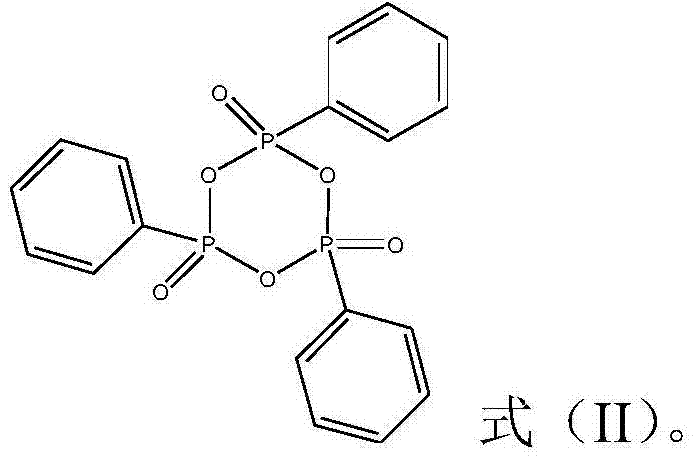
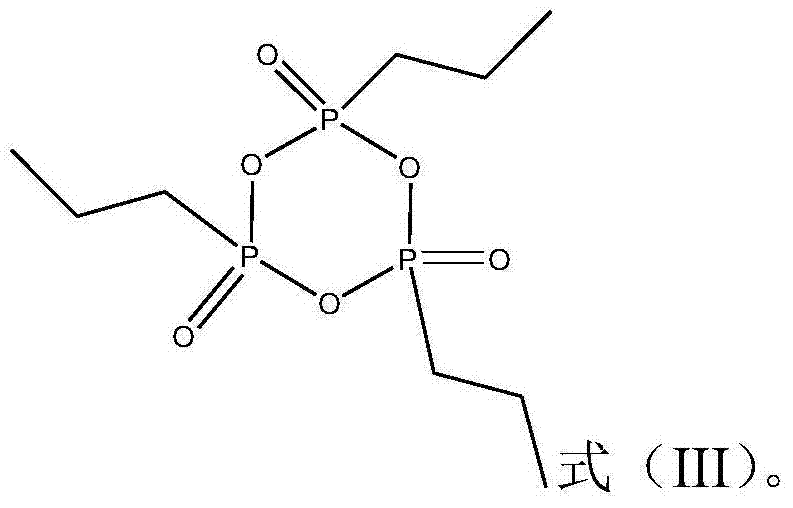
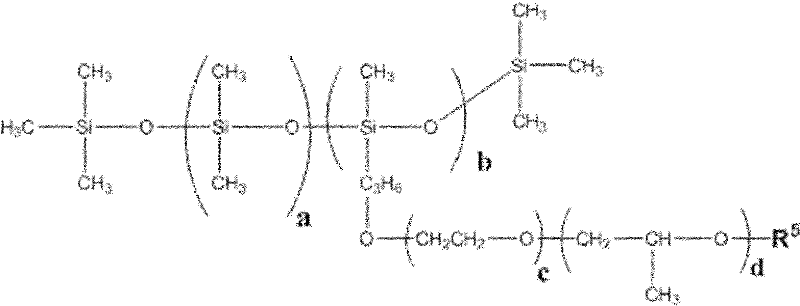
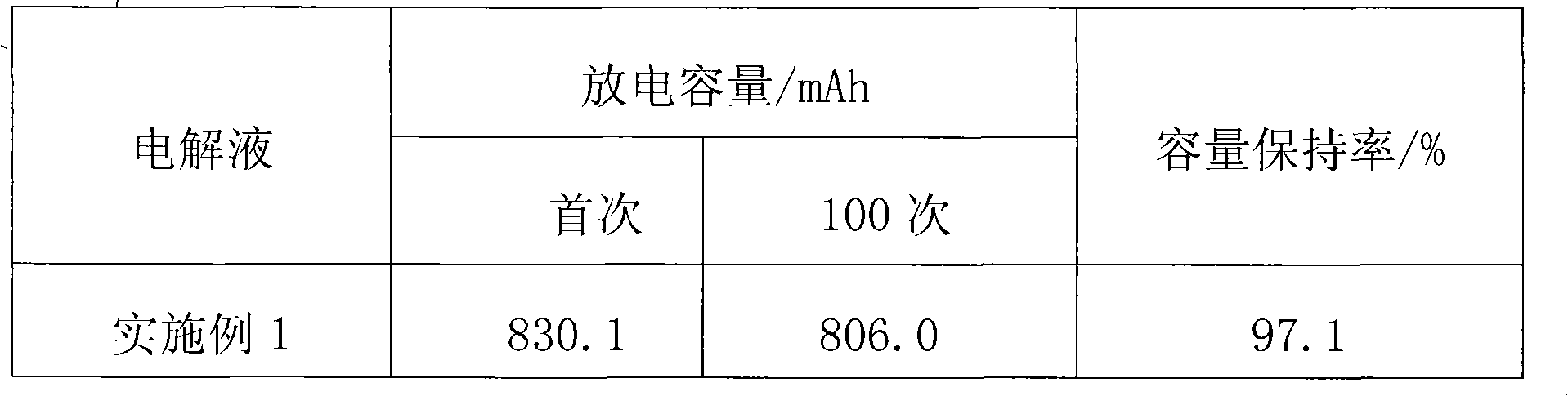
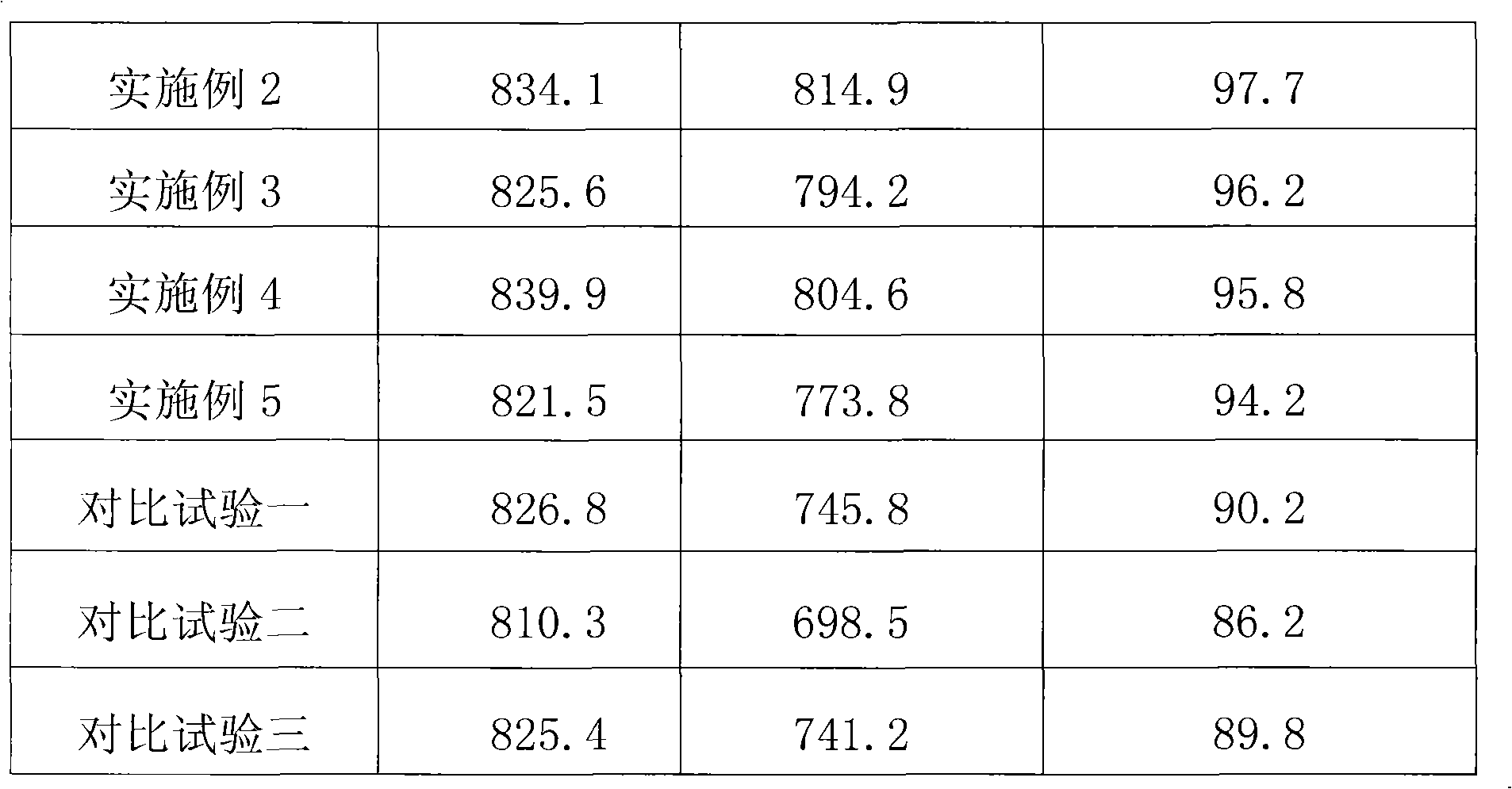
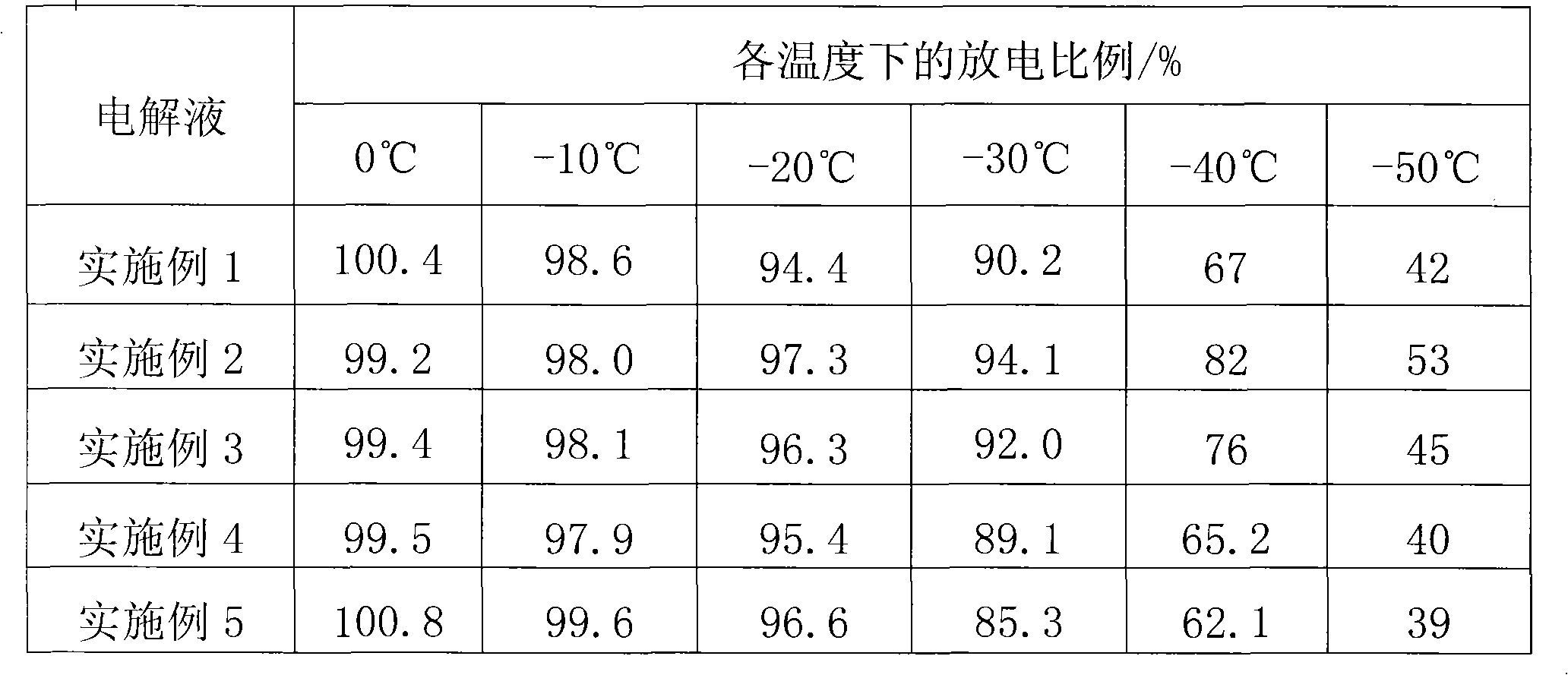
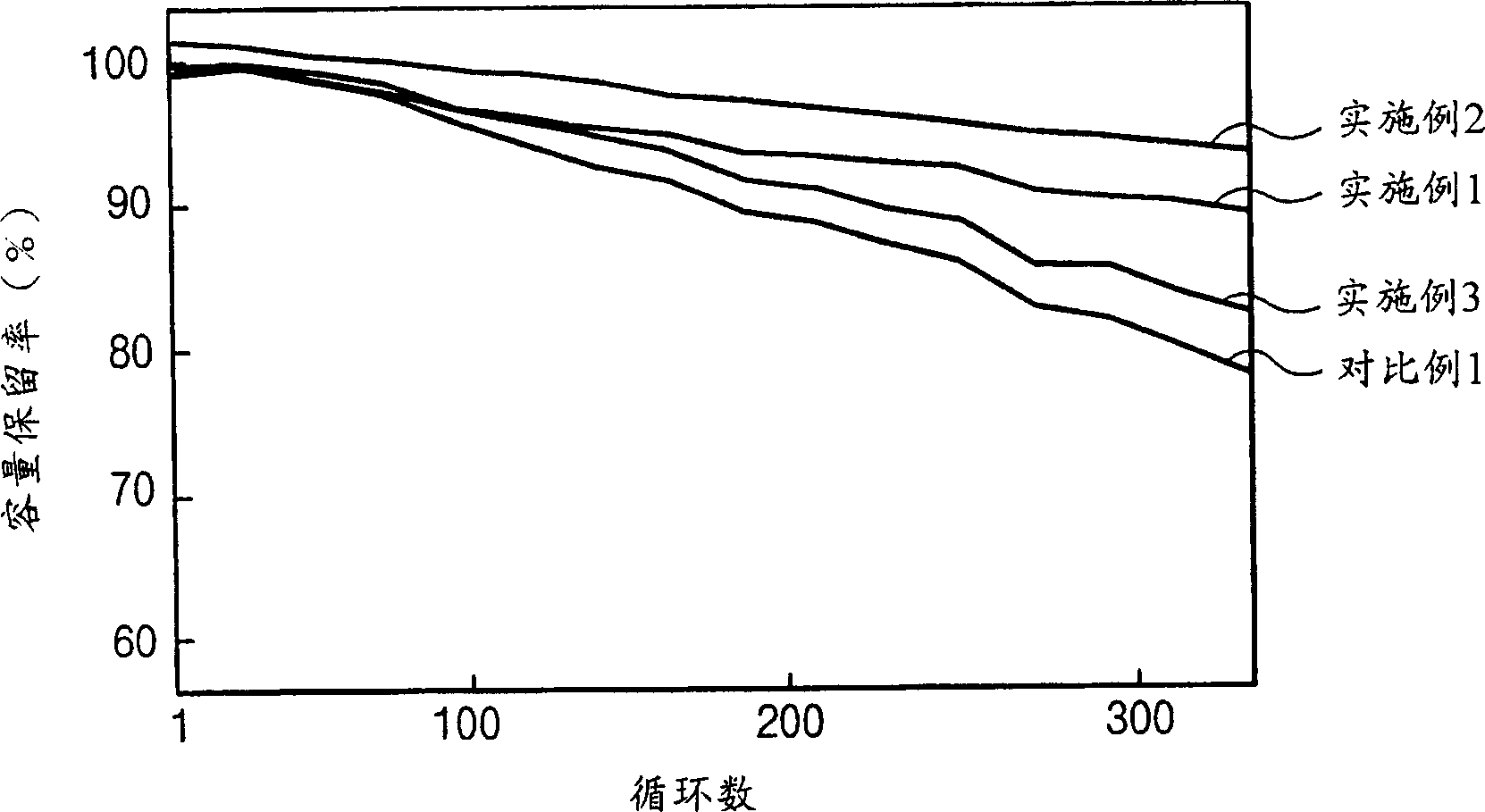
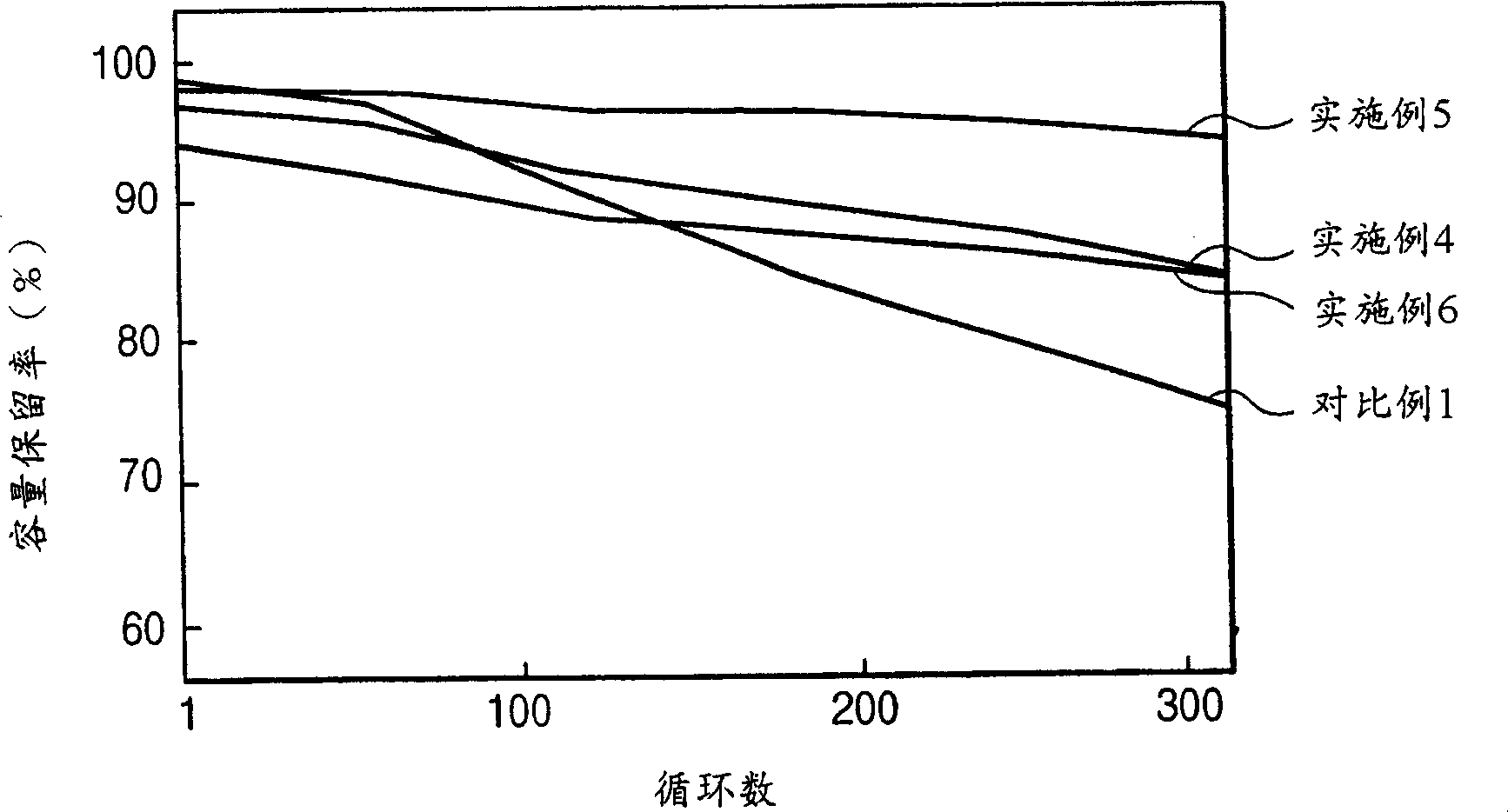
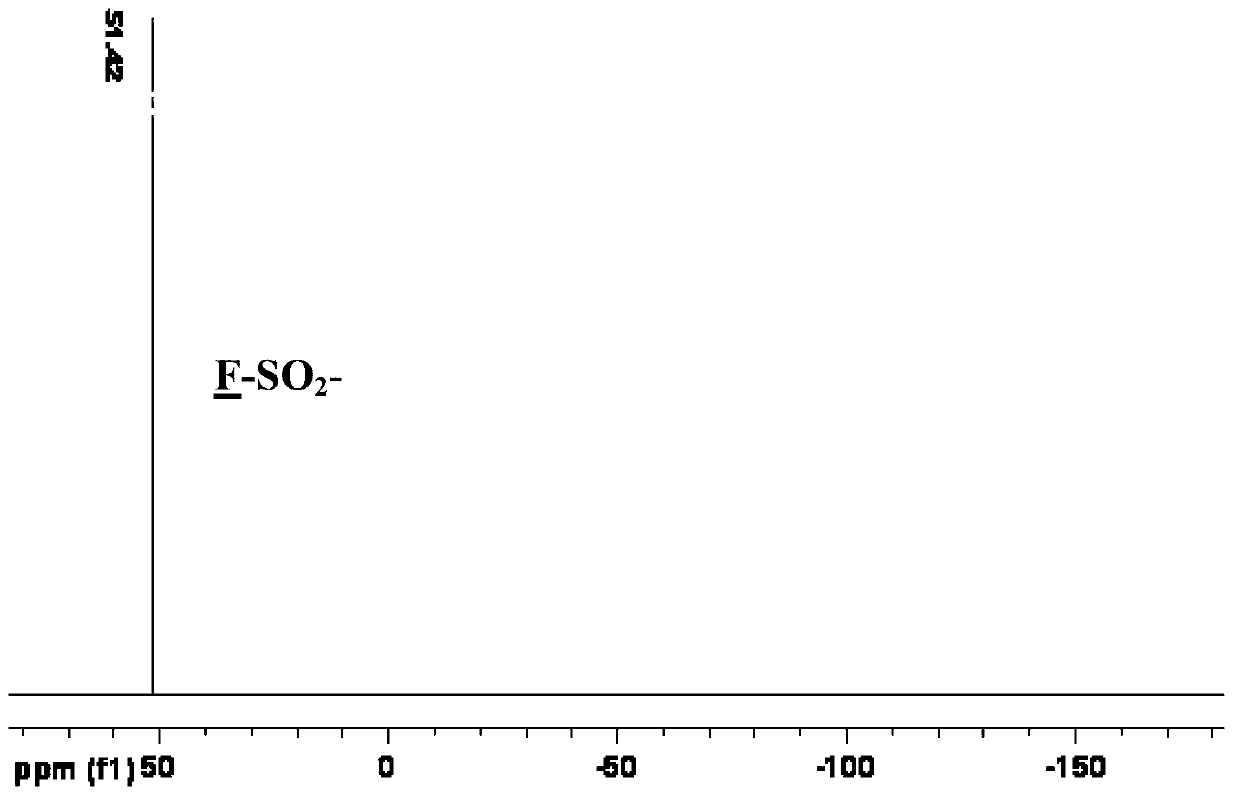
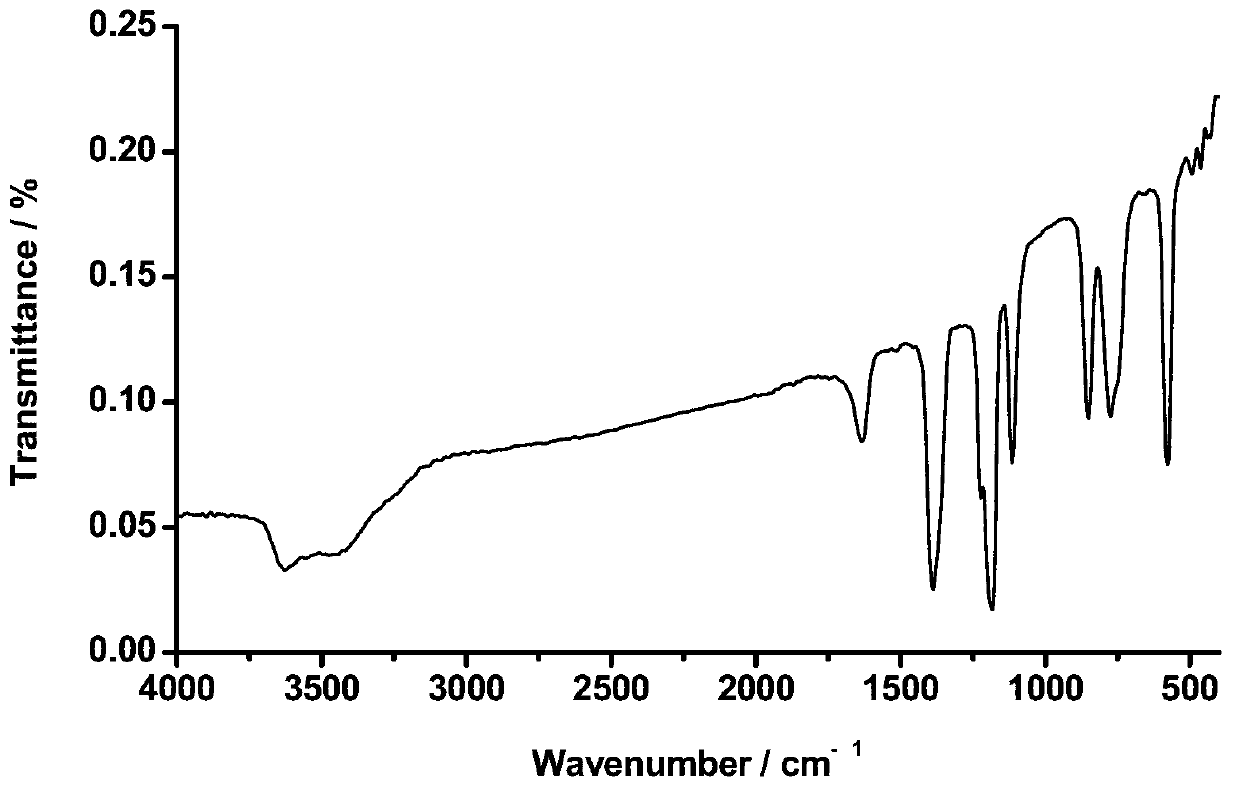
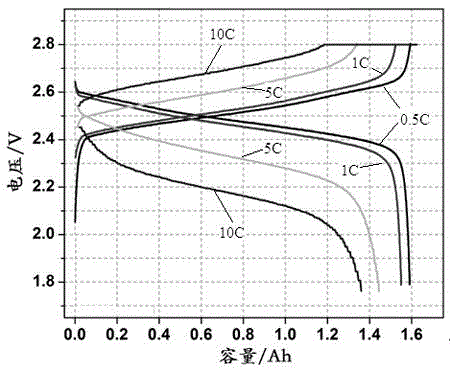
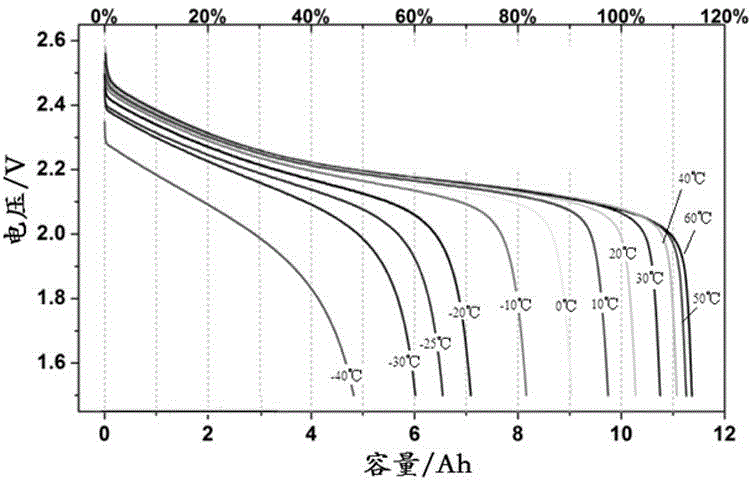
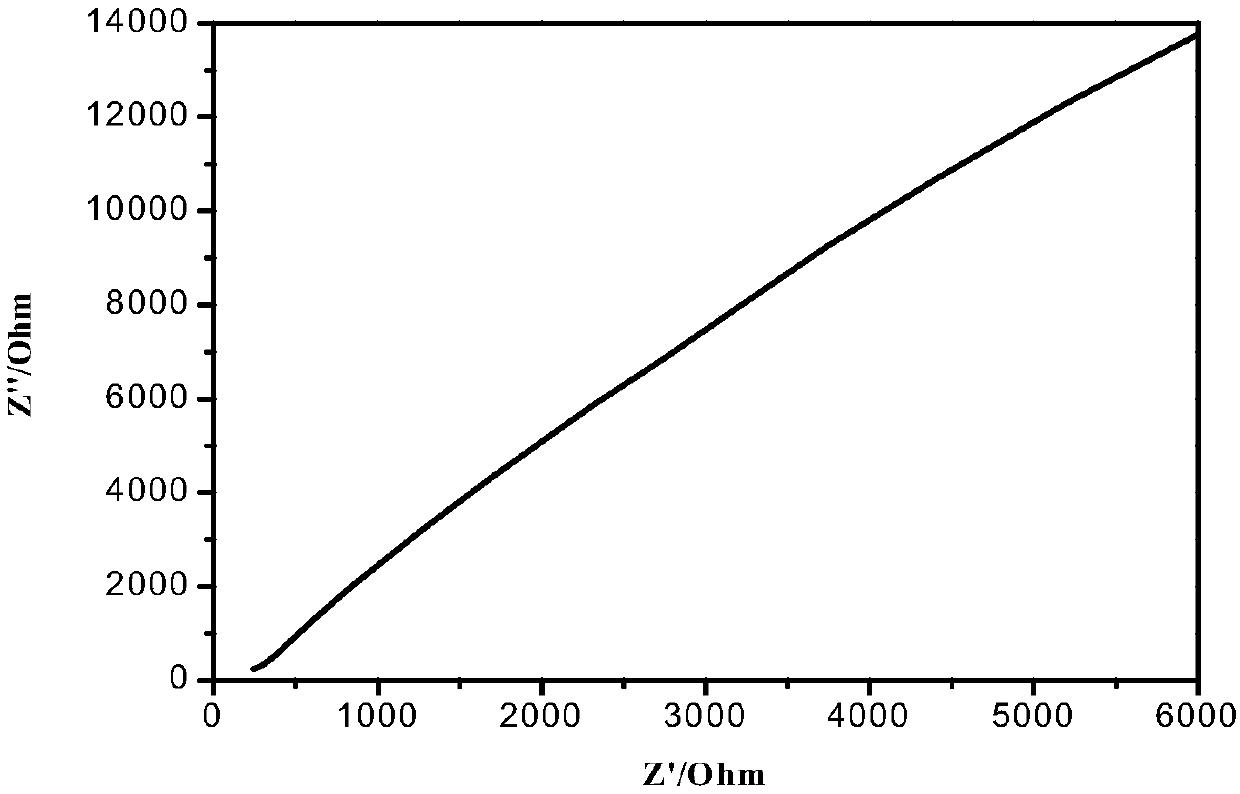
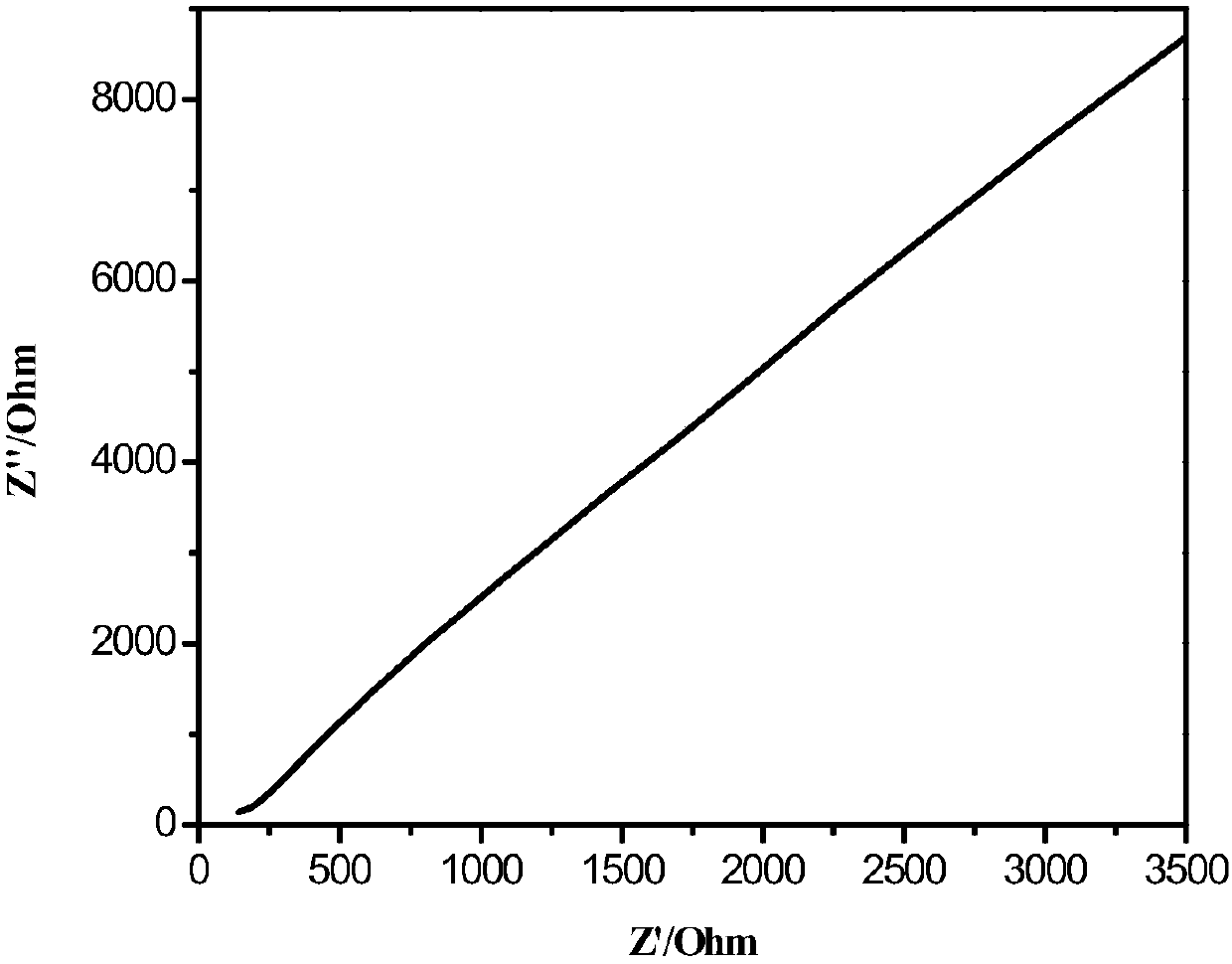
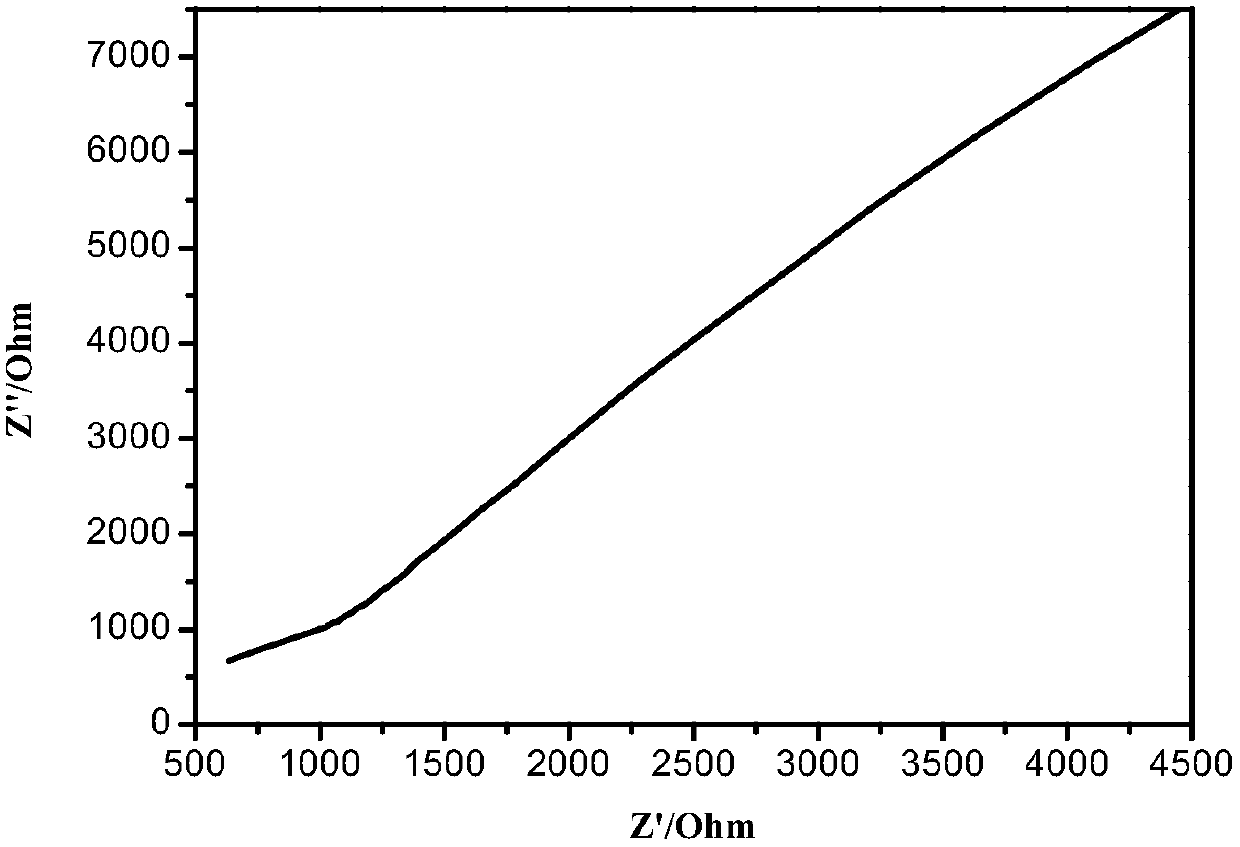
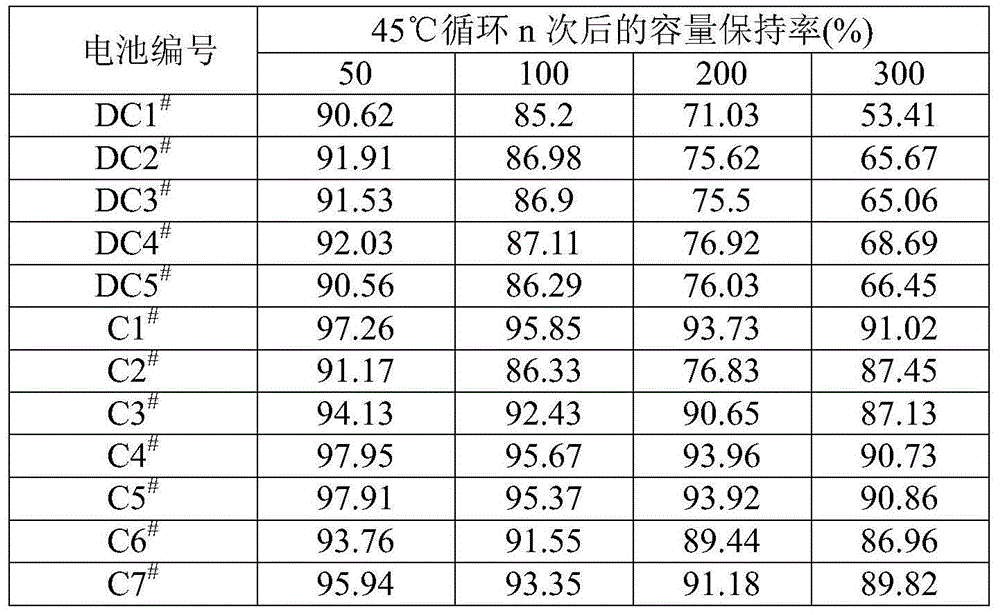
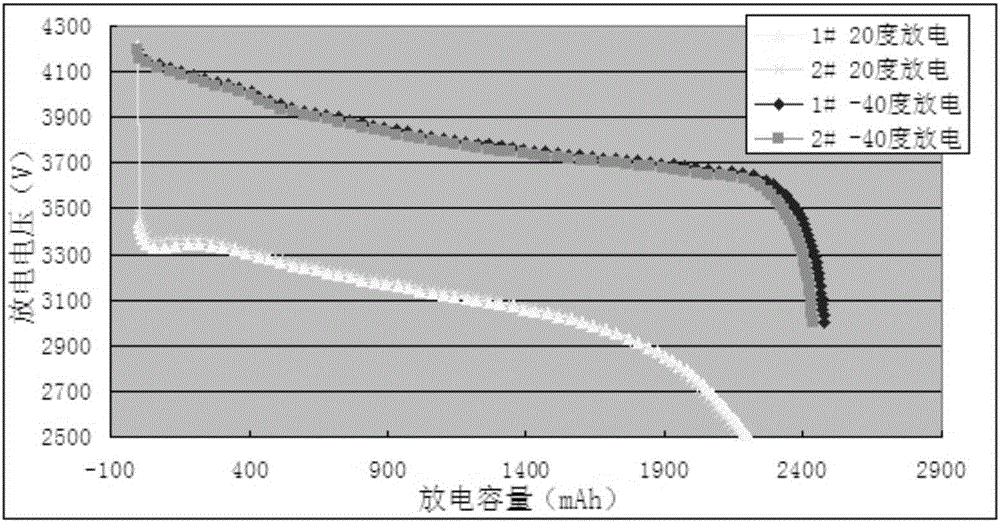
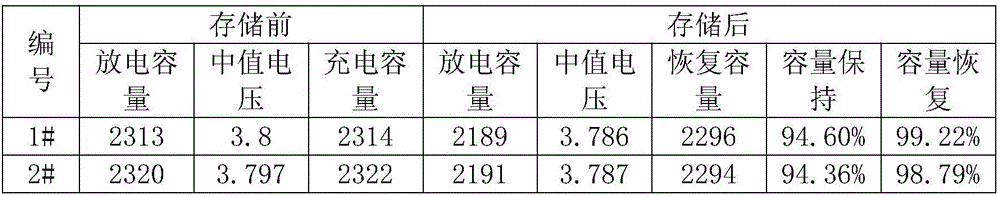
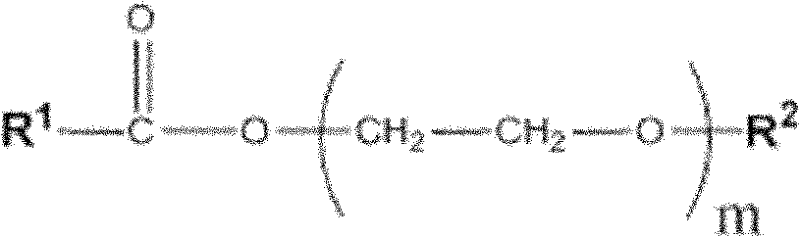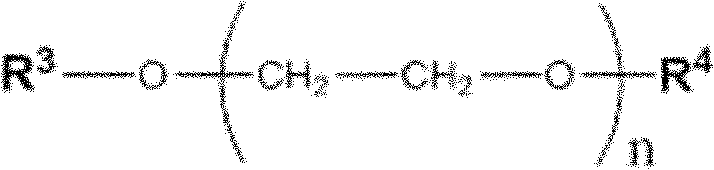Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
262 results about "Lithium tetrafluoroborate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium tetrafluoroborate is an inorganic compound with the formula LiBF₄. It is a white crystalline powder. It has been extensively tested for use in commercial secondary batteries, an application that exploits its high solubility in nonpolar solvents.
All-solid polymer electrolyte, and preparation method and application of all-solid polymer electrolyte
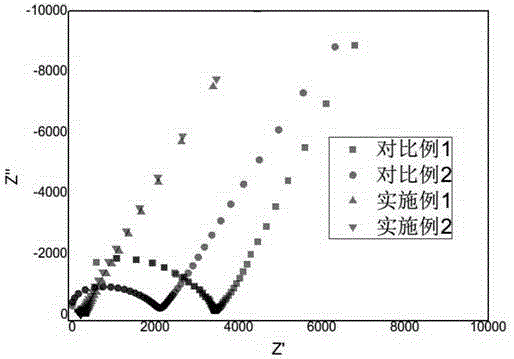
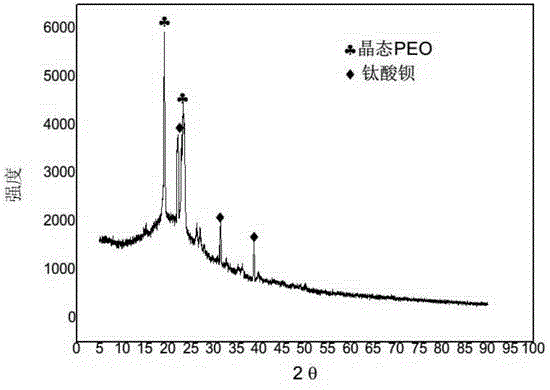
InactiveCN104538670AImprove conductivityHigh mechanical strengthSolid electrolytesFinal product manufacturePolyethylene oxideBarium titanate
The invention discloses an all-solid polymer electrolyte, and a preparation method and an application of the all-solid polymer electrolyte, and belongs to the field of lithium ion batteries. The all-solid polymer electrolyte comprises polyethylene oxide, lithium salt, inorganic nano particles and ion liquid, wherein a mass ratio of the lithium salt to polyethylene oxide is 0.1-0.5; the mass sum of the inorganic nano particles and the ion liquid is 10-30% of the mass of the all-solid polymer electrolyte; the lithium salt comprises one or several of bistrifluoromethane sulfonimide lithium salt, lithium tetrafluoroborate, lithium perchlorate, lithium hexafluorophosphate, lithium hexafluoroarsenate, lithium trifluoromethanesulfonate and lithium bis borate; and the inorganic nano particles comprise one or several of nano aluminum oxide, nano silicon oxide, nano zirconium oxide and nano barium titanate. The all-solid polymer electrolyte has better mechanical strength and higher ion conductivity. The method is simple in technology and low in cost; and raw materials are easy to obtain.
Owner:HUAZHONG UNIV OF SCI & TECH RES INST SHENZHEN
Synthesizing process for obtaining lithium difluoro-oxalato-borate and lithium tetrafluoroborate
InactiveCN101648963AImprove performanceSimple processGroup 3/13 element organic compoundsBoron halogen compoundsOrganic solventPhysical chemistry
The invention discloses a synthesizing process for simultaneously obtaining lithium difluoro-oxalato-borate and lithium tetrafluoroborate with favorable performance, which comprises the following steps: (1) leading a fluorine-contained compound, a boron-contained compound, a lithium-contained compound and an oxalate-contained compound to react in a reaction medium at the reaction pressure of 0.1-1MPa and the temperature of 0-100 DEG C, wherein the molar ratio of lithium element, fluorine element, boron element and oxalate ion is (2-3):(5-6):6:2:1; generating reaction liquid containing the lithium difluoro-oxalato-borate and the lithium tetrafluoroborate; (2) carrying out initial separation on the lithium difluoro-oxalato-borate and the lithium tetrafluoroborate in the reaction liquid and then carrying out further extraction separation by an organic solvent which can extract the lithium difluoro-oxalato-borate or the lithium tetrafluoroborate; and (3) respectively carrying out recrystallization and vacuum drying to obtain the battery-grade lithium difluoro-oxalato-borate and the lithium tetrafluoroborate. The invention is suitable for industrially producing two lithium salts which have favorable performance and are used for a lithium ion battery.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
Low-temperature organic electrolyte taking gamma-butyrolactone as base solvent and application thereof
ActiveCN101916878AImprove low temperature performanceImprove cycle lifeHybrid capacitor electrolytesElectrolytic capacitorsCapacitanceGraphite
The invention discloses low-temperature organic electrolyte taking gamma-butyrolactone as a base solvent and a preparation method thereof. The organic electrolyte comprises a solvent and lithium tetrafluoroborate (LiBF4), wherein the solvent is a mixture of cyclic gamma-butyrolactone, chain carbonic ester and chain carboxylic ester. The organic electrolyte is suitable to be used by a non-graphite-based cathode lithium ion capacitor, a non-graphite-based cathode lithium ion battery and a non-graphite-based cathode lithium ion system. Compared with the formula of the traditional electrolyte, the organic electrolyte greatly improves the low-temperature performance of an electrochemical device and prolongs the cyclic service life of the electrochemical device on the basis of ensuring the electrochemical performance, and meanwhile is favorable for the safety of the electrochemical device; and the formula of the electrolyte is suitable for the non-graphite-based cathode lithium ion capacitor, the non-graphite-based cathode lithium ion battery and the non-graphite-based cathode lithium ion capacitance battery.
Owner:SHANGHAI AOWEI TECH DEV
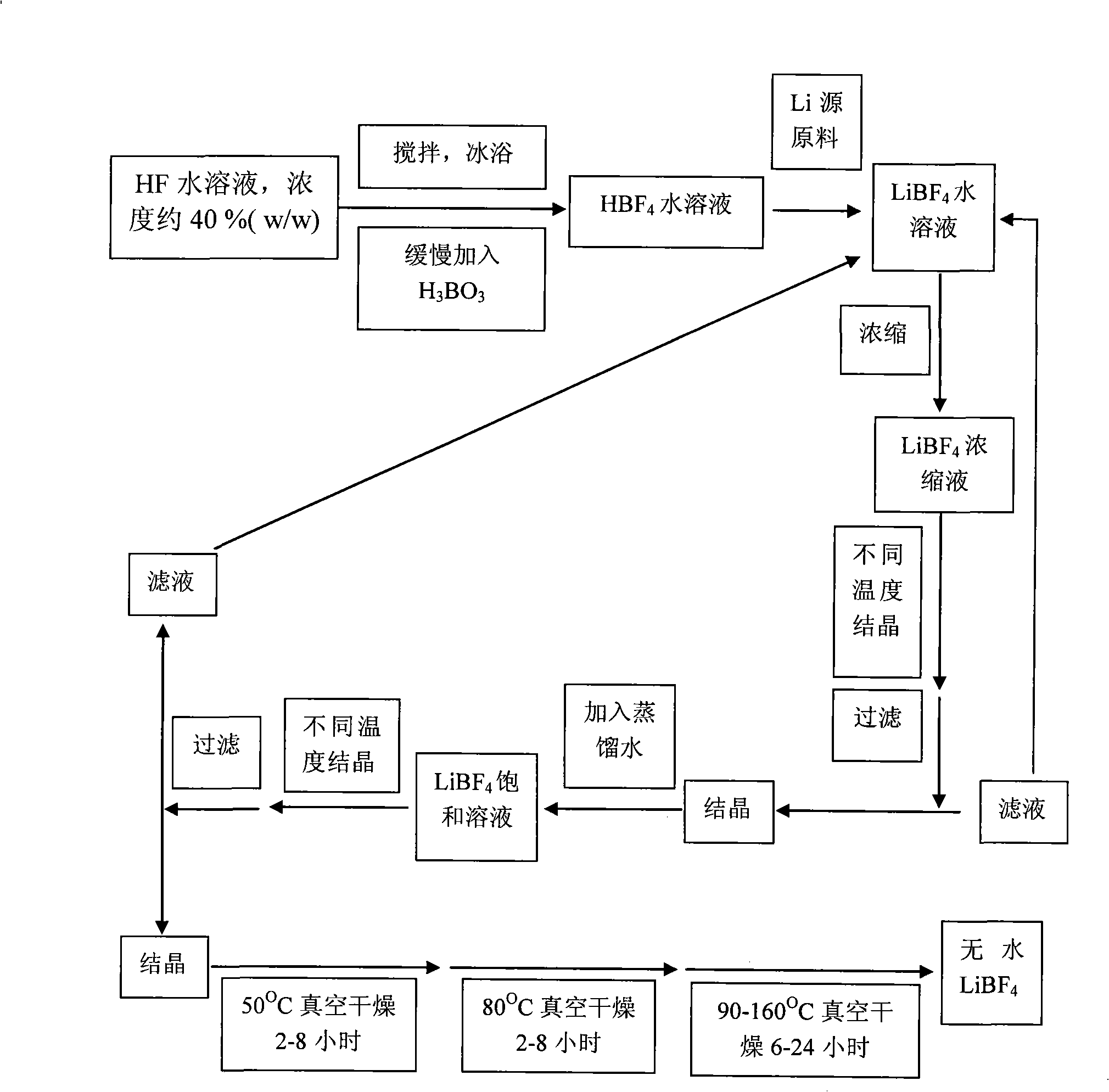
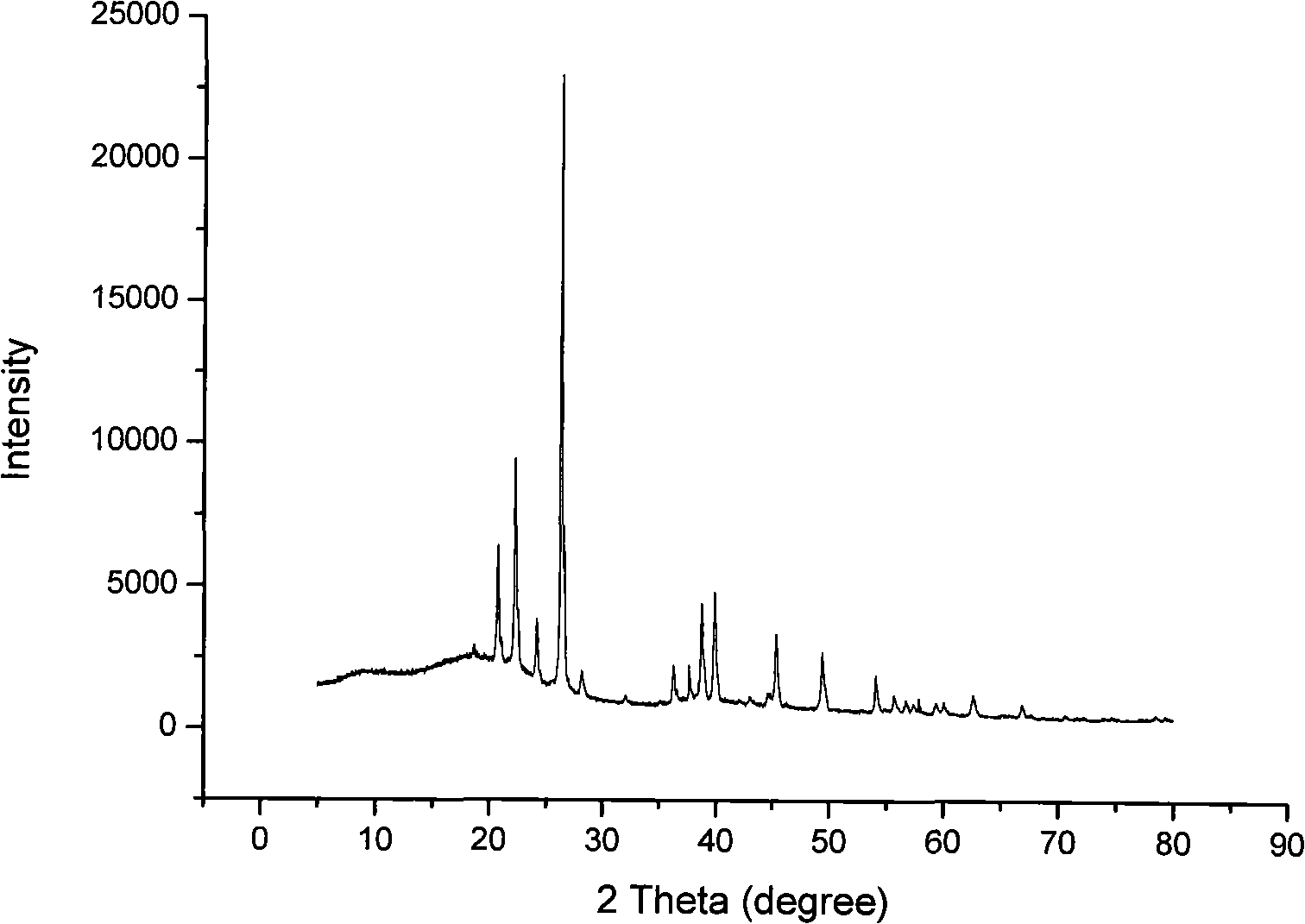
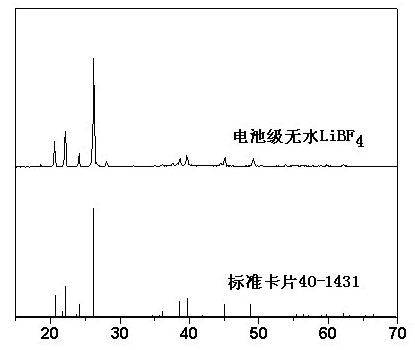
Method of preparing waterless lithium terafluoroborate
InactiveCN101318664AThe product quality is quiteEfficient removalTretrafluoboric acidLithium hydroxideX-ray
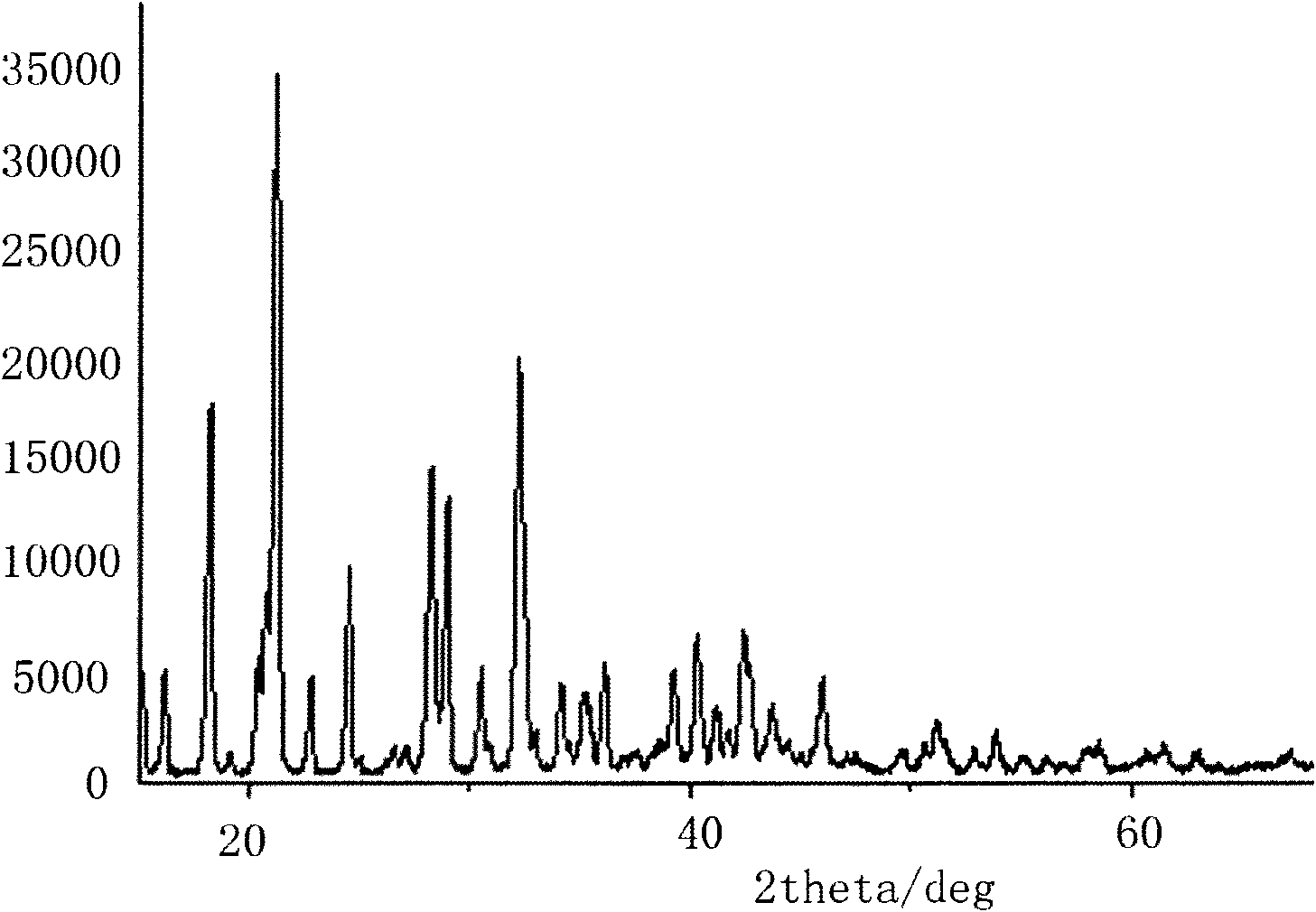
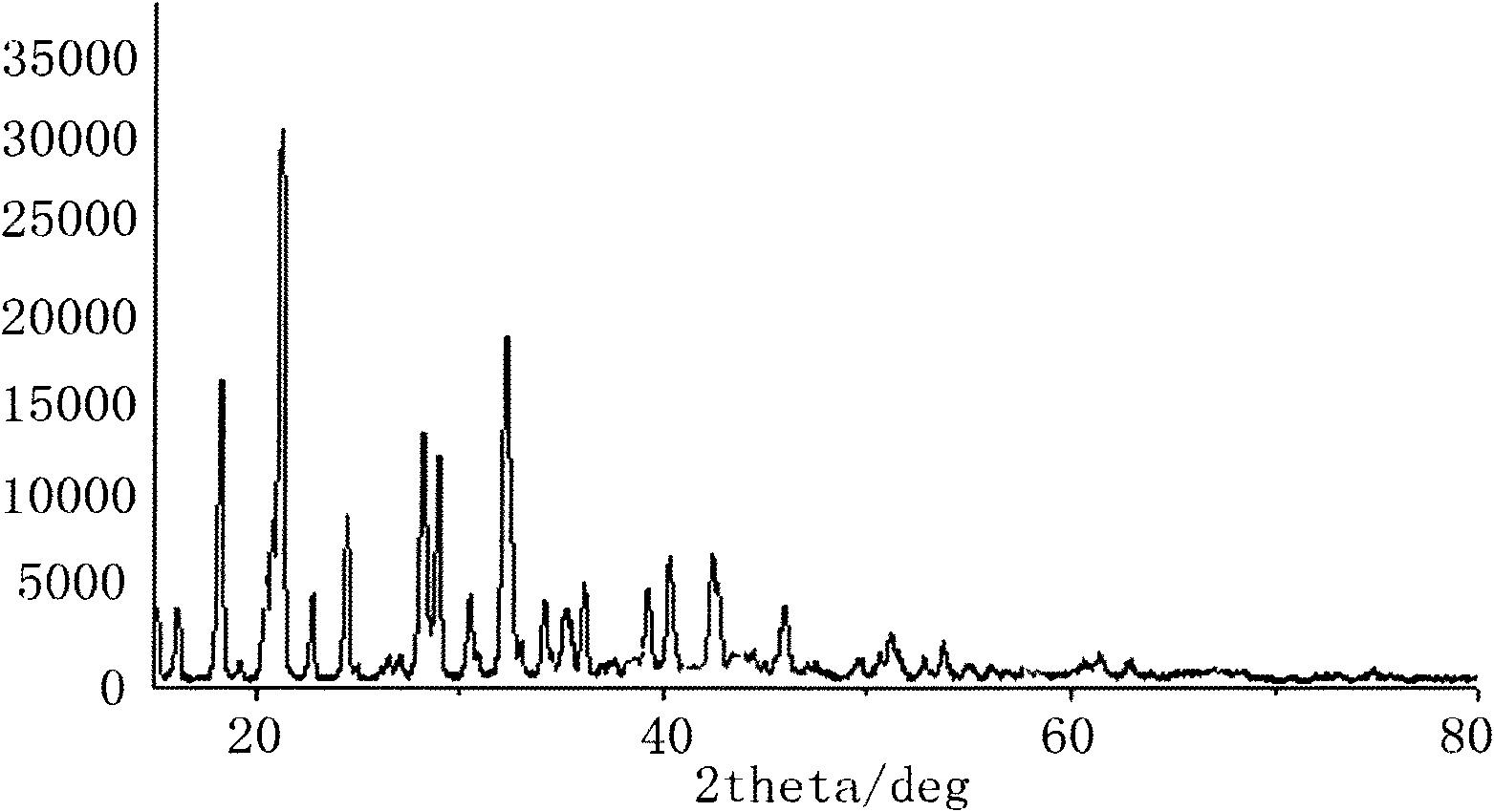
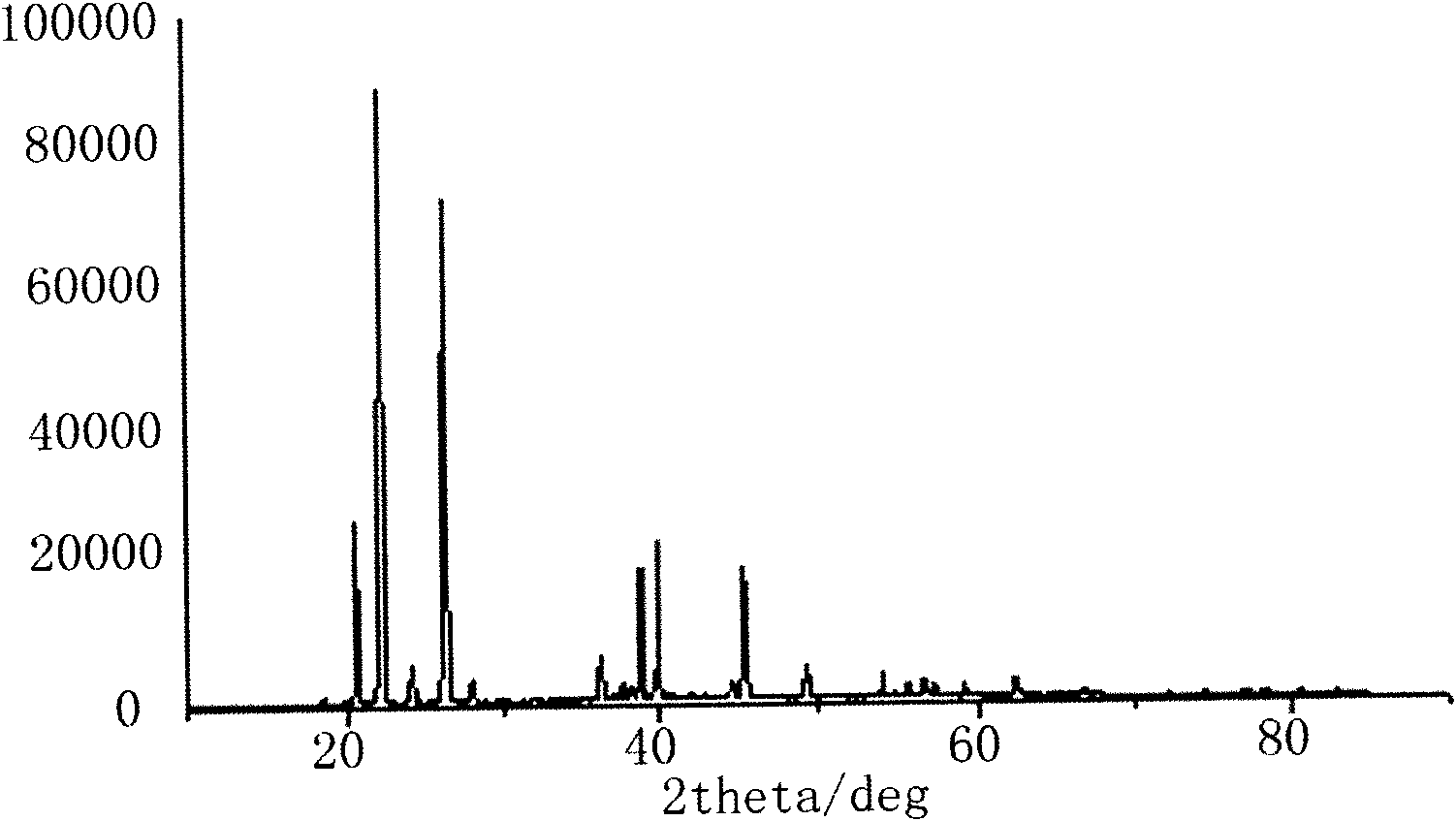
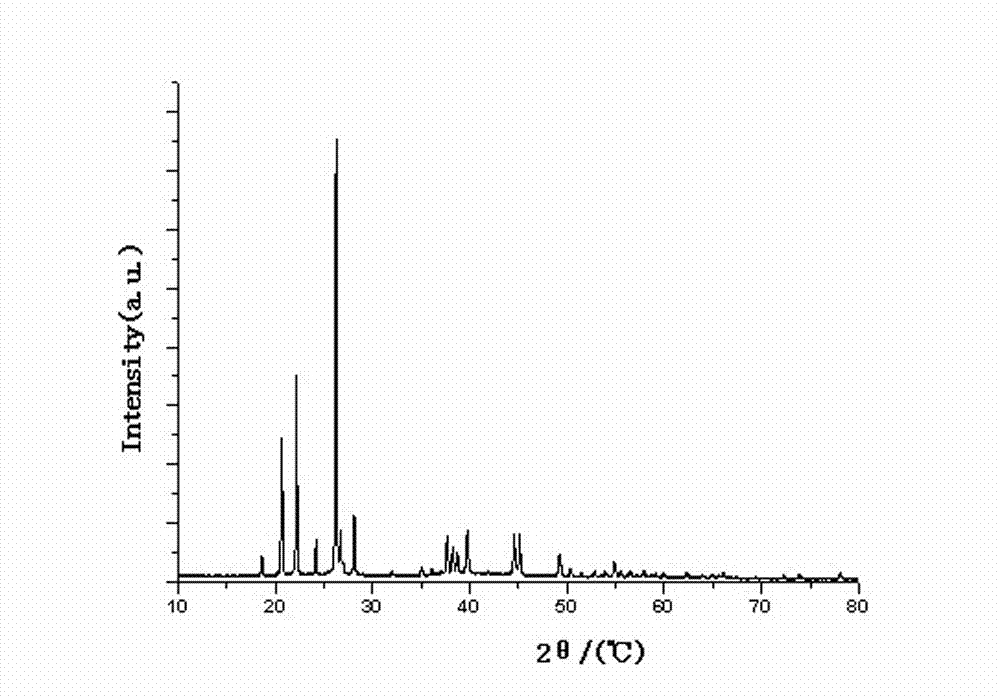
The invention relates to a method for preparing an anhydrous lithium tetrafluoroborate which comprises the following steps: a lithium source including lithium hydroxide, lithium carbonate, and the like, reacts with fluorine hydride and boric acid to obtain lithium tetrafluoroborate solution, then the lithium tetrafluoroborate solution is condensed, crystallized and recrystallized, ground, and vacuum dried to obtain the anhydrous lithium tetrafluoroborate. The method of the invention adopts a staged temperature rise control, the process of the preparation is simple, raw materials are cheap and the preparation cost is low, no organic solvent is used during the synthetic process, no poison is produced, therefore, the method accords with the concept of green environmental protection, the anhydrous lithium tetrafluoroborate prepared by the method is determined by a X-ray diffraction map, the diffraction peak is clear and sharp and completely matches the standard card, which shows that the product prepared by the method of the invention is anhydrous LiBF4 with complete crystal form, the quality of the product is equal to the quality of the anhydrous lithium tetrafluoroborate prepared by the reaction of lithium fluoride and boron trifluoride.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Non-aqueous electrolyte and lithium ion battery using the same
ActiveCN104332653AGuaranteed high temperature performanceImprove low temperature cycle performanceSecondary cellsHigh rateOrganic solvent
The invention relates to a non-aqueous electrolyte and a lithium ion battery using the same. The non-aqueous electrolyte comprises a non-aqueous organic solvent, lithium salts, and an additive, and is characterized in that the additive comprises phosphonic acid cyclic anhydride compounds and lithium tetrafluoroborate, the mass percentage of phosphonic acid cyclic anhydride compounds in electrolyte is 0.05% to 3%, and the mass percentage of lithium tetrafluoroborate in electrolyte is 0.01% to 2%. Compared to the prior art, the lithium ion battery using the electrolyte has the characteristics of excellent low temperature cycle performance and high-rate discharge performance.
Owner:DONGGUAN AMPEREX TECH
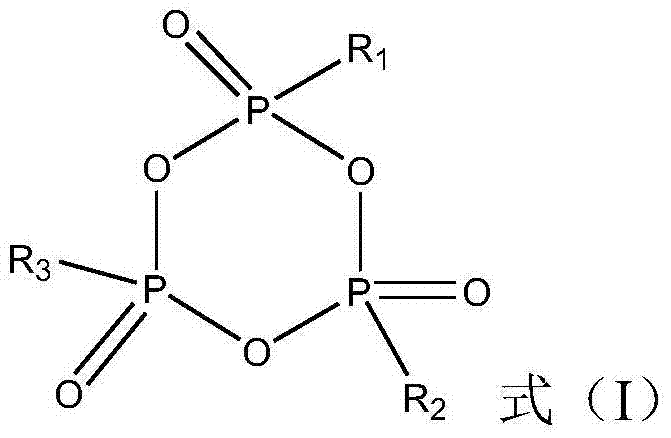
High temperature-resistant electrolyte solution of lithium ion battery
ActiveCN103825049AImprove cycle stabilitySmall capacity attenuationSecondary cellsOrganic baseSolvent
The invention provides a high temperature-resistant electrolyte solution of a lithium ion battery. The high temperature-resistant electrolyte solution of the lithium ion battery comprises the raw materials of lithium electrolyte salt, an organic solvent, a high temperature-resistant additive, a film forming additive and a circulatory stability additive, wherein the concentration of the lithium electrolyte salt in the organic solvent is 0.5-2 mol / L; the organic solvent comprises the following components in parts by volume: 5-30 parts of a high-dielectric-constant organic base solvent, 40-65 parts of a high-boiling-point organic solvent, and 5-55 parts of a low-viscosity organic solvent; the high temperature-resistant additive is at least one of lithium tetrafluoroborate, lithium difluoroborate, lithium bis(malonato)borate, lithium bis(oxalate)borate and lithium malonato oxalate borate, the mass of the high temperature-resistant additive accounts for 0.1-8% of the total mass of the electrolyte solution, the mass of the film forming additive accounts for 0.2-4% of the total mass of the electrolyte solution, and the mass of the circulatory stability additive accounts for 0.5-5% of the total mass of the electrolyte solution. According to the invention, the high temperature resistance and circulatory stability of the lithium ion battery are effectively improved.
Owner:DONGFENG COMML VEHICLE CO LTD
Electrolyte for power lithium ion battery
InactiveCN102544582AImprove wettabilityStable electrochemical propertiesSecondary cellsPolyolefinSolvent
The invention discloses high-wettability and high-pourability electrolyte for a power lithium ion battery. The electrolyte comprises a wetting additive, a lithium salt, a non-aqueous organic solvent and other additives, wherein the wetting additive is a nonionic surfactant, and the wetting additive accounts for 0.001 to 5 percent of the mass of the electrolyte; the non-aqueous solvent is one or a mixture of more of ethylene carbonate, propylene carbonate, methyl ethyl carbonate, dimethyl carbonate, diethyl carbonate and gamma-butyrolactone, and the non-aqueous solvent accounts for 50 to 90 percent of the mass of the electrolyte; the lithium salt at the concentration of 0.6 to 1.5 mol / L is at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(oxalato)borate and lithium trifluoromethanesulfonate; and the other additives accounts for 0.5 to 8 percent of the mass of the electrolyte. The electrolyte has high wettability for a polyolefin diaphragm and electrode active materials, and has long cycle life.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
Low temperature type lithium ion battery electrolyte with high temperature property and lithium ion battery
InactiveCN101867064AEasy to manufactureEasy to operateSecondary cellsCell component detailsOxalateOrganic solvent
The invention discloses a low temperature type lithium ion battery electrolyte with high temperature property and lithium ion battery. The electrolyte is made by uniformly mixing and confecting electrolytic salt, non-water organic solvent, alkali additive and film-forming additive, the electrolytic salt is mixed lithium salt made by uniformly mixing lithium tetrafluoroborate and lithium bis(oxalate)borate, and the mol ratio of the two is 96-73: 4-27, the concentration of the mixed lithium salt solution formed by the electrolytic salt and the non-water organic solvent is 0.8-1.2mol / L; the alkali additive is heptamethyldisilazane and the mass ratio of the hepatmethyldisilazane and the non-water organic solvent is 0.1-8%; the mass ratio of the film-forming additive and the non-water organic solvent is 0.1-5%; and the lithium ion battery comprises an anode, a cathode, a diaphragm and low temperature type lithium ion battery electrolyte with high temperature property. The invention has reasonable design, simple preparation method steps and convenient implementation, and the prepared electrolyte and lithium ion battery have favourable comprehensive performances.
Owner:XIAN SAFTY ENERGY TECH
Electrolyte
InactiveCN102185156AThe group ratio is reasonableReasonable ratioSecondary cellsHigh energyMethyl carbonate
The invention relates to electrolyte, belonging to the technical field of material chemistry and high energy batteries. The electrolyte consists of an organic solvent, an additive and lithium salt, wherein the organic solvent is one or a mixture of ethylene carbonate, ethyl methyl carbonate, methyl-carbonate or diethyl carbonate; the additive is sulfurous ester; and the lithium salt is selected from lithium perchlorate, lithium hexafluorophosphate, lithium tetrafluoroborate, perfluoroalkyl lithium sulfonate, perfluoroalkyl sulfoacid imide lithium, annular perfluoroalkyl di(sulfonyl)lithium imide, perfluoroalkyl acyl sulfonate lithium methide, organic boric acid ester lithium, organic lithium phosphate or organic aluminic acid ester lithium. Due to the adoption of the electrolyte, a layer of stable solid electrolyte phase boundary face film can be formed on the surface of a graphite electrode, the compatibility between the electrolyte and an electrode material is improved, the temperature suitability of the electrolyte material is expanded through proportion optimization, and the cycle performance, rate capability and temperature suitability of a lithium secondary battery using the electrolyte can be effectively improved.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Electrolyte for lithium secondary battery and lithium secondary battery comprising same
PURPOSE: Provided are a non-aqueous electrolyte capable of improving the electrochemical property, and a lithium secondary cell excellent in electrochemical property. CONSTITUTION: The electrolyte comprises a lithium salt; a non-aqueous, organic solvent; and at least one of organic compound selected from compounds of formula 1 to 4(wherein the formula 3 is R9-SO3-Si-(CmH2m+1)3 and formula 4 is CnX2n+1-SO3-Si(CmH2m+1)3). In the formulae, each of R1 to R9 independently represents primary, secondary, or tertiary alkyl group, alkenyl group or aryl group, X is hydrogen or halogen atom, and each of n and m is 0-3. The lithium salt is selected from the group consisting of lithium hexafluorophosphate(LiPF6), lithium tetrafluoroborate(LiBF4), lithium perchlorate(LiClO4), lithium trifluoromethanesulfonate(LiCF3SO3), lithium hexafluoroarsenate(LiAsF6), and mixture thereof.
Owner:SAMSUNG SDI CO LTD +1
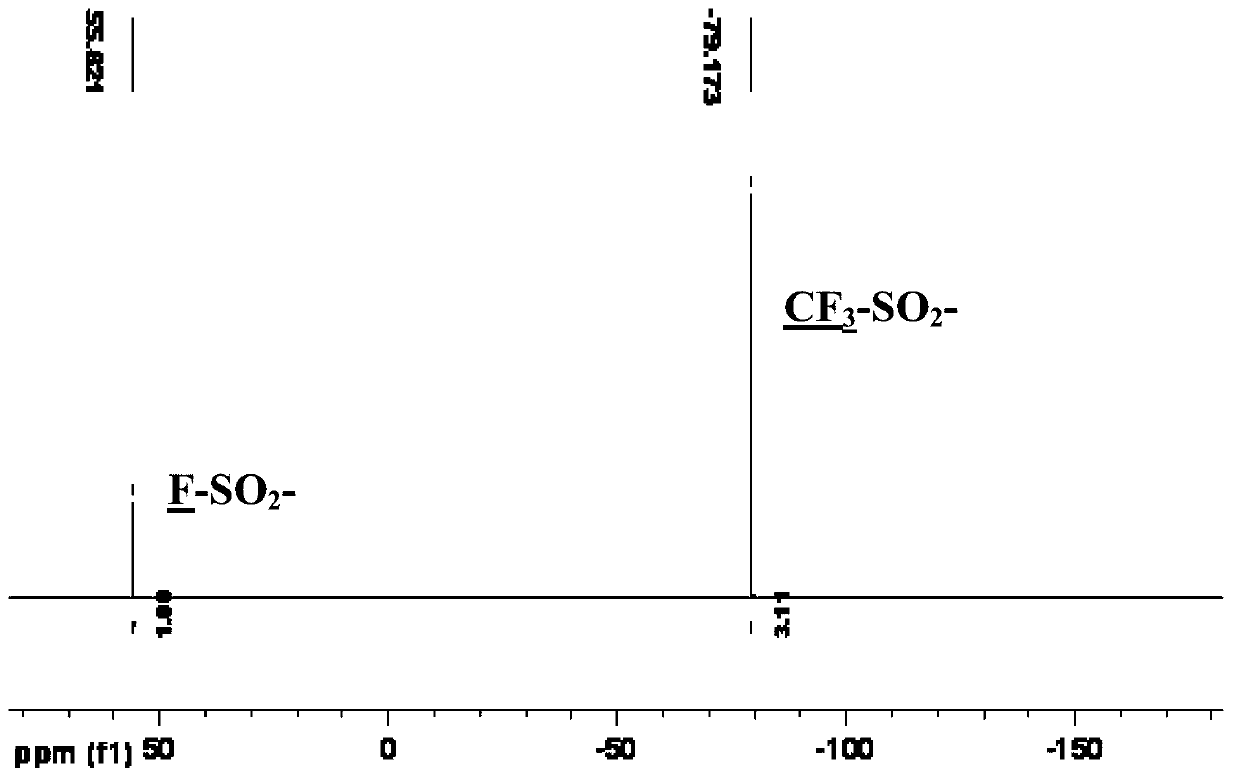
Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
ActiveCN102786452AOvercome operabilityOvercome fatal shortcomings such as difficult product purificationSulfonic acid amide preparationTetrafluoroborateDecomposition
The invention discloses a method for preparing bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. According to the method, sulfamide is utilized to take reaction with thionyl chloride and chlorosulfonic acid for preparing bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine, then, the bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine takes reaction with antimony trifluoride and potassium (rubidium or caesium and the like) carbonate, and corresponding high-purity bis(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt can be obtained; and the double decomposition exchange reaction of the potassium (rubidium or caesium) salt and lithium (or sodium) perchlorate or lithium (or sodium) tetrafluoroborate and the like in aprotic polar solvents is utilized to obtain corresponding high-purity lithium (or sodium) salt. The method provided by the invention has the characteristics that the operation step is simple, the products can be easily separated and purified, the purity and the yield are high, the environment pollution is avoided, the method is suitable for industrial mass production, and the like.
Owner:武汉市瑞华新能源科技有限公司
Primary fluorinated carbon lithium battery and preparation method thereof
InactiveCN103000915AImprove electrode conductivityLow viscosityNon-aqueous electrolyte cellsPrimary cell electrodesMetallic lithiumElectrolytic agent
The invention discloses a primary fluorinated carbon lithium battery and a preparation method thereof. The battery comprises a positive electrode, a negative electrode with metallic lithium as active materials, diaphragms, a non-aqueous electrolyte, pole lugs and a case, wherein the positive electrode comprises fluorinated carbon active materials and electric conduction additives, and the non-aqueous electrolyte comprises one component of lithium tetrafluoroborate (LIBF4), propylene carbonate(PC), 1,2dimethoxyethane (DME), gamma-butyrolactone (BL), dimethyl tetrahydrofuran (2Me-THF) and dioxolane (DOL). According to the primary fluorinated carbon lithium battery, the positive electrode is made of fluorinated carbon material with high fluoride content, various electric conduction additives are used for improving electric conductivity of the electrodes, and the electrolyte is low in viscosity, good in liquidity, and large in quantity of solid liquid interfaces, so that conductivity of the solid liquid interfaces and ions can be improved effectively, a liquid injection volume is reduced, electric discharge with different rates from 50h-rate to 5h-rate can be achieved, gravimetric specific energy of the battery achieves 400-610Wh / kg, and volumetric specific energy of the battery achieves 700-950Wh / L. Furthermore, the primary fluorinated carbon lithium battery is strong in processing property, and suitable for large-scale production and application.
Owner:天津蓝天特种电源科技股份公司
Preparation method of battery-grade anhydrous lithium tetrafluoroborate
The invention discloses a preparation method of battery-grade anhydrous lithium tetrafluoroborate. The preparation method comprises the following steps of: making hydrofluoric acid and boric acid react and adding lithium carbonate or lithium hydroxide to obtain a lithium tetrafluoroborate solution; then, filtering, evaporating, concentrating, crystallizing, separating and drying; and adding absolute ethanol to dissolve in a glove box and evaporating the ethanol to obtain a lithium tetrafluoroborate product. The invention improves an aqueous solution method, synthesizes in an inorganic medium, extracts with an organic solvent, further dries to obtain an anhydrous product, overcomes the difficulties in dehydration, extraction and separation because of the inorganic medium and has low cost and convenient operation.
Owner:XINJIANG RES INST OF NON FERROUS METALS
Preparation method of lithium ion battery electrolyte salt LiODFB (lithium oxalyldifluroborate)
InactiveCN104628754AAvoid wastingIncrease profitGroup 3/13 element organic compoundsFiltrationSolvent
The invention relates to a preparation method of lithium ion battery electrolyte salt LiODFB (lithium oxalyldifluroborate). The method comprises the following steps: performing catalytic synthesis of lithium oxalate and boron trifluoride diethyl ether in a solvent of DMC (dimethyl carbonate) and the like to obtain a liquid-phase mixture and a little unreacted lithium oxalate solid, performing filtration, and then performing evaporative crystallization to obtain crude LiODFB; recrystallizing the crude LiODFB to meet the requirement of lithium ion battery electrolyte salt; and collecting filtered mother liquid and crystallization mother liquid, adding oxalate and a catalyst, performing catalytic conversion to obtain a liquid-phase mixture which mainly contains LiODFB, and performing evaporative crystallization again. The technological process is low in cost, the purity of the prepared LiODFB is above 99.9%, the yield is above 90%, and the method is convenient to operate, has favorable economic benefits and environmental benefits and is suitable for industrial production.
Owner:HUNAN ZHENGYUAN ENERGY STORAGE MATERIALS & DEVICE INST +1
Co-production method for lithium oxalyldifluoroborate and lithium tetrafluoroborate
InactiveCN103374023AHigh purityHigh yieldGroup 3/13 element organic compoundsBoron halogen compoundsProtonSolvent
The invention discloses a co-production method for lithium oxalyldifluoroborate and lithium tetrafluoroborate. The co-production method comprises the following steps of: (1) uniformly mixing compounds containing lithium salts and BF3 in an aprotic nonpolar solvent or a solvent with a low aprotic polarity in a molar ratio of (1: 1: 3)-(1: 1.25: 3.75) of lithium to boron to fluorine; (2) refluxing for 1-48 hours at a temperature of 30-100 DEG C, then performing solid-liquid separation, and drying the obtained solid substance to obtain crude products LiODFB and LiBF4; and (3) performing purification and separation on the obtained crude products for one time, or performing purification for many times.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
Electrolyte and application thereof
InactiveCN103000945AImprove cycle performanceHigh densitySecondary cellsElectrolytic agentOrganosolv
The invention relates to an electrolyte. The electrolyte comprises an organic solvent, lithium hexafluorophosphate and additives, wherein the additives comprise lithium tetrafluoroborate, 1,3-propane sultone, lithium bis(oxalate) borate, adiponitrile, vinylene carbonate and fluoroethylene carbonate, and in the electrolyte, the mass concentration of the lithium tetrafluoroborate is 0.5%-2%, the mass concentration of the 1,3-propane sultone is 1%-4%, the mass concentration of the lithium bis(oxalate) borate is 1%-5%, the mass concentration of the adiponitrile is 1%-3%, the mass concentration of the vinylene carbonate is 0.5%-2%, and the mass concentration of the fluoroethylene carbonate is 2%-5%. The electrolyte can be used for improving the cycle performance, the high-temperature storage performance and the initial energy density of a lithium-ion battery. The invention further provides an application of the electrolyte in the lithium-ion battery.
Owner:EVE HYPERPOWER BATTERIES INC +1
Method for synthesizing an electrolyte lithium salt: lithium difluoro(oxalate)borate
ActiveCN107698611AHigh reaction conversion rateEasy to realize industrial productionGroup 3/13 element organic compoundsSilane compoundsOxalate
The invention relates to a method for synthesizing an electrolyte lithium salt: lithium difluoro(oxalate)borate. The method comprises the following steps: 1) performing reaction on a silane compound with oxalic acid to obtain a condensation compound of silane and oxalic acid; 2) performing reaction on lithium tetrafluoroborate with the condensation compound of the silane and oxalic acid in a solvent to obtain a crude product; and 3) performing recrystallization on the crude product to the obtain lithium difluoro(oxalate)borate. The synthesizing method not only can be used to obtain the high-purity lithium difluoro(oxalate)borate, but also can effectively reduce the acidity and Cl content of the lithium difluoro(oxalate)borate.
Owner:浙江圣持新材料科技有限公司 +1
Preparation method of high-purity lithium tetrafluoroborate
The invention provides a preparation method of high-purity lithium tetrafluoroborate; and the method comprises the following steps that high-purity lithium fluoride and a boron trifluoride coordination compound react in chain carbonate organic solvent, and lithium tetrafluoroborate is obtained after filtration, concentration, extractive crystallization, washing and drying. In the preparation method of high-purity lithium tetrafluoroborate, the boron trifluoride coordination compound has wide sources of raw materials and is low in price, the linear carbonate organic solvent has low toxin and is environment-friendly, the synthetic reaction conditions are mild, the operation is simple, the equipment investment is low, the lithium tetrafluoroborate productivity is high, the yield is high, the energy consumption is low, the post-treatment is simple, and the method is applicable to large-scale production.
Owner:GUANGZHOU TINCI MATERIALS TECH +1
Electrolyte of the lithium ion battery for ultra-low temperature discharge and its lithium ion battery
InactiveCN101017918AImprove performanceExcellent cycle indexOrganic electrolyte cellsSecondary cellsDimethoxyethaneEthyl carbonate
The related Li-ion cell fit to discharge at ultra-low temperature comprises an anode, a cathode, a membrane, and the electrolyte. Wherein, the electrolyte comprises: lithium hexafluorophosphate and lithium tetrafluoroborate with weight ratio as 1:5-10:1, and the solvent (including ethylene carbonate, dimethyl carbonate, methyl- ethyl carbonate, and dimethoxyethane). Wherein, the dimethoxyethane is 0.5-10wt%. Compared with prior art, this product can work well at -40deg.
Owner:HANGZHOU SKYRICH POWER CO LTD
Rechargeable lithium battery
ActiveUS20080206650A1Large capacityImprove cycle lifeOrganic electrolyte cellsSolid electrolyte cellsOrganic solventLithium imide
A rechargeable lithium battery includes a positive electrode including a positive active material being capable of intercalating and deintercalating lithium ions; a negative electrode including a negative active material being capable of intercalating and deintercalating lithium ions; and an electrolyte including a non-aqueous organic solvent and a lithium salt. The positive electrode has a positive active mass density of 3.65 g / cc or more, and the lithium salt includes lithium hexafluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4), and a lithium imide-based compound. The rechargeable lithium battery has high capacity, excellent cycle-life, and reliability at a high temperature.
Owner:SAMSUNG SDI CO LTD
Superlow-temperature lithium ion battery electrolyte and lithium ion battery using same
ActiveCN108511800ALow melting pointLow viscositySecondary cellsOrganic electrolytesDifluorophosphateN dimethylformamide
The invention discloses a superlow-temperature lithium ion battery electrolyte. The electrolyte is prepared from the following raw materials of organic solvents, lithium salt and a film forming additive, wherein the organic solvents comprise carbon disulfide, ethyl butyrate, diglyme and N,N-dimethylformamide; the lithium salt is lithium tetrafluoroborate; the film forming additive consists of vinylene carbonate and any one of ethylene sulfate, lithium difluorophosphate and imidodisulfuryl fluoride. Compared with t traditional carbonate solvents, the electrolyte has the advantages that the melting point of the used organic solvent is very low, and the higher ion conductivity is still maintained at superlow temperature of -40 DEG C; the additive forms a film at the surface of a cathode, andcan form a stable SEI (solid electrolyte interface) film with low impedance, so as to quickly intercalate and de-intercalate the lithium ions at low temperature. The invention also discloses the lithium ion battery using the electrolyte. The lithium ion battery can reach excellent low-temperature discharge and cycle properties under the superlow-temperature environment.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Lithium ion battery electrolyte and lithium ion battery containing electrolyte
InactiveCN107546415AImprove cycle lifeImprove thermal stabilitySecondary cellsOrganic electrolytesHigh temperature storageOxalate
The invention provides a lithium ion battery electrolyte and a lithium ion battery containing the electrolyte. The lithium ion battery electrolyte includes lithium salt, a multi-element organic solvent, a film-forming additive, an SEI film shape modifier and an impregnating additive, the lithium salt is formed by selecting and mixing at least two of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium difluoroborate, lithium imidodisulfuryl fluoride and lithium bis(oxalate)borate, and the concentration of the lithium salt in the electrolyte is 0.75-1.5 mol / L. The lithium ion batteryis long in cycle life, good in high-temperature storage performance and long in cycle life at 55 DEG C, and excellent in low-temperature performance, and meets the requirements for various performances of power batteries in the actual running process of electric buses.
Owner:CHAOYANG GUANGDA CHEM
Method for preparing anhydrous high-purity lithium tetrafluoroborate
InactiveCN101863489ASimplify the steps of removing crystal waterReduce manufacturing costSecondary cellsBoratesAcetic acidBoron trifluoride
The invention discloses a method for preparing anhydrous high-purity lithium tetrafluoroborate, which is characterized by comprising the following steps of: mixing 1 to 3mol of 98 to 99.55 percent of industrial boric acid and 2.5 to 12mol of excessive 150 to 300 percent fuming sulfuric acid with 9 to 20 percent of SO3 to obtain boron-containing mixed acid solution, adding 1 to 3mol of fluorine-containing compound into the boron-containing mixed acid solution, heating the solution to 80 DEG C to obtain high-purity gaseous boron trifluoride (BF3), compressing the obtained boron trifluoride gas into lithium fluoride-containing ethyl acetate by using a compressor to perform contact reaction for 2 to 7 hours till the suspension is clarified, distilling and crystallizing the reaction solution at the temperature of between 60 and 70 DEG C under reduced pressure to obtain a wet lithium tetrafluoroborate product, washing and purifying the wet lithium tetrafluoroborate product by using washing and purifying agents, and drying the product to obtain the anhydrous high-purity lithium tetrafluoroborate.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Electrolyte for lithium titanate lithium ion battery
InactiveCN104900917ASolve or relieve gas problemsSecondary cellsNon-aqueous electrolytesLithium hexafluorophosphateLithium electrode
The invention discloses electrolyte for a lithium titanate lithium ion battery. The electrolyte for the lithium titanate lithium ion battery comprises main electrolyte lithium salts, auxiliary electrolyte lithium salts, non-aqueous solvent and additives, wherein main electrolyte lithium salts are lithium hexafluorophosphate, and the concentration of the lithium hexafluorophosphate in the electrolyte is 0.5 to 1.5mol / L; the concentration of the auxiliary electrolyte lithium salts in the electrolyte is 0.005 to 0.5mol / L and the auxiliary electrolyte lithium salts are selected from any one or two or more than two kinds of lithium tetrafluoroborate, lithium bisoxalatoborate, lithium bisfluoroxalatoborate, lithium bis(trifluoromethane sulfonimide) and lithium bis(fluorosulfonyl)imide. According to the electrolyte provided by the invention, the electrolyte lithium salts approximately count for 8 to 15 percent of the total mass percent and are formed by compounding the main electrolyte lithium salts and the auxiliary electrolyte lithium salts; the electrolyte additives are optimized. The lithium titanate lithium ion battery adopting the electrolyte disclosed by the invention has excellent cycle performance, rate performance and high-and-low temperature performance; in addition, an effective interfacial film is formed on a cathode lithium titanate interface, so that the gas expansion phenomenon of the lithium titanate lithium ion battery is greatly relieved.
Owner:SHANGHAI POWER ENERGY STORAGE BATTERY SYST ENG TECH +1
Preparation method of ion liquid crystal/polyimidazole semi-interpenetrating network polymer electrolyte
ActiveCN107946641AFast aggregationLess quantitySolid electrolytesSecondary cellsCross-linkPolymer science
The invention relates to the technical field of a battery material, and provides a preparation method of an ion liquid crystal / polyimidazole semi-interpenetrating network polymer electrolyte. The method comprises the steps of taking an imidazole liquid (MOBIm-BF4), polyethylene glycol diacrylate (PEGDA), ion liquid crystal ([Cmin]BF4) and lithium tetrafluoroborate (LiBF4) as raw materials, performing ultraviolet irradiation curing to form a film in an organic solvent under a condition that a photoinitiator participates, and obtaining the cross-linked polymerization semi-interpenetrating network solid-state polymer electrolyte after drying, wherein the MOBIm-BF4 can be photo-initiated and polymerized to obtain polyimidazole, and then photo-initiation polymerization is performed after the polyimidazole, the PEGDA, the [Cmin]BF4 and the LiBF4 are mixed, or photo-initiation polymerization is performed on the MOBIm-BF4, the [Cmin]BF4 and the LiBF4 and then photo-initiation polymerization isperformed after the PEGDA is added for mixing. The method is simple and efficient and is suitable for industrial application. The maximum electrical conductivity of the ion liquid crystal / polyimidazole semi-interpenetrating network polymer electrolyte prepared by the method can reach 10<-5>S cm<-1>.
Owner:NANCHANG HANGKONG UNIVERSITY
Electrolyte of lithium ion battery and lithium ion battery utilizing same
ActiveCN104466248AReduce oxidationIncrease loopSecondary cellsOrganic electrolytesOrganic solventInternal resistance
The invention discloses electrolyte of a lithium ion battery. The electrolyte comprises an organic solvent, electrolyte lithium salt and additives, wherein the additives include butanedinitrile, fluorobenzene and lithium tetrafluoroborate; the mass percentage of the fluorobenzene in the electrolyte is 0.1 to 15 percent; the mass percentage of the butanedinitrile in the electrolyte is 0.1 to 10 percent; the mass percentage of the lithium tetrafluoroborate in the electrolyte is 0.01 to 1 percent. By adopting the electrolyte, while the charging upper limit voltage and high temperature intermittent cycling performance of the lithium ion battery are improved, simultaneously the expansion rate of the battery is reduced, the internal resistance is reduced, and the stability and the safety of the lithium ion battery can be improved.
Owner:DONGGUAN AMPEREX TECH +1
Method for producing lithium tetrafluoroborate solution
ActiveCN103733416AEasy to controlReduced concentration of acidic impuritiesFinal product manufactureLi-accumulatorsBoron trifluorideWater concentration
This method for producing a lithium tetrafluoroborate solution for lithium battery electrolyte solutions comprises: a reaction step wherein lithium fluoride and boron trifluoride are reacted with each other in a chain carbonic acid ester that serves as a solvent, thereby producing lithium tetrafluoroborate and obtaining a reaction solution that is obtained by dissolving the lithium tetrafluoroborate in the solvent; a water removal step wherein a water remover is added into the reaction solution; an acidic impurity removal step wherein acidic impurities are removed by concentrating the reaction solution after the water removal step; and a dilution step wherein the concentrated solution after the acidic impurity removal step is diluted. This production method is capable of providing a method for producing a lithium tetrafluoroborate solution for lithium battery electrolyte solutions, said lithium tetrafluoroborate solution having an acidic impurity concentration reduced to 50 ppm by mass or less and a water concentration reduced to 15 ppm by mass or less.
Owner:ZHEJIANG SINO NITROGEN KANGPENG CHEM CO LTD
Method for preparing bis(fluorosulfonyl)lithium imine
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Low-temperature lithium-ion battery
ActiveCN106531984AImprove conductivityHigh specific surface areaFinal product manufactureCell electrodesLithium iron phosphatePhysical chemistry
The invention relates to a low-temperature lithium-ion battery, which comprises a positive pole piece, a negative pole piece, a membrane, a low-temperature electrolyte and a housing, wherein each of the positive pole piece and the negative pole piece comprises a positive active material, a negative active material, a binder and a conductive agent; the positive active material is one or a mixture of more of a lithium cobalt oxide, lithium manganate and lithium iron phosphate and accounts for 80%-97% of total mass of a coating of the positive pole piece, the compaction density of the coating is 3.2-3.5 and the single-sided surface density of the coating of the positive pole piece is 50-210g / m<2>; the negative active material is synthetic graphite and accounts for 85%-95% of total mass of the coating of the negative pole piece, the compaction density of the coating is 1.2-1.45, the single-sided surface density of the coating of the negative pole piece is 40-110g / m<2>; and the low-temperature electrolyte is prepared from the following components in percentage by mass: 5%-20% of lithium hexafluorophosphate, 1%-5% of lithium tetrafluoroborate, 20%-30% of ethylene carbonate, 20%-30% of methyl ethyl carbonate, 20%-40% of methyl acetate, 0.5%-2% of glycol sulfite and 0.5%-2% of N,N-dimethyl trifluoroacetamide.
Owner:GUANGDONG JIUZHOU SOLAR ENERGY TECH CO LTD
Electrolyte capable of suppressing gas production of lithium ion batteries
InactiveCN109378524AReduce gas productionImprove securitySecondary cellsOrganic electrolytesDifluorophosphatePhosphate
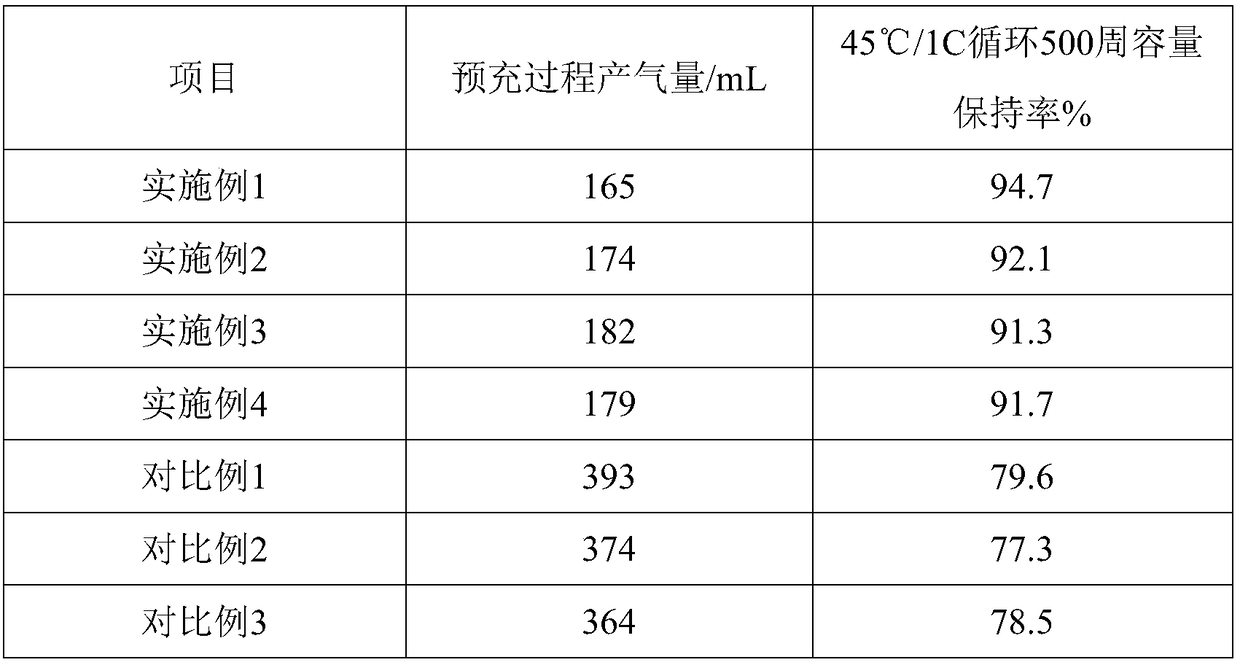
The invention relates to the field of lithium ion batteries, in particular to an electrolyte capable of suppressing gas production of lithium ion batteries. The electrolyte comprises lithium salt, organic solvents and additives; the lithium salt is selected from one or multiple of lithium hexafluoroarsenate, lithium tetrafluoroborate, lithium hexafluorophosphate and lithium bis(fluorosulfonyl)imide, and the concentration of the lithium salt substance ranges from 0.5 mol / L to 2.0 mol / L; the additives are selected from one or multiple of lithium difluoroborate, tetrafluoroethylene oxalic acid phosphate lithium,1,3-propane suhone, DTD, vinylethylene carbonate, propylene sulfite, 1,4-butane sultone, tris(trimethylsilyl) phosphate, fluoroethylene carbonate and lithium difluorophosphate, and theusage of the additives is equal to 2-2.5% of the total mass of the lithium salt and the organic solvents. The electrolyte is used for preparation of the lithium ion batteries, can suppress gas production rate of the lithium ion batteries during precharging, and accordingly improves production efficiency of the lithium ion batteries and performance thereof.
Owner:福建冠城瑞闽新能源科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com