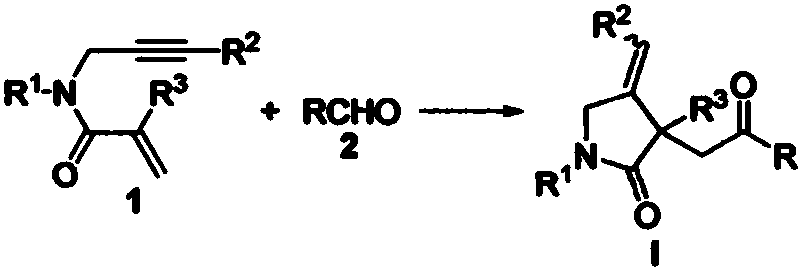
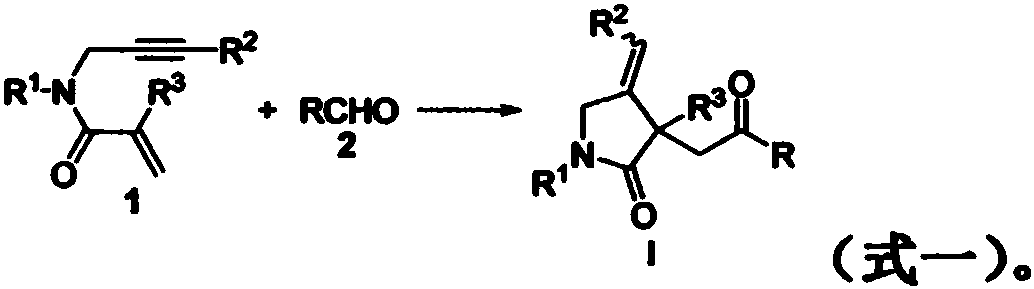
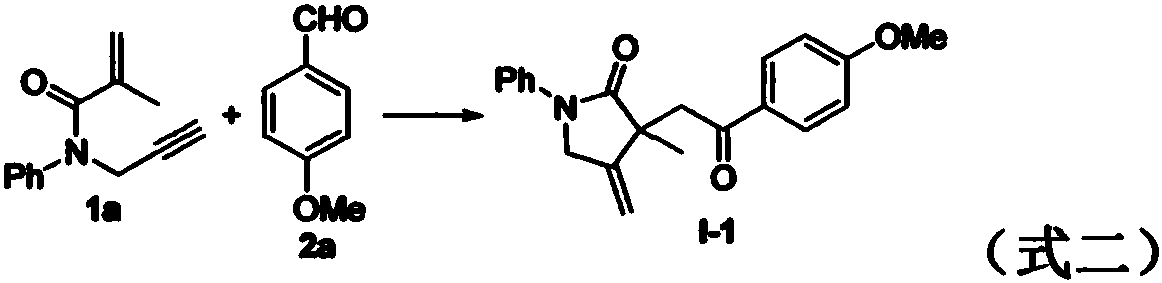
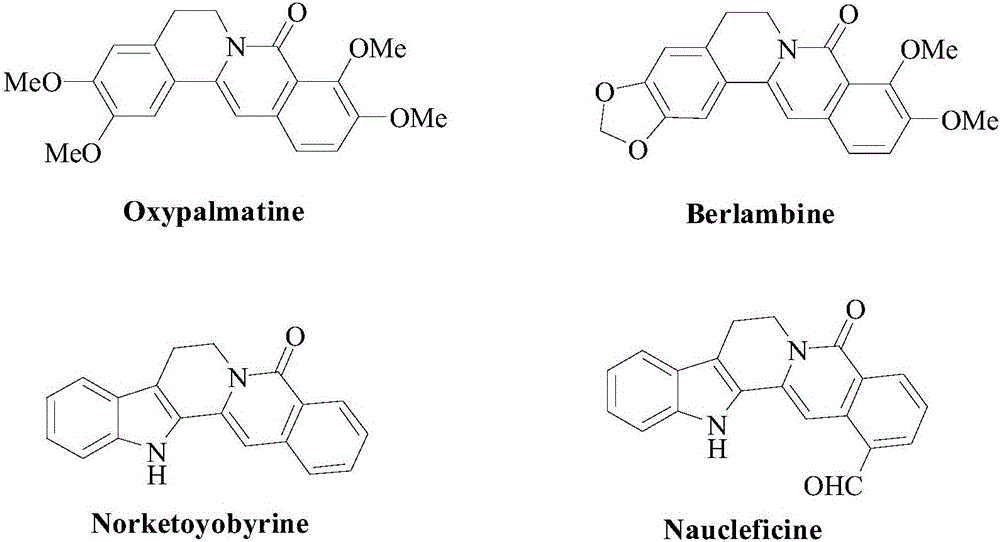
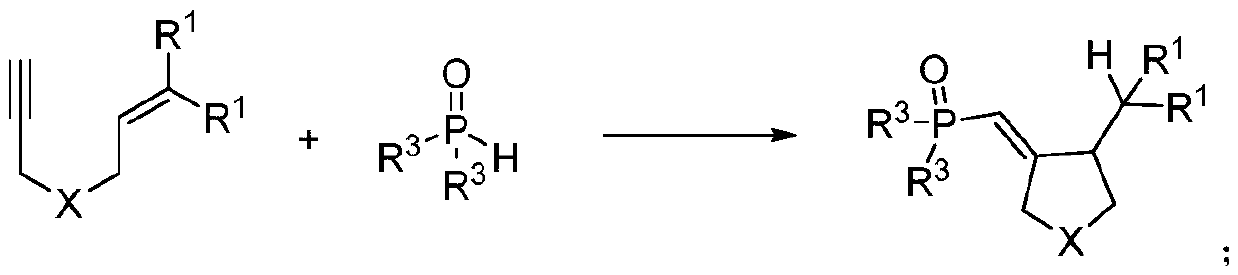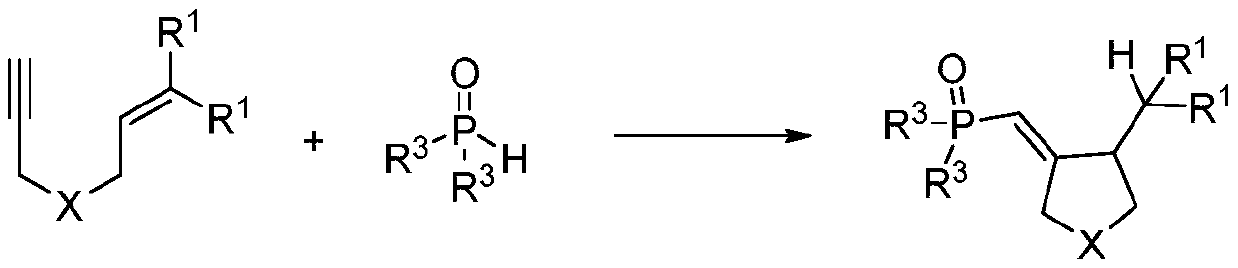Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
172 results about "Enyne" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
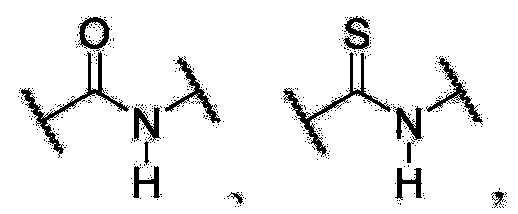
An enyne is an organic compound consisting of a double bond (alkene) and triple bond (alkyne). Its called a conjugated enyne when the double and triple bonds are conjugated. The term is a contraction of the terms alkene and alkyne.
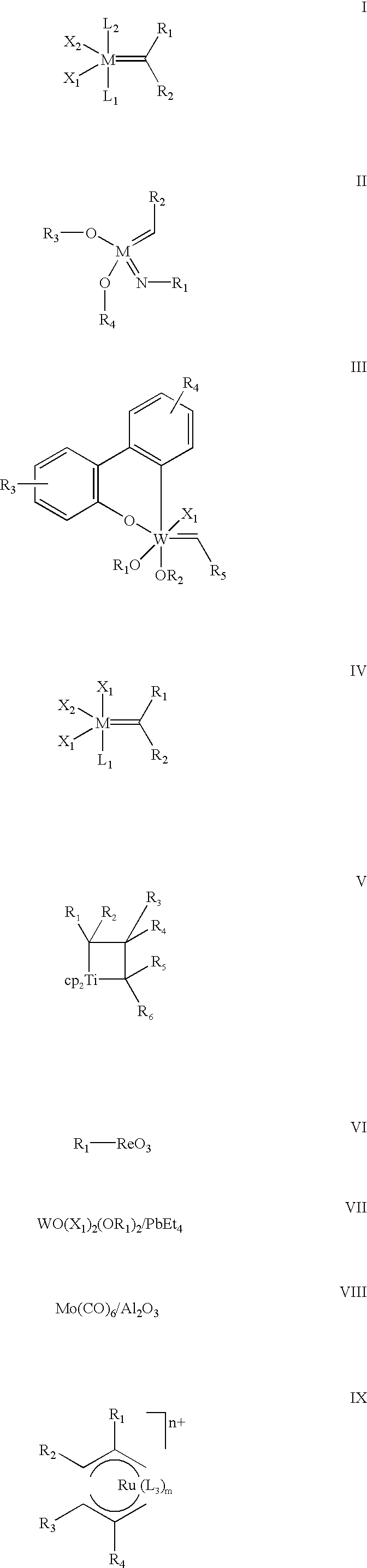
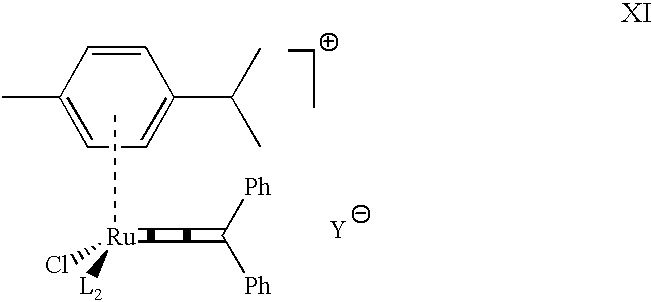
Highly active cationic ruthenium and osmium complexes for olefin metathesis reactions
InactiveUS6590048B1Hydrocarbon by isomerisationOrganic chemistry methodsRing-closing metathesisDepolymerization
The present invention describes the use of cationic vinylidene, allenylidene and higher cumulenylidene complexes of ruthenium or osmium as catalysts or catalyst precursors for olefin metathesis reactions of all types, as well as to new cationic allenylidene complexes of ruthenium and osmium which can be used as metathesis catalysts with preferred embodiment. These catalysts or catalyst precursors are easy to prepare from well accessible, stable and essentially non toxic starting materials, can be isolated and stored, they exhibit a high catalytic activity, a good compatibility with functional groups, solvents, water and additives, and they need not to be activated by any additive. Olefins of all types can be used as the substrates in the present invention in ring closing metathesis (RCM) of acyclic dienes and polyenes, the metathesis of enynes and dienynes, the ring opening metathesis polymerization (ROMP) of cyclic olefins, the acyclic diene metathesis polymerization (ADMET) of acyclic dienes or polyenes, the depolymerization of olefinic polymers, and the cross metathesis of two or more olefins. The present invention also applies to combinations of these types of metathetic reactions and domino processes thereof.
Owner:STUDIENGES KOHLE MBH
Catalysts and methods for catalytic oxidation
InactiveUS6906189B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetal catalystCross bridge
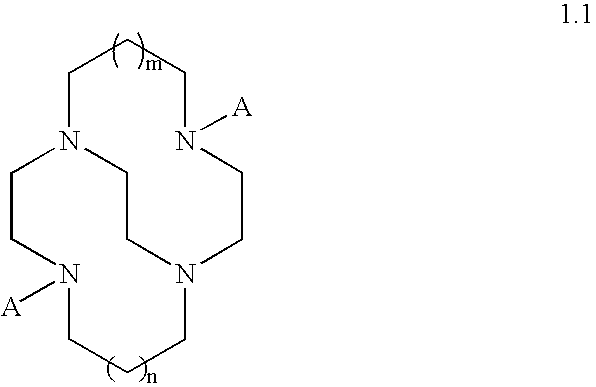
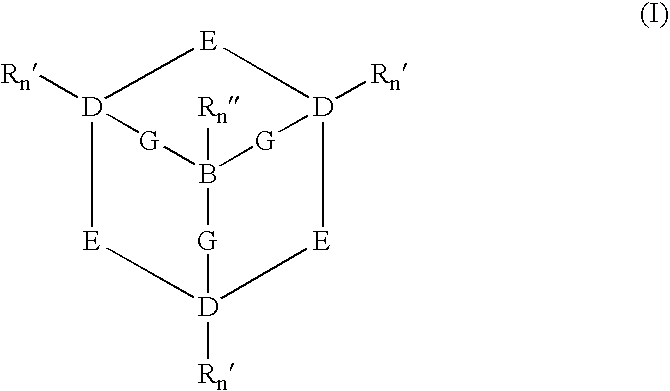
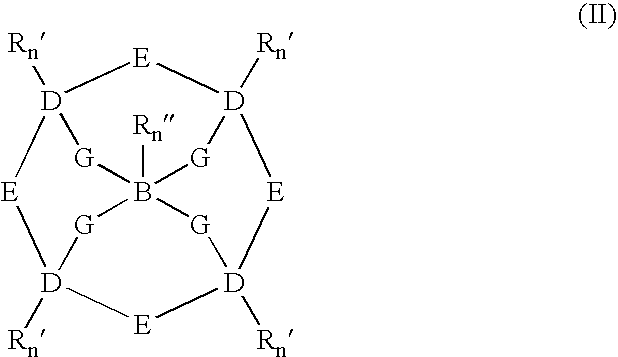
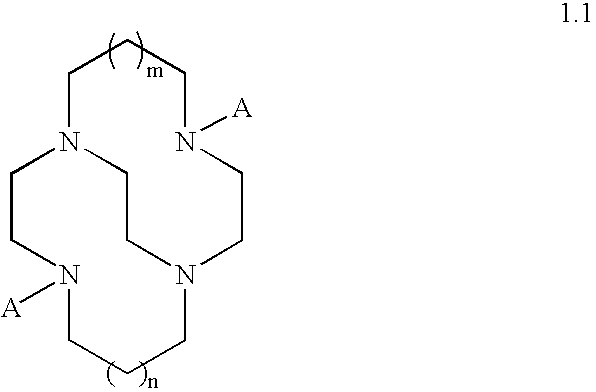
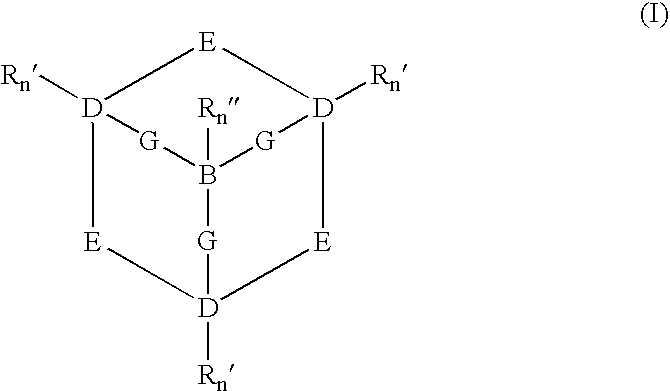
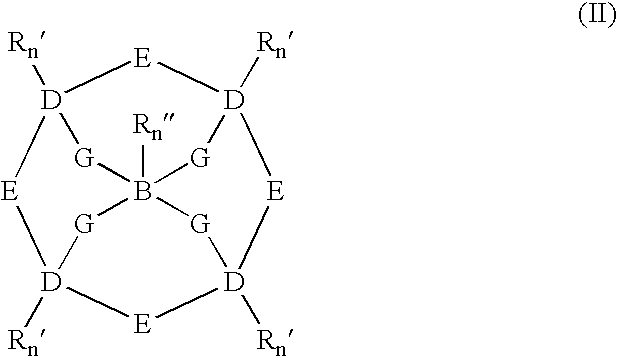
Catalytic systems and methods for oxidizing materials in the presence of metal catalysts (preferably manganese-containing catalysts) complexed with selected macropolycyclic rigid ligands, preferably cross-bridged macropolycyclic ligands. Included are using these metal catalysts in such processes as: synthetic organic oxidation reactions such as oxidation of organic functional groups, hydrocarbons, and heteroatoms, including enantiomeric epoxidation of alkenes, enynes, sulfides to sulfones and the like; oxidation of oxidizable compounds (e.g., stains) on surfaces such as fabrics, dishes, countertops, dentures and the like; oxidation of oxidizable compounds in solution, dye transfer inhibition in the laundering of fabrics; and further in the bleaching of pulp and paper products.
Owner:THE PROCTER & GAMBLE COMPANY
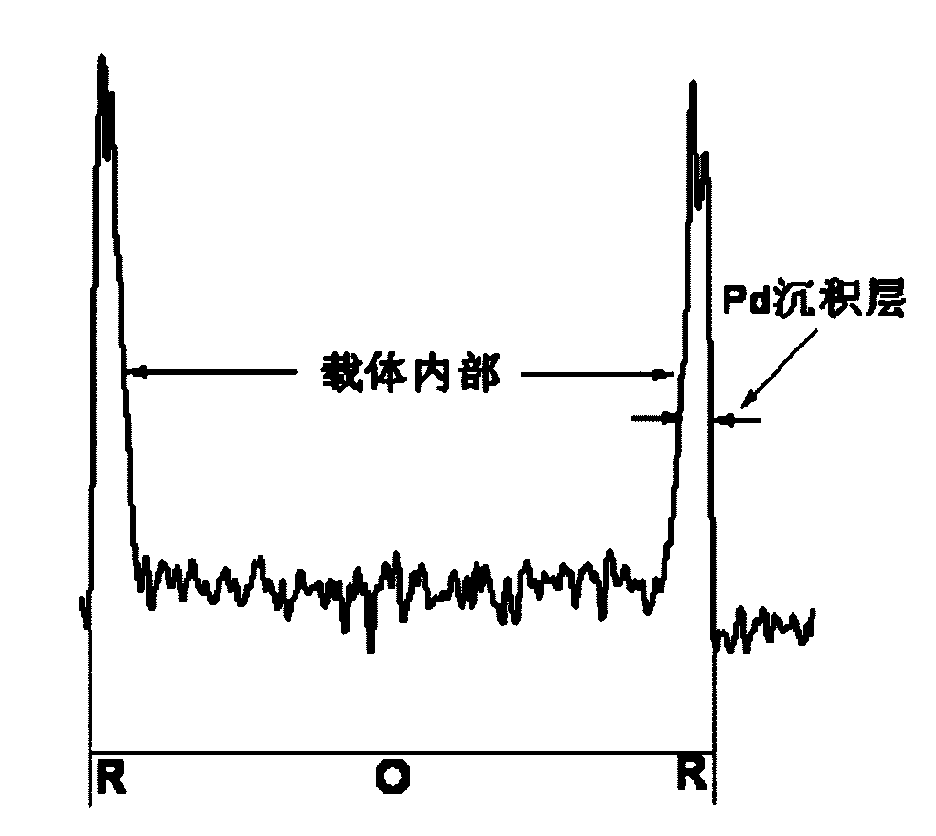
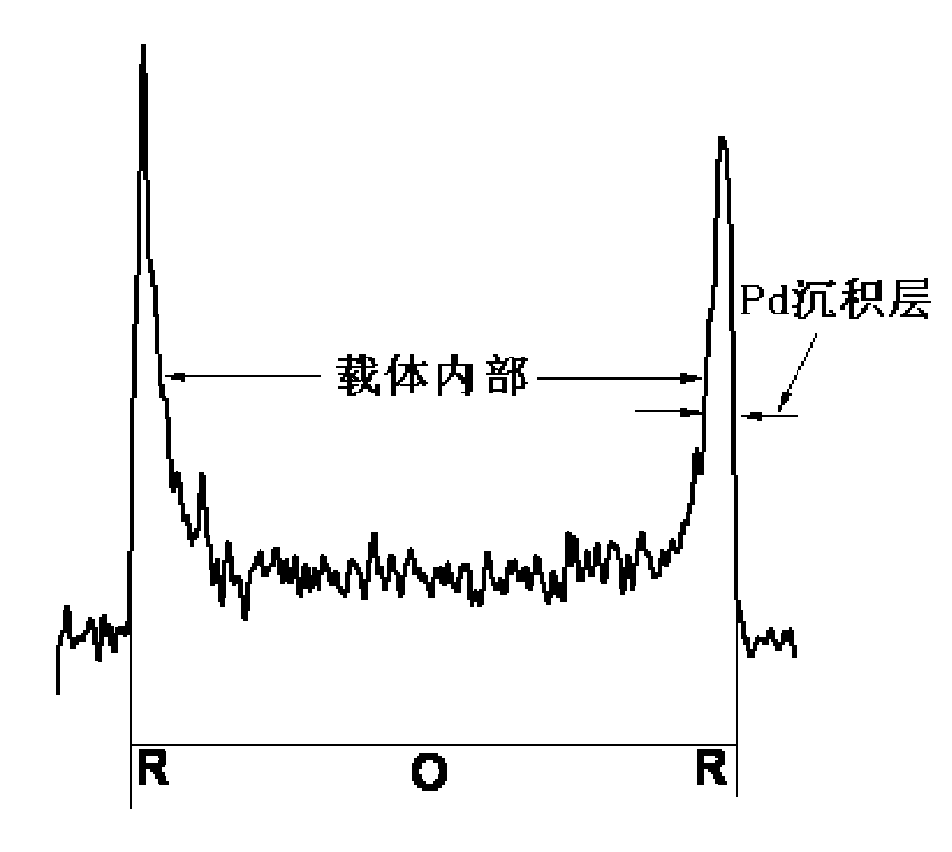
Eggshell type Pd catalyst prepared by reaction deposition method
ActiveCN101618320AImprove performanceReduce dosageOrganic compound preparationPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesEggshellMetal salts
The invention discloses an eggshell type Pd catalyst prepared by a reaction deposition method, which belongs to the technical field of industrial catalysts. The method comprises the following steps: adding a carrier into Pd metal salt solution; controlling the deposition of Pd on the surface of the carrier by a rapid reduction reaction; and carrying out filtering, washing as well as heating and drying in inert atmosphere to form a stable eggshell type Pd catalyst. The eggshell type Pd catalyst has the advantages of simple process, controllable structure, low energy consumption process and the like; Pd particles in the prepared eggshell type Pd catalyst are evenly distributed on the surface of the carrier, thus the eggshell type Pd catalyst reduces the consumption of noble metal Pd, effectively reduces the cost of the catalyst and has favorable industrial application prospect. The eggshell type Pd catalyst can be used for enyne hydrocarbon selective hydrogenation, cracking ethylene C9 fraction selective hydrogenation, olefin aldehyde selective hydrogenation, anthraquinone catalytic hydrogenation in H2O2 synthesis and other reactions.
Owner:成都大研科技产业发展有限公司
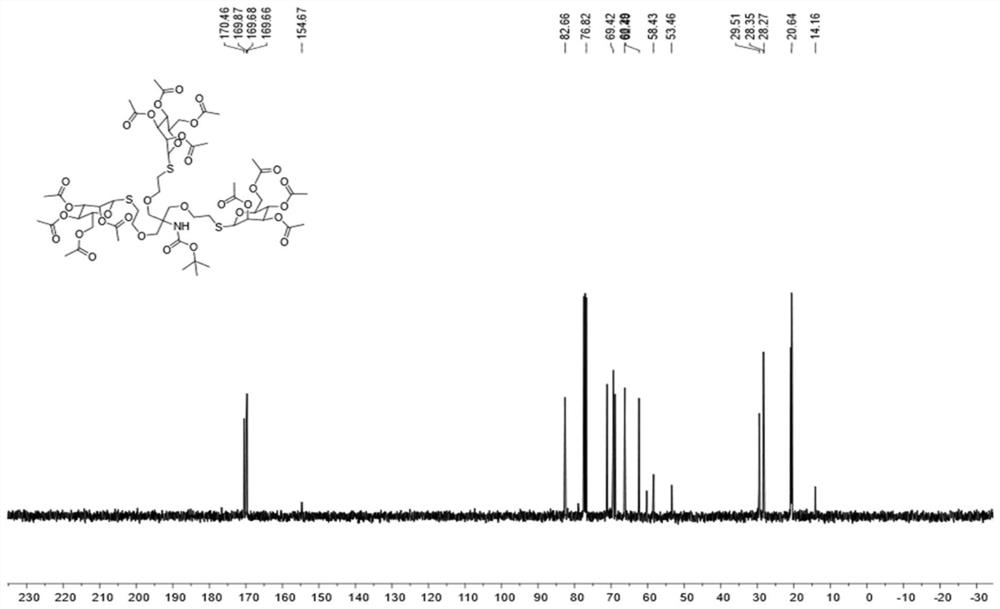
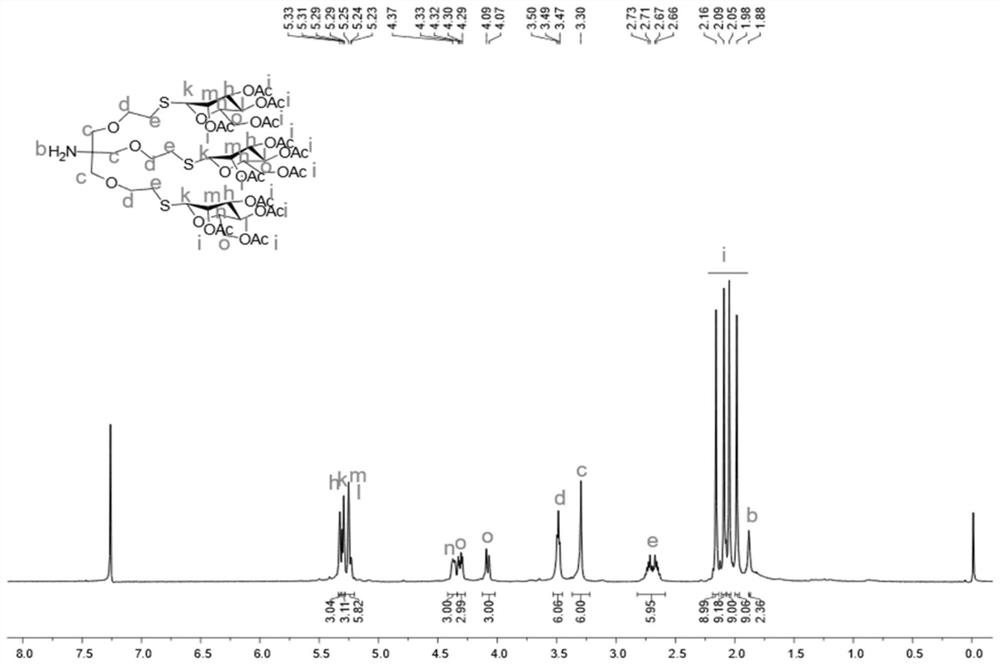
Self-repairing solid-state hybrid dynamic polymer and application thereof
PendingCN109666168ARich dynamic reversible characteristicsVarious forms of activationDevice materialBackbone chain
The invention discloses a self-repairing solid-state hybrid dynamic polymer. The self-repairing solid-state hybrid dynamic polymer contains sulfydryl-Michael addition bond dynamic covalent crosslinking and supermolecular hydrogen bond interaction, wherein the sulfydryl-Michael addition bond dynamic covalent crosslinking reaches above a gel point in at least one crosslinking network; sulfydryl-Michael addition bonds are obtained through sulfydryl-Michael addition reaction between sulfydryl groups and electron-deficient conjugate alkenes / enynes; the supermolecular hydrogen bond interaction contains hydrogen bond interaction involving at least one from side hydrogen bond groups, backbone hydrogen bond groups and other end hydrogen bond groups; with dynamic reversibility, the sulfydryl-Michaeladdition bonds and the supermolecular hydrogen bonds endow the self-repairing solid-state hybrid dynamic polymer with good plasticity, self-repairability, repeatability, reusability and recoverability and ensure that the self-repairing solid-state hybrid dynamic polymer can be widely applied to self-repairing materials, flexible materials, shape memory materials, energy storage device materials and the like.
Owner:厦门天策材料科技有限公司
Catalysts and methods for catalytic oxidation
InactiveUS20070093379A1Good bleachingLow tendency to damageGroup 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsMetal catalystHeteroatom
Catalytic systems and methods for oxidizing materials in the presence of metal catalysts (preferably manganese-containing catalysts) complexed with selected macropolycyclic rigid ligands, preferably cross-bridged macropolycyclic ligands. Included are using these metal catalysts in such processes as: synthetic organic oxidation reactions such as oxidation of organic functional groups, hydrocarbons, and heteroatoms, including enantiomeric epoxidation of alkenes, enynes, sulfides to sulfones and the like; oxidation of oxidizable compounds (e.g., stains) on surfaces such as fabrics, dishes, countertops, dentures and the like; oxidation of oxidizable compounds in solution, dye transfer inhibition in the laundering of fabrics; and further in the bleaching of pulp and paper products.
Owner:BUSCH DARYLE HADLEY +2
Catalysts and methods for catalytic oxidation
InactiveUS20070298962A1Good bleachingLow tendency to damageOrganic-compounds/hydrides/coordination-complexes catalystsOrganic/inorganic per-compounds compounding agentsMetal catalystHeteroatom
Catalytic systems and methods for oxidizing materials in the presence of metal catalysts (preferably manganese-containing catalysts) complexed with selected macropolycyclic rigid ligands, preferably cross-bridged macropolycyclic ligands. Included are using these metal catalysts in such processes as: synthetic organic oxidation reactions such as oxidation of organic functional groups, hydrocarbons, and heteroatoms, including enantiomeric epoxidation of alkenes, enynes, sulfides to sulfones and the like; oxidation of oxidizable compounds (e.g., stains) on surfaces such as fabrics, dishes, countertops, dentures and the like; oxidation of oxidizable compounds in solution, dye transfer inhibition in the laundering of fabrics; and further in the bleaching of pulp and paper products.
Owner:PROCTER & GAMBLE CO
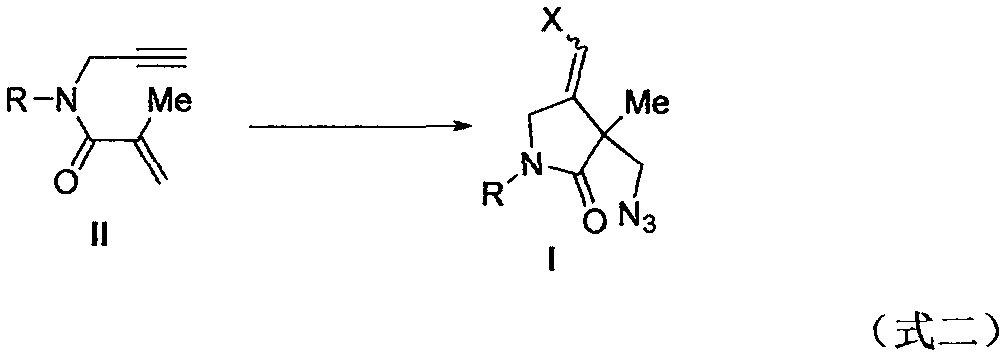
Preparation method of 2-pyrrolidinone compound
ActiveCN108409625AImprove efficiencyAvoid Metal ResidueOrganic chemistryOrganic solventTrimethylsilyl
The invention relates to a preparation method of a 2-pyrrolidinone compound. According to the method, a 1, 6-enyne compound serves as a raw material, iodosobenzene diacetate serves as an oxidizing agent and reacts with trimethyl silicon-based azide and N-chlorosuccinimide in organic solvents, and the 2-pyrrolidinone compound is conveniently prepared by excellent yield.
Owner:NINGBO UNIV
Fluorenone derivative, preparation method of fluorenone derivative and redox method of synthetic fluorenone
ActiveCN104370724AOxygen-containing compound preparationOrganic compound preparationOxidation-Reduction AgentRedox
The invention relates to a fluorenone derivative, a preparation method of the fluorenone derivative and a redox method of synthetic fluorenone. The preparation method includes a, performing precursor synthesis; b, performing target product synthesis; c, performing purifying. The fluorenone derivative is obtained by the redox method of four alkyne and polyenynes and serves as non-silver sensitive material applied widely. The fluorenone serving as a medical intermediate is used for synthesis of various drugs.
Owner:ANHUI NORMAL UNIV
Catalysts and methods for catalytic oxidation
InactiveUS20050192195A1Organic detergent compounding agentsOrganic-compounds/hydrides/coordination-complexes catalystsMetal catalystCatalytic oxidation
Catalytic systems and methods for oxidizing materials in the presence of metal catalysts (preferably manganese-containing catalysts) complexed with selected macropolycyclic rigid ligands, preferably cross-bridged macropolycyclic ligands. Included are using these metal catalysts in such processes as: synthetic organic oxidation reactions such as oxidation of organic functional groups, hydrocarbons, and heteroatoms, including enantiomeric epoxidation of alkenes, enynes, sulfides to sulfones and the like; oxidation of oxidizable compounds (e.g., stains) on surfaces such as fabrics, dishes, countertops, dentures and the like; oxidation of oxidizable compounds in solution, dye transfer inhibition in the laundering of fabrics; and further in the bleaching of pulp and paper products.
Owner:BUSCH DARYLE HADLEY +2
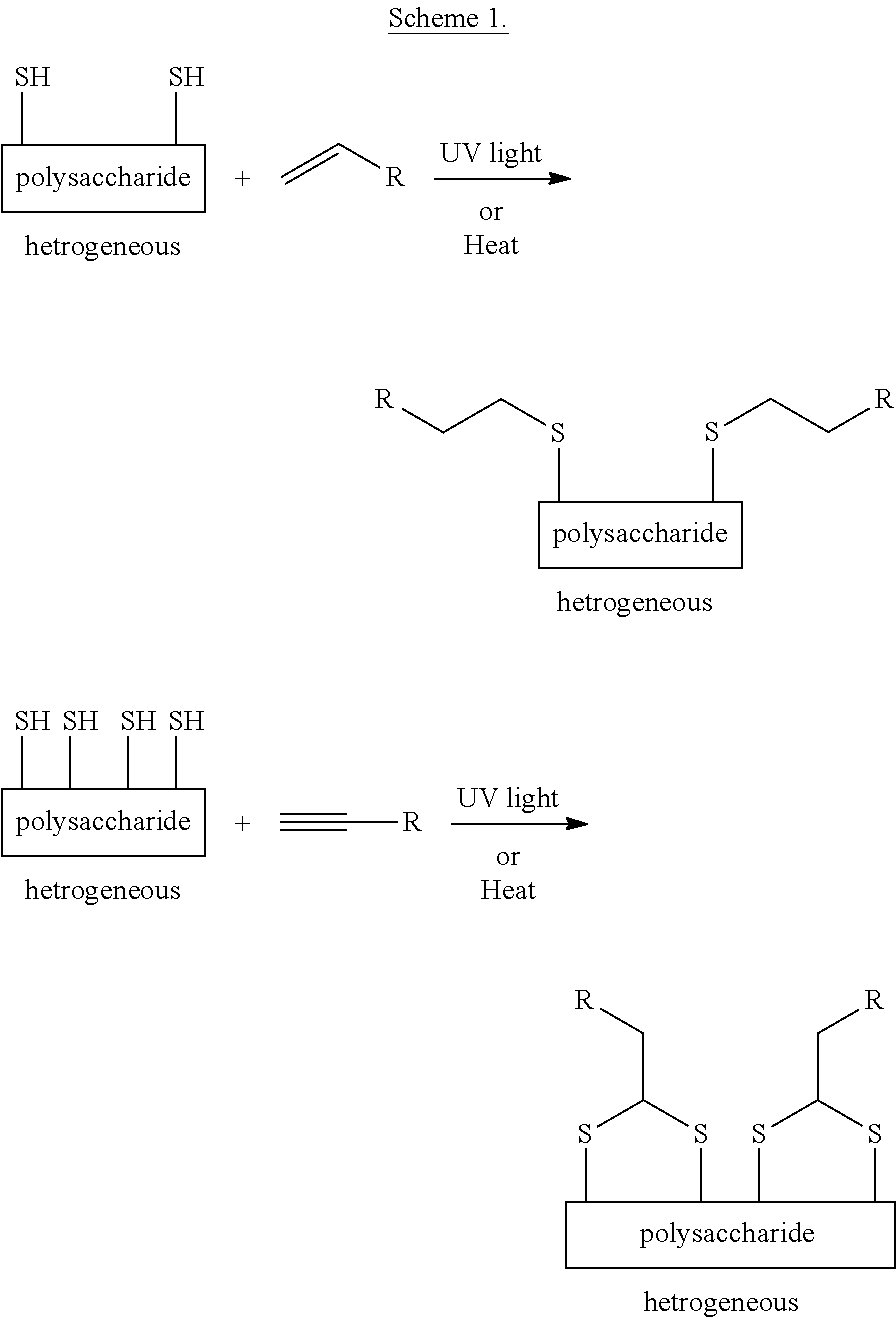
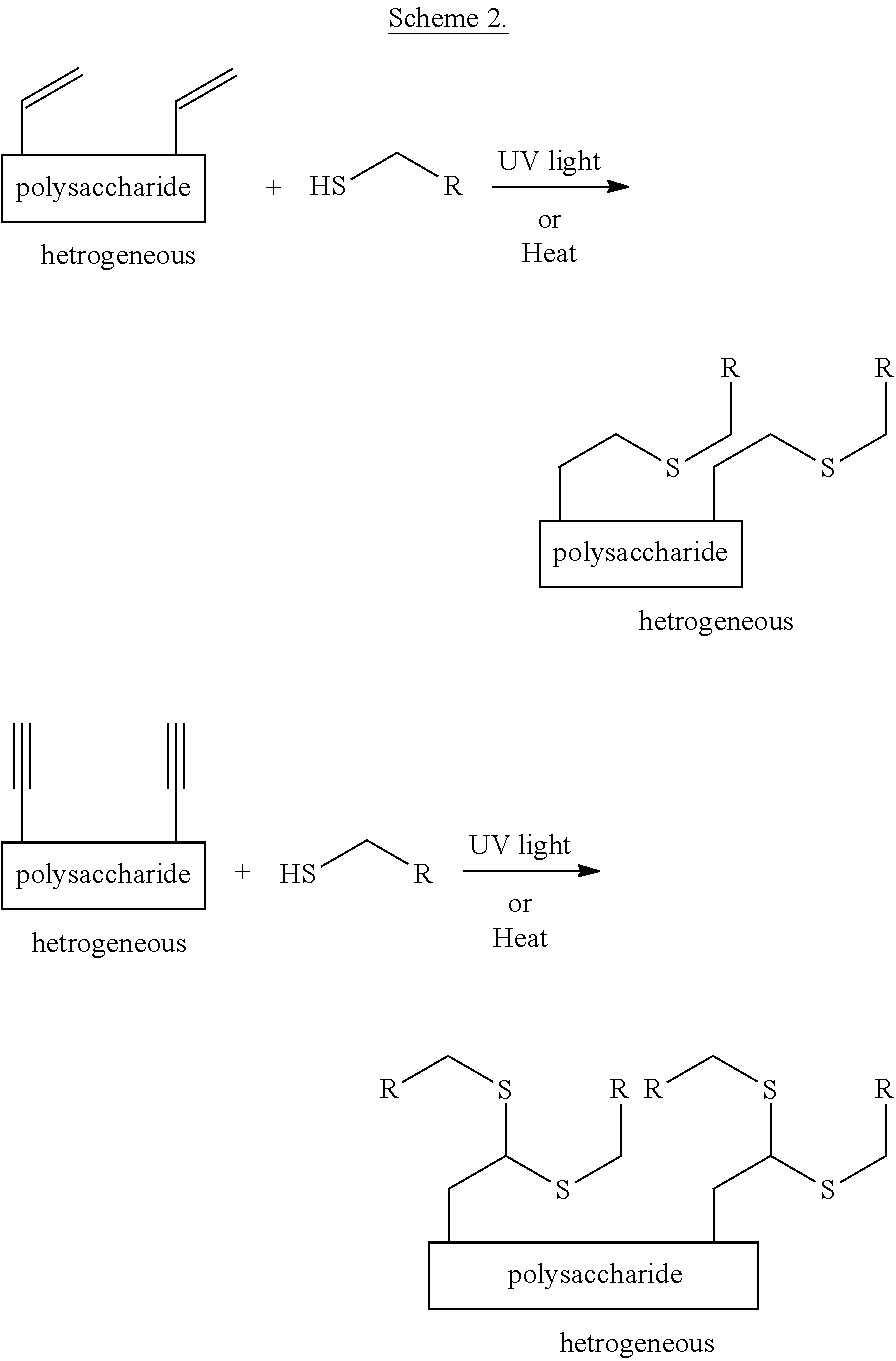
Heterogeneous thiol-ene click modifications of solid polysaccharide-based materials
This invention concerns the first environmentally benign heterogeneous modification of polysaccharide-based material in native solid state by thiol-ene “click chemistry”. The direct reaction of a thiol with an un-activated double or triple bond by thiol-ene and thiol-enyne click modification is thermally or photochemically catalyzed and is completely metal-free and allows for a highly modular approach to modifications of fibers and fiber-based materials.
Owner:ORGANOCLICK AB
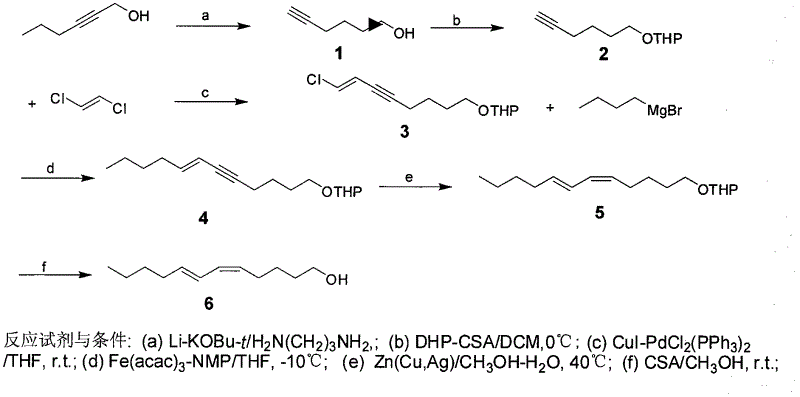
Process for synthesizing sex pheromone of pine caterpillar
ActiveCN102613177AEasy to operateSynthetic raw materials are readily availableBiocidePreparation by isomerisationGrignard reagentIodide
The invention discloses a process for synthesizing sex pheromone of pine caterpillar, which employs 2-hexyne-1-alcohol as an initial raw material, three-bond positional transference is carried out under the effect of lithium and propane diamine to obtain 5-hexyne-1-alcohol; under acidic condition, 5-hexyne-1-alcohol is reacted with dihydropyran to obtain 1-THP-5-hexyne-1-alcohol protected by THP on hydroxyl, Under co-catalysis of metal palladium and cuprous iodide, 1-THP-5-hexyne-1-alcohol and trans-dichloroethylene are subjected to coupling reaction to generate conjugate enyne(7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol; under the catalysis of metallic iron, (7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol and a n-Butyl bromide grignard reagent are further subjected to coupling reaction to obtain (7E)-1-THP-5-alkyne-7-alkene-1-dodecanol, under the catalytic reduction of metal zinc, (5Z, 7E)-1-THP-dodecanol dienol; under the camphor sulfonic acid condition, (5Z, 7E)-1-THP-dodecanol dienol removes the THP protective group to obtain the final target product (5Z, 7E)-dodecanol dienol. The method of the invention has the advantages of easily available synthesis raw materials, low cost, mild reaction condition, easy operation, high yield and good stereoselectivity.
Owner:WENZHOU MEDICAL UNIV +1
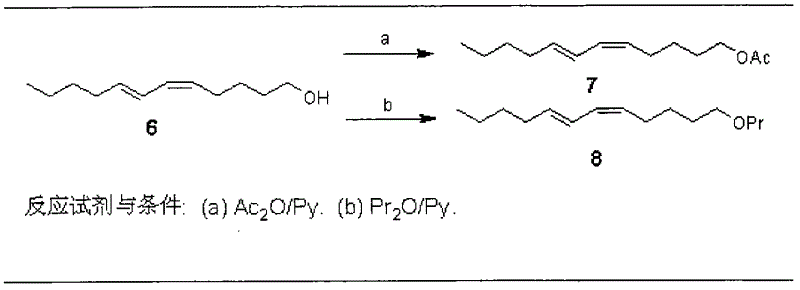
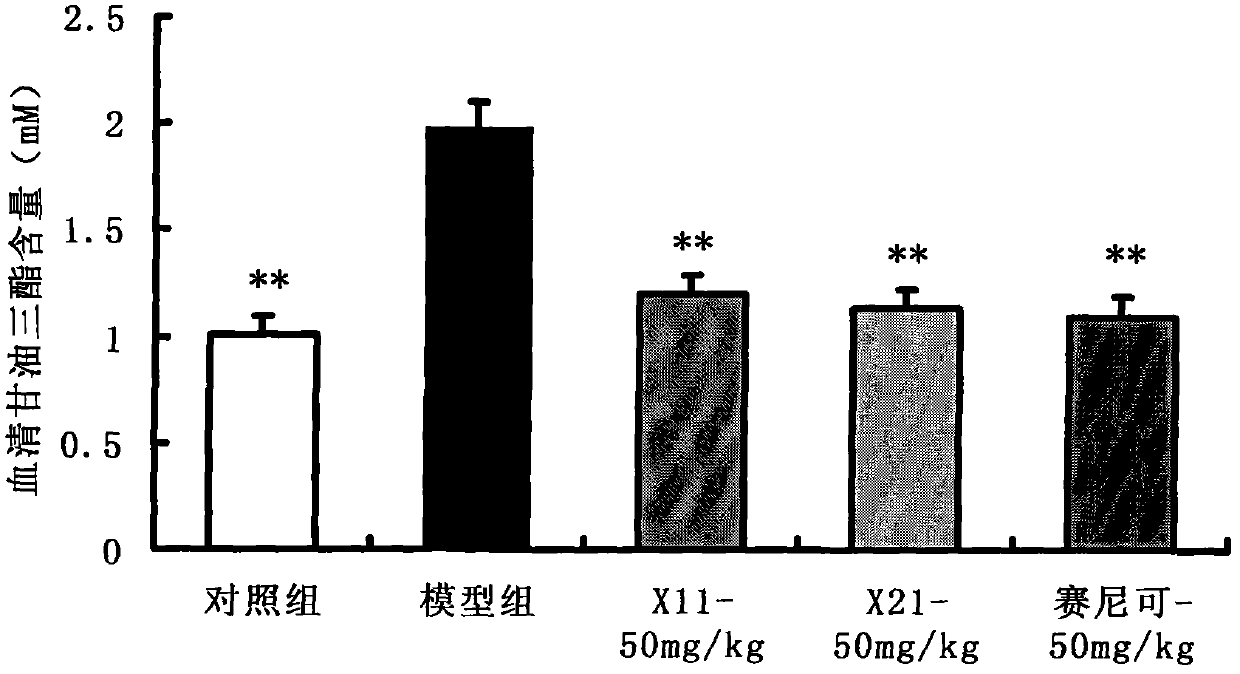
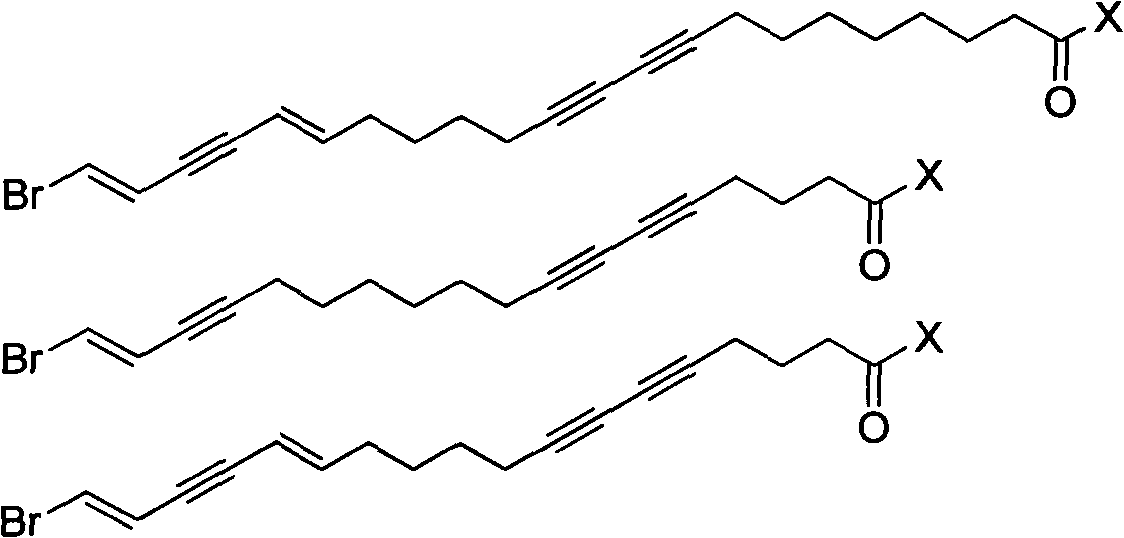
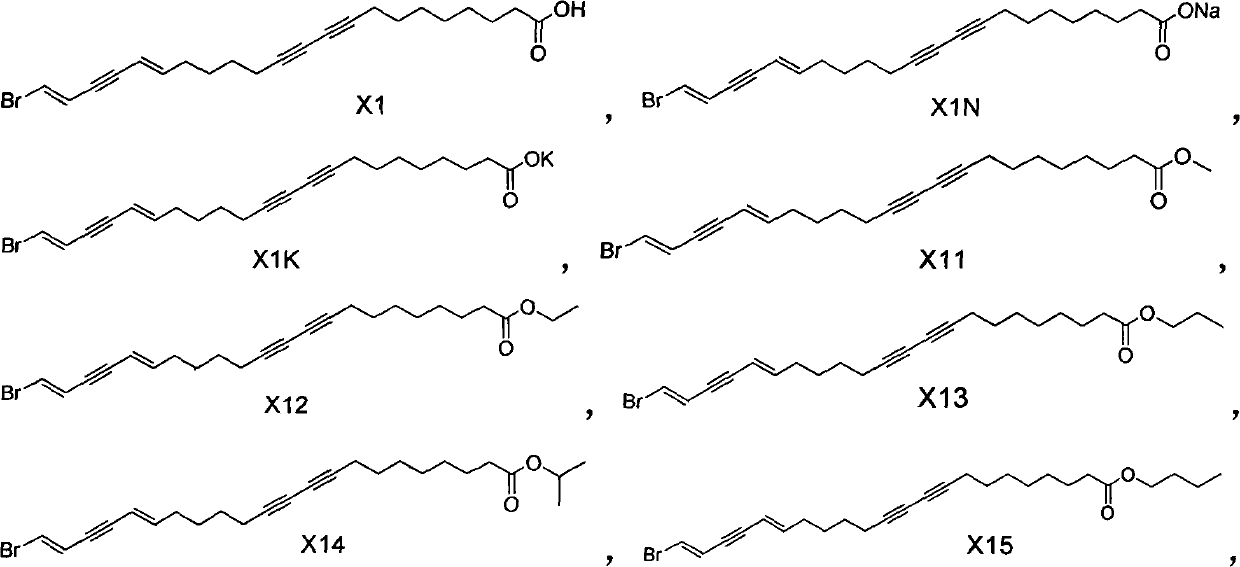
Enyne bromide compounds and preparation method and use thereof
InactiveCN102850208APreparation from carboxylic acid halidesOrganic compound preparationStructural formulaTherapeutic treatment
The present invention relates to enyne bromide compounds (with structural formula shown below) and preparation method and use thereof. Vitro biological activity test shows such compounds significantly inhibit the activity of pancreatic lipase, and can be used as a new drug lead compound for prevention or therapeutic treatment of obesity and related metabolic disorders and used for the preparation of the drug for clinical treatment of common obesity and related metabolic diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Process for producing 1,3-enyne compounds
InactiveCN101492340ANo pollutionThe synthesis method is simpleOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsRoom temperature
The invention discloses a method for preparing a 1, 3-eneyne compound. The method is characterized in that at the room temperature and under the protection of nitrogen, an alkenyl iodine compound, substituent end alkyne, catalyst, cesium carbonate and a ligand are mixed in the mol proportion of 1-1.5:1:1-3:0.1-0.15:0.1-0.3; then a mixture is put into the solvent of toluene and dimethylbenzene to react for 36 to 72 hours with the temperature increased to 110 DEG C; and the 1, 3-eneyne compound is obtained after separating and purifying. Compared with the prior art, the invention has simple synthesis method, easily obtained raw materials, low reaction cost, high yield and no environmental pollution, and a generated product is kept with a double-bond configuration.
Owner:EAST CHINA NORMAL UNIV
Derivatives of 4-polyfluoroalkyl-2,4-disubstituted pyrrole and preparation method thereof
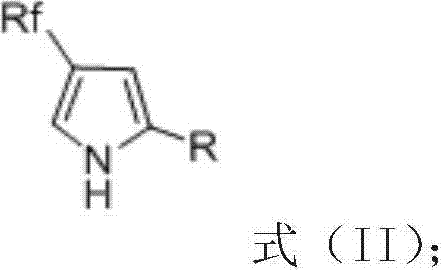
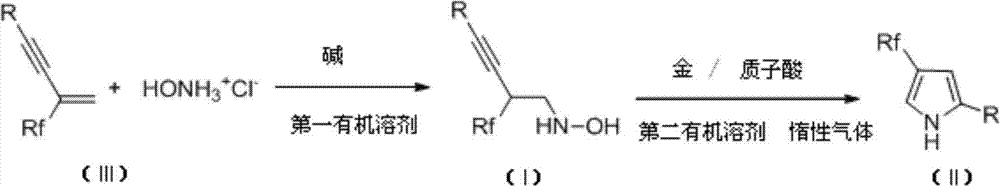
InactiveCN103755587ARaw materials are easy to getMild reaction conditionsOximes preparationHydroxylamine HydrochlorideSolvent
The invention discloses derivatives of 4-polyfluoroalkyl-2,4-disubstituted pyrroles as shown in a formula (II) and a preparation method thereof. The preparation method comprises the steps of dissolving fluorine-containing conjugated enyne, hydroxylamine hydrochloride and an alkali in an organic solvent, sufficiently reacting at a temperature ranging from 0 to the room temperature, removing the solvent, and obtaining fluorine-containing hydroxylamine compounds with an alkynyl group as shown in a formula (I) through column chromatography, next, dissolving the fluorine-containing hydroxylamine compounds with the alkynyl group in the organic solvent, cyclizing under co-catalysis of a gold catalyst and a protonic acid catalyst under the room temperature condition, removing the solvent, and obtaining the derivatives of the 4-polyfluoroalkyl-2,4-disubstituted pyrroles through column chromatography. The preparation method is capable of getting raw materials easily, high in yield, mild in reaction conditions, and simple to operate. The invention also provides the fluorine-containing hydroxylamine compounds with an alkynyl group as shown in the formula (I) and a preparation method thereof. The invention further provides a 2,4-disubstituted polyfluoroalkyl-containing pyrrole compound framework which plays an important role in synthesizing the substituted fluorine-containing pyrrole compounds.
Owner:EAST CHINA NORMAL UNIV
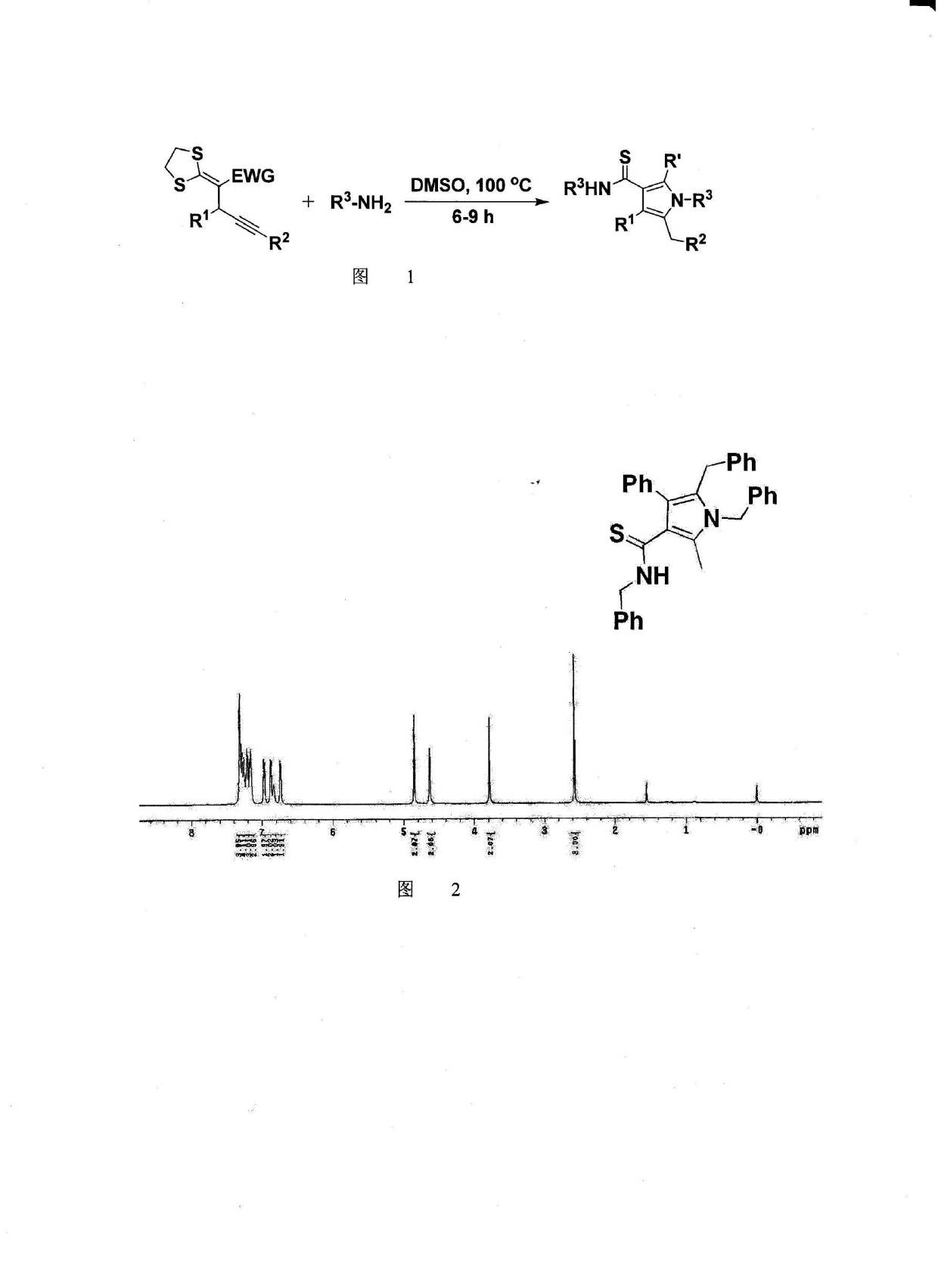
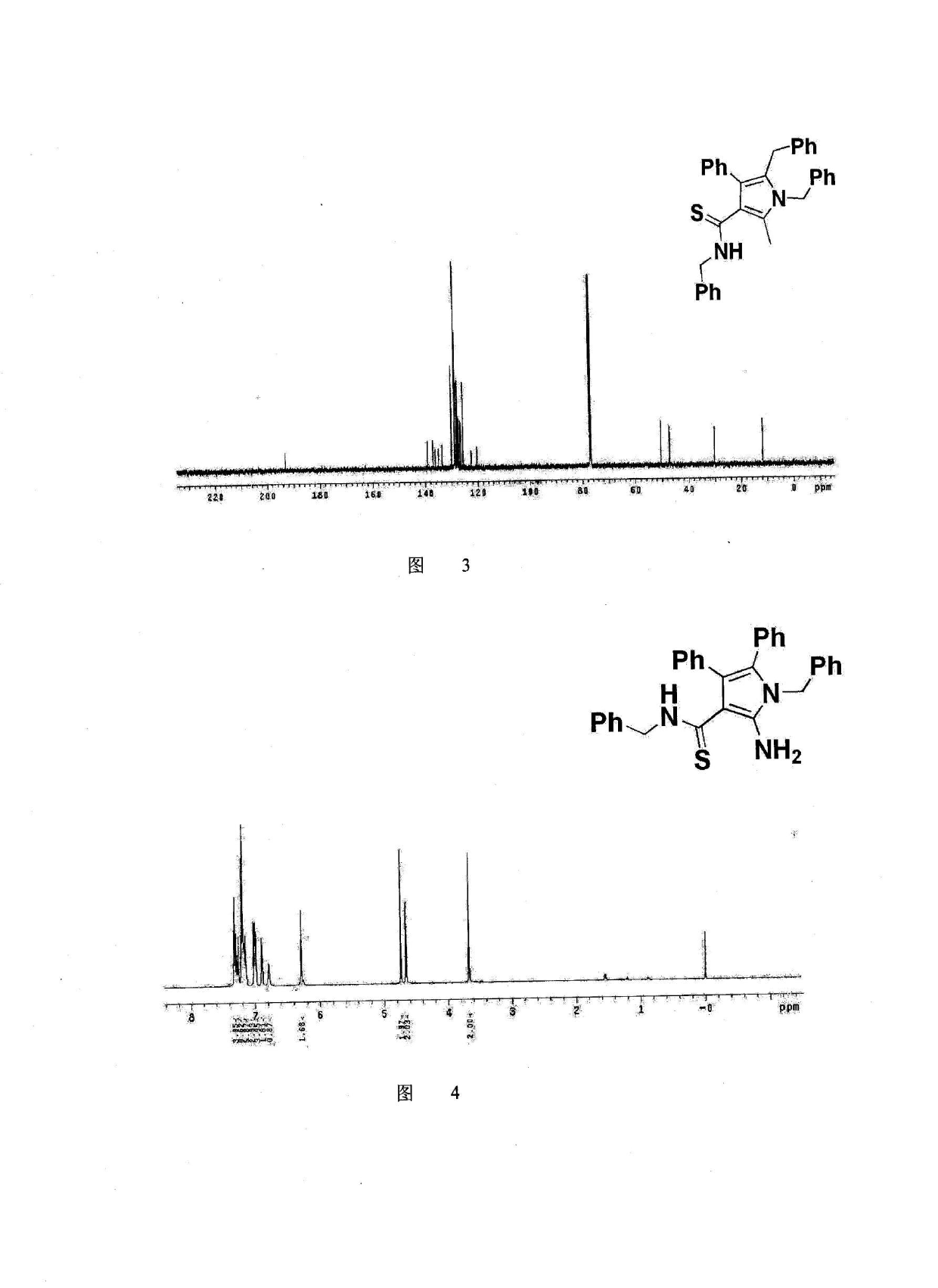
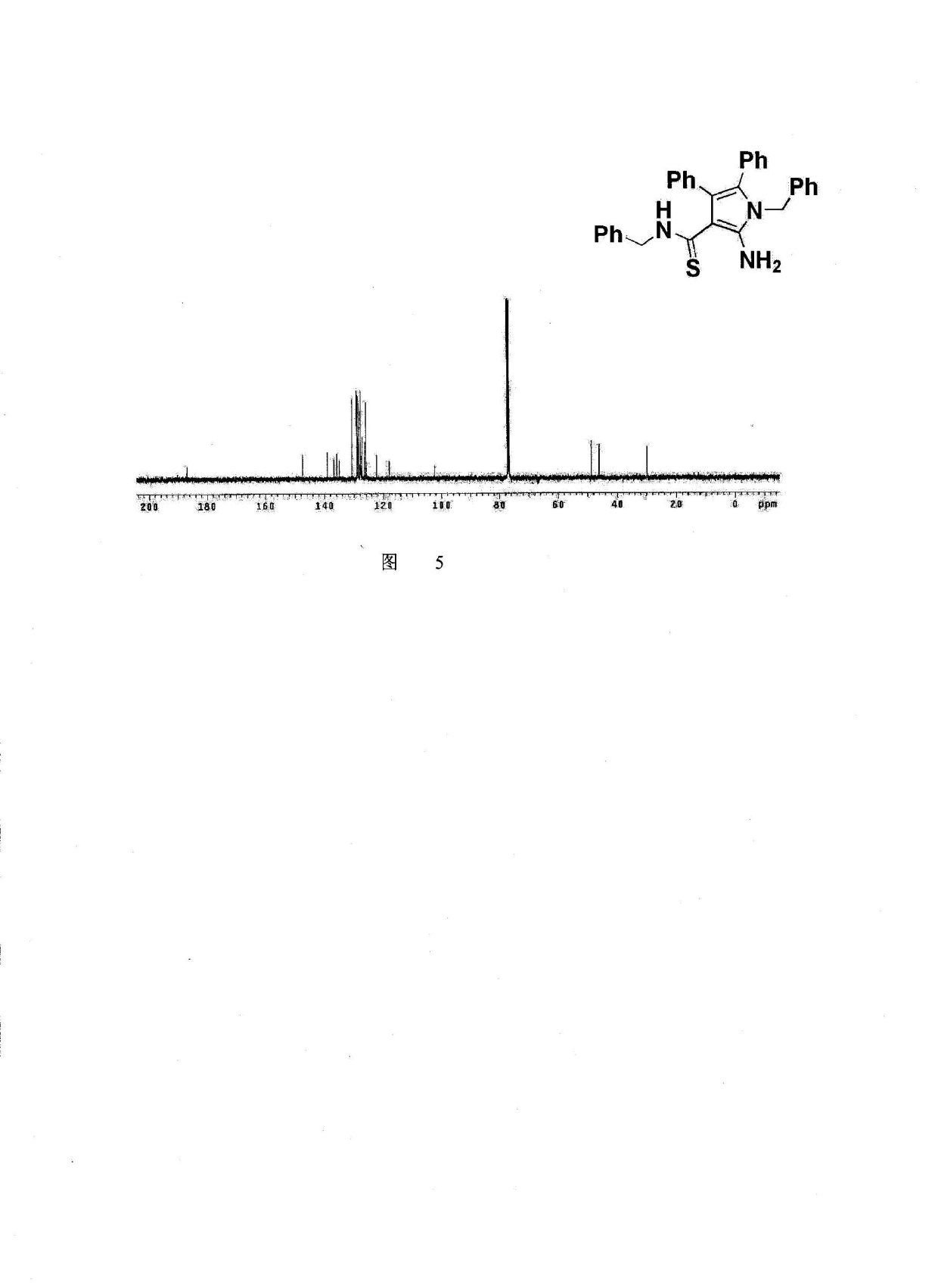
Method for synthesis of highly functionalized pyrrole compound
The invention belongs to the technical field of organic synthetic chemistry and especially relates to a method for synthesis of a highly functionalized pyrrole compound. The highly functionalized pyrrole compound is obtained by two reaction processes. The method for synthesis of the highly functionalized pyrrole compound comprises that functionalized ketene dithioacetal and alkynol as raw materials are synthesized into a dialkylthio-substituted enyne compound and the dialkylthio-substituted enyne compound and primary amine are prepared into the highly functionalized pyrrole compound. The method for synthesis of the highly functionalized pyrrole compound has simple processes, adopts easily available raw materials and reagents, realizes simultaneous introduction of multiple substituent groups and easy separation purification of products, and is suitable for synthesis of various highly-functionalized pyrrole compounds.
Owner:NORTHEAST NORMAL UNIVERSITY
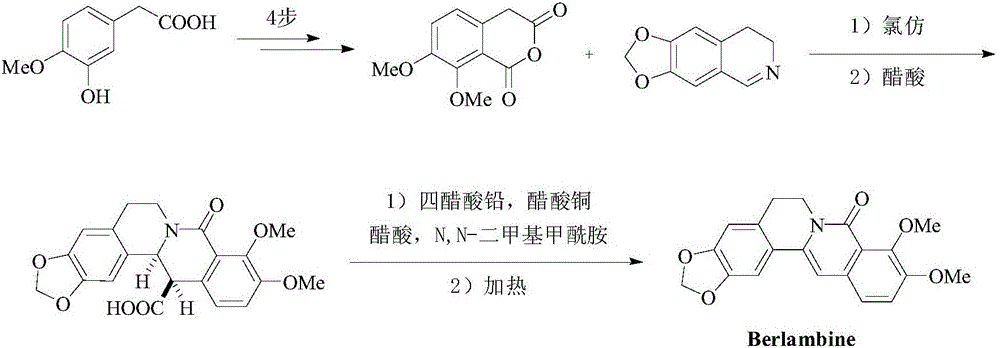
Method for synthesizing 6-6 fused ring structure in berberine and ebony natural product
InactiveCN105801573AEasy to operateReduce manufacturing costOrganic chemistryBulk chemical productionArylBerberine
The invention discloses a method for synthesizing a 6-6 fused ring structure in berberine and ebony natural products. The method comprises the step of carrying out intramolecular tandem cyclization reaction on conjugated enyne ester with a structure shown in a formula II in a solvent, so that the 6-6 fused ring structure shown in a formula I is obtained, a specific reaction formula is described in the specification, wherein R1 and R2 are respectively independently selected from hydrogen, alkyl, naphthenic base, aryl and heterocyclic radical; or R1 and R2 form a saturated or unsaturated carboatomic ring or heterocyclic ring together; R3 and R4 are respectively independently selected from hydrogen, alkyl, naphthenic base, aryl and heterocyclic radical; or R3 and R4 form a saturated or unsaturated carboatomic ring or heterocyclic ring together; R is selected from C1-C4 alkyl; P is an amino-protecting group. The method disclosed by the invention has the advantages of simple operation, safety, environmental friendliness, low production cost, high yield and adaptability to large scale production and has important value in promotion of extensive use of the compound in the filed of medicines.
Owner:EAST CHINA NORMAL UNIV
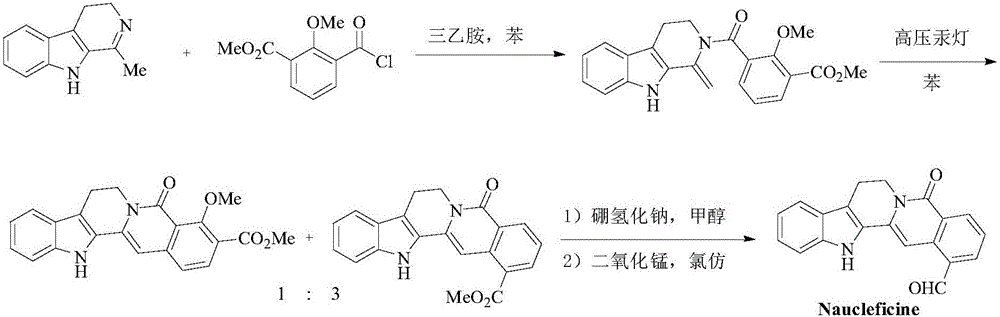
Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device
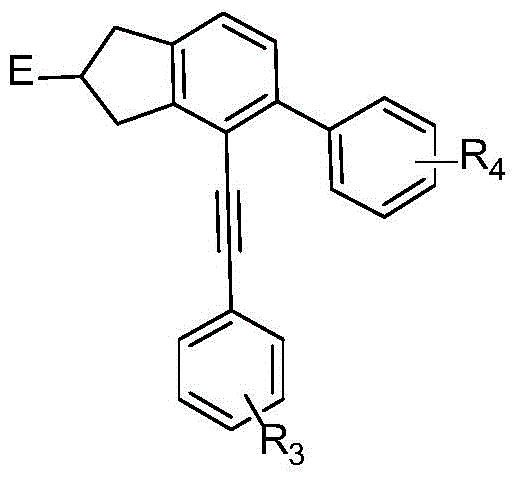
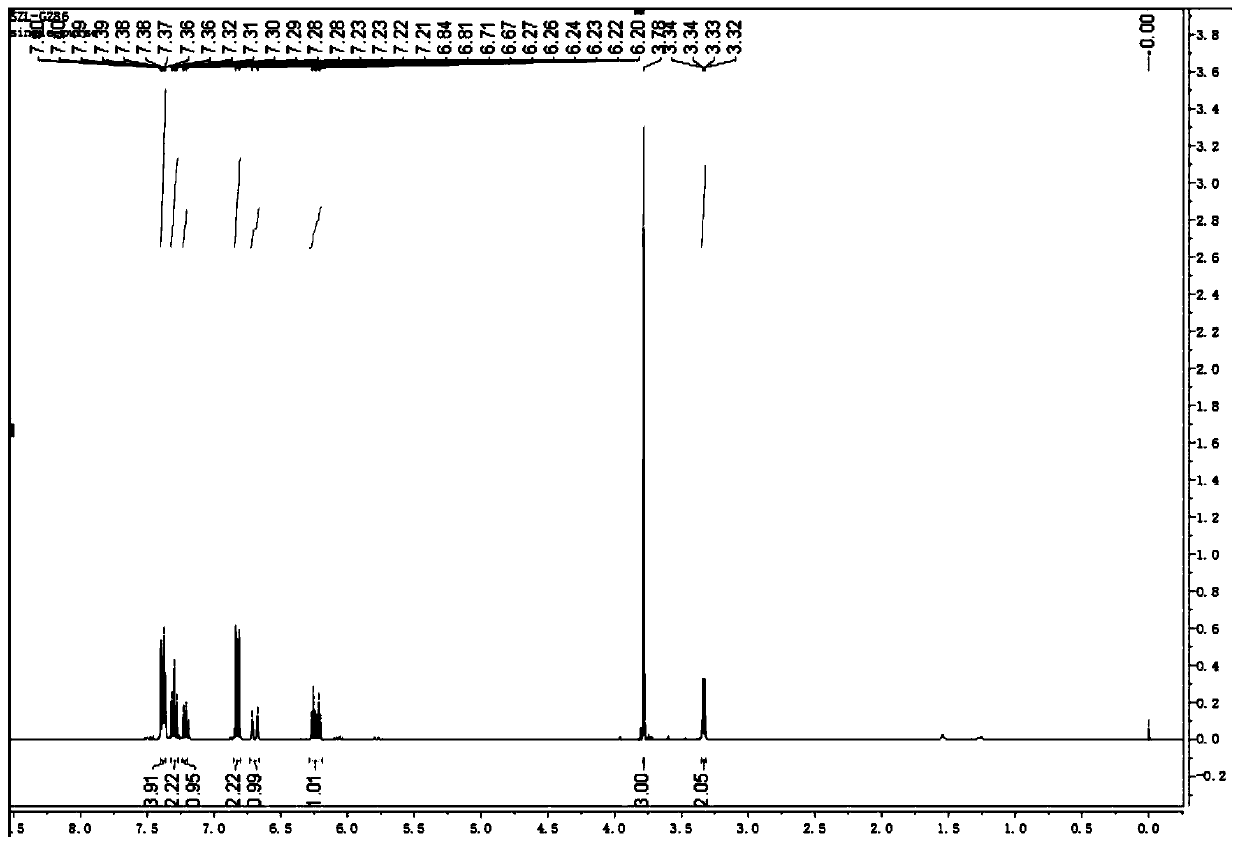
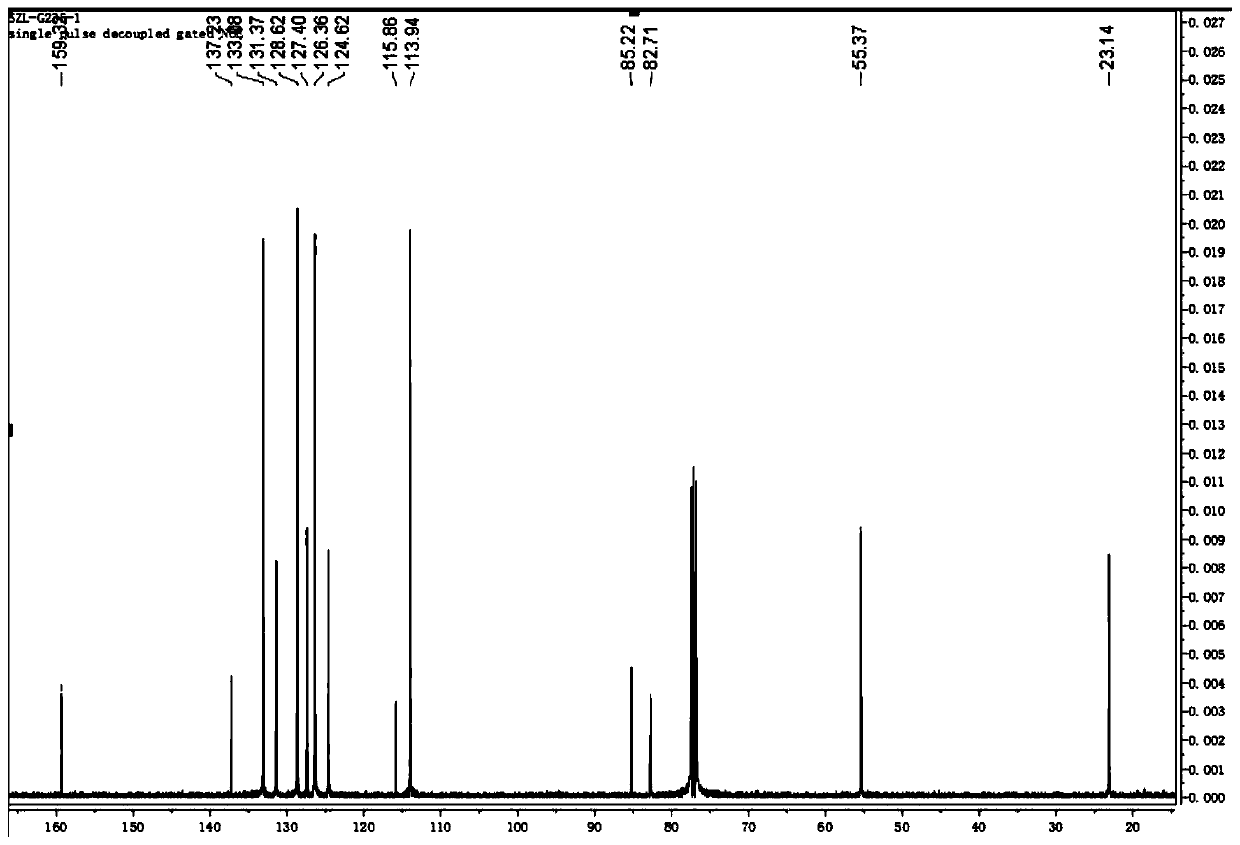
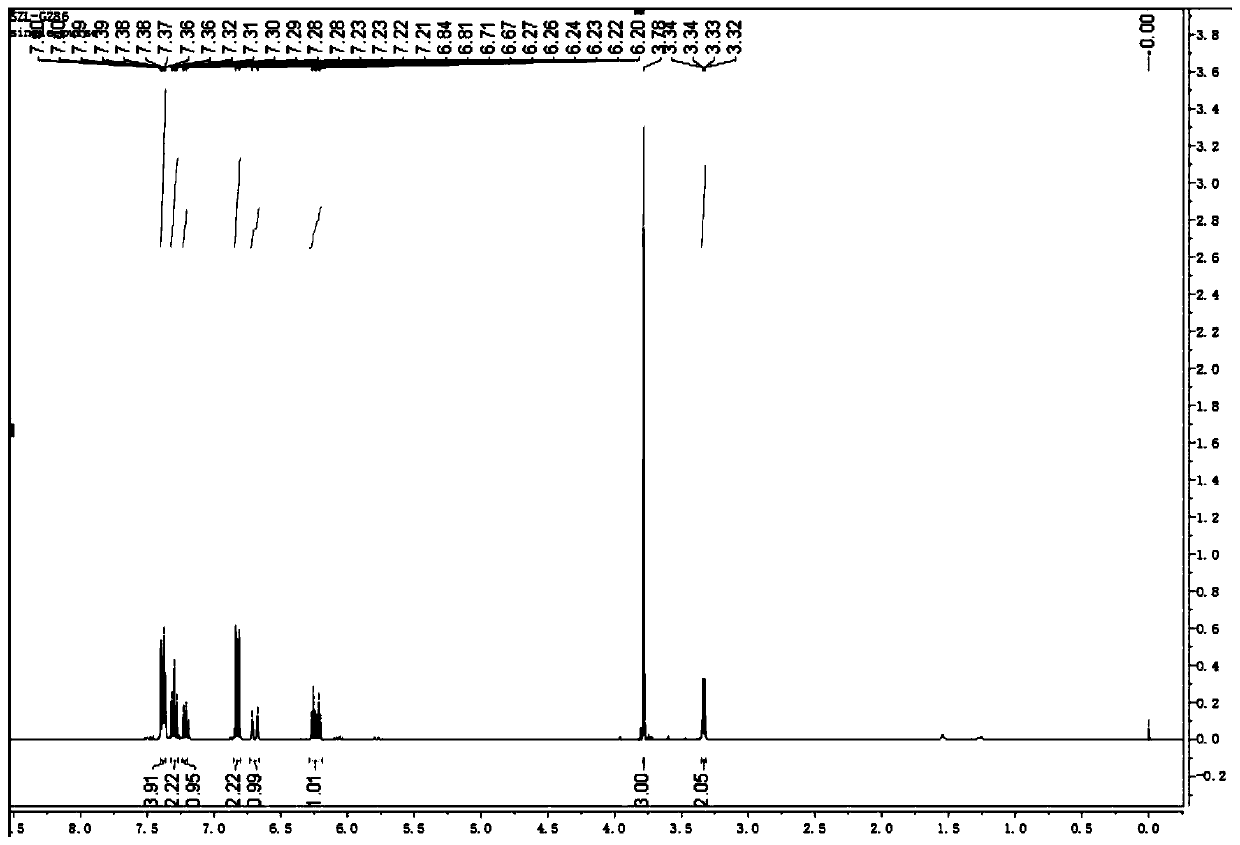
The invention discloses a method for continuously preparing a dihydrobenzo [j] phenanthridine compound containing a trifluoromethyl functional group by using a micro-channel reaction device, which comprises the following steps: (1) dissolving a 1,7-eneyne compound and alkali in a proper solvent to obtain a material I; (2) dissolving a trifluoromethyl reagent and a photocatalyst in a proper solventto obtain a material II; (3) respectively pumping the material I and the material II into a micro-channel reaction device, fully mixing, and carrying out a photocatalytic trifluoromethylation reaction to obtain a reaction solution; and (4) quenching the reaction liquid, adding a corresponding organic solvent for extraction, collecting an organic phase, drying, concentrating and recrystallizing toobtain a target product. The micro-channel reaction device is used for preparing the 1,7-eneyne trifluoromethylation product, the reaction conditions are milder, the reaction rate can be effectivelycontrolled, the reaction time is shortened, continuous production is achieved, side reactions are reduced, the maximum product yield can reach 99.3%, the amplification effect is basically avoided, andindustrial amplification is facilitated.
Owner:NANJING UNIV OF TECH
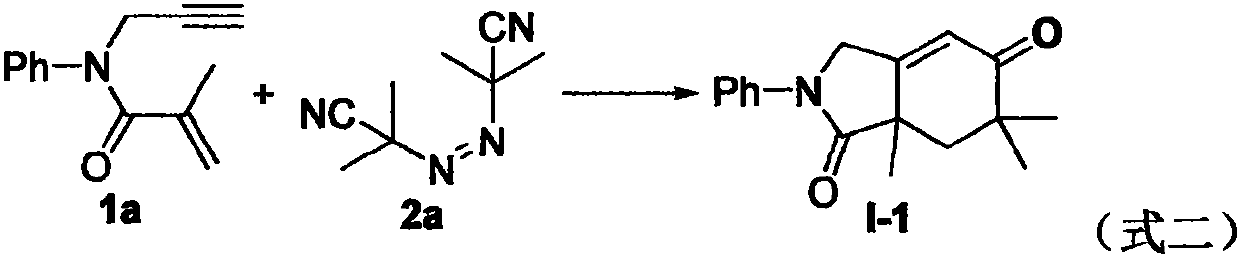
Free radical cyclization reaction method of 1, 6-enyne compound and azoalkyl nitrile
The invention relates to a regioselective free radical cyclization reaction method of a 1, 6-enyne compound and azoalkyl nitrile under a mild condition. According to the method, the 1, 6-enyne compound, an azoalkyl nitrile compound, a catalyst, alkali and a solvent are added into a Schlenk reaction bottle for stirring reaction under certain temperature and air atmosphere conditions, thus obtaininga cyclization product.
Owner:NINGBO UNIV
Synthesis method of azepine anthraquinone
InactiveCN101712648AAchieve synthesisHas antibacterial activityOrganic chemistryPlatinum saltsIsomerization
The invention relates to a synthesis method of azepine anthraquinone. The azepine anthraquinone is obtained by carrying out intramolecular 6-endo-dig cycloisomerisation reaction shown as in formula (1) on N-propargyl quinine with a 1,5-eneyne structure under the action of a metal catalyst, and purifying, wherein the intramolecular 6-endo-dig cycloisomerisation reaction is homogeneous phase metal catalytic reaction, the metal catalyst is gold salt, platinum salt or univalent gold complex; and the use level of the metal catalyst is 0.01-0.5 equivalent weight of the N--propargyl quinine. The gold slat is auri chloridum (AuCl3) or aurous chloride (AuCl); the platinum salt is platinum tetrachloride, platinum bichloride or potassium chloroplatinate; and the univalent gold complex is PPh3AuOTf, PPh3AuSbF6, PPh3AuNTf2 or LAuNTf2, wherein L is nitrogen heterocyclic ring carbene ligand. The invention realizes the synthesis of the azepine anthraquinone by utilizing metal catalytic intramolecular eneyne cyclization reaction, and has the advantages of simple and easy-accessible raw materials and moderate reaction conditions.
Owner:NANJING UNIV
DNA-cleaving antitumor agents
A chemical composition and method of use of the composition is described. The chemical composition includes an aza-enediyne, aza-enyne allene, or an aza-diallene. These compound are preferably non-hydrolyzable, cationic compounds that bind to nucleic acids. In addition it is believed that these compounds may undergo chemical reactions in the presence of a nucleic acid to generate reactive intermediates that cleave nucleic acids.
Owner:RES DEVMENT FOUND
Novel fluorocyclopentenone preparation method and product thereof
InactiveCN108276260AMild conditionsReduce energy consumptionOrganic compound preparationCarboxylic acid esters preparationNatural productAcetonitrile
The invention discloses a novel fluorocyclopentenone preparation method and a product thereof. The novel fluorocyclopentenone preparation method is characterized by comprising the following step: methyl tert-butyl ether, enynic ester, gold (acetonitrile)[(2-biphenyl)di-tert-butylphosphine] hexafluoroantimonate (I) and N-fluorobenzenesulfonimide are added to react, so that fluorocyclopentenone is obtained. The novel method for preparing a fluorocyclopentenone compound which is provided by the invention ensures that an enynic ester compound can be converted into the fluorocyclopentenone compound. The whole reaction is carried out under normal temperature and normal pressure, conditions are mild, and energy consumption is low. The whole reaction is carried out by utilizing a one-pot method, operation is easy, yield is high, and the purity of the product is 98 percent or more. The reaction substrate range is wide, and not only the simple enynic ester compound but also complex compounds containing natural product groups are applicable. The developed fluorocyclopentenone compound has potential bioactivity, and can become a drug by subsequent testing or modification.
Owner:NANJING FORESTRY UNIV
Emulsion containing crosslinked and non-cross linked silicone polyethers
Emulsions are prepared by heating and polymerizing a mixture containing (i) a non-crosslinked silicone polyether; (ii) optionally, a cosurfactant which can be a monohydroxy alcohol, diol, triol, or glycol ether; (iii) an =Si-H containing polysiloxane; (iv) a mono-alkenyl polyether; (v) an alpha,omega-diene, alpha,omega-diyne, or alpha,omega-ene-yne; (vi) optionally, a silicone oil such as (a) a low molecular weight linear or cyclic volatile methyl siloxane, or (b) a low molecular weight linear or cyclic volatile or non-volatile alkyl or aryl siloxane; (vii) a platinum catalyst; and (viii) water.
Owner:DOW CORNING CORP
Anticancer active molecular skeleton 1,4-enyne compound, and preparation method and application thereof
ActiveCN109704926AReduce dosageGrowth inhibitionSilicon organic compoundsOrganic compound preparationArgon atmospherePalladium catalyst
The invention discloses an anticancer active molecular skeleton 1,4-enyne compound, and a preparation method and application thereof. The preparation method comprises the following steps: an allyl alcohol raw material, terminal alkyne, tetrakis (triphenylphosphine)palladium, calcium bis(trifluoromethyl sulfonyl)imide and an additive are added into a reaction solvent in sequence; a catalytic reaction is carried out for 12-48 hours at an argon atmosphere at a temperature of 100 DEG C and under a stirring state; a reaction solvent in the reaction solution is removed; and purifying is carried outto obtain the anticancer active molecular skeleton 1,4-enyne compound. The 1,4-enyne compound can be used for medicines inhibiting human esophageal carcinoma cells. The usage amount of a palladium catalyst in the method is 1%, the usage amount of a calcium catalyst is 5%, the usage amounts are extremely small, but an expected effect can be achieved. According to the method disclosed by the invention, the substrate application range is wide, and the allyl alcohol can contain various substituted phenyls, heterocyclic rings and alkyls. The method disclosed by the invention is suitable for different types of allyl alcohol, and the 1,4-enyne compound can be synthesized in a scale of 10 g. The 1,4-enyne compound disclosed by the invention has relatively good anti-cancer activity.
Owner:NANJING UNIV OF TECH
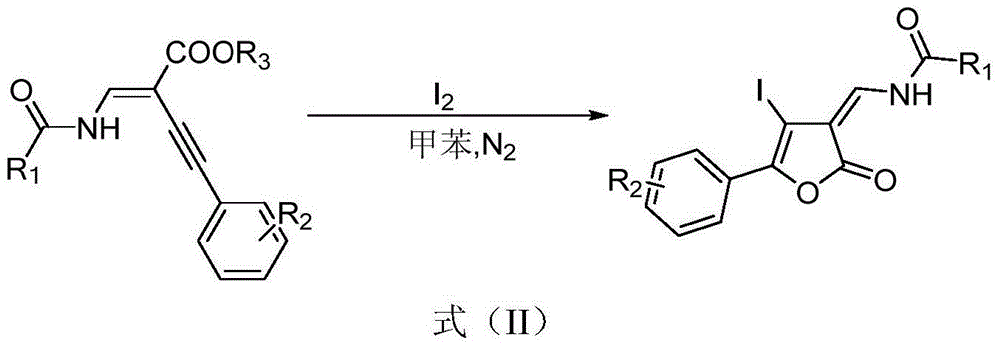
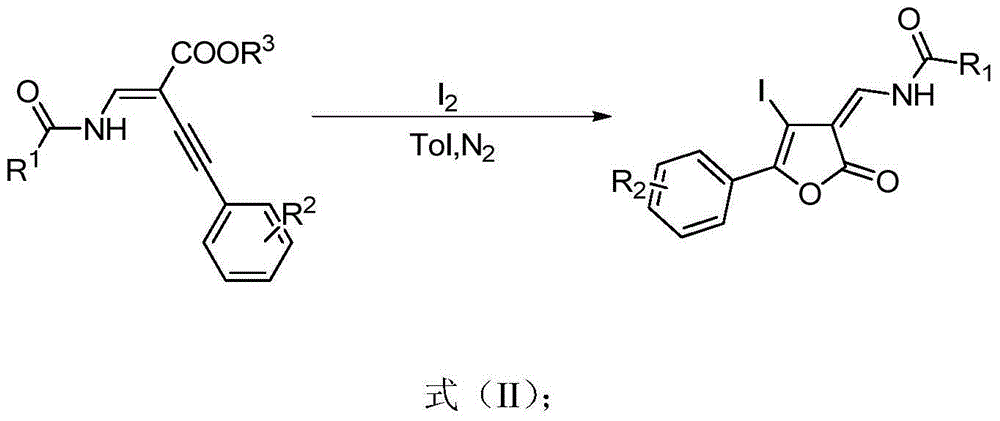
Furanone derivative and synthetic method thereof
The invention discloses a furanone derivative shown in a formula (I) and a synthetic method thereof. By taking an acylated amine substituted enyne ester compound as a raw material, under the action of a catalyst, the furanone derivative shown in the formula (I) is synthesized. The preparation method disclosed by the invention has the advantages of simple and easily available raw materials, simple and convenient post-treatment, cheap catalyst, good yield, environmental friendliness, and the like. The formula (I) is as shown in the specification.
Owner:EAST CHINA NORMAL UNIV
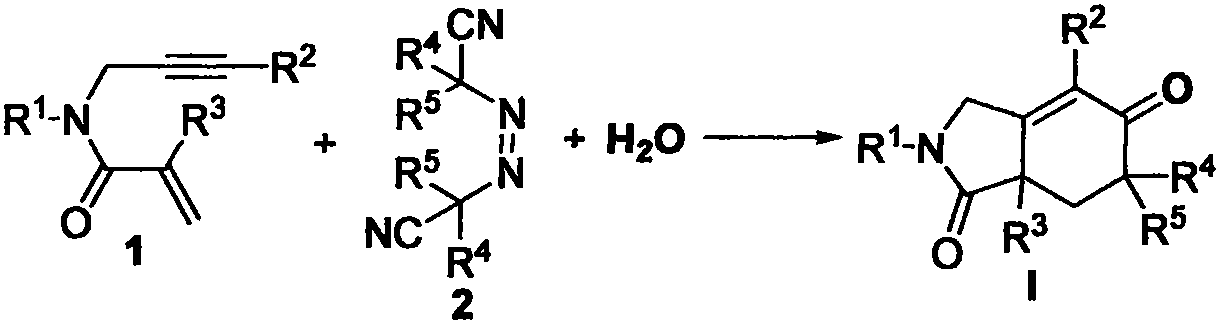
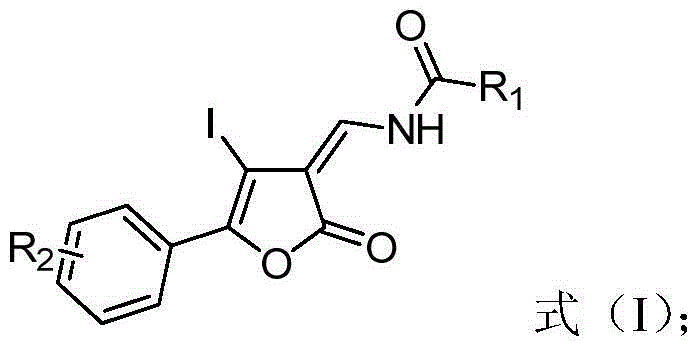
Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one
The invention discloses a method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one. The method comprises the following steps of: performing intramolecular tandem cyclization reaction on 2-[3-(2-amidophenyl)-2-propynylamino]quinone with a 1,5-eneyne structure under the action of a metal catalyst, and purifying to obtain the benzo[c]pyridine[4,3,2-mn]acridine-8-one, wherein R refers to hydroxyl or methoxy; R1 and R2 refer to halogen atoms or alkyl with 1 to 6 carbon atoms; the intramolecular tandem cyclization reaction is homogeneous metal catalysis reaction, and the metal catalyst is gold salt or univalent gold coordination complex; the using amount of the catalyst is equivalent to 0.01 to 0.5 of the quinone raw material; and after the intramolecular tandem cyclization reaction is finished, purification, namely quick column chromatography, is carried out and the benzo[c]pyridine[4,3,2-mn]acridine-8-one is obtained. The raw material is simple and readily available, the reaction conditions are mild, and the chemical regioselectivity is high.
Owner:NANJING UNIV
Preparation method of 2-pyrrolidone derivative
The invention relates to a preparation method of a 2-pyrrolidone derivative. According to the method, 1,6-eneyne compounds, carbonyl compounds, oxidizing agents and organic solvent are added into a Schlenk reaction flask, the mixture is stirred on the conditions of certain temperature and the air atmosphere to carry out free radical cyclization reaction.
Owner:NINGBO UNIV
Synthetic method of phosphonyl methylene substituted five-membered cyclic compound
ActiveCN111072720ARaw materials are cheap and easy to getIn line with the concept of green environmental protectionGroup 5/15 element organic compoundsOrganic dyeAcyl group
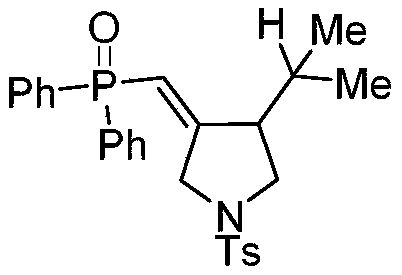
The invention discloses a synthetic method of a phosphonyl methylene substituted five-membered cyclic compound. According to the method, 1,6-enyne and diaryloxy phosphorus are taken as reaction raw materials, an organic dye is taken as a photocatalyst, a reaction solvent is added, an illumination reaction is carried out under a nitrogen protection condition, a target product is synthesized, and the structure of the target product is represented and analyzed through IR, 1H NMR, 13C NMR, HRMS and X-ray single crystal diffraction. The method does not need any additive, high temperature and otherharsh and complex reaction conditions, and has the advantages of mild reaction conditions, simple operation, convenient subsequent treatment, easily available raw materials, easy derivatization to obtain different types of organic compounds, and the like.
Owner:YANGZHOU UNIV
Bi-triazole compound, preparation method and application thereof
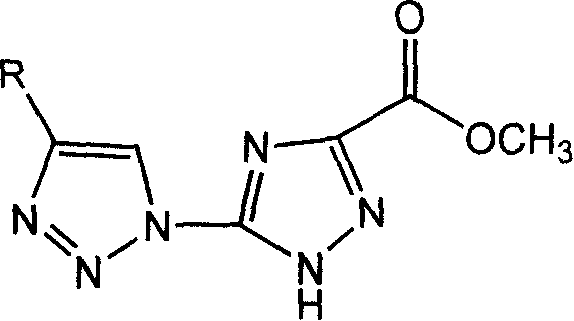
The present invention relates to a triazole compound. Said invention provides its general formula. Its preparation method includes the following steps: using 5-triazo-1,2,4 triazol-3-acyl methyl ester and propiolic alcohol ester acetate, p-amyl phenylacetylene, syclohexanol alkyne, cyclohexene alkyne or methoxyphenyl acetylene, and making them be reacted in the tetrahydrofuran or mixed solvent of tetrahydrofuran and water at 10-100 deg.C under the action of Cu(I) catalyst so as to obtain the required triazole compound. Said triazole compound has the activity for directly inhibiting plant virus, in particular inhibiting tobacco mosaic virus.
Owner:WUHAN UNIV
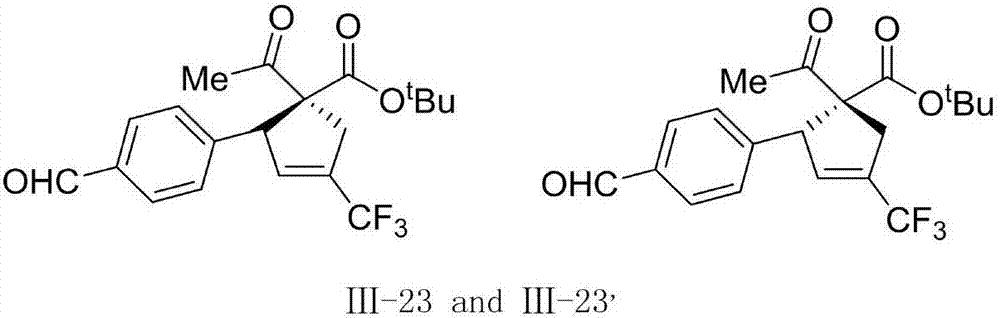
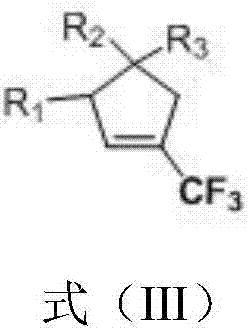
1-trifluoromethyl-tetrasubstituted cyclopentene derivatives as well as preparation method and application thereof
ActiveCN107324982AEfficient synthesisSimple structureCarboxylic acid nitrile preparationOrganic compound preparationCyclopenteneOrganic solvent
The invention discloses 1-trifluoromethyl-tetrasubstituted cyclopentene derivatives shown as the formula (III) in the description as well as a preparation method and an application thereof. The 1-trifluoromethyl-tetrasubstituted cyclopentene derivatives are prepared from 2-trifluoromethyl-1,3-conjugated enyne compounds in the formula (I) in the description and 1,3-dicarbonyl compounds in the formula (II) in the description through a cyclization reaction in an organic solvent under the action of a base and a catalyst. The method adopts mild reaction conditions, is simple to operate and has great significance in synthesis of polyfunctional group substituted fluorine-containing cyclopentene compounds in the formula (III).
Owner:EAST CHINA NORMAL UNIVERSITY
Three-arm mannose derivative and preparation method thereof through combination with double-click chemistry
ActiveCN113248550APromote aggregationClick chemical reaction is efficientSugar derivativesSugar derivatives preparationSide chainTert-Butyloxycarbonyl protecting group
The invention relates to a three-arm mannose derivative and a preparation method thereof through combination with double-click chemistry. Firstly, a three-arm terminal alkene / alkyne compound and [alpha]-D-azide mannose ([alpha]-Man-N3) are used for carrying out a CuAAC reaction between end group alkyne and azide to generate a rigid triazole group; then, the rigid triazole group and [alpha]-D-sulfydryl mannose ([alpha]-Man-SH) carry out a Thiol-ene reaction between end group alkene and the sulfydryl to generate a flexible thioether bond; and then, under an acidic condition of trifluoroacetic acid, protection of t-butyloxycarboryl is removed to obtain one series of three-arm mannose derivatives. Compared with the prior art, the invention utilizes the advantages of the click chemistry reaction to innovatively synthesize three kinds of three-arm mannose derivatives with different structures so as to be favorable for jointly utilizing RAFT polymerization in the later stage to prepare a sugar-containing polymer of which the side chain contains tri-functional mannose and perform an important guidance meaning for researching the influence of the rigidity and the flexibility of different branched chains of the polymer of which the side chain contains three-arm mannose for the biological characteristics.
Owner:SHANGHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

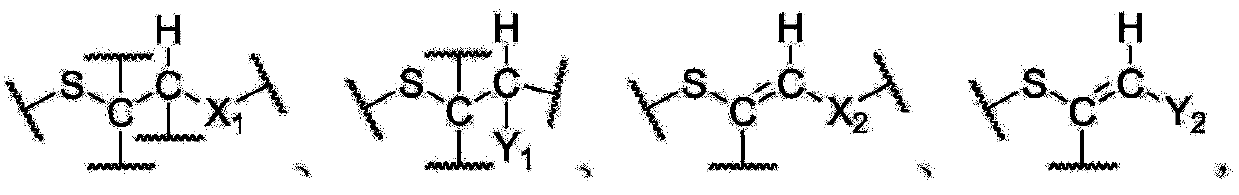
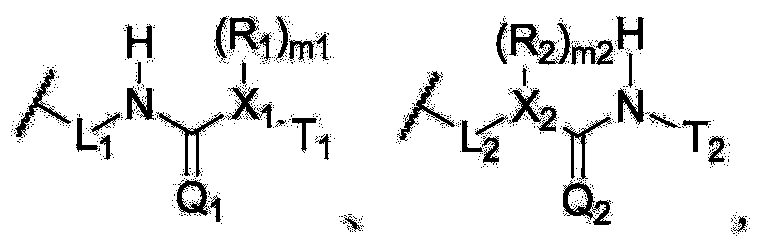
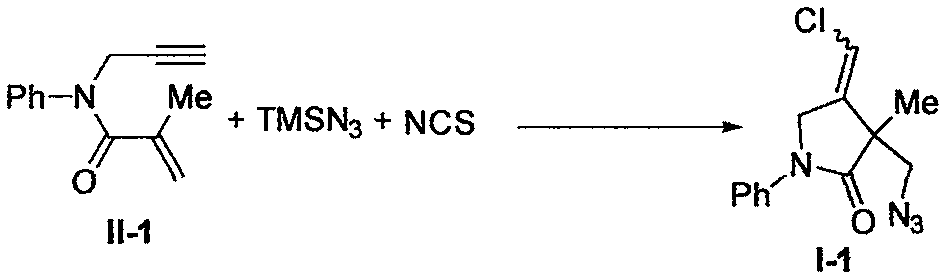

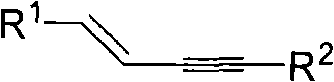
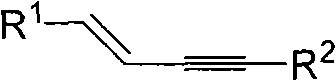

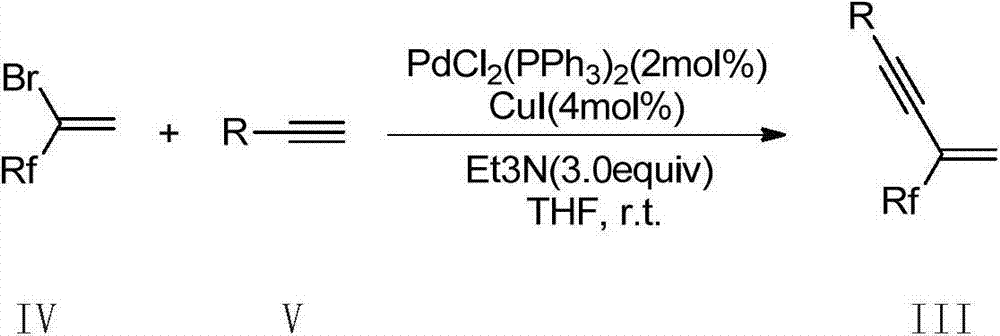
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000011.png)
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000012.png)
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000021.png)
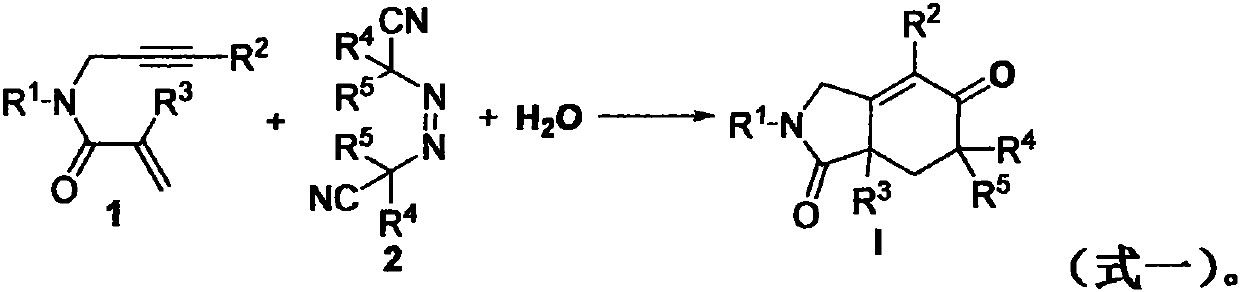



![Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ca20112e-62b2-4e09-8994-1aae8aa30c19/BDA0000098450610000011.PNG)
![Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ca20112e-62b2-4e09-8994-1aae8aa30c19/BDA0000098450610000021.PNG)
![Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one Method for synthesizing benzo[c]pyridine[4,3,2-mn]acridine-8-one](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ca20112e-62b2-4e09-8994-1aae8aa30c19/BDA0000098450610000022.PNG)