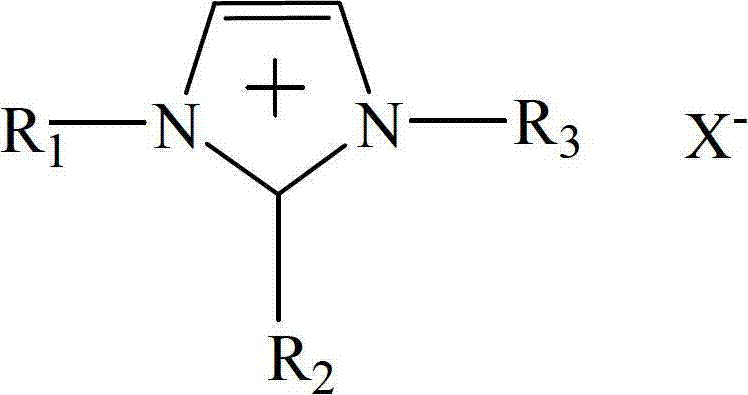
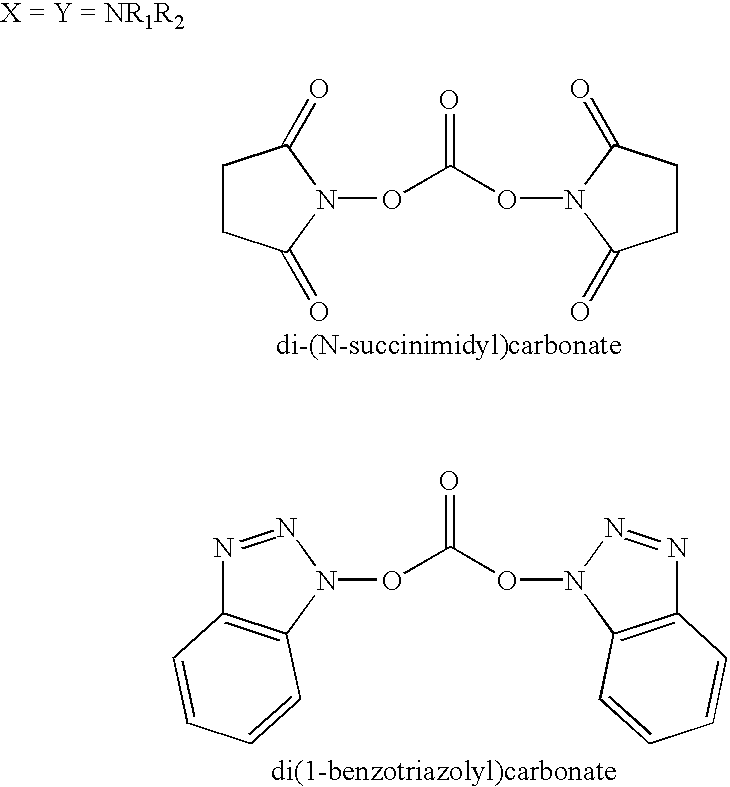
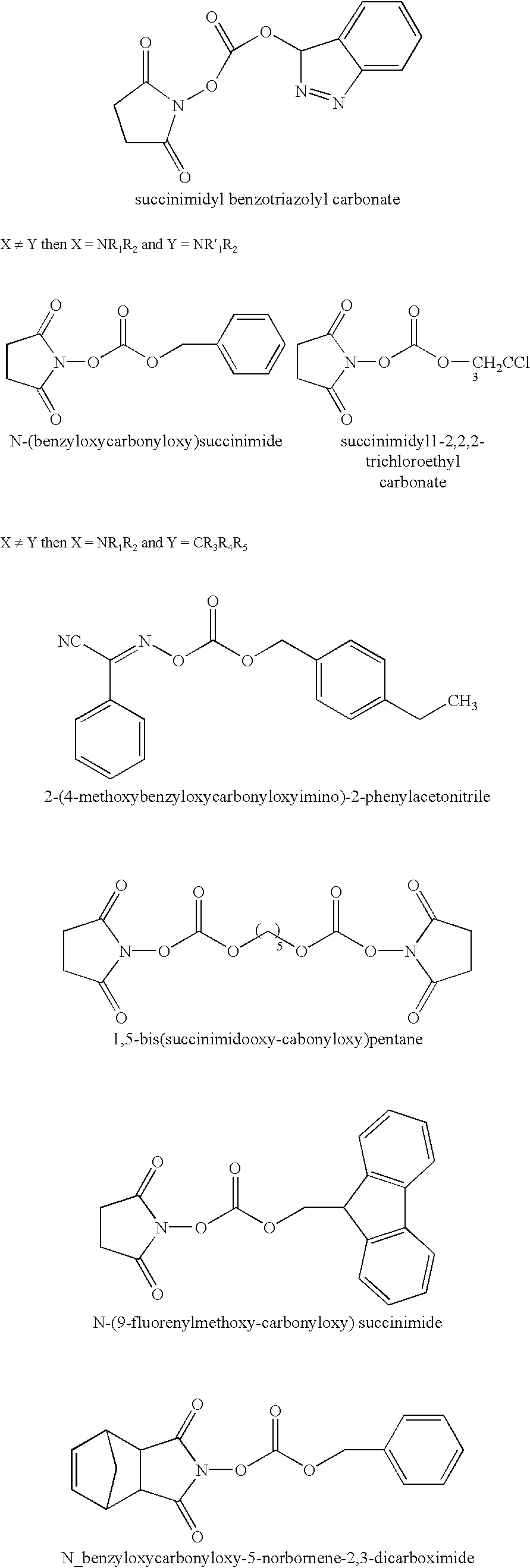
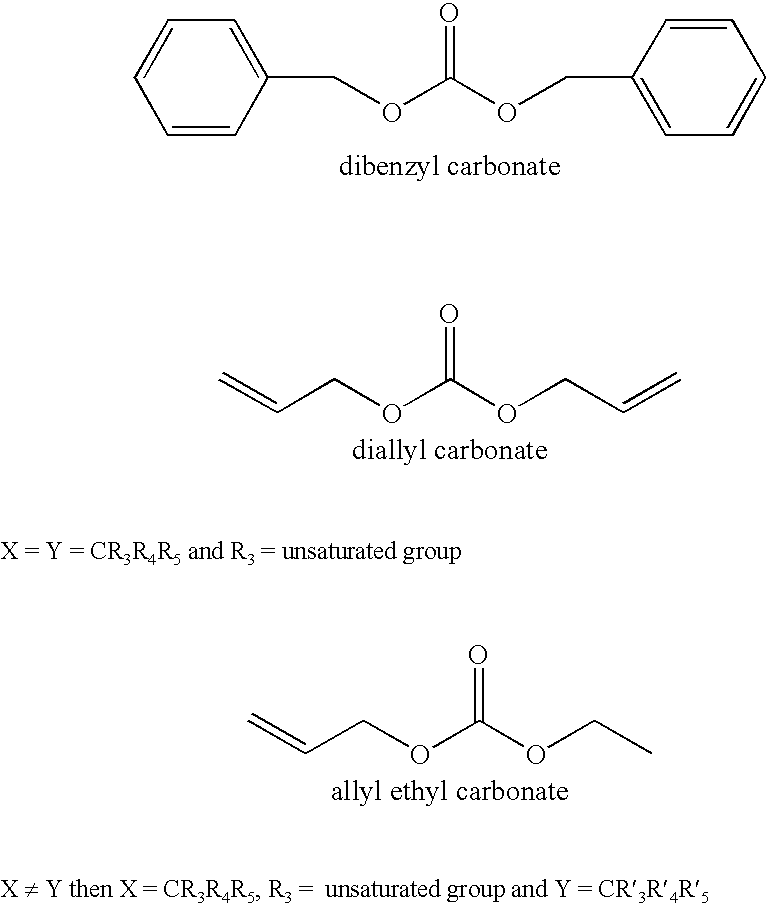
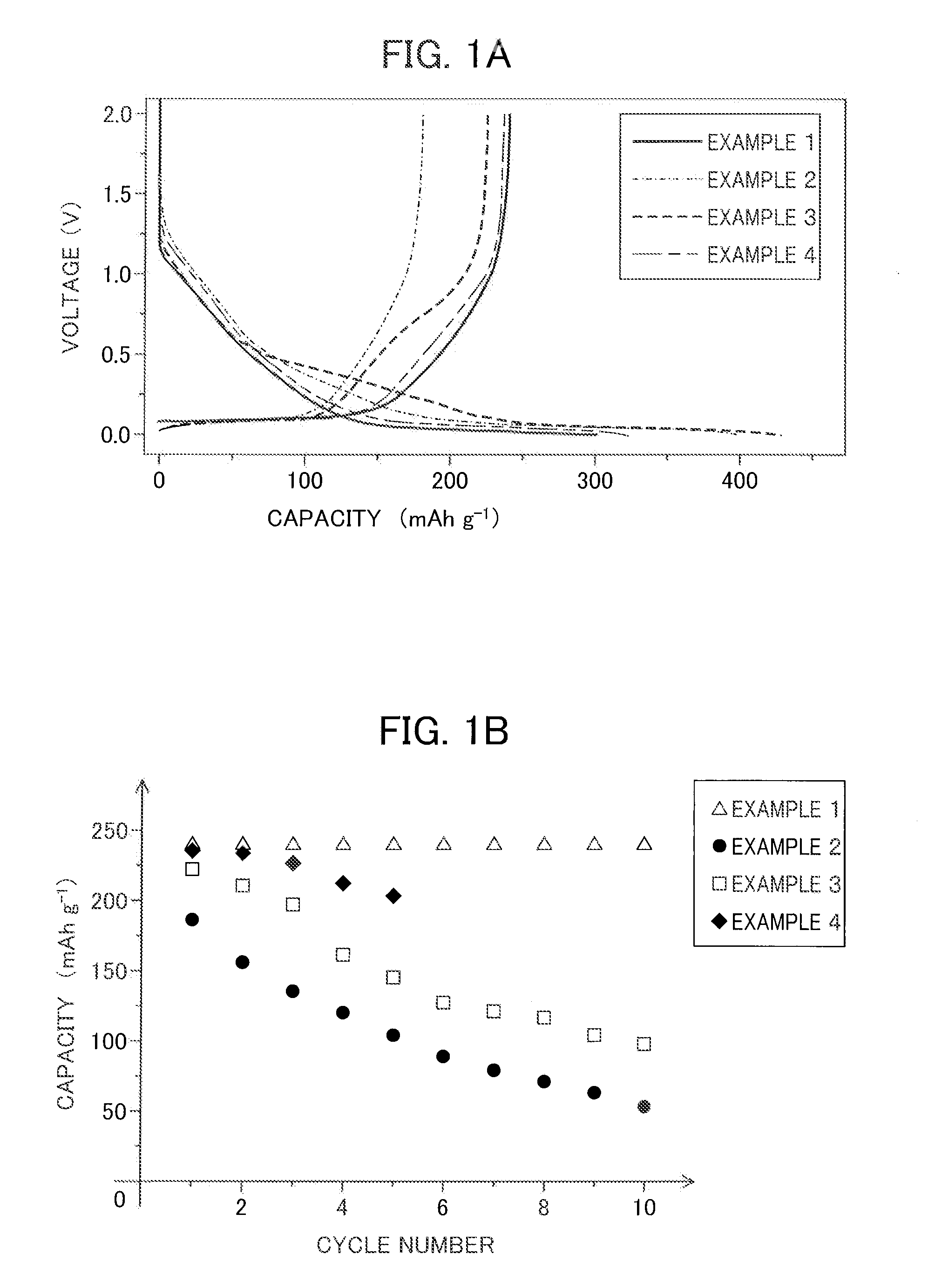
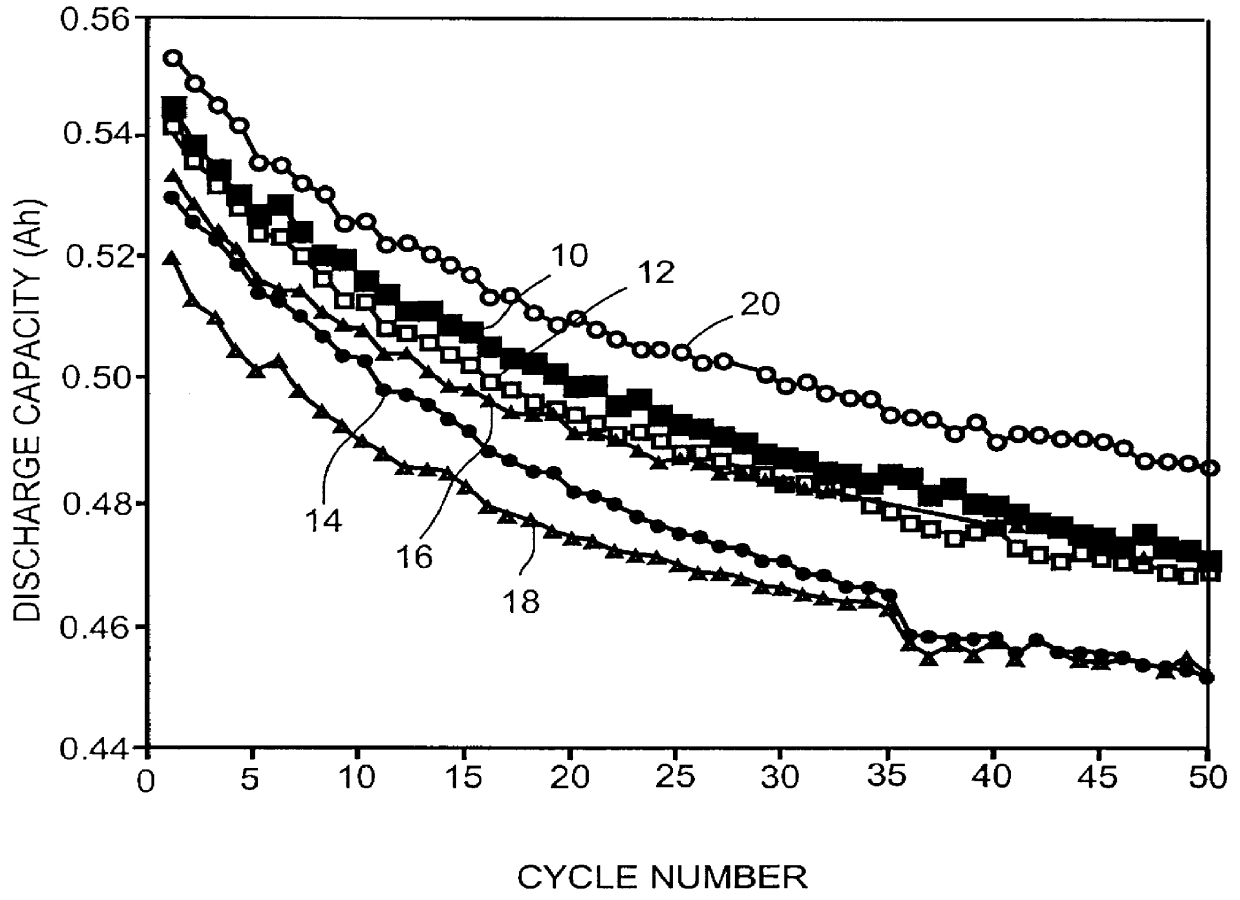
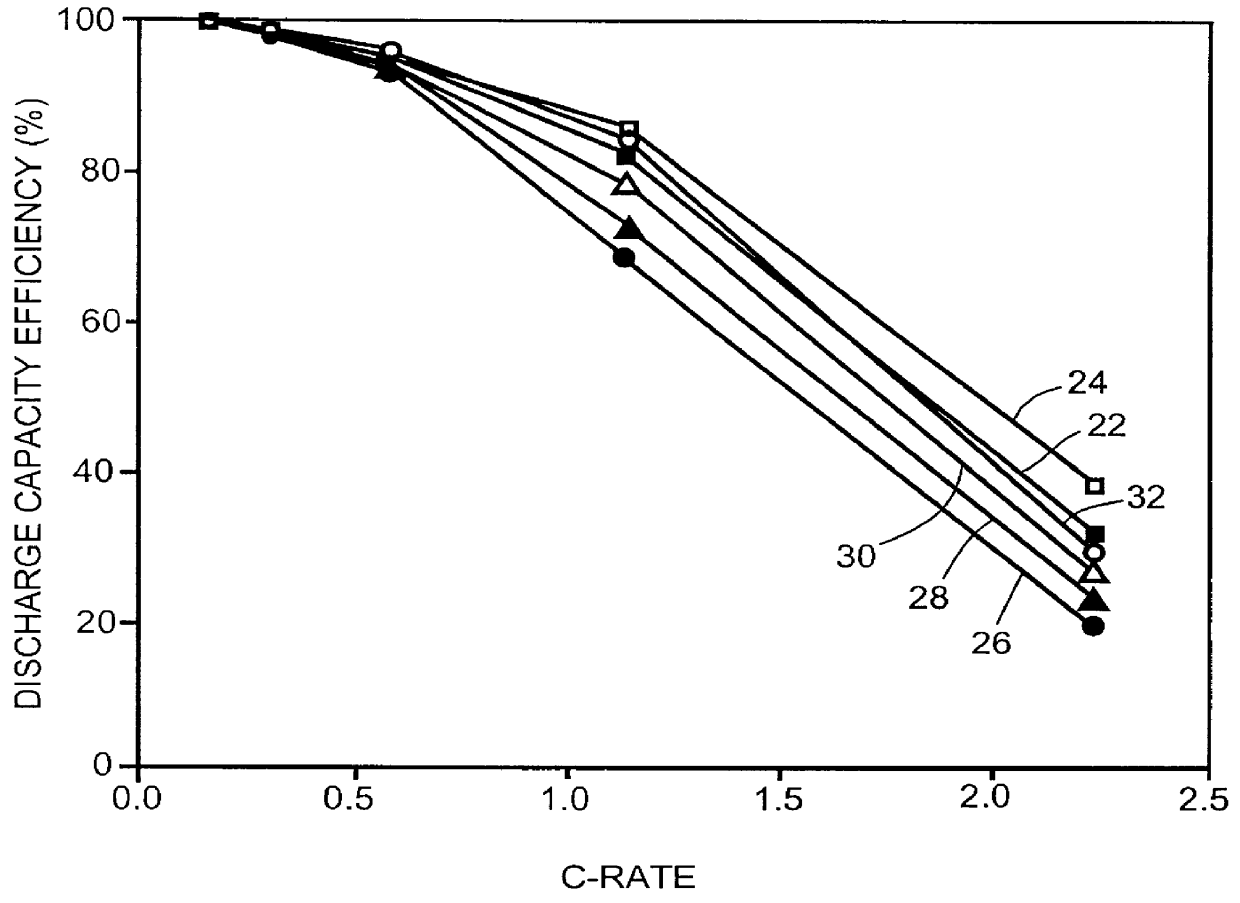
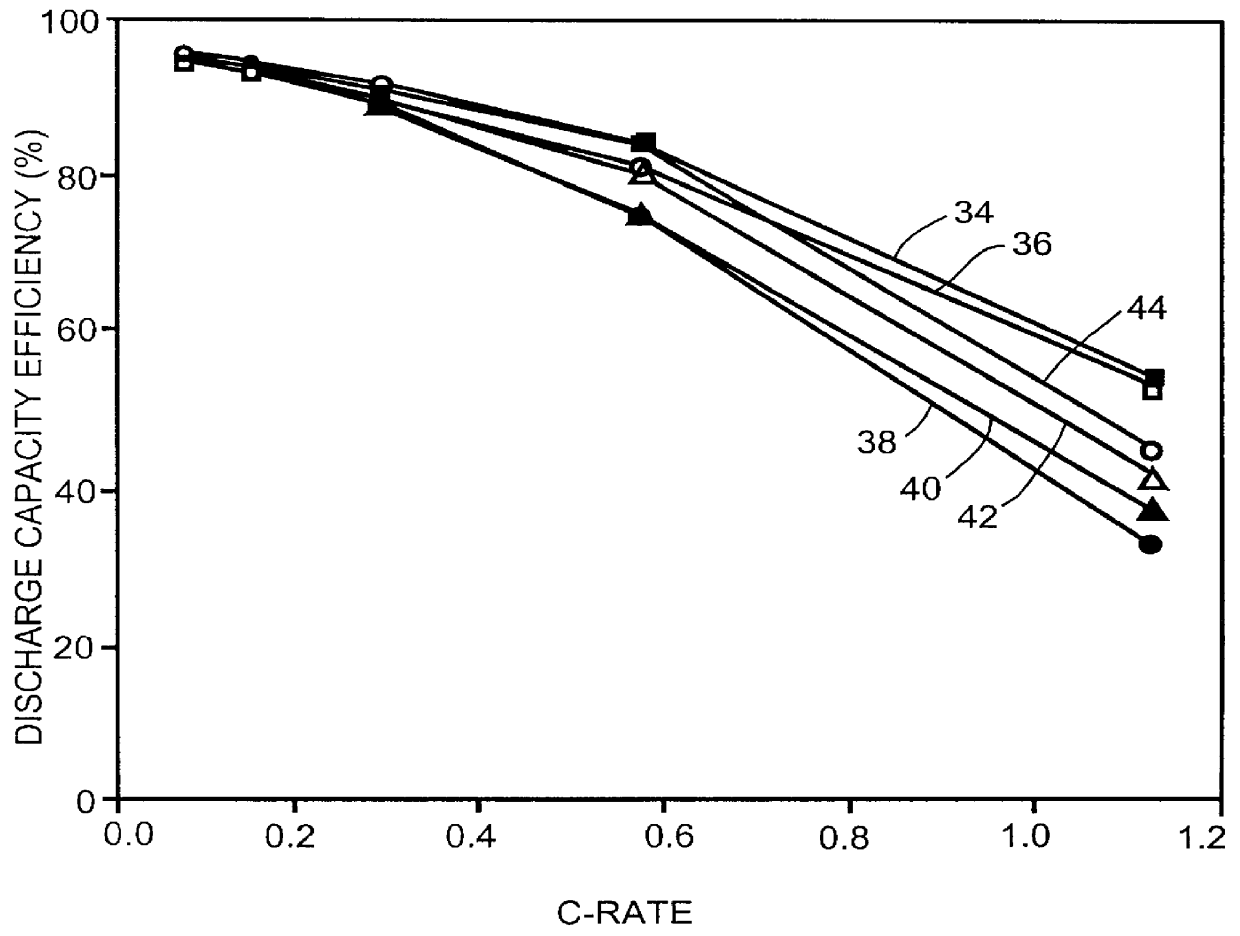
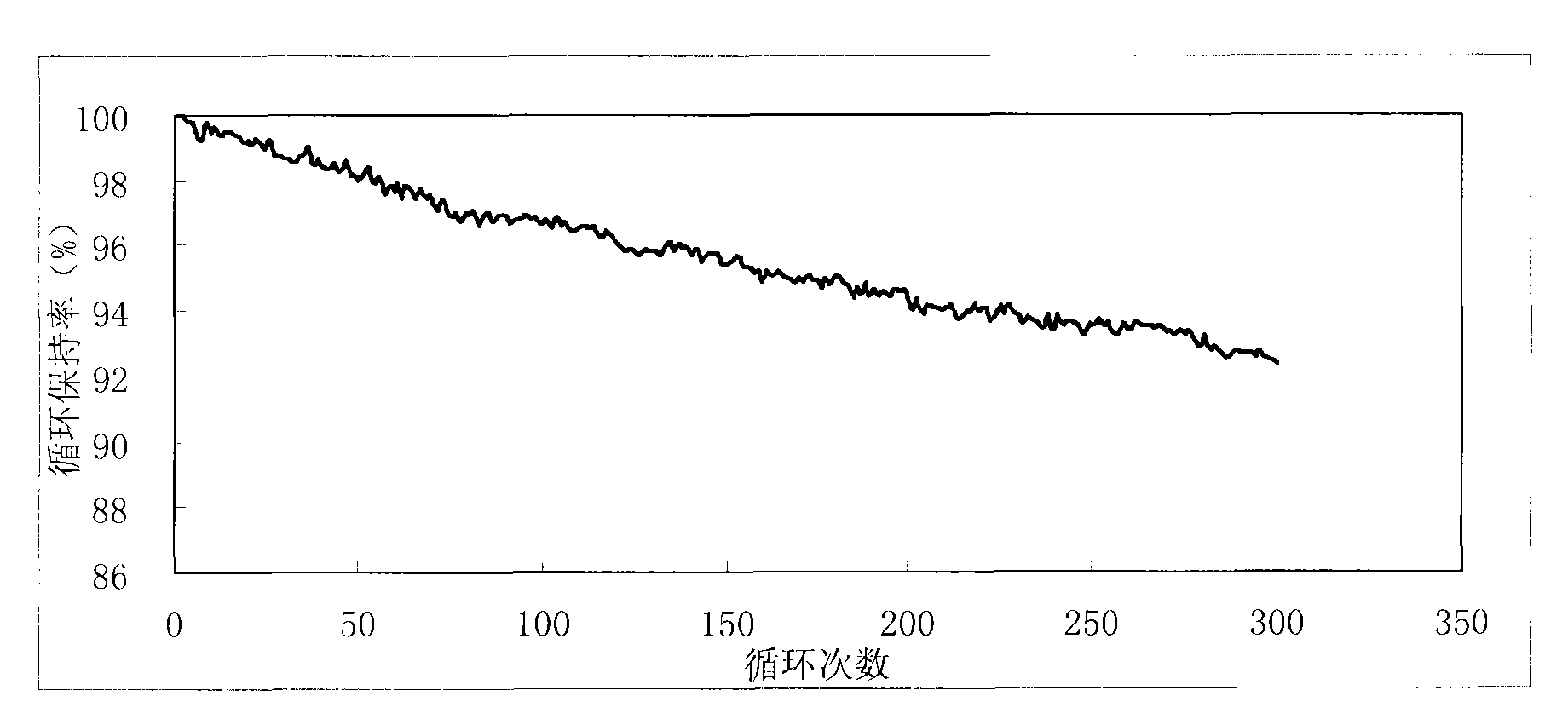
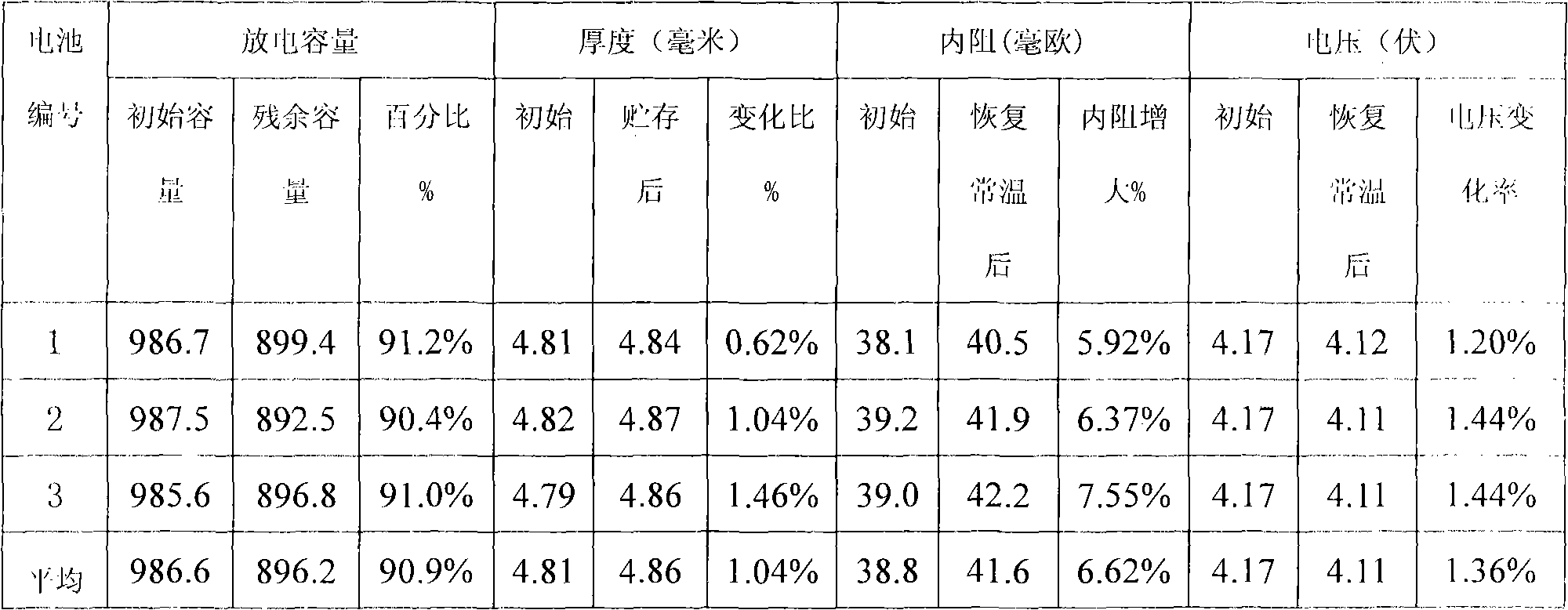
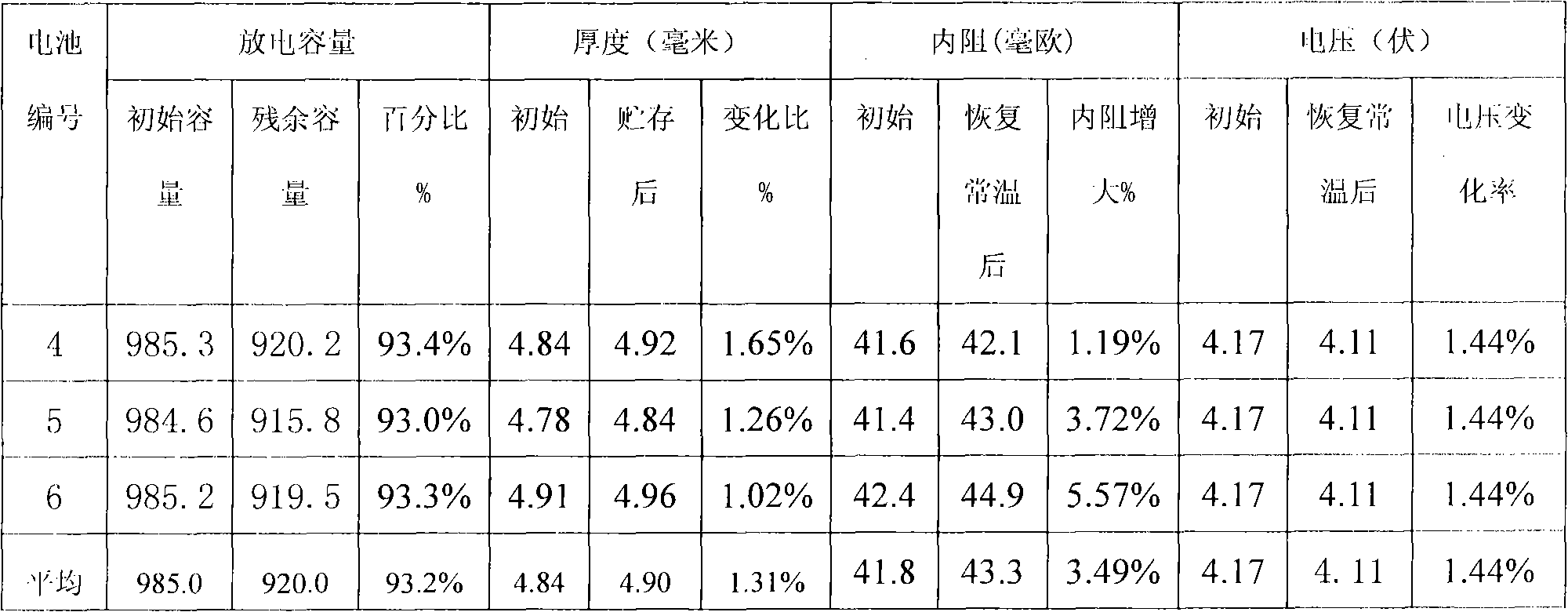
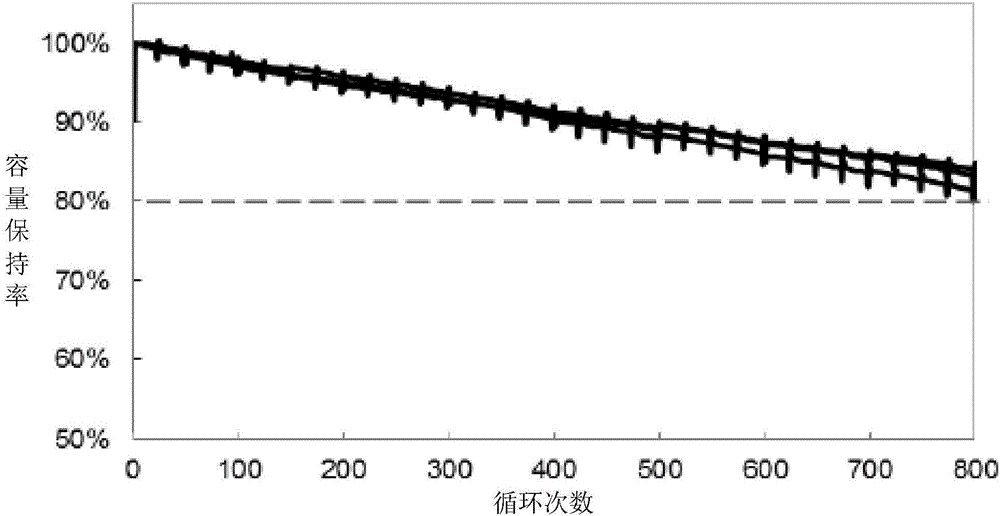
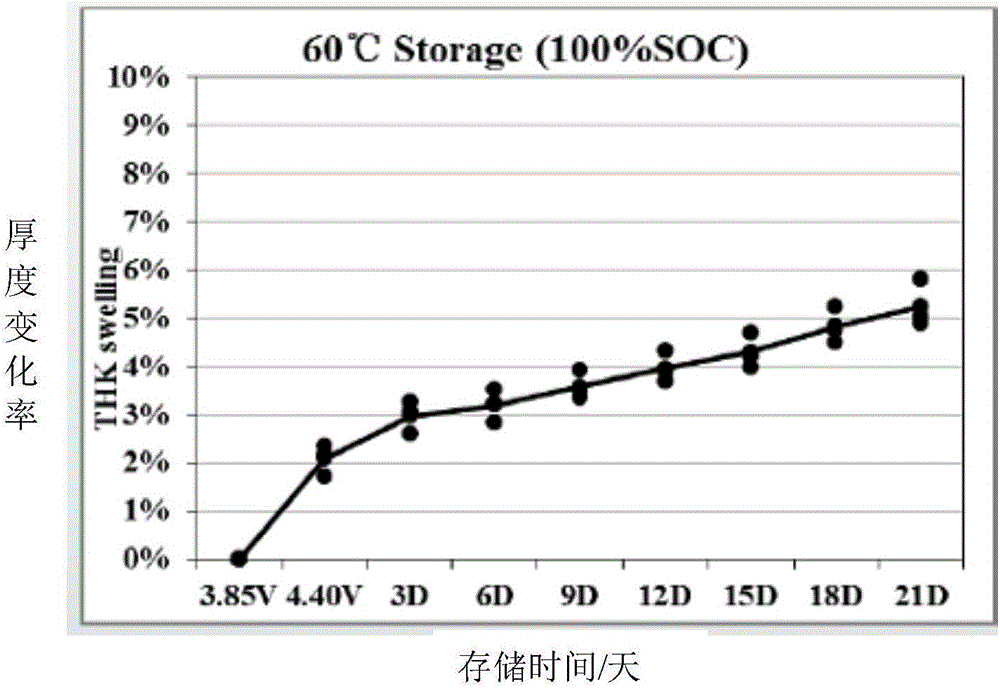
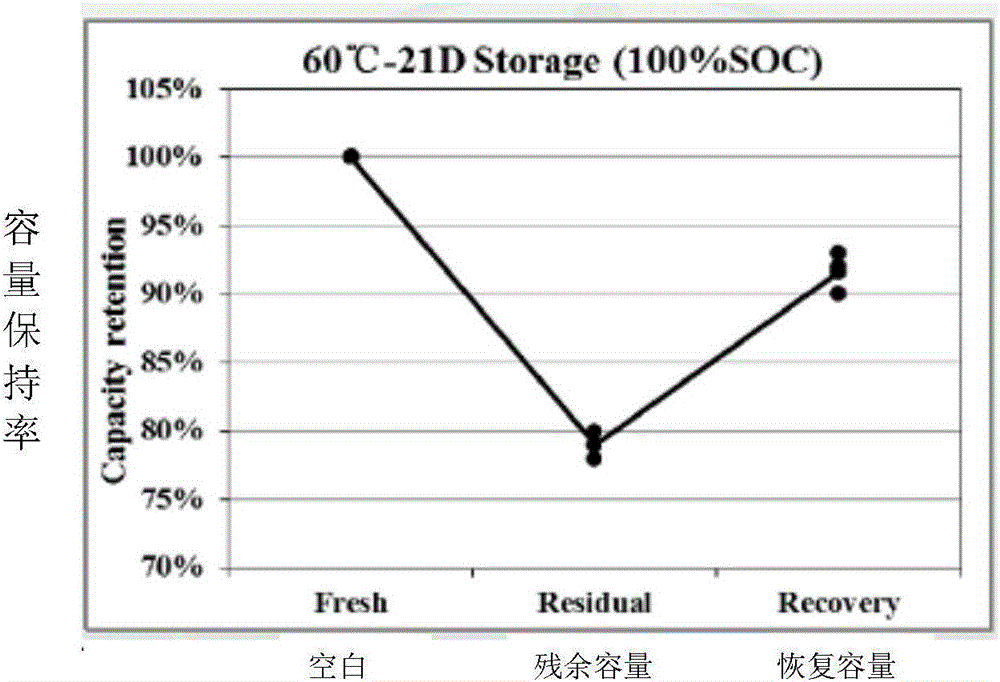
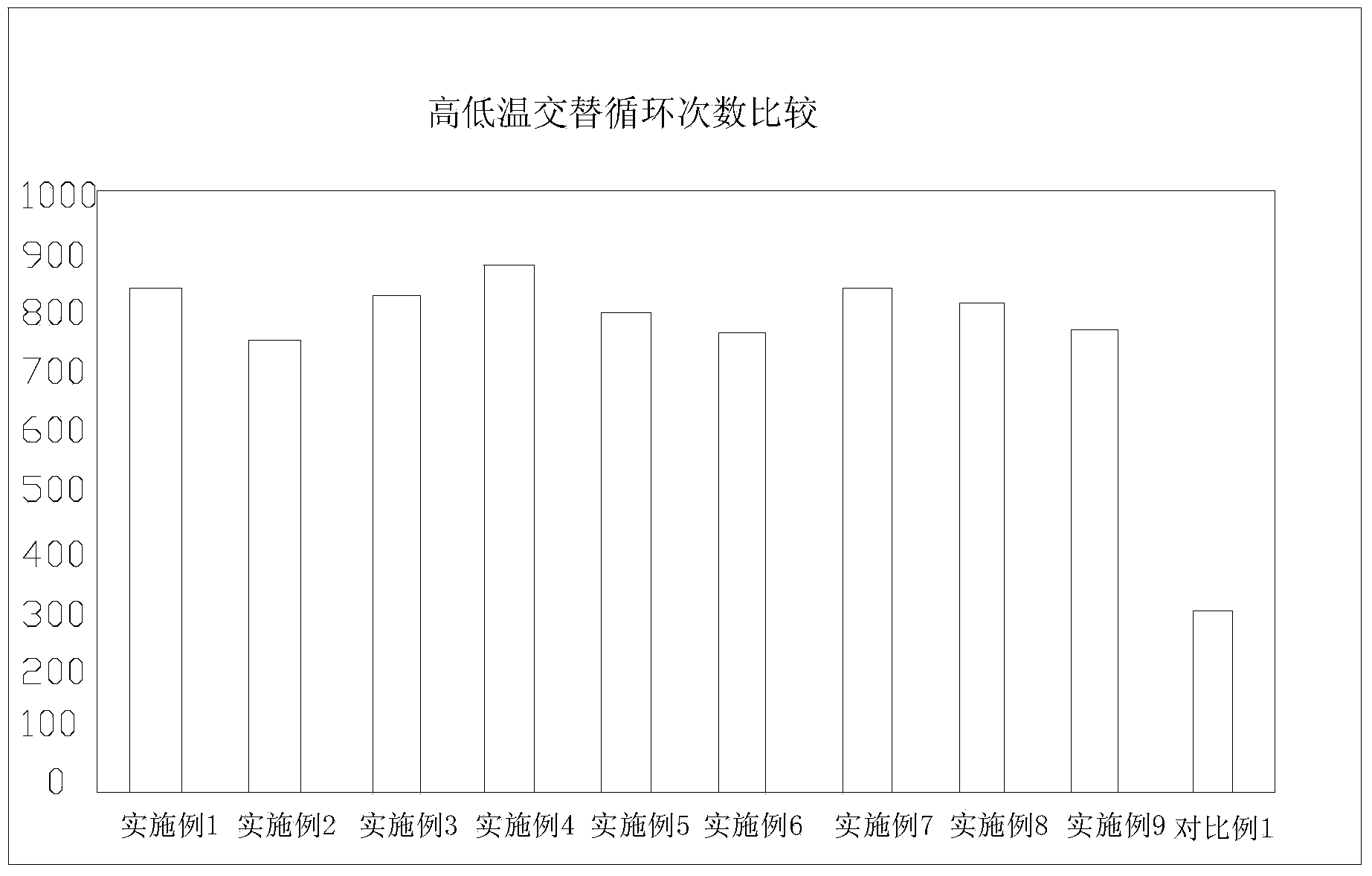
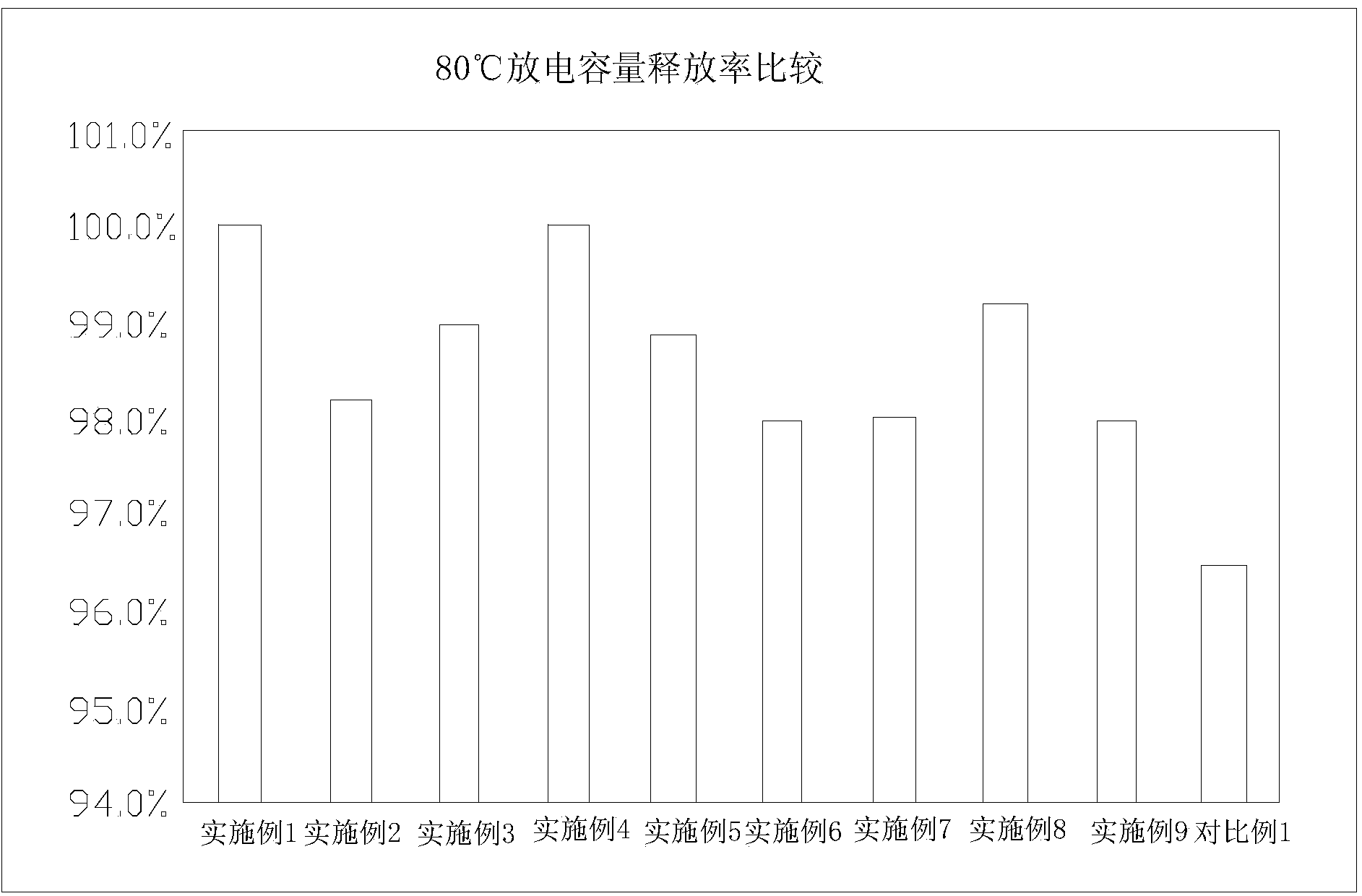
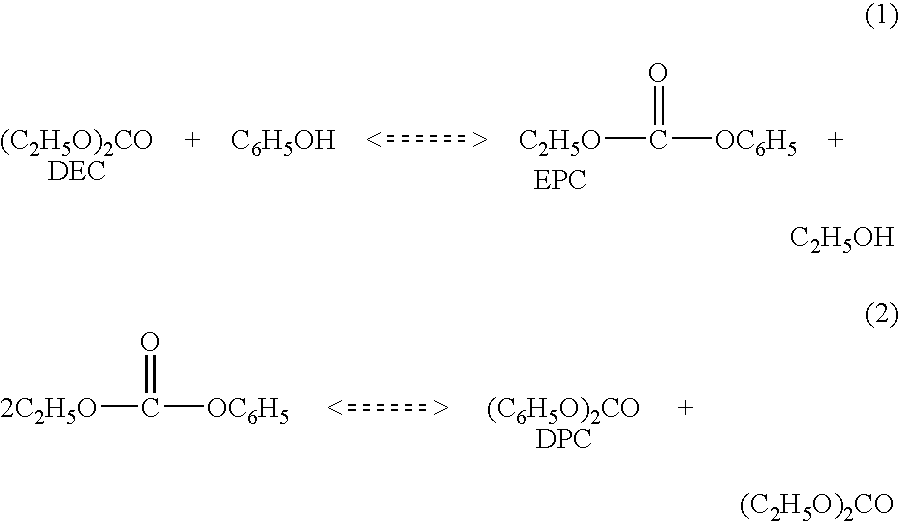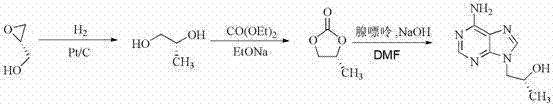Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
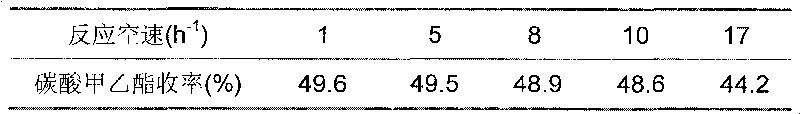
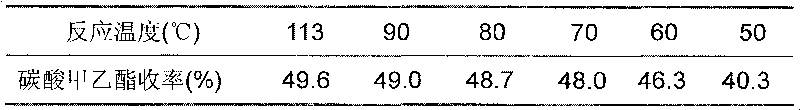
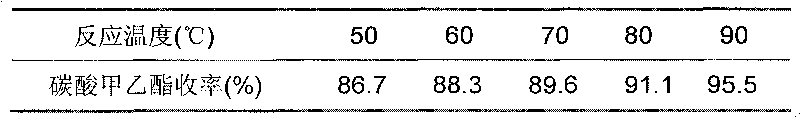
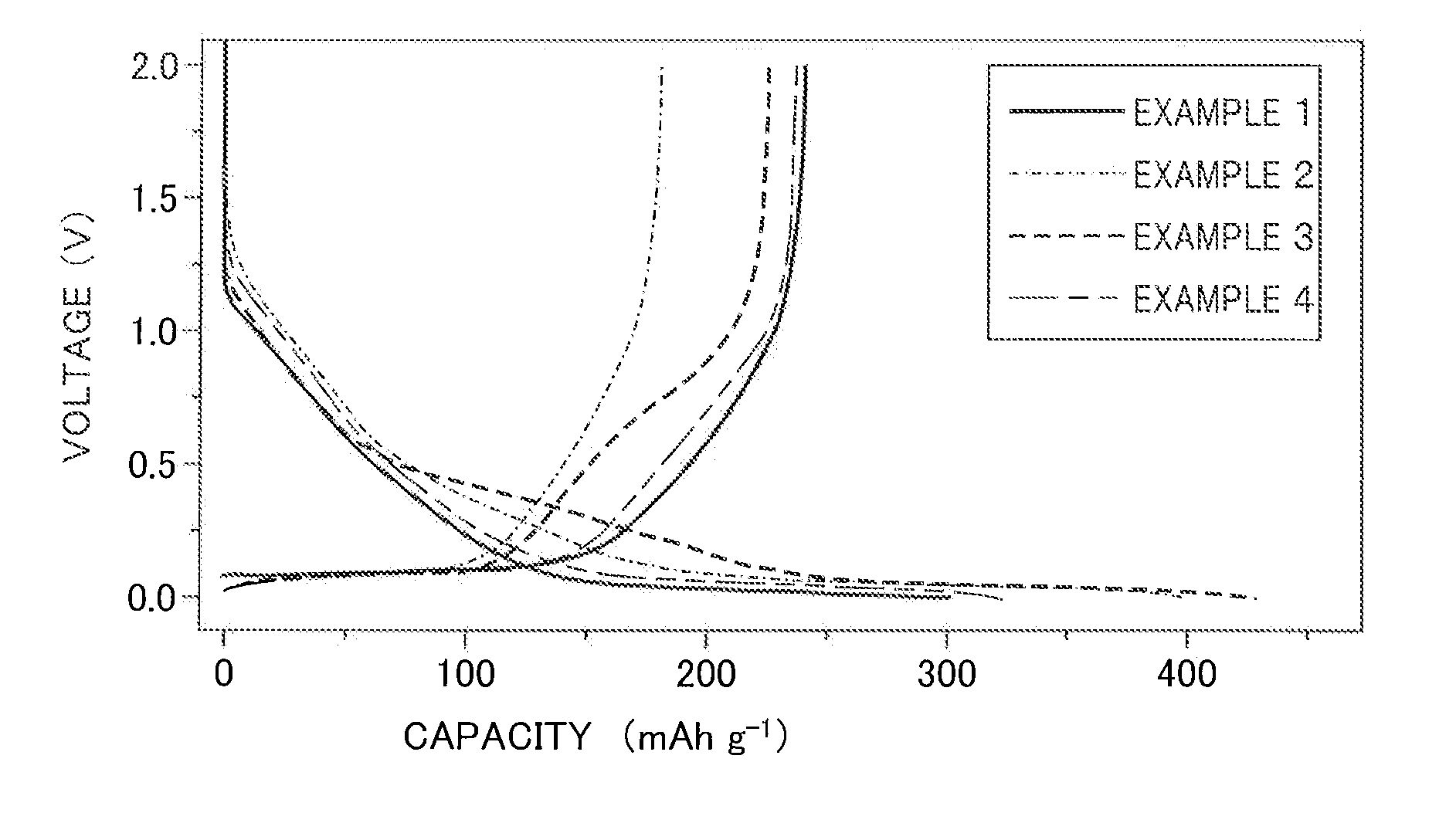
706 results about "Diethyl carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
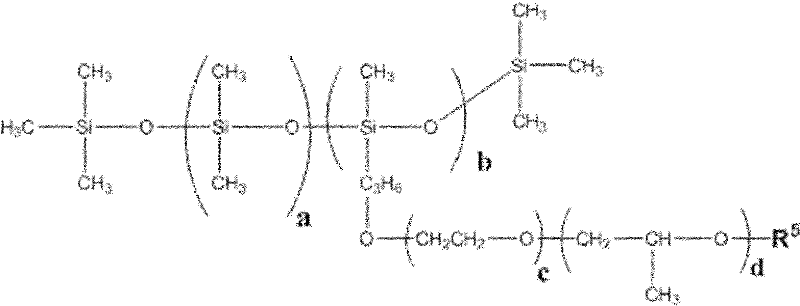
Diethyl carbonate (sometimes abbreviated DEC) is a carbonate ester of carbonic acid and ethanol with the formula OC(OCH₂CH₃)₂. At room temperature (25 °C) diethyl carbonate is a clear liquid with a low flash point.
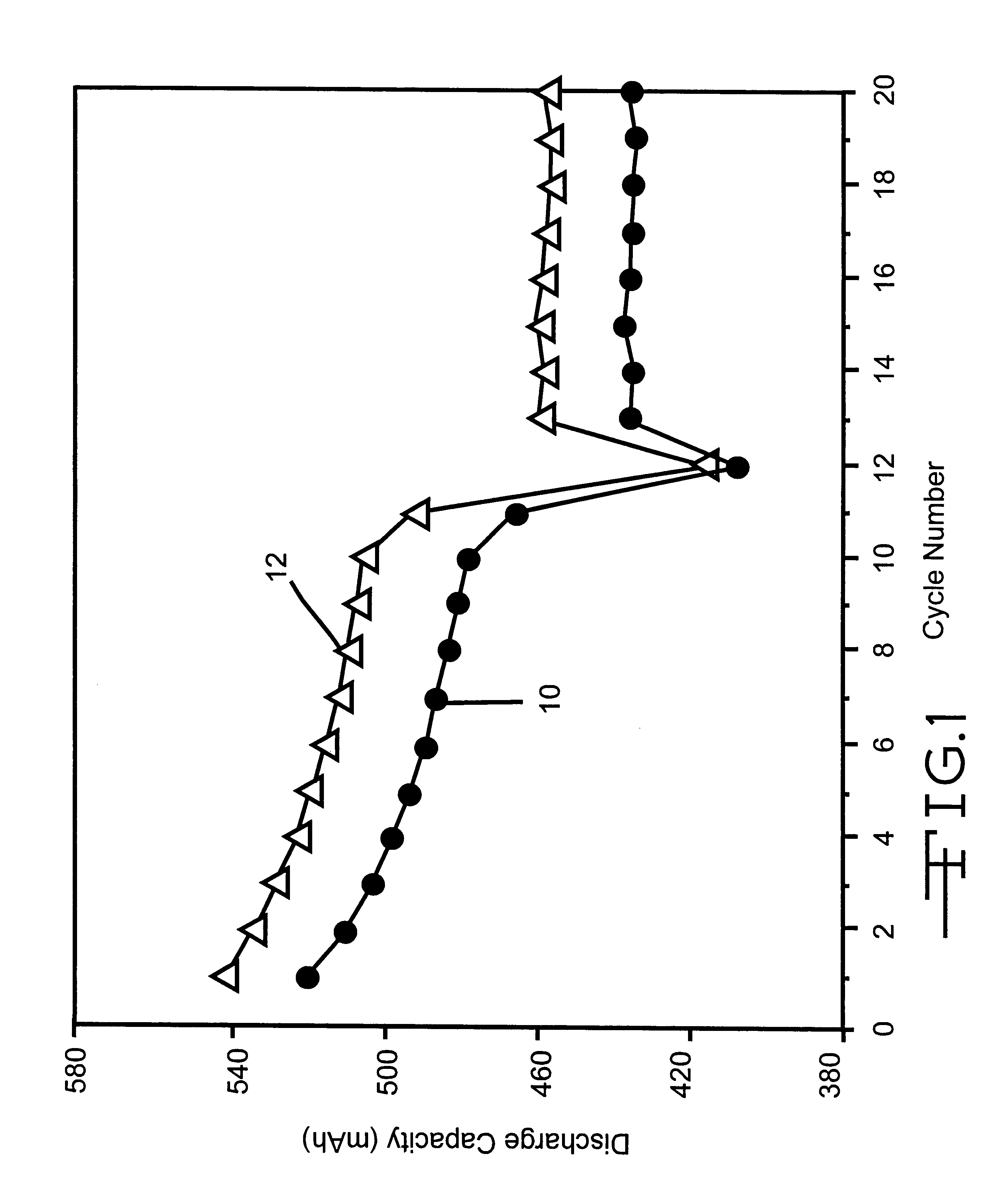
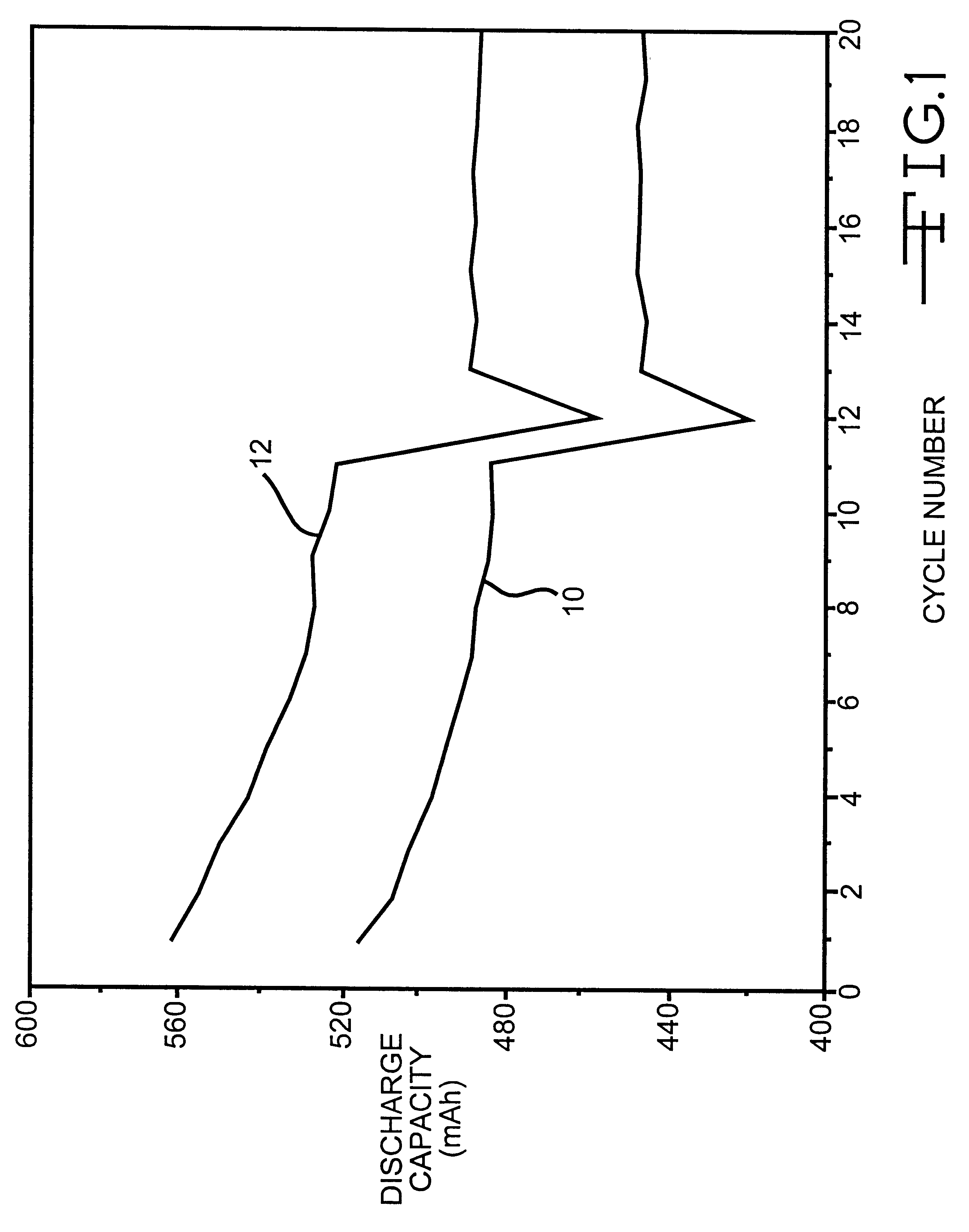
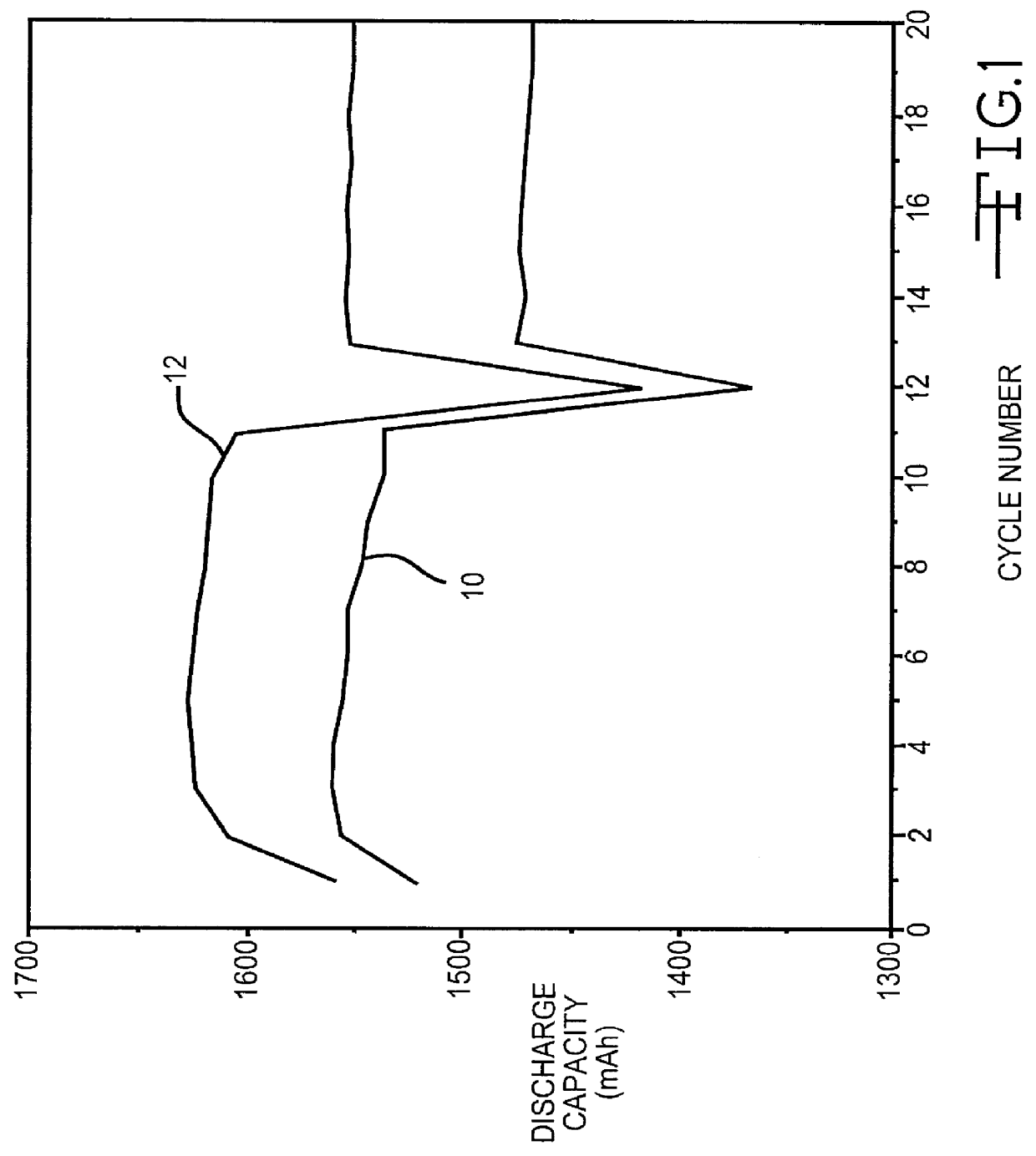
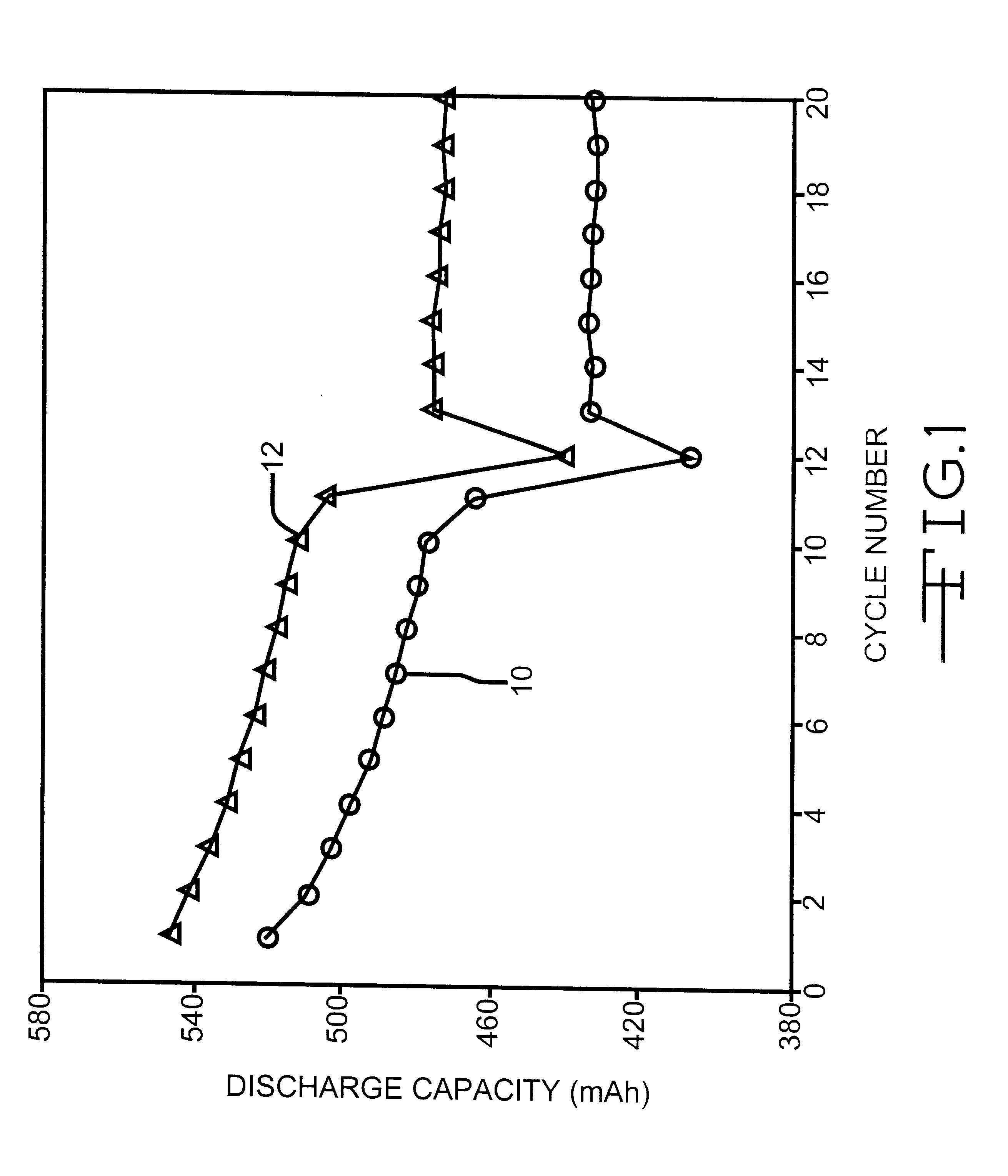
Phosphonate additives for nonaqueous electrolyte in rechargeable cells
InactiveUS6200701B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsRechargeable cellPhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphonate compound.
Owner:WILSON GREATBATCH LTD
Phosphate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6203942B1Improve oxidation stabilityImprove dynamic stabilityPrimary cell maintainance/servicingOrganic electrolyte cellsAlkylphosphateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphate compound.
Owner:WILSON GREATBATCH LTD
Nitrate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6136477AImprove oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsNitrateRechargeable cell
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an organic alkyl nitrate compound.
Owner:WILSON GREATBATCH LTD
Nitrite additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6210839B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSolid electrolyte cellsNitritePhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrite additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl nitrite compound.
Owner:WILSON GREATBATCH LTD
Nonaqueous electrolyte secondary battery
ActiveUS20060046155A1Excellent in high-temperature cycle capabilityOrganic electrolyte cellsActive material electrodesMetallic lithiumElectrode potential
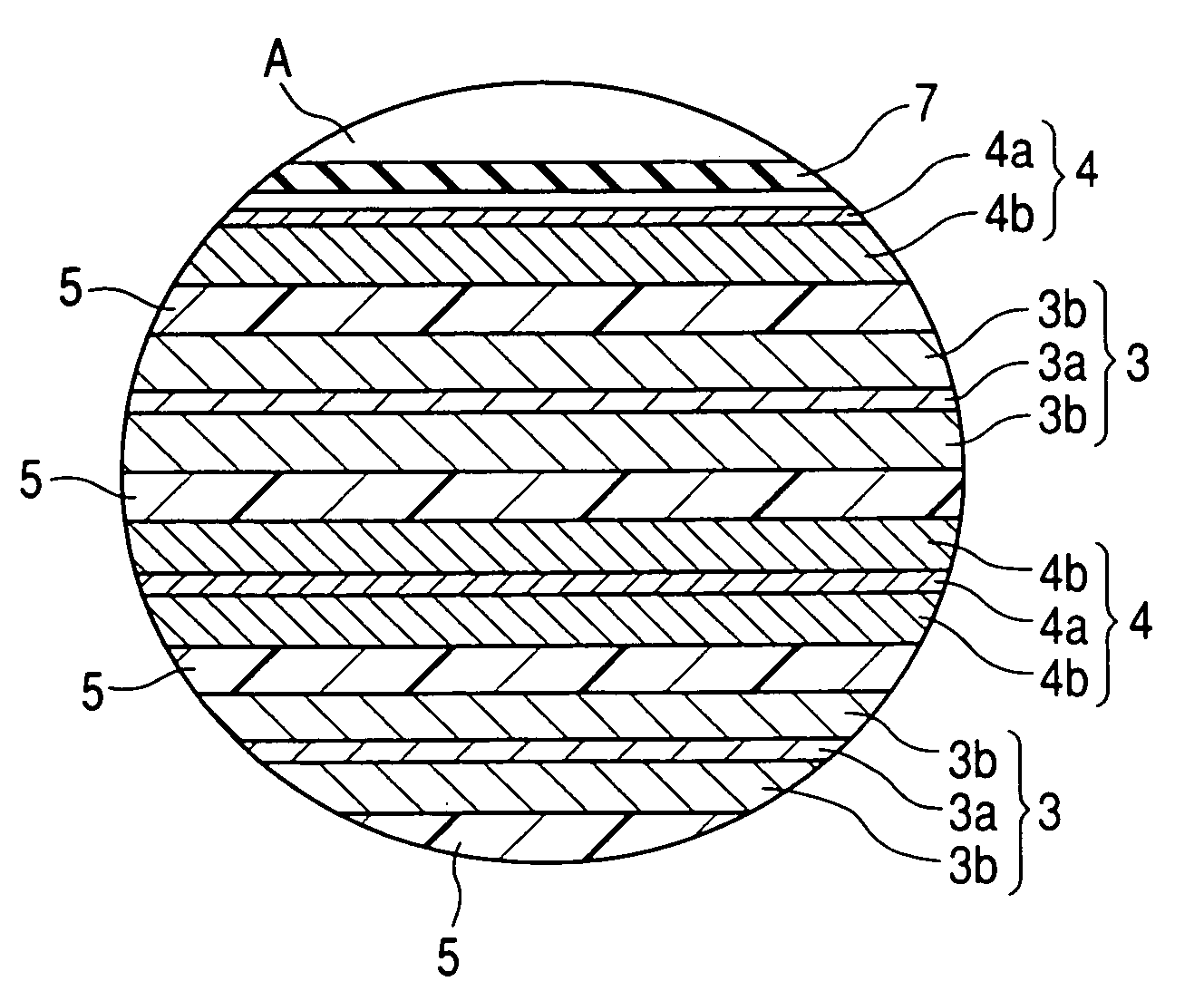
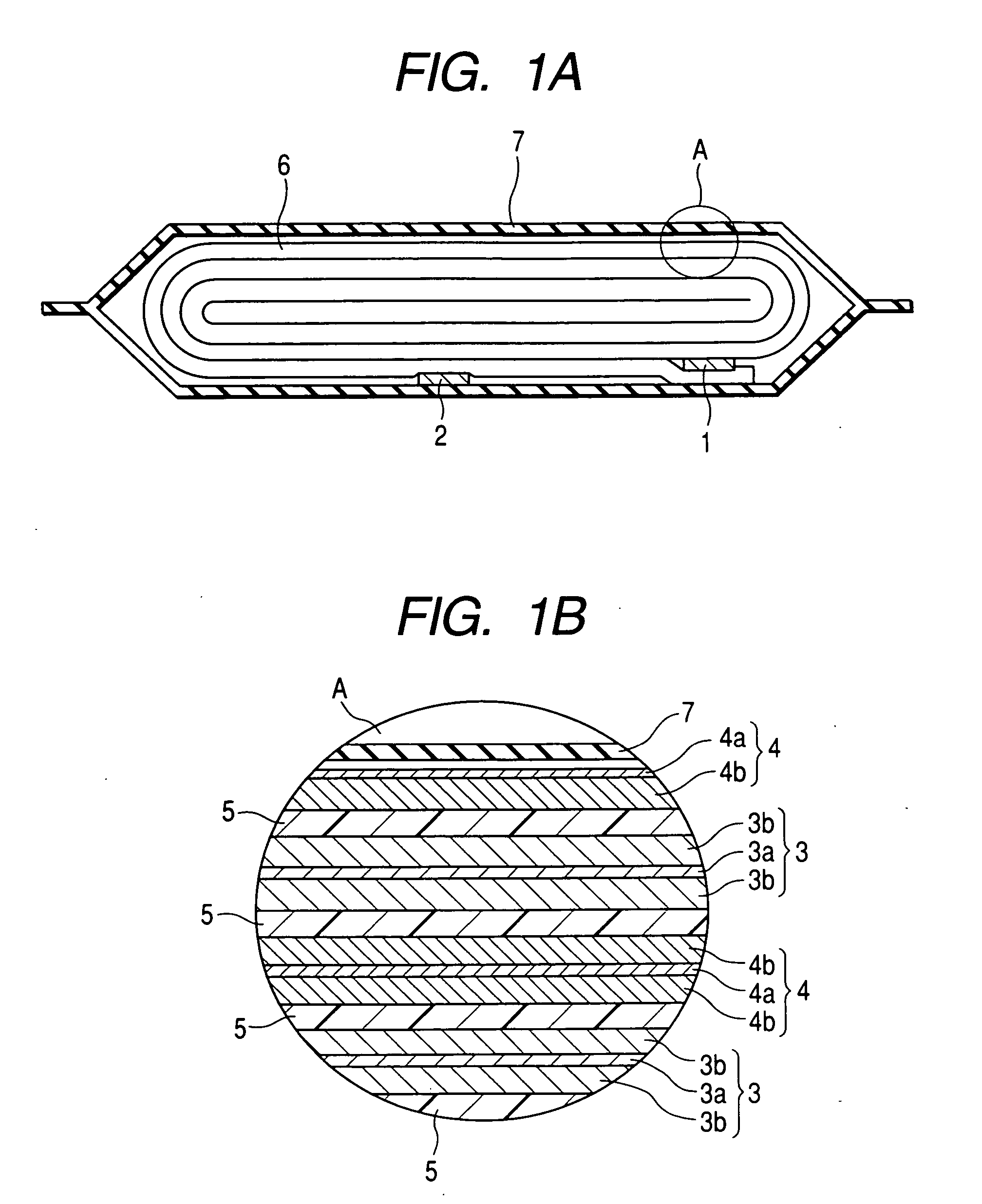
A nonaqueous electrolyte secondary battery includes: an outer housing; a nonaqueous electrolyte filled in the outer housing, a positive electrode housed in the outer housing, a negative electrode housed in the outer housing and a separator disposed between the negative electrode and the positive electrode. The nonaqueous electrolyte comprises a nonaqueous solvent including diethyl carbonate and at least one of ethylene carbonate and propylene carbonate, and the nonaqueous electrolyte has a content of the diethyl carbonate of from 80 to 95% by volume. The positive electrode comprises a positive electrode active substance having a positive electrode potential in a full charged state of 4.4 V or higher with respect to a potential of metallic lithium. The negative electrode comprises a negative electrode active substance having a negative electrode potential in a full charged state of 1.0 V or higher with respect to a potential of metallic lithium.
Owner:KK TOSHIBA
Non-aqueous electrolyte for high-voltage lithium ion batteries
ActiveCN103268956ASimple compositionPromote circulationSecondary cellsHigh voltage batteryPropylene carbonate
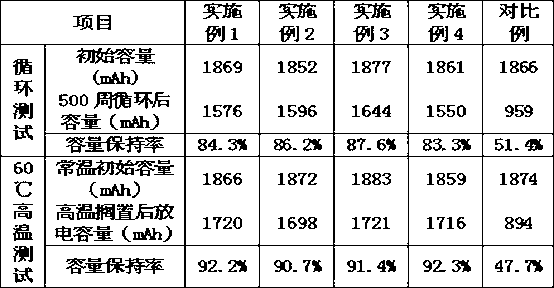
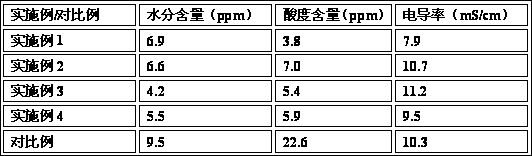
The invention relates to a non-aqueous electrolyte for high-voltage lithium ion batteries, which is prepared from the following raw materials in percentage by weight: 70-85% of carbonate, 3-20% of functional additive and 11-17% of lithium hexafluorophosphate. The carbonate is one or mixture of more of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, dipropyl carbonate, methylethyl carbonate, methyl propyl carbonate and methyl butyl carbonate; and the functional additive is one or mixture of more of 0.5-10% of negative pole film-forming additive, 0.5-10% of high-temperature additive, 0.5-10% of positive pole film-forming additive, 0.5-10% of high-voltage additive and 0.001-2% of stability additive. The invention solves the problem of adaptation of the lithium ion battery electrolyte to the 4.35V high-voltage battery positive / negative pole, and provides an electrolyte for high-voltage batteries, which has the advantages of high cycle life, low inflation rate and favorable high-temperature properties.
Owner:广东金光高科股份有限公司
Lithium-ion secondary battery and electrolyte thereof
InactiveCN103078141AFacilitated DiffusionImproved magnification performanceSecondary cellsNon-aqueous electrolyte accumulator electrodesMethyl carbonateCarbonate
The invention discloses a lithium-ion secondary battery and an electrolyte thereof. The electrolyte comprises a solvent, lithium salt and a film forming additive, wherein the solvent comprises a first solvent and a second solvent; the first solvent comprises linear carboxylic ester and ethylene carbonate; the second solvent is one or more of ethyl methyl carbonate, diethyl carbonate, dimethyl carbonate and propylene carbonate; and the film forming additive is one or more of fluoroethylene carbonate, vinylene carbonate, 1,3-propane suhone, succinonitrile, adiponitrile, lithium bis(oxalato)borate, and lithium oxalyldifluoroborate. Due to the collocation of linear carboxylic ester and ethylene carbonate, a solvent system with a higher dielectric constant and low viscosity is obtained; the film forming additive improves poor compatibility between linear carboxylic ester and graphite; and finally the lithium-ion secondary battery adopting the electrolyte presents high-power discharge capacity, excellent high-temperature cycling stability and low-temperature charge and discharge properties.
Owner:NINGDE AMPEREX TECH
Rechargeable lithium electrochemical cell
InactiveUS6942949B2Organic electrolyte cellsNon-aqueous electrolyte accumulator electrodesMethyl carbonatePhosphate
A secondary battery is comprised of a positive electrode, a negative electrode formed from a lithium storage material, and a non-aqueous electrolyte. The non-aqueous electrolyte includes a lithium salt, non-aqueous aprotic solvent(s), such as ethylen carbonate, propylene carbonate, dimethyl carbonate, ethymethyl carbonate and diethyl carbonate, and a small percentage of at least one organic additive. The negative electrode may comprise a carbon such as graphite, and the positive electrode may comprise a lithiated metal oxide or phosphate, such as LiCoO2, LiNiO2, LiMn2O4, LiFePO4, or mixtures thereof. The organic additives have one or more unsaturated bonds activated with respect to oxidation by electron-pushing alkyl groups. They are in most cases known to be able to undergo polymerization reactions, such as an anodically induced polymerization especially under certain conditions. The additives are oxidized at the cathode at a potential of more than 4.3 V vs. Li / Li+. With these additives in amounts of 0.001 to 10%, a passivation layer is formed on the cathodes, and the sensitiveness of the battery against overcharge is reduced. The electrolyte mixtures do not deteriorate the properties of the battery anodes.
Owner:LG ENERGY SOLUTION LTD
Binder composition for secondary battery positive electrode, slurry composition for secondary battery positive electrode, secondary battery positive electrode, and secondary battery
ActiveUS20150050554A1Improve stabilityImprove smoothnessFinal product manufactureSecondary cellsCarbon numberSlurry
Provided are a binder composition which has high electrolyte resistance characteristics, and a secondary battery which uses a positive electrode using the binder composition for high-temperature cycle characteristics. [Solution] The binder composition for the secondary battery positive electrode according to the present invention contains a polymerized unit which contains a nitrile group; a polymerized unit of (meth) acrylic acid ester; a polymerized unit which contains a hydrophilic group; and a polymerized unit of linear alkylene having a carbon number of at least four. In a mixed solvent in which a volume ratio EC:DEC between ethylene carbonate (EC) and diethyl carbonate (DEC) at 20° C. is 1:2, a degree of swelling with respect to an electrolyte in which LiPF6 is dissolved to have a concentration of 1.0 mol / L is between 100% and 500%.
Owner:ZEON CORP
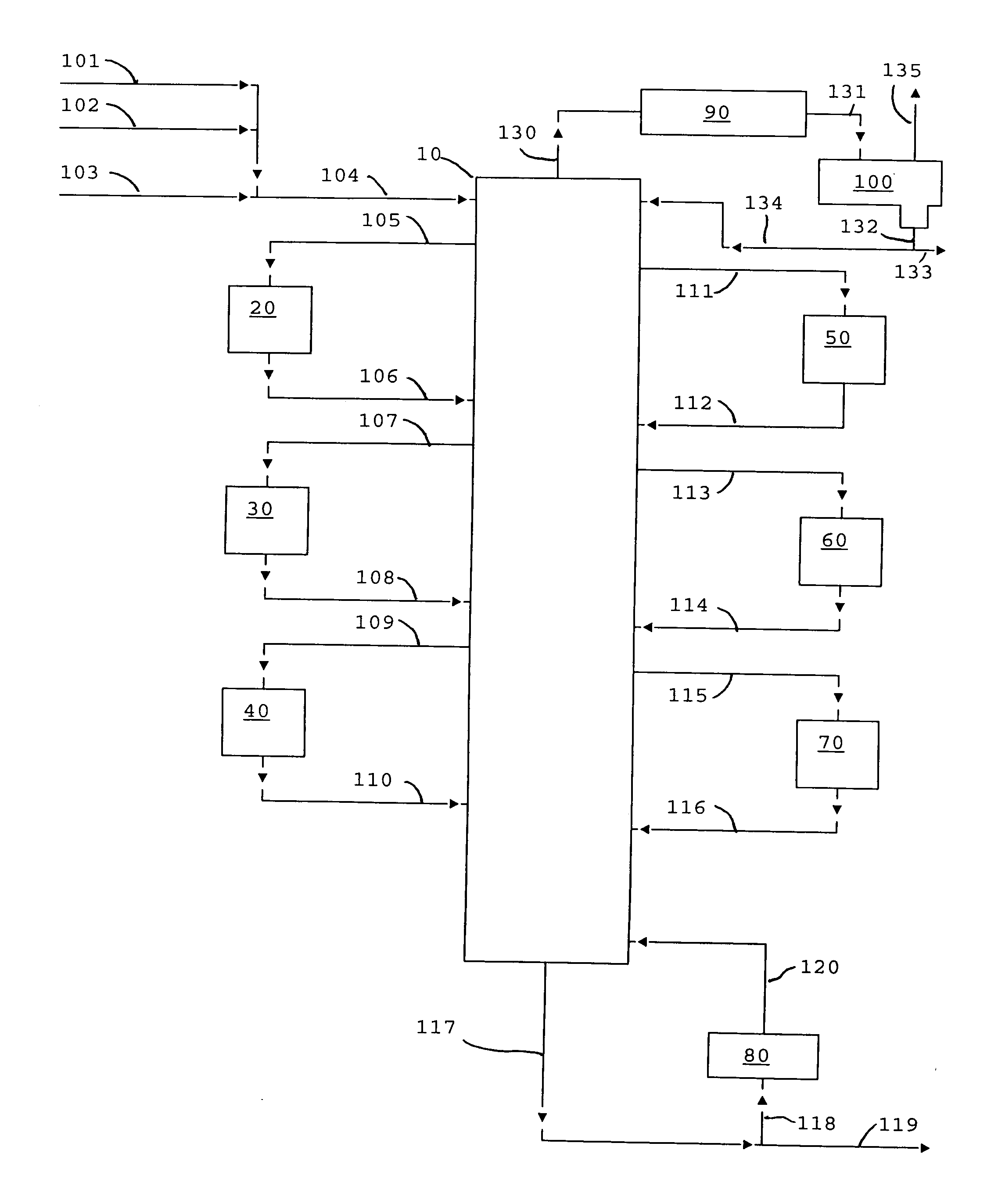
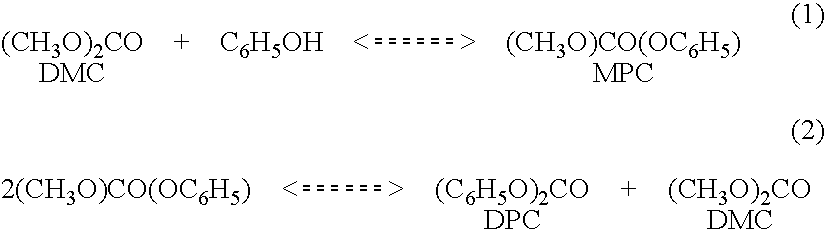
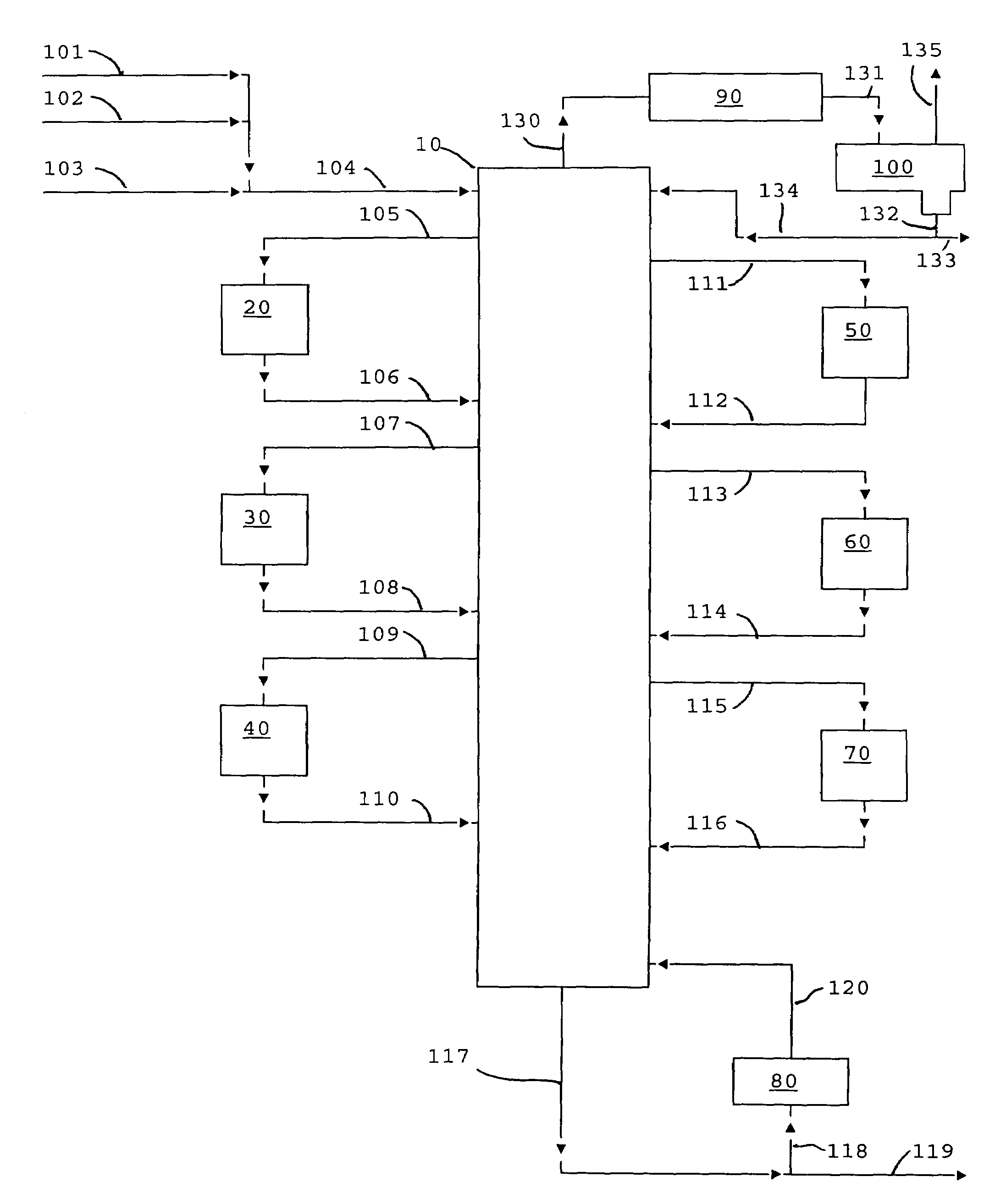
Process for making diaryl carbonate
Diphenyl carbonate is produced by reacting phenol with diethyl carbonate in a series of fixed bed reactors each of which is connected at different position on a distillation column via side draw and return streams. The composition of material in a distillation column varies along the length of the column, which is predictable under a given set of conditions of temperature and pressure, thus withdrawing streams at different stages in the column, allows the reactor receiving the feed from a particular stage to be operated under conditions to maximize the desired reaction, while allowing the unreacted or byproduct to go back into the distillation and be sent to a stage (by the equilibrium of the distillation) where they are favorably treated in a reactor.
Owner:SHELL USA INC
Non-flammable Quasi-Solid Electrolyte and Lithium Secondary Batteries Containing Same
PendingUS20180277913A1Reduce electrical conductivityLow ionic conductivityFuel and secondary cellsCell electrodesSolid state electrolyteCelsius Degree
A rechargeable lithium cell comprising a cathode, an anode, a non-flammable quasi-solid electrolyte containing a lithium salt dissolved in a mixture of a liquid solvent and a liquid additive having a salt concentration from 1.5 M to 5.0 M so that said electrolyte exhibits a vapor pressure less than 0.01 kPa, a vapor pressure less than 60% of the vapor pressure of the liquid solvent alone, a flash point at least 20 degrees Celsius higher than the flash point of the liquid solvent alone, a flash point higher than 150° C., or no flash point, wherein the liquid additive is selected from Hydrofluoro ether (HFE), Trifluoro propylene carbonate (FPC), Methyl nonafluorobutyl ether (MFE), Fluoroethylene carbonate (FEC), Tris(trimethylsilyl)phosphite (TTSPi), Triallyl phosphate (TAP), Ethylene sulfate (DTD), 1,3-propane sultone (PS), Propene sultone (PES), Diethyl carbonate (DEC), Alkylsiloxane (Si—O), Alkylsilane (Si—C), liquid oligomeric silaxane (—Si—O—Si—), Tetraethylene glycol dimethylether (TEGDME), or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Dicarbonate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6174629B1Improve oxidation stabilityImprove dynamic stabilityAlkaline accumulatorsOrganic electrolyte cellsRechargeable cellPhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one dicarbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl dicarbonate compound.
Owner:WILSON GREATBATCH LTD
Non-aqueous electrolytic solution and non-aqueous electrolyte battery
InactiveCN101454938AIncrease capacityImprove performancePrimary cellsLi-accumulatorsHigh voltage batteryCarbonate
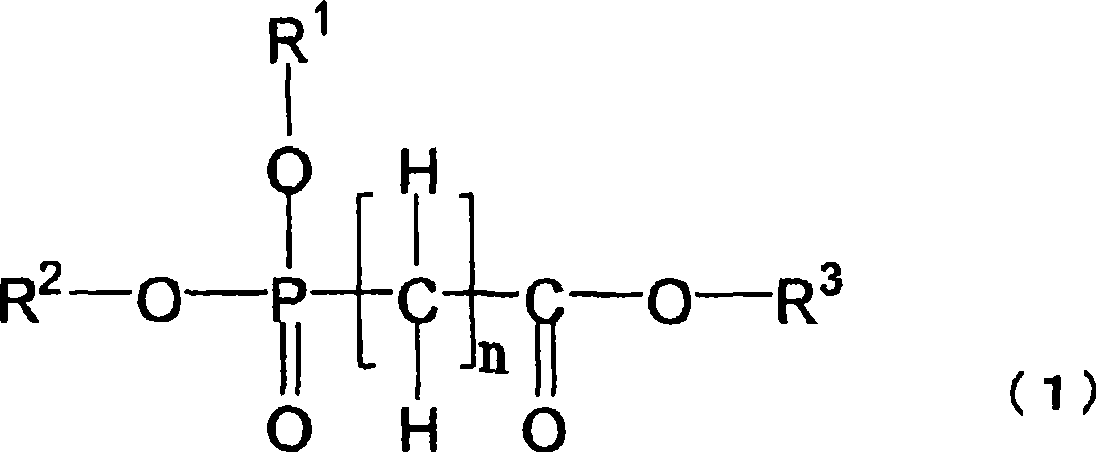
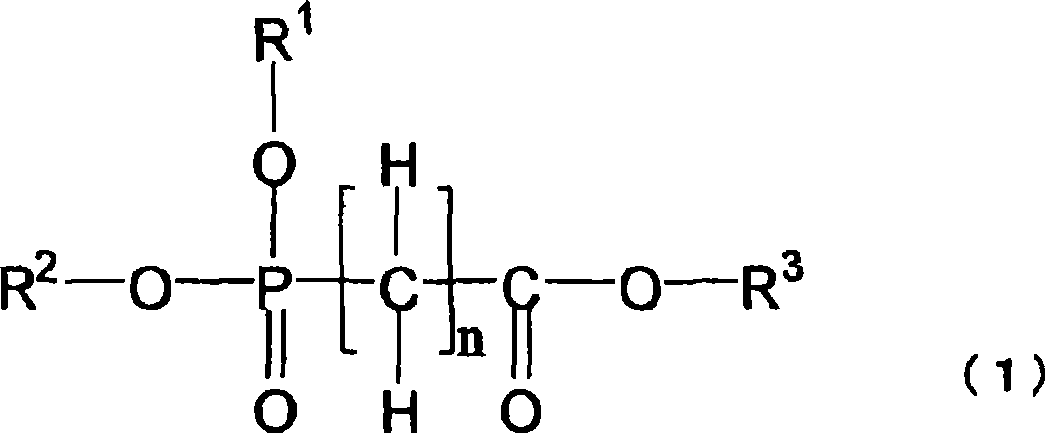
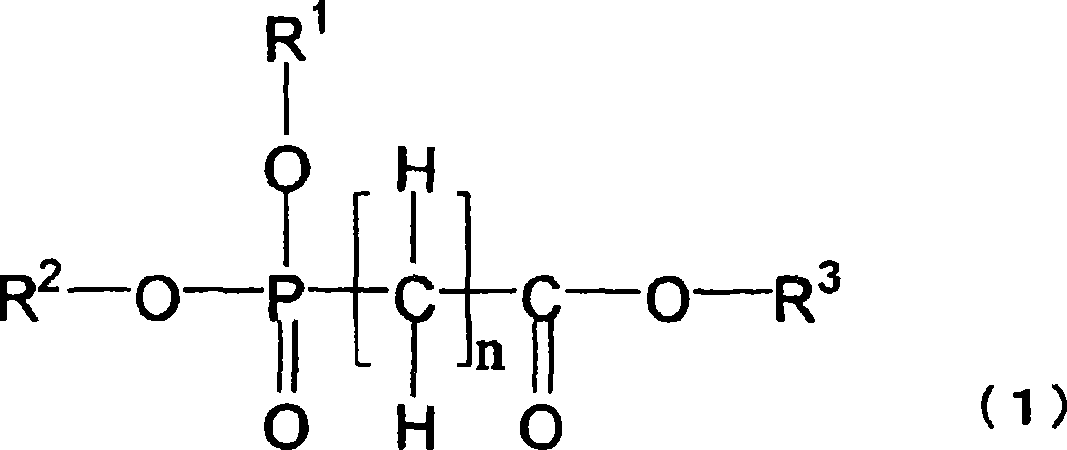
Nonaqueous electrolytes capable of providing a battery having a high capacity and excellent in storability and cycle characteristics are provided. Also provided are nonaqueous-electrolyte batteries produced with the electrolytes. The nonaqueous electrolytes include ones which (1) contains a cyclic carbonate having an unsaturated bond and further contains a fluorinated cyclic carbonate having two or more fluorine atoms, (2) contains an aromatic compound having 7-18 carbon atoms in total and further contains a fluorinated cyclic carbonate having two or more fluorine atoms, (3) contains diethyl carbonate and further contains a fluorinated cyclic carbonate having two or more fluorine atoms, or (4) contains at least one compound selected from the group consisting of cyclic sulfonic acid ester compounds, di-sulfonic acid ester compounds, nitrile compounds, and compounds represented by general formula (1) and further contains a fluorinated cyclic carbonate having two or more fluorine atoms. The electrolytes further include (5) a nonaqueous electrolyte characterized by being an electrolytic solution for use in a high-voltage battery having a final charge voltage of 4.3 V or higher and containing a fluorinated cyclic carbonate having two or more fluorine atoms.
Owner:MITSUBISHI CHEM CORP
Process for making diaryl carbonate
Diphenyl carbonate is produced by reacting phenol with diethyl carbonate in a series of fixed bed reactors each of which is connected at different position on a distillation column via side draw and return streams. The composition of material in a distillation column varies along the length of the column, which is predictable under a given set of conditions of temperature and pressure, thus withdrawing streams at different stages in the column, allows the reactor receiving the feed from a particular stage to be operated under conditions to maximize the desired reaction, while allowing the unreacted or byproduct to go back into the distillation and be sent to a stage (by the equilibrium of the distillation) where they are favorably treated in a reactor.
Owner:SHELL USA INC
Non-aqueous electrolyte for lithium iron phosphate battery
The invention discloses a non-aqueous electrolyte for a lithium iron phosphate battery. The non-aqueous electrolyte comprises 70 to 85 weight percent of carbonic ester compound, 3 to 20 weight percent of various function additives and 11 to 17 weight percent of lithium hexafluorophosphate, wherein the carbonic ester compound is one of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate and diethyl carbonate or a mixture of more of the ethylene carbonate, the propylene carbonate, the butylene carbonate, the dimethyl carbonate and the diethyl carbonate; and the additives comprise one of 0.5 to 10 percent of film-forming additive, 0.5 to 10 percent of high-temperature additive, 0.5 to 10 percent of low-temperature additive, 0.5 to 10 percent of overcharge-preventing additive and 0.001 to 2 percent of stability additive, and a mixture of more of the additives. The non-aqueous electrolyte for the lithium iron phosphate battery has the advantages that the solubility and dissociation of the lithium hexafluorophosphate are improved, and electric conductivity is improved; the low temperature resistance of a solid electrolyte interphase (SEI) is reduced; the overall stability of the battery is improved, the overall service life of the battery is prolonged, the compatibility of an electrolyte and a cathode is improved, circulation of the battery is improved, and the service life is prolonged; and the non-aqueous electrolyte can have high performance at high temperature.
Owner:广东金光高科股份有限公司
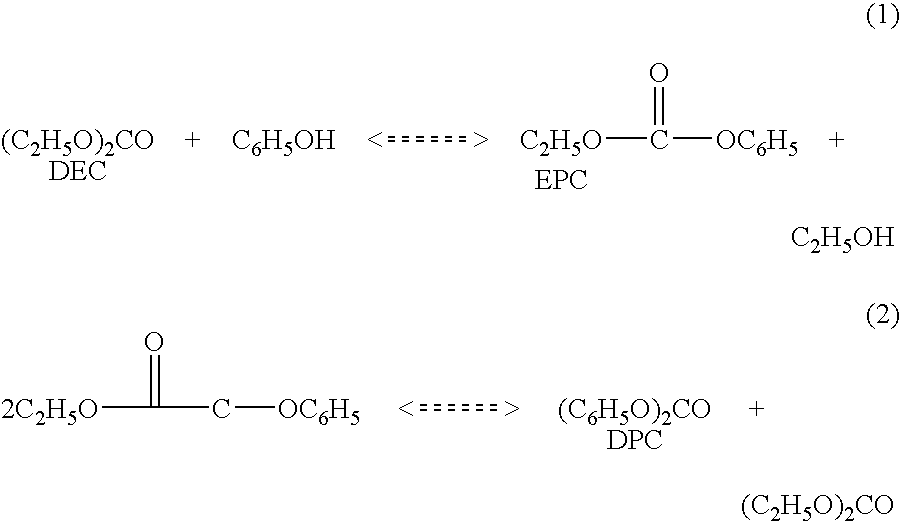
Method for synthesizing methylethyl carbonate by ester exchange of dimethyl carbonate and diethyl carbonate
ActiveCN102863339AHigh catalytic activityLow costChemical recyclingPreparation from organic carbonatesReaction temperatureIonic liquid
The invention relates to a method for synthesizing methylethyl carbonate by ester exchange of dimethyl carbonate and diethyl carbonate, which is implemented by carrying out synthetic reaction of methylethyl carbonate on raw materials dimethyl carbonate and diethyl carbonate by using an imidazole ionic liquid as a catalyst at certain reaction temperature under ordinary pressure for some reaction time. The catalyst consumption is only 0.2-2 wt% of the dimethyl carbonate; the ionic liquid catalyst has high catalytic activity in the reaction process, the maximum yield of methylethyl carbonate is up to 62.31%, and the selectivity is 100%; and the catalyst can be recycled for cyclic utilization after being subjected to simple treatment after reaction, has the advantages of long service life and no pollution, and greatly lowers the preparation cost of methylethyl carbonate.
Owner:灯塔森佳新能源合伙企业(有限合伙)
Binder for electrode of lithium ion secondary battery
InactiveUS20060228627A1Heightening density of active materialEasy to removeActive material electrodesSecondary cellsSolventPolymer chemistry
A binder for electrode of lithium ion secondary battery, comprised of a copolymer composed of 15 to 80 weight % of units from ethylenically unsaturated monomer (A) whose homopolymerization yields a polymer soluble in N-methylpyrrolidone (NMP) and 20 to 85 weight % of units from ethylenically unsaturated monomer (B) whose homopolymerization yields a polymer insoluble in NMP, which copolymer exhibits a swelling degree of 4 or below, in an electrolyte obtained by dissolving LiPF6 in the concentration of 1 mol / liter into a solvent of 1:2 (volume ratio at 20° C.) mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) . This binder for electrode of lithium ion secondary battery enables obtaining an electrode having a flexible electrode layer excelling in binding properties with industrial advantage.
Owner:ZEON CORP
Organic carbonate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS20030129500A1Improve oxidation stabilityImprove dynamic stabilityElectrode carriers/collectorsOrganic electrolyte cellsMethyl carbonateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one carbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture including ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. The preferred additive is either a linear or cyclic carbonate containing covalent O-X and O-Y bonds on opposite sides of a carbonyl group wherein at least one of the O-X and the O-Y bonds has a dissociation energy less than about 80 kcal / mole.
Owner:WILSON GREATBATCH LTD
Synthesis method of methyl ethyl carbonate
InactiveCN101704751AStable in natureHigh yieldPreparation from organic carbonatesSynthesis methodsFixed bed
The synthesis method of methyl ethyl carbonate belongs to the technical field of organic compounds synthesized by ester exchange. Dimethyl carbonate and diethyl carbonate, or dimethyl carbonate and ethanol are used as raw materials to perform ester exchange in the presence of catalyst. The reaction mode comprises two kinds of fixed bed continuous reaction and kettle-type reaction. The catalyst issolid alkali catalyst in which the carrier is active carbon, carbon molecular sieve or mesoporous carbon and the active component is Na2O, K2O, MgO, CaO, SrO or BaO. In the process of fixed bed continuous synthesis, the catalyst is stable without inactivation after long-term use, thereby retaining the high yield of the methyl ethyl carbonate, simplifying the production process, and achieving environmental friendliness and energy conservation. In the kettle-type reaction, the catalytic efficiency is high, the catalyst can be recovered by simple filtering and re-sintering, and the target product can be obtained in short time with high yield.
Owner:JILIN UNIV
Sodium ion secondary battery
ActiveUS20120015256A1Lower performance requirementsCell electrodesOrganic electrolyte cellsDischarge efficiencyPropylene carbonate
Disclosed is a sodium-ion secondary battery having excellent charge and discharge efficiencies as well as excellent charge and discharge characteristics, wherein charging and discharging can be repeated without causing problems such as deterioration in battery performance. Specifically disclosed is a sodium ion secondary battery which is provided with a positive electrode, a negative electrode having a negative electrode active material, and a nonaqueous electrolyte solution containing a nonaqueous solvent. The nonaqueous solvent is substantially composed of a saturated cyclic carbonate (excluding the use of ethylene carbonate by itself), or a mixed solvent of a saturated cyclic carbonate and a chain carbonate, and a hard carbon is used as the negative electrode active material. It is preferable that the nonaqueous solvent used for the sodium-ion secondary battery is substantially composed of propylene carbonate, a mixed solvent of ethylene carbonate and diethyl carbonate, or a mixed solvent of ethylene carbonate and propylene carbonate.
Owner:TOKYO UNIVERSITY OF SCIENCE
Nonaqueous organic electrolytes for low temperature discharge of rechargeable electrochemical cells
InactiveUS6153338AImprove low temperature charge/discharge performanceImprove cycle lifeElectrolytic capacitorsOrganic electrolyte cellsRoom temperaturePhysical chemistry
An alkali metal secondary electrochemical cell, and preferably a lithium ion cell, activated with a quaternary solvent system, is described. The solvent system comprises a quaternary mixture of dialkyl carbonates and cyclic carbonates, and preferably dimethyl carbonate, diethyl carbonate, ethylmethyl carbonate and ethylene carbonate. Lithium ion cells activated with this electrolyte have good room temperature cycling characteristics and excellent low temperature discharge behavior.
Owner:WILSON GREATBATCH LTD
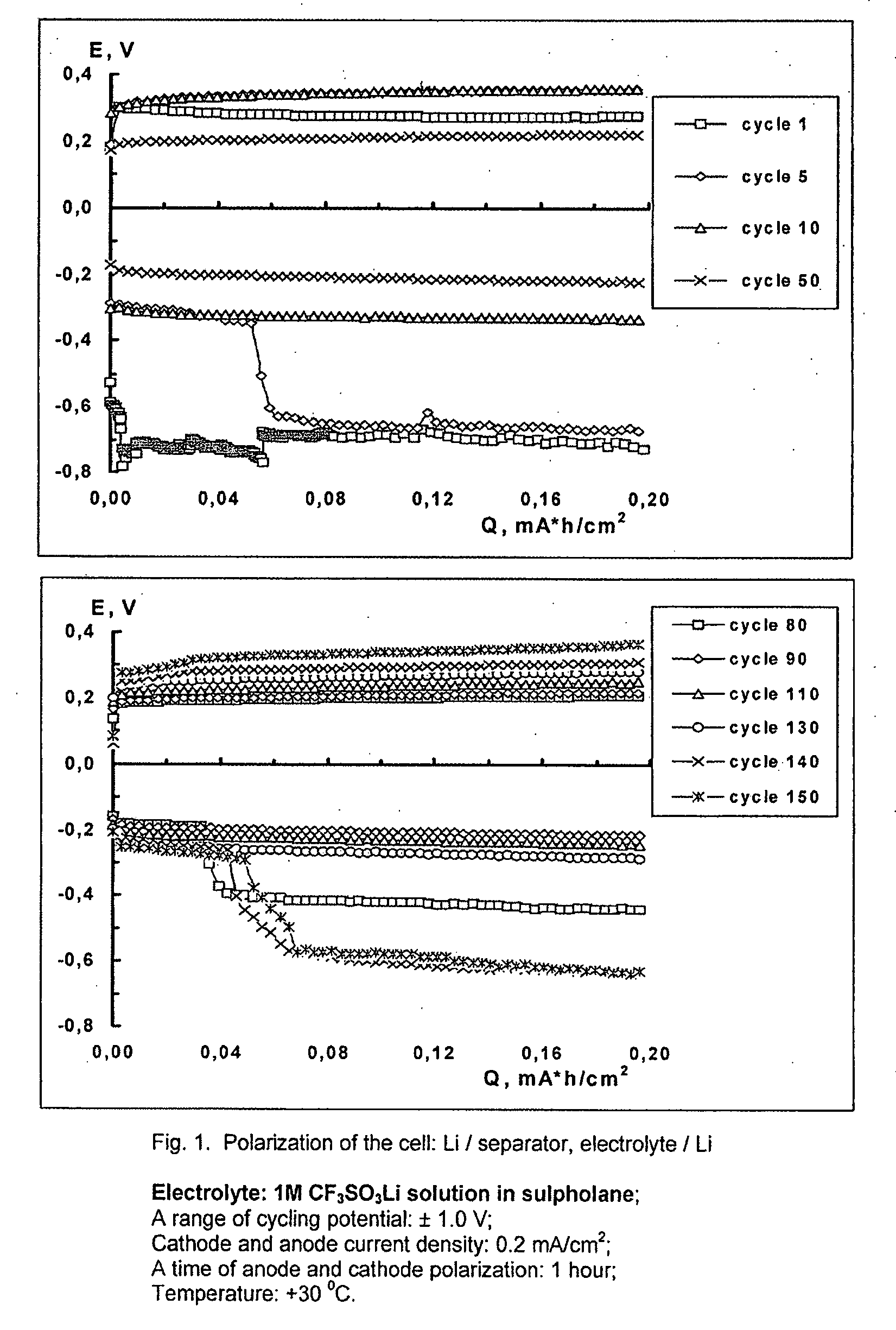
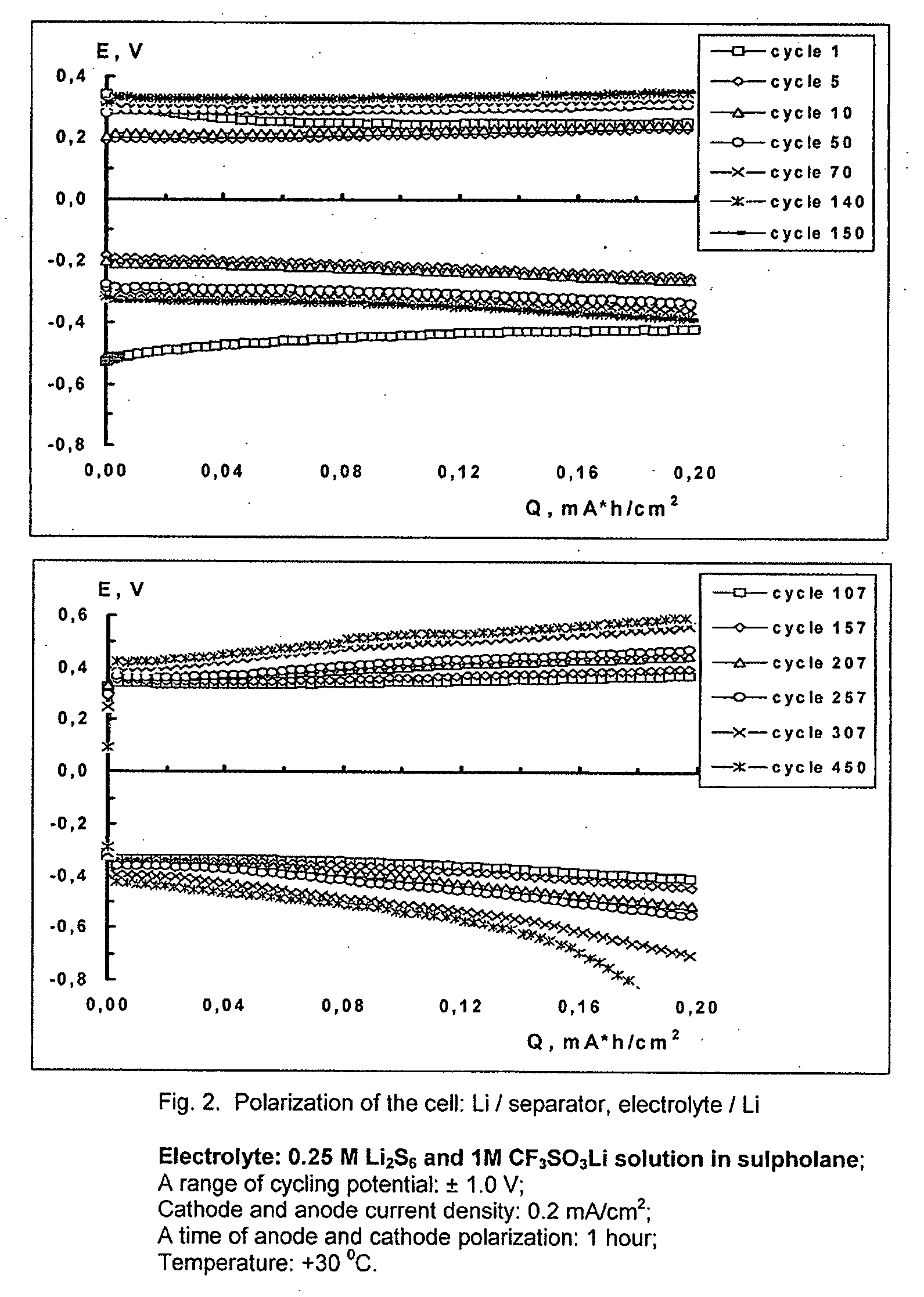
Cell or battery with a metal lithium electrode and electrolytes therefor
InactiveUS20080038645A1Improve cycle lifeOrganic electrolyte cellsLi-accumulatorsSulfolaneMetallic lithium
An electrolyte for rechargeable batteries with a negative electrode of lithium or lithium containing alloys comprising: one or several non-aqueous organic solvents, one or several lithium salts and one or several additives increasing the cycle life of the lithium electrode. The electrolyte solution may comprise one or several solvents selected from the group comprising: tetrahydrofurane, 2-methyltetrahydrofurane, dimethylcarbonate, diethylcarbonate, ethylmethylcarbonate, methylpropylcarbonate, methylpropylpropyonate, ethylpropylpropyonate, methylacetate, ethylacetate, propylacetate, dimetoxyethane, 1,3-dioxalane, diglyme (2-methoxyethil ether), tetraglyme, ethylenecarbonate, propylencarbonate, γ-butyrolactone, and sulfolane. The electrolyte solution may further comprise at least one salt or several salts selected from the group consisting of lithium hexafluorophosphate (LiPF6), lithium hexafluoroarsenate (LiAsF6), lithium perchlorate (LiClO4), lithium sulfonylimid trifluoromethane (LiN(CF3SO2)2)) and lithium trifluorosulfonate (CF3SO3Li) or other lithium salts or salts of another alkali metal or a mixture thereof. Also disclosed is an electrochemical cell or battery with an anode of metallic lithium or a lithium-containing alloy, and such an electrolyte.
Owner:OXIS ENERGY
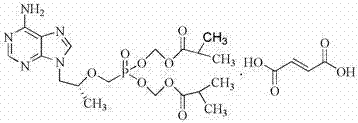
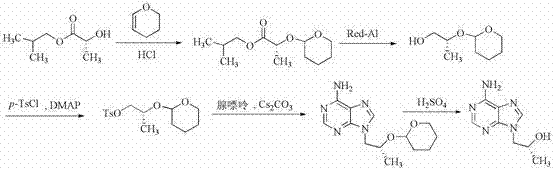
Synthetic process of tenofovir
InactiveCN102295660AHigh yieldEasy post-processingGroup 5/15 element organic compoundsDiethyl phosphatePurine
The invention relates to a synthesis technique of tenofovir, and relates to the field of medicinal chemistry. Using natural (R)-lactate as the starting material, chiral R-1,2-propanediol is obtained through reduction, and then reacted with diethyl carbonate under the action of alkali to obtain the key reaction intermediate R-propylene carbonate ester. Use adenine and R-propylene carbonate to prepare R-9-(2-hydroxypropyl)adenine; Gained R-9-(2-hydroxypropyl)adenine and diethyl p-toluenesulfonyloxyphosphate Under the catalysis of magnesium tert-butoxide, the condensation reaction is carried out to obtain R-9[(diethylphosphorylmethoxy)propyl]purine; the obtained R-9[2-(diethylphosphorylmethoxy) Propyl]purine is hydrolyzed to obtain Tenofovir. The process has short reaction steps, short required reaction time, high quality yield and good product quality, and is suitable for industrialized production.
Owner:CHANGZHOU UNIV
Positive Electrode and Non-Aqueous Electrolyte Secondary Battery
ActiveUS20090053613A1Low costAlkaline accumulatorsNon-aqueous electrolyte accumulatorsElectrical batterySodium phosphates
A material (hereinafter referred to as “positive electrode material”) including sodium manganate powder as a positive electrode active material, carbon black powder as a conductive agent, and polytetrafluoroethylene as a binder is prepared. The positive electrode material is mixed in an N-methylpyrrolidone solution to produce slurry as a positive electrode mixture. A working electrode is produced by applying the slurry on a positive electrode collector. A negative electrode containing tin or germanium is produced. The non-aqueous electrolyte is produced by adding sodium hexafluorophosphate as an electrolyte salt in a non-aqueous solvent produced by mixing ethylenecarbonate and diethyl carbonate.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Preparation method of polymer lithium ion battery
ActiveCN101685878AStable structureMeet capacityCell electrodesFinal product manufactureHigh temperature storagePolymer science
The invention belongs to a preparation method of a polymer lithium ion battery in the technical field of battery product development, which is characterized in that the polymer lithium ion battery comprises an anode system, a cathode system and gel polymer electrolyte, wherein, the anode system adopts lithium manganate and lithium nickelate cobaltate manganate in a weight ratio of 1:1-4, the cathode system adopts natural graphite and / or artificial graphite in a weight ratio of 0-100:100-0, the gel polymer electrolyte comprises a monomer / an initiator / organic electrolyte, the monomer is methyl acrylate / tetraethenol diacrylate, the initiator is a radical initiator, the organic electrolyte is ethylene carbonate / methyl ethyl carbonate / diethyl carbonate. The product prepared by the invention canmeet the requirements of the battery on capacity and overcharge protection, is excellent in high temperature storage and cyclicity and is widely applied to such electronic equipment as MP3, MP4, portable DVD and the like.
Owner:ZHENGZHOU BAK BATTERY CO LTD
Lithium-ion battery electrolyte, lithium-ion battery and electronic instrument
The invention provides a lithium-ion battery electrolyte, comprising a solvent and an additive, wherein the solvent comprises propyl propionate and two or three selected from a group consisting of vinyl carbonate, diethyl carbonate and propylene carbonate, and the additive comprises vinyl sulfate, fluoroethylene carbonate, adiponitrile, glycol bis(propionitrile) ether, 1,3-propanesultone and fluorobenzene. A lithium-ion battery prepared from the lithium-ion battery electrolyte has stable usability under the condition of 4.40 V or 4.45 V, has energy density of 750 Wh / L or above, cycle life of 800 times or above and a capacity retention ratio of 80% or above, so the service life of a novel high-voltage high-energy-density polymer lithium-ion battery is substantially improved.
Owner:LENOVO (BEIJING) CO LTD
Lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging
ActiveCN103367803AFacilitate quick migrationImprove ionic conductivitySecondary cellsMethyl carbonateEthyl butyrate
The invention relates to a lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging. The lithium ion battery electrolyte comprises lithium salt, a multi-element organic solvent and additives, wherein the additives comprise a low-melting-point additive, a film forming additive and a high-temperature additive; the multi-element organic solvent contains at least three of ethylene carbonate, diethyl carbonate, dimethyl carbonate, methyl ethyl carbonate, propylene carbonate and butylene carbonate; the low-melting-point additive contains at least one of 4-methyl-1,3-dioxolane, methyl acetate, methyl propionate, methyl butyrate, ethyl butyrate, propyl butyrate and butyl acetate; the high-temperature additive is at least one of methyl ester, di-n-propyl carbonate and 1,3-propane sultone. The lithium iron phosphate battery with the electrolyte can charge and discharge at an ultralow temperature and in a high-temperature environment, and is stable in performance and long in recycling life.
Owner:HANGZHOU LIAO TECH
Non-aqueous electrolyte for lithium manganate power battery
ActiveCN102610859APrevent high temperature swellingAvoid capacity lossSecondary cellsElectrolytic agentMethyl carbonate
The invention discloses non-aqueous electrolyte for a lithium manganate power battery. The non-aqueous electrolyte comprises 70-90% of carbonic ester compound, 3-20% of various functional additives and 11-17% of lithium hexafluorophate, wherein the carbonic ester compound is one of or mixture of ethylene carbonate (EC), propylene carbonate (PC), butane carbonate (BC), dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate (DPC), methyl ethyl carbonate (EMC), methyl propyl carbonate (MPC) and methyl butyl carbonate (BMC); and the additives comprise 0.5-10% of a film forming additive, 0.5-10% of a high-temperature additive, 0.5-10% of an anti-overcharge additive, 0.5-10% of a flame retardant additive and 0.001-2% of a stability additive. In the non-aqueous electrolyte for the lithium manganate power battery of the invention, the performance of a solid phase interfacial film in the battery is improved, the compatibility of the electrolyte with negative electrode material is enhanced, and the cycle performance as well as the safety performance of the battery is greatly improved.
Owner:广东金光高科股份有限公司
Electrolyte for power lithium ion battery
InactiveCN102544582AImprove wettabilityStable electrochemical propertiesSecondary cellsPolyolefinSolvent
The invention discloses high-wettability and high-pourability electrolyte for a power lithium ion battery. The electrolyte comprises a wetting additive, a lithium salt, a non-aqueous organic solvent and other additives, wherein the wetting additive is a nonionic surfactant, and the wetting additive accounts for 0.001 to 5 percent of the mass of the electrolyte; the non-aqueous solvent is one or a mixture of more of ethylene carbonate, propylene carbonate, methyl ethyl carbonate, dimethyl carbonate, diethyl carbonate and gamma-butyrolactone, and the non-aqueous solvent accounts for 50 to 90 percent of the mass of the electrolyte; the lithium salt at the concentration of 0.6 to 1.5 mol / L is at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(oxalato)borate and lithium trifluoromethanesulfonate; and the other additives accounts for 0.5 to 8 percent of the mass of the electrolyte. The electrolyte has high wettability for a polyolefin diaphragm and electrode active materials, and has long cycle life.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
Low-temperature lithium ion battery
InactiveCN110739485AReduce DC internal resistanceImprove low temperature performanceCell electrodesSecondary cellsElectrolytic agentPropanoic acid
The invention belongs to the field of lithium batteries, in particular to a low-temperature lithium ion battery. The positive electrode sheet comprises a positive electrode current collector and a positive electrode active material layer. The positive electrode active material layer comprises 4.4 V lithium cobaltate material, a positive electrode conductive agent and a positive electrode binder. The negative electrode sheet comprises a negative electrode current collector and a negative electrode active material layer. The negative electrode active material layer comprises a hard carbon coatedsecondary particle artificial graphite material, a negative electrode conductive agent, a dispersant and a negative electrode binder. The electrolyte comprises 10-20% of lithium hexafluorophosphate,10-20% of ethyl propionate, 10-20% of diethyl carbonate, 15-30% of vinyl carbonate, 15-30% of propyl propionate, 3-10% of fluorovinyl carbonate, 2-3% of propylene sulfite, 0.5-2.5% of ethylene glycolmonobutyl ether and 0.5-2.5% of 2, 2'-dithiopyridine. Compared with the batteries in the prior art, the lithium ion battery has good charging and discharging performance and higher energy density under low temperature condition.
Owner:东莞维科电池有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com