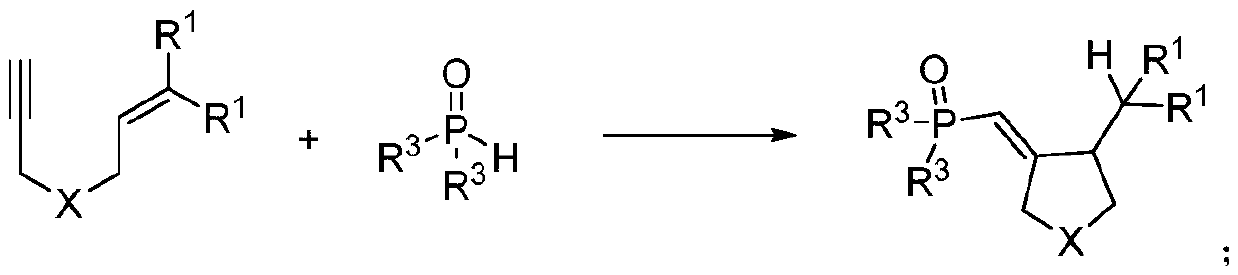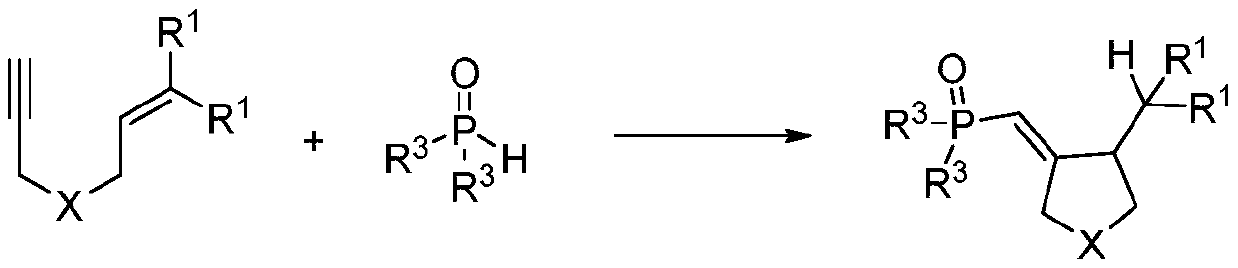Synthetic method of phosphonyl methylene substituted five-membered cyclic compound
A technology for phosphonomethylene and cyclic compounds, which is applied in the field of simple synthesis of phosphono-containing heterocyclic compounds, can solve the problems of unavoidable heavy metal residues, and achieve the effect of simple and convenient operation and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
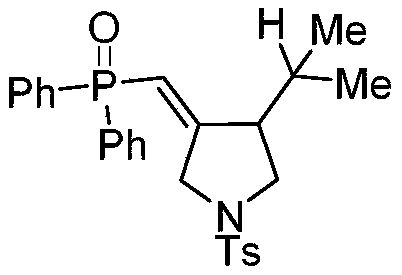
[0016] Weigh 27.7mg (0.1mmol) of 4-methyl-N-(3-methyl-2-buten-1-yl)-N-propargylbenzenesulfonamide, 60.7mg (0.3mmol) of diphenylphosphine ) and organic dye photocatalyst eosin 3.4mg (5%mmol) in a 10mL colorless transparent reaction tube, add 2mL tetrahydrofuran solvent, under nitrogen, room temperature light stirring reaction, TLC detection reaction progress, after about 24h, after the reaction is complete, After separation and purification by column chromatography (petroleum ether: ethyl acetate = 1:1), a white solid was obtained with a yield of 83%.
[0017]
[0018] White solid (39.8mg, 83% yield); m.p.198-202℃; 1 H NMR (600MHz, CDCl 3 ): δ7.58(s,6H),7.43(s,2H),7.37(s,4H),7.19(s,2H),5.89(d,J=22.3Hz,1H),4.09(dd,J= 124.5,17.0Hz,2H),3.20–3.04(d,J=3.1Hz,2H),2.62(s,1H),2.33(s,3H),1.87(s,1H),0.90(s,3H), 0.71(s,3H); 13 C NMR (CDCl 3 ,150MHz): δ162.7,143.6,133.8(d,J=17.9Hz),133.1(d,J=18.9Hz),132.1,131.8(d,J=7.4Hz),130.7(q,J=6.2Hz), IR( neat):ν=2923,2860,2308,1639,1447,1338...
Embodiment 2
[0041] Using the same method and reaction conditions as in Example 1, when 1,6-enyne is 2-(3-methyl-2-buten-1-yl)-2-propargyl dimethyl malonate, The following target product 2 was obtained, yield: 94%.
[0042] The structure of product 2 is characterized as follows:
[0043]
[0044] colorless liquid; 1 H NMR (400MHz, CDCl 3 ):δ7.82–7.71(m,4H),7.58–7.46(m,6H),5.99(d,J=23.7Hz,1H),3.72(s,3H),3.66(s,3H),3.05( d,J=18.5Hz,1H),2.84(d,J=3.0Hz,1H),2.46(dd,J=12.9,8.0Hz,1H),2.12(td,J=13.2,6.6Hz,1H), 1.98(t, J=12.2Hz, 1H), 1.30(s, 1H), 1.03(d, J=6.8Hz, 3H), 0.88(d, J=6.7Hz, 3H); 13 C NMR (100MHz, CDCl 3)δ171.8, 171.5, 168.0, 131.4 (d, J = 2.3Hz), 130.9 (dd, J = 19.5, 9.8Hz), 128.5 (dd, J = 12.0, 6.7Hz), 113.0 (d, J = 104.9Hz) ,58.5,52.7,51.5(d,J=13.9Hz),40.6,40.5,32.8,29.6,21.3,16.3; 31 P NMR (243MHz, CDCl 3 )δ21.6; IR (neat): ν=2957,1736,1632,1439,1267,1192,1115,703cm -1 ; HRMS (ESI) Exact mass calculated for [C 25 h 29 NaO 5 P] + [M+Na] + :463.1645,found:463.1664.
Embodiment 3
[0046] Adopt the same method and reaction condition of embodiment 1, when R 3 When it is 3,5-dimethylphenyl, the following different target products 3 are obtained with a yield of 36%.
[0047] The structure of product 3 is characterized as follows:
[0048]
[0049] colorless liquid; 1 H NMR (400MHz, CDCl 3 ): δ7.65(d, J=8.1Hz, 2H), 7.26(d, J=6.8Hz, 4H), 7.22(s, 2H), 7.12(s, 2H), 5.93(d, J=22.0Hz ,1H),4.23(d,J=17.0Hz,1H),4.04(d,J=17.8Hz,1H),3.25–3.12(m,2H),2.70(s,1H),2.39(s,3H) ,2.30(s,12H),2.01–1.93(m,1H),0.96(d,J=6.8Hz,3H),0.83(d,J=6.8Hz,3H); 13 C NMR (CDCl 3 ,100MHz): δ162.1,143.5,138.3(dd,J=12.7,1.7Hz),133.5(dd,J=5.4,2.9Hz),129.6,128.267(d,J=17.8Hz),128.266(d,J=17.8Hz) 1.9Hz), 127.9, 114.3(d, J=101.0Hz), 51.8(d, J=13.5Hz), 51.5(d, J=6.3Hz), 47.8, 30.7(d, J=1.4Hz), 21.4, 21.214 (d, J=45.3Hz), 21.211, 21.0, 17.4; 31 P NMR (243MHz, CDCl 3 )δ21.7; IR (neat): ν=2959,2923,2866,1639,1458,1345,1170,1039,860cm -1 ; HRMS (ESI) Exact mass calculated for [C 31 h 38 NNa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com