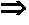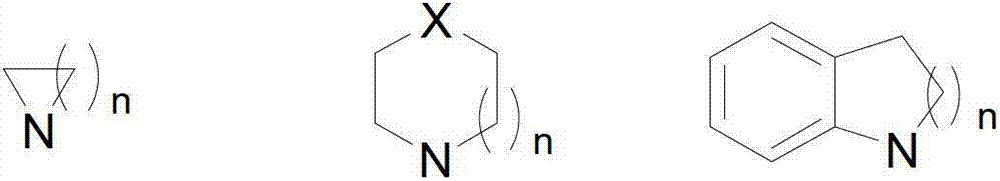Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
580 results about "Propargyl" patented technology
Efficacy Topic
Property
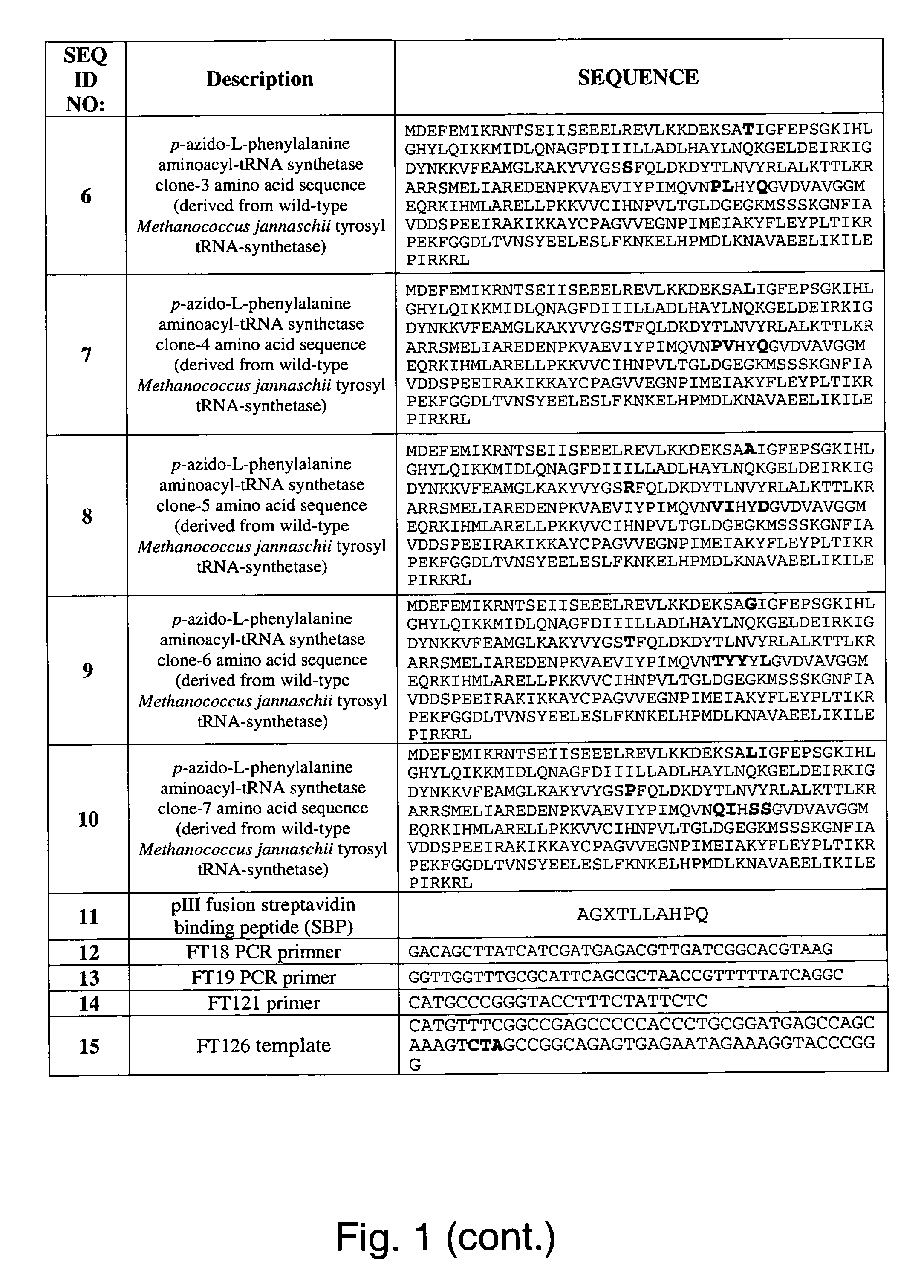
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, propargyl is an alkyl functional group of 2-propynyl with the structure HC≡C−CH₂−, derived from the alkyne propyne. The term propargylic refers to a saturated position (sp³-hybridized) on a molecular framework next to an alkynyl group. The name comes from mix of propene and argentum, which refers to the typical reaction of the terminal alkynes with silver salts. The term homopropargylic designates in the same manner a saturated position on a molecular framework next to a propargylic group and thus two bonds from an alkyne moiety. a 3-butynyl fragment, HC≡C-CH₂CH₂-, or substituted homologue.
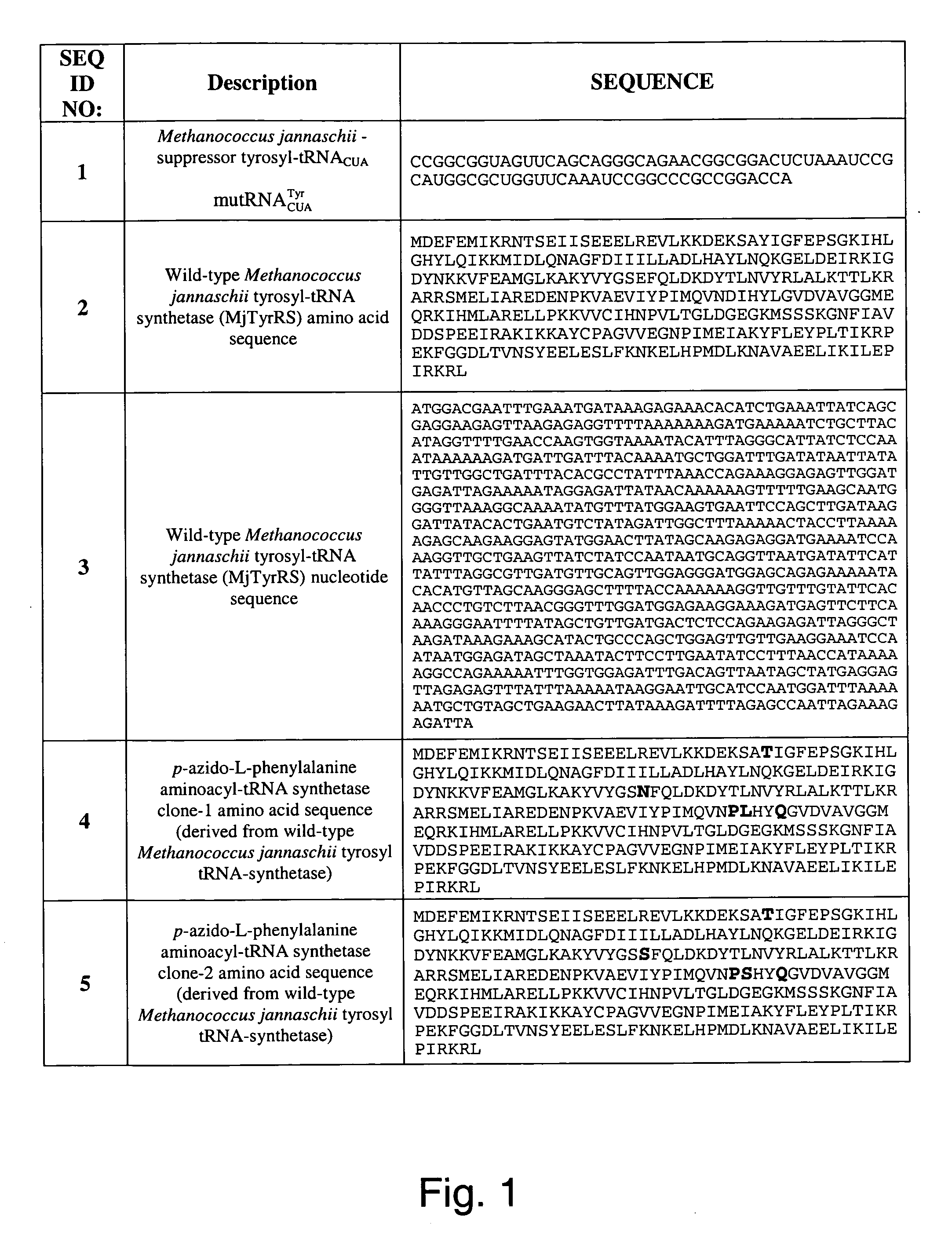
Selective posttranslational modification of phage-displayed polypeptides
ActiveUS20070178448A1Easy to detectEasy to quantifyAntibody mimetics/scaffoldsVirus peptidesArylCycloaddition
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such as p-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2] cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Aminoindan derivatives
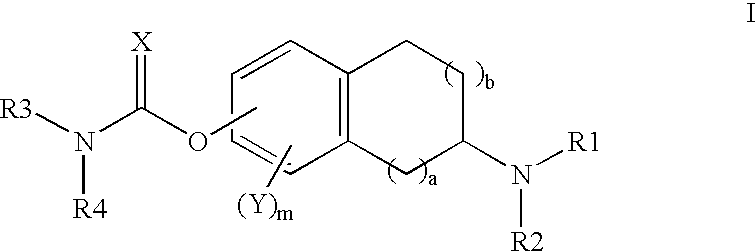
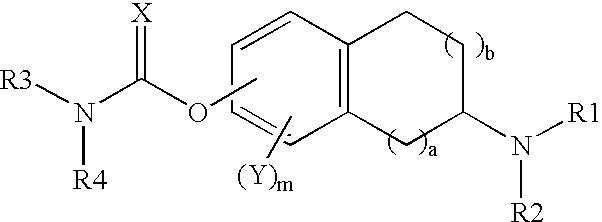
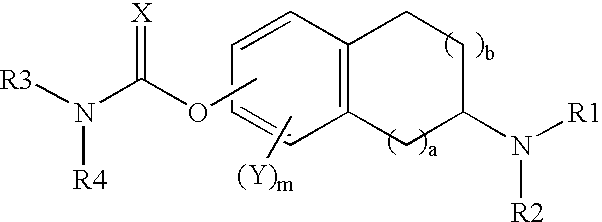
This invention is directed to compounds of the following formula:wherein when a is 0, b is 1 or 2; when a is 1, b is 1, m is from 0-3, X is 0 or S, Y is halogeno, R1 is hydrogen C1-4 alkyl, R2 is hydrogen, C1-4 alkyl, or optionally substituted propargyl and R1 and R4 are each independently hydrogen, C1-6 alkyl, C6-12 aryl, C6-12 aralkyl each optionally substituted.This invention is also directed to the use of these compounds for treating depression, Attention Deficit Disorder (ADD), Attention Deficit and Hyperactivity Disorder (ADHD), Tourette's Syndrome. Alzheimer's Disease and other dementia's such as senile dementia, dementia of the Parkinson's type, vascular dementia and Lewy body dementia.This invention is further directed to a pharmaceutical composition comprising a therapeutically effective amount of the above-defined compounds and a pharmaceutically acceptable carrier.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
In vivo incorporation of alkynyl amino acids into proteins in eubacteria
Owner:THE SCRIPPS RES INST
Methods for isolating propargylated aminoindans
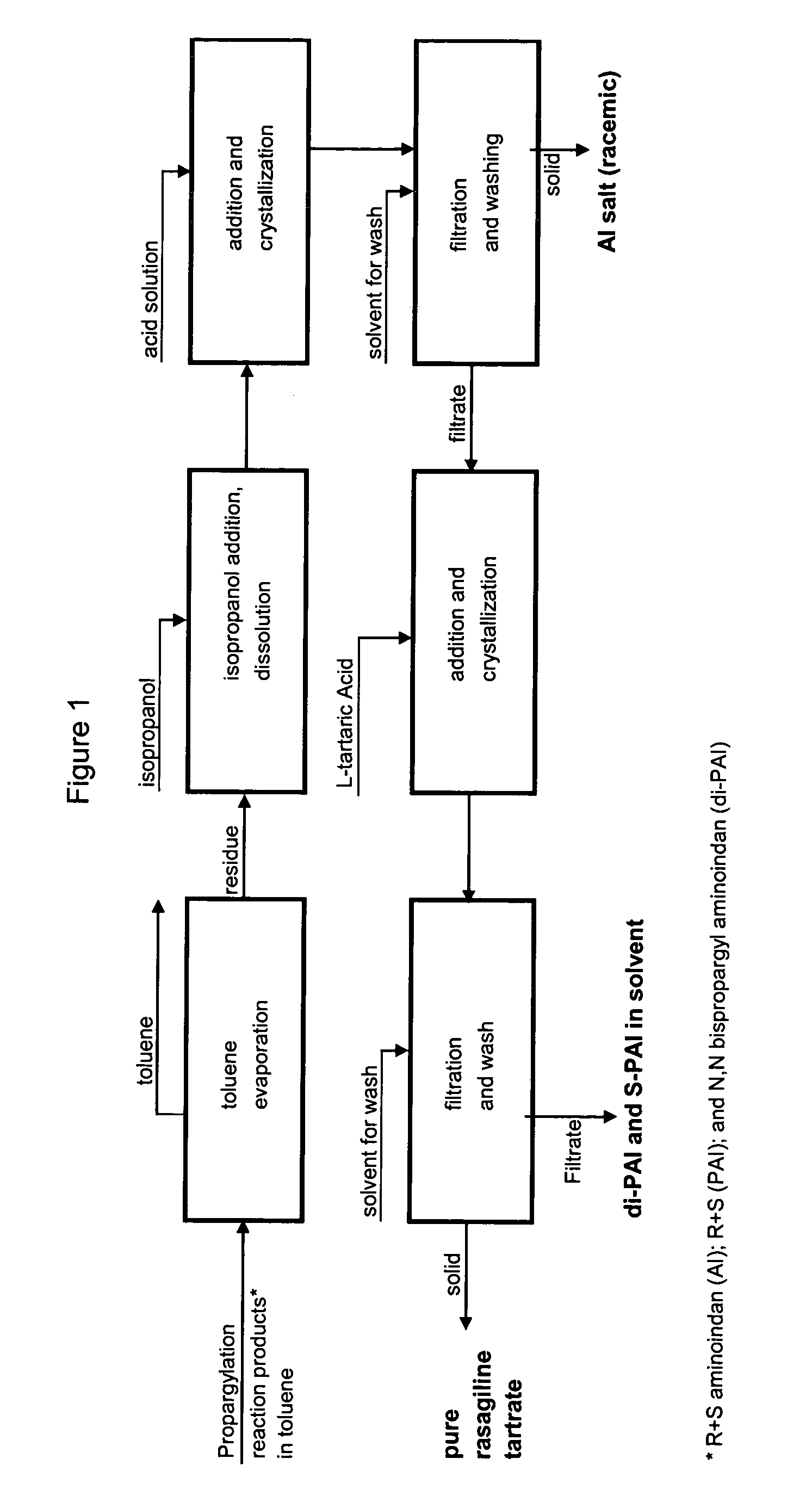
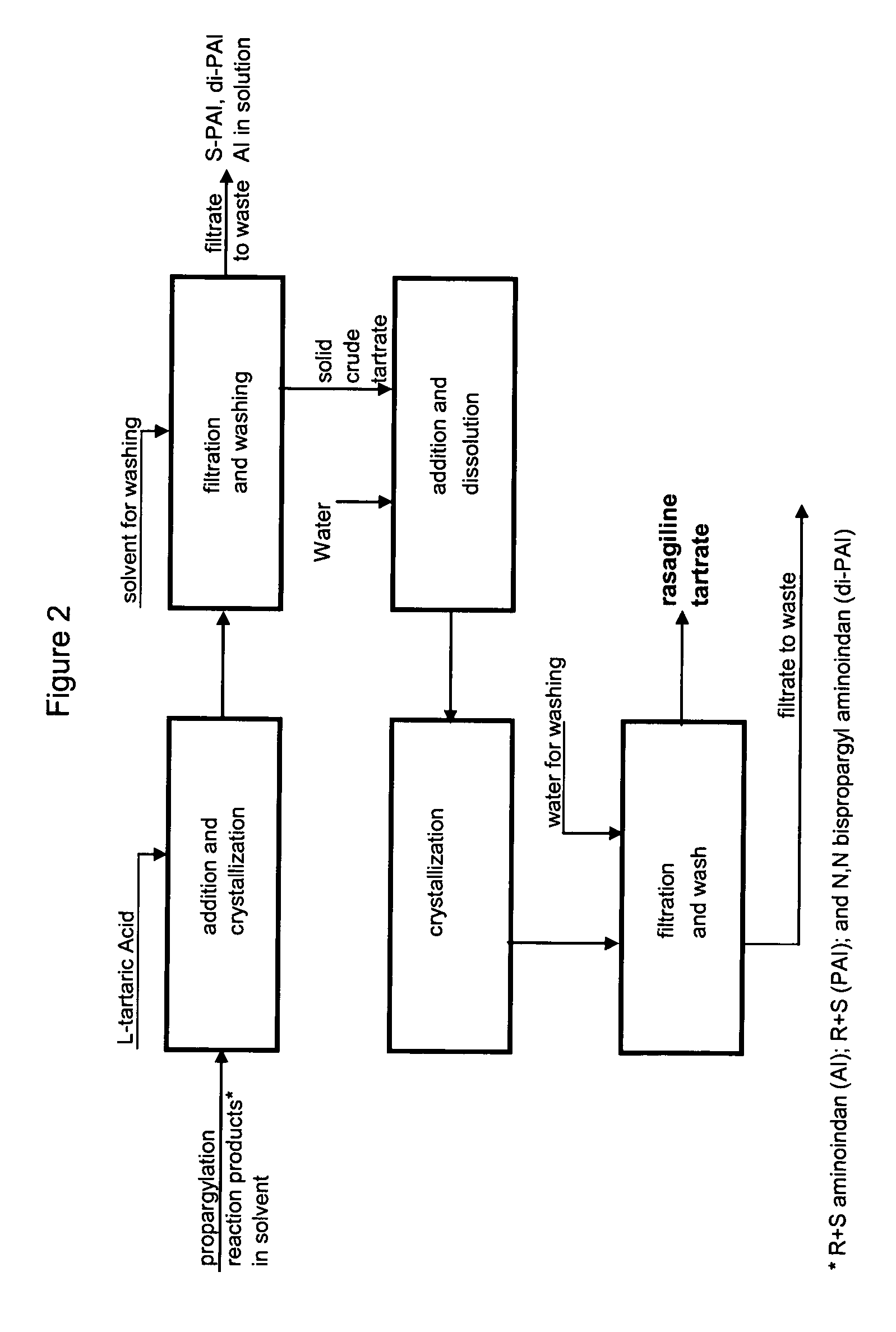
A process for isolating from a reaction mixture a salt of a mono-propargylated aminoindan, a process for isolating from a reaction mixture a crystalline diastereomeric salt of a mono-propargylated aminoindan, and a process for isolating from a reaction mixture a salt of enantiomerically pure N-propargyl-1-aminoindan or a salt of enantiomerically pure 6-(N-methyl, N-ethyl-carbamoyloxy)-N′-propargyl-1-aminoindan. The corresponding products are also disclosed.
Owner:TEVA PHARMA IND LTD
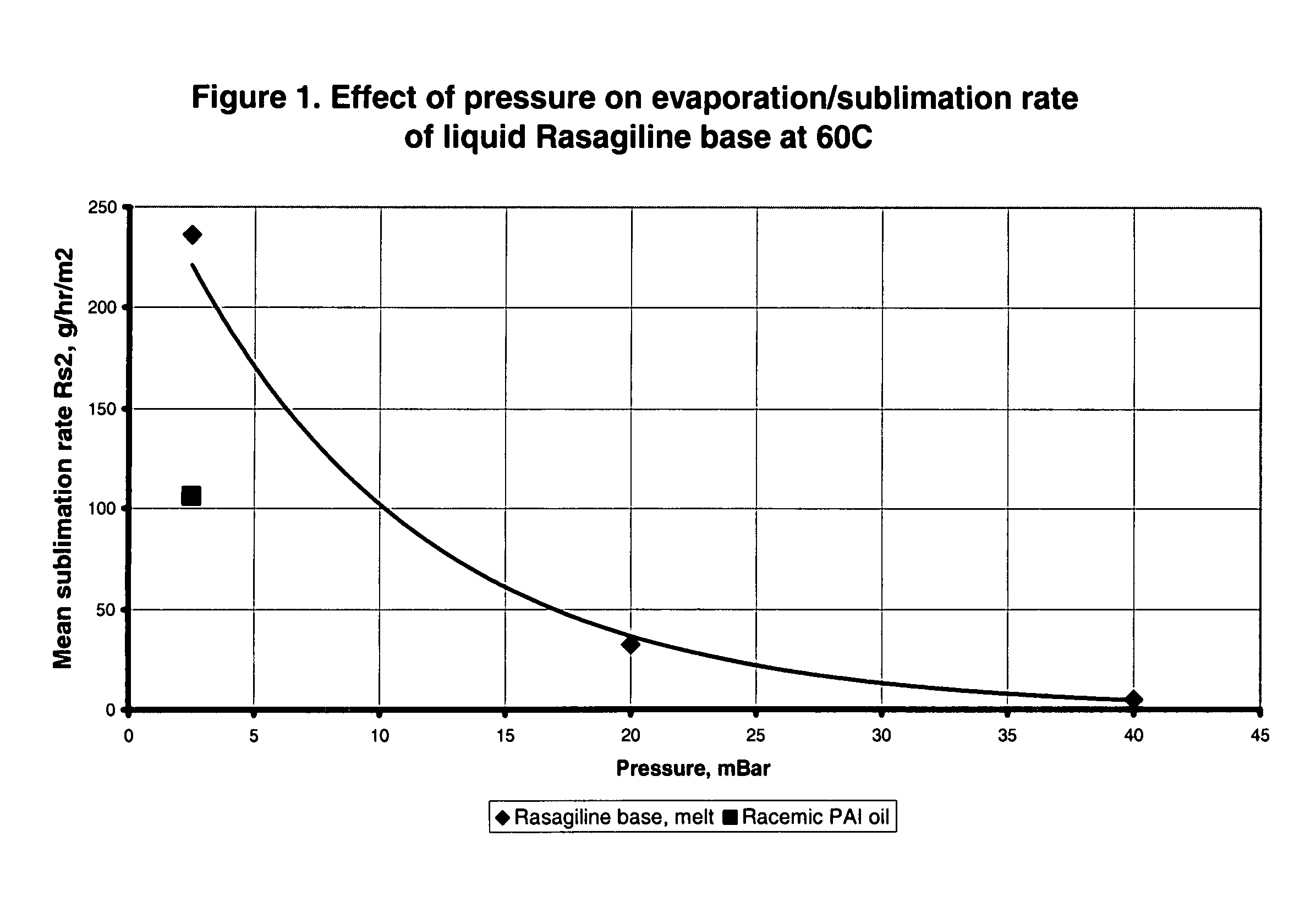
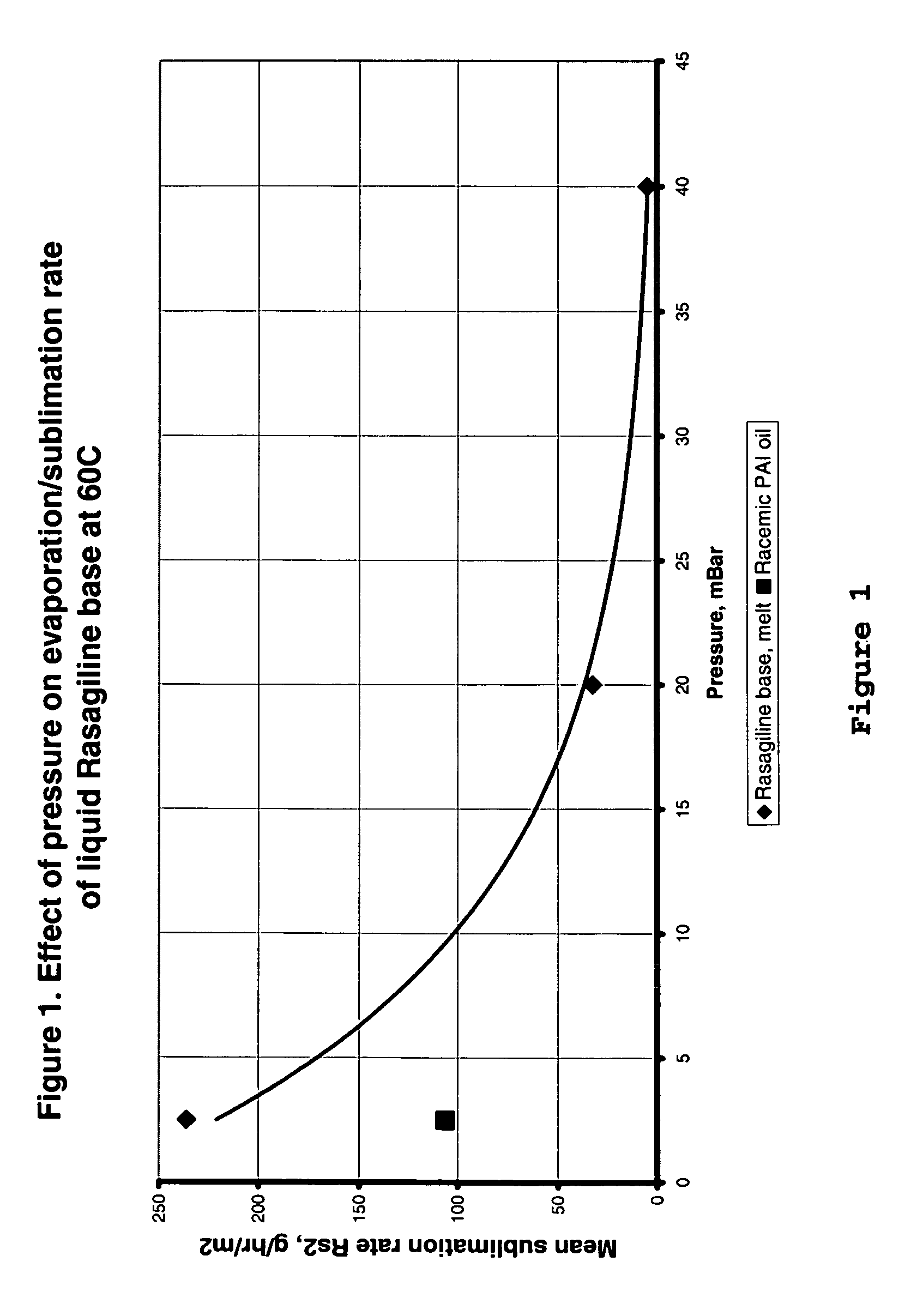
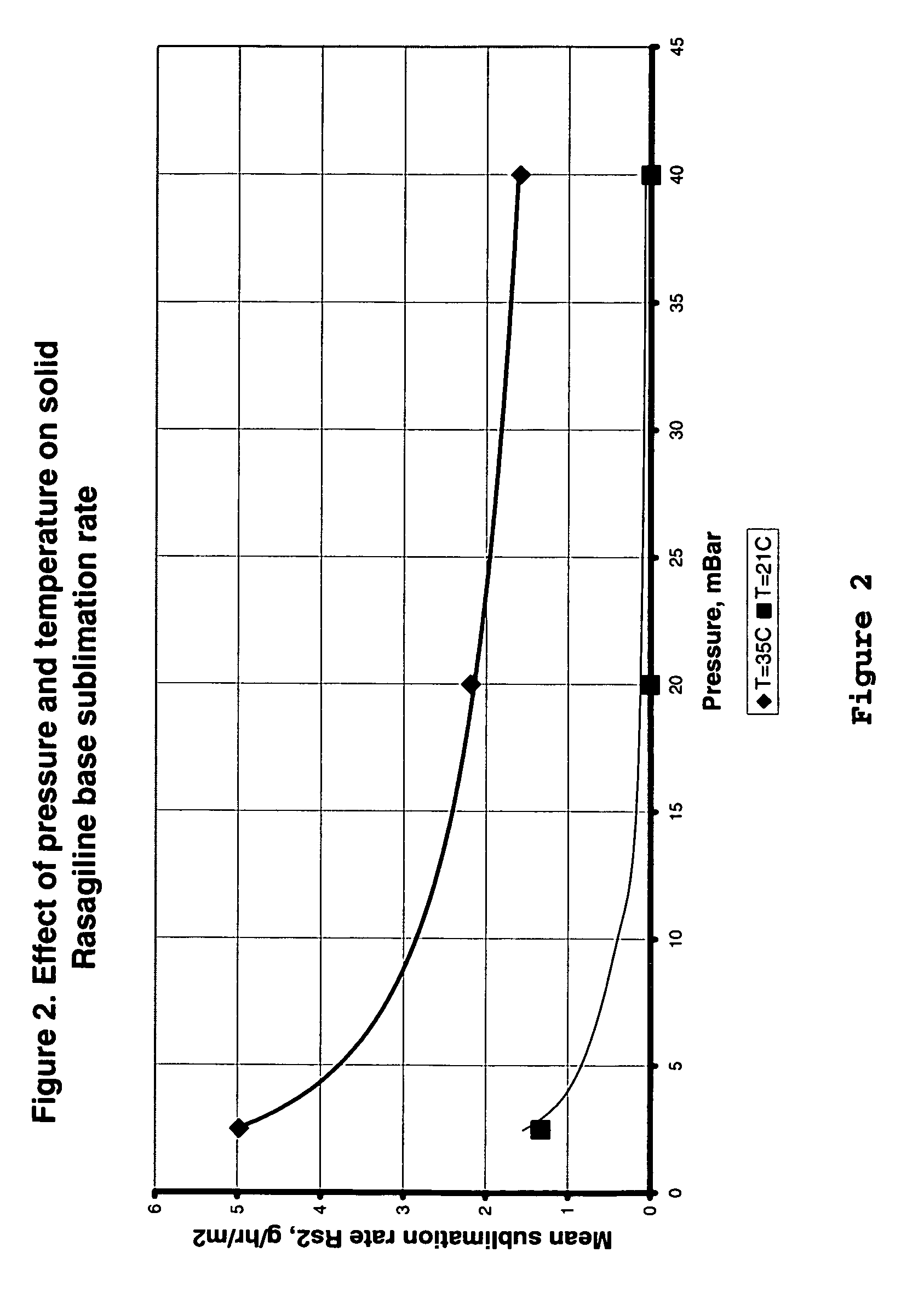
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Rasagiline formulations of improved content uniformity
Disclosed are pharmaceutical preparations of R(+)-N-propargyl-1-aminoindan salts having enhanced content uniformity, processes for preparation of the compositions, and their uses.
Owner:TEVA PHARMA IND LTD
Nonaqueous electrolyte solution for lithium secondary battery and lithium secondary battery using the same
ActiveUS20100092872A1Suppress gas productionExcellent battery characteristicsOrganic chemistryElectrolytic capacitorsSolventLithium-ion battery
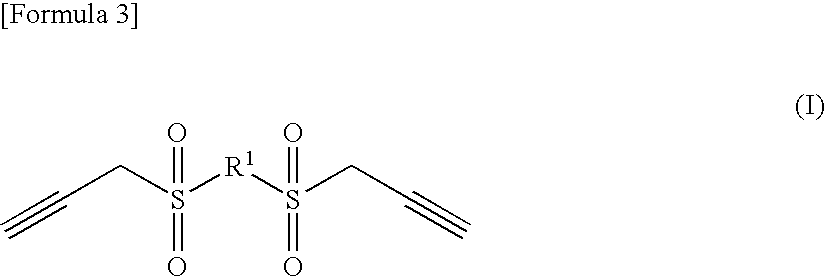
The present invention provides (1) a sulfone compounds having a propargyl group, (2) a nonaqueous electrolytic solution for lithium secondary batteries, which comprises an electrolyte salt dissolved in a nonaqueous solvent and contains a sulfone compound having a specific structure that has an SO2 group with a propargyl group or a vinyl group bonding thereto, in an amount of from 0.01 to 10% by weight of the nonaqueous electrolytic solution, and which can prevent gas generation and is excellent in battery characteristics such as cycle property and the like, and (3) a lithium secondary battery comprising a positive electrode, a negative electrode and a nonaqueous electrolytic solution of an electrolyte salt dissolved in a nonaqueous solvent, wherein the nonaqueous electrolytic solution contains a sulfone compound having a specific structure, in an amount of from 0.01 to 10% by weight of the nonaqueous electrolytic solution.
Owner:MU IONIC SOLUTIONS CORP
Crystalline solid rasagiline base
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
InactiveUS20100145101A1Organic active ingredientsAmino compound purification/separationN-propargyl1-aminoindan
The subject invention provides crystalline R(+)-N-propargyl-1-aminoindan, pharmaceutical compositions and methods of manufacture thereof.
Owner:TEVA PHARMA IND LTD
Neuroprotective iron chelators and pharmaceutical compositions comprising them
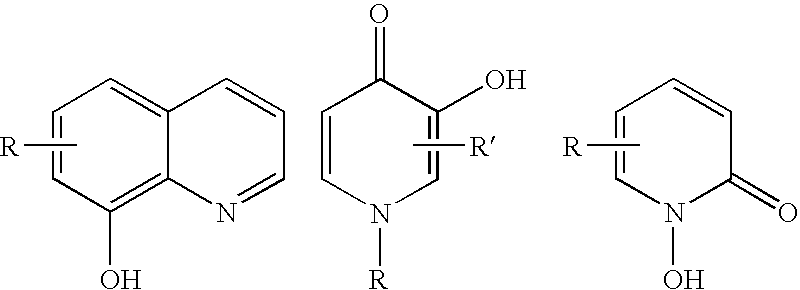
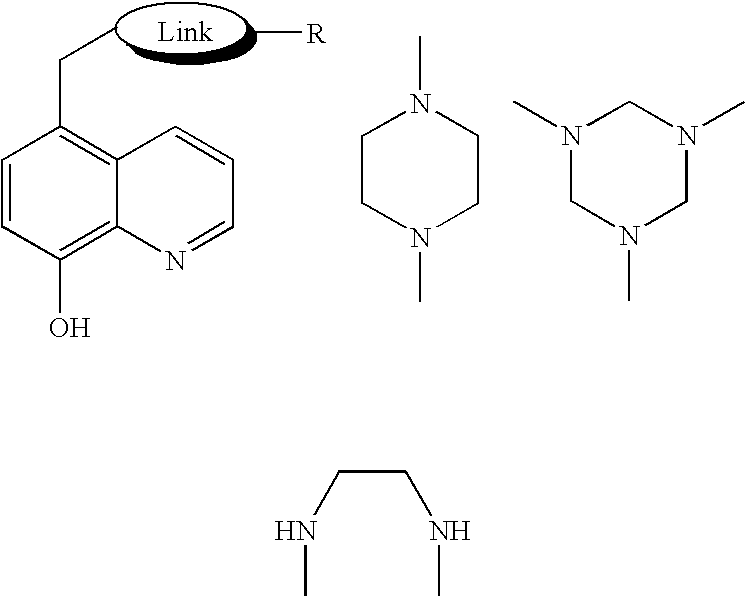
Novel iron chelators exhibiting neuroprotective and good transport properties are useful in iron chelation therapy for treatment of a disease, disorder or condition associated with iron overload and oxidative stress, eg. a neurodegenerative or cerebrovascular disease or disorder, a neoplastic disease, hemochromatosis, thalassemia, a cardiovascular disease, diabetes, a inflammatory disorder, anthracycline cardiotoxicity, a viral infection, a protozoal infection, a yeast infection, retarding ageing, and prevention and / or treatment of skin ageing and skin protection against sunlight and / or UV light. The iron chelator function is provided by a 8-hydroxyquinoline, a hydroxypyridinone or a hydroxamate moiety, the neuroprotective function is imparted to the compound e.g. by a neuroprotective peptide, and a combined antiapoptotic and neuroprotective function by a propargyl group.
Owner:TECHNION RES & DEV FOUND LTD +1
Preparation method and application of acid sensitive doxorubicin prodrug based on polyethylene glycol
InactiveCN103834002ASimple purification technologyEasy to getOrganic active ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingPolyethylene glycol
The present invention provides an acid sensitive doxorubicin prodrug based on polyethylene glycol, and a method and an application of the acid sensitive doxorubicin prodrug based on polyethylene glycol. The preparation method comprises: preparing a polyethylene glycol whose end contains an acid sensitive acetal group and a p-nitrophenyl ester azide group by a reaction of a polyethylene glycol whose double ends contain diazido ethyl diacetal groups with a p-nitro phenyl propargyl carbonate; preparing the acid sensitive doxorubicin prodrug based on the polyethylene glycol by the reaction of the prepared polyethylene glycol with doxorubicin hydrochloride. The acid sensitive and water soluble doxorubicin prodrug has good biocompatibility and acid sensitivity, thereby being used as a stimulation sensitive antineoplastic prodrug.
Owner:SUZHOU UNIV
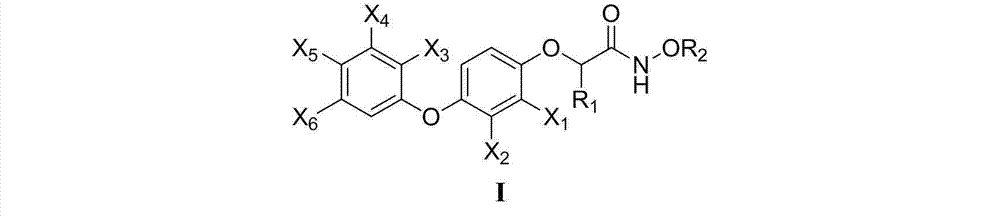
2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof
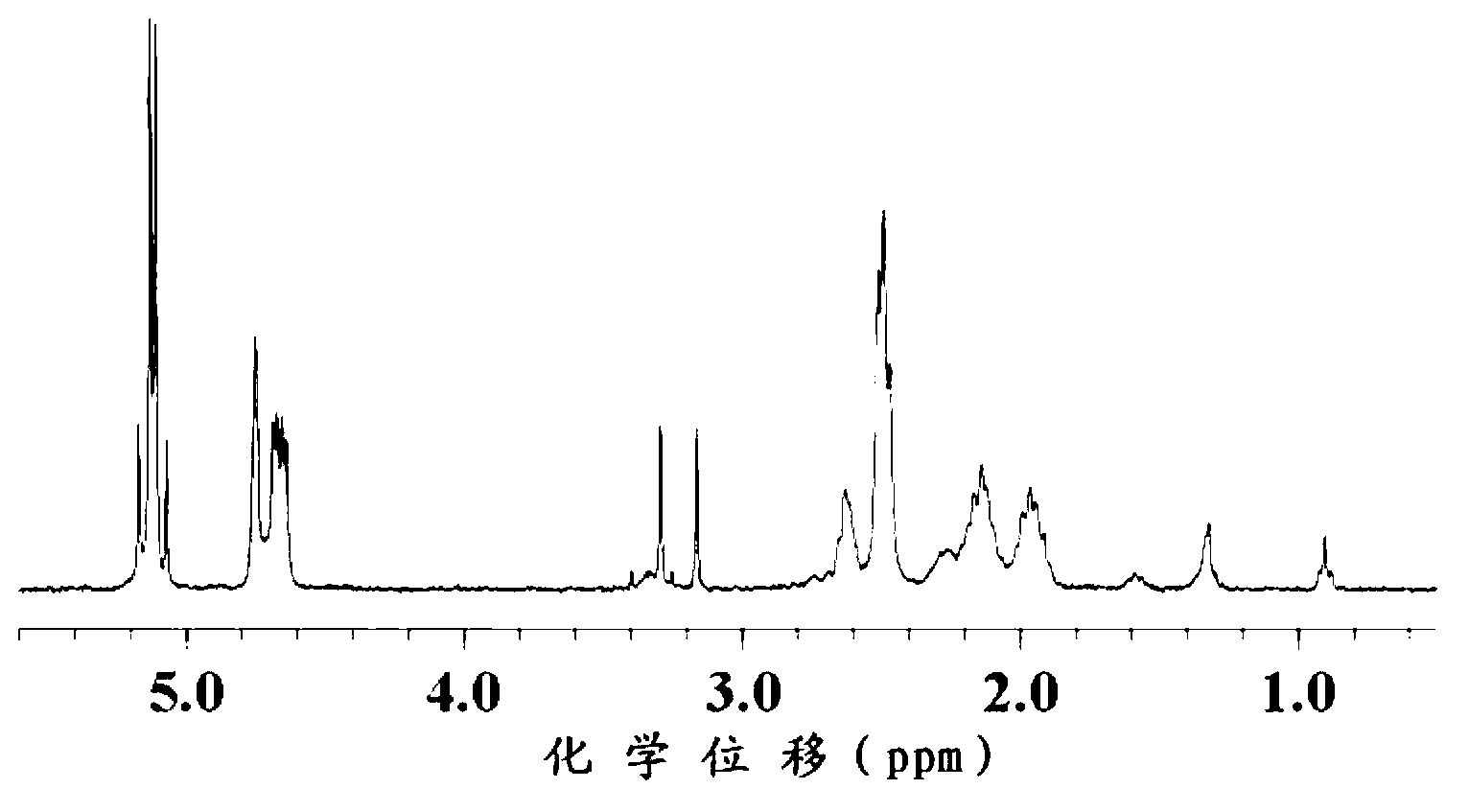
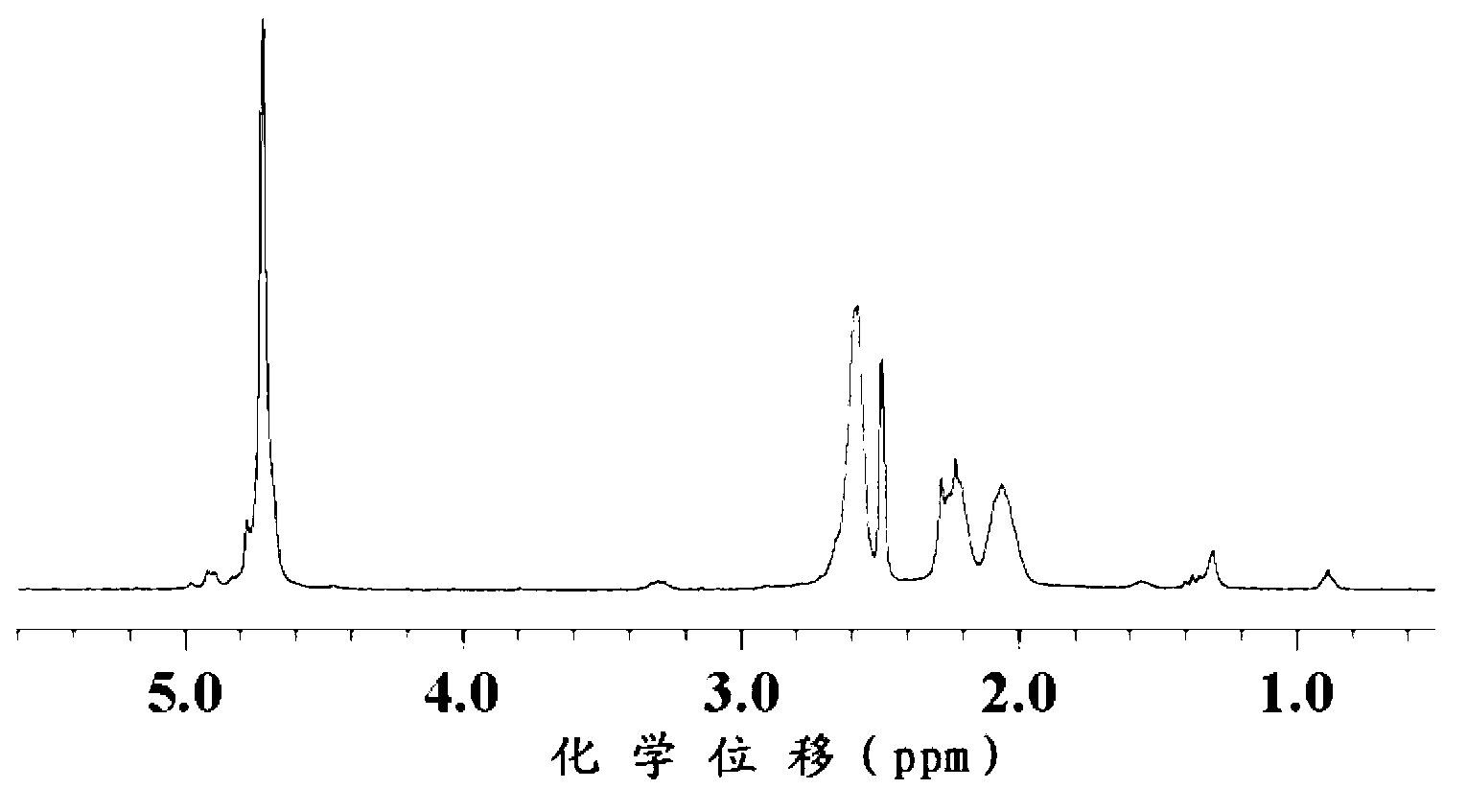
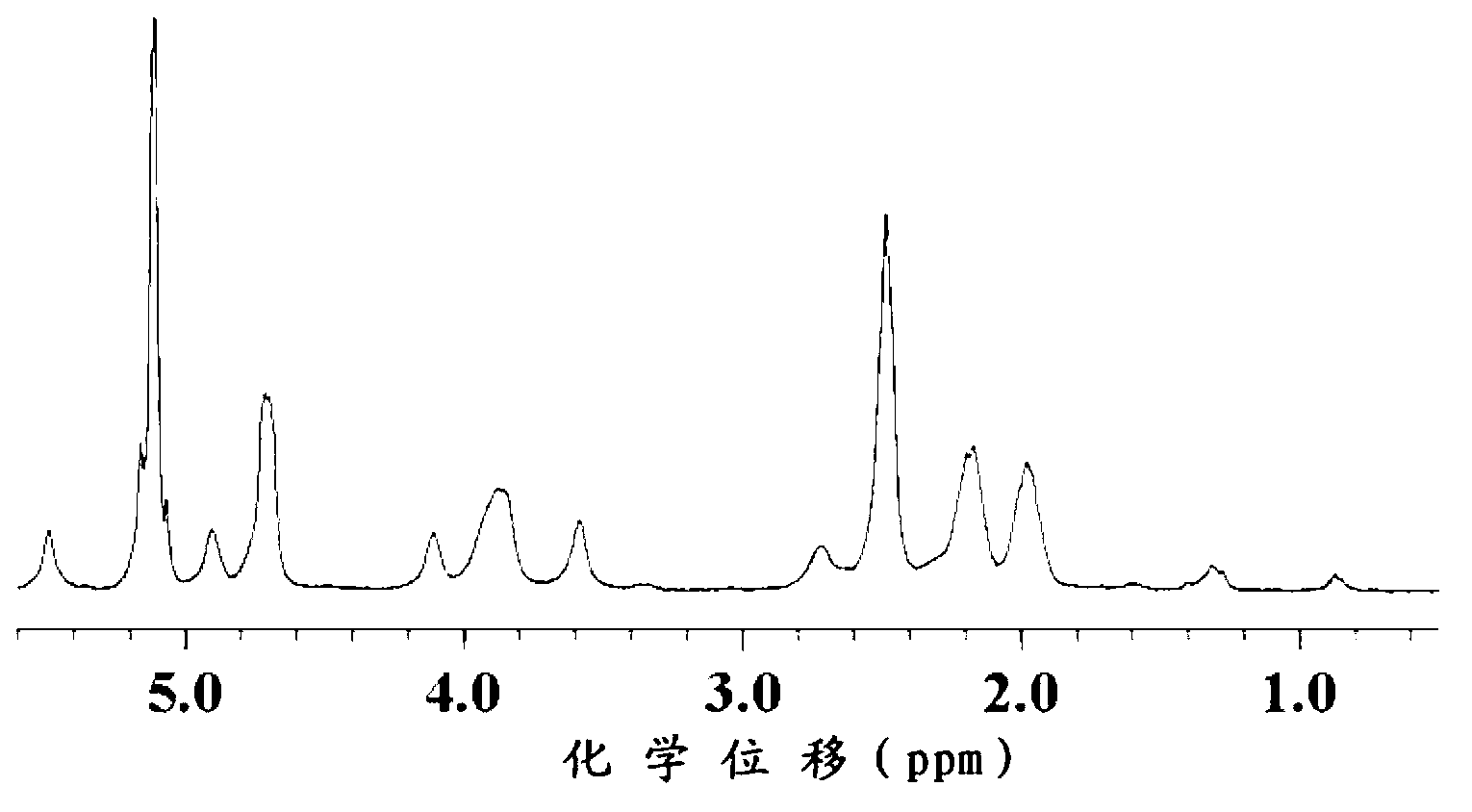
The invention relates to 2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide shown as a chemical structural formula I or an optical isomer thereof. In the formula, R1 is selected from H, C1-C1alkyl, C3-C4 straight-chain or branch-chain alkyl; R2 is selected from allyl, propargyl, halogenated allyl, halogenated propargyl; and X1-X6 are selected from hydrogen, C1-C2 alkyl, trifluoromethyl, fluorine, chlorine, bromine, nitryl or trifluoromethoxy. The invention further discloses an application of 2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide or the optical isomer thereof in preparation of anti-lung adenocarcinoma drugs.
Owner:HUNAN UNIV
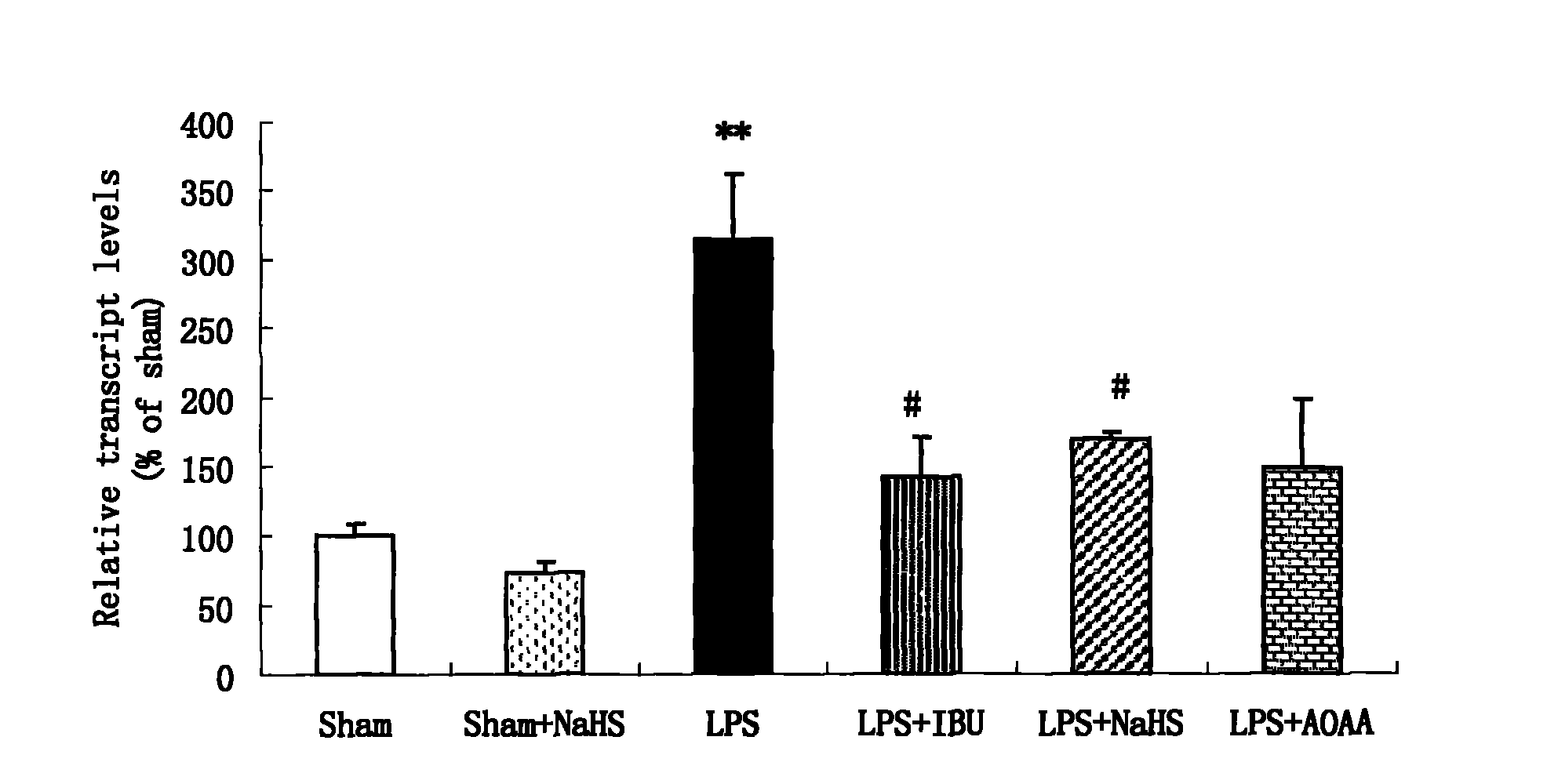
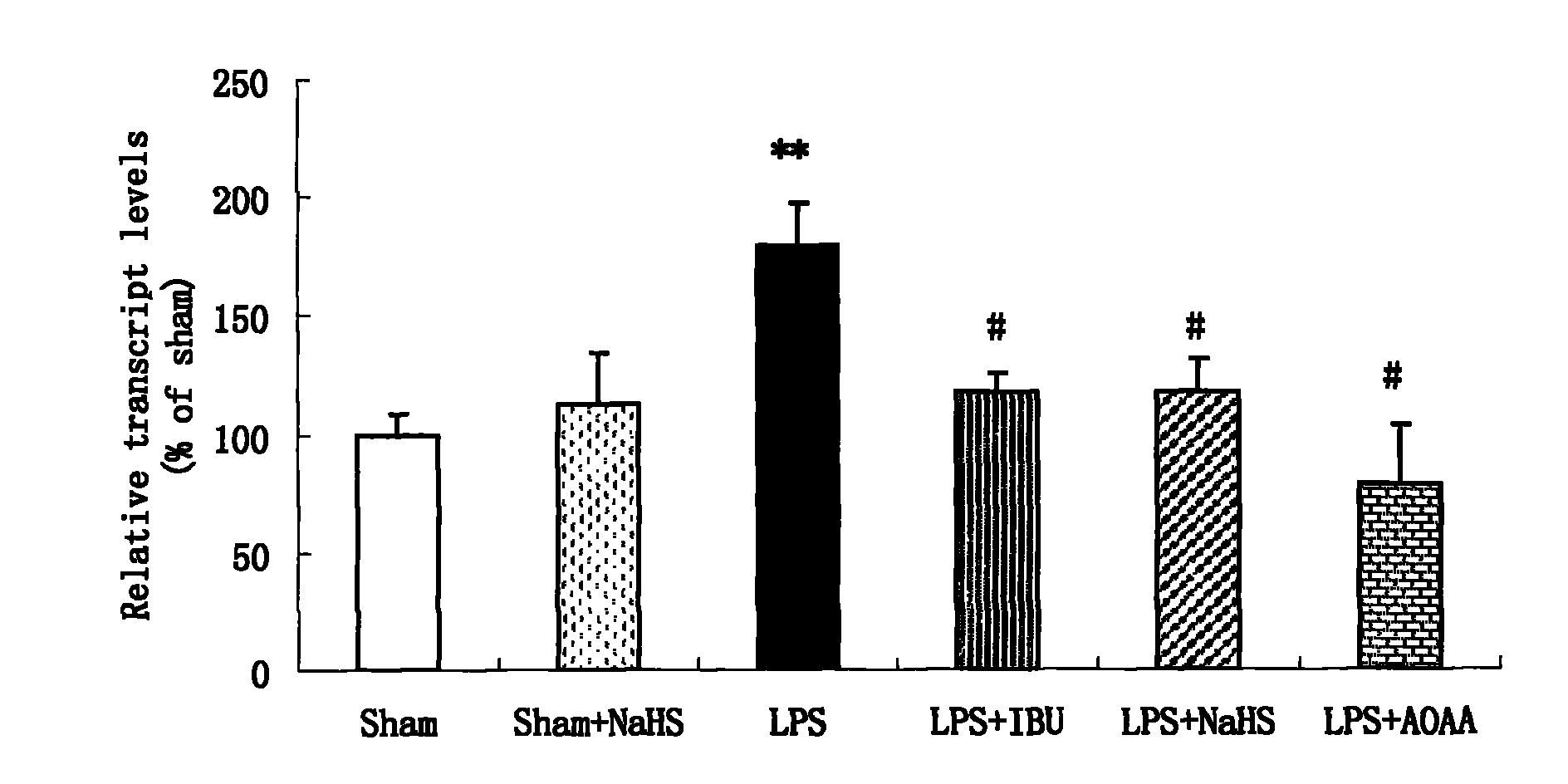
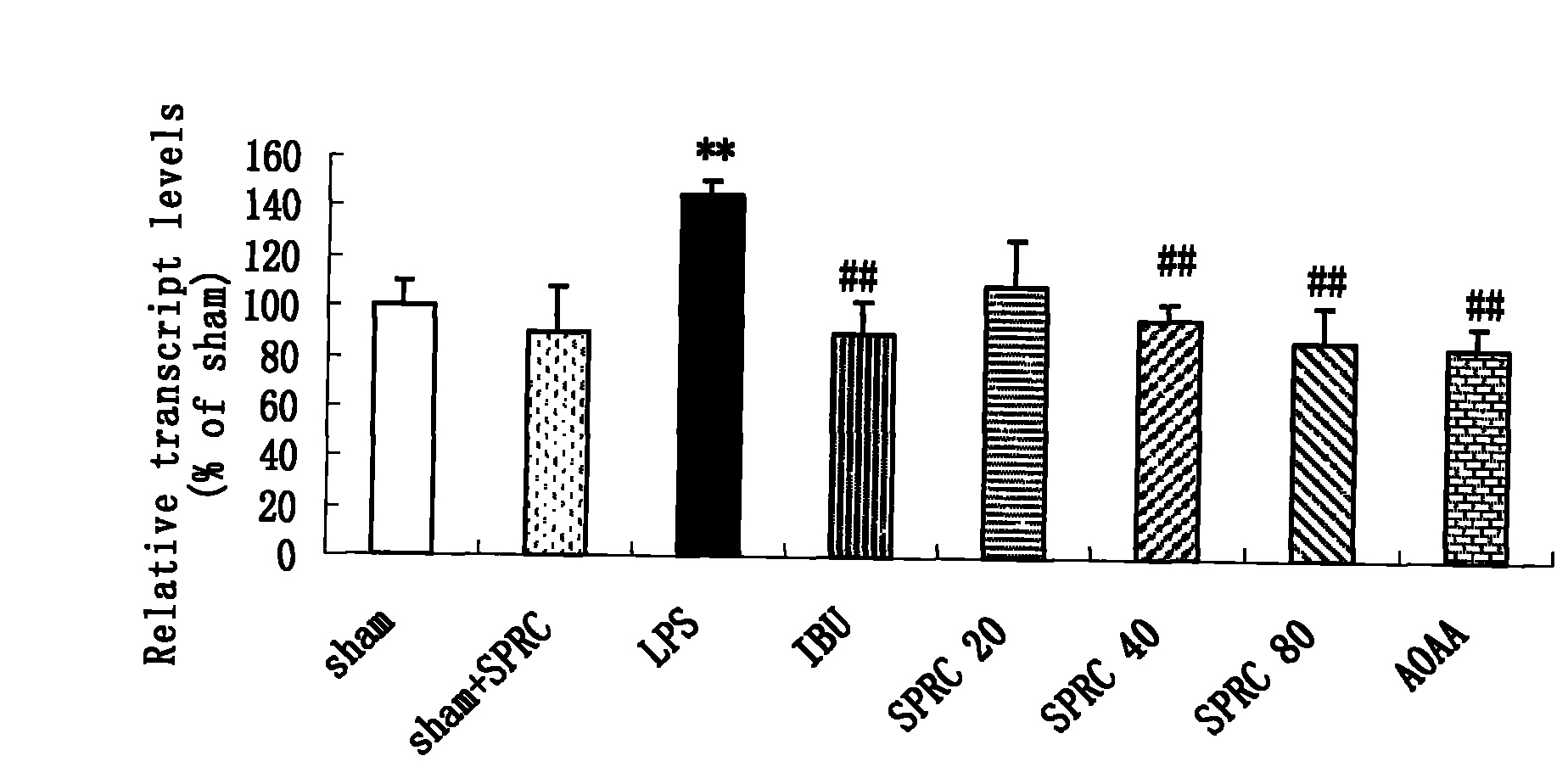
Application of hydrogen sulfide donor to preparation of medicine for treating central nervous system disease
ActiveCN102078327AAlleviate learning and memory lossPromote learning and memoryOrganic active ingredientsNervous disorderTumor necrosis factor alphaSodium hydrosulfide
The invention belongs to the field of pharmacy, and relates to application of a hydrogen sulfide donor to the preparation of a medicine for treating central nervous system disease, in particular to application of the hydrogen sulfide donor, namely sodium hydrosulfide, or allyl cysteine and analogues thereof to the preparation of the medicine for treating the central nervous system disease. Integral animal model experiments prove that the sodium hydrosulfide and propargyl cysteine can reduce the learning and memory impairment of rats, increase the content of hydrogen sulfide in hippocampus tissues of the rats, inhibit the messenger ribonucleic acid (mRNA) expression of a tumor necrosis factor alpha and a receptor I thereof, inhibit an inflammatory mediator in the hippocampus tissues of rats with dementia and inhibit inflammation-related enzymes in the hippocampus tissues of the rats, and can be used as a medicine for treating Alzheimer disease and other inflammation-related central nervous system degenerative diseases such as Parkinson disease.
Owner:FUDAN UNIV
Composition, preparation of polycarbosilanes and their uses
The invention provides branched copolymers as precursors for preparing silicon carbide (SiC) ceramics represented by the general formulae:[Si(˜)RC(˜)H]xn[SiR1R2CH2]yn,Formula Type-Iwherein n is the degree of polymerization, 0.1≦x<0.8, 0.2≦y<0.9 and x+y=1; and R=methyl or H, R1 and R2 are randomly composed of hydrogen (H), allyl, methyl (Me), phenyl (Ph), propargyl or vinyl. Another branched copolymer is represented by the general formula:[Si(˜)RC(˜)H]xn[SiR1R2CH2]yn[SiR3R4CH2]znFormula Type-IIwherein n is the degree of polymerization, 0.1≦x<0.8, 0≦y<0.8, 0.2≦z<0.8 and x+y+z=1; and R=methyl or H, R1 and R2 are randomly composed of hydrogen (H), methyl (Me) and phenyl; R3 and R4 are randomly composed of H, allyl, methyl, phenyl (Ph), propargyl, and vinyl. The invention also provides methods for the preparation of such branched copolymers.
Owner:STARFIRE SYST
Methods for Assessing Cancer for Increased Sensitivity to 10-Propargyl-10-Deazaaminopterin by Assessing Egfr Levels
InactiveUS20100248249A1Organic active ingredientsMicrobiological testing/measurement10-deazaminopterinPropargyl
The present invention relates to a method for assessing the sensitivity of a patient's cancer to treatment with 10-propargyl-10-deazaminopterin and a method for selecting a patient for treatment of cancer with 10-propargyl-10-deazaminopterin, by determining the amount of a EGFR or other growth factor expressed by the cancer and comparing the amount with the amount of EGFR or other growth factor expressed by a reference cancer.
Owner:ALLOS THERAPEUTICS
2-(4-aryloxyphenoxy)alkylamide and application thereof
The invention relates to 2-(4-aryloxyphenoxy)alkylamide represented by a chemical structural formula I or an optical isomer thereof, wherein R1 is selected from H, C1-C1 alkyl, and C3-C4 straight-chain or branched-chain alkyl; R2 is selected from allyl, propargyl, halogenated allyl, and halogenated propargyl; X1-X6 are selected from hydrogen, C1-C2 alkyl, fluoro, chloro, bromo, or cyan. The invention also relates to the application of the 2-(4-aryloxyphenoxy)alkylamide or the optical isomer thereof in preparing anti-lung-adenocarcinoma medicines.
Owner:HUNAN UNIV
Method for preparing 1-propone-1,3-sultone
The invention relates to a method for preparing 1-propene-3-sultone, and the technical field of preparation of organic materials. The method mainly comprises the processes of addition, acidation and cyclization: a, addition, namely an addition reaction of propargyl ethanol and alkali metal sulphite or hydrosulfite is carried out in an aqueous solution, the molar ratio of the propargyl alcohol to the alkali metal sulphite or the hydrosulfite is 1:1-10, and inorganic acid or organic acid is added to stop reaction after the addition reaction is ended; and b, a product of the addition reaction isseparated out, and then the separated product is subjected to cyclization and separation to obtain a 1-propene-3-sultone product. The PST product can be refined. The method overcomes the defect of the prior art, and has the remarkable advantages of unique method, simple manufacturing process, easy operation, mild reaction conditions, cheap and easily-obtained raw materials, higher yield, good selectivity, high and stable quality of products, few three wastes, lower cost, remarkable economic benefit, and the like.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Process for the preparation of unsaturated 4,5-allene ketones, 3,5-diene ketones and the corresponding saturated ketones
InactiveUS6380437B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetonePropargyl
Process for the preparation of unsaturated 4,5-allene ketones by reaction of tertiary propargyl alcohols with alkenyl alkyl ethers or ketals in the presence of aliphatic sulfonic acids or sulfonic acid salts.
Owner:DEGUSSA AG
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Method for asymmetrically synthesizing chiral beta-acetenyl ketone from beta-ketonic acid
InactiveCN104513146AHigh reactivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetonic acidsKetone
The invention relates to a method for synthesizing chiral beta-acetenyl ketone by catalytic intermolecular decarboxylation from beta-ketonic acid and a propargyl compound. A chiral copper catalyst adopted in the invention is synthesized in stiu from a copper salt and a chiral P,N,N-tridentate ligand in various polar solvents and nonpolar solvents. According to the invention, various chiral beta-acetenyl ketone compounds with substituent groups can be synthesized conveniently, and can obtain a percent enantiomeric excess as high as 95%. The method in the invention is advantaged by operational simplicity, available raw materials, wide substrate application range, high enantioselectivity, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing 1,6-eneyne compounds
InactiveCN101591275AHigh enantioselectivityMild reaction conditionsGroup 4/14 element organic compoundsOrganic compound preparationIridiumRegioselectivity
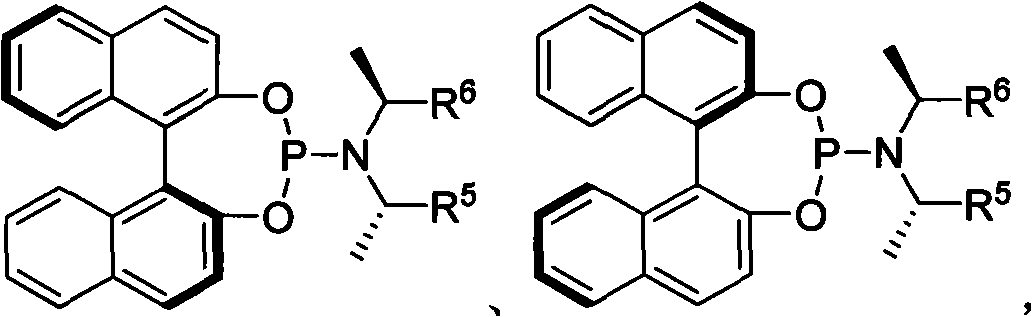
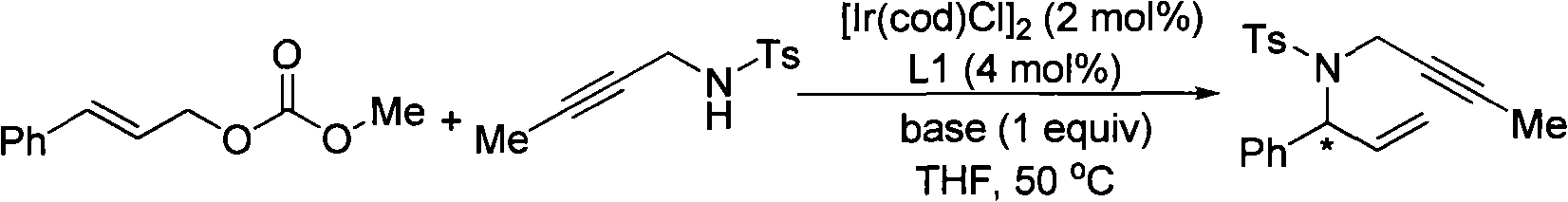
The invention provides a method for synthesizing 1,6-eneyne compounds. The method is an effective method which synthesizes the optically active 1,6-eneyne compounds with high regioselectivity and enantioselectivity by using iridium complex as a catalyst as well as allyl carbonate compounds and a propargyl nucleophilic reagent as raw materials. The method has the advantages of readily available catalyst, high catalytic activity, mild reaction temperature, wide substrate application range and high product regioselectivity and enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Composition, preparation of polycarbosilanes and their uses
Owner:STARFIRE SYST
Propargyl nitroxides and indanyl nitroxides and their use for the treatment of neurologic diseases and disorders
Owner:TEVA PHARMA IND LTD
Poly (gamma-propargyl-L-glutamate)-polyamino acid segmented copolymer, functional segmented copolymer and preparation method
ActiveCN102936337AHigh graft densityGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsBonding processBiocompatibility Testing
The invention provides poly (gamma-propargyl-L-glutamate)-polyamino acid segmented copolymer. The poly (gamma-propargyl-L-glutamate)-polyamino acid segmented copolymer is presented as the formula (I). Small molecules containing primary amino radical serve as an initiator, and the poly (gamma-propargyl-L-glutamate)-polyamino acid segmented copolymer is obtained through the gradual ring cleavage reaction of gamma- propargyl-L-glutamate-N-carboxylic acid anhydride and amino acid-N- carboxylic acid anhydride. The side chain of the segmented copolymer is propargyl which is easy to modify, has high reactivity and is beneficial to functional small molecules which are subjected to bonded azide and have biological activity or environmental responsiveness, and the bonding process cannot lead to the breakage of amino acid backbones and is not affected by the amount and steric hindrance of functional small molecules. The segmented copolymer has good biocompatibility after being functionalized and can be applied in fields such as targeted drug delivery, organizational projects and protein separation and detection.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Method for catalytic synthesis of chiral propargylamine compound by chiral copper catalyst
InactiveCN102826947AHigh enantioselectivityHigh reactivityOrganic compound preparationGroup 5/15 element organic compoundsAlcoholDisplacement reactions
The invention discloses a method for catalytic synthesis of a chiral propargylamine compound by a chiral copper catalyst. The method comprises the following steps that a copper salt and a chiral P, N, N-ligand undergo an in-suit reaction in the presence of an alcohol solvent to produce a chiral copper catalyst; and a propargyl compound and primary amine or secondary amine undergo a displacement reaction in the presence of the chiral copper catalyst to produce the chiral propargylamine compound. The method can realize convenient synthesis of various propargylamine compounds, and has the maximum yield of 92% and enantioselectivity (ee) of 97%. The method has simple processes, strong catalytic system universality and high enantioselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
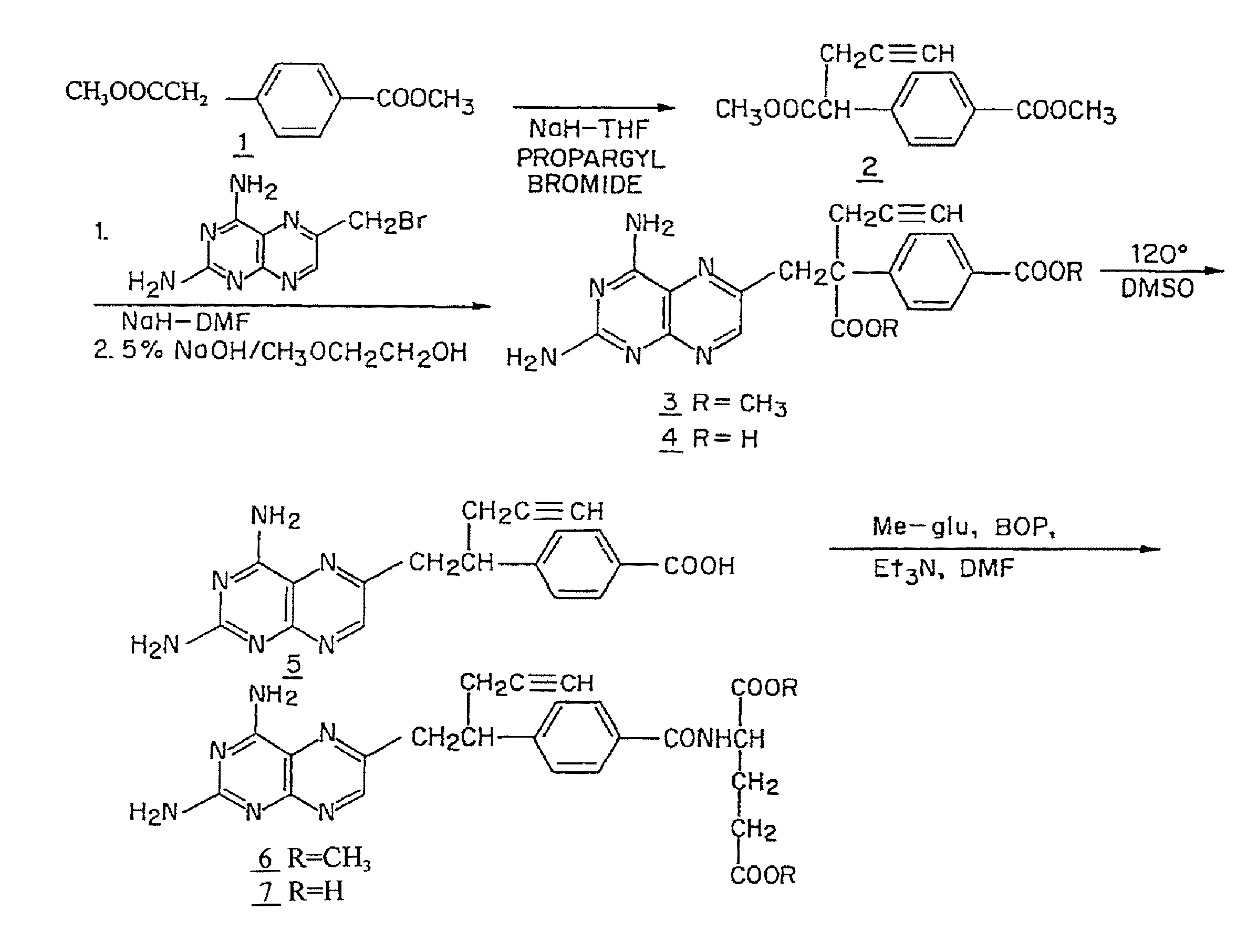
Methods for assessing cancer for increased sensitivity to 10-propargyl-10-deazaaminopterin
ActiveUS20110111436A1Disease diagnosisGlycinamide Ribonucleotide FormyltransferaseThymidylate synthase
Sensitivity of a patient's cancer to treatment with 10-propargyl-10-deazaaminopterin is assessed and patients are selected for treatment of cancer with 10-propargyl-10-deazaaminopterin, by determining the amount of a selected polypeptide expressed by the cancer and comparing the amount with the amount of the selected polypeptide expressed by a reference cancer. The polypeptide includes a member of a folate pathway polypeptide within a cell and may include at least one of reduced folate carrier-1 enzyme (RFC-1), dihydrofolate reductase (DHFR), folylpoly-gamma-glutamate synthetase (FPGS), thymidylate synthase (TS), γ-glutamyl hydrolase (GGH), and glycinamide ribonucleotide formyltransferase (GARFT).
Owner:SLOAN KETTERING INST FOR CANCER RES
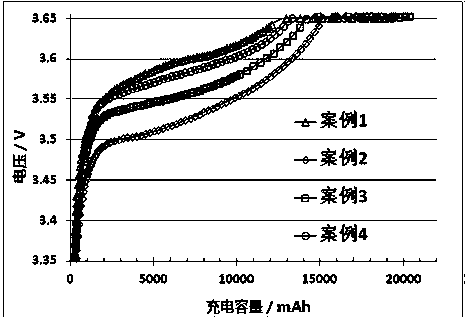
Lithium iron phosphate power battery with improved low temperature charge performance
InactiveCN108306018AImprove low temperature performanceNo negative impact on energy densityElectrode carriers/collectorsSecondary cellsDifluorophosphateElectrical battery
The invention discloses a lithium iron phosphate power battery with an improved low temperature charge performance, belongs to the technical field of lithium iron phosphate power batteries, and aims to solve the problem that the negative pole can easily reach a cut-off voltage, and the charge capacity is reduced therefore. The lithium iron phosphate power battery is characterized by comprising a positive plate, a negative plate, and electrolyte. The lithium salt is lithium hexafluorophosphate (11.5-15.0%). The organic solvent is a four component mixed solvent, which is composed of propylene carbonate, methyl carbonate, ethyl carbonate, and diethyl carbonate according to a ratio of 10-40: 1-10: 20-50: 10-30. The additive is one or more of vinylene carbonate, fluoroethylene carbonate, propargyl sulfonate, lithium difluorophosphate, lithium difluoro(oxalato)borate, and lithium tetrafluoro(oxalato)phosphate. The interface impedance of the negative pole can be reduced, the low temperature properties of the lithium iron phosphate power battery are obviously improved, and the low temperature charge performance of the lithium iron phosphate power battery is improved.
Owner:CAMEL GRP WUHAN OPTICS VALLEY R&D CENT CO LTD
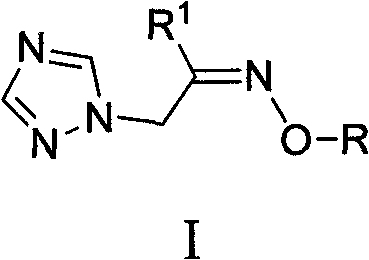
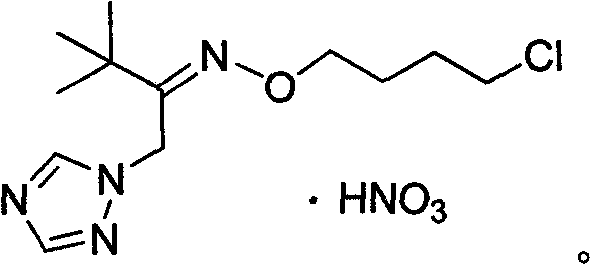
1-(1,2,4-triazole-1-group)ketoxime ethers and its application in preparation of bactericide
The invention discloses 1-(1,2,4-triazole triazole-1- group)ketoxime ether or its nitrate as shown in the chemical structure formula I, wherein R<1> is selected from the group consisting of C1-2alkyl, C3-4straight chain or branched alkyl; R is selected from the group consisting of C1-C2alkyl, C3-C18 straight chain or branched alkyl or alicyclic groups; allyl, propargyl, 2-methyl allyl, 2-chlorallyl, (Z)-3-chlorallyl, (E)-3-chlorallyl, or ArCH2; or Y(CH2)n:Y equals to fluorine, chlorine, bromine or iodine, n equals to 1,2,3,4,5 or 6. The application of 1-(1,2,4-triazole triazole-1- group)ketoxime ether or its nitrate as shown in the formula I in the preparation of a bactericide for killing wheat powdery mildew or Botrytis cinerea.
Owner:HUNAN UNIV
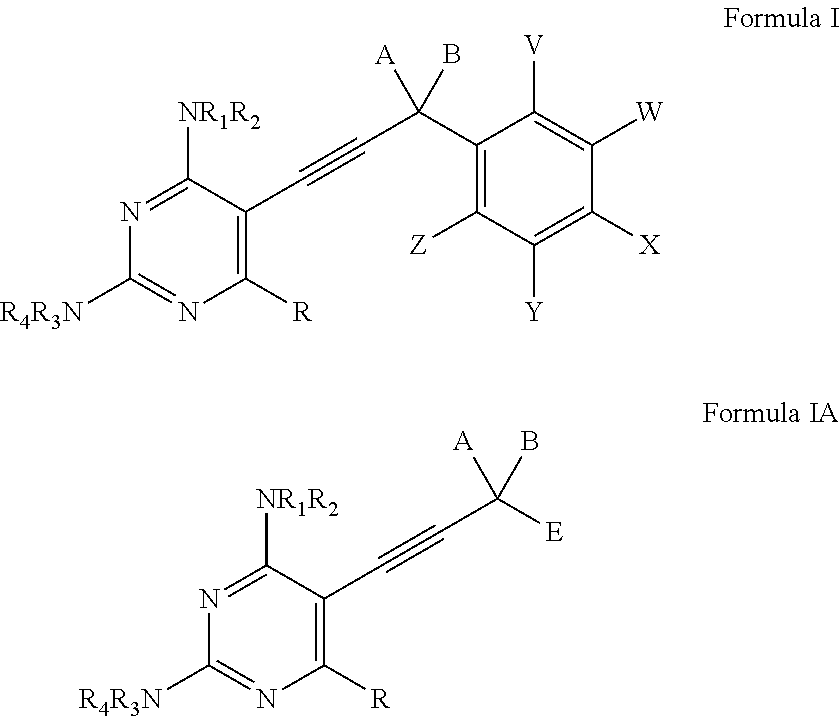
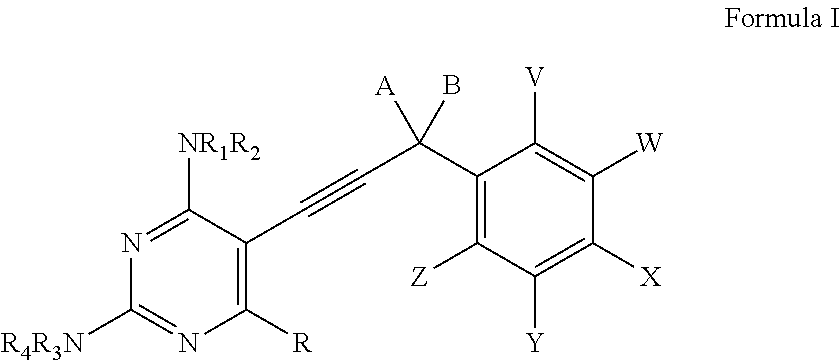
Heterocyclic and Cyclic Analogs of Propargyl-Linked Inhibitors of Dihydrofolate Reductase
ActiveUS20150225353A1BiocideOrganic active ingredientsDihydrofolate reductase inhibitorDihydrofolate reductase deficiency
Compounds of Formula I and Formula IA are inhibitors of dihydrofolate reductase and are suitable for use in compositions and methods for dihydrofolate reductase inhibition or, more specifically, treatment of a fungal infection, a bacterial infection or a protozoal infection, and, in specific embodiments, treatment of a fungal infection caused by C. albicans or C. glabrata:wherein R, R1, R2, R3, R4, A, B, E, V, W, X, Y and Z are as defined herein.
Owner:PROMILIAD BIOPHARMA INC +1
Quaternary ammonium salt type hyperbranched polythioether modified polymer microspheres and preparation method thereof
ActiveCN110918014ANot easy to fall offPoor solubilityBiocideFungicidesSodium methoxidePolymer science
The invention discloses quaternary ammonium salt type hyperbranched polythioether modified polymer microspheres and a preparation method thereof. The preparation method comprises the following steps of: under a photo-initiation condition, taking polyethylene glycol as a pore-foaming agent, taking alkyl dithiol, propargyl glycidyl ether and 1,7-octadiyne as raw materials, and preparing epoxy groupmicroporous polymer spheres by using a sulfydryl-alkyne addition suspension polymerization method; then, under the condition of 0 DEG C, taking methanol as a solvent, and initiating allyl glycidyl ether and a cysteamine compound by ultraviolet light to react to synthesize an [alpha]-epoxy-[omega]-ammonium-based intermediate; and, in a solvent and under the condition of 60 DEG C, synthesizing the hyperbranched ammonium polythioether modified polymer microspheres from the epoxy group microporous polymer spheres, the [alpha]-epoxy-[omega]-ammonium-based intermediate and sodium methoxide in one step. The obtained product has a good killing effect on escherichia coli, and the modified polymer microspheres can be recycled through simple filtration.
Owner:YANTAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com










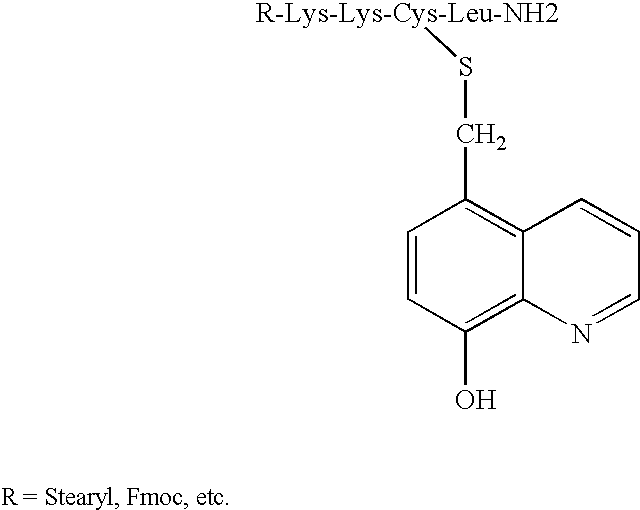


![2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof 2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e1f67f92-9d08-4a02-9c4e-f8fcd010772f/BDA0000280795931.PNG)
![2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof 2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e1f67f92-9d08-4a02-9c4e-f8fcd010772f/BDA00002807959310.PNG)
![2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof 2-[4-(quinoxaline-2-oxygroup) phenoxy] alkylamide and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e1f67f92-9d08-4a02-9c4e-f8fcd010772f/BDA00002807959311.PNG)