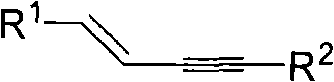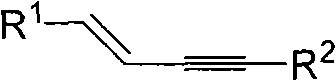Process for producing 1,3-enyne compounds
A compound and enyne technology, applied in 1 field, can solve problems such as expensive metal catalysts, increased cost, environmental pollution, etc., and achieve the effects of low reaction cost, no environmental pollution, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The alkenyl iodide compound used in the present invention is (E)-1-(2-iodovinyl)benzene, the substituted terminal alkyne is phenylacetylene, and the catalyst is FeCl 3 , The ligand is 1,10-phenanthroline (1,10-phenanthroline).
[0016] Substituted terminal alkyne 1.0mmol, alkenyl iodide 1.0mmol, cesium carbonate 3.0mmol, catalyst 0.15mmol, ligand 0.3mmol, placed in 5ml of toluene solvent at room temperature and under the protection of nitrogen, heated to 110°C, reacted for 48 hours, after After separation and purification, (E) 1,4-diphenyl-but-1-en-3-yne was obtained with a yield of 82%. Its structural formula is as follows:
[0017]
Embodiment 2
[0019] The alkenyl iodide compound used in the present invention is (E)-1-(2-iodovinyl)benzene, the substituted terminal alkyne is phenylacetylene, and the catalyst is FeCl 3 , The ligand is 1,10-phenanthroline (1,10-phenanthroline).
[0020] 1.0mmol of substituted terminal alkyne, 1.5mmol of alkenyl iodide, 3.0mmol of cesium carbonate, 0.10mmol of catalyst, and 0.2mmol of ligand were put into 5ml of toluene solvent at room temperature and under the protection of nitrogen and heated to 110°C for 48 hours of reaction. After separation and purification, (E) 1,4-diphenyl-but-1-en-3-yne was obtained with a yield of 60%. Its structural formula is as follows:
[0021]
Embodiment 3
[0023] The alkenyl iodide compound used in the present invention is (E)-1-(2-iodovinyl)benzene, the substituted terminal alkyne is phenylacetylene, and the catalyst is FeCl 3 , The ligand is 1,10-phenanthroline (1,10-phenanthroline).
[0024] Substituted terminal alkyne 1.0mmol, alkenyl iodide 1.0mmol, cesium carbonate 2.0mmol, catalyst 0.15mmol, ligand 0.3mmol, placed in 5ml of toluene solvent at room temperature and under the protection of nitrogen, heated to 110°C, reacted for 48 hours, after After separation and purification, (E) 1,4-diphenyl-but-1-en-3-yne was obtained with a yield of 78%. Its structural formula is as follows:
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com