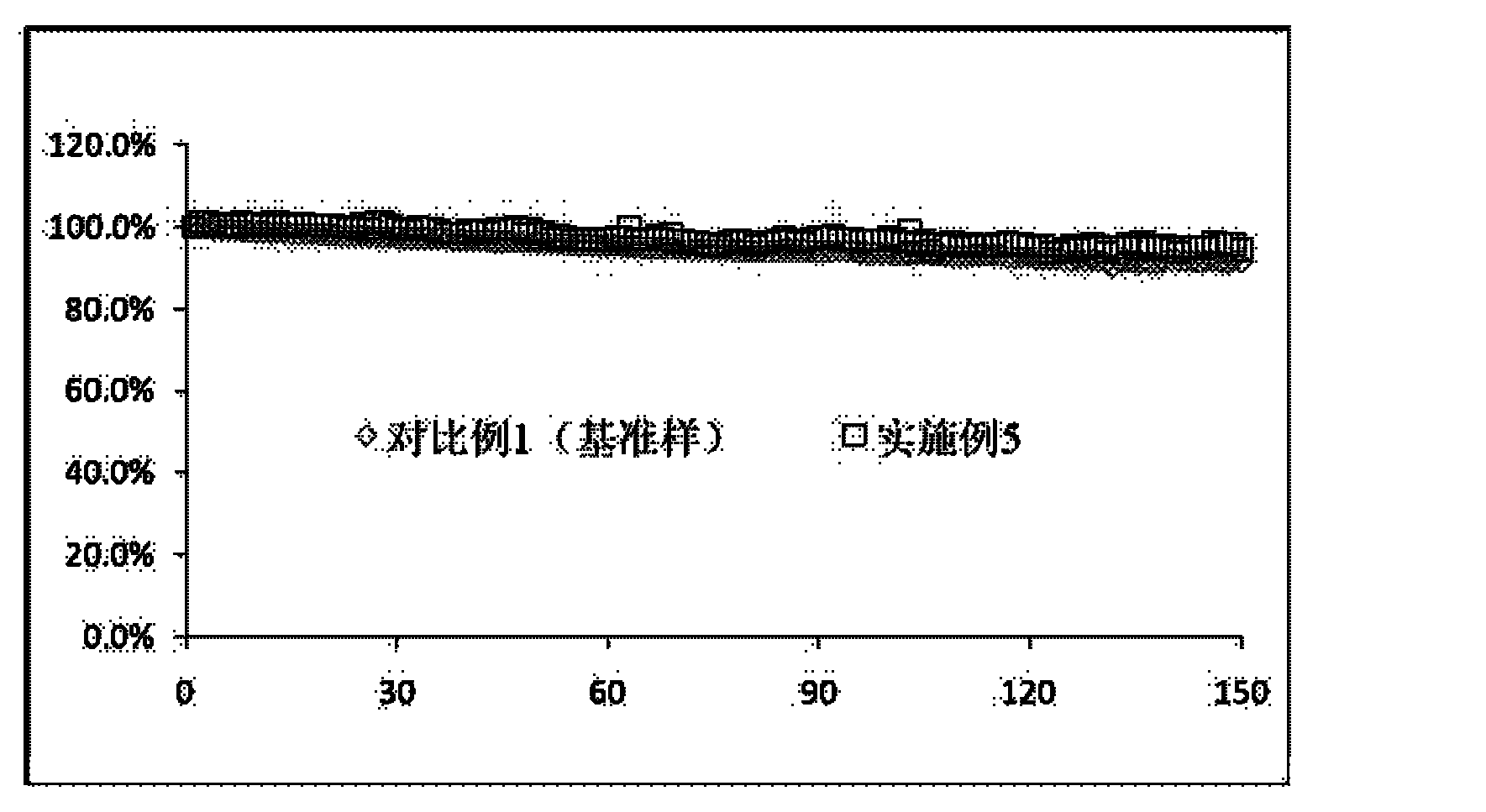
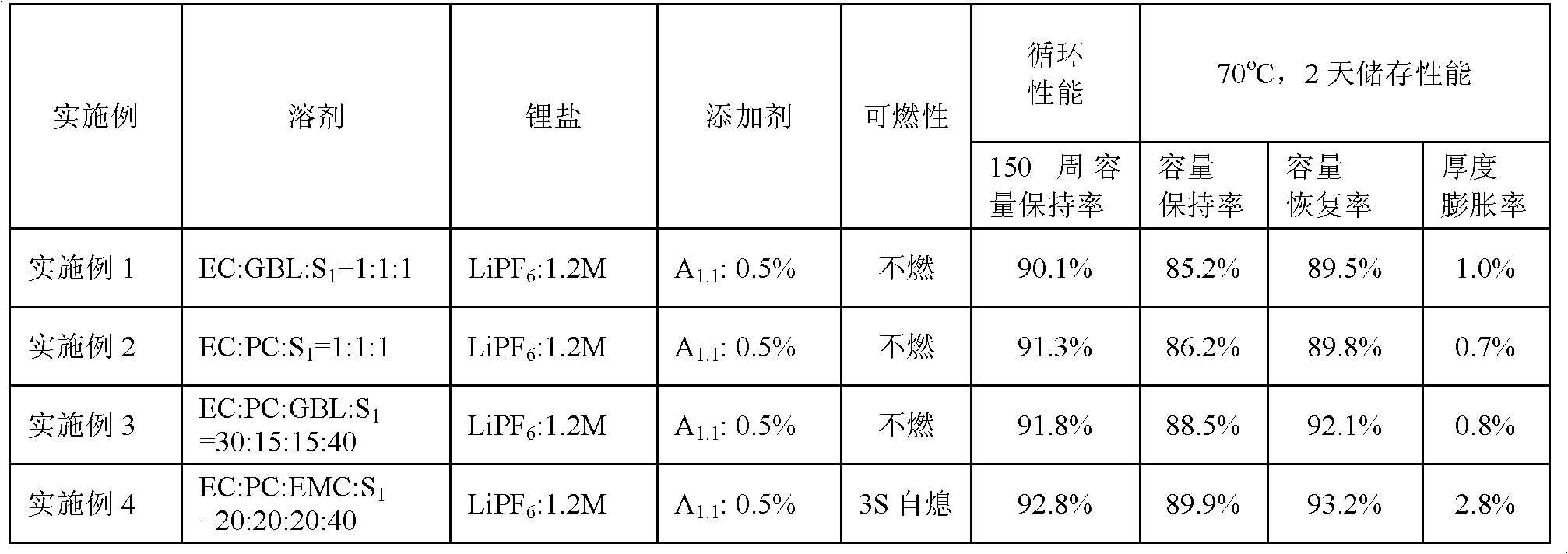
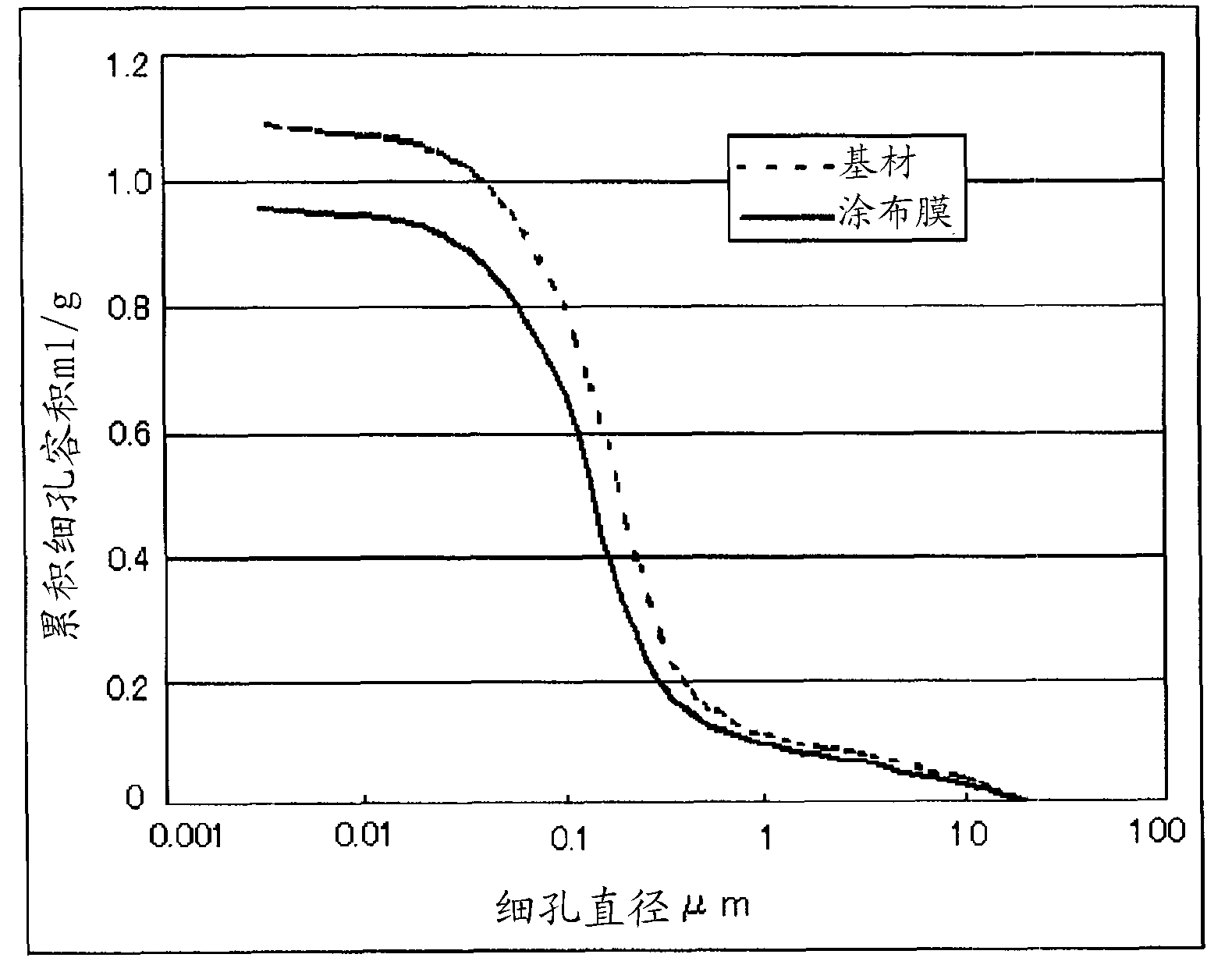
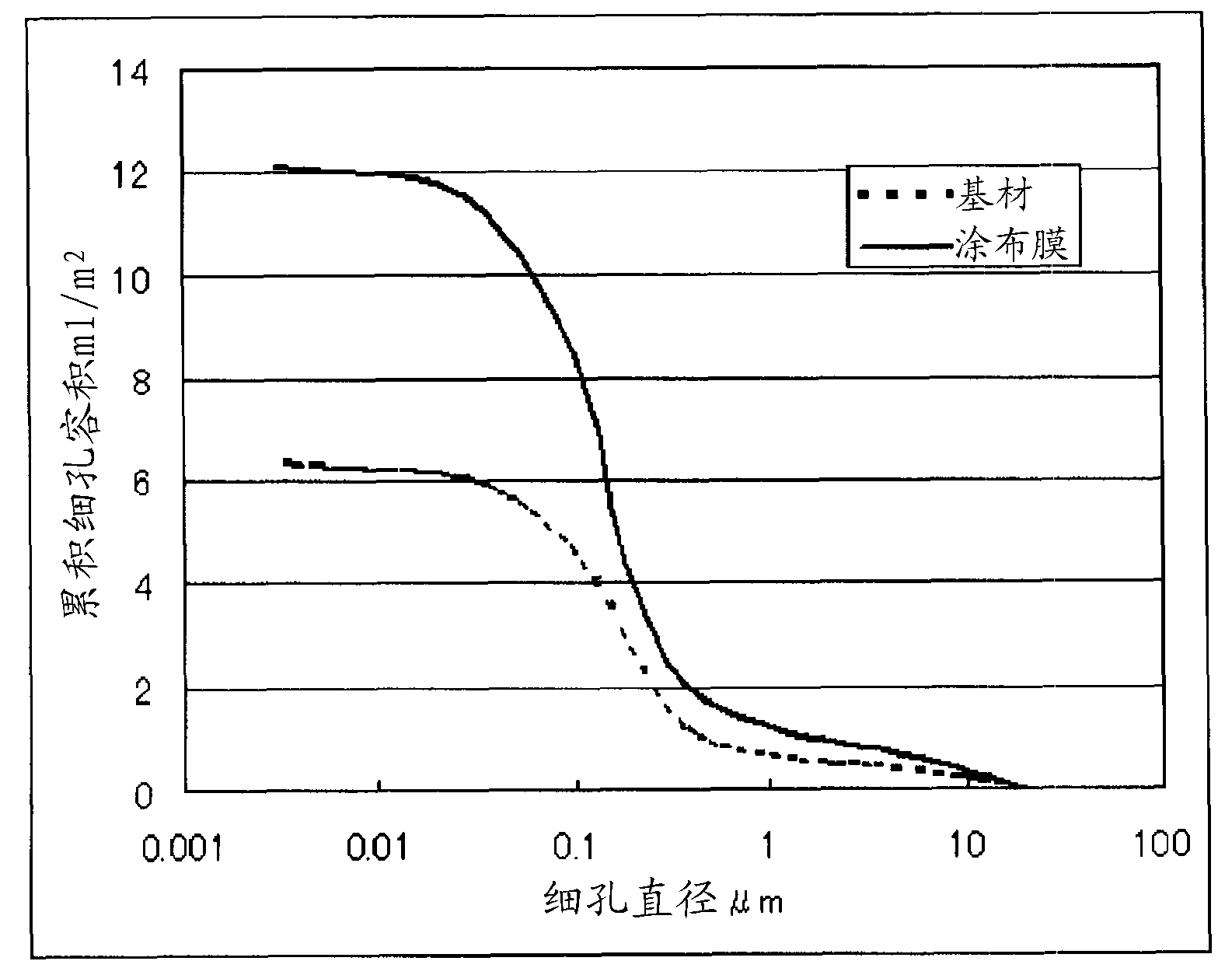
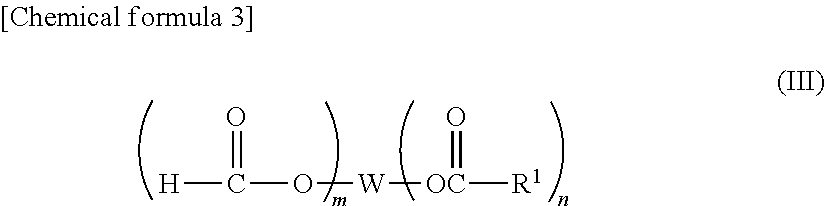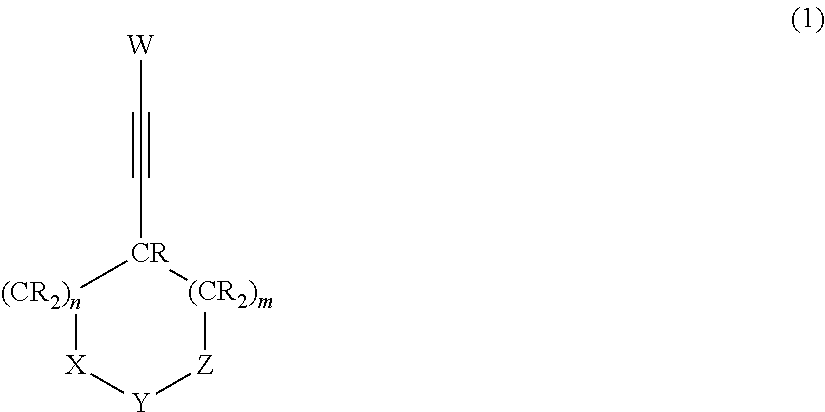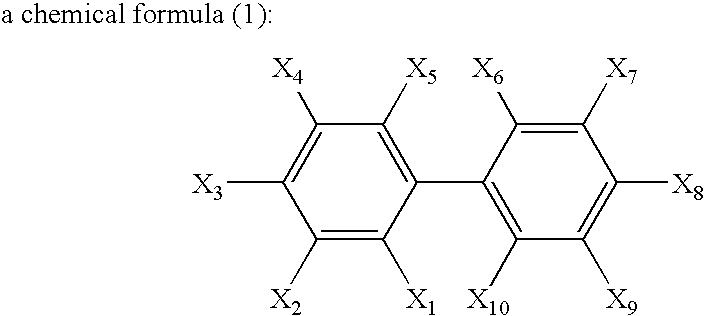Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
289results about How to "Excellent battery characteristics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
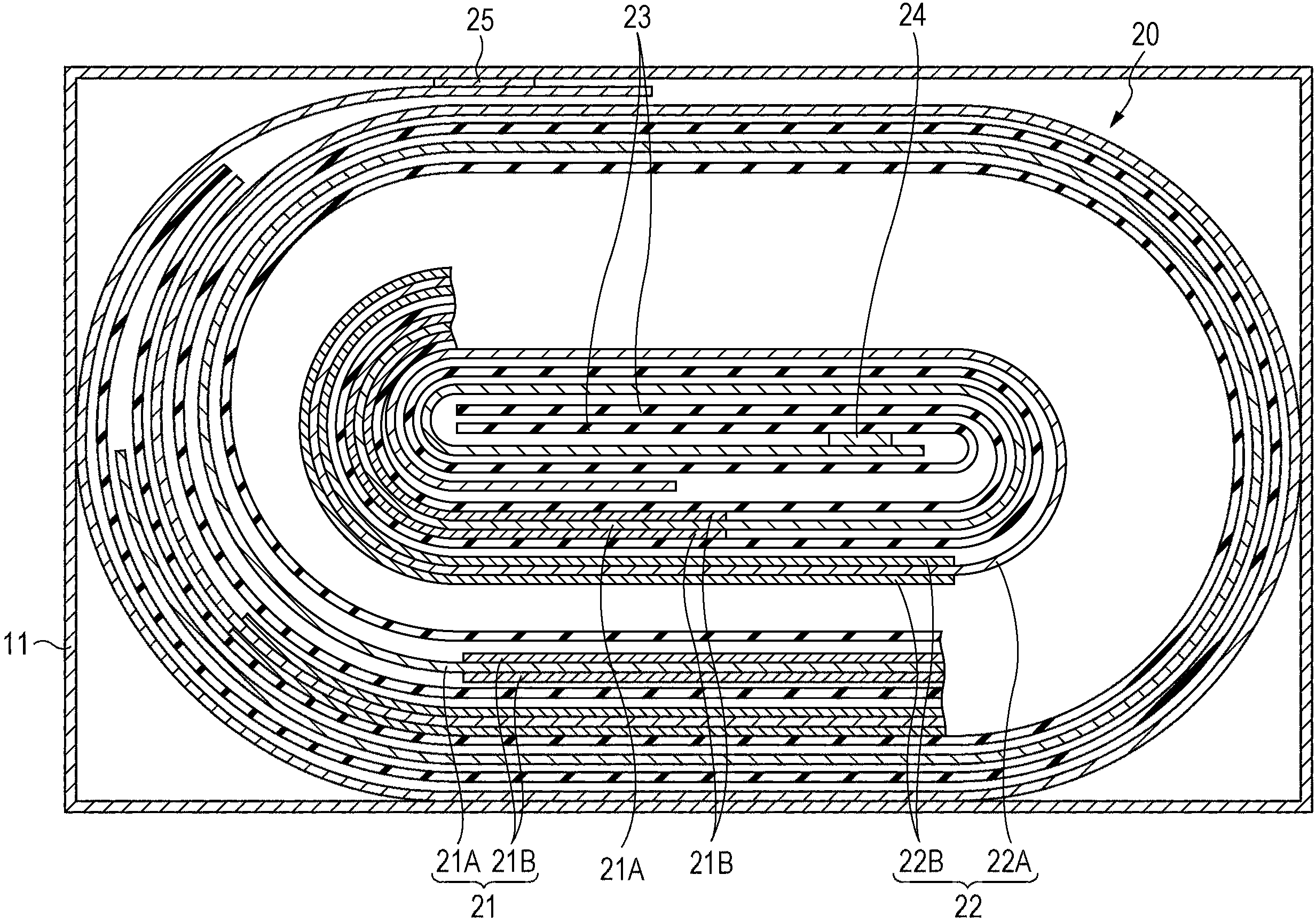
Electrolyte solution and battery
ActiveUS20050196670A1Improve ionic conductivityHigh suppression characteristicsJoints with sealing surfacesOrganic electrolyte cellsDecompositionAlloy
An electrolyte solution and a battery which are capable of improving cycle characteristics are provided. An anode includes a simple substance, an alloy or a compound of a metal element or a metalloid element capable of forming an alloy with lithium as an anode active material. A separator is impregnated with an electrolyte solution formed through dissolving an electrolyte salt in a solvent. The electrolyte salt includes a first electrolyte salt including LiB(C2O4)2 and a second electrolyte salt including at least one kind selected from the group consisting of LiPF6, LiBF4, LiN(CF3SO2)2, LiN(C2F5SO2)2, LiClO4, LiAsF6 and LiC(CF3SO2)3. In the solvent, 4-fluoroethylene carbonate is included. A coating is formed on the anode by the first electrolyte salt, and high ionic conductivity can be obtained by the second electrolyte salt. Further an oxidation-decomposition reaction of the electrolyte solution which occurs in a cathode can be prevented by 4-fluoroethylene carbonate.
Owner:MURATA MFG CO LTD
Glass film for lithium ion battery
InactiveUS20120040211A1Excellent in insulating property and heat resistance and surface smoothnessGood in battery characteristicPrinted batteriesGlass forming apparatusLithium electrodeGlass film
A glass film for a lithium ion battery has a thickness of 300 μm or less and a surface roughness (Ra) of 100 Å or less.
Owner:NIPPON ELECTRIC GLASS CO LTD
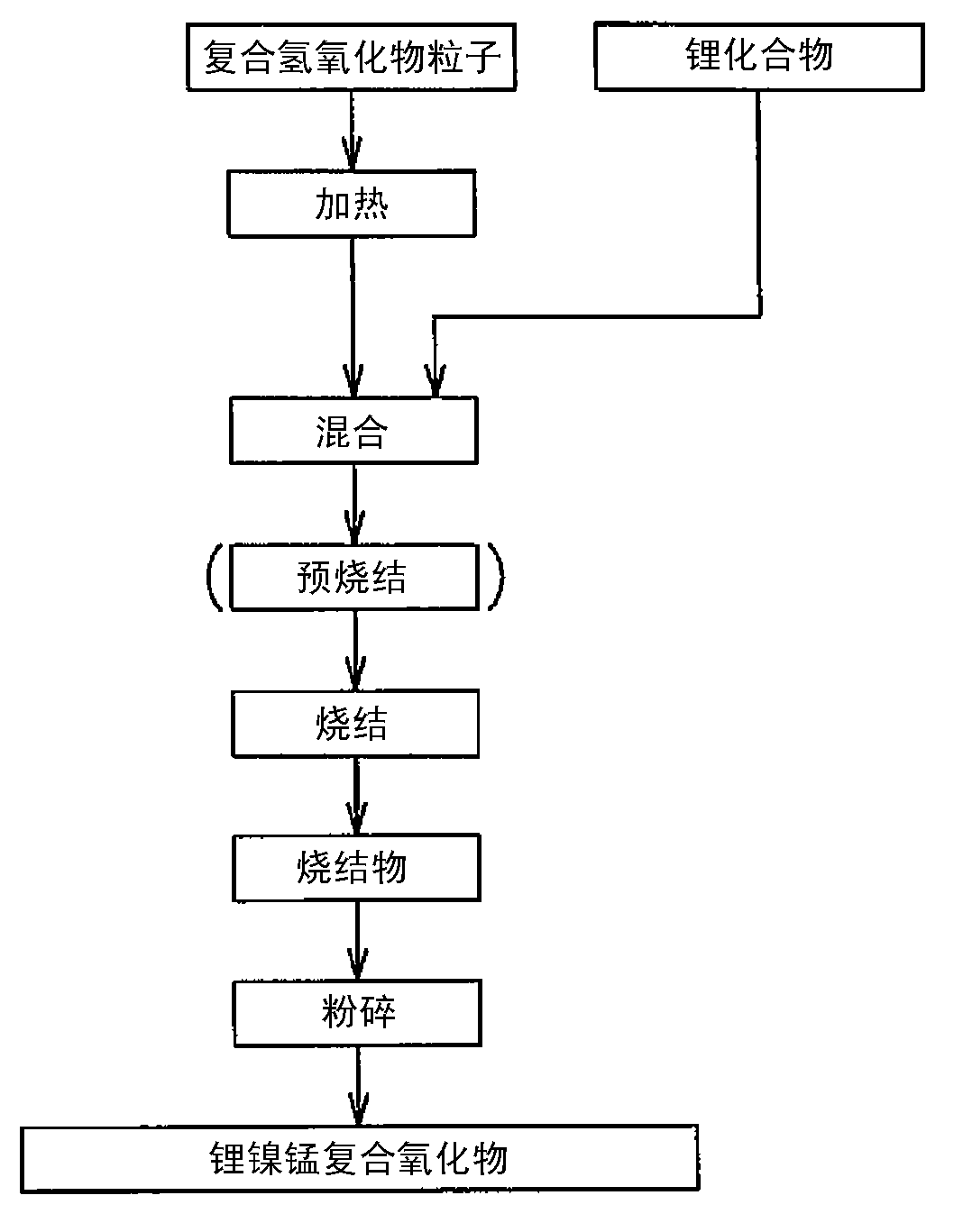
Nickel-manganese composite hydroxide particles, method for producing same, positive electrode active material for nonaqueous electrolyte secondary batteries, method for producing said positive electrode active material, and nonaqueous electrolyte secondary battery
ActiveCN102884659ASmall particle sizeImprove particle size uniformityFinal product manufactureCylindrical casing cells/batteryComplex ionsComposite oxide
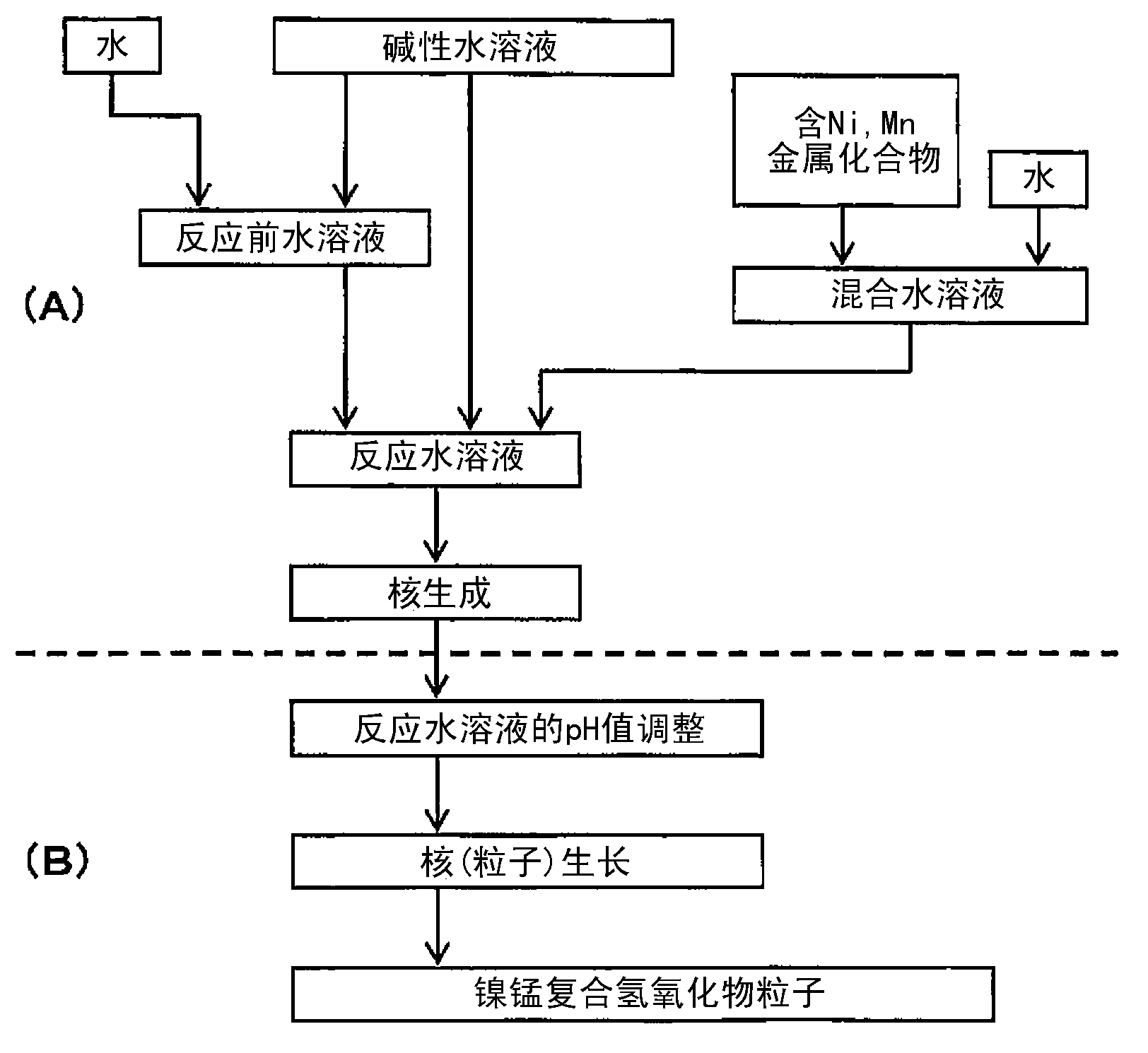
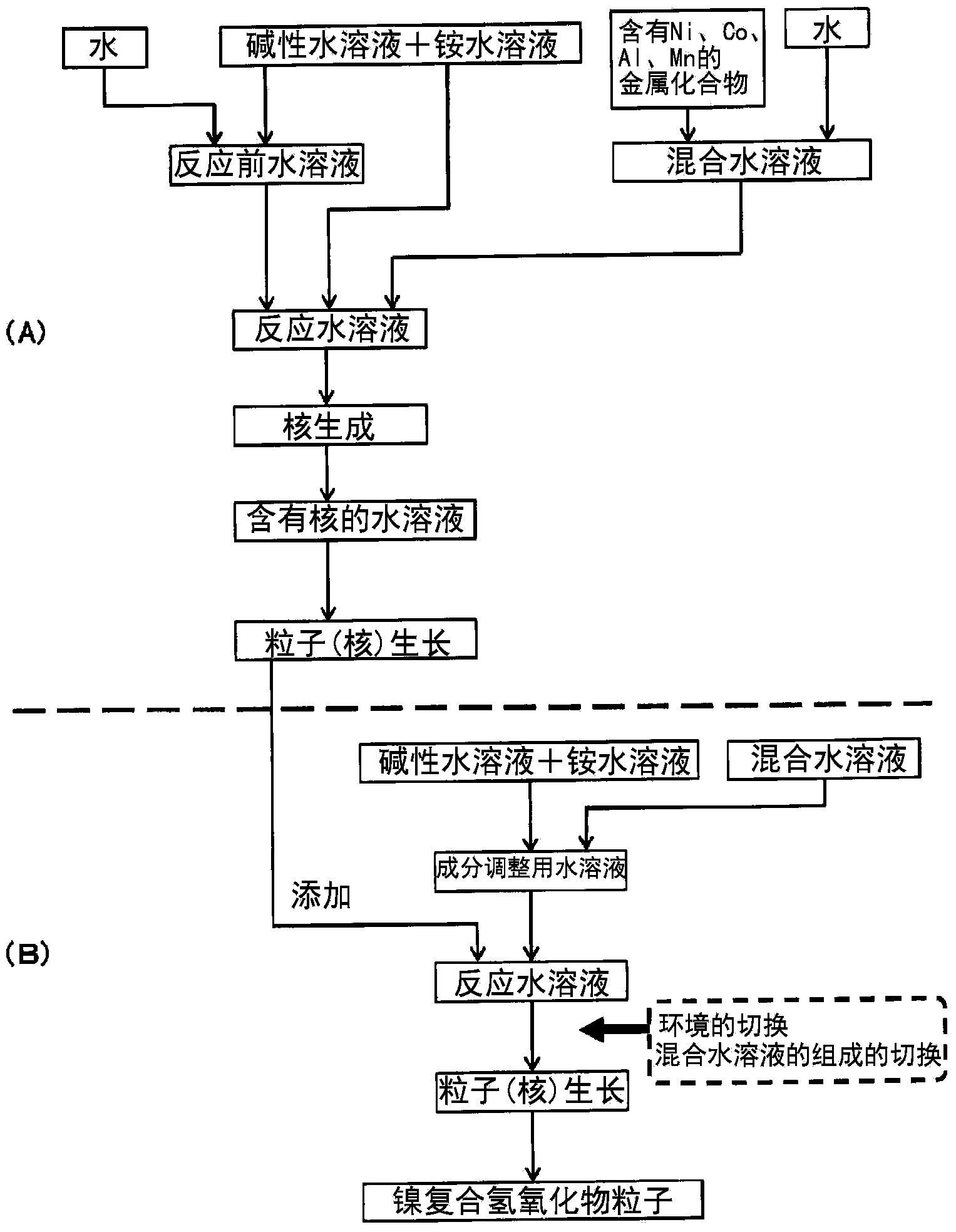
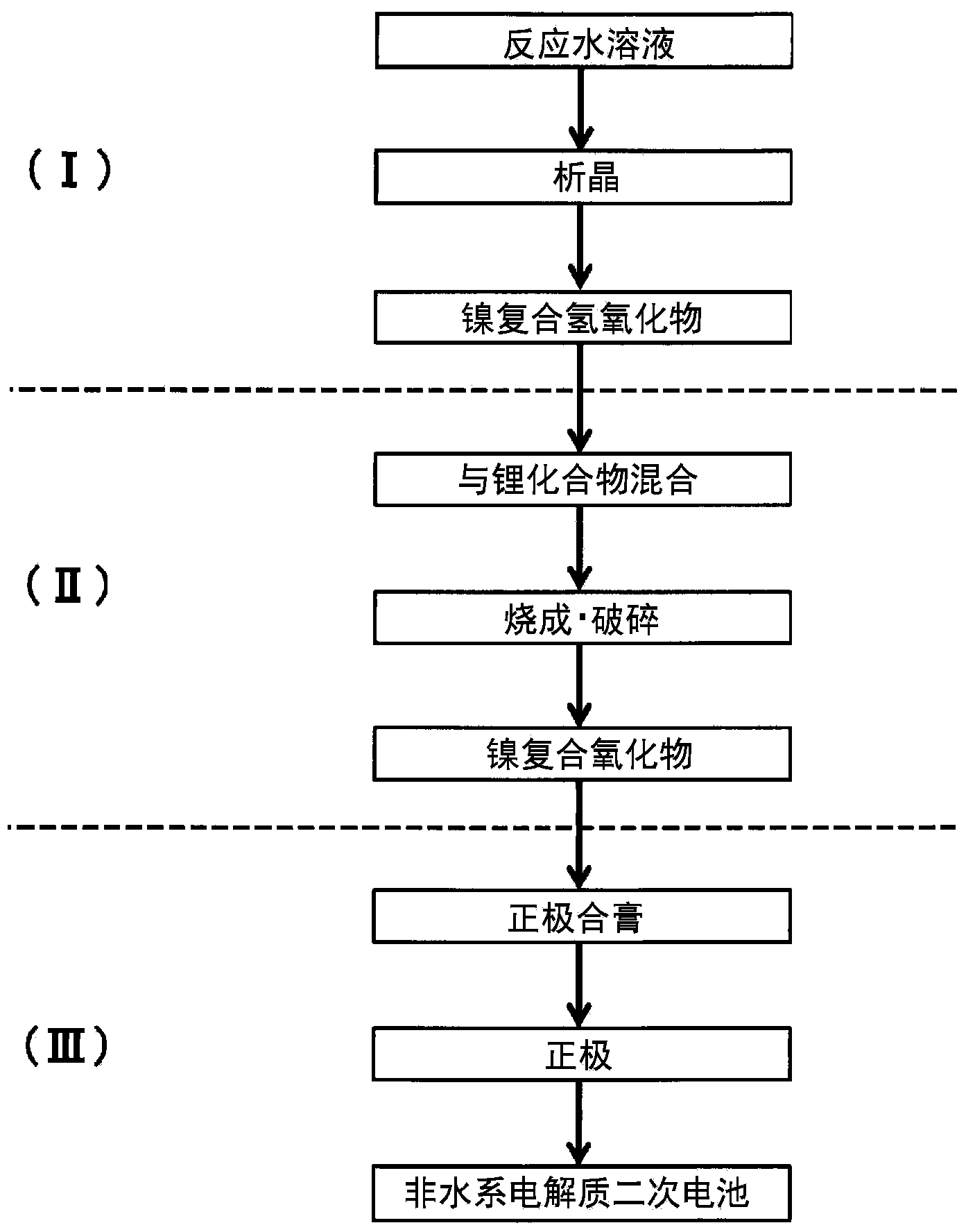
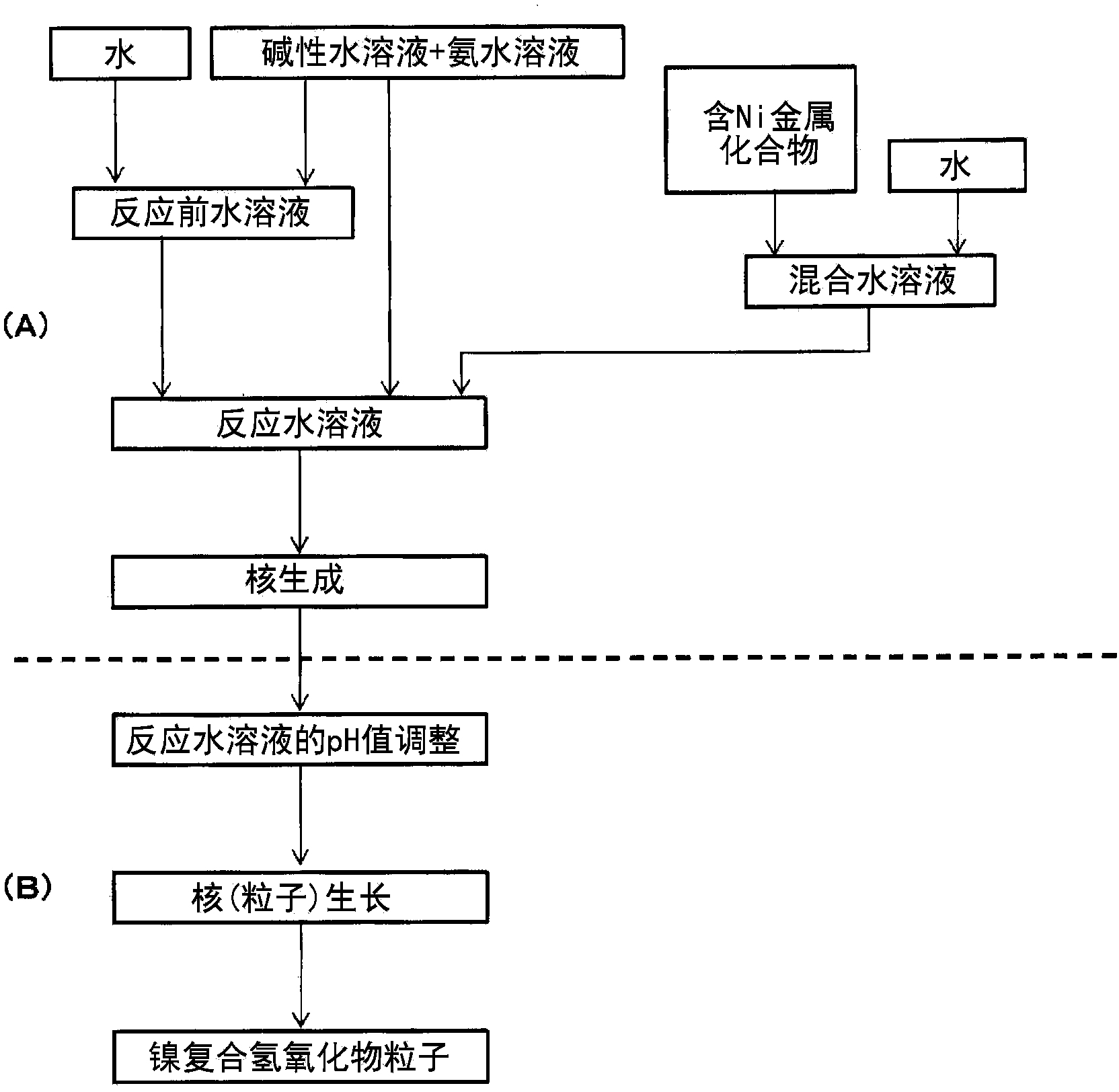
Provided are nickel manganese composite hydroxide particles that are a precursor for forming cathode active material comprising lithium nickel manganese composite oxide having hollow structure of particles having a small and uniform particle size for obtaining a non-aqueous electrolyte secondary battery having high capacity, high output and good cyclability. When obtaining the nickel manganese composite hydroxide particles from a crystallization reaction, an aqueous solution for nucleation, which includes at least a metallic compound that contains nickel and a metallic compound that contains manganese, and does not include a complex ion formation agent that forms complex ions with nickel, manganese and cobalt, is controlled so that the temperature of the solution is 60 DEG C or greater, and so that the pH value that is measured at a standard solution temperature of 25 DEG C is 11.5 to 13.5, and after nucleation is performed, an aqueous solution for particle growth, which includes the nuclei that were formed in the nucleation step and does not substantially include a complex ion formation agent that forms complex ions with nickel, manganese and cobalt, is controlled so that the temperature of the solution is 60 DEG C or greater, and so that the pH value that is measured at a standard solution temperature of 25 DEG C is 9.5 to 11.5, and is less than the pH value in the nucleation step.
Owner:SUMITOMO METAL MINING CO LTD
Electroactive material and use thereof
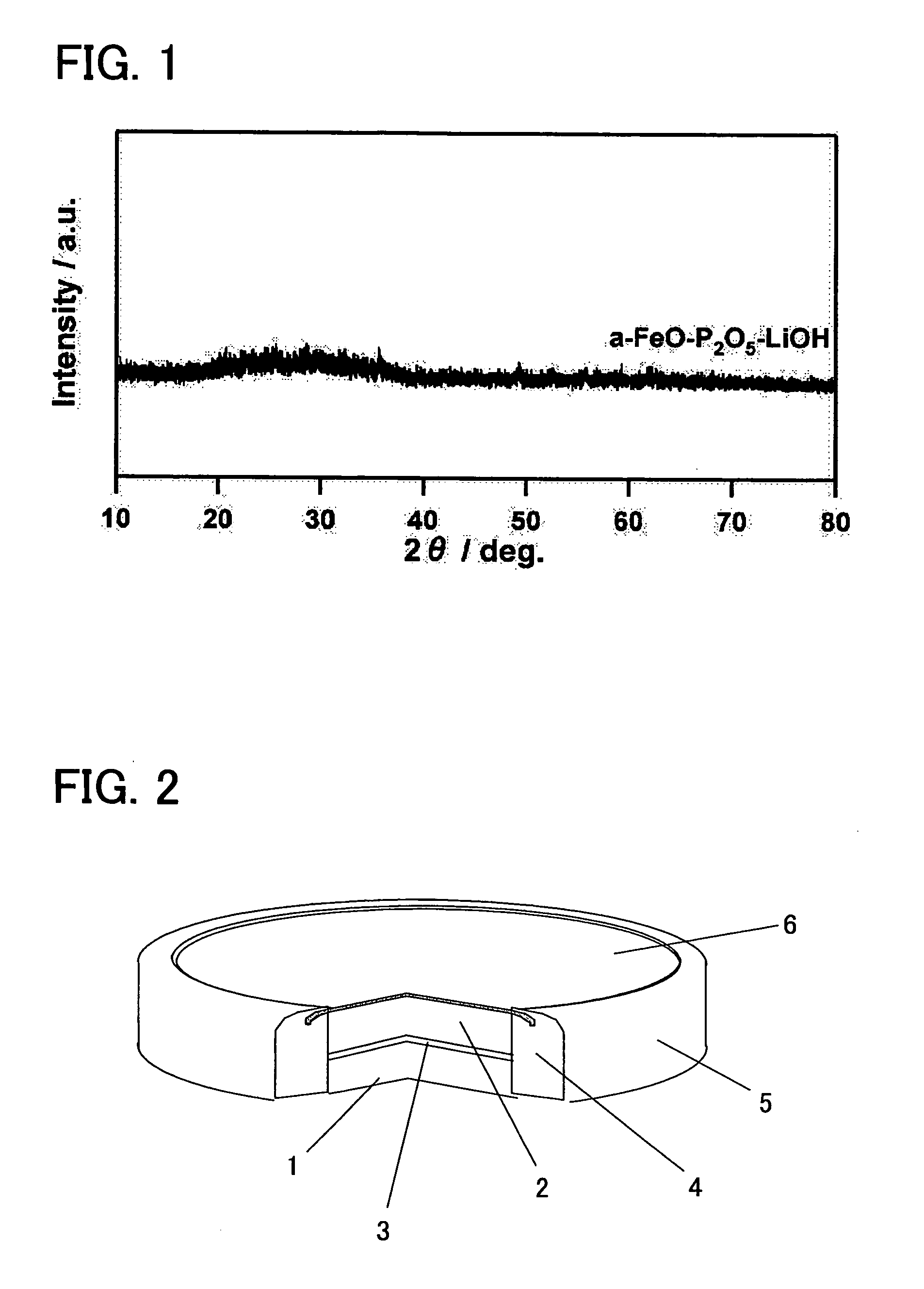
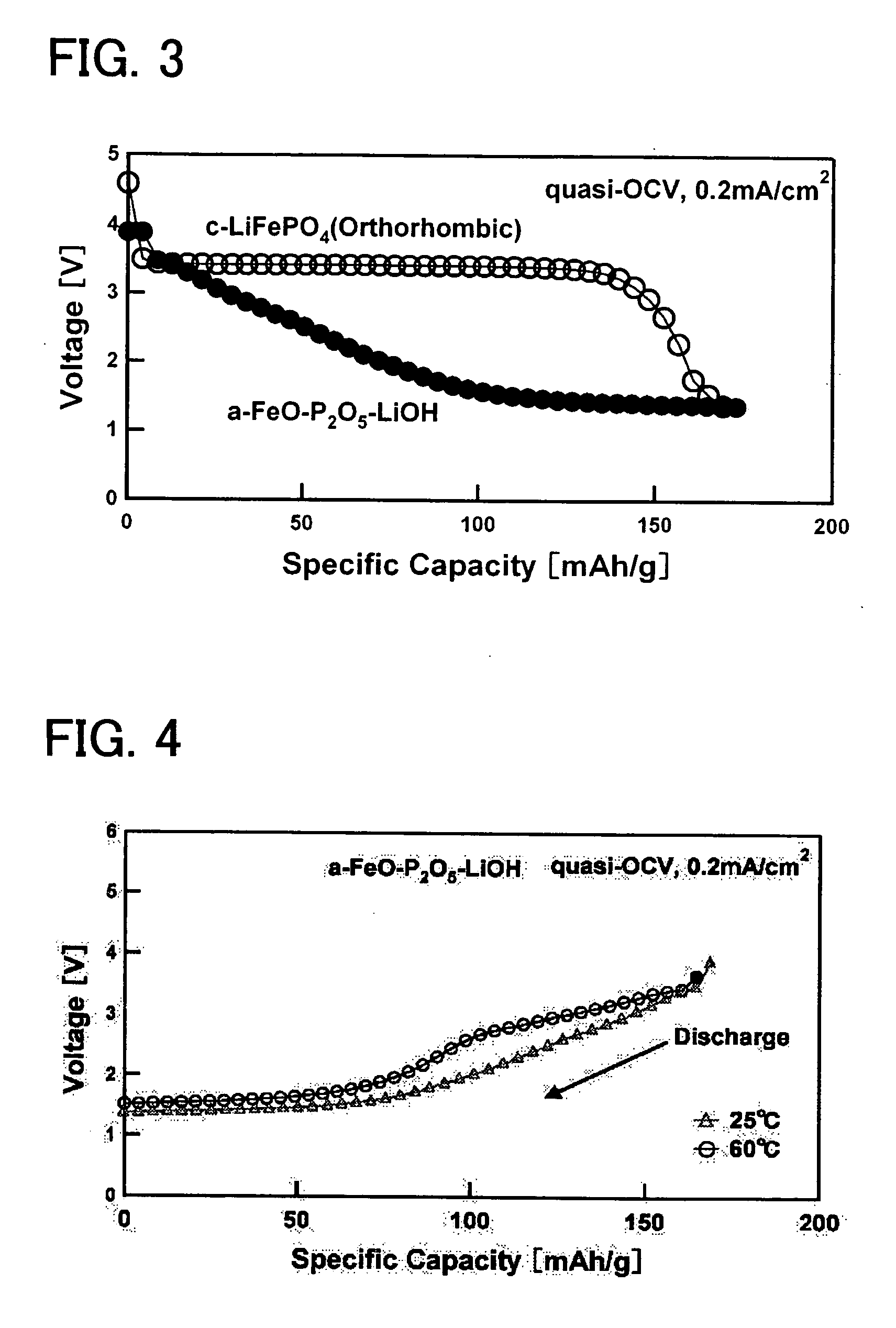
InactiveUS20060194113A1Excellent battery characteristicsExcellent charge and discharge characteristicsElectrode manufacturing processesPhosphatesPhosphateAmorphous metal
An electroactive material and a method of manufacturing the same is provided, in which the primary component of the electroactive material is a metal phosphate complex, and the electroactive material exhibits excellent charge / discharge characteristics. The electroactive material of the present invention is primarily composed of an amorphous metal complex represented by the general formula AxM(PO4)y. Here, A is an alkali metal, and M is one or two or more elements selected from the transition metals. In addition, 0≦x≦2, 0<y ≦2. The electroactive material described above can be manufactured more inexpensively and in a shorter amount of time than a conventional electroactive material which employs a crystalline metal complex, and can exhibit the same battery characteristics as the aforementioned conventional electroactive material.
Owner:TOYOTA JIDOSHA KK
Electrode for a lithium battery and lithium battery
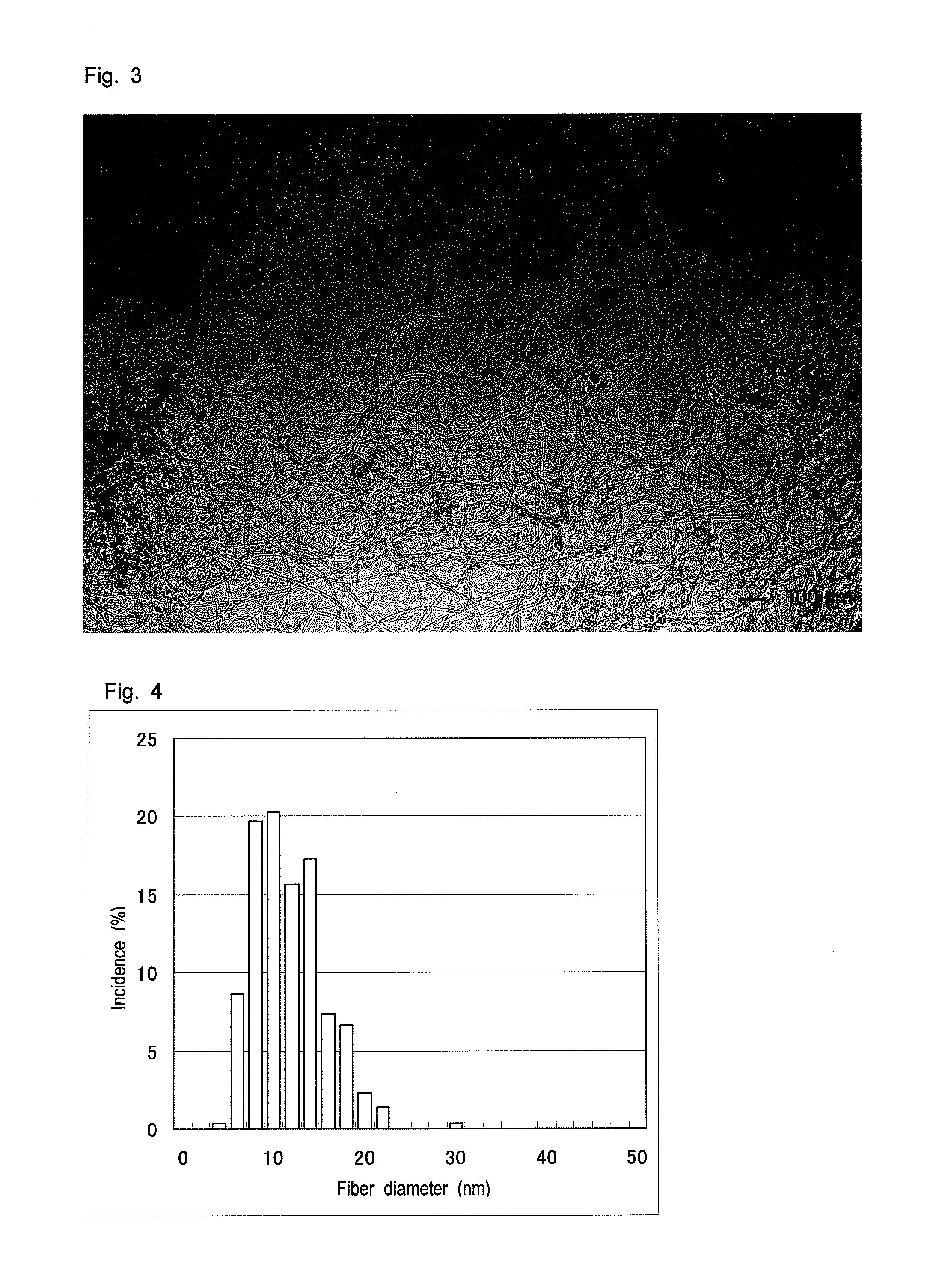
ActiveUS20120214070A1Excellent battery characteristicsInhibition decreasedMaterial nanotechnologyLi-accumulatorsCarbon fibersFiber diameter
An electrode for a lithium battery, which electrode includes an electrode active material which can charge and discharge lithium ions (A), a carbonaceous conductive additive (B) and a binder (C). The carbonaceous conductive additive contains carbon fiber, the carbon fiber including a mixture of two kinds of carbon fibers having different diameter distributions on a number basis; and the fiber diameter distribution of the carbon fiber in the electrode has one or more maximum values at 5-40 nm and at 50-300 nm, respectively. Also disclosed is a lithium battery using the electrode. The electrode enables production of a lithium battery having a reduced discharge capacity decline.
Owner:RESONAC CORP
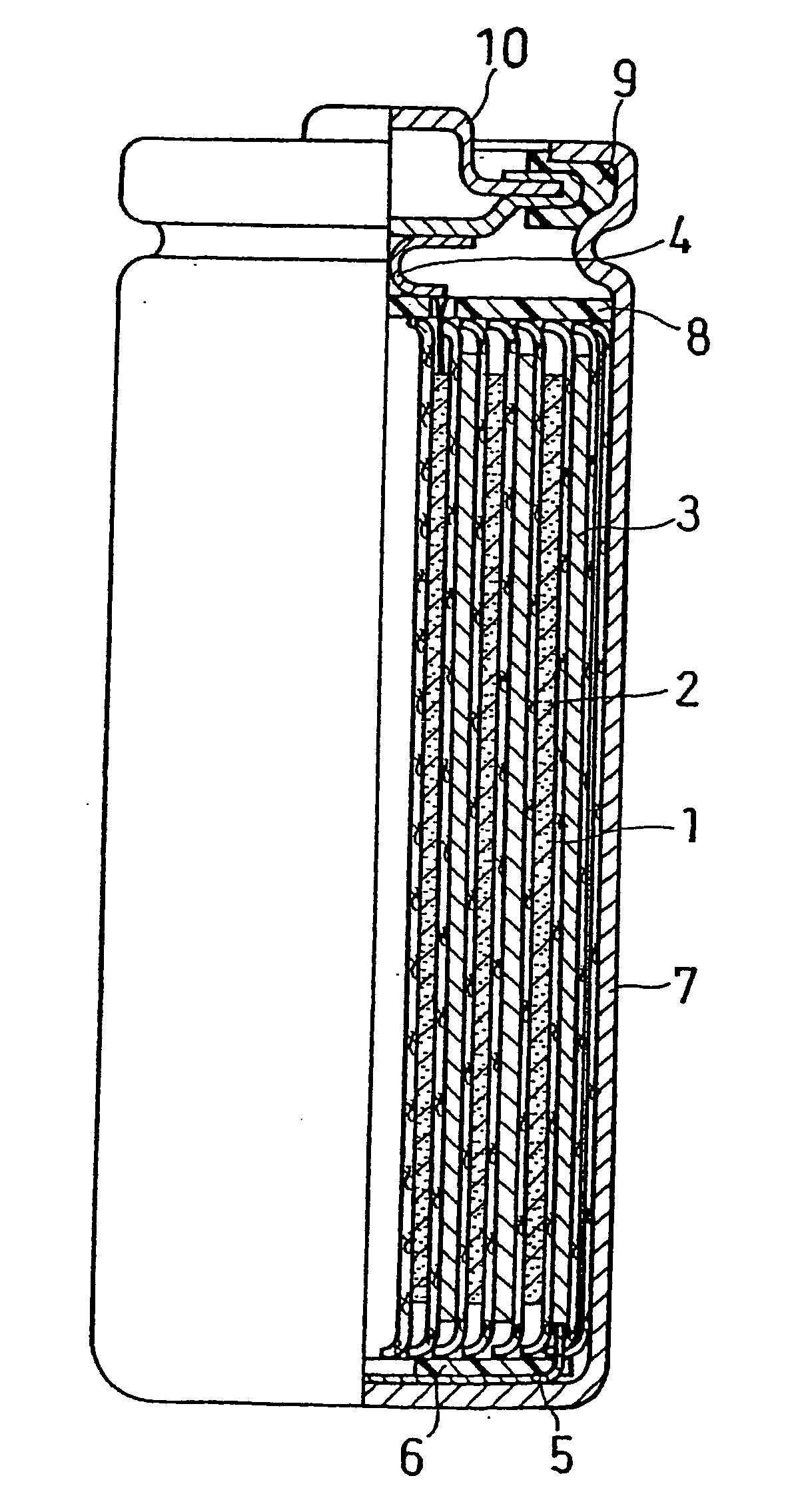
Nonaqueous-electrolyte battery
InactiveCN101373848AInhibitory responseImprove securityActive material electrodesSecondary cellsSimple Organic CompoundsLithium
The invention provides a nonaqueous-electrolyte battery which produces few gas and has good battery characteristic. The nonaqueous-electrolyte battery has a positive electrode 3 including a positive active material, a negative electrode 4 including a negative active material having a lithium insertion / release potential higher than 1.0 V (vs. Li / Li+), and a nonaqueous electrolyte, wherein an organic compound having one or more isocyanato groups has been added to the nonaqueous electrolyte.
Owner:KK TOSHIBA
Flame retardant type non-aqueous electrolyte solution and battery thereof
InactiveCN102306833AImprove securityImprove electrochemical performanceSecondary cellsPhysical chemistrySolvent
The invention relates to an organic composition, in particular to a flame retardant type non-aqueous electrolyte solution and a lithium ion battery prepared by the electrolyte solution. The flame retardant type non-aqueous electrolyte solution comprises electrolyte salt, a non-aqueous solvent, 10 to 50 mass percent of flame retardant and 0.001 to 2 mass percent of surfactant, wherein the non-aqueous solvent comprises cyclic carbonic ester and / or cyclic carboxylic ester; the flame retardant comprises an isoflurane organic matter; and the surfactant comprises fluorocarbon surfactant. The electrolyte solution can greatly improve the safety performance of the lithium ion battery; simultaneously, the electrochemistry performance such as cycle performance and high-temperature preservation performance of the lithium ion battery can be obviously improved.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Separator for nonaqueous secondary battery
ActiveCN102160211AWith off featureImprove heat resistanceSecondary cellsCell component detailsElectrical resistance and conductancePolyolefin
The invention aims to provide a separator for nonaqueous secondary batteries, which has both excellent heat resistance and ion permeability in addition to shutdown characteristics. The first embodiment of the separator is a separator for nonaqueous secondary batteries which is composed of a porous composite membrane wherein a heat-resistant porous layer containing a heat-resistant resin is formed on at least one side of a base which is composed of a polyolefin microporous membrane, said separator being characterized in that the membrane resistance (A) of the base, the Gurley value (B) of the base, the membrane resistance (C) of the porous composite membrane and the Gurley value (D) of the porous composite membrane satisfy a specific relation. The second embodiment of the separator is characterized in that the heat-resistant porous layer has an average pore diameter as determined by a mercury intrusion method of 0.1-0.2 [mu]m. The third embodiment of the separator is characterized in that heat-resistant resin fibrils have an average fibril diameter of 10-80 nm and the pores in the heat-resistant porous layer have an average pore diameter of 50-250 nm.
Owner:TEIJIN LTD
Polyolefin microporous membrane
ActiveCN101331178AExcellent battery characteristicsImprove battery safetyMembranesSemi-permeable membranesPorosityPolyolefin
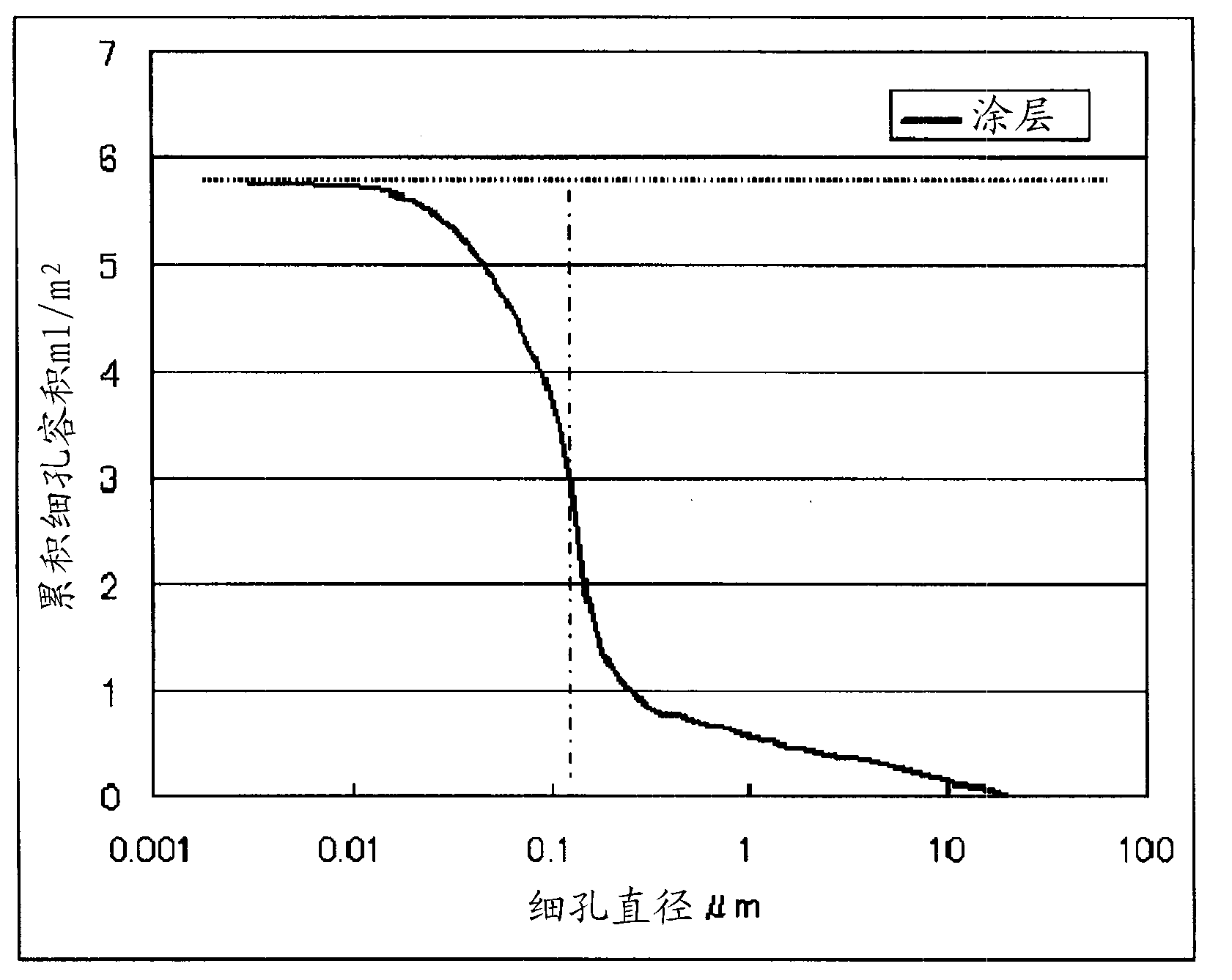
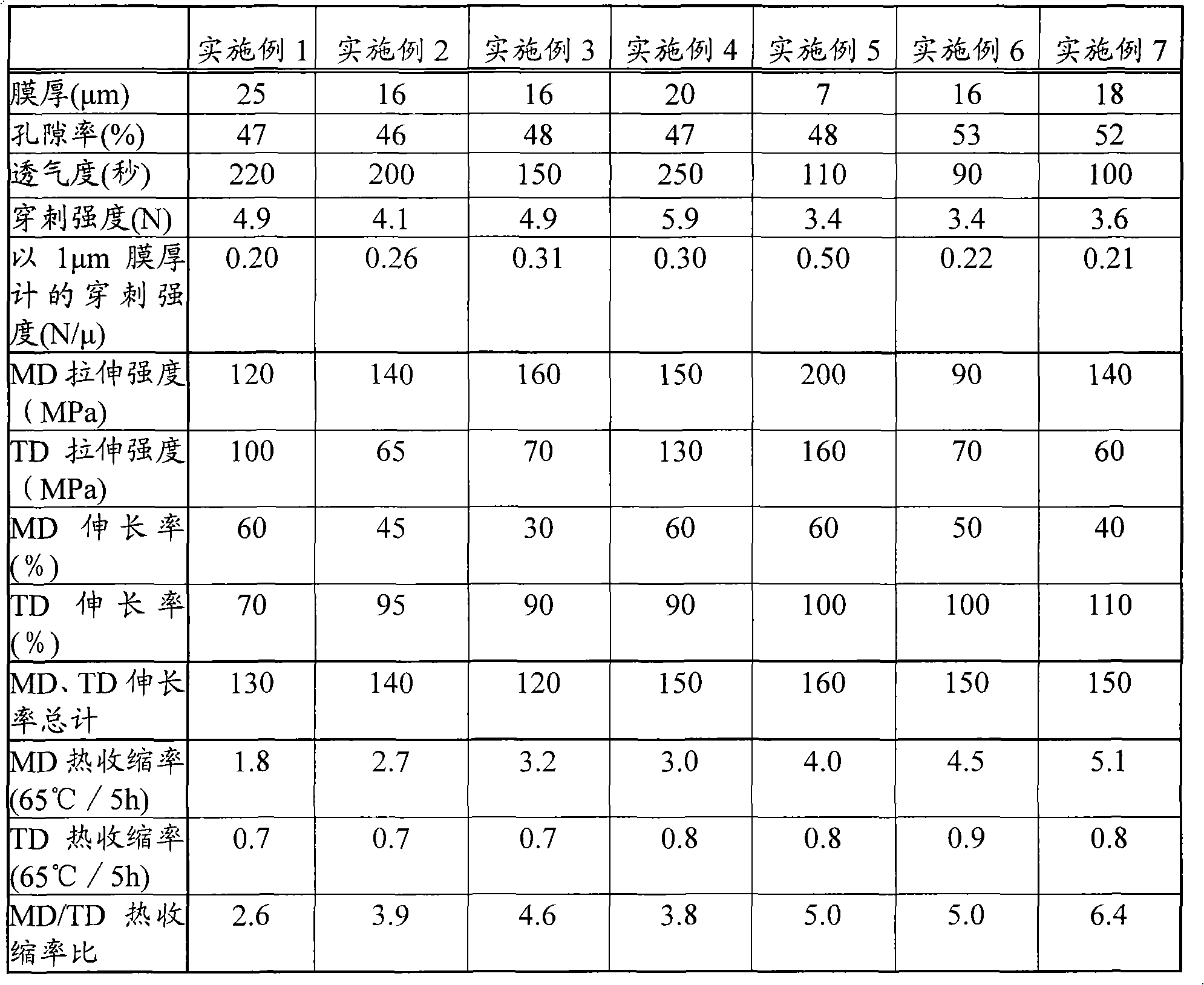
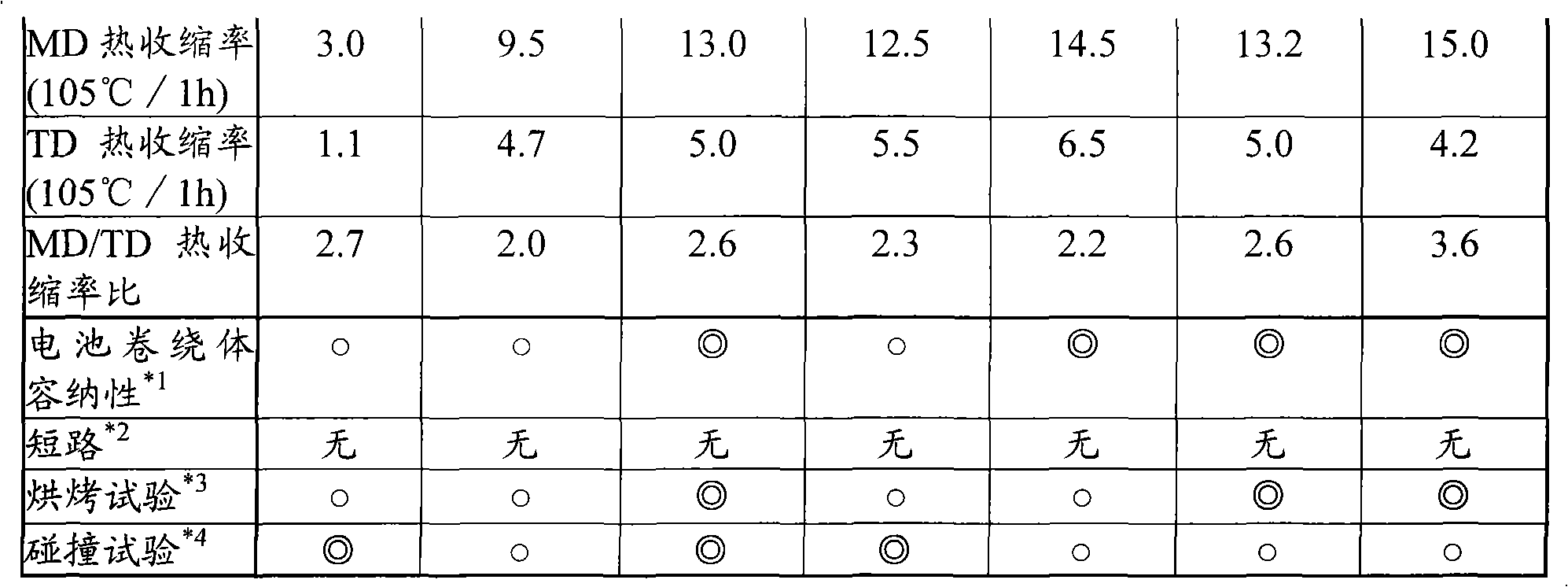
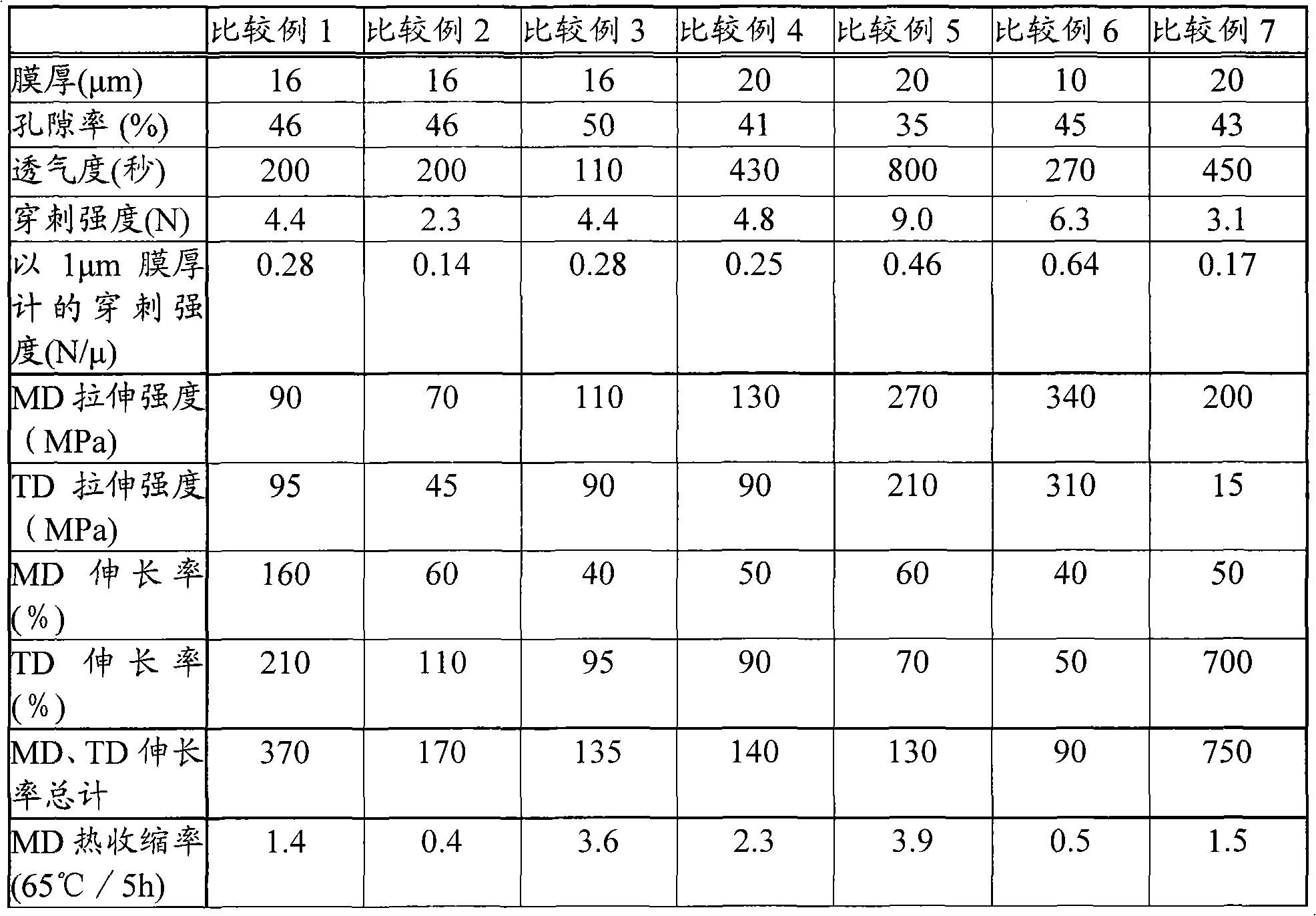
Disclosed is a polyolefin microporous membrane having a thickness of not less than 1[mu]m and not more than 50 [mu]m, a porosity of not less than 30 percent and not more than 70 percent, a puncture strength in terms of a 1[mu]m thick film of not less than 0.15 N / [mu]m, a tensile strength in the machine direction (MD tensile strength) of not less than 30 MPa, a tensile strength in the transverse direction (TD tensile strength) of not less than 30MPa, a thermal shrinkage at 65 DEG C in the transverse direction (TD thermal shrinkage) of not more than 1 percent, and a ratio between the thermal shrinkage at 65 DEG C in the machine direction and the thermal shrinkage at 65 DEG C in the transverse direction (MD thermal shrinkage / TD thermal shrinkage) of more than 2. Also disclosed are a method for producing such a polyolefin microporous membrane and a nonaqueous electrolyte secondary battery using such a polyolefin microporous membrane.
Owner:ASAHI KASEI KK
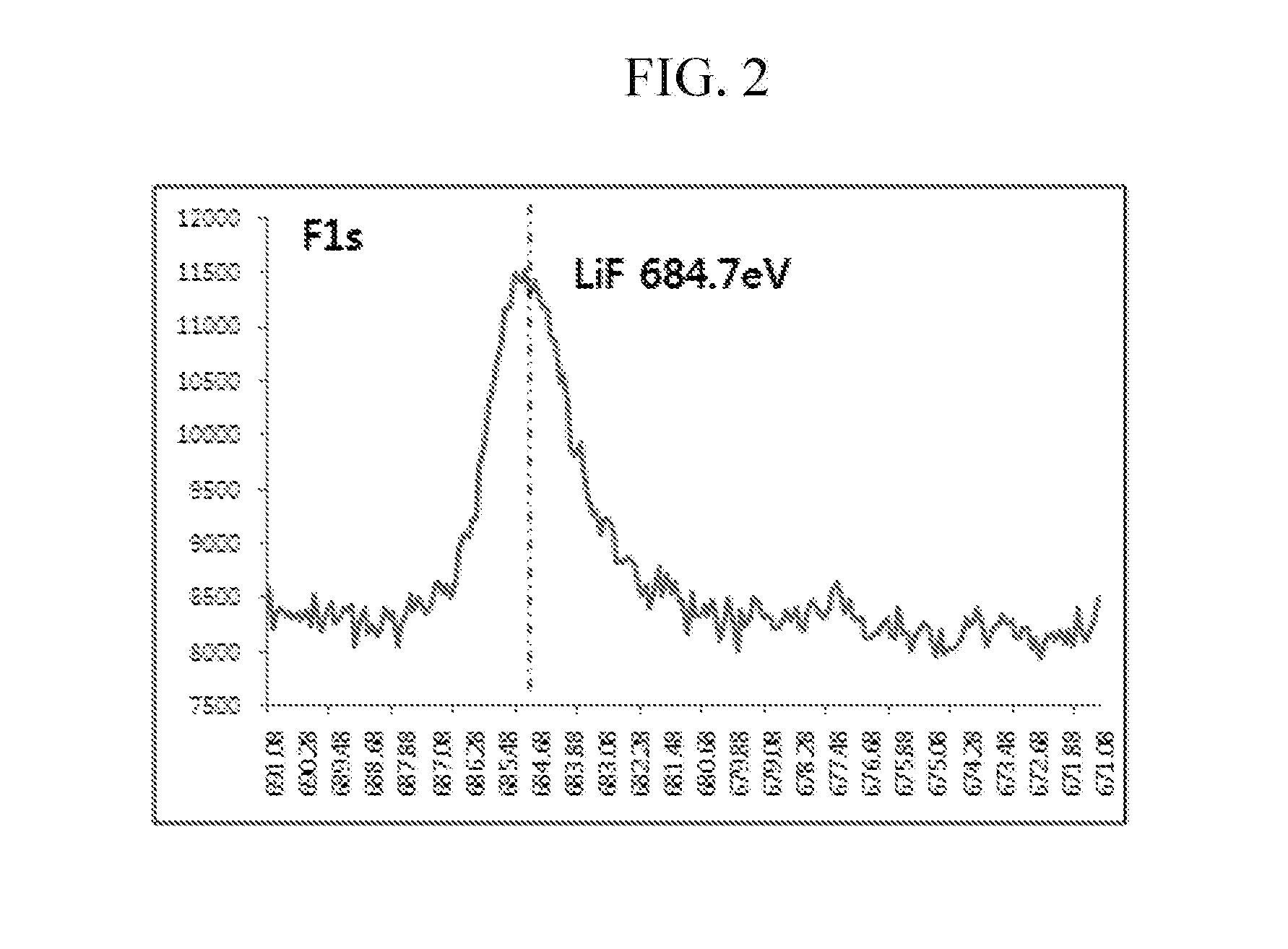
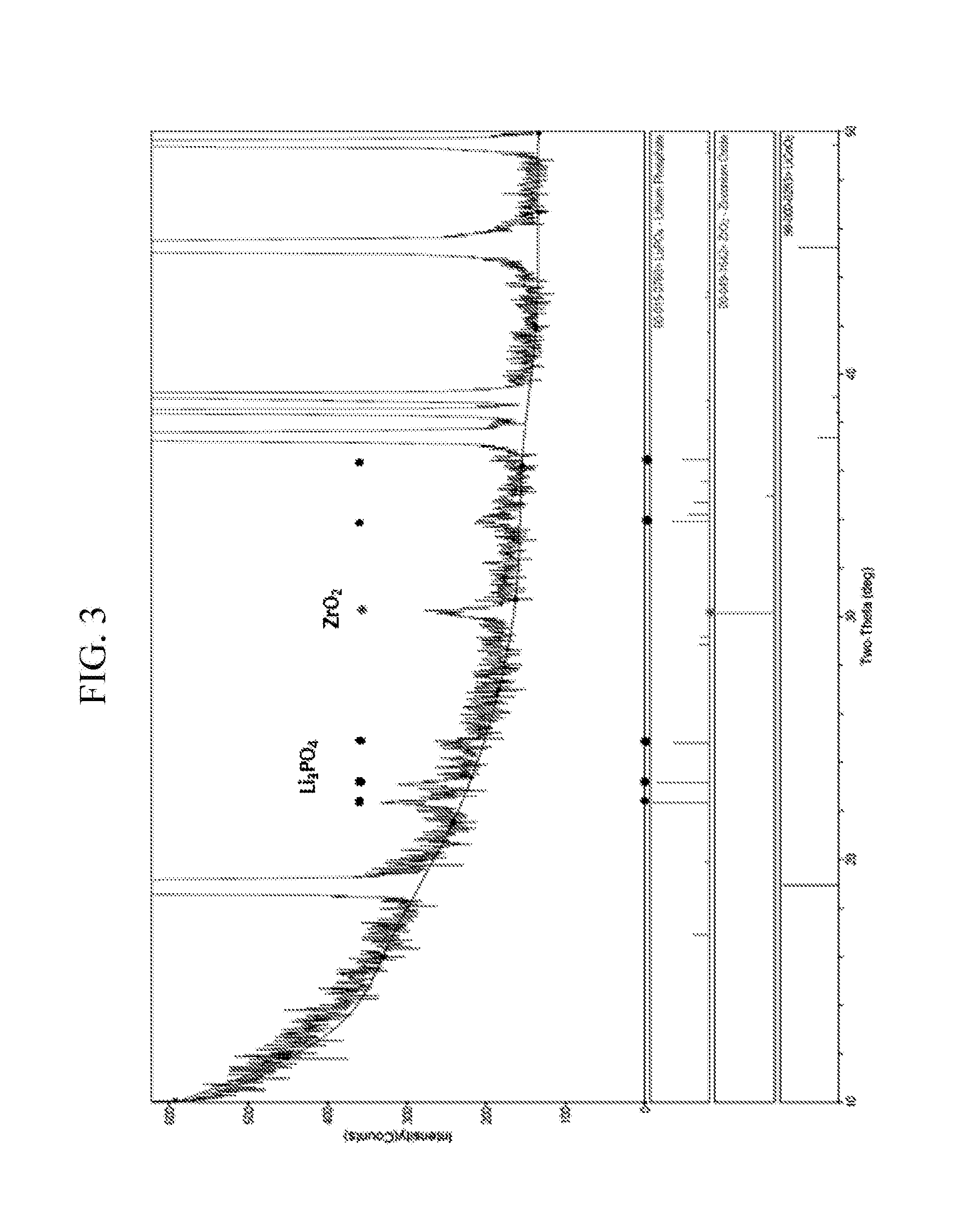
Cathode active material for lithium secondary battery, method of preparing the same, and lithium secondary battery containing the same
ActiveUS20160276659A1Excellent battery characteristicsElectrode thermal treatmentPositive electrodesOxideMetal
There are provided a cathode active material for a lithium secondary battery, a method of preparing the same, and a lithium secondary battery containing the same. The cathode active material for a lithium secondary battery includes: a core made of a compound reversibly intercalating and deintercalating lithium; and a coating layer positioned on at least a portion of a surface of the compound, wherein the coating layer is a composite coating layer containing Li3PO4 and LiF, and further containing a lithium metal compound, a metal oxide, a metal fluoride compound, and / or a combination thereof, and the core is doped with fluorine.
Owner:L & F
Nickel compound hydroxide and method for producing same, positive pole active substance for nonaqueous electrolyte secondary cell and method for producing same, and nonaqueous electrolyte secondary cell
ActiveCN104136376AIncrease capacityExcellent cycle characteristicsElectrode thermal treatmentFinal product manufactureElectrical batteryManganese
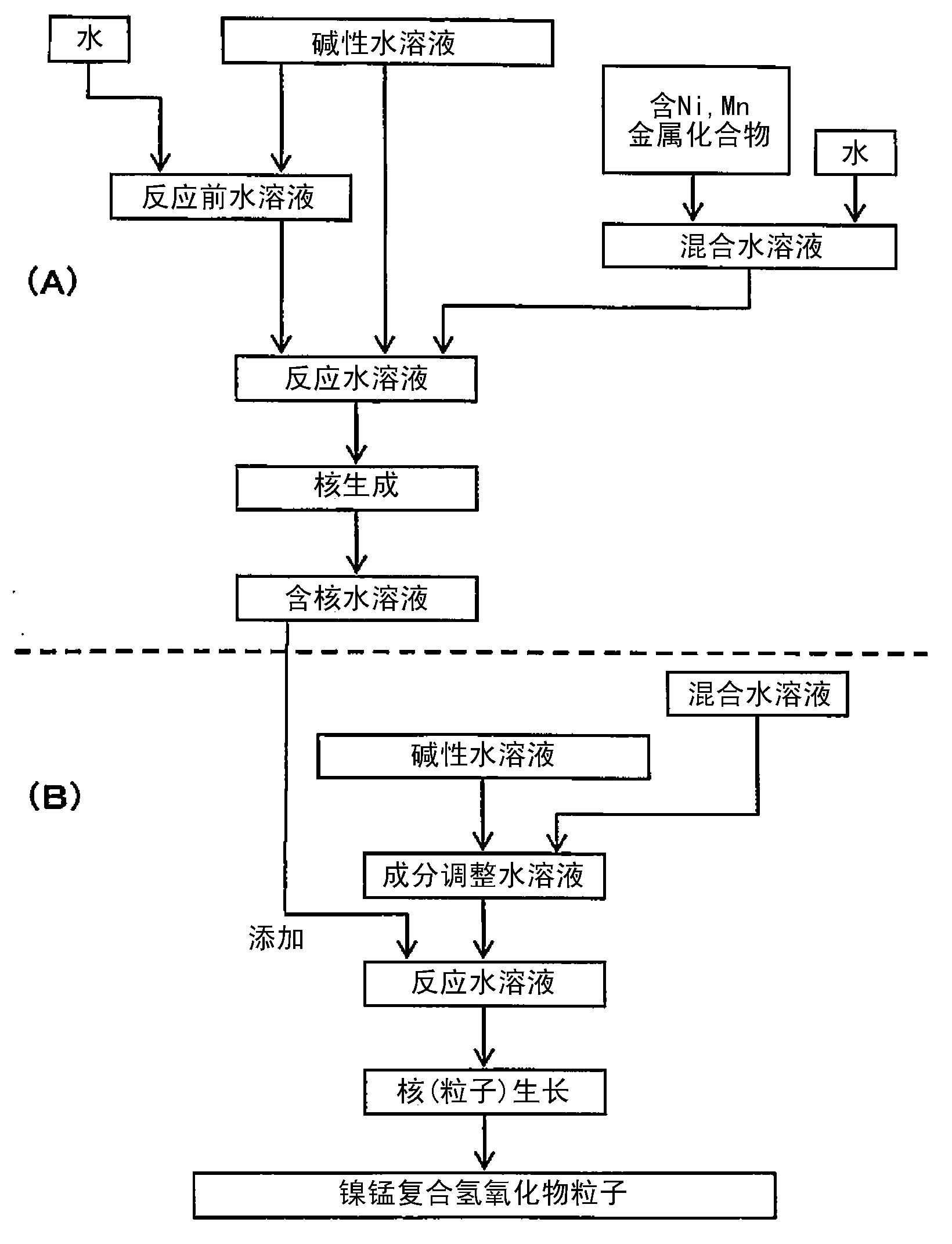
Provided is a lithium composite oxide having a uniform and suitable particle size and high specific surface area due to a hollow structure that can be produced on an industrial scale. A nickel composite hydroxide as a raw material thereof is obtained controlling the particle size distribution of the nickel composite hydroxide, the nickel composite hydroxide having a structure comprising a center section that comprises minute primary particles, and an outer-shell section that exists on the outside of the center section and comprises plate shaped primary particles that are larger than the primary particles of the center section, by a nucleation process and a particle growth process that are separated by controlling the pH during crystallization, and by controlling the reaction atmosphere in each process and the manganese content in a metal compound that is supplied in each process.
Owner:SUMITOMO METAL MINING CO LTD
Battery including an electrolyte solution comprising a halogenated carbonate derivative
ActiveUS7754389B2Excellent battery characteristicsImprove ionic conductivityJoints with sealing surfacesOrganic electrolyte cellsLithiumDecomposition
Owner:MURATA MFG CO LTD
Lithium ion secondary battery and a solid electrolyte thereof
InactiveCN101040401AIncrease capacityHigh outputSolid electrolyte cellsActive material electrodesLithiumPolyethylene oxide
A solid electrolyte comprising powder of an inorganic substance comprising a lithium ion conductive crystal or powder of a lithium ion conductive glass-ceramic and an organic polymer added with an inorganic or organic lithium salt, and being free of an electrolytic solution. The organic polymer is a copolymer, a bridge structure or a mixture thereof of polyethylene oxide and other organic polymer or polymers. A lithium ion secondary battery comprises this solid electrolyte.
Owner:OHARA
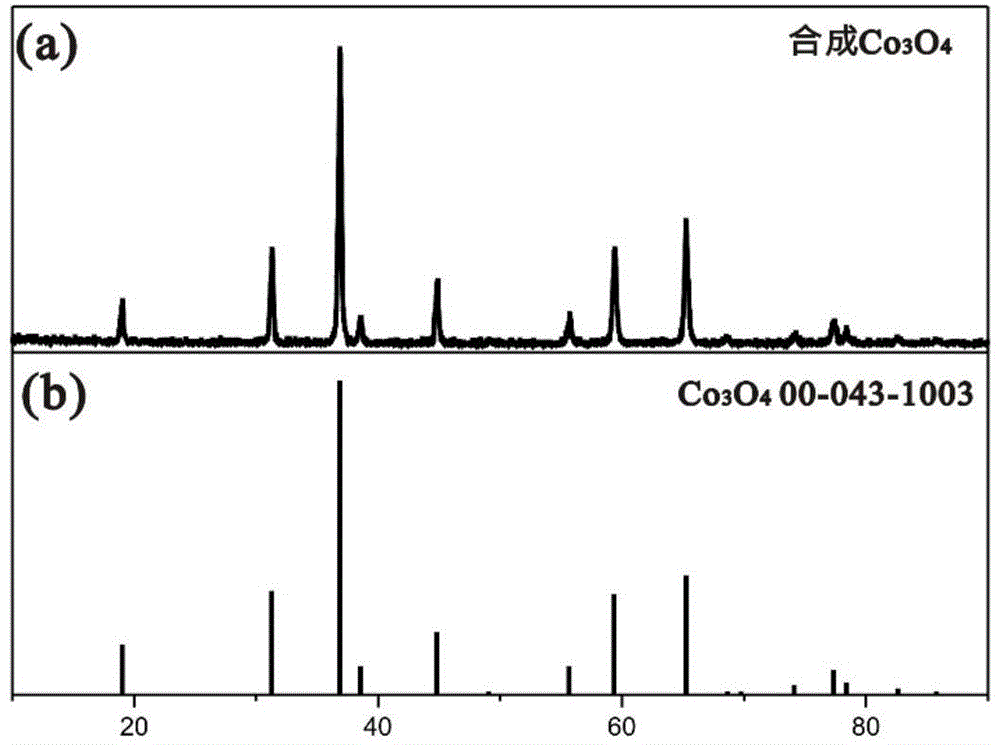
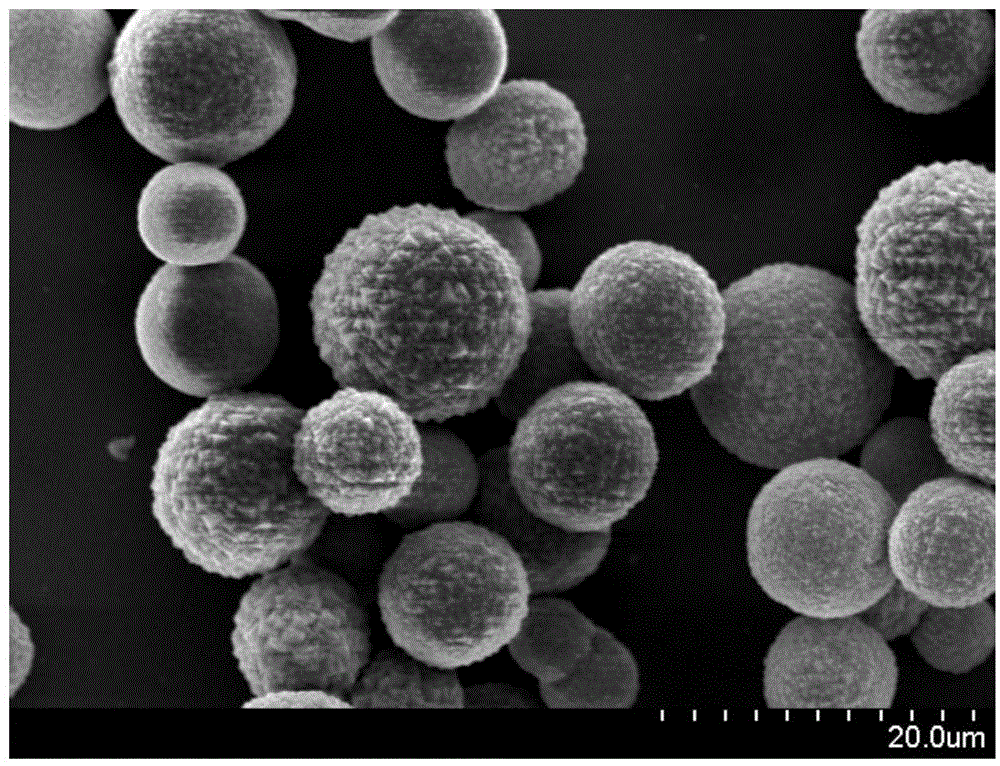
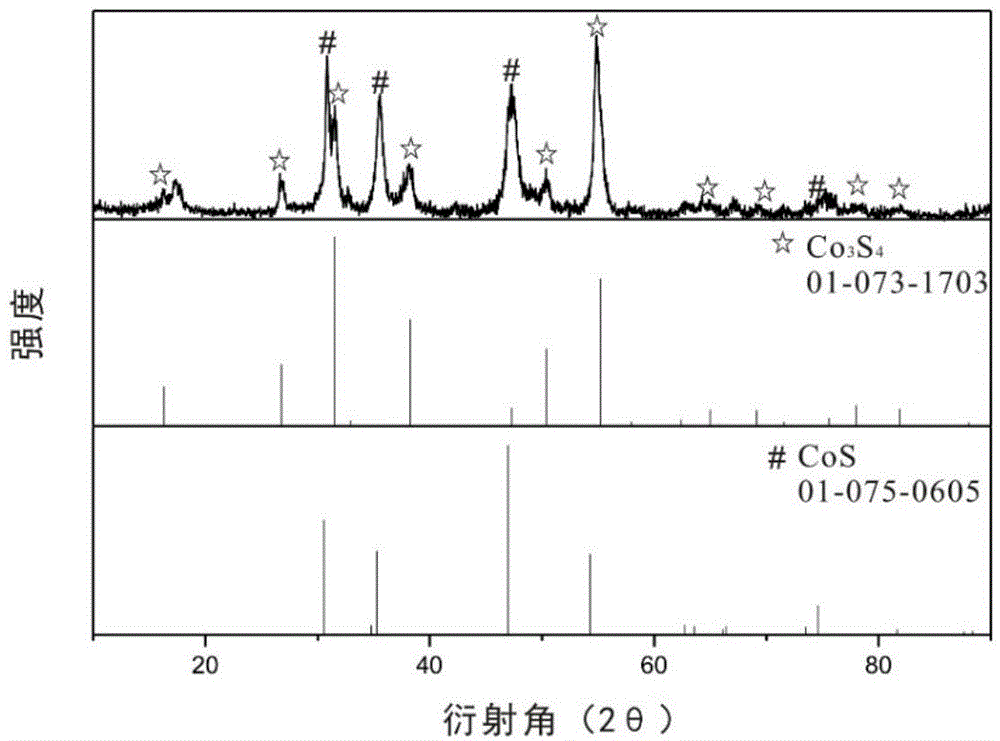
Preparation method and application of cobalt sulfur compound
ActiveCN104993132AWide range of sourcesHigh specific capacityCobalt sulfidesCell electrodesSemiconductor materialsSolar cell
Relating to cobalt sulfur compounds, the invention provides a preparation method and application of a cobalt sulfur compound. The method includes: dissolving a water soluble cobalt source and urea in a mixed solvent to form a solution, carrying out reaction to obtain cobalt carbonate, performing calcinations to obtain a cobalt oxide, and reacting the cobalt oxide with a sulfur source in a reducing atmosphere to obtain a micrometer-scale cobalt sulfur compound. The micrometer-scale cobalt sulfur compound can be spherical cobalt sulfur compound or lamellar square-like cobalt sulfur compound, and the obtained cobalt sulfur compound can be Co9S8, CoS, Co3S4 and CoS2, etc. The cobalt sulfur compound prepared by the preparation method provided by the invention can be applied as an electrode active material in preparation of secondary battery electrodes. According to the invention, specific shape cobalt sulfide can be prepared, is low in synthesis cost and has high tap density, and can be applied to secondary battery electrode materials, optical parametric oscillators, semiconductor materials, solar cells and other aspects.
Owner:XIAMEN UNIV
Non-aqueous electrolyte for lithium ion battery and lithium ion secondary battery
ActiveCN102569885AAvoid decompositionReduce gas productionSecondary cellsElectrical batteryAqueous electrolyte
The invention provides non-aqueous electrolyte for a lithium ion battery and a lithium ion secondary battery prepared from the non-aqueous electrolyte. The non-aqueous electrolyte for the lithium ion battery comprises cyclic carboxylic ester and / or cyclic carbonate ester, cyclic sulfate, an electrolyte salt, fluoroether with the following structural formula: Rf1-O-Rf2, and a fluorocarbon surfactant, wherein the Rf1 is a fluorine-containing alkyl group of which the carbon atom number is 3 to 4; the Rf2 is a fluorine-containing alkyl group of which the carbon atom number is 2 to 5; the mass percentage of the fluoroether in the electrolyte is 10 to 50 percent; and the fluorocarbon surfactant is used for further improving the performances of the battery. The high temperature performance and the safety of the lithium ion secondary battery prepared from the non-aqueous electrolyte can be kept at a higher level as a whole, and the cycling performance is improved.
Owner:SHENZHEN CAPCHEM TECH
Transition metal sulfide coated with carbon, preparation method and application
InactiveCN104835961AGood electrical conductivityGood cycle stabilityCell electrodesSecondary cellsCarbon coatingCarbon source
A transition metal sulfide coated with carbon, a preparation method and an application relate to transition metal sulfide. The transition metal sulfide coated with the carbon comprises a nucleus and a coating layer on the surface of the nucleus, wherein the nucleus is a transition metal sulfide nucleus, and the coating layer is a carbon coating layer. The preparation method comprises the steps as follows: dissolving the transition metal sulfide in water, adding a carbon source, and coating the surface of the transition metal sulfide with carbon. The transition metal sulfide coated with the carbon is applied to preparation of an electrode material, and the electrode material could be a battery electrode material or the like, and is specifically used as an electrode active material to be applied in a secondary battery. The transition metal sulfide coated with the carbon is greatly improved in conductive performance, further improved in charge and discharge capacity and rate capability, greatly improved in coulombic efficiency and cycle performance, and low in material cost and simple in preparation process. The composite material used as an electrode material of a secondary lithium battery has high energy density, excellent cyclicity, and especially excellent rate capability, and is safe and reliable.
Owner:XIAMEN UNIV
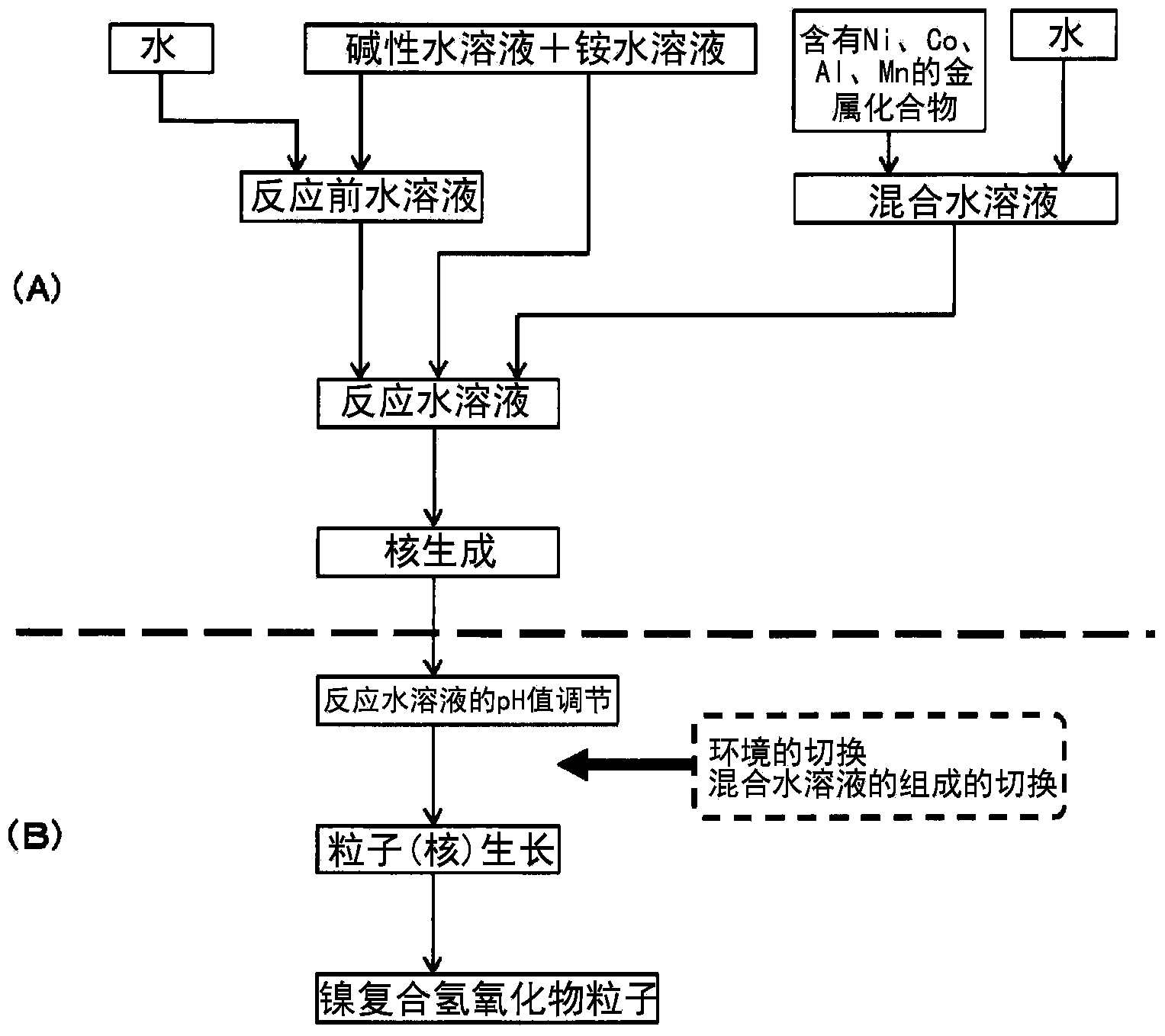
Nickel composite hydroxide and process for producing same, positive active material for nonaqueous-electrolyte secondary battery and process for producing same, and nonaqueous-electrolyte secondary battery
ActiveCN103797623AImprove particle size uniformityLarge specific surface areaPositive electrodesLi-accumulatorsOxygenElectrolyte
To provide a positive active material which has a moderate particle diameter and has high evenness in particle diameter and a nickel composite hydroxide which is for use as a precursor for the positive active material. When a nickel composite hydroxide is obtained through a crystallization reaction, an aqueous solution for nucleation which contains both a metal compound at least containing nickel and an ammonium ion source is used to conduct nucleation after having been regulated so as to have a pH of 12.0-14.0 at a solution temperature of 25 degree centigrade, and thereafter the resultant aqueous solution, which is for particle growth and which contains formed nuclei, is regulated so as to have a pH that, at a solution temperature of 25 degree centigrade, is 10.5-12.0 and lower than in the nucleation step, thereby growing the particles. This particle growth is conducted in a non-oxidizing atmosphere having an oxygen concentration of 1 vol.% or lower, over a period which ranges from initiation of the particle growth step and which accounts for more than 40% of the whole period of the particle growth step. Furthermore, the stirring power per unit volume in at least the nucleation step is regulated to 0.5-4 kW / m3.
Owner:SUMITOMO METAL MINING CO LTD
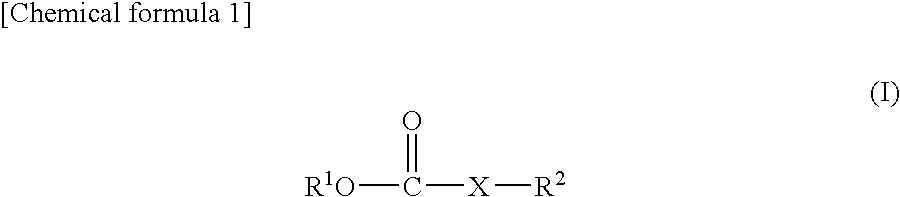
Nonaqueous electrolyte solution and lithium secondary battery using same
InactiveUS7985502B2Improve battery cycle performanceImprove featuresOrganic electrolyte cellsNon-aqueous electrolyte accumulator electrodesLithiumPhysical chemistry
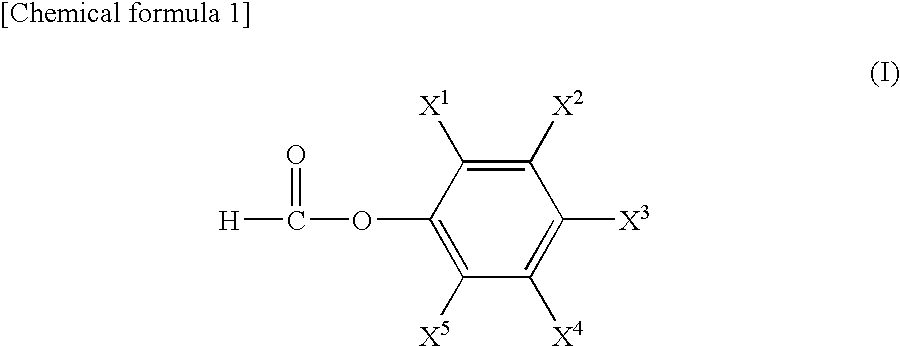
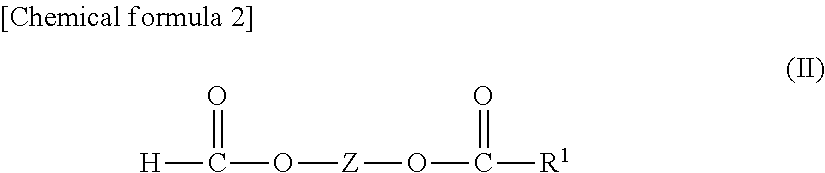
The present invention provides a lithium secondary battery having excellent battery characteristics such as battery cycling property, electrical capacity and storage property.The present invention relates to a nonaqueous electrolytic solution for lithium secondary batteries in which an electrolyte salt is dissolved in a nonaqueous solvent, the nonaqueous electrolytic solution comprising a formic ester compound having a specific structure in an amount of 0.01 to 10% by weight of the nonaqueous electrolytic solution, and a lithium secondary battery using the same.
Owner:UBE IND LTD
Nonaqueous electrolytic solution and nonaqueous electrolyte secondary battery
ActiveUS20130216919A1Improving durability of batteryImprove stabilityOrganic electrolyte cellsLi-accumulatorsHigh current densityLithium
Provided are a nonaqueous electrolyte solution having improved durability properties in terms of cycling, storage and the like and improved discharge characteristic at a high current density, and a nonaqueous electrolyte battery that uses that nonaqueous electrolyte solution. The nonaqueous electrolyte solution containing a lithium salt and a nonaqueous solvent that dissolves the lithium salt, wherein the nonaqueous electrolyte solution contains a compound represented by formula (1) and at least one compound selected from the group consisting of a compound having a cyano group, a cyclic ester compound having a sulfur atom and a compound having an isocyanate group.
Owner:MU IONIC SOLUTIONS CORP +1
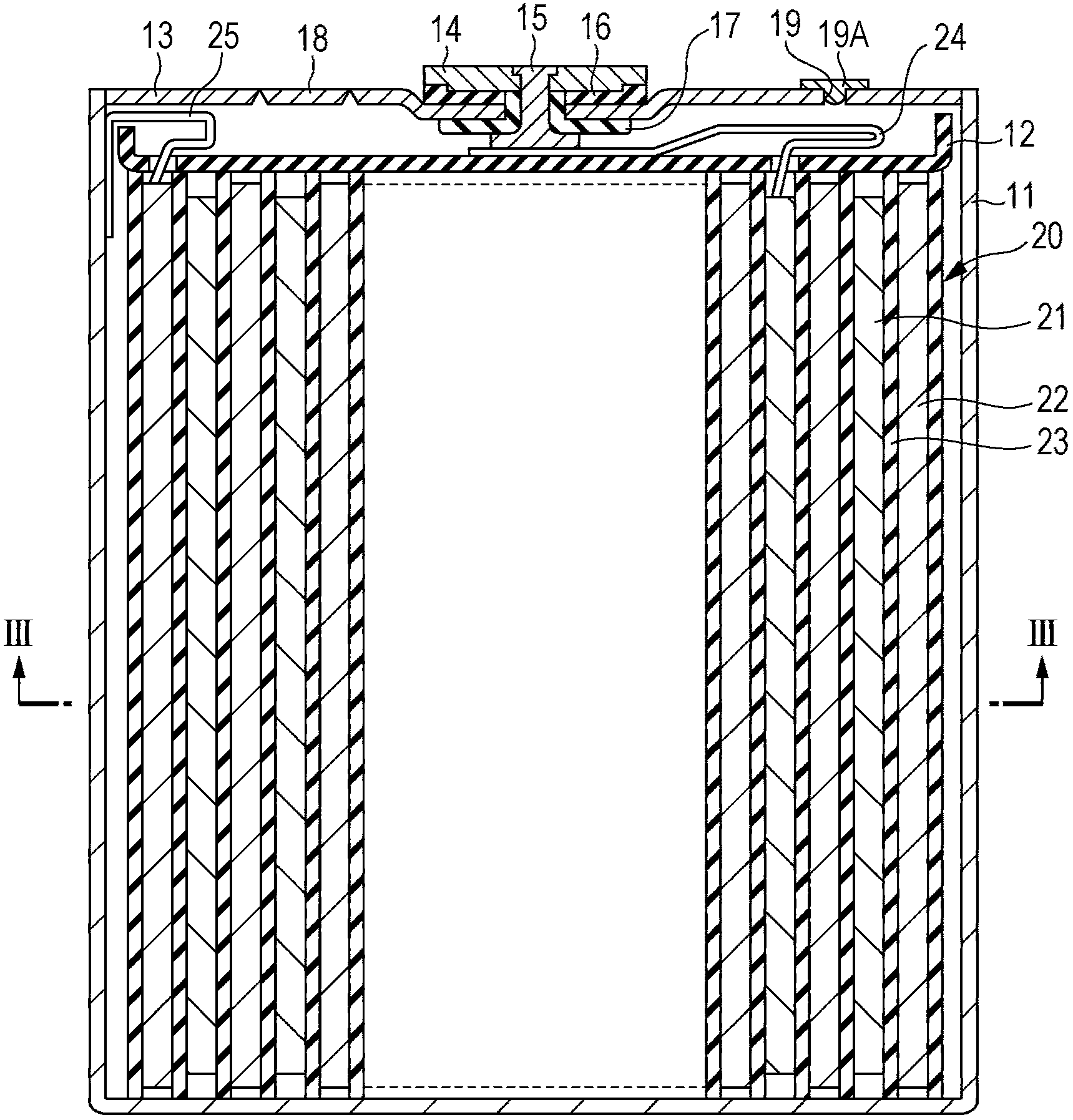
Metallic bipolar plate for fuel cells, and fuel cell comprising the same
InactiveCN101123313AReduce contact resistanceImprove corrosion resistanceCell electrodesCollectors/separatorsFuel cellsMetallic substrate
The present invention relates to a metallic bipolar plate for fuel cells, including: a plate-like metallic substrate containing a metal capable of being passivated; and a noble-metal layer disposed partially on both surfaces of the metallic substrate to coat the metallic substrate, the metallic bipolar plate having a concave-convex portion formed at least in a region facing an electrode of a membrane electrode assembly, in which both surfaces of the metallic bipolar plate respectively have a contact surface which contacts with the electrode and a non-contact surface which does not contact with the electrode, and in which both surfaces of the metallic bipolar plate respectively have a coating area ratio of the noble-metal layer to the contact surface within a range of from 20% to 100%, and a coating area ratio of the noble-metal layer to the non-contact surface within a range of from 5% or more but less than 100%.
Owner:DAIDO STEEL CO LTD
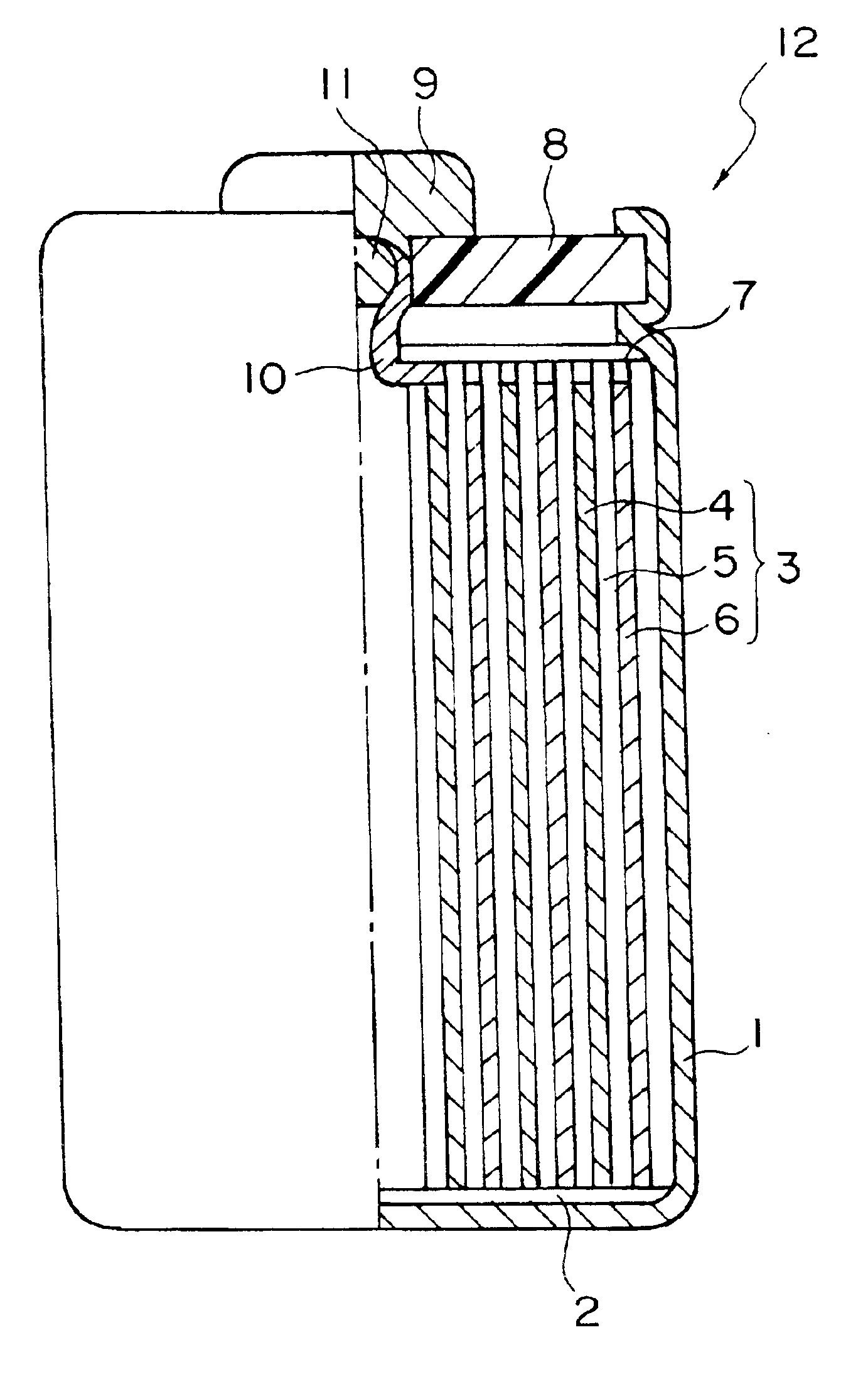
Electrolyte, negative electrode and battery
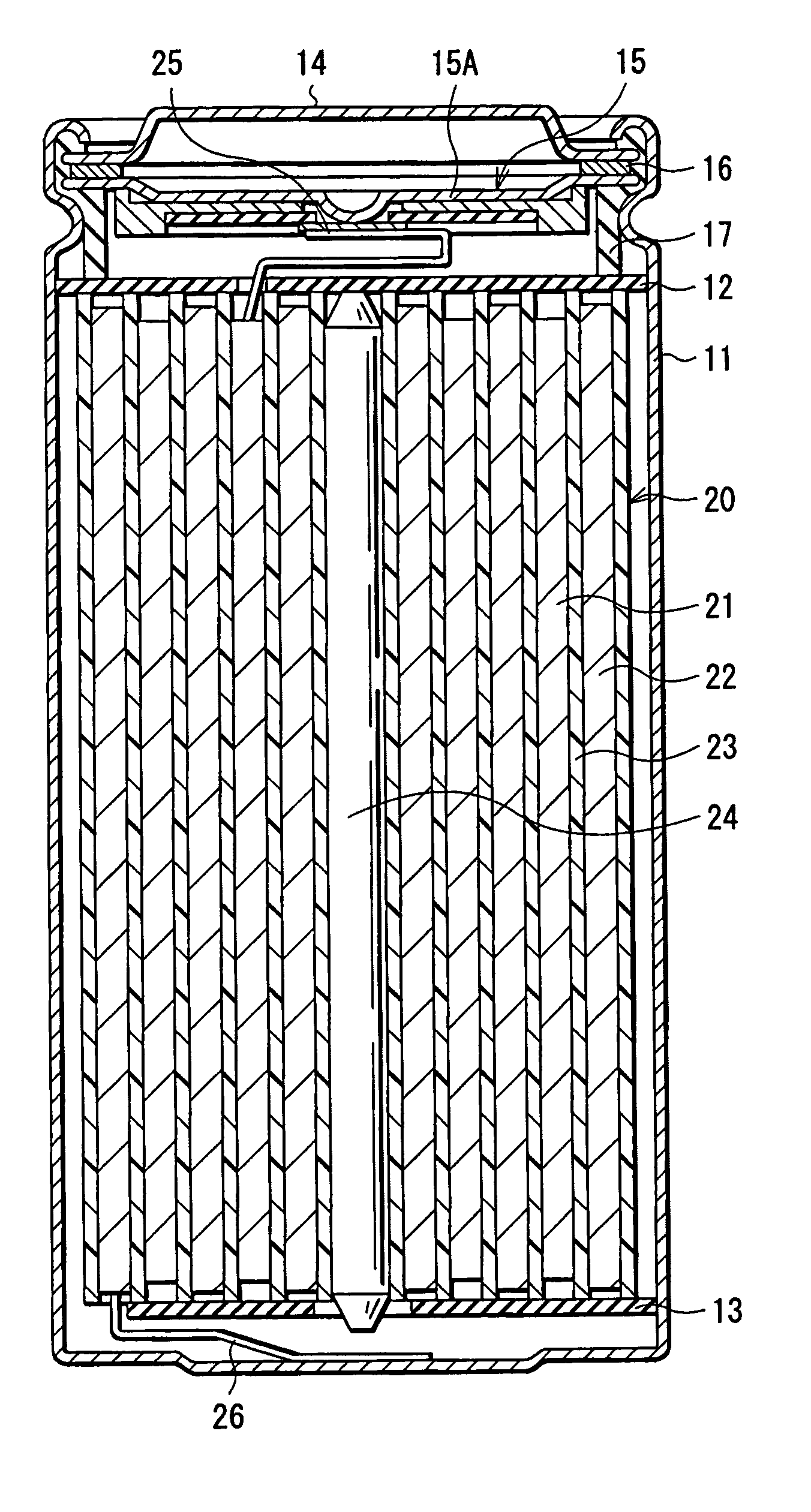
InactiveUS20050147883A1Inhibit side effectsExcellent battery characteristicsElectrochemical processing of electrodesElectrode carriers/collectorsMetal sheetDissolution
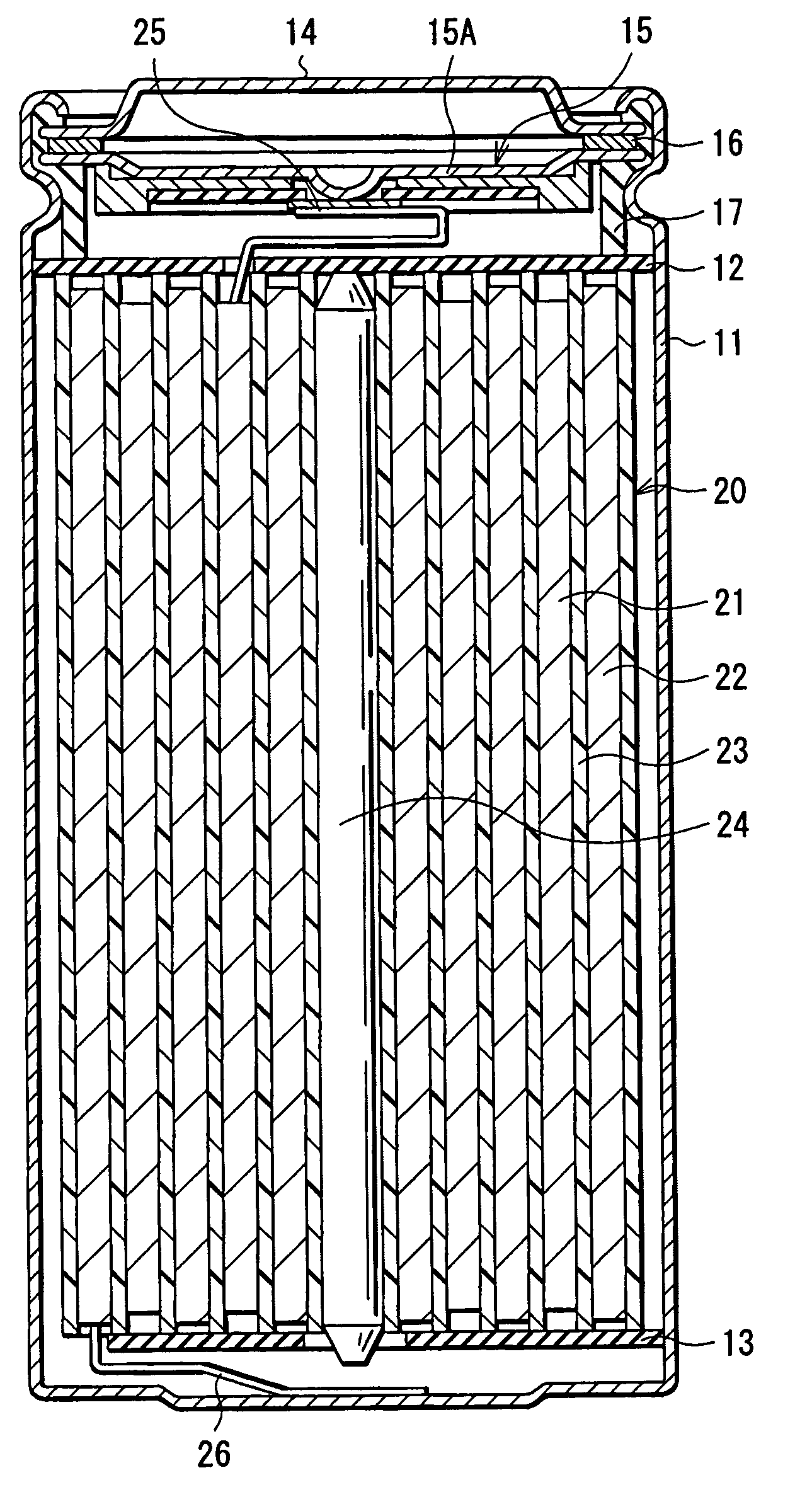
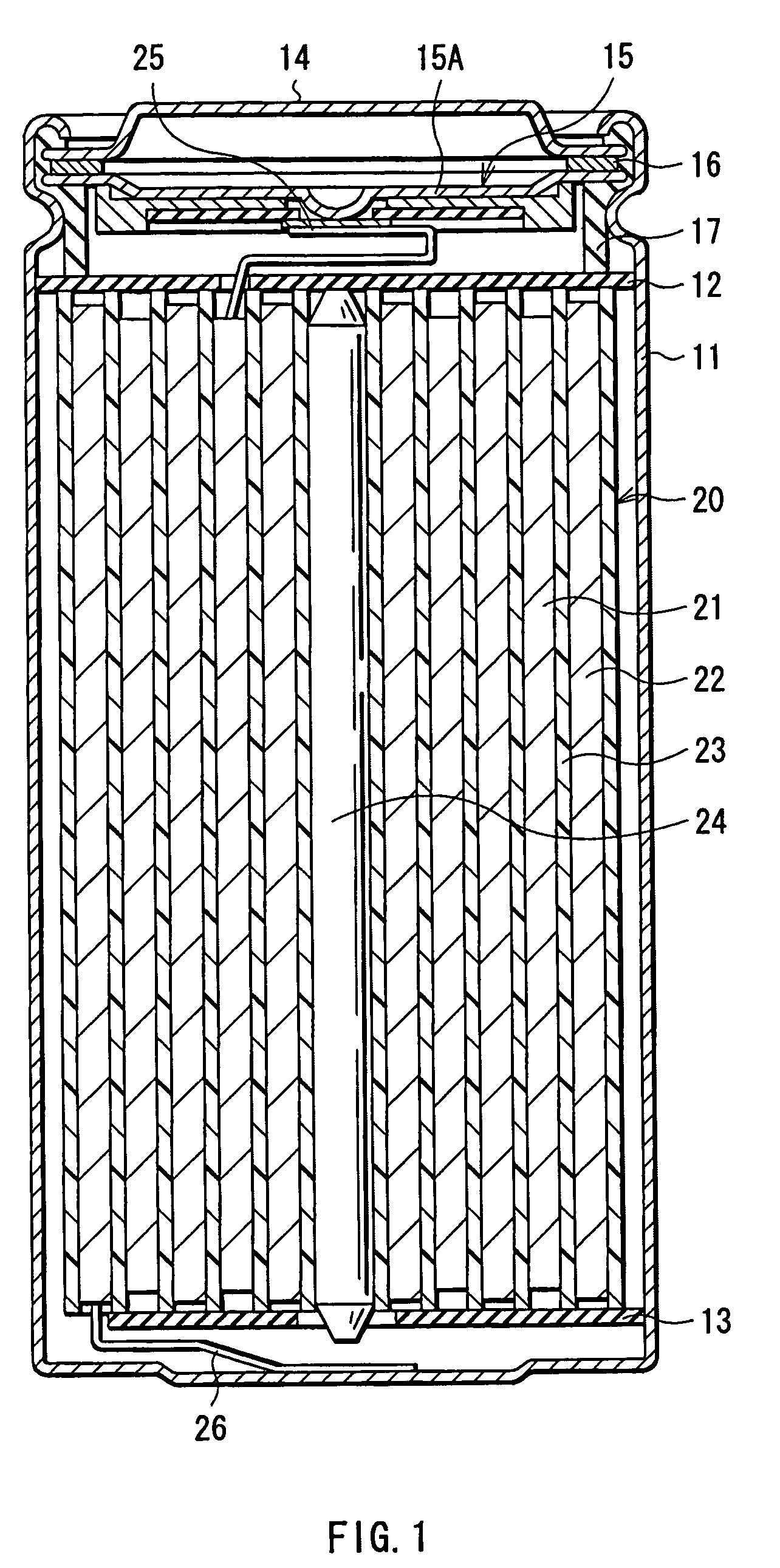
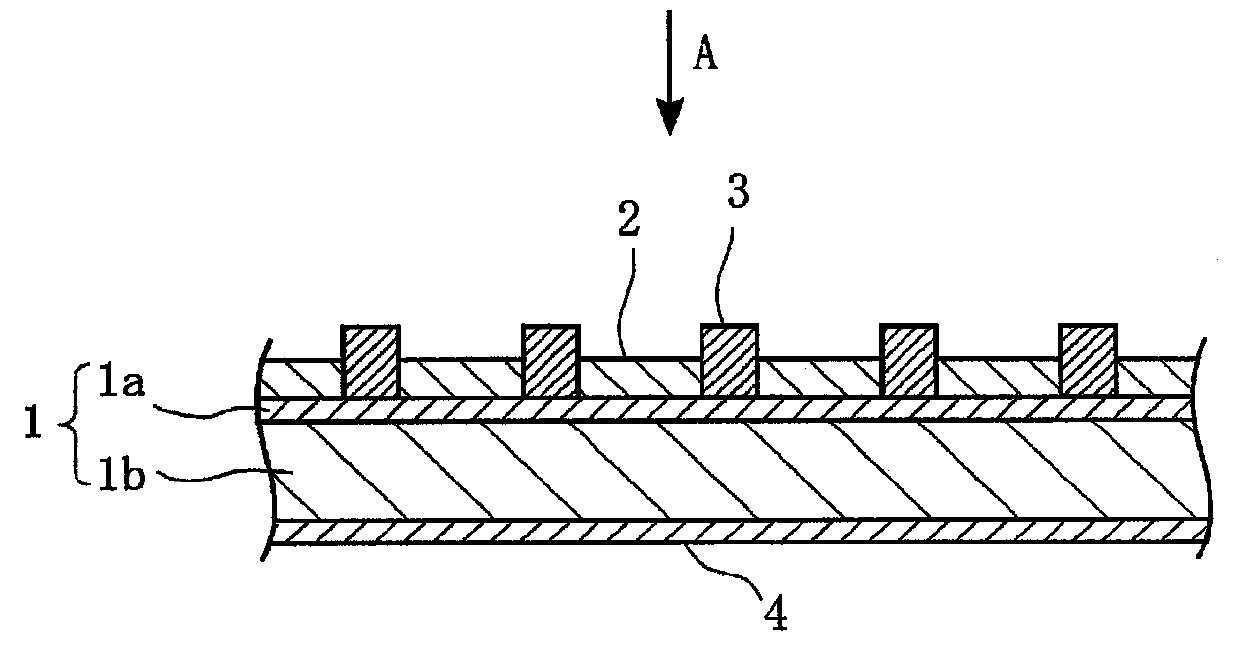
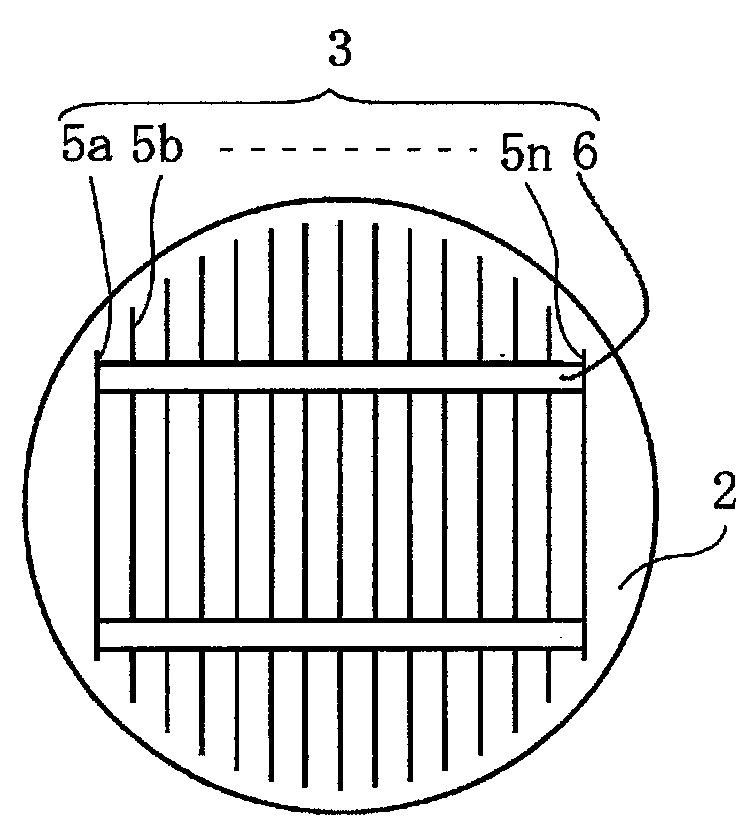
Provided are an electrolyte, an anode and a battery capable of improving the efficiency of precipitation and dissolution of Li, and cycle characteristics. A metal sheet (12A) which is made of Cu and does not contain Li is used as the anode (12) to deposit Li metal. An aromatic compound having an —OX group (X is H or alkali metal) such as catechol is added to an electrolytic solution (16) and a precipitation film (12C) is deposited on the surface thereof with Li metal in the initial charge. The precipitation film (12C) prevents a dendrite growth of Li and a reaction between Li and the electrolytic solution (16).
Owner:SONY CORP
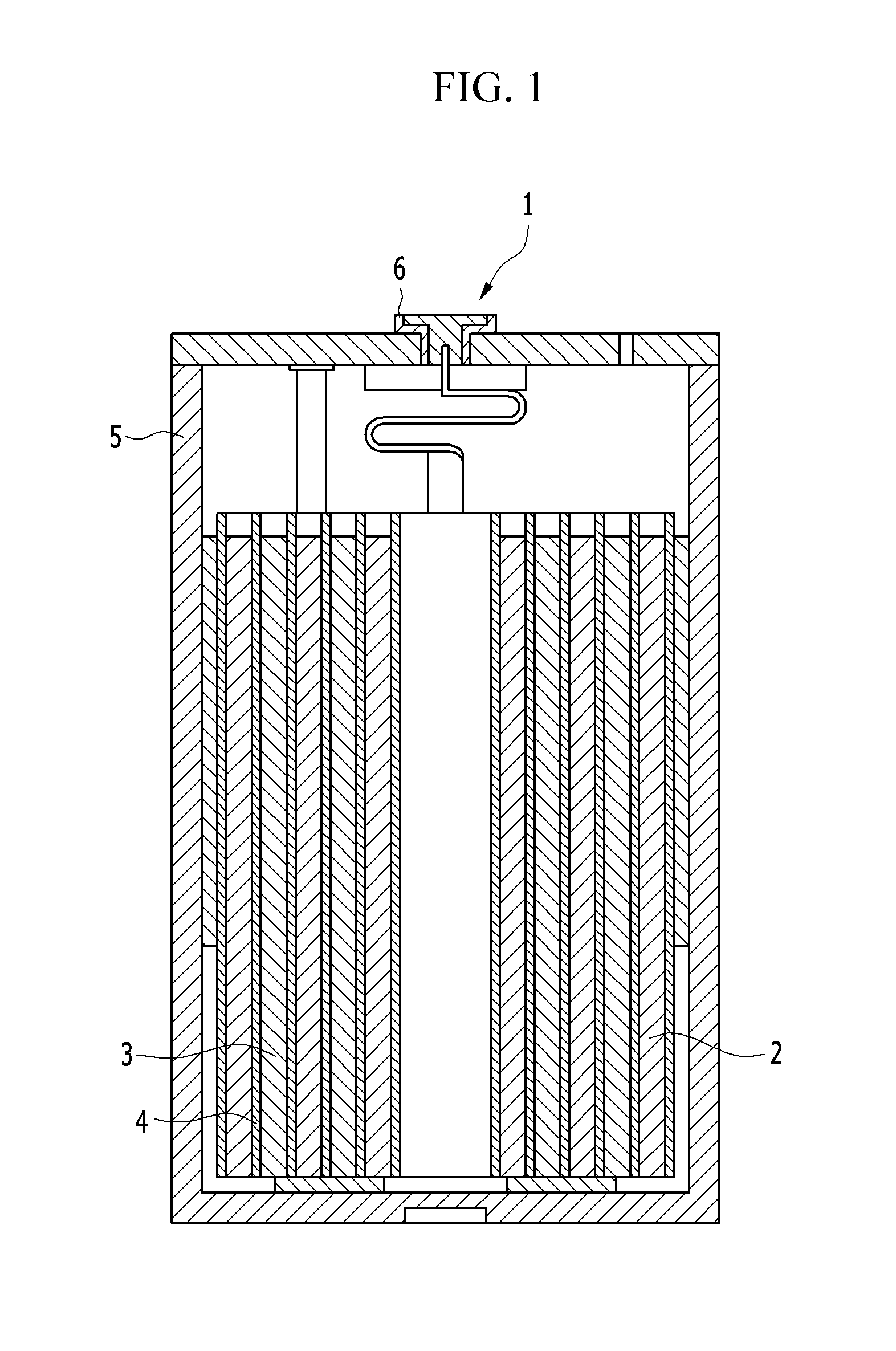
All-solid-state thin-film battery
ActiveUS20150340727A1Excellent battery characteristicsFinal product manufacturePrinted batteriesAll solid stateEngineering
An all-solid-state thin-film battery according to an aspect of the present disclosure includes: a solid electrolyte layer; a cathode active material layer; a cathode current collector layer including a first portion and a second portion; an anode terminal layer; and an anode layer including a third portion and a fourth portion. The first contact surface between the solid electrolyte layer and the cathode active material layer, the second contact surface between the solid electrolyte layer and the first portion of the cathode current collector layer, and the third contact surface between the third portion of the anode layer and the anode terminal layer are located within a single plane.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Nonaqueous electrolyte composition and nonaqueous electrolyte secondary battery
ActiveUS20100273048A1High strengthReduced characteristicsSolid electrolytesNon-aqueous electrolyte accumulatorsElectrolyte compositionPhysical chemistry
A nonaqueous electrolyte composition includes: a nonaqueous solvent; an electrolyte salt; a matrix resin; a filler; and a surfactant.
Owner:MURATA MFG CO LTD
Battery cooling device, battery attached with cooling device, and vehicle
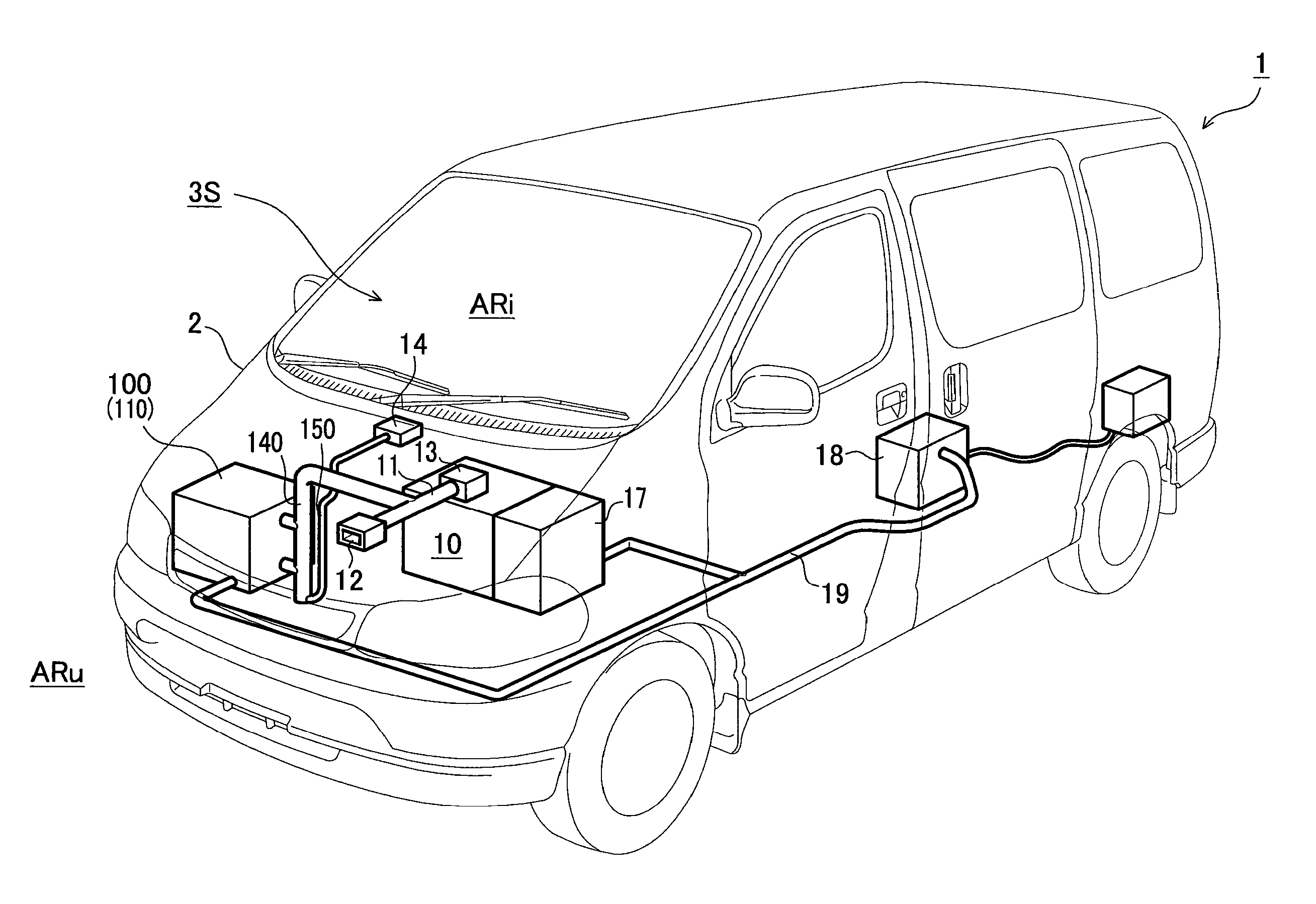
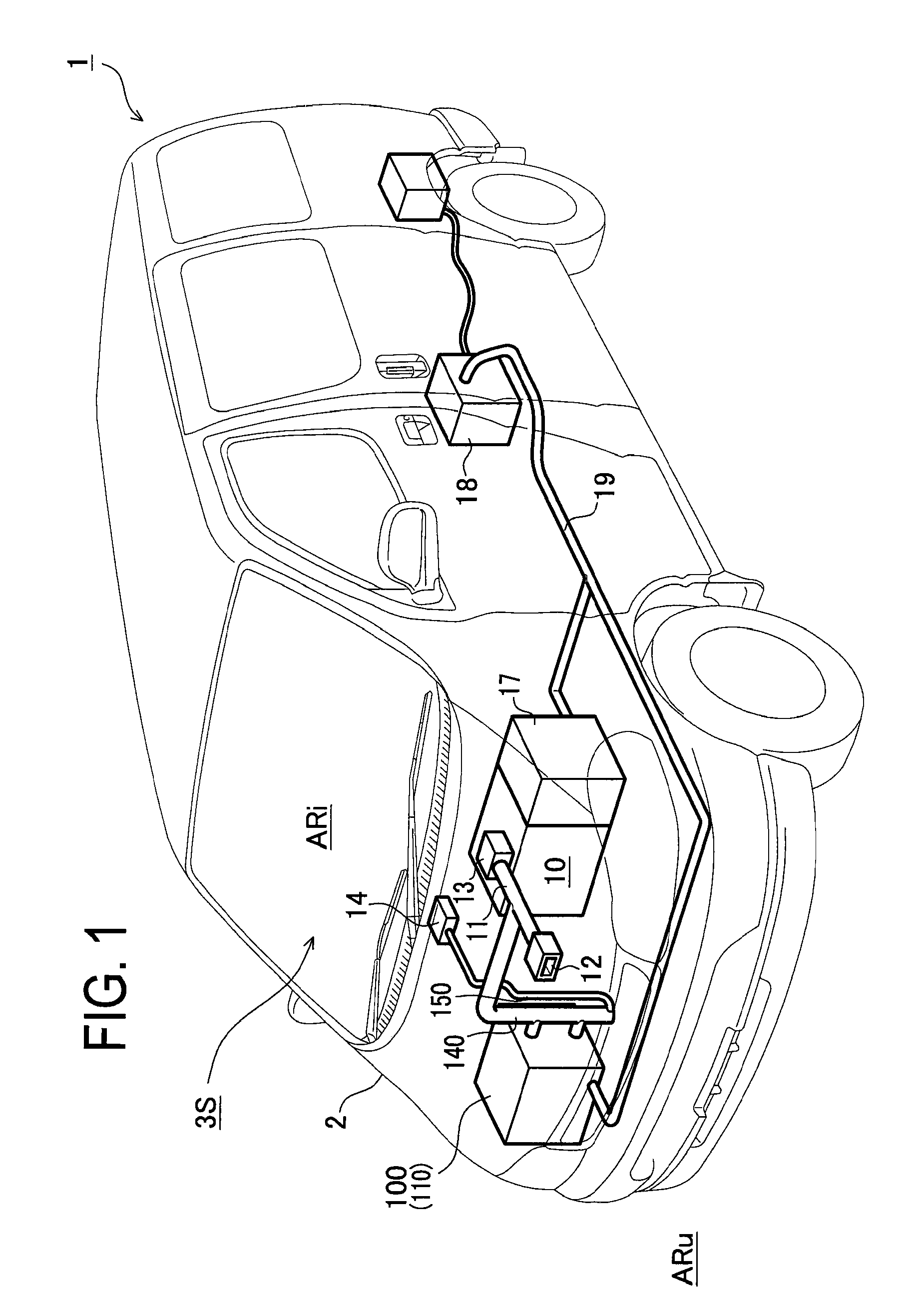
InactiveUS20100055553A1Improve cooling effectExcellent battery characteristicsCell temperature controlElectric propulsion mountingTurbineRefrigerant
Provided are a battery cooling device utilizing intake air or exhaust gas of an engine, a battery having a cooling device attached to it, and a vehicle mounted with the battery cooling device and an engine. The battery cooling device has a battery housing chamber capable of housing a battery and filled with refrigerant, a turbine to be rotated by exhaust gas of the engine, and a refrigerant flow part including a compressor, a heat exchanger a pipe, etc.
Owner:TOYOTA JIDOSHA KK
Nonaques Electrolyte Solution And Lithium Secondary Battery Using Same
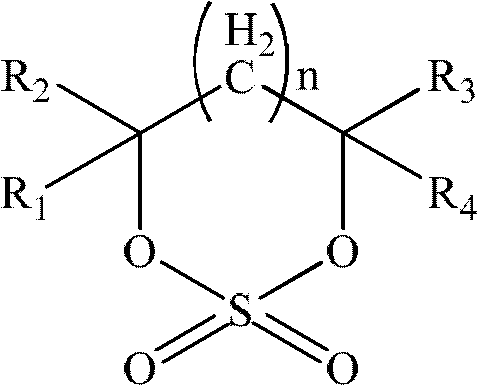
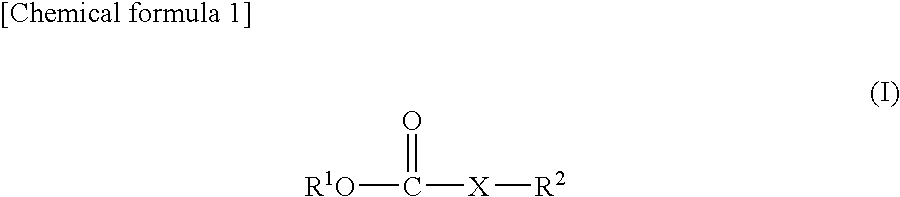
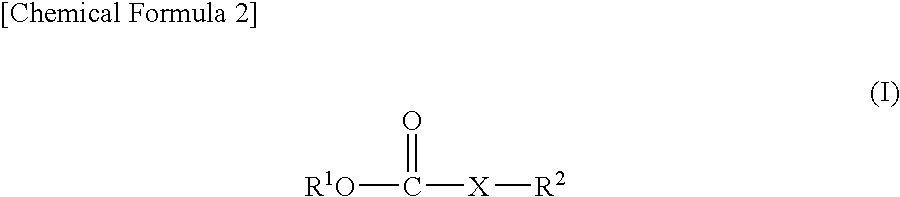
ActiveUS20080038644A1Cycle characteristic of be improveLong-term in capacityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsSolventPropane sultone
Disclosed is a lithium secondary battery which is excellent in battery characteristics such as long-term cycle characteristics, capacity and shelf life characteristics. Also disclosed is a nonaqueous electrolyte solution which can be used for such a lithium secondary battery. Specifically disclosed is a nonaqueous electrolyte solution for lithium secondary batteries obtained by dissolving an electrolyte salt in a nonaqueous solvent which is characterized by containing 0.01-10% by weight of a carboxylate compound represented by the general formula (I) below and 0.01-10% by weight or 0.01-10% by volume of a vinylene carbonate and / or 1,3-propane sultone. Also disclosed is a lithium secondary battery using such a nonaqueous electrolyte solution. (In the formula, R2 represents a hydrogen atom or COOR3 group, R1 and R3 respectively represent an alkyl group, a cycloalkyl group, an alkenyl group, an alkynyl group or a phenyl group, and X represents an alkynylene group or an alkenylene group.)
Owner:MU IONIC SOLUTIONS CORP
Active material for anode of secondary cell and method for production thereof and non-aqueous electrolyte secondary cell, and recycled electronic functional material and method for recycling electronic functional material
InactiveUS6811923B1Easy to separateEasy to removeActive material electrodesWaste accumulators reclaimingHigh densityElectron
Positive electrode active material for nonaqueous electrolytic solution secondary batteries is made of a metal oxide. A content of coarse particles, of which particles sizes are 600% or more with respect to an average particle size of the metal oxide, is 1% or less by volume, and a content of high density particles, of which densities are 150% or more with respect to an average density of the metal oxide, is 1000 ppm or less. By using the positive electrode active material, battery characteristics and a manufacturing yield may be improved.
Owner:KK TOSHIBA
Conductive paste and solar cell
ActiveCN104798209AReduce contact resistanceImprove energy conversion efficiencyNon-conductive material with dispersed conductive materialPhotovoltaic energy generationElectrical resistance and conductanceConductive paste
The conductive paste contains a conductive powder, a glass frit, and an organic vehicle. The glass frit contains 35-90 mol% of Te in terms of TeO 2 , 5-50 mol% of Zn in terms of ZnO, and 1-20 mol% of Bi in terms of Bi 2 O 3 , and contains 0.1-15 mol% of at least one element selected from the group of Li, Na, and K in terms of oxides thereof. The conductive paste is used to form light-receiving surface electrodes (3). A conductive paste appropriate for formation of electrodes of a solar cell capable of reducing contact resistance between the electrode (3) and a semiconductor substrate (1) is thereby achieved, the use of the conductive paste obtaining high energy conversion efficiency and attaining a solar cell having excellent cell characteristics.
Owner:HERAEUS PRECIOUS METALS NORTH AMERICA CONSHOHOCKEN
Lithium-ion secondary battery, negative electrode for lithium-ion secondary battery, battery pack, electric vehicle, power storage system, electric tool, and electronic device
ActiveCN102842707AExcellent battery characteristicsImprove featuresCell electrodesSecondary cellsPower storageElectric vehicle
The invention provides a lithium-ion secondary battery, a negative electrode for the same and a battery. The lithium-ion secondary battery includes a positive electrode, a negative electrode containing an active material, and an electrolytic solution, in which the active material contains, as constituent elements, Si, O, and at least one element Ml selected from Li, C, Mg, Al, Ca, Ti, Cr, Mn, Fe, Co, Ni, Cu, Ge, Zr, Mo, Ag, Sn, Ba, W, Ta, Na, and K, and the atomic ratio x (O / Si) of O to Si is 0.5<=x<=1.8.
Owner:MURATA MFG CO LTD
Non-aqueous electrolyte secondary battery
InactiveUS20060292450A1Avoid temperature riseExcellent battery characteristicsFinal product manufactureOrganic electrolyte cellsBromineNon aqueous electrolytes
The present invention provides a highly-reliable non-aqueous electrolyte secondary battery excellent in safety. The non-aqueous electrolyte secondary battery includes an electrode group, a non-aqueous electrolyte and a case accommodating the electrode group and the non-aqueous electrolyte. The non-aqueous electrolyte contains a specific bromine compound having an aromatic ring.
Owner:PANASONIC CORP
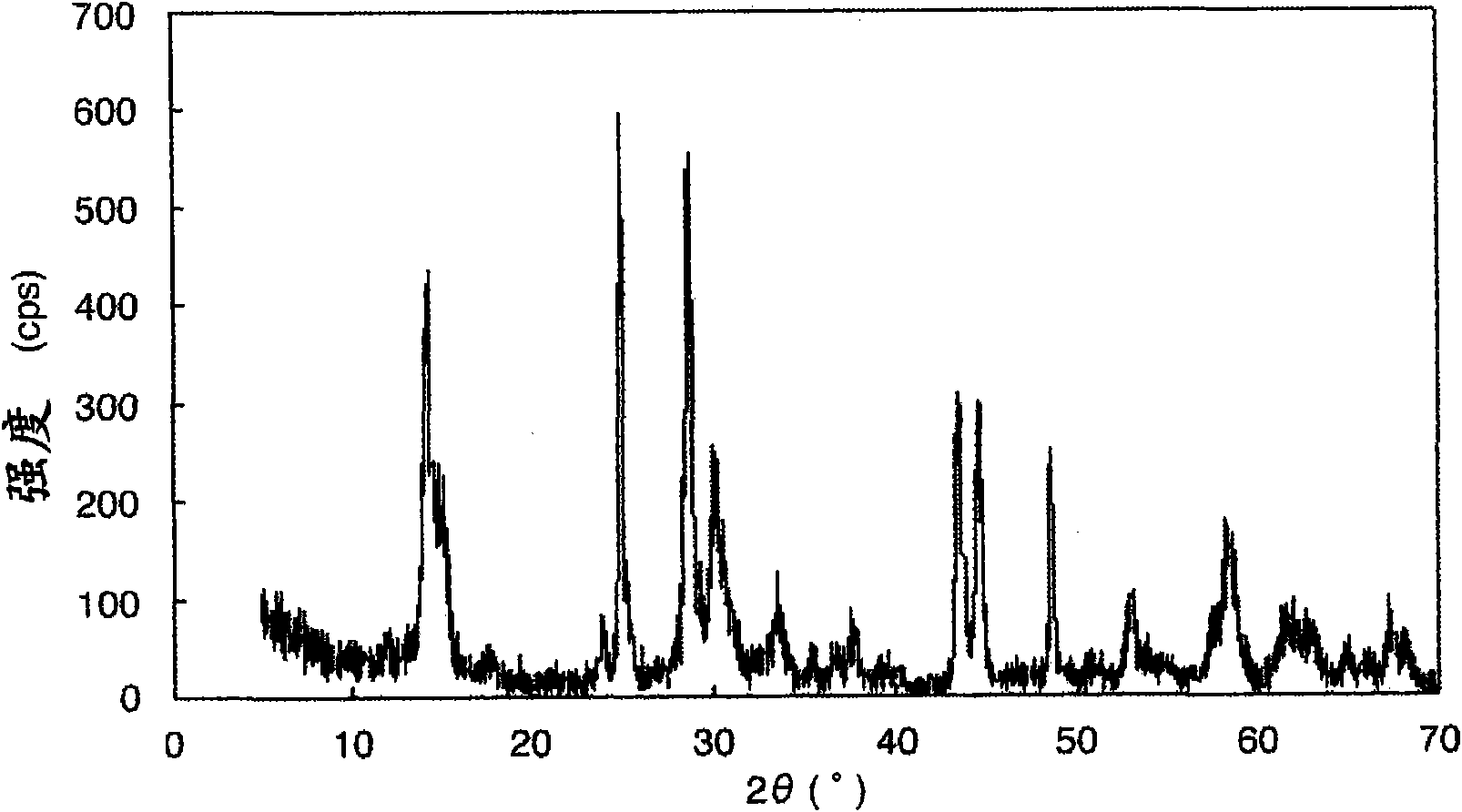
Titanic acid compound, process for producing the titanic acid compound, electrode active material containing the titanic acid compound, and storage device using the electrode active material
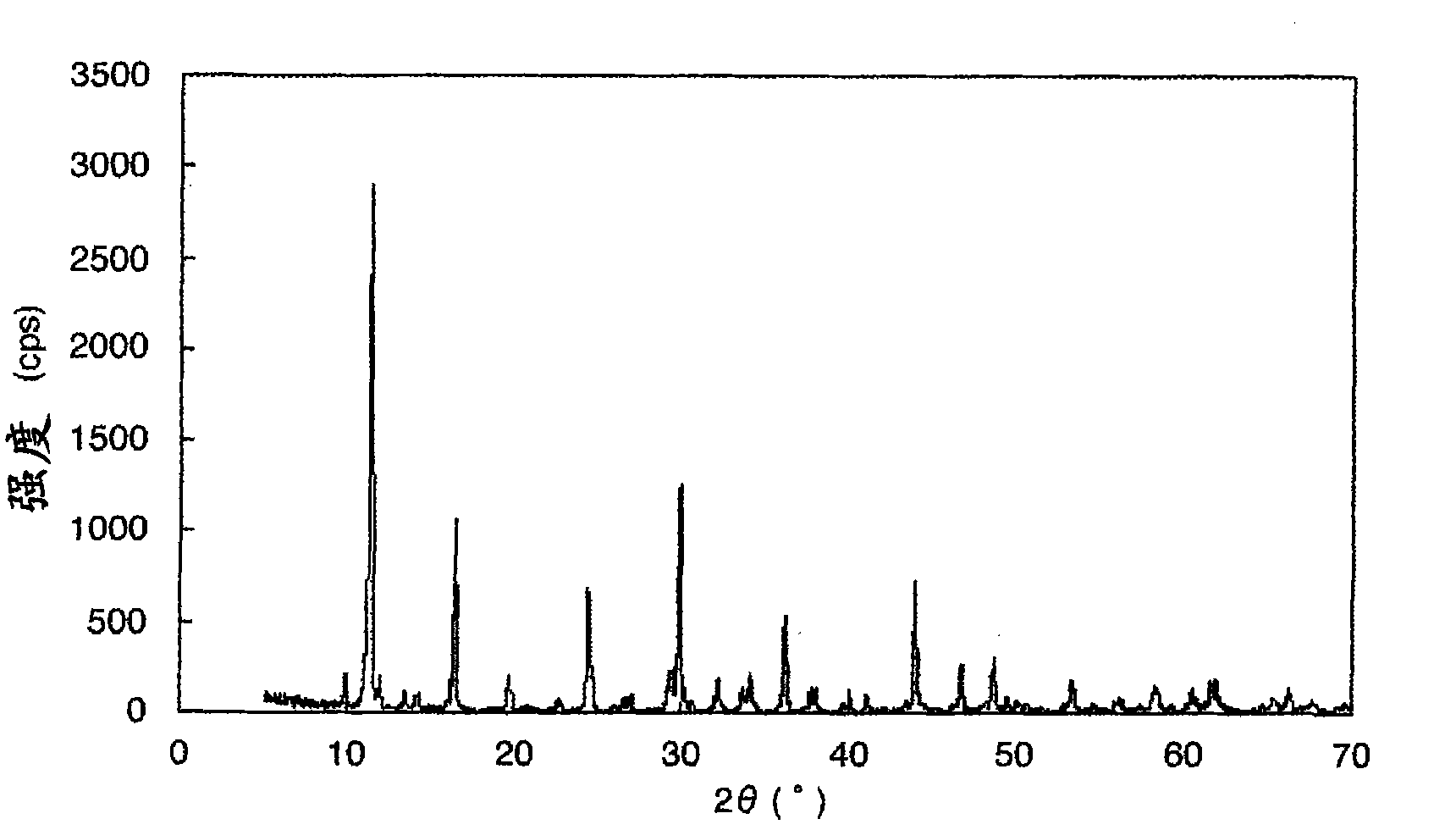
InactiveCN101842923AExcellent battery characteristicsIncrease capacityPigmenting treatmentAlkali titanatesX-rayUltimate tensile strength
This invention provides a titanic acid compound-type electrode active material having a high battery capacity and, at the same time, having excellent cycle characteristics. The titanic acid compound exhibits an X-ray diffraction pattern corresponding to a bronze-type titanium dioxide except for a peak for a (200) plane and having a peak intensity ratio between the (001) plane and the (200) plane,i.e., I(200) / I(001), of not more than 0.2. The titanic acid compound may be produced by heat dehydrating H2Ti3O7 at a temperature in the range of 200 to 330 DEG C, by heat dehydrating H2Ti4O9 at a temperature in the range of 250 to 650 DEG C, or by heat dehydrating H2Ti5O11at a temperature in the range of 200 to 600 DEG C.
Owner:ISHIHARA SANGYO KAISHA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com