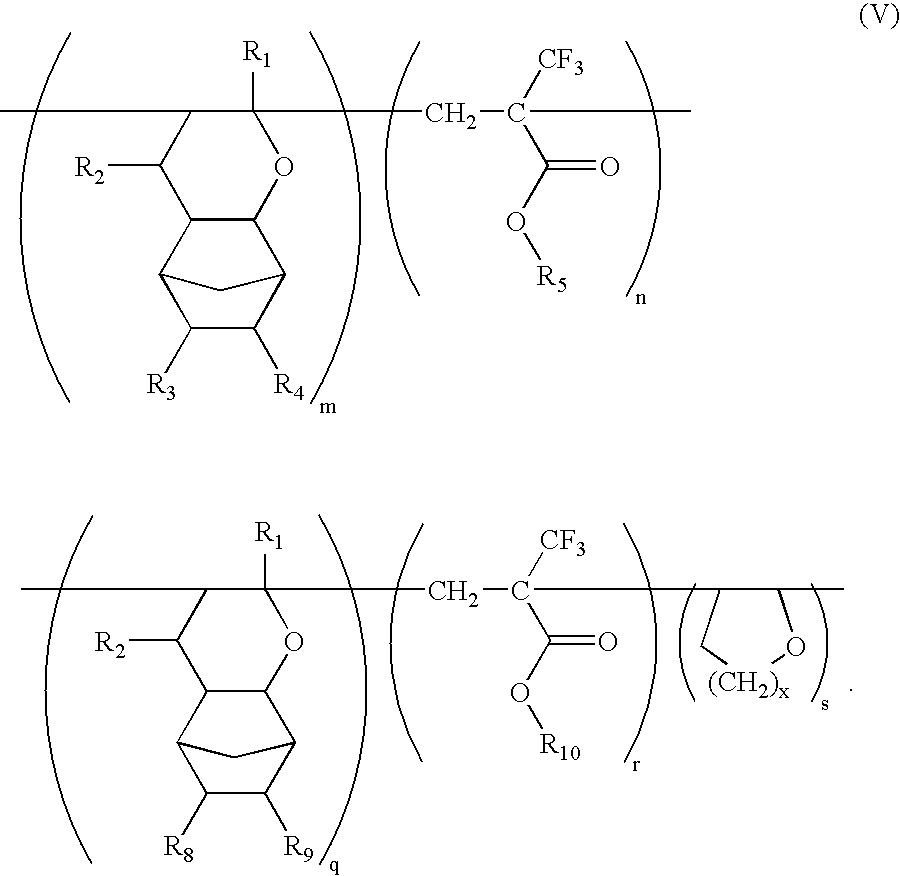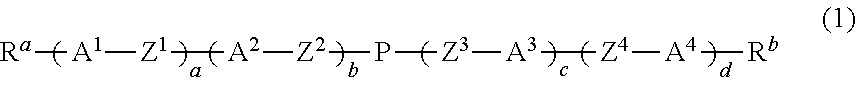Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
268 results about "Dihydropyran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
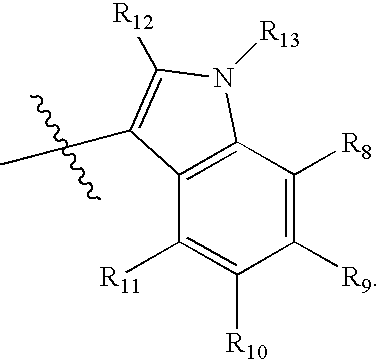
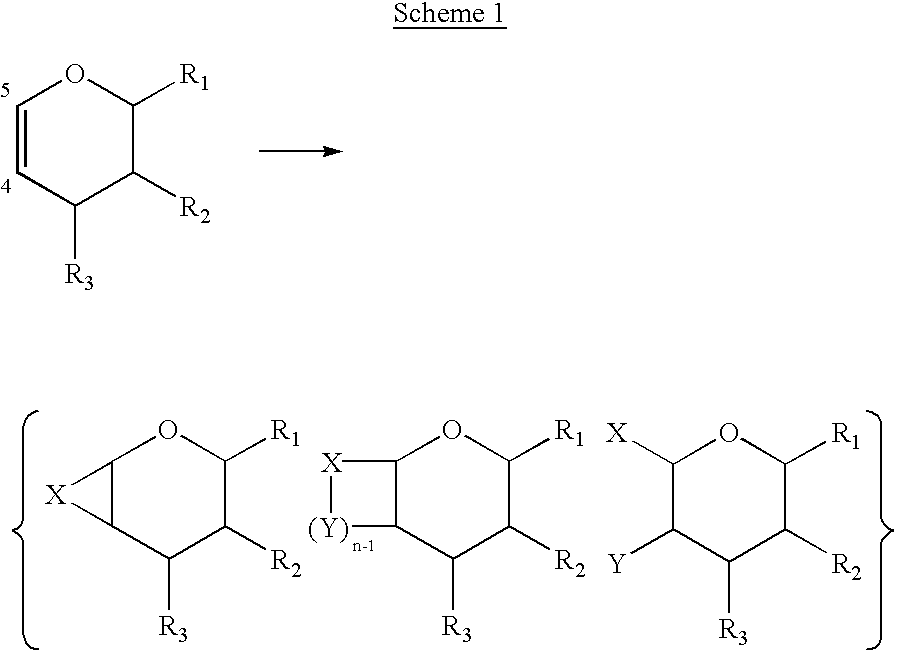
Dihydropyran is a heterocyclic compound with the formula C₅H₈O. The six-membered, not aromatic, ring has five carbon atoms and one oxygen atom. It contains one double bond. There are two isomers of dihydropyran that differ by the location of the double bond. 3,4-Dihydro-2H-pyran has a double bond at position 5; 3,6-dihydro-2H-pyran has the double bond at position 4.
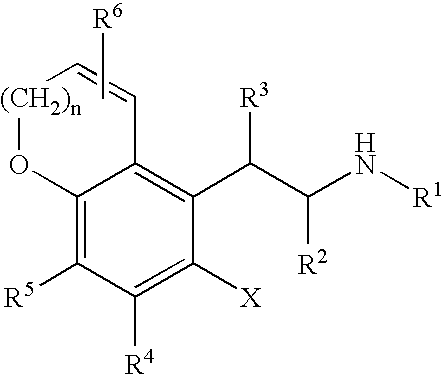
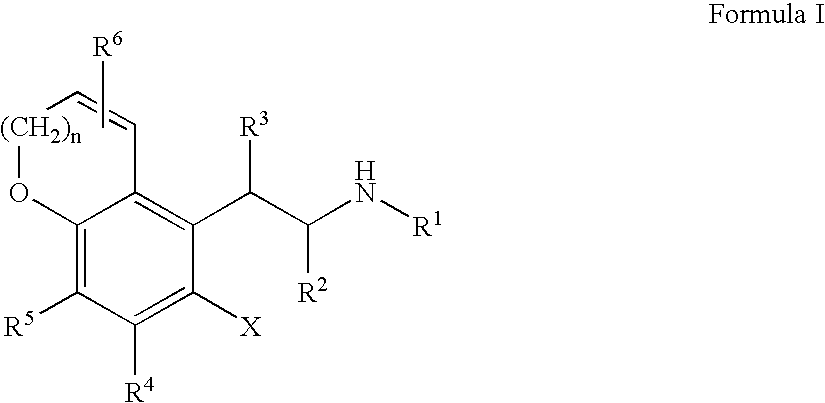
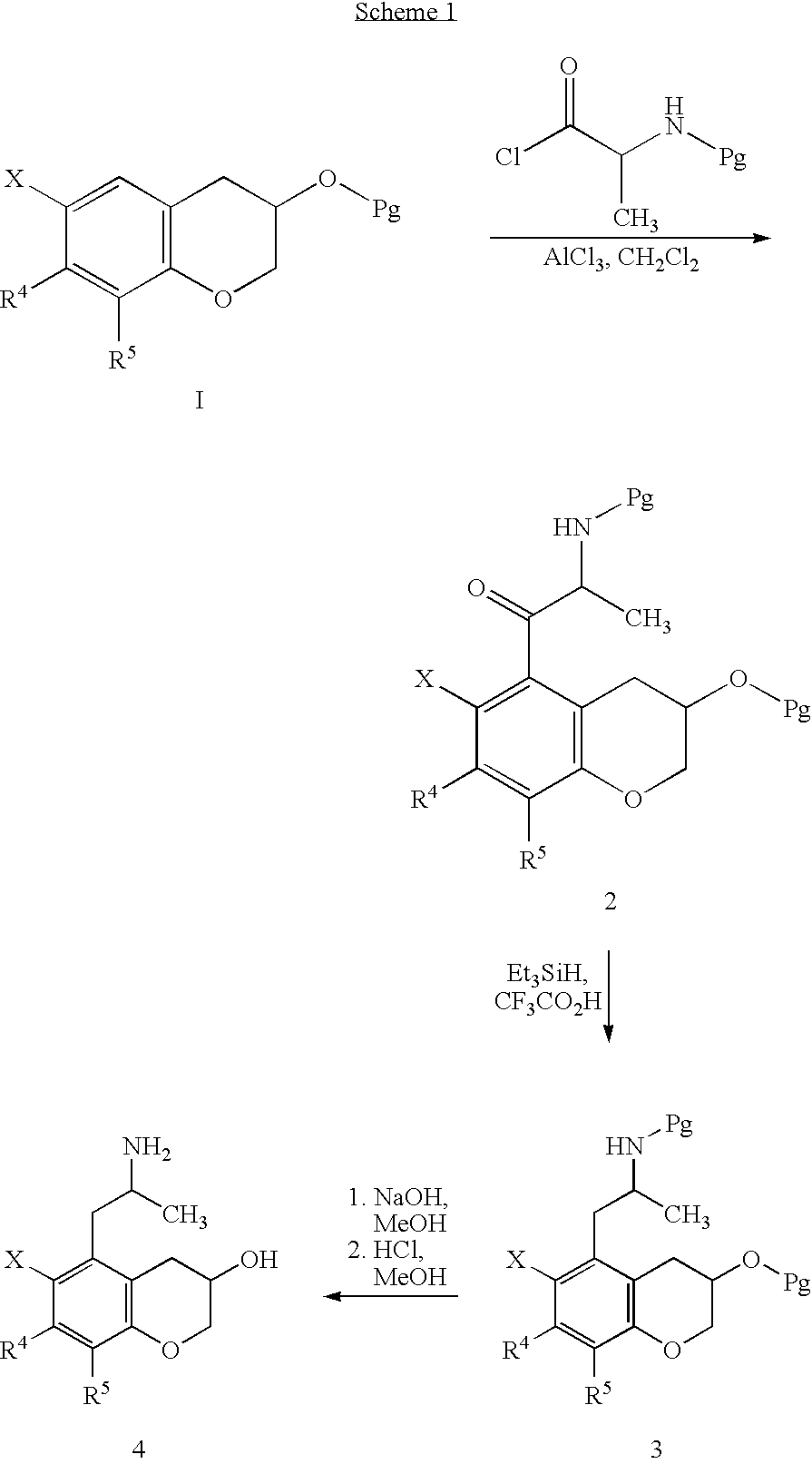
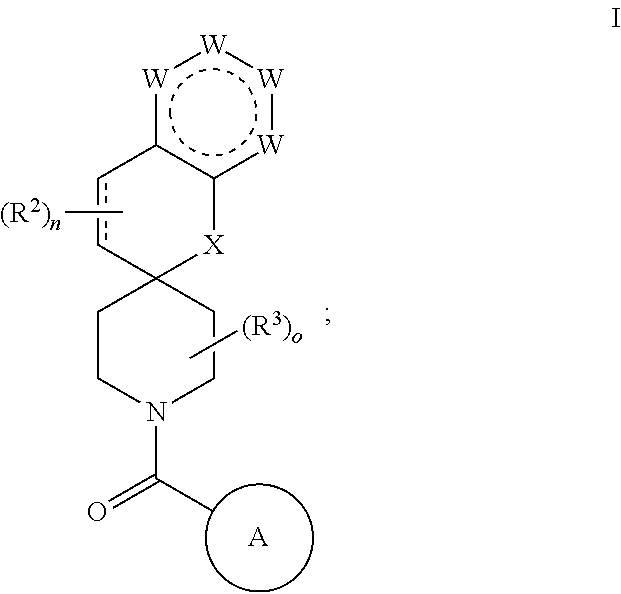
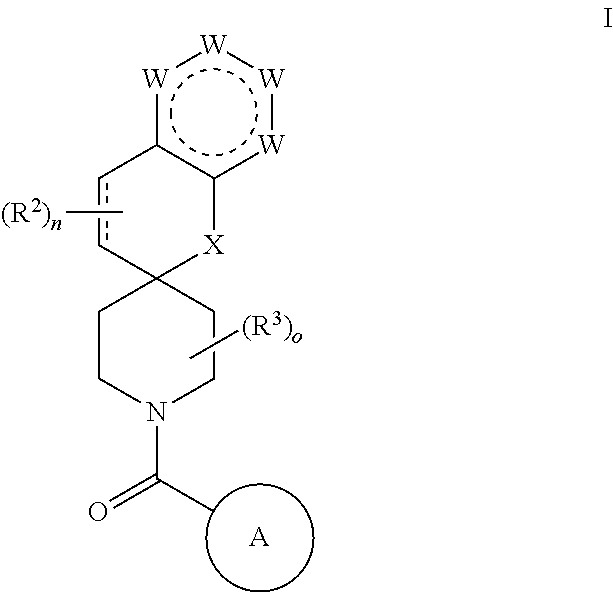
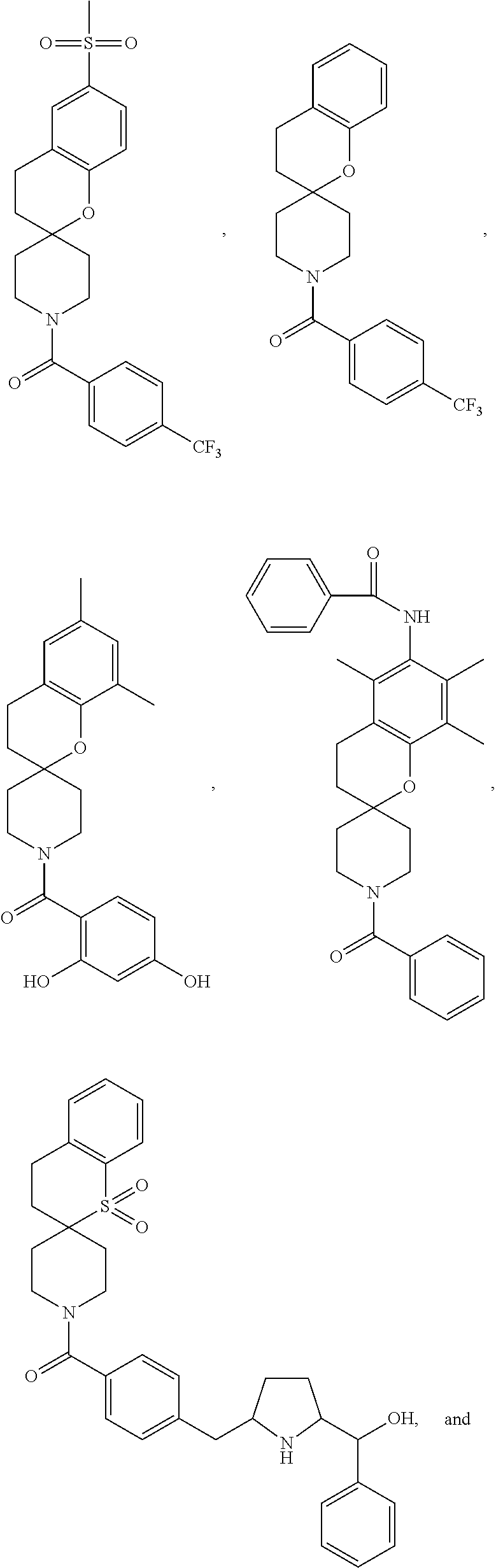
Chroman-spirocyclic piperidine amides as modulators of ion channels
The invention relates to chroman spirocyclic piperidine amide derivatives useful as inhibitors of ion channels. The invention also provides pharmaceutically acceptable compositions comprising the compounds of the invention and methods of using the compositions in the treatment of various disorders.
Owner:VERTEX PHARMA INC
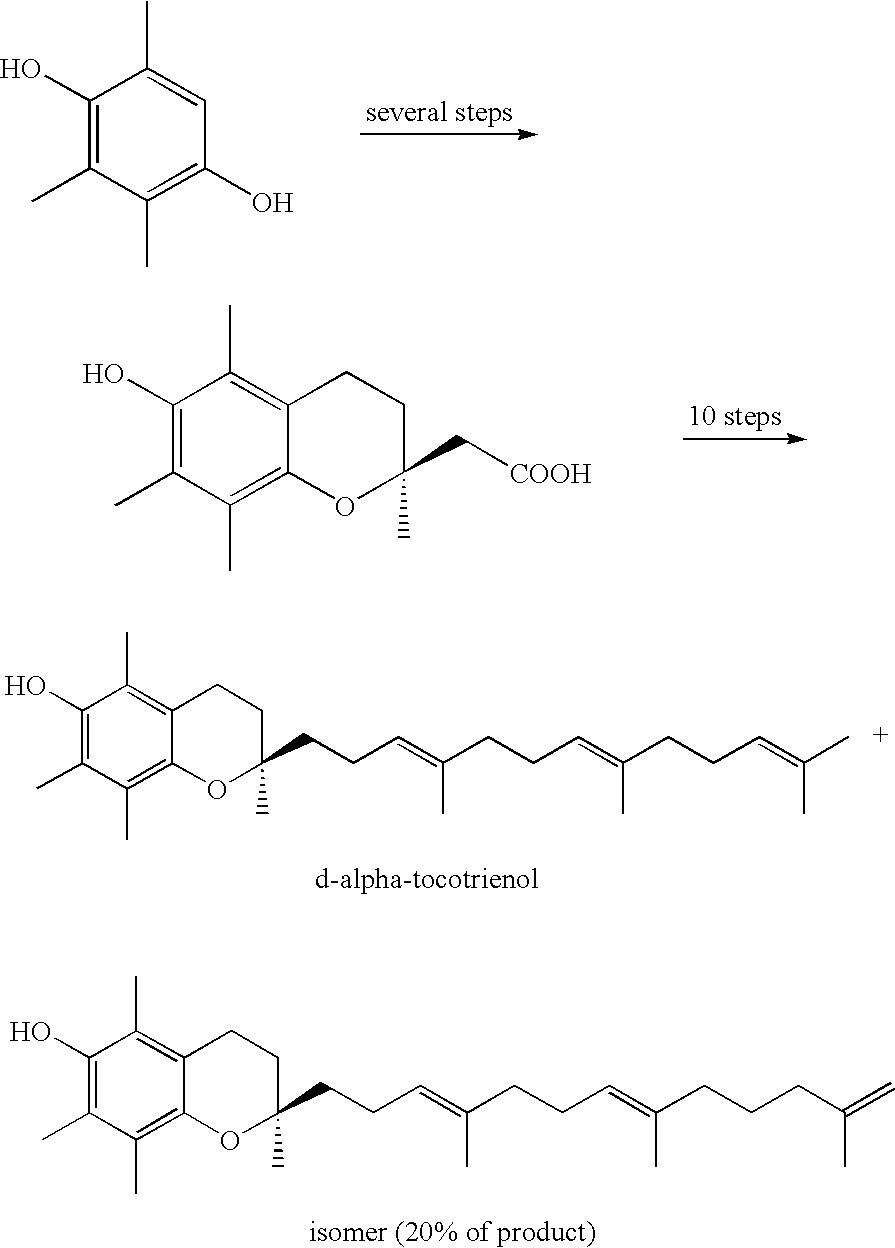
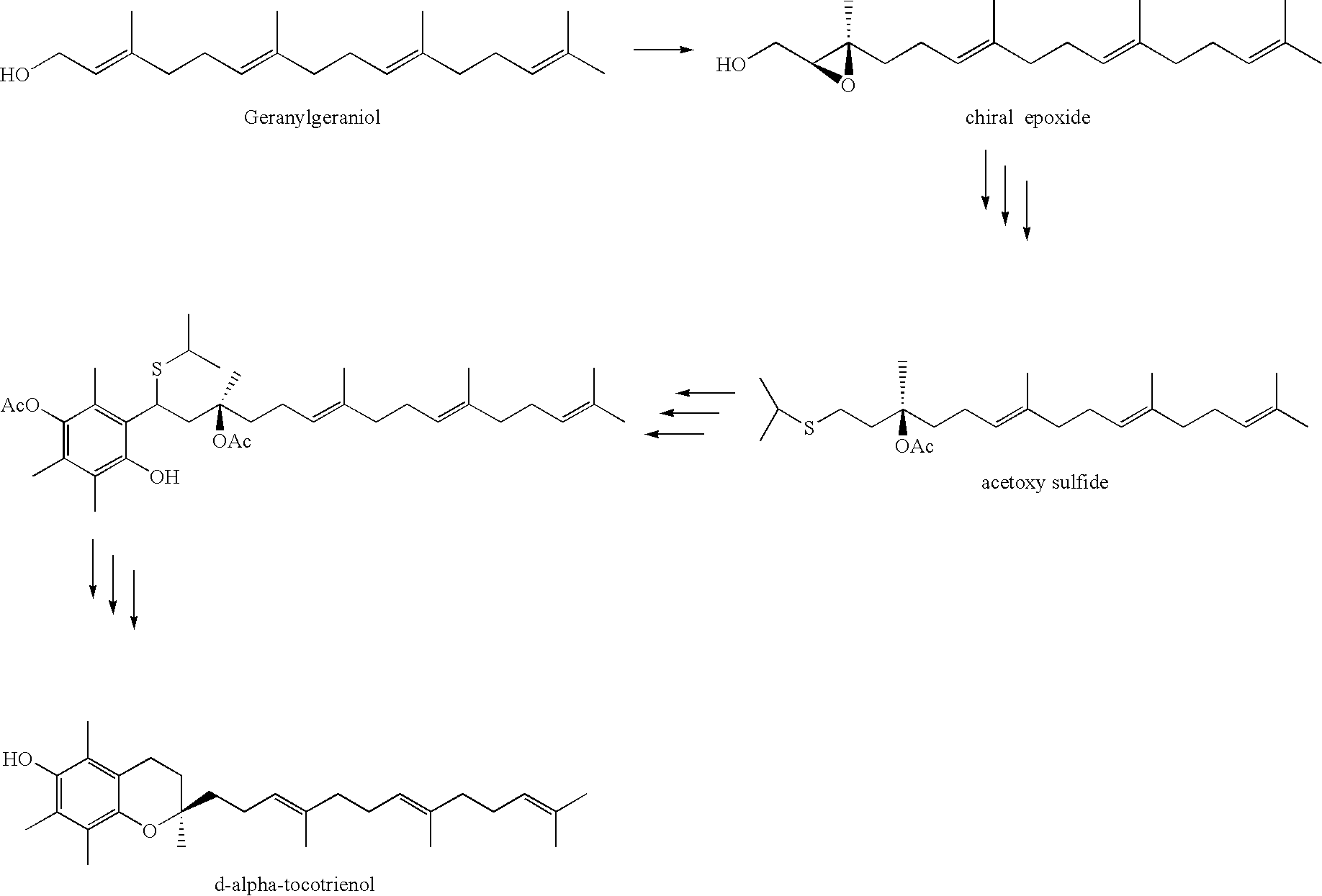
Process for synthesizing d-tocotrienols from 2-vinylchromane compound
A process of forming a d-tocotrienol from a (2S)-vinylchromane compound, through hydroboration of the (2S)-vinylchromane to provide an organoborane, followed by coupling the organoborane with a halogenated C-14 sidechain compound under conditions of palladium-catalyzed cross-coupling is taught. Methods for providing the (2S)-vinylchromane compound and the halogenated C-14 compound are disclosed.
Owner:YASOO HEALTH
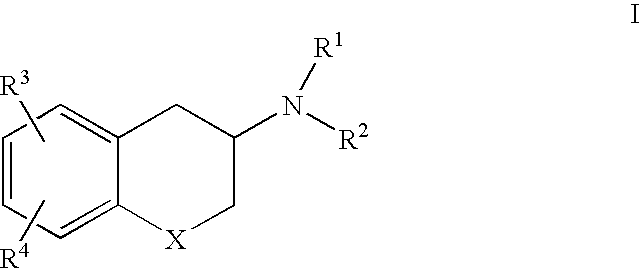
3-Amino chroman and 2-amino tetralin derivatives
3-Amino chroman and 2-amino tetralin derivatives and compositions containing such compounds are disclosed. Methods of using the 3-amino chroman and 2-amino tetralin compounds and compositions containing such compounds in the treatment of serotonin disorders, such as depression and anxiety, are also disclosed.
Owner:WYETH
Synthesis of ester ether compound with high acidolysis activity
InactiveCN1347869AReduce the discharge of three wastesSlow responseOrganic compound preparationCarboxylic acid esters preparationSolventActive point
The ester ether compound with high acidolysis activity is synthesized through the reaction between benzil acid (2,2-diphenyl glycolic acid) and phenols, each of which has at residual active point in its benzene ring, in the presence of p-toluene sulfonic-acid catalyst in 0.1-2 % and methyl benzene, dimethyl benzene and other benzene compound as solvent to synthesize a serial addition compound of benzil acid and phenols which may be further dewatered to produce lactone compound; and the etherification of the said addition compound or the lactone compound the ether compound, such as vinyl ethylether, dihydropyran, dihydrofuran, dihyidropyrrole, etc. at below 70 deg.c. The ester ether compound may be used as photothermal imaging information record material.
Owner:BEIJING NORMAL UNIVERSITY
Stabilization of radiopharmaceutical compositions using hydrophilic thioethers and hydrophilic 6-hydroxy chromans
InactiveUS6989138B2Simple compositionImprove efficiencyBiocideContainer/bottle contructionCombinatorial chemistryChroman derivative
Radiopharmaceutical compositions which are stabilized by addition of a mixture of a hydrophilic thioether and a hydrophilic 6-hydroxy-chroman derivative.
Owner:CIS BIO INT
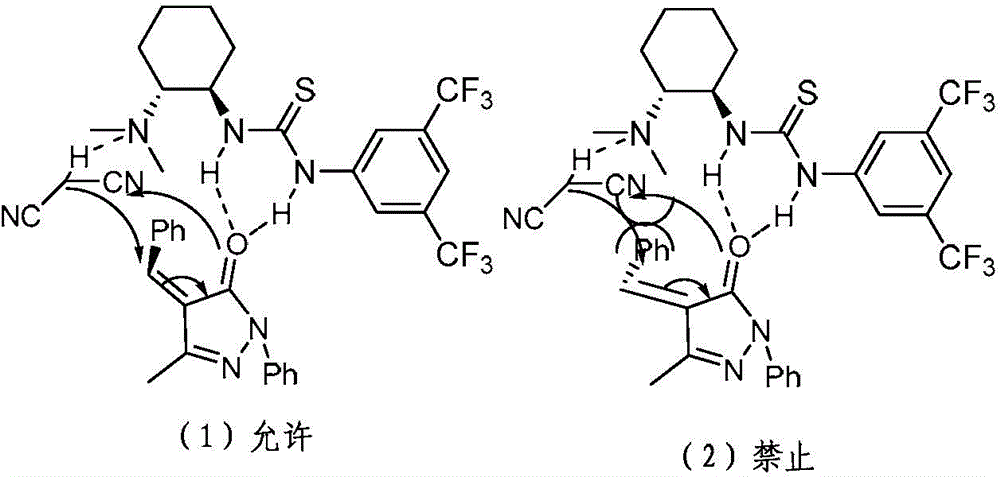
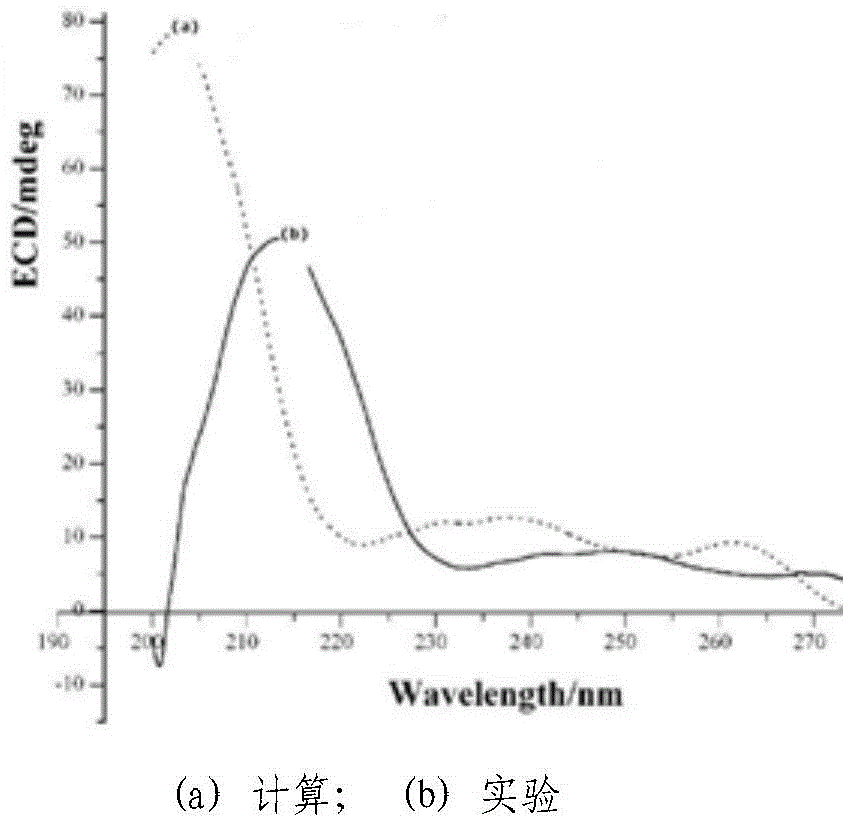
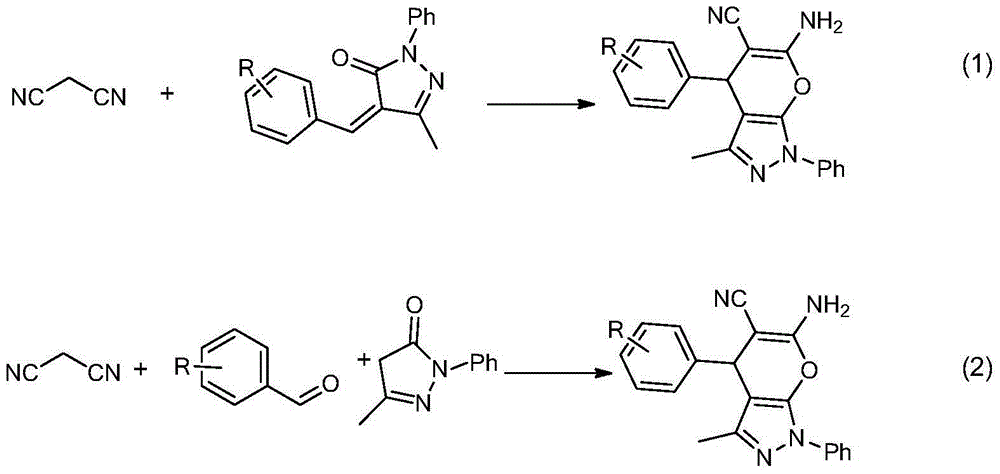
Chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative as well as synthesis method and application of chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative
ActiveCN103910737AFlexible response timeHigh yieldOrganic active ingredientsOrganic chemistrySynthesis methodsSolvent
The invention discloses a chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative as well as a synthesis method and application of the chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative. The synthesis method comprises the steps of with methylbenzene as a solvent, enabling a substituted or unsubstituted imidazolone derivative and propanedinitrile to react under the analysis of a Takemoto catalyst, and carrying out after-treatment on a reaction product to obtain the chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative. The method has the advantages of flexible reaction time, relatively high yield, simplicity and convenience in operation and the like, and is wide in application range. The chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative has an inhibiting effect on gram positive bacteria and gram negative bacteria.
Owner:惠州拓康生物科技有限公司
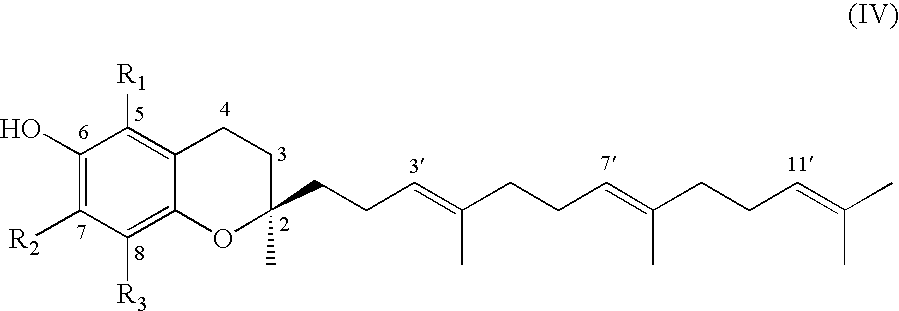
Tocopherols, tocotrienols, other chroman and side chain derivatives and uses thereof
The present invention provides an antiproliferative compound having the structural formulawherein X is oxygen, nitrogen or sulfur; Y is selected from the group consisting of oxygen, nitrogen and sulfur wherein when Y is oxygen or nitrogen, n is 1 and when Y is sulfur, n is 0. Also provided is a method for inducing apoptosis in a cell comprising administering a composition comprising a compound having said structural formula.
Owner:RES DEVMENT FOUND
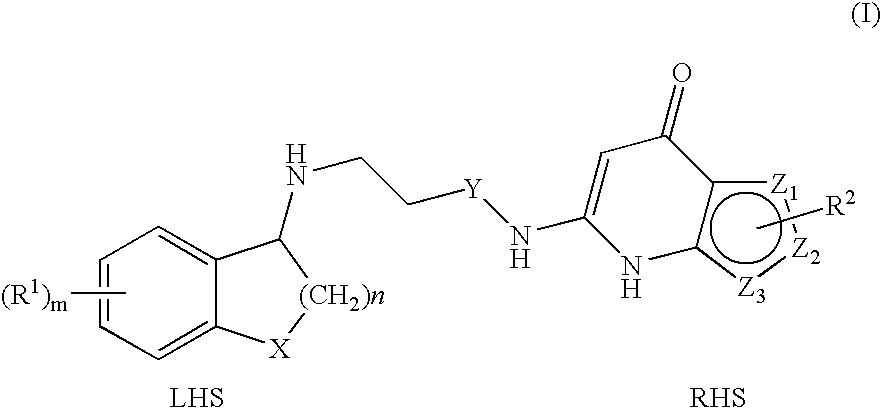
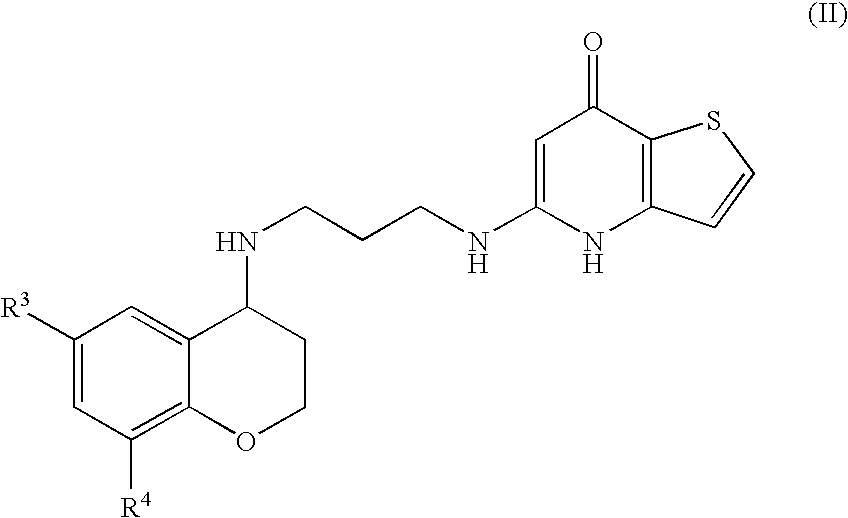
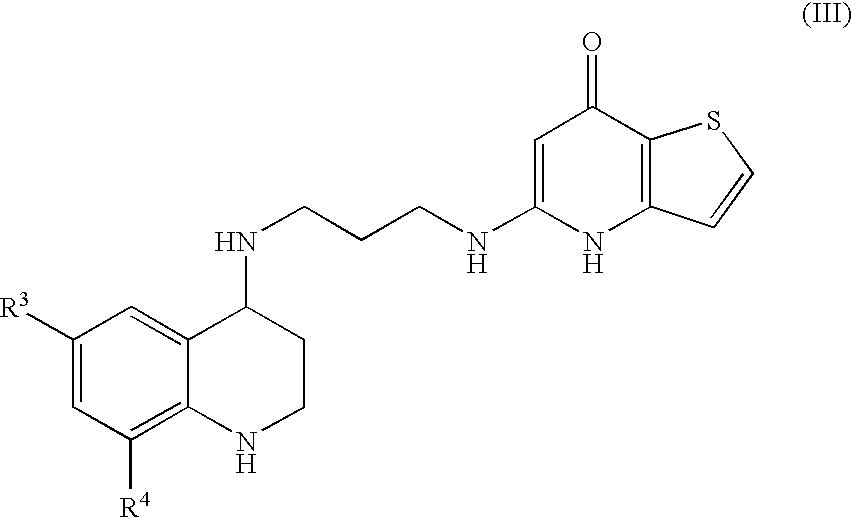
Substituted Thienopyridone Compounds With Antibacterial Activity
Novel bicyclic heteroaromatic compounds are provided that are inhibitors of bacterial methionyl tRNA synthetase (MetRS). Compounds of the invention generally have a left hand side chroman group or left hand side tetrahydroquinoline group and a right hand side thienopyridone group. Also disclosed are methods for their preparation and their use in therapy as antibacterial agents, particularly as anti-Clostridium difficile agents.
Owner:REPLIDYNE
Method for preparing intermediate of axitinib and application of intermediate in preparation of axitinib
The invention relates to a method for preparing an intermediate of axitinib and application of the intermediate in preparation of axitinib. The preparation method for the intermediate of axitinib 3-iodine-6-nitro-1-(teralin-2H-pyran-2-base)-1H-indazole comprises the following steps that 6-nitroindazole and 3,4-dihydro-2H-pyran react under the action of catalyst so as to protect perssad tetralin-2H-pyran-2-base at N-H site, and the key intermediate with high yield is obtained through iodination at site 3. The application of the intermediate in preparation of axitinib is as follows: Heck coupled reaction is carried out on the intermediate and 2-vinyl pyridine, then nitro reduction and diazo-reaction for iodination are sequentially carried out, finally, the axitinib is obtained after docking of 2-sulfydryl-N-methyl benzamide and deprotection. The related main initial raw materials are easy to purchase in markets, and the method has high yield and high molecule economic efficiency, is efficient and environment-friendly, and is suitable for industrial mass production.
Owner:湖南欧亚药业有限公司
Top coating composition for photoresist and method of forming photoresist pattern using same
Owner:SAMSUNG ELECTRONICS CO LTD
Enantiopure 4-deoxypentenosides, dihydropyrans and tetrahydropyrans and syntheses thereof
Owner:PURDUE RES FOUND INC
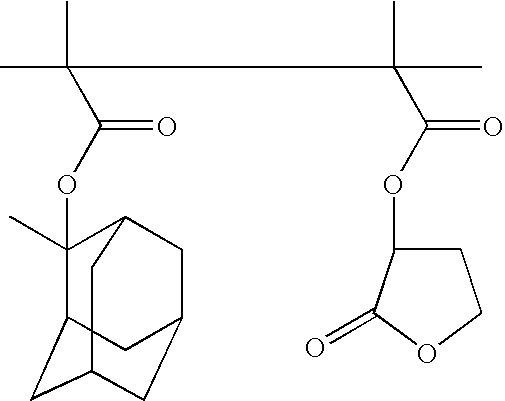
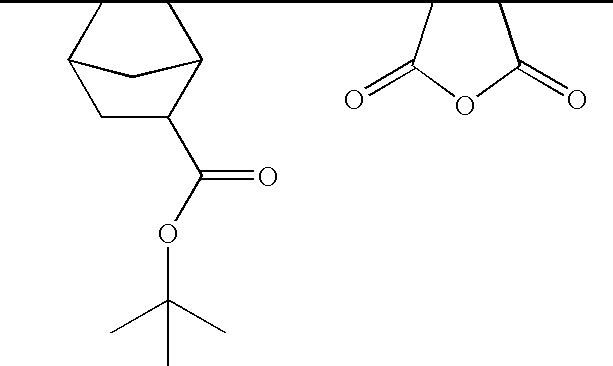
Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative
The invention discloses a method for catalytically preparing a 4,5-dihydropyran[c]chromene derivative, and belongs to the technical field of chemical material preparation.In the preparation reaction, the molar ratio of aromatic aldehyde to 4-hydroxycoumarin to malononitrile is 1:1:(1-1.2), the molar weight of an alkaline ionic liquid catalyst is 8%-15% of that of aromatic aldehyde, the volume dose of a 50% ethanol water solution serving as reaction solvent on a milliliter basis is 8-10 times of the molar weight of aromatic aldehyde on a millimole basis, the reflux reaction time ranges from 10 min to 28 min, after the reaction is finished, cooling is conducted to room temperature, extraction filtration is conducted, filter residues are washed with ethyl alcohol and vacuum-dried, and the 4,5-dihydropyran[c]chromene derivative is obtained.Compared with a preparation method adopting other alkaline ionic liquid catalysts, the method for catalytically preparing the 4,5-dihydropyran[c]chromene derivative has the advantages of being high in catalytic activity of the catalyst, free of treatment during the reuse process, high in raw material utilization rate, easy and convenient to operate in the whole preparation process and the like, and large-scale industrial application is convenient.
Owner:日照新睿招商发展有限公司
Enantioselective syntheses of heteroyohimbine natural product intermediates
Enantioselective syntheses of cis- and trans-bicyclic dihydropyran compounds, and other intermediates, en route to heteroyohimbine alkaloids.
Owner:NORTHWESTERN UNIV
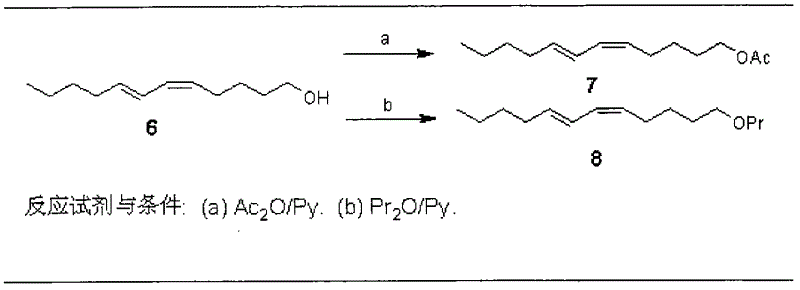
Process for synthesizing sex pheromone of pine caterpillar
ActiveCN102613177AEasy to operateSynthetic raw materials are readily availableBiocidePreparation by isomerisationGrignard reagentIodide
The invention discloses a process for synthesizing sex pheromone of pine caterpillar, which employs 2-hexyne-1-alcohol as an initial raw material, three-bond positional transference is carried out under the effect of lithium and propane diamine to obtain 5-hexyne-1-alcohol; under acidic condition, 5-hexyne-1-alcohol is reacted with dihydropyran to obtain 1-THP-5-hexyne-1-alcohol protected by THP on hydroxyl, Under co-catalysis of metal palladium and cuprous iodide, 1-THP-5-hexyne-1-alcohol and trans-dichloroethylene are subjected to coupling reaction to generate conjugate enyne(7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol; under the catalysis of metallic iron, (7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol and a n-Butyl bromide grignard reagent are further subjected to coupling reaction to obtain (7E)-1-THP-5-alkyne-7-alkene-1-dodecanol, under the catalytic reduction of metal zinc, (5Z, 7E)-1-THP-dodecanol dienol; under the camphor sulfonic acid condition, (5Z, 7E)-1-THP-dodecanol dienol removes the THP protective group to obtain the final target product (5Z, 7E)-dodecanol dienol. The method of the invention has the advantages of easily available synthesis raw materials, low cost, mild reaction condition, easy operation, high yield and good stereoselectivity.
Owner:WENZHOU MEDICAL UNIV +1
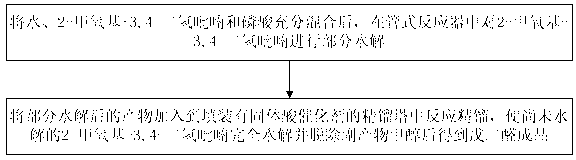
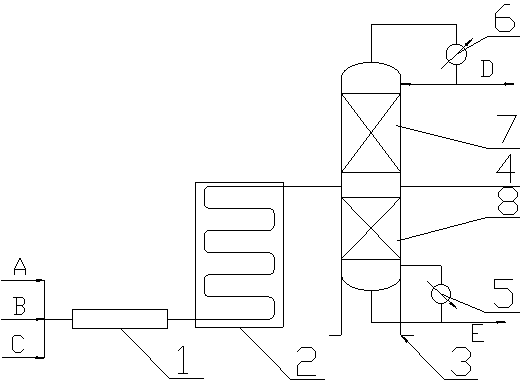
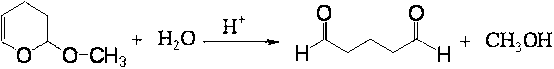
Method and device for continuously preparing glutaraldehyde
ActiveCN102992975ASimple structureLess investmentCarbonyl compound separation/purificationPreparation from heterocyclic compoundsPartial hydrolysisSolid acid
The invention discloses a method and a device for continuously preparing glutaraldehyde and belongs to the field of synthesis of chemical products. The method comprises the following steps of: fully mixing water, 2-methoxy-3,4-dihydropyran and phosphoric acid, partially hydrolyzing the 2-methoxy-3,4-dihydropyran in a tubular reactor, wherein the mass ratio of the water to 2-methoxy-3,4-dihydropyran to phosphoric acid is 1000-10000:1500-2500:1 during partial hydrolysis; adding the product of partial hydrolysis into a rectifying tower filled with a solid acid catalyst, reacting and rectifying, so that the 2-methoxy-3,4-dihydropyran is completely hydrolyzed; and removing the by-product methanol, thus obtaining the finished glutaraldehyde product. According to the method and the device, 2-methoxy-3,4-dihydropyran serves as a raw material, high-purity glutaraldehyde is prepared through partial hydrolysis, reaction and rectification; the glutaraldehyde is high in yield, high in purity, almost does not have impurities; and the cost is low and blending operation is omitted.
Owner:湖北微控生物科技有限公司
Method for producing optically active chroman-carboxylate
InactiveUS20060141591A1Facilitates separation and recoveryHigh purityOrganic chemistryFermentationAlcoholEnantiomer
In the method for producing an optically active chromancarboxylate of the present invention, one of the enantiomers of racemic chromancarboxylic acid is esterified in a solvent containing an alcohol in the presence of a biocatalyst. After the esterification, the other enantiomer, i.e., the non-reacted chromancarboxylic acid is separated out of the reaction mixture to obtain the aimed optically active ester. The optically active chromancarboxylate is useful as the material for medicines, agricultural chemicals, etc. The invention provides an efficient production method thereof which is industrially applicable.
Owner:MITSUBISHI GAS CHEM CO INC
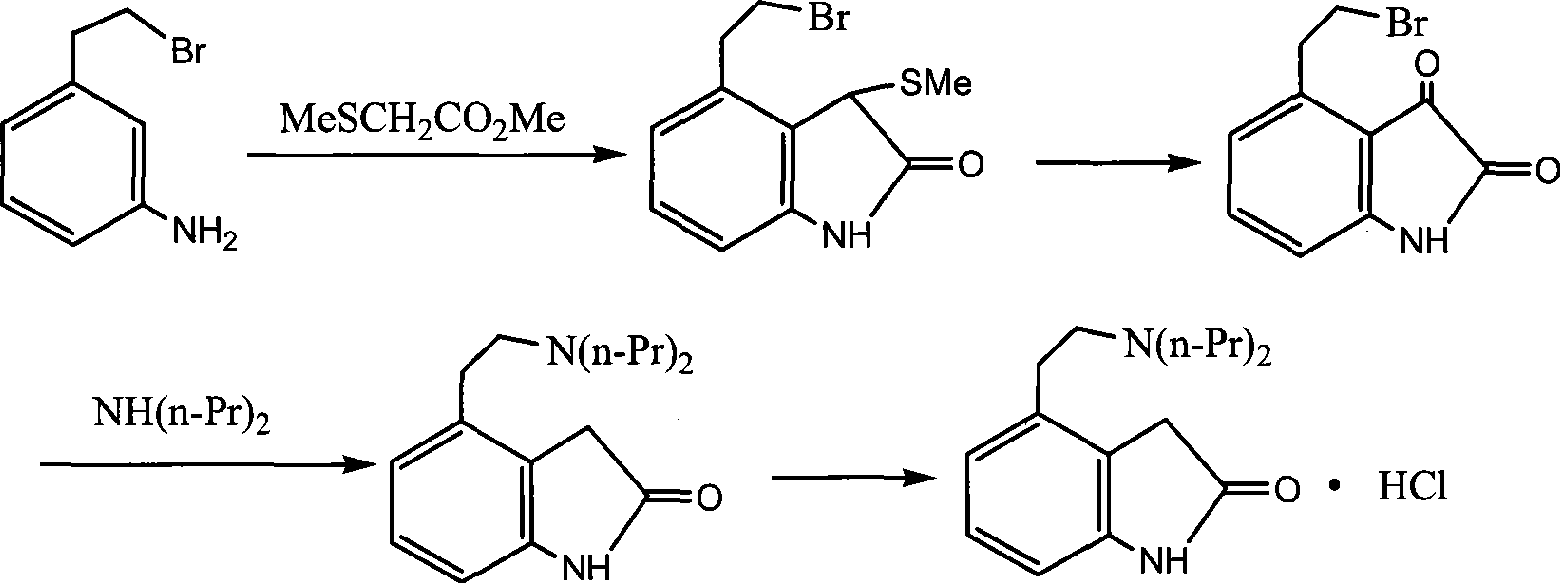
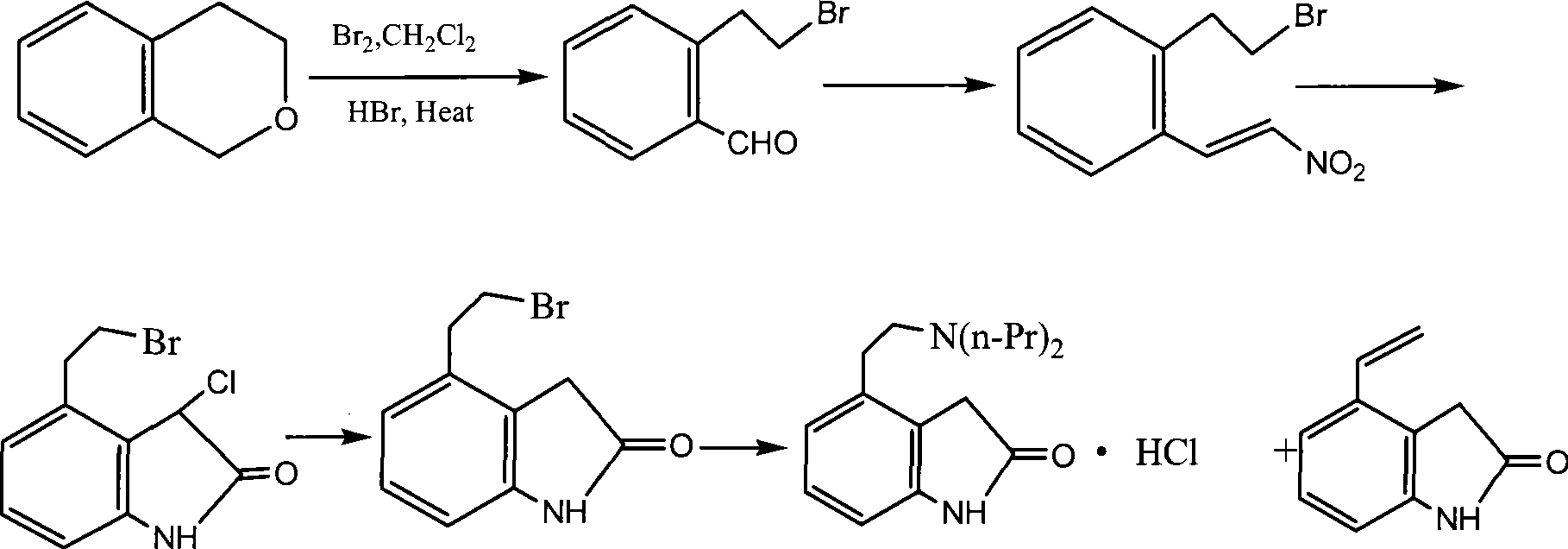
Preparation of ropinirole hydrochloride
InactiveCN101481347ARaw materials are easy to getLow costNervous disorderOrganic chemistryBenzaldehydeKetone
The invention relates to a method for preparing ropinirole hydrochloride, comprising the followings in turn: (1) beta-phenylethyl alcohol reacts with paraformaldehyde to produce isochroman; (2) the product obtained in step (1) reacts with bromide to produce 2-bromoethyl benzaldehyde; (3) the product obtained in step (2) reacts with nitromethane to produce 2-bromoethyl nitrostyrolene; (4) the product obtained in step (3) reacts with acetyl chloride to produce 4-bromoethyl-3-chloride-1, 3-dihydro-2H-indole-2-ketone; (5) 4-bromoethyl-1, 3- dihydro-2H-indole-2-ketone is obtained after catalytic hydrogenation of the product obtained in step (4); (6) 4-ethoxyl-1, 3-dihydro-2H-indole-2-ketone is obtained after acetylation and hydrolysis of the product obtained in step (5); (7) the product obtained in step (6) reacts with toluene sulfonic acid to produce toluene sulfonic acid-2-(2-oxygen-1, 3-dihydro-4-indole) ethyl ester; (8) the product obtained in step (7) and di-n-propylamine undergo reflux reaction in water and pH value is regulated by hydrochloric acid to 1-2 to obtain the ropinirole hydrochloride. In the method, the raw materials are easily acquired; the target products enjoy high selectivity and yield, thus being suitable for industrial production.
Owner:太仓浦源医药原料有限公司
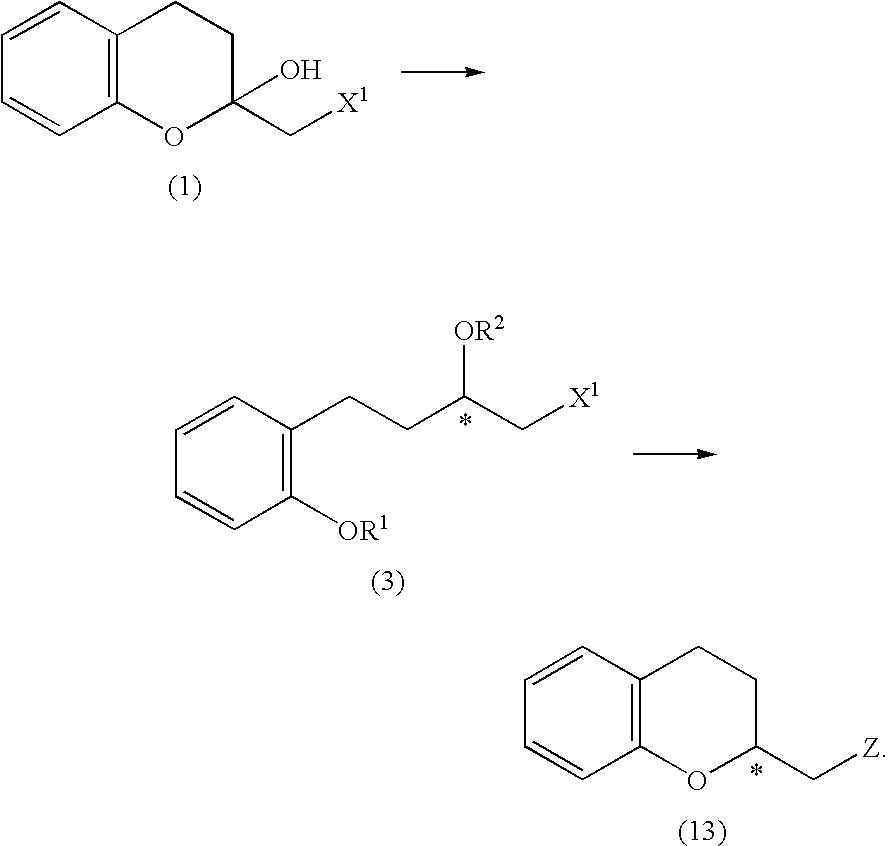
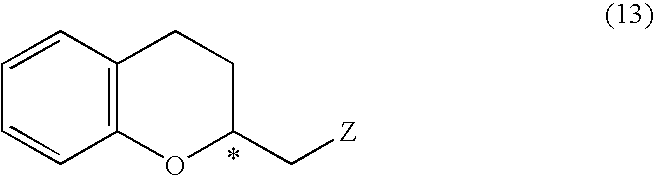
Process for producing optically active chroman derivative and intermediate
InactiveUS20050014818A1Efficient productionHighly practicalBiocideSulfonic acid esters preparationHemiacetalHalohydrin
A process for easily producing various optically active chroman derivatives that are useful as pharmaceutical intermediates from inexpensive starting materials is provided. Cyclic hemiacetal (1) obtained from dihydrocoumarin through one step is asymmetrically reduced to produce an optically active halohydrin derivative (3), and the optically active halohydrin derivative (3) is cyclized to produce an optically active chroman derivative (13):
Owner:KANEKA CORP
Method for synthesizing (E,E) Geranyl linalool
InactiveCN101070270AOrganic compound preparationHydroxy compound preparationSulfonyl chlorideNerolidol
This invention relates to synthetic method of a ( E, E) - geranyl linalool. The invention takes (E) - nerolidol as raw material. The hydroxyl is shield by dihydropyrane, gain ( E) - nerolidol tetrahydropyrane aether; selenium dioxide and teri-butyl hydroperoxide selectively oxidize the anti-form methyl of ( E) - nerolidol tetrahydropyrane aether to gain anti-form allyl position hydroxylated oxidative product ( E, E) - 12 - hydroxy nerolidol tetrahydropyrane aether, transit halogenating reaction to gain ( E, E) - 12 - halogeno- nerolidol tetrahydropyrane aether, then take reaction with isopropyl methyl ketone that is selectively divested one proton by diisopropyl amido lithium, generate ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - ketone, use sodium borohydride to reduce to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - alcohol, takes reaction with sulfonyl chloride or sulphonic acid ester with alkali presence to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 oxygen) -16 - 6, 10, 15 - triene - 3 - alcoholic sulphonic acid ester, then divide sulphonic acid ester group under base catalysis to gain ( E, E) - geranyl linalool tetrahydropyrane aether, and by deprotection to gain ( E, E) - geranyl linalool. ËFor the configuration of ( E) - nerolidol 3 position tertiary carbon is not influenced in the course of reaction, if use ( E) - nerolidol that has optical activity as raw material, should gain optical active ( E, E) - geranyl linalool. ((E, E)-geranyl linalool can replace Teprenone and such type medicament intermediate, natural product intermediate, insect pheromone and spice etc.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
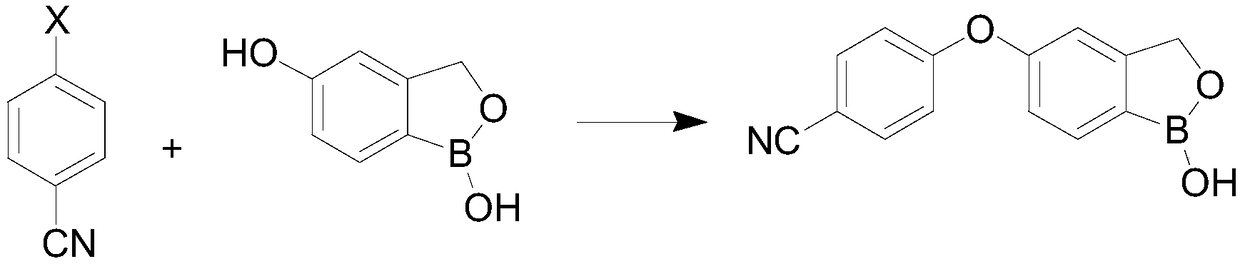
Preparation method of crisaborole
InactiveCN108659024ALow priceMild reaction conditionsGroup 3/13 element organic compoundsTert-butyldimethylsilyl chlorideTrimethyl borate
The invention discloses a preparation method of crisaborole. The method comprises the steps as follows: a compound shown in a formula I and alkali metal borohydride are subjected to a contact reaction, and a compound shown in a formula II is obtained; the compound shown in the formula II and a compound a are subjected to a contact reaction, and a compound III is obtained; or the compound shown inthe formula II and dihydropyran are subjected to a contact reaction, and a compound III is obtained, wherein the compound a is trimethylchlorosilane, tert-butyldimethylsilyl chloride or chloromethyl methyl ether; the compound shown in the formula III and an isopropylmagnesium chloride solution are subjected to a contact reaction, and a standby solution is obtained; then, the standby solution is added to a mixed solution of a compound b and a third organic solvent for a contact reaction, hydrochloric acid is added for a contact reaction, and crisaborole, namely, a compound shown in a formula IV, is obtained, wherein the compound b is 2-alkoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, triisopropyl borate or trimethyl borate.
Owner:WUHAN POLYTECHNIC UNIVERSITY
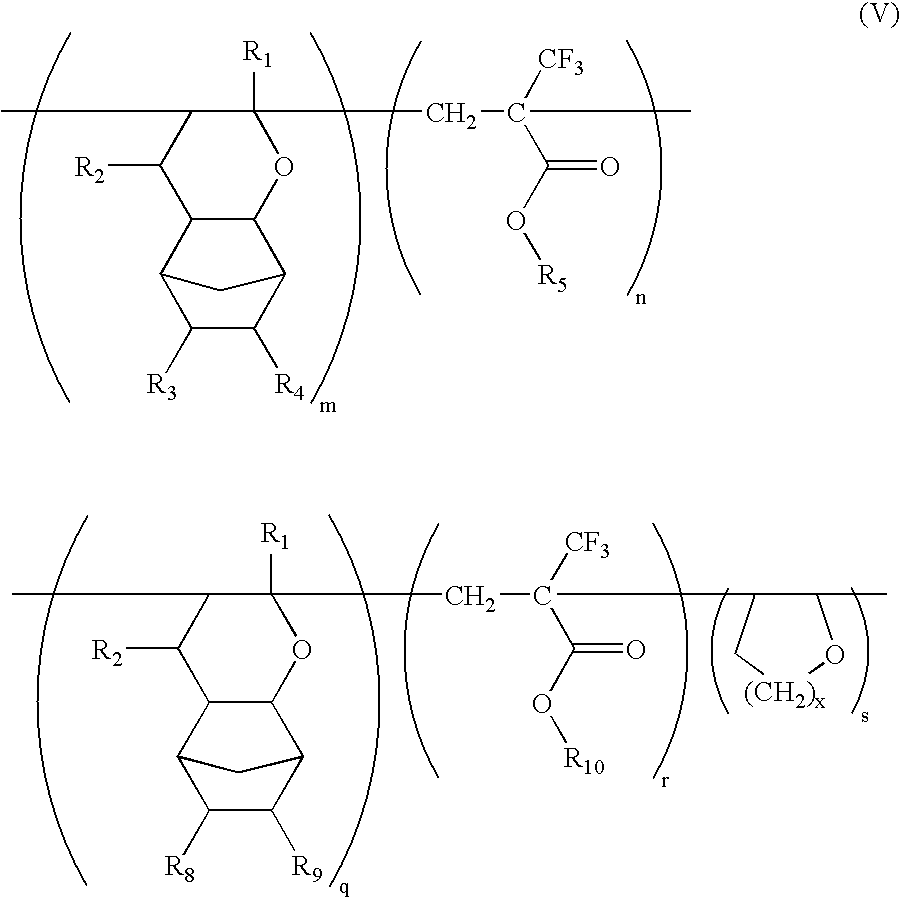
Ether monomers and polymers having multi-ring structures, and photosensitive polymers and resist compositions obtained from the same
InactiveUS6962768B2Satisfactory resistanceFree from pollutionOrganic chemistryPhotosensitive materialsResistPolymer science
Provided are a variety of monomers suitable of producing photosensitive polymers, that are in turn, useful in photoresist compositions, through radical (cationic) polymerization including at least one multi-ring alkenyl ethers and one α-fluorinated acrylate. The resulting photoresist compositions exhibit both acceptable resistance to dry etching processing and light transmittance suitable for use with various light sources such as KrF excimer lasers, ArF excimer lasers or F2 excimer lasers, in a photolithography process to produce fine photoresist patterns. In addition to the multi-ring alkenyl ethers and α-fluorinated acrylates, additional monomers comprising one or more cyclic aliphatic and heterocyclic compounds, both unsubstituted and substituted, in particular dihydropyrans, may be incorporated into the photosensitive polymers. Photosensitive polymers can then be produced by combining these various monomer units to form copolymers, terpolymers and higher order polymers, an exemplary embodiment of which may be generally represented by the formula V:
Owner:SAMSUNG ELECTRONICS CO LTD
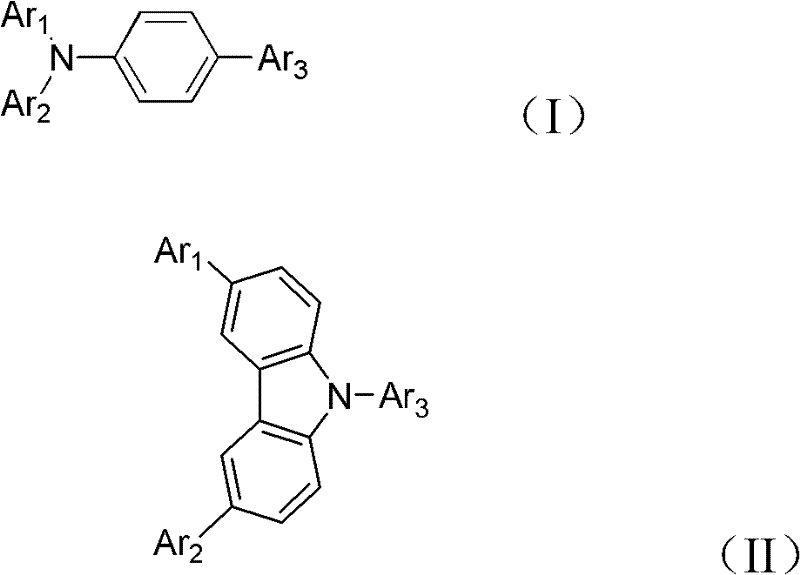
Alcohol-soluble hole-transporting molecular material and preparation method thereof
InactiveCN102649766AGood hole transport propertiesGood alcohol solubilityOrganic compound preparationLuminescent compositionsAlkaneSolubility
The invention discloses an alcohol-soluble hole-transporting molecular material and a preparation method thereof. A plurality of hydroxyl groups are introduced into aromatic amine molecules, so that the prepared material has excellent hot-transporting performance and alcohol solubility, and is insoluble in other types of solvents such as toluene, chlorobenzene, dichloromethane, chloroform and the like. During the preparation, the alcohol-soluble hole-transporting molecular material is prepared by the following steps: protecting the hydroxyl groups of alkane, aromatic hydrocarbon, alkane derivative or aromatic hydrocarbon derivative containing the hydroxyl groups through dihydropyran; obtaining an aromatic amino precursor through reaction such as Suzuki coupling, Ullmann coupling, Stille coupling and the like; and finally removing the protective groups to obtain the target product. The material can perform film formation in alcohol solution, can resist corrosion by other solvents such as toluene, chlorobenzene, dichloromethane, chloroform and the like, and has application prospect in solution processing of multi-layer organic photoelectric devices.
Owner:SOUTH CHINA UNIV OF TECH
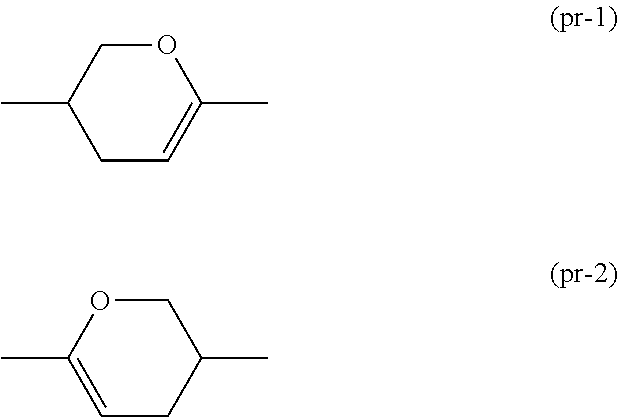
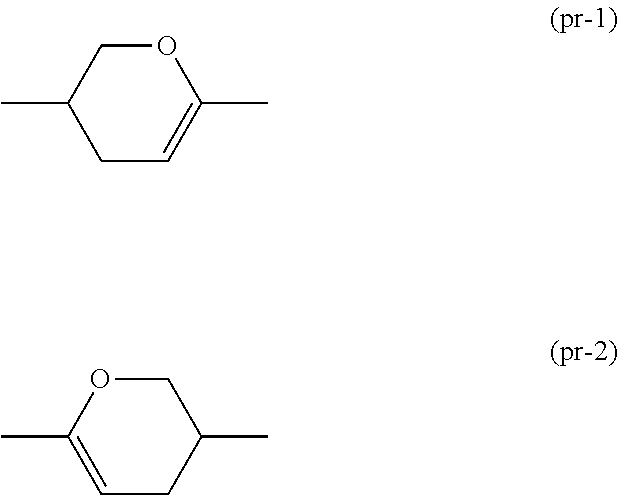
Dihydropyran compound, liquid crystal composition and liquid crystal display device
ActiveUS20150361344A1Stable environmentSmall viscosityLiquid crystal compositionsOrganic chemistryDielectric anisotropyUltraviolet lights
To provide a liquid-crystal compound satisfying at least one physical property such as high stability to ultraviolet light and heat, a high clearing point, low minimum temperature of a liquid crystal phase, small viscosity, suitable optical anisotropy, large dielectric anisotropy and excellent compatibility with other liquid-crystal compounds, a liquid-crystal composition containing the compound, and a liquid-crystal display device including the composition.The compound is represented by formula (1), the composition contains the compound, and the liquid-crystal display device includes the composition:wherein, for example, Ra and Rb are alkyl; rings A1, A2, A3 and A4 are 1,4-cyclohexylene or 1,4-phenylene; and P is a divalent group represented by formula (pr-1) or (pr-2):wherein, for example, Z1, Z2, Z3 and Z4 are a single bond or alkylene; and a, b, c and d are 0, 1 or 2, and a sum of a, b, c and d is 5 or less.
Owner:JNC CORP +1
Substituted 5-chroman-5-YL-ethylamine compounds and their use for the treatment of glaucoma
InactiveUS20050171190A1Good chemical stabilityDesired length of therapeutic activityBiocideSenses disorderMedicineElevated intraocular pressure
Substituted 5-chroman-5-yl-ethylamine compounds are disclosed. Also disclosed are methods for the lowering and controlling of normal or elevated intraocular pressure as well as a method for the treatment of glaucoma using compositions containing one or more of the compounds of the present invention.
Owner:ALCON INC
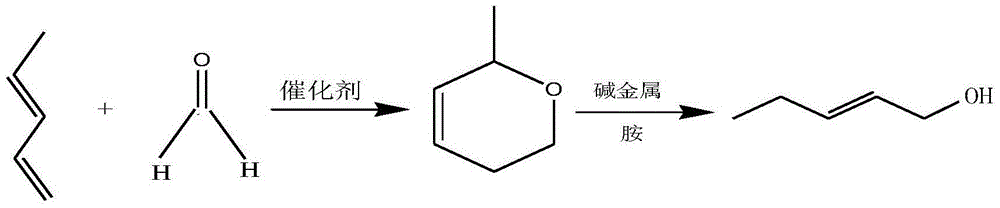
Preparation method of Fe/Mo-Al2O3 catalyst and method of synthesizing leaf alcohol by virtue of piperylene
ActiveCN104689824AEasy to prepareMild process conditionsMetal/metal-oxides/metal-hydroxide catalystsPreparation by hydrolysisSolventMethyl group
The invention relates to a preparation method of a Fe / Mo-Al2O3 catalyst and a method of synthesizing leaf alcohol by virtue of piperylene. The method comprises the following steps: with Fe / Mo as the active component, gamma-Al2O3 as a carrier and formaldehyde or acetaldehyde as a solvent, under the conditions of heating and stirring, catalytically synthesizing 2-methyl-5,6-dihydropyran; and converting 2-methyl-5,6-dihydropyran into the leaf alcohol under the catalysis of alkali metal and amine. According to the method disclosed by the invention, Fe / Mo-Al2O3 is firstly adopted as a catalyst for synthesizing 2-methyl-5,6-dihydropyran through piperylene; according to the process for catalyzed synthesis of 2-methyl-5,6-dihydropyran, conditions are mild, and the equipment requirements are low; Diels-Alder reaction is carried out on piperylene under the action of the catalyst without changing the catalyst; 2-methyl-5,6-dihydropyran, serving as a synthetic intermediate, is subjected to reaction under the catalysis of alkali metal and amine to easily produce leaf alcohol serving as a target product, and purity and yield are high.
Owner:TIANJIN UNIV
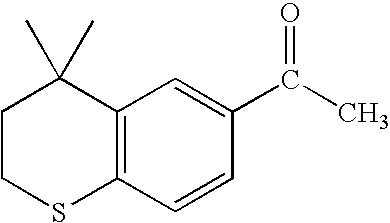
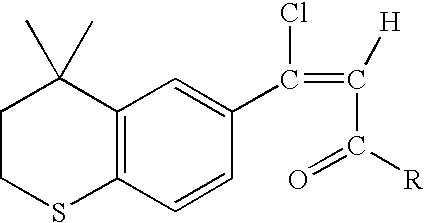
Process for the preparation of 4,4-dimethyl-6-ethynylthiochroman
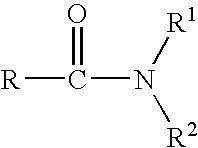
An improved process for the preparation of key intermediates for tazarotene, 4,4-dimethyl-6-ethynylthiochroman, is provided comprising (a) reacting 4,4-dimethyl-6-acetylthiochroman of the formula with an acid chloride and an amido-group containing compound of the general formula wherein R is hydrogen or a hydrocarbyl of from 1 to 15 carbon atoms and R1 and R2 can be the same or different and are hydrocarbyls of from 1 to 15 carbon atoms or R1 and R2 together with the nitrogen atom to which they are bonded are joined together to form a heterocyclic group, optionally containing one or more additional heterocyclic atoms, or one of R1 and R2 together with the nitrogen atom to which it bonded are joined together with the carbonyl radical to form a heterocyclic group, optionally containing one or more additional heterocyclic atoms to form a β-chloro vinyl carbonyl compound intermediate of the general formula wherein R has the aforestated meanings; and (b) reacting the β-chloro vinyl carbonyl compound intermediate with an alkali metal to provide the 4,4-dimethyl-6-ethynylthiochroman.
Owner:GLENMARK GENERRICS LTD
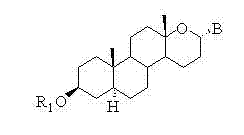
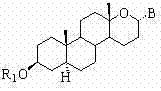
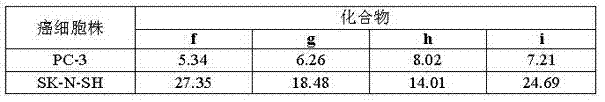
Steroid n-glycoside analogue taking dihydro-pyranoid ring as D ring and preparation and application thereof
ActiveCN102391356AGood inhibitory effectEasy to prepareOrganic active ingredientsSteroidsGlycosideMedicine
The invention belongs to the technical field of organic chemistry, relates to an n-glycoside analogue and in particular relates to a steroid n-glycoside analogue taking a dihydro-pyranoid ring as a D ring and a preparation and an application thereof. The steroid n-glycoside analogue taking the dihydro-pyranoid ring as the D ring has significant inhibition effects against a variety of tumor cell strains, such as the cell strains of prostatic cancer, human neuroblastoma and the like, and can be used for preparing anti-tumor medicaments. The steroid n-glycoside analogue taking the dihydro-pyranoid ring as the D ring is prepared trough five-step reactions, namely oxidation, hydroxyl protection, reduction, selective oxidation and glycosidation, the preparation method is simple and the conditions are mild.
Owner:PHARMARON BEIJING
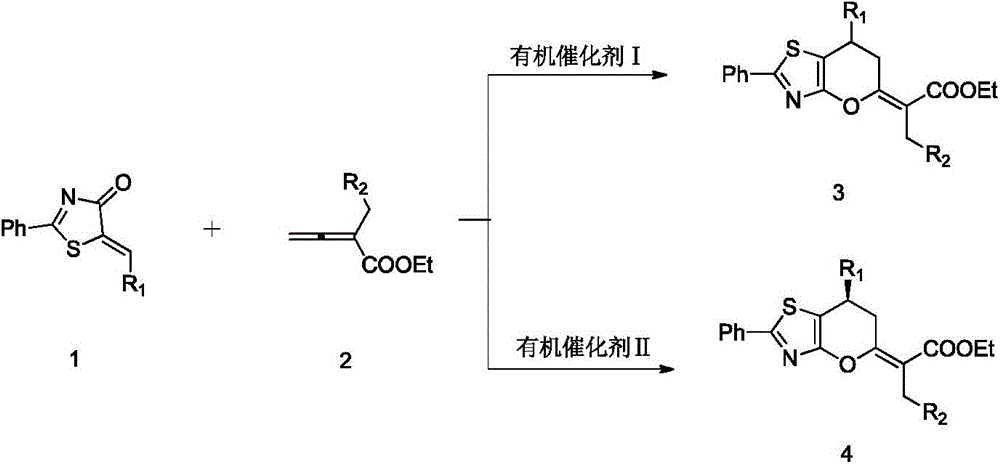
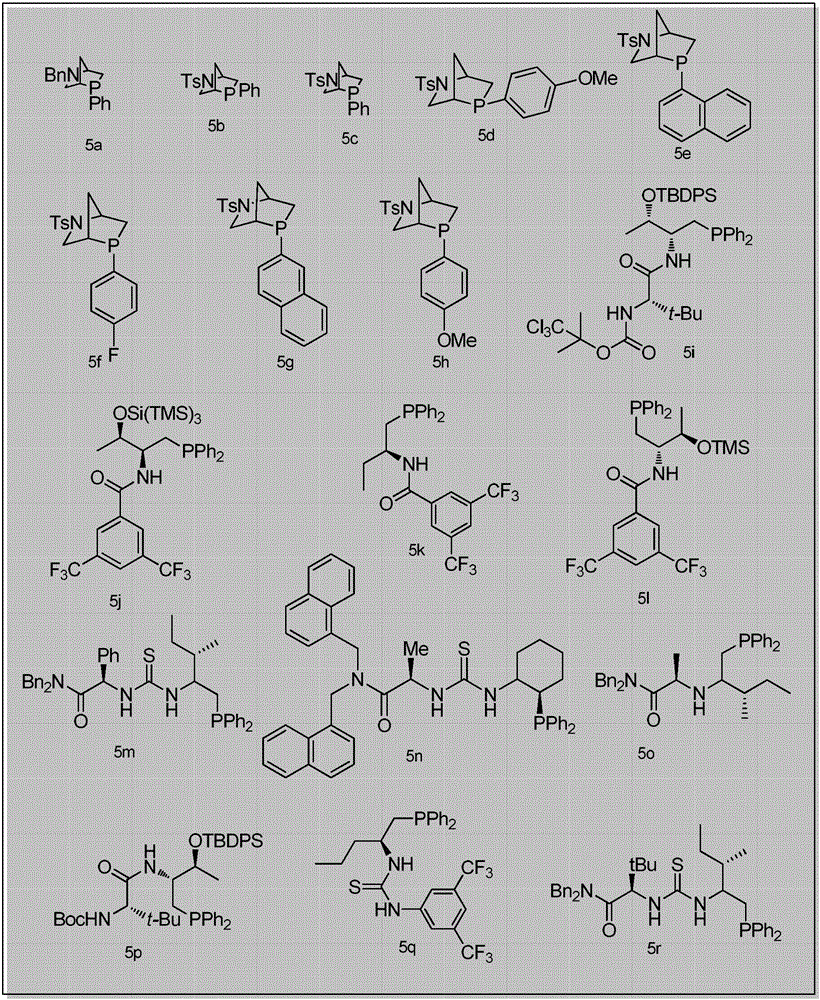
2,3-dihydropyranothiazole derivatives and preparation method thereof
InactiveCN105669702ANo heavy metal residuesIncrease diversityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolventDihydropyran
The invention discloses 2,3-dihydropyranothiazole derivatives and a preparation method thereof. The preparation method comprises the following steps: in a dry Schlenk tube, uniformly mixing a first compound and allenoate in a solvent, adding an organic phosphine catalyst, stirring, and carrying out cycloaddition reaction to obtain the derivatives. The invention provides a simple and feasible novel method for synthesizing pyranothiazole derivatives. The required raw materials can be prepared by simple reaction, and thus, are low in price. The synthetic compounds are not reported in the documents, and are all new compounds. The method is implemented by carrying out cycloaddition reaction under the catalytic action of phosphine without the action of transition metals, and thus, avoids metal residues. Meanwhile, the cycloaddition reaction belongs to atomic economic reaction.
Owner:CHINA AGRI UNIV
Ether monomers and polymers having multi-ring structures, and photosensitive polymers and resist compositions obtained from the same
InactiveUS20040170919A1Improved resistance to dry etchingImprove adhesionOrganic chemistryPhotosensitive materialsResistPolymer science
Provided are a variety of monomers suitable of producing photosensitive polymers, that are in turn, useful in photoresist compositions, through radical (cationic) polymerization including at least one multi-ring alkenyl ethers and one alpha-fluorinated acrylate. The resulting photoresist compositions exhibit both acceptable resistance to dry etching processing and light transmittance suitable for use with various light sources such as KrF excimer lasers, ArF excimer lasers or F2 excimer lasers, in a photolithography process to produce fine photoresist patterns. In addition to the multi-ring alkenyl ethers and alpha-fluorinated acrylates, additional monomers comprising one or more cyclic aliphatic and heterocyclic compounds, both unsubstituted and substituted, in particular dihydropyrans, may be incorporated into the photosensitive polymers. Photosensitive polymers can then be produced by combining these various monomer units to form copolymers, terpolymers and higher order polymers, an exemplary embodiment of which may be generally represented by the formula V:
Owner:SAMSUNG ELECTRONICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/BDA0000991840970000031.PNG)
![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/BDA0000991840970000032.PNG)
![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/FDA0000991840960000011.PNG)