Nickel compound hydroxide and method for producing same, positive pole active substance for nonaqueous electrolyte secondary cell and method for producing same, and nonaqueous electrolyte secondary cell
A composite hydroxide and cathode active material technology, which is applied in the direction of secondary batteries, nickel compounds, battery electrodes, etc., can solve the problem of battery capacity reduction, failure to consider the particle size and particle size distribution of lithium composite oxides, and the selective degradation of fine particles And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0112] a) Nucleation process
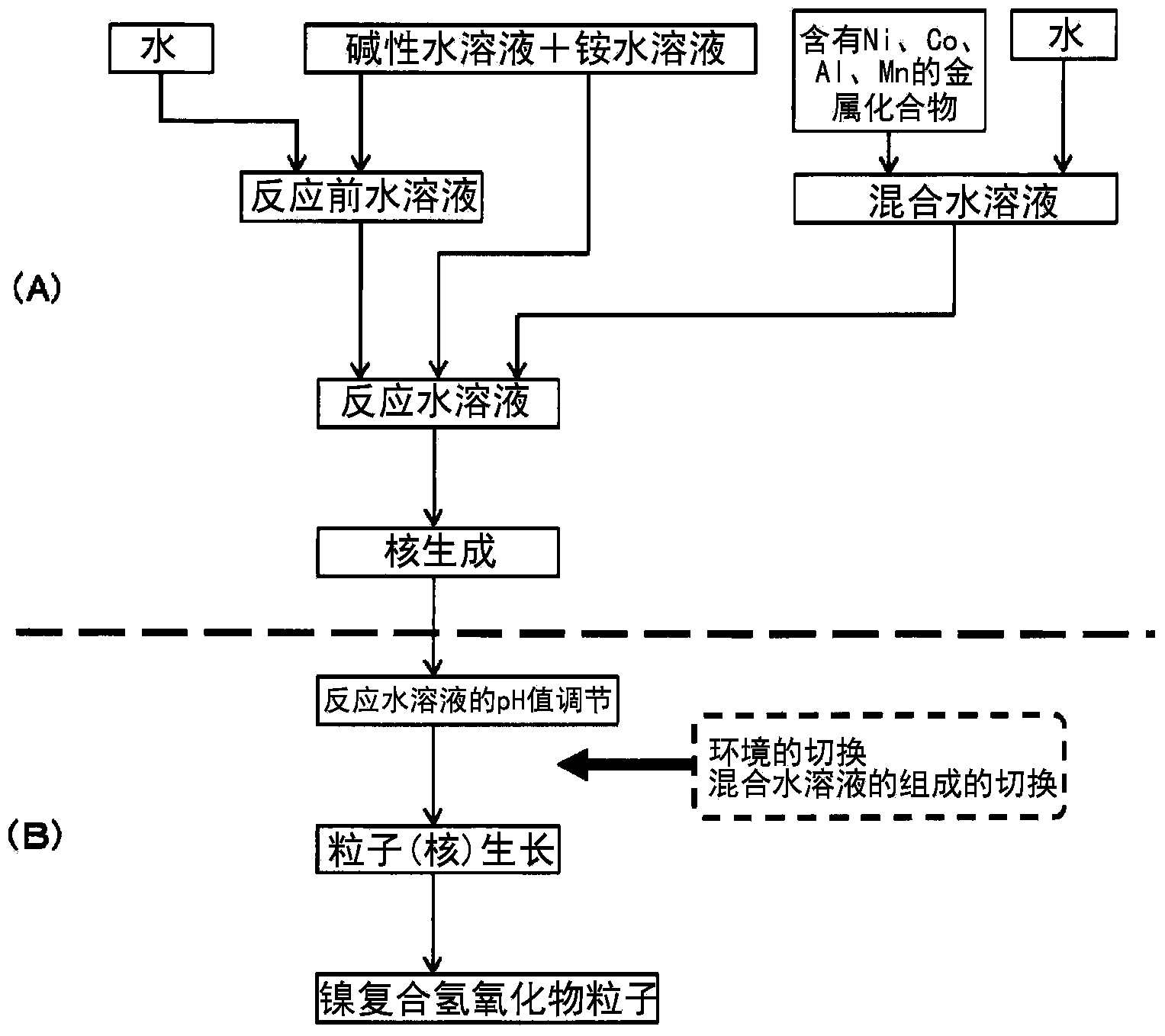
[0113] Such as figure 1 As shown, first, the general formula (2) related to the metal composition ratio and the composite hydroxide becomes the general formula (a): Ni x co y al z mn t m s (x+y+z+t+s=1, 0≤y≤0.8, 0≤z≤0.1, 0.1≤t≤0.8, 0≤s≤0.05, M is selected from Mg, Ca, Ti, V , Cr, Zr, Nb, Mo, and W composition ratio of at least one additional element in the group consisting of), a plurality of metal compounds are dissolved in water at a predetermined ratio to prepare a mixed aqueous solution.
[0114] In particular, the manganese content in the mixed aqueous solution needs to be controlled so that t in the general formula (a) is in the range of 0.1 to 0.8, preferably 0.2 to 0.7, more preferably 0.3 to 0.8. 0.6 below the way to control. If t is less than 0.1, fine primary particles cannot be obtained; if it exceeds 0.8, shrinkage during firing decreases and a sufficient hollow structure cannot be obtained.
[0115] In addition, in order to ...
no. 2 approach
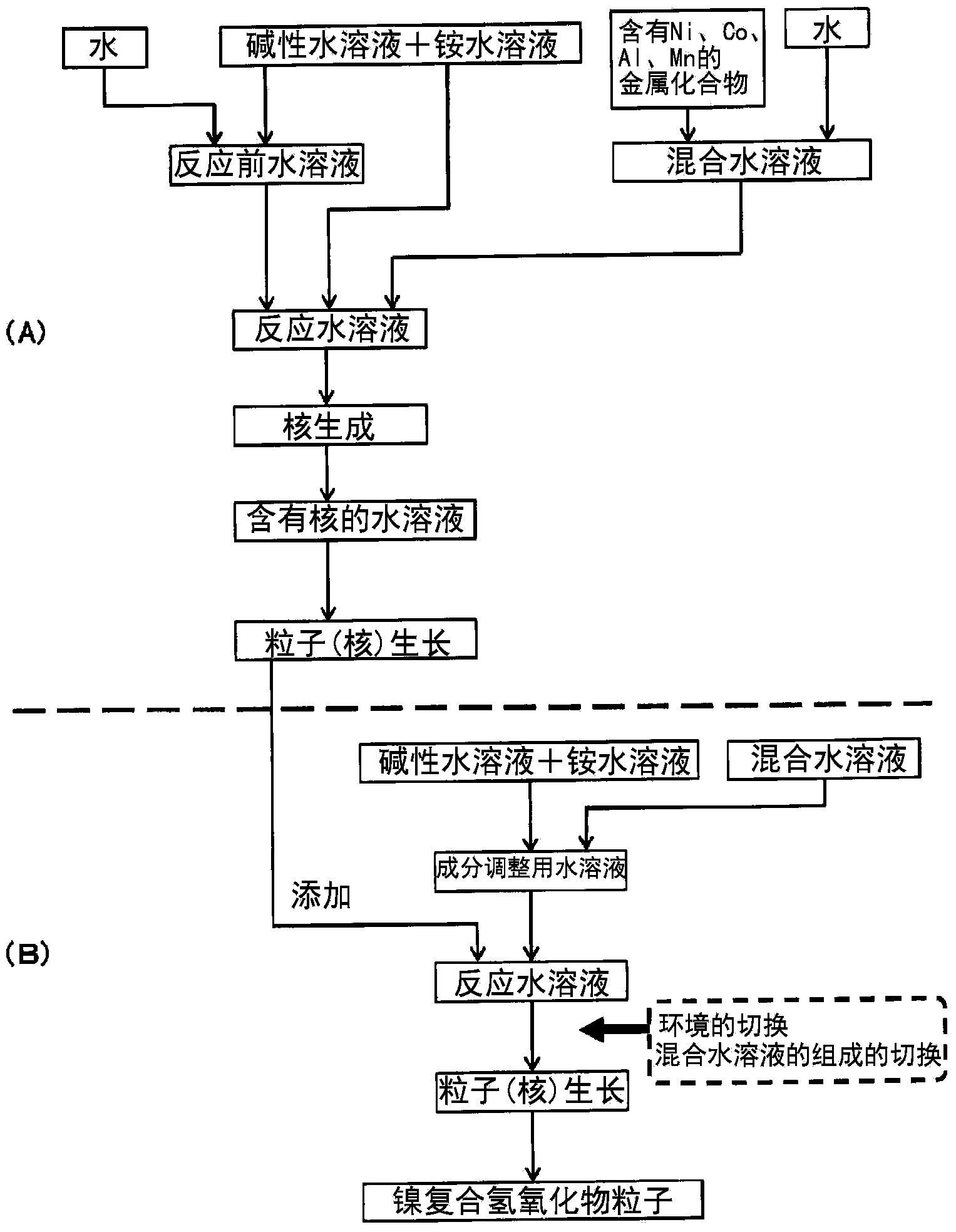
[0138] Such as figure 2 In another embodiment shown, in addition to the aqueous solution for nucleation, a component-adjusted aqueous solution adjusted to a pH value and an ammonium ion concentration suitable for the particle growth process may be formed in advance, and another reaction tank may be added to the component-adjusted aqueous solution. The aqueous solution containing nuclei (aqueous solution for nucleation, preferably an aqueous solution after removing a part of the liquid component from the aqueous solution for nucleation) generated by carrying out the nucleation step in the process can be used as the reaction aqueous solution, and the reaction aqueous solution can also be used as the particle growth solution. aqueous solution to perform the particle growth process.
[0139] In this case, since the separation of the nucleation step and the particle growth step can be performed more reliably, the state of the reaction aqueous solution in each step can be made into...
Embodiment 1
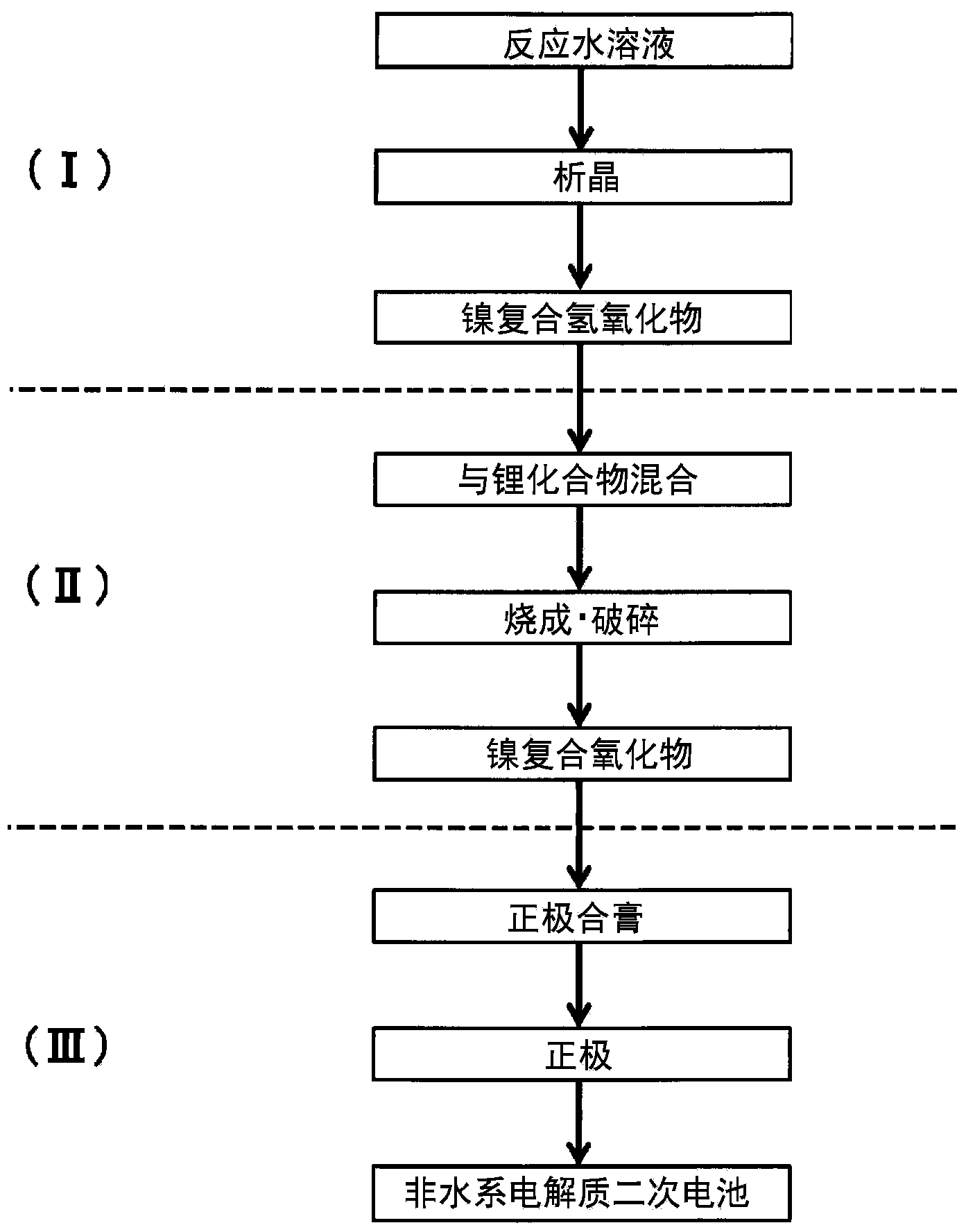
[0270] [Manufacture of composite hydroxide]
[0271] Composite hydroxides were prepared as described below. In addition, when making the composite hydroxide, the positive electrode active material and the secondary battery in all examples, all used various special grade products (special grade chemicals manufactured by Wako Pure Chemical Industry Co., Ltd.) unless otherwise specified. ) reagents.
[0272] (nucleation process)
[0273] First, water was added to half the capacity of the reaction tank (34 L), and the temperature in the tank was set to 40° C. while stirring. At this time, the inside of the reaction tank was an atmospheric environment (oxygen concentration: 21% by volume). By adding an appropriate amount of 25% by mass aqueous sodium hydroxide solution and 25% by mass ammonia water to the water in the reaction tank, the pH of the reaction solution in the reaction tank was adjusted to be 13.0 based on a liquid temperature of 25°C. Furthermore, the ammonia concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com