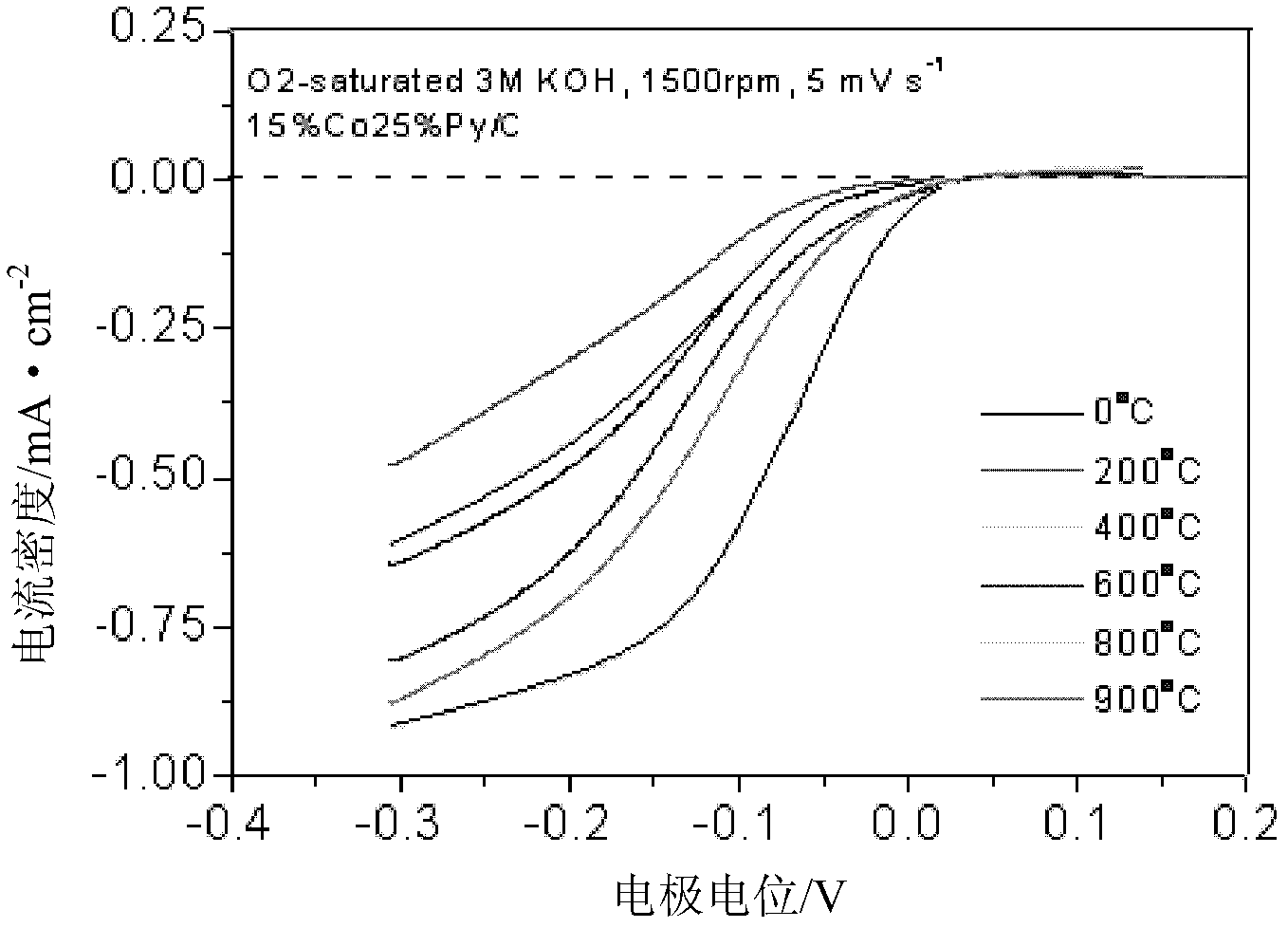
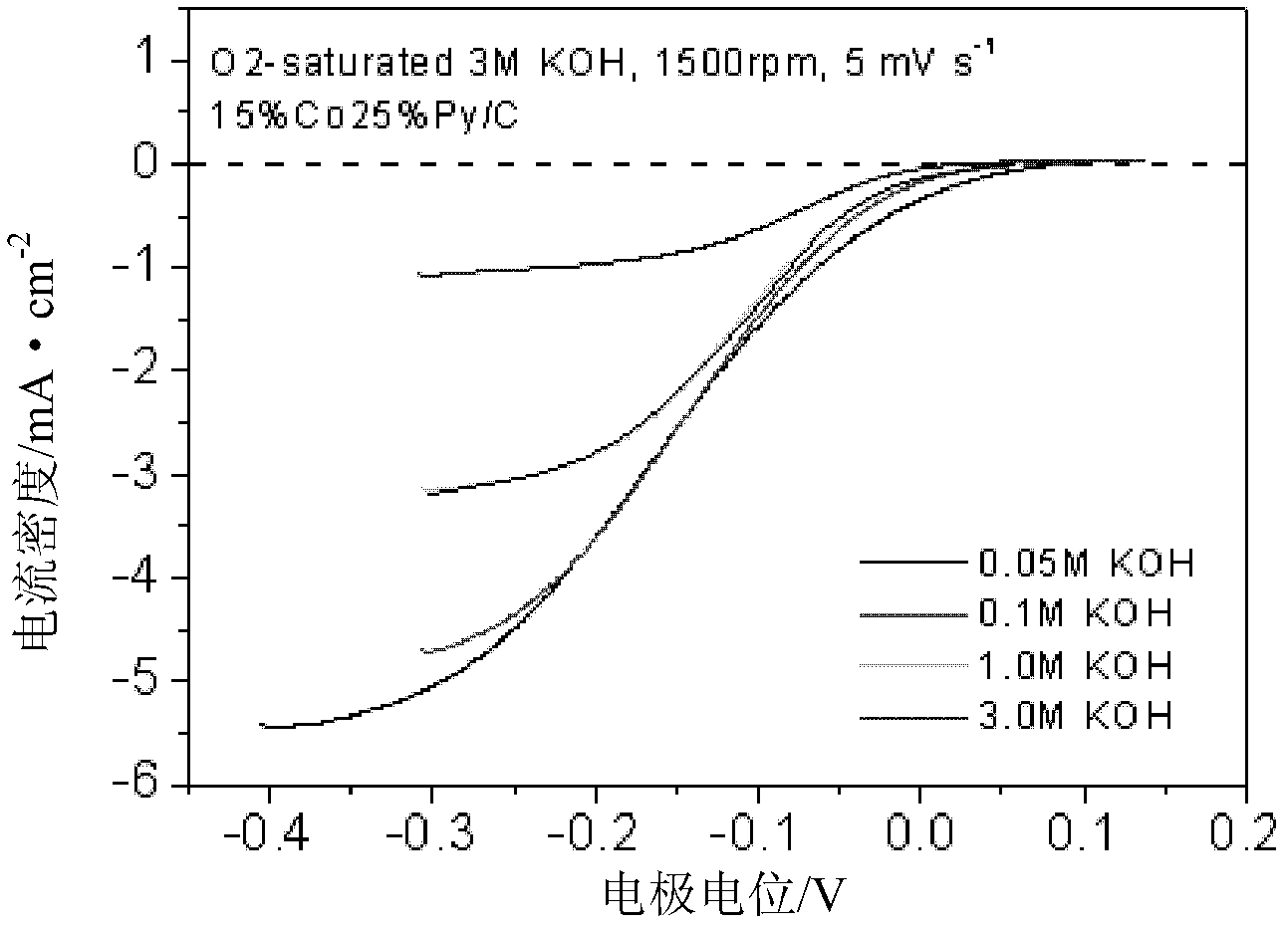
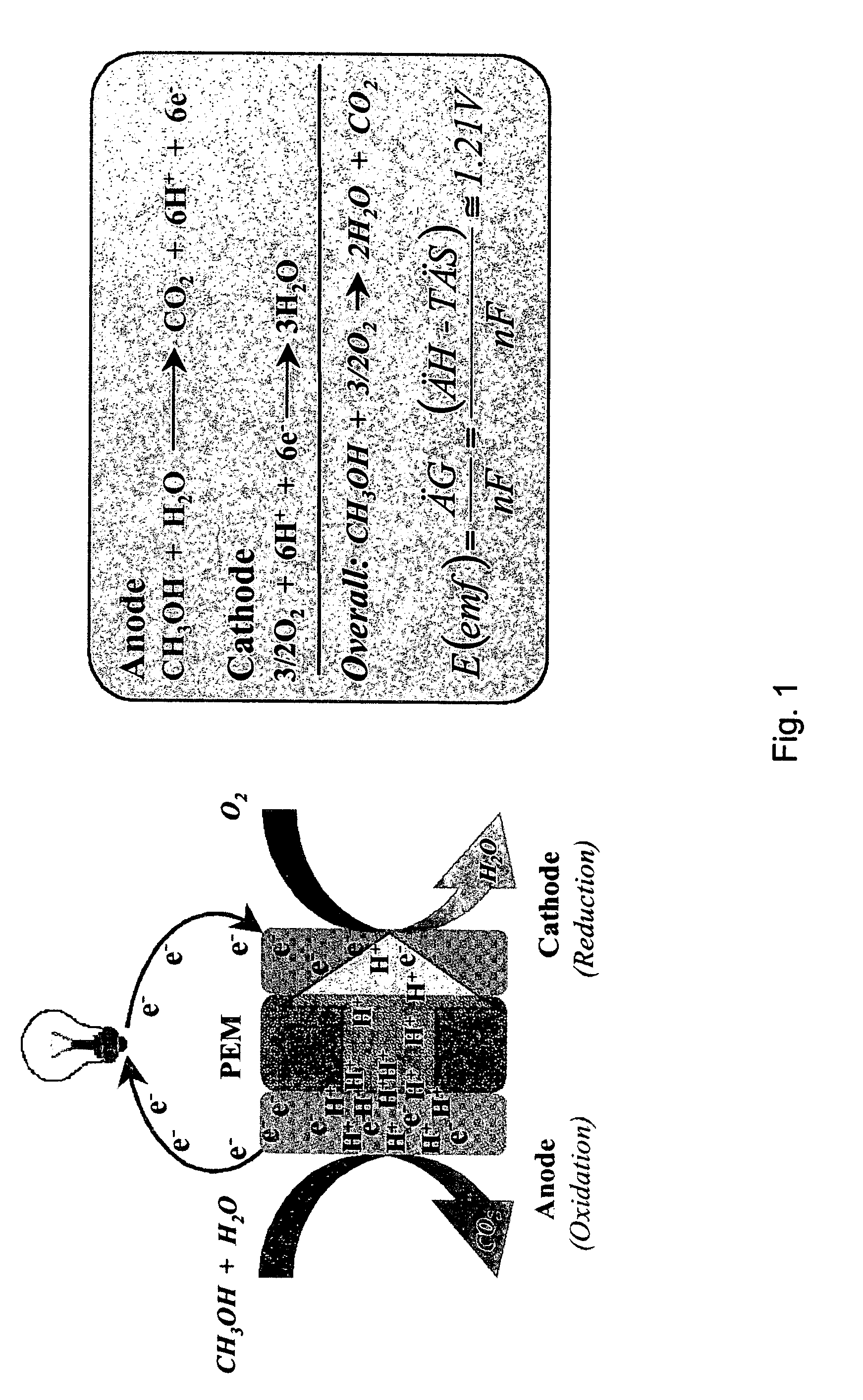
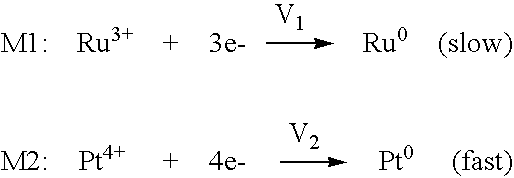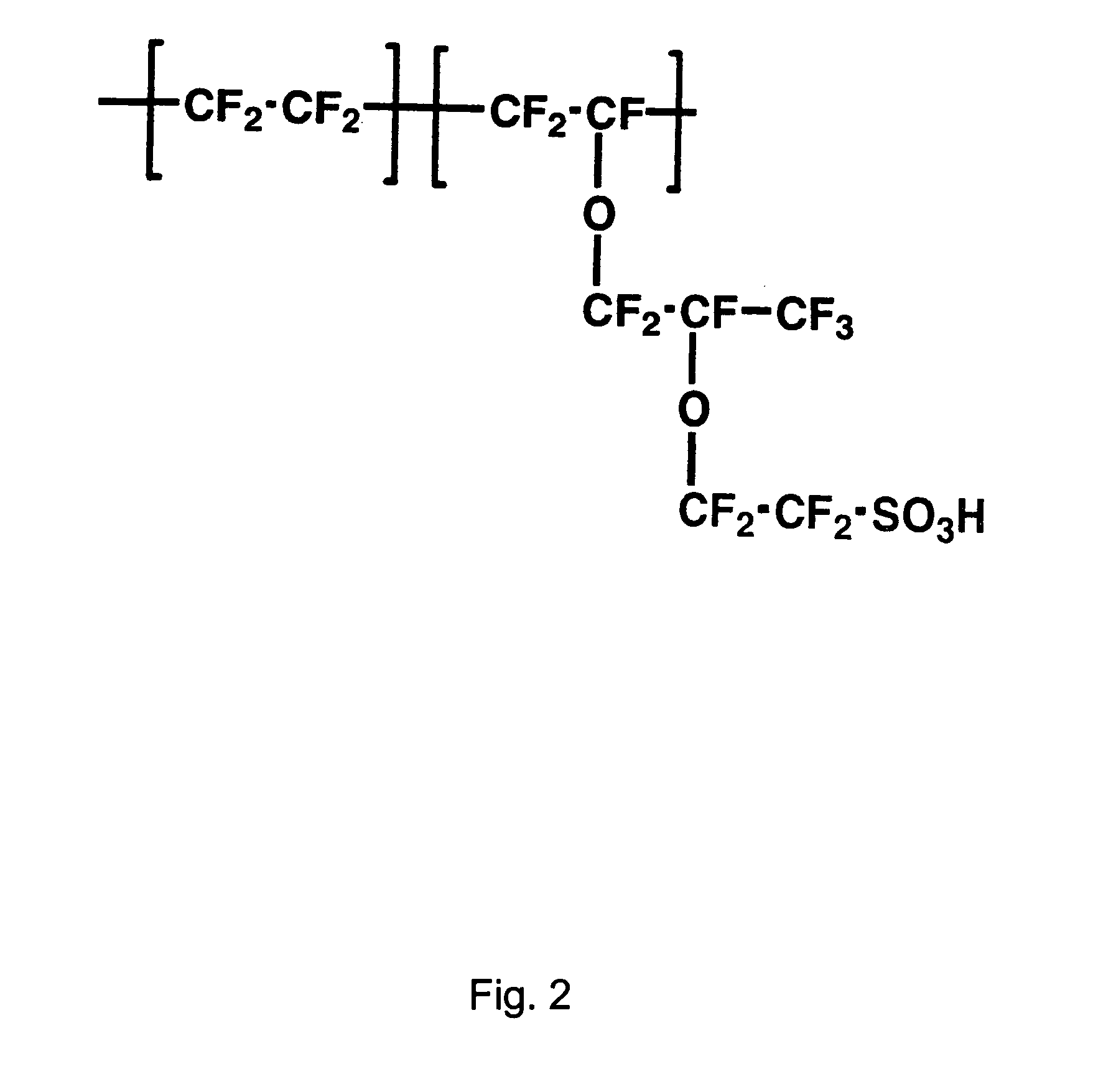Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
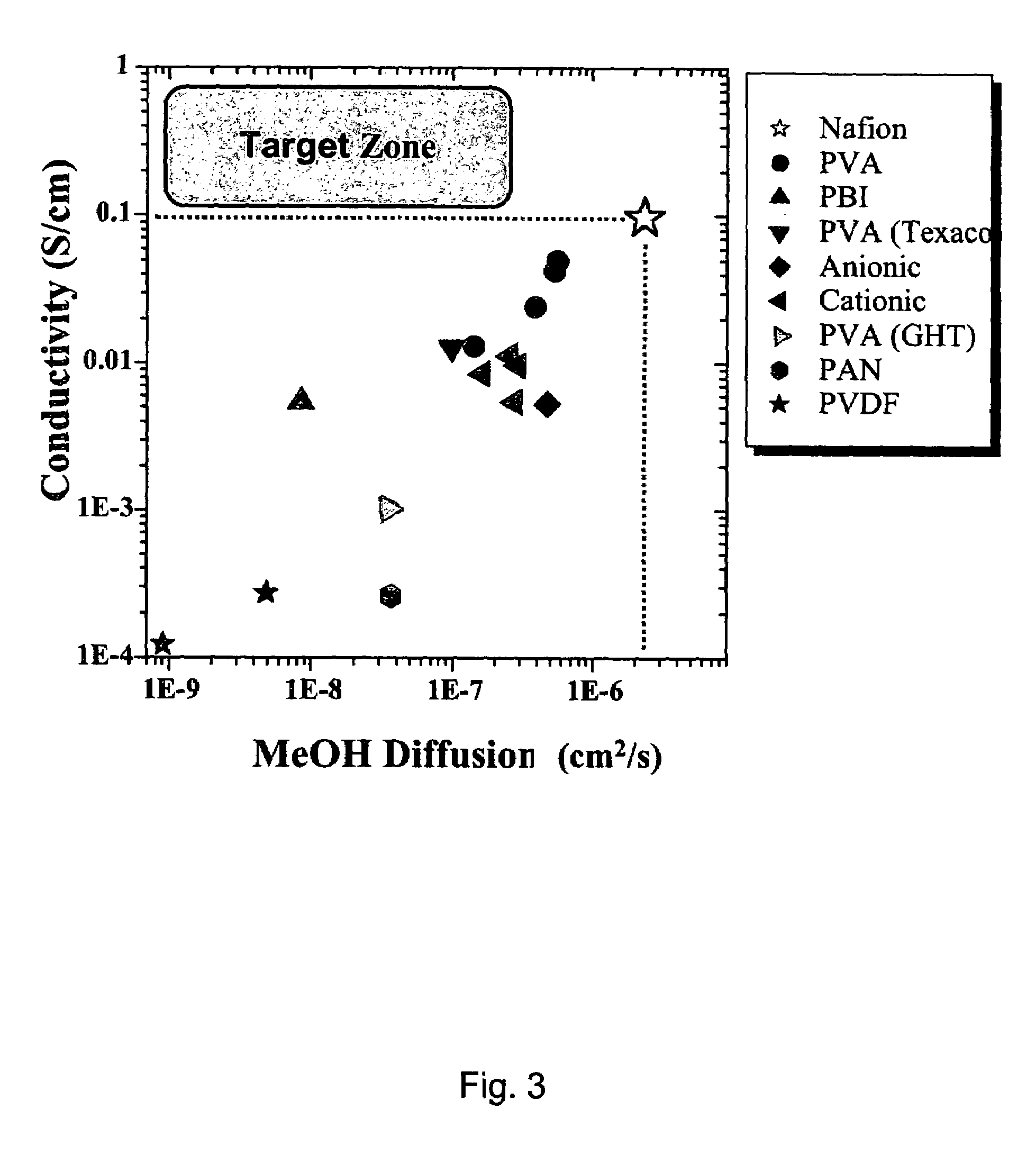
1224 results about "Methanol fuel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methanol fuel is an alternative biofuel for internal combustion and other engines, either in combination with gasoline or independently. Methanol is less expensive to produce sustainably than ethanol fuel, although it is generally more toxic and has lower energy density. For optimizing engine performance and fuel availability, however, a blend of ethanol, methanol and petroleum is likely to be preferable to using any of these alone. Methanol may be made from hydrocarbon or renewable resources, in particular natural gas and biomass respectively. It can also be synthesized from CO₂ (carbon dioxide) and hydrogen. Methanol fuel is currently used by racing cars in many countries but has not seen widespread use otherwise.
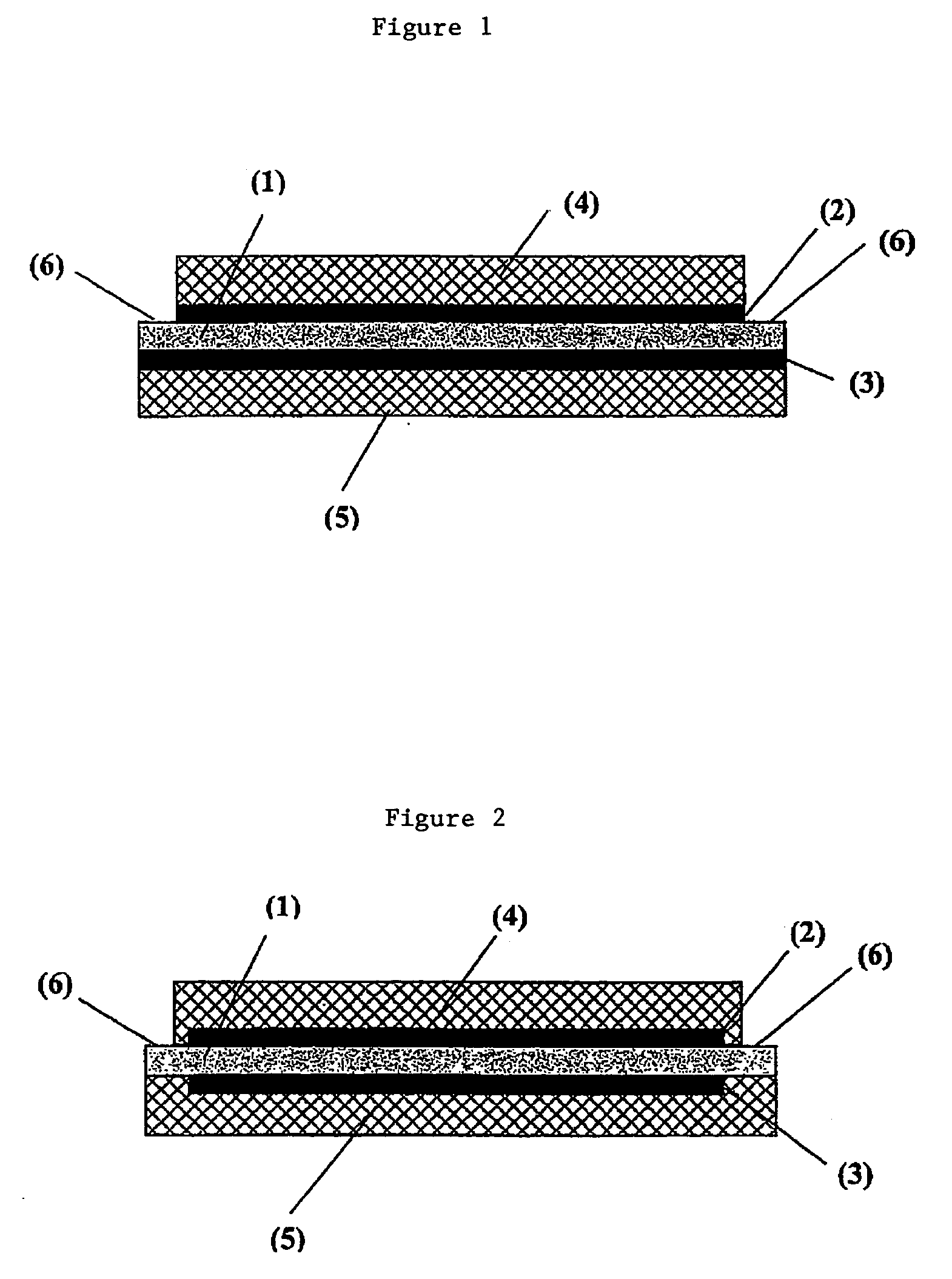
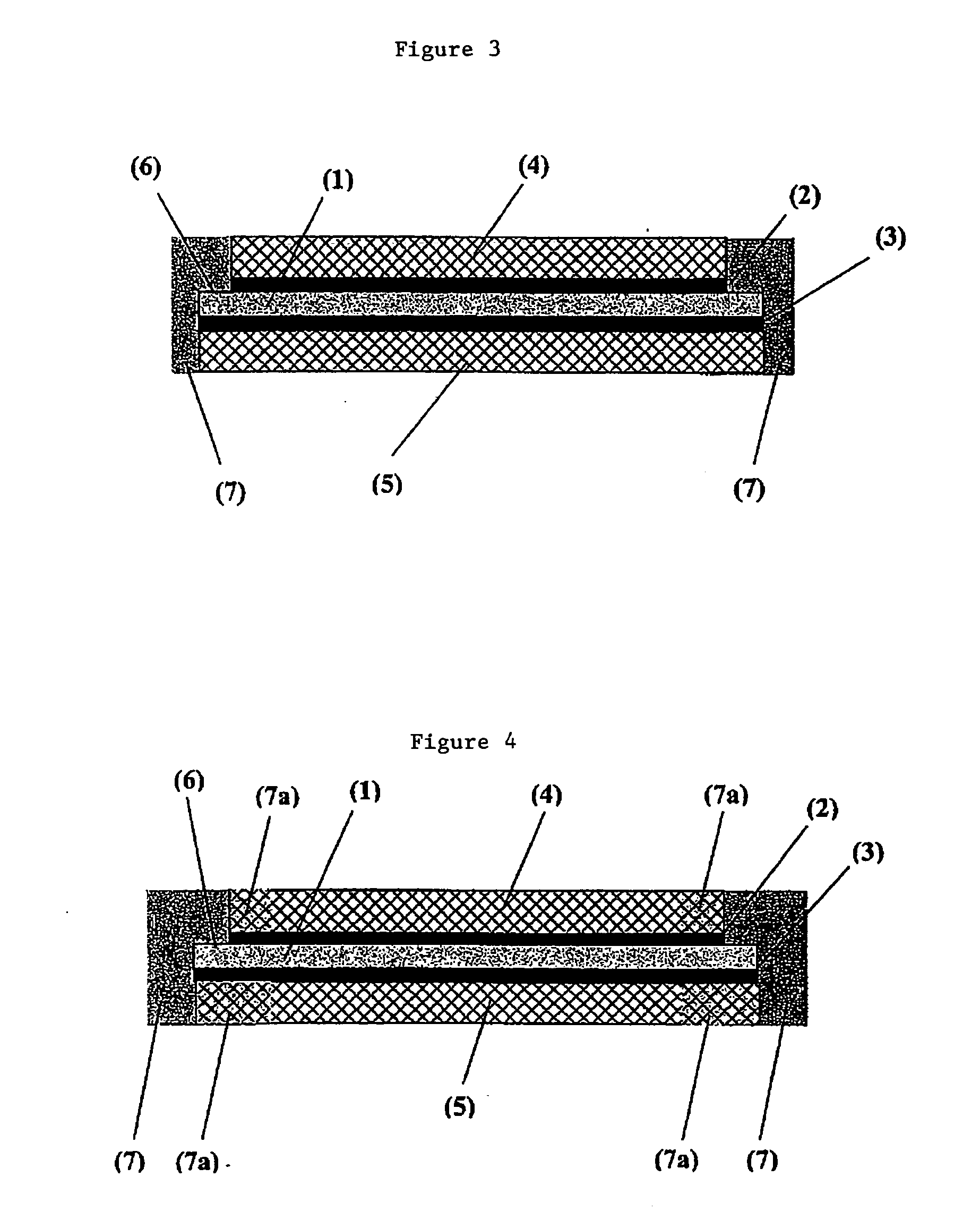
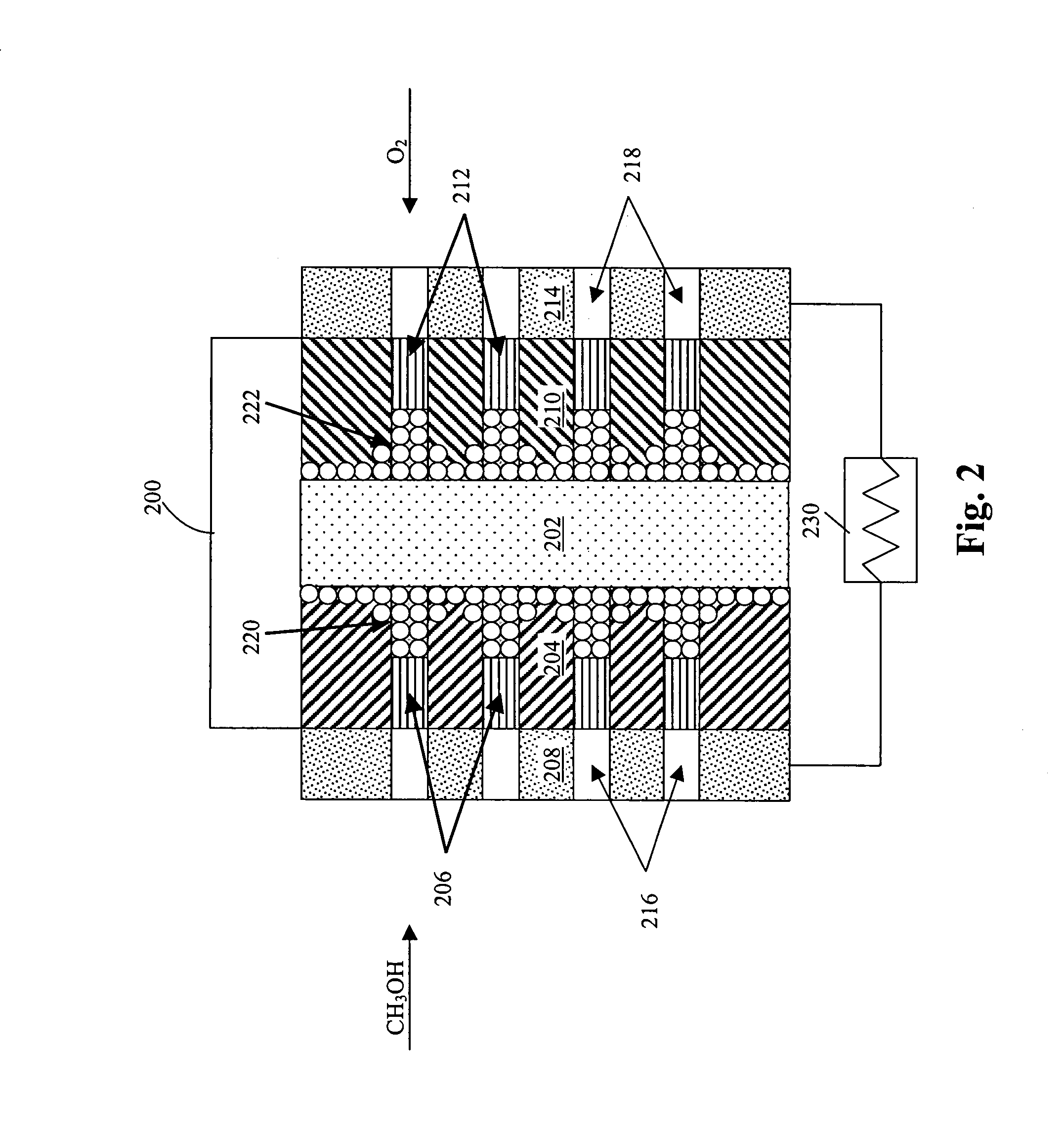
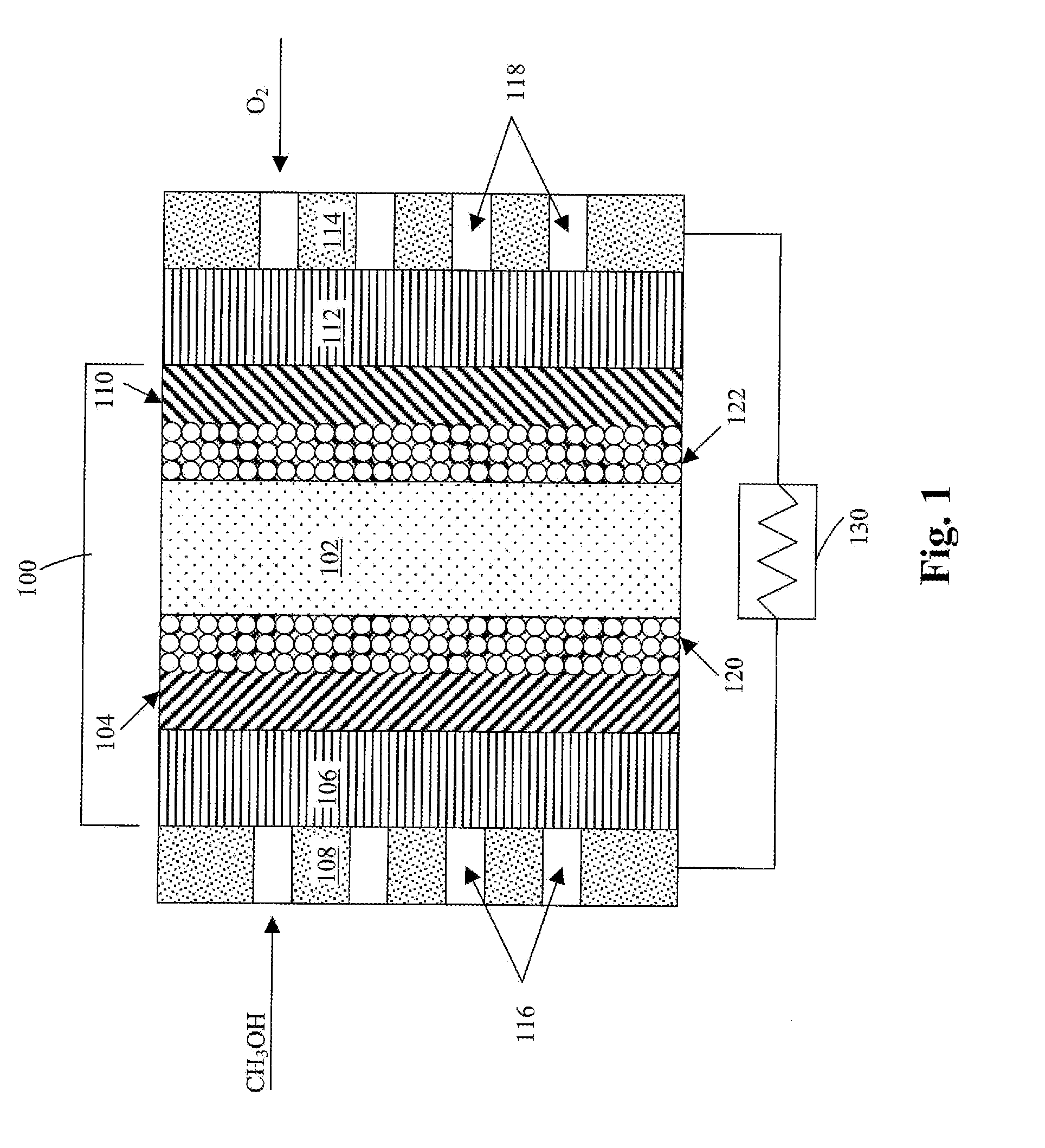
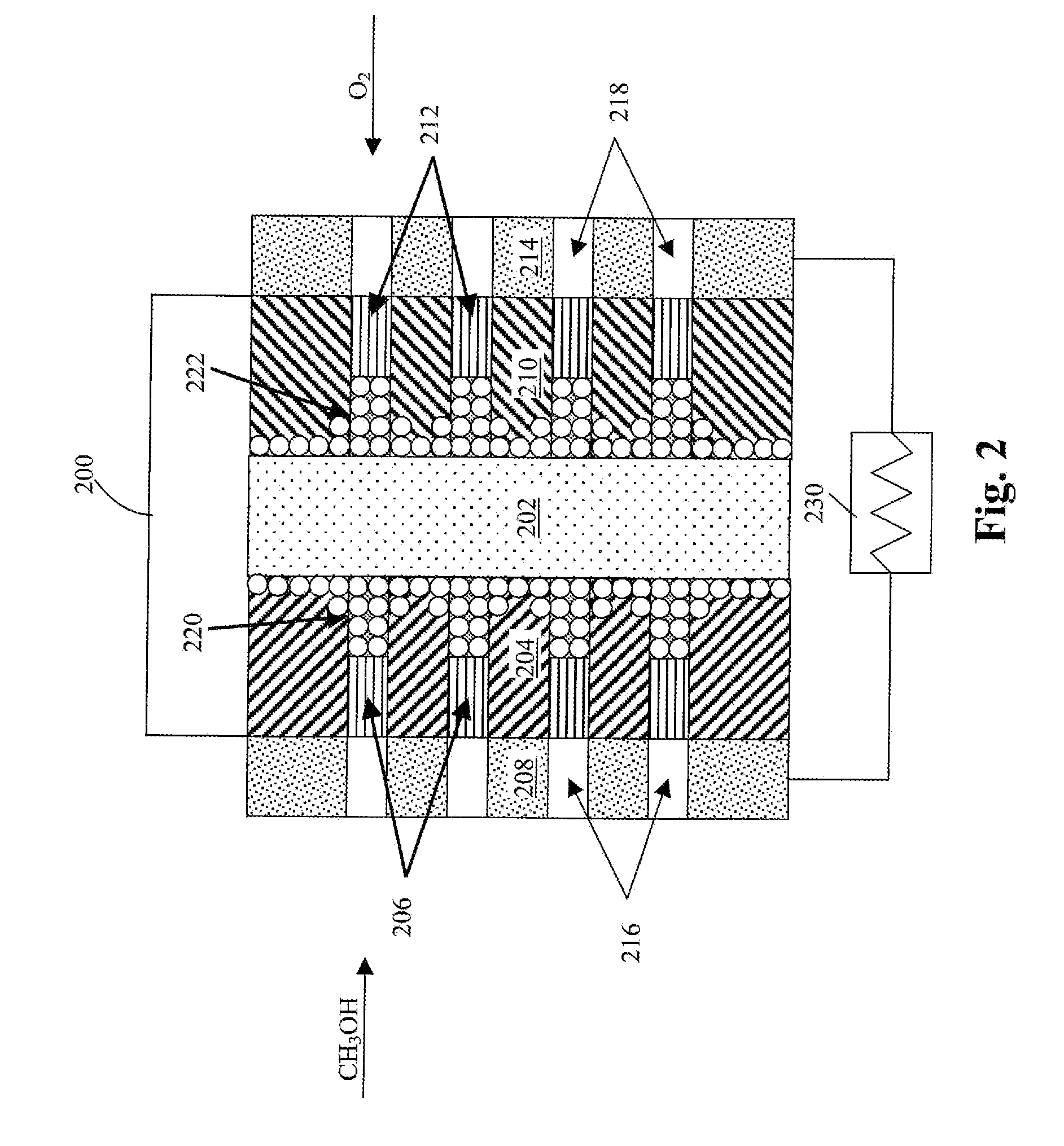
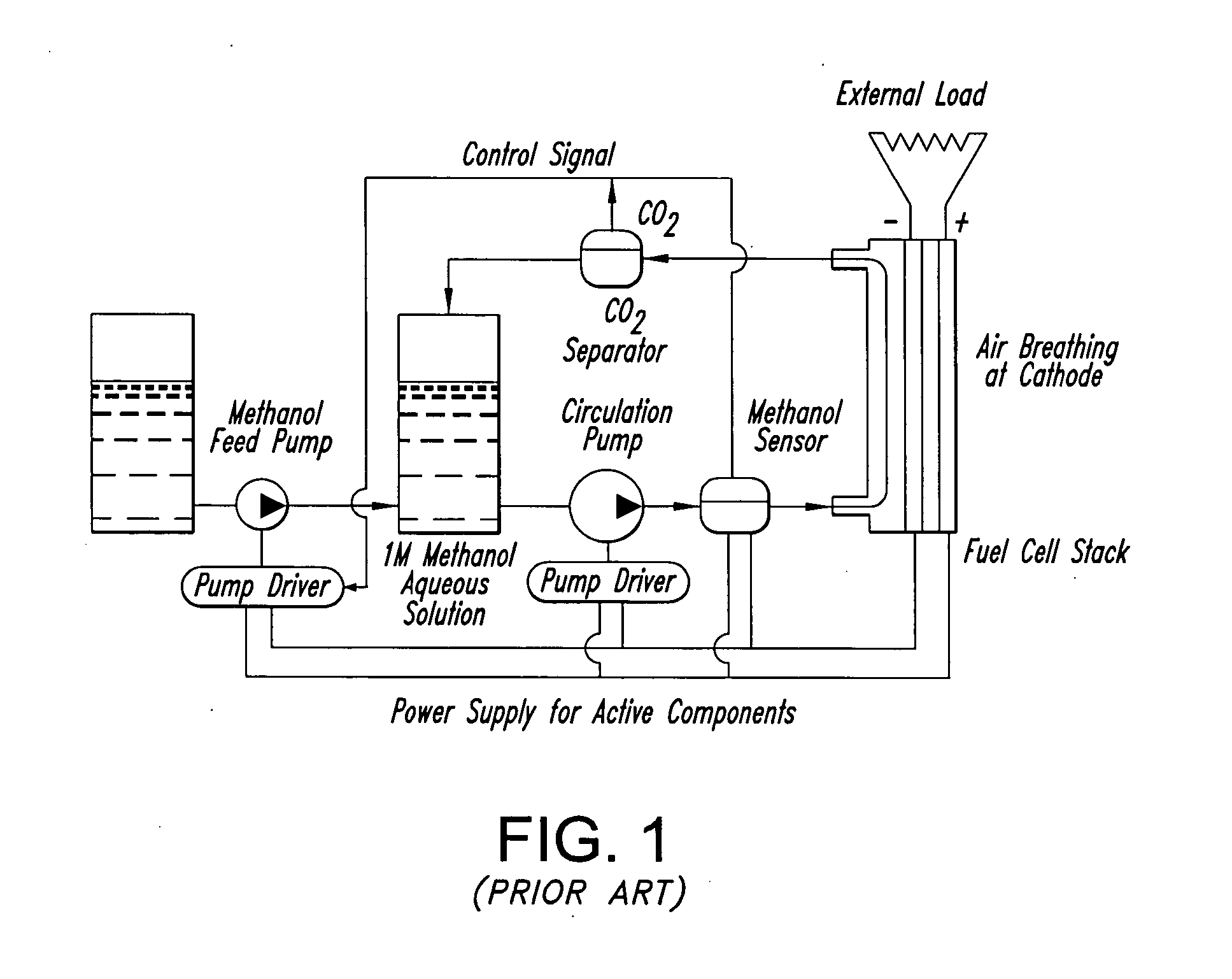
Membrane electrode unit for electrochemical equipment
InactiveUS20050014056A1Good design conceptOvercome disadvantagesCellsFuse device manufactureSpecial designEngineering
The invention concerns a membrane electrode unit (MEU) for electrochemical equipment, especially for membrane fuel cells. The membrane electrode unit has a “semi-coextensive” design and contains an ionically conductive membrane, two catalyst layers, and gas distributor substrates of different sizes on the front and back sides. The first gas distributor substrate has smaller surface dimensions than the ionically conductive membrane, while the second gas distributor substrate has the same area as the ionically conductive membrane. The membrane electrode unit has, because of its special design, a stable structure that can be handled well, and which exhibits advantages for sealing the reactive gases off from each other and in its electrical properties. In particular, the hydrogen penetration current is distinctly reduced. The membrane electrode unit is used in PEM fuel cells, direct methanol fuel cells, electrolyzers, and other electrochemical equipment.
Owner:UMICORE AG & CO KG
Method of producing membrane electrode assemblies for use in proton exchange membrane and direct methanol fuel cells
Compositions and methods for the manufacture of electrodes for fuel cells. The compositions and methods are particularly useful for the manufacture of anodes and cathodes for proton exchange membrane fuel cells, particularly direct methanol fuel cells. The methods can utilize direct-write tools to deposit ink compositions and form functional layers of a membrane electrode assembly having controlled properties and enhanced performance.
Owner:CABOT CORP
Method of producing membrane electrode assemblies for use in proton exchange membrane and direct methanol fuel cells
InactiveUS20060269824A1Improve performanceCell electrodesFinal product manufactureMethanol fuelProton
Compositions and methods for the manufacture of electrodes for fuel cells. The compositions and methods are particularly useful for the manufacture of anodes and cathodes for proton exchange membrane fuel cells, particularly direct methanol fuel cells. The methods can utilize direct-write tools to deposit ink compositions and form functional layers of a membrane electrode assembly having controlled properties and enhanced performance.
Owner:CABOT CORP
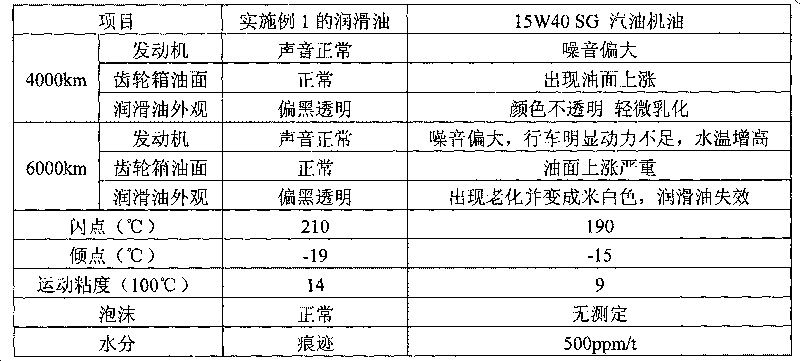
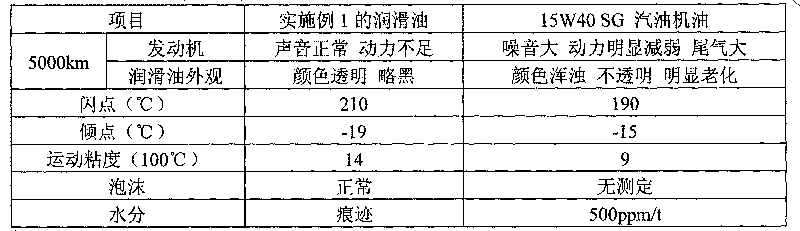
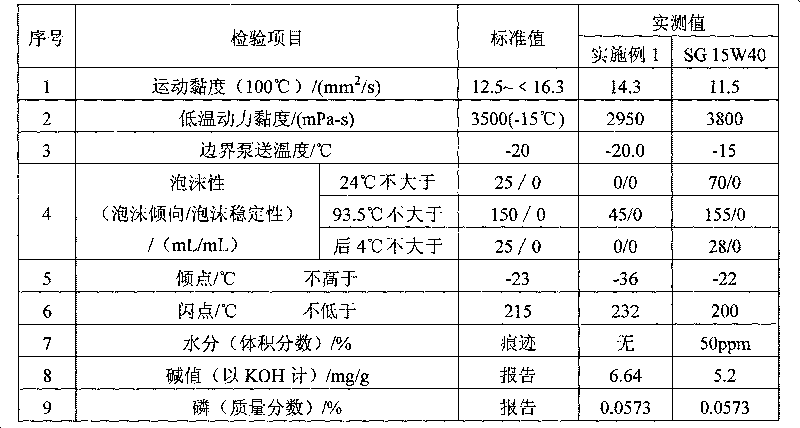
Lubricating oil for methanol fuel engine and preparation method thereof
InactiveCN101705144AAnti-agingResistance to emulsification damageAdditivesAntioxidantBULK ACTIVE INGREDIENT
The invention provides lubricating oil for a methanol fuel engine, which comprises the following components in percentage by weight: metal detergent 2.2-5.2, ashless dispersant 3.5-6.8, antioxidant and anticorrosion agent 0.5-1.6, high-temperature antioxidant 0.3-0.8, viscosity index improver 6-11, oiliness solvent 2.8-6, anti-foaming agent 120ppm, metal anti-rust agent 0.3-0.7, base oil 62.7-83, demulsifying agent 0.1-0.3, metal extreme pressure anti-wear agent 1.0-4 and pour point depressant 0.3-0.9. The lubricating oil of the invention has functions of preventing wear and rust of the engine, can prevent the active ingredients thereof from being extracted and emulsified by methanol and is particularly suitable for engines using M85 or M100 methanol gasoline as a fuel.
Owner:王恩臣 +2
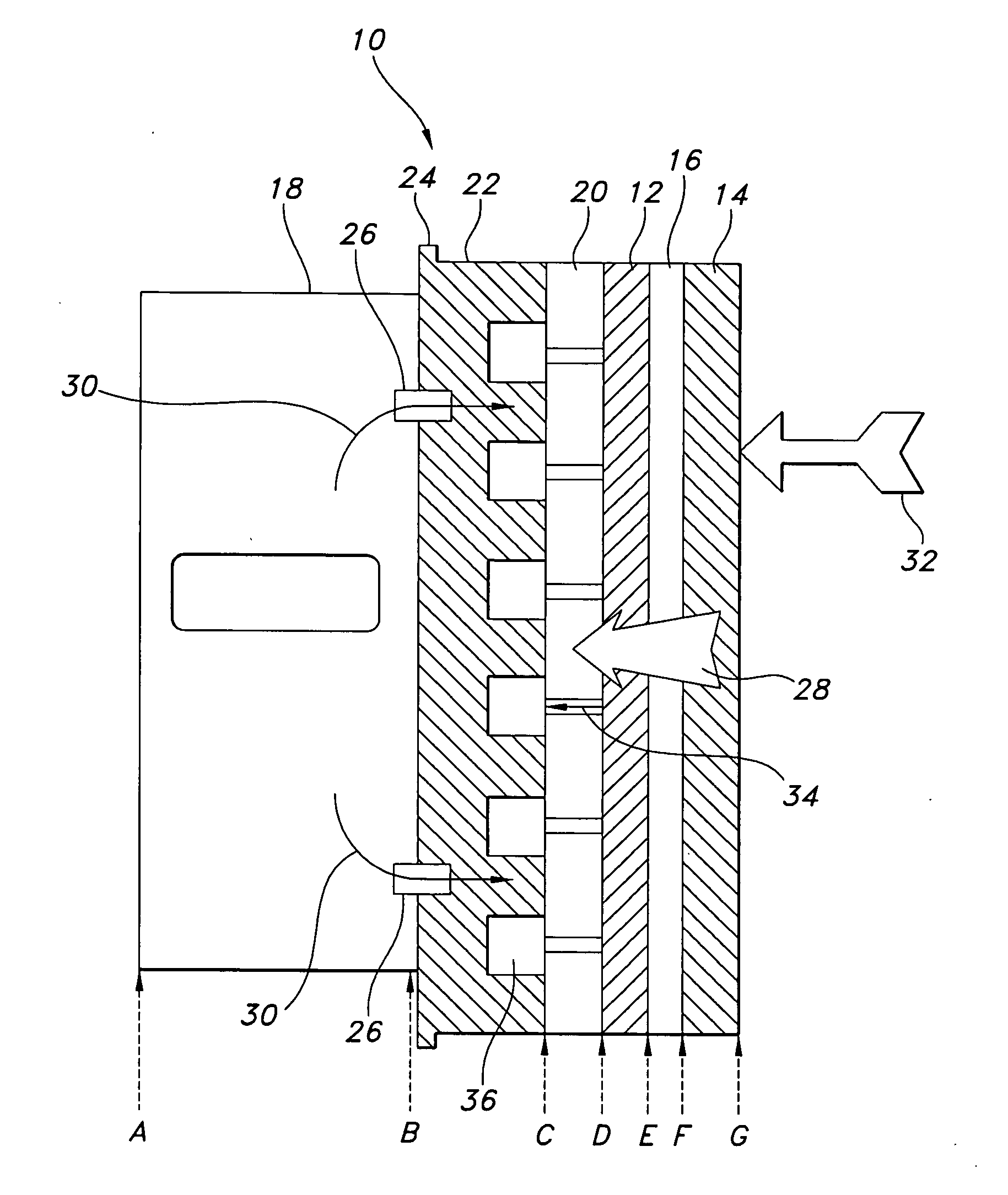
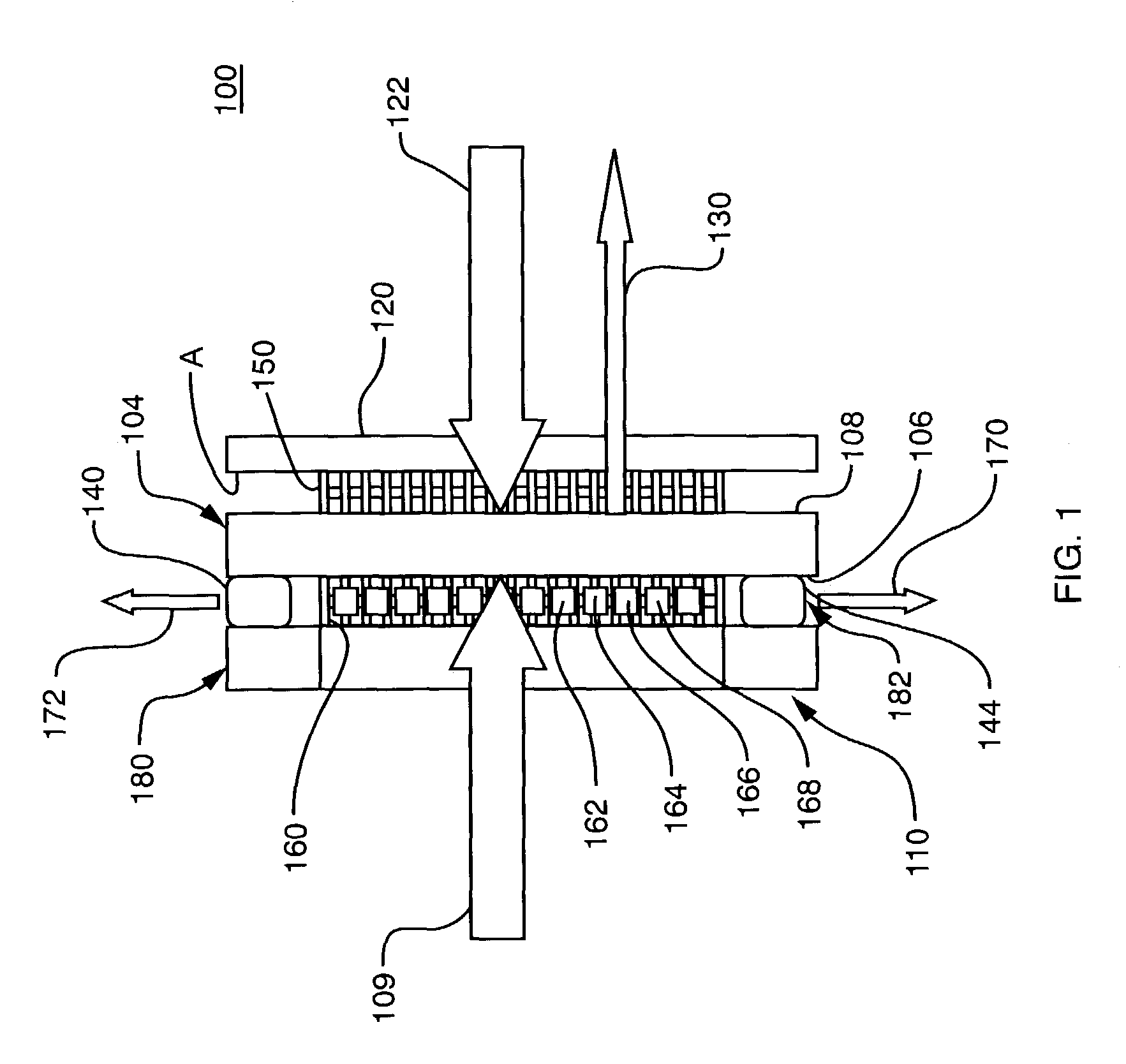
Compact direct methanol fuel cell
InactiveUS20020127451A1Eliminate needImprove the stability of actionReactant parameters controlFuel cells groupingSafety valveElectron
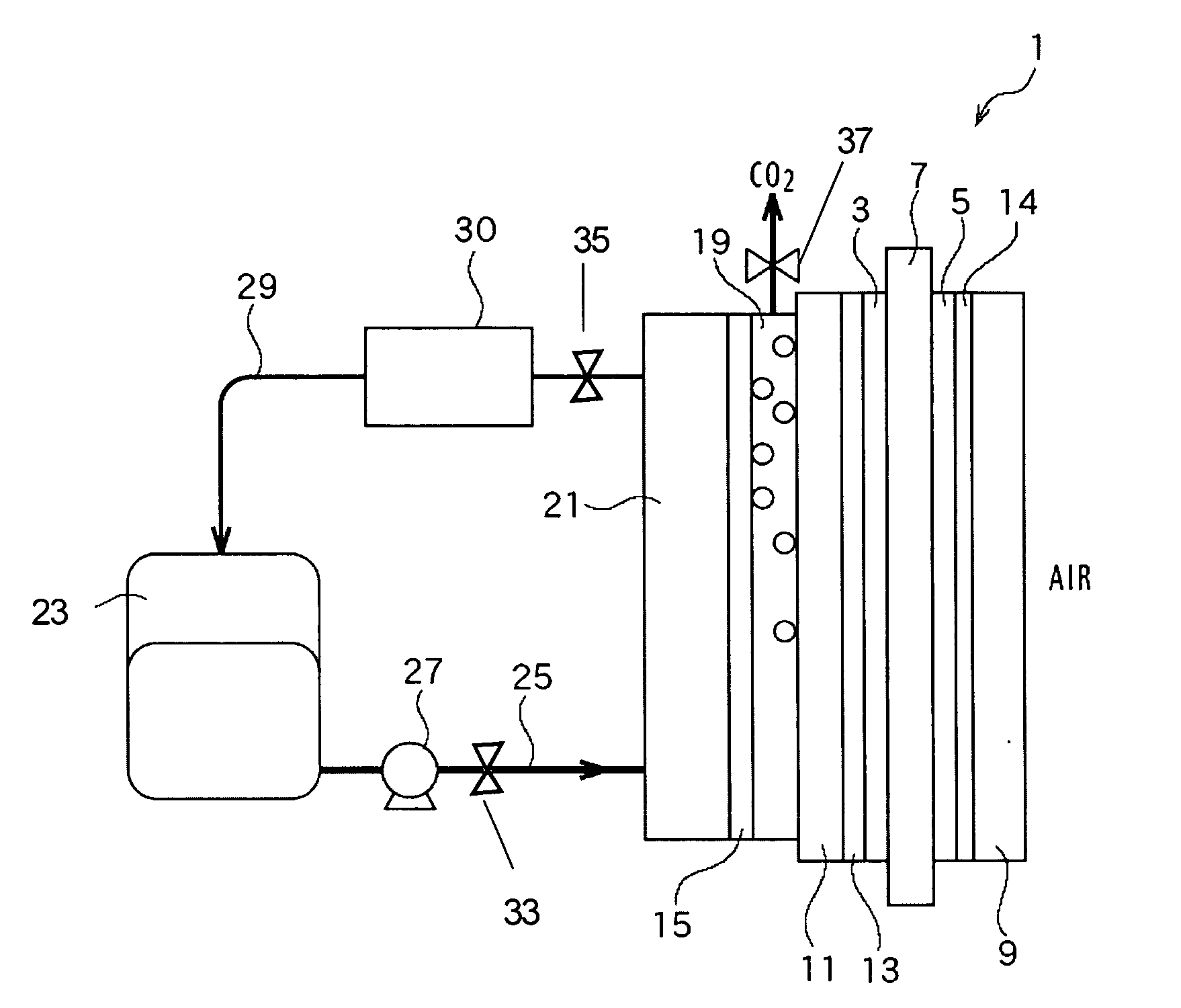
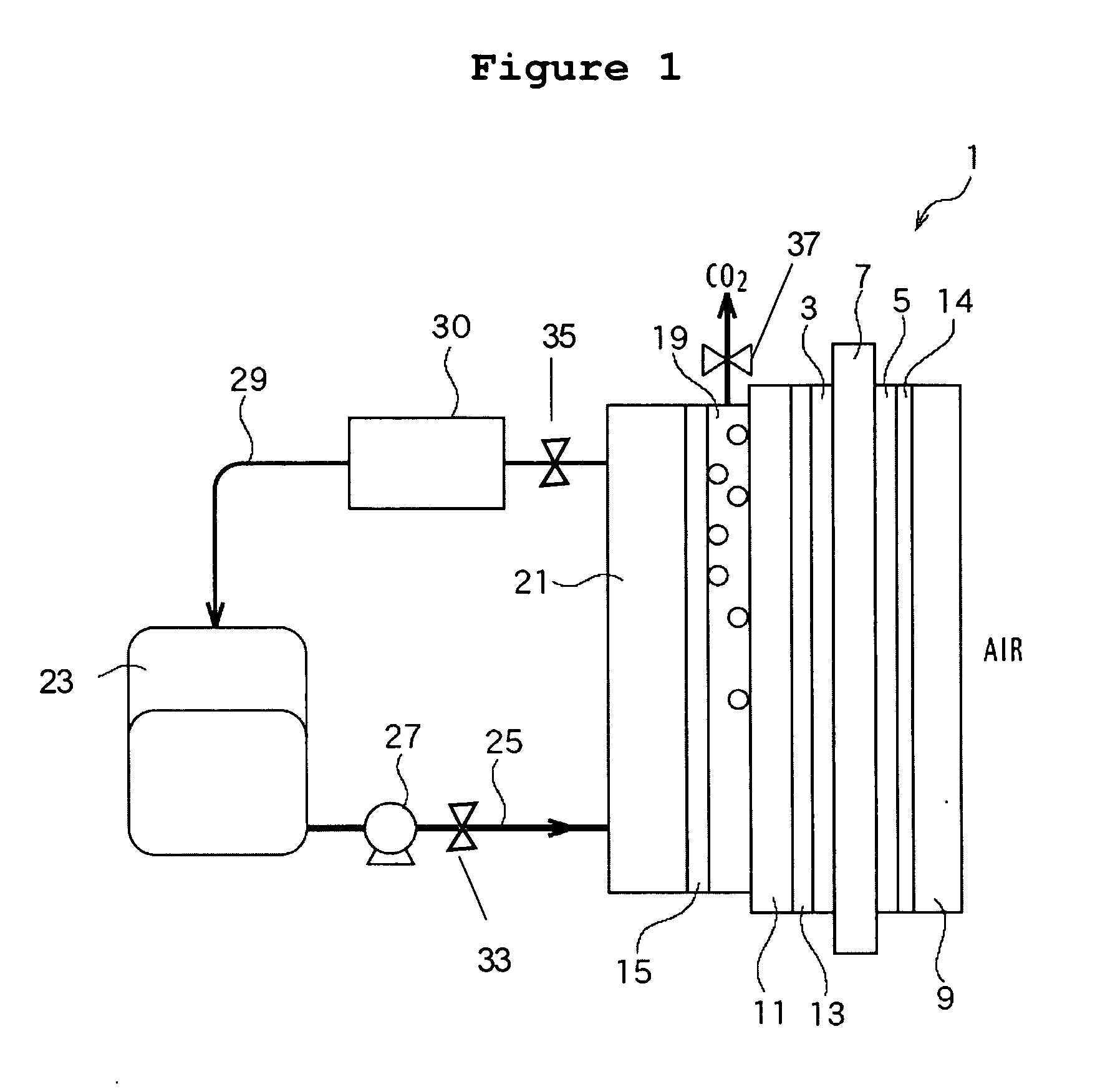
A compact, lightweight direct methanol fuel cell unit includes a circular membrane electrode assembly (MEA) having a cathode, a proton exchange membrane (PEM), and an anode, an air flow duct on the cathode side of the MEA, an annular fuel reservoir on the anode side of the MEA which contains a mixture of methanol and water as fuel, a carbon dioxide (CO.sub.2) relief valve communicating with the fuel reservoir, and a control unit. Due to the CO.sub.2 release mechanism, the fuel cell is completely self-sustaining without the need for a mechanical auxiliary system. Additionally, the control unit improves the stability of the fuel cell power output and the catalysis activity of the MEA. Another embodiment of the present invention is a direct methanol fuel cell system which incorporates a number of MEAs and a fuel cell reservoir. All the cathodic electrical terminals of the MEAs are serially connected together while they are electronically communicating with the control unit. The anodic electrical terminals similarly communicate electronically with the control unit. Higher power and voltage are thus attained while maintaining a compact size of the fuel cell system.
Owner:CAO YIDING +1
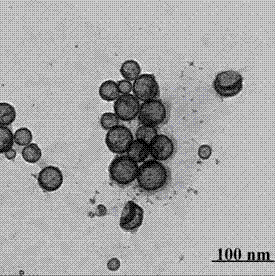
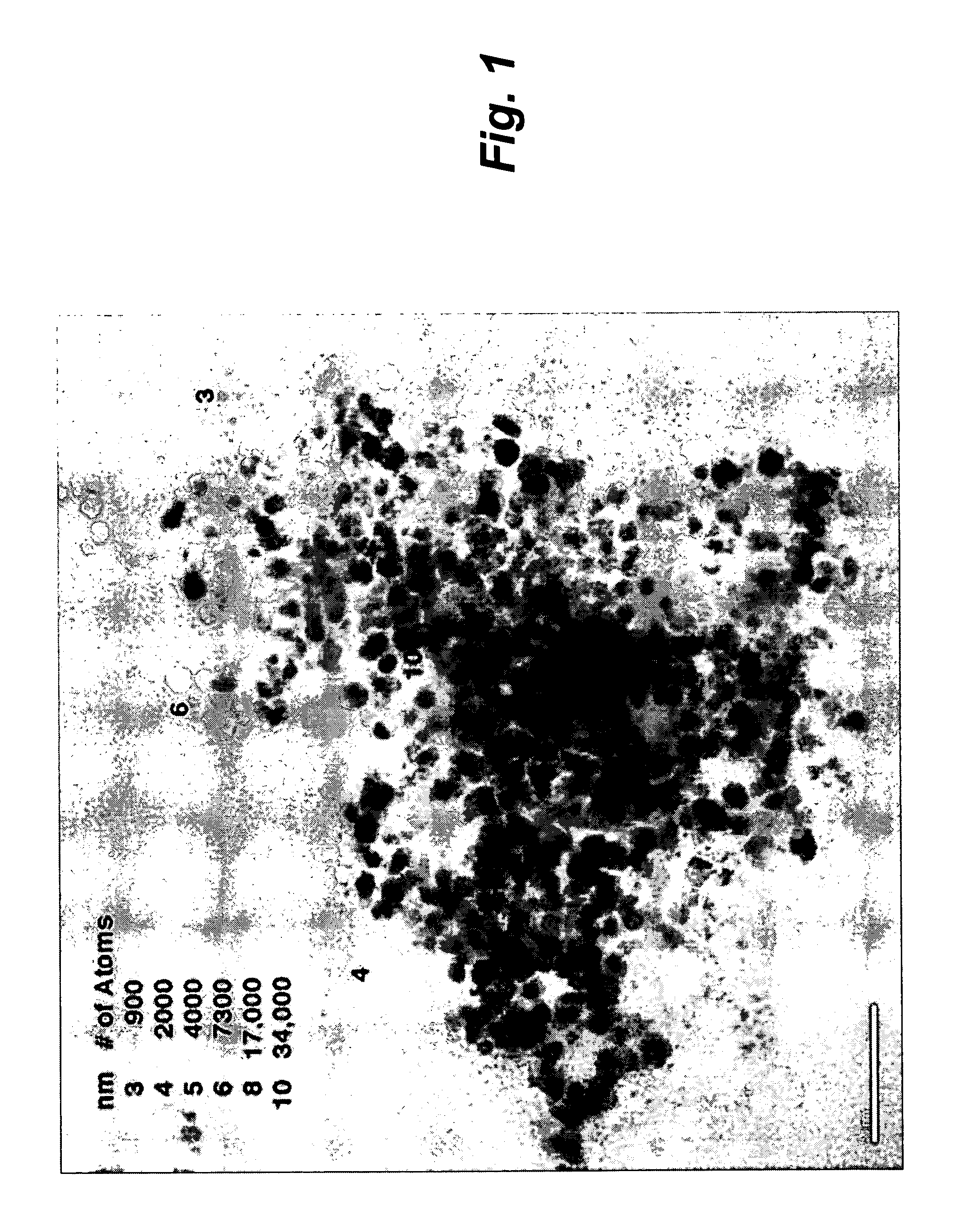
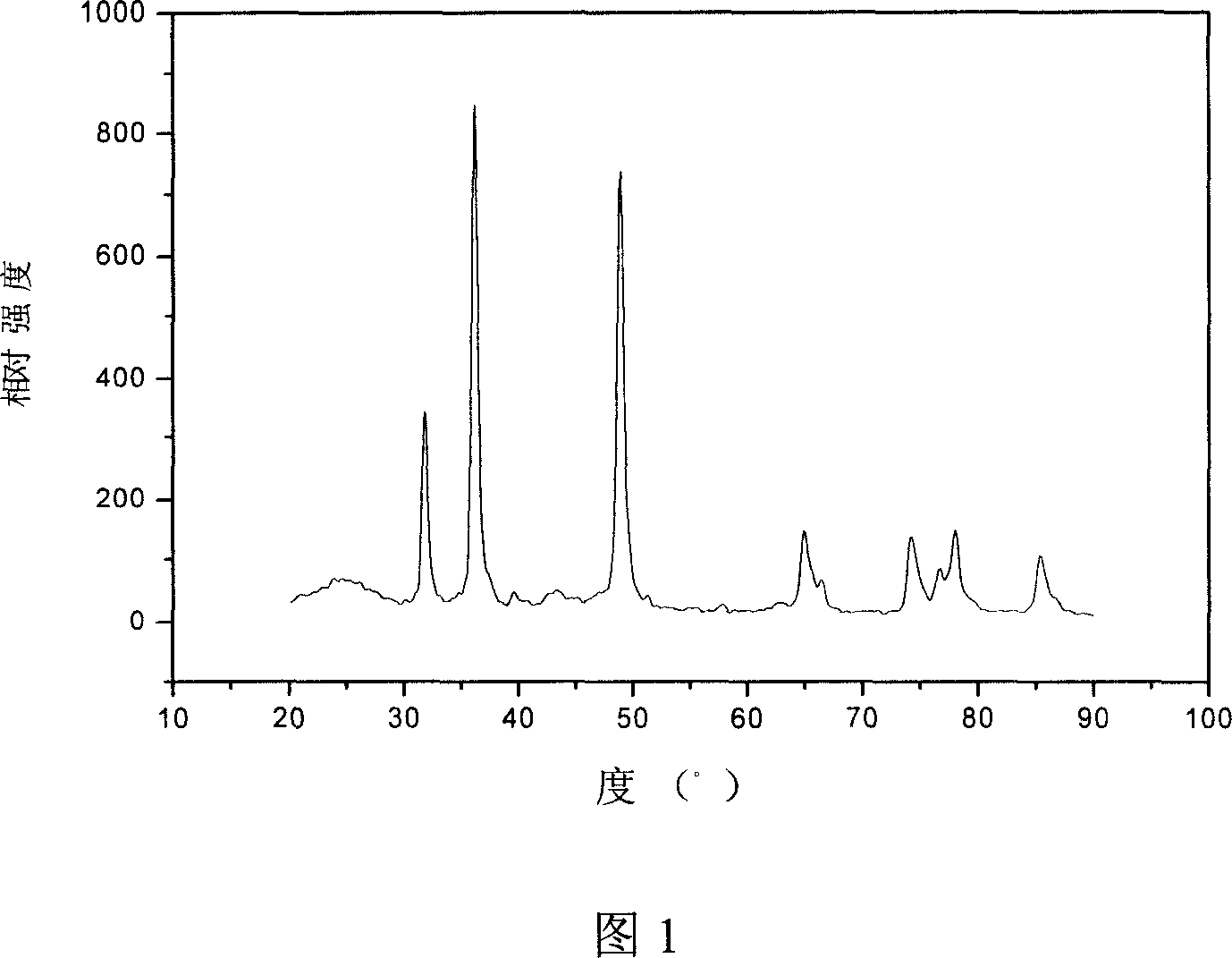
Island-shaped porous tri-metal nano rod with gold core/silver-platinum alloy shell structure and method for preparing same
InactiveCN101623762AEasy to achieve topographyEasy to achieve component contentCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsPlatinumMethanol fuel
The invention relates to an island-shaped porous tri-metal nano rod with a gold core / silver-platinum alloy shell structure, which adopts the gold core / silver-platinum alloy shell structure formed by a cylindrical gold nano rod core and an island-shaped porous silver-platinum alloy shell coated on the outer surface of the cylindrical gold nano rod core. The preparation method comprises the following steps of preparation of gold crystal seed solution, preparation and purification of gold nano rod solution, preparation of solution of a platinum coated gold core / platinum shell nano rod, preparation of island-shaped porous tri-metal nano rod with the gold core / silver-platinum alloy shell structure and the like. The tri-metal nano rod has the advantages of stronger ability of catalyzing the methanol oxidation, stronger CO poisoning resistant ability, lower cost and the like, and can be widely used for preparing a methanol fuel battery catalyst; and the preparation method has the advantages of simplicity, low consumption, environmental protection, and high efficiency, and by the method, the island-shaped porous tri-metal nano rod with the gold core / silver-platinum alloy shell structure with high yield and narrow size distribution can be obtained.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
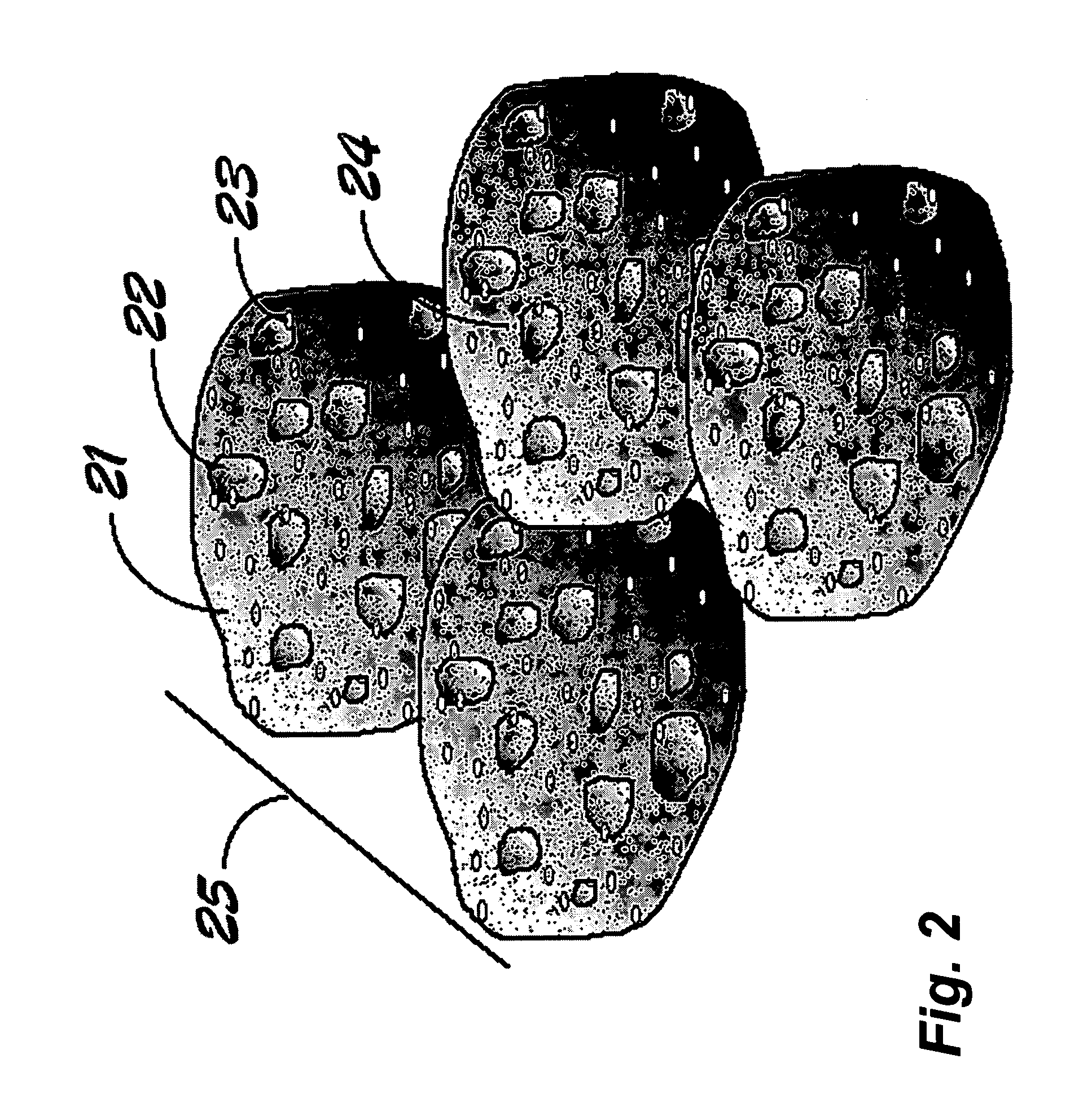
PtNi alloy/graphene combined nanometer catalyst with hollow structure and preparation method thereof
InactiveCN102430413AUnique hollow structureReduce dosageCell electrodesMetal/metal-oxides/metal-hydroxide catalystsNano catalystPlatinum salts
A PtNi alloy / graphene combined nanometer catalyst with a hollow structure adopts the graphene as a carrier and loads the PtNi alloy nanometer particles of which the grain diameter is 10-50 nm on the surface of the graphene, wherein the PtNi alloy nanometer particles are hollow spherical structures. The method for preparing the combined catalyst comprises the following steps: dispersing the graphene oxide in water liquor to which surfactant is added through ultrasound; uniformly mixing with soluble nickel salt (II); adding the reducing agent in inert gas atmosphere; adding soluble platinum salt (IV); stirring at 0-50 DEG C to execute the reduction reaction; centrifuging, washing and drying the reaction product to obtain the PtNi alloy / graphene combined nanometer catalyst with the hollow structure. The PtNi alloy / graphene combined nanometer catalyst with the hollow structure in the invention has good electro-catalytic property on electrochemical oxidation of methyl alcohol, and can be widely applied in methyl alcohol fuel cells.
Owner:NANJING NORMAL UNIVERSITY
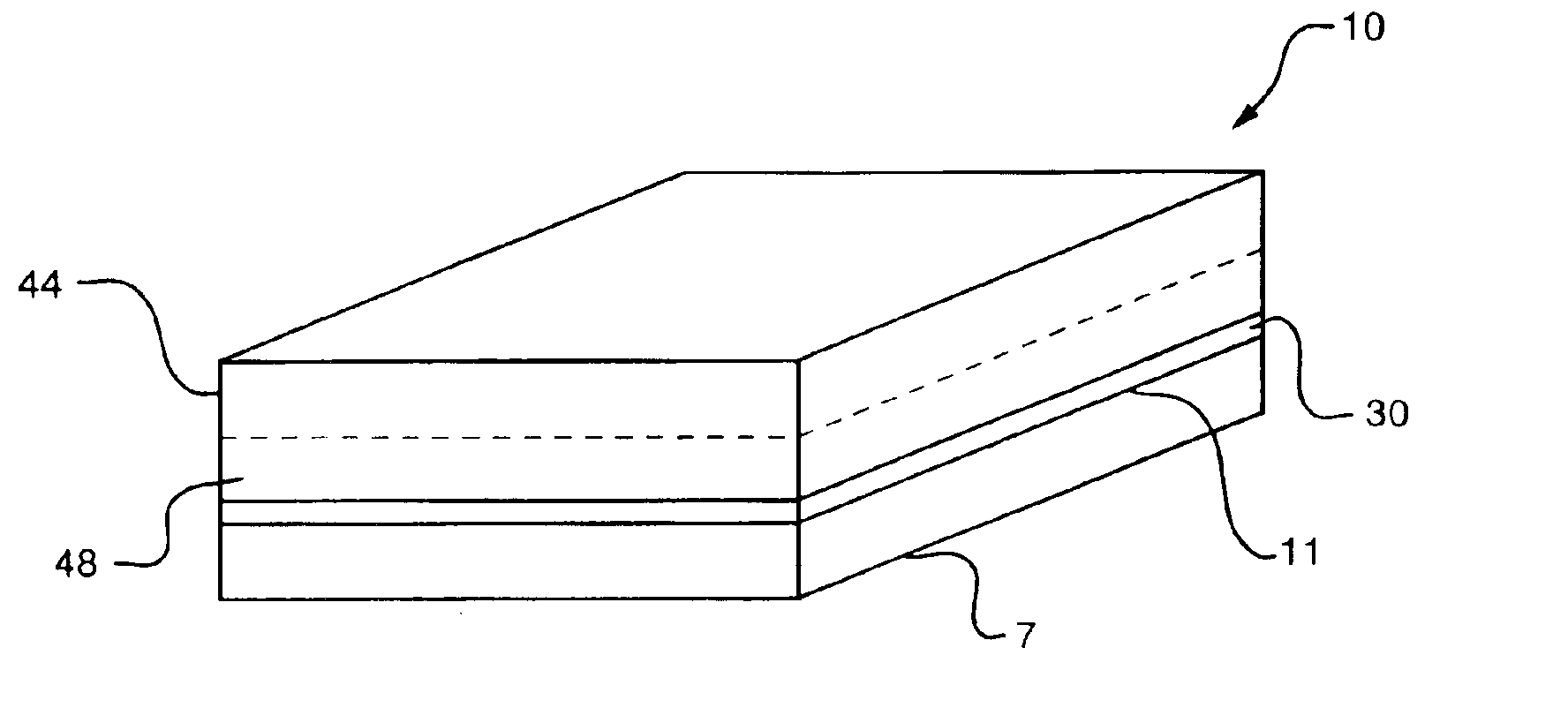
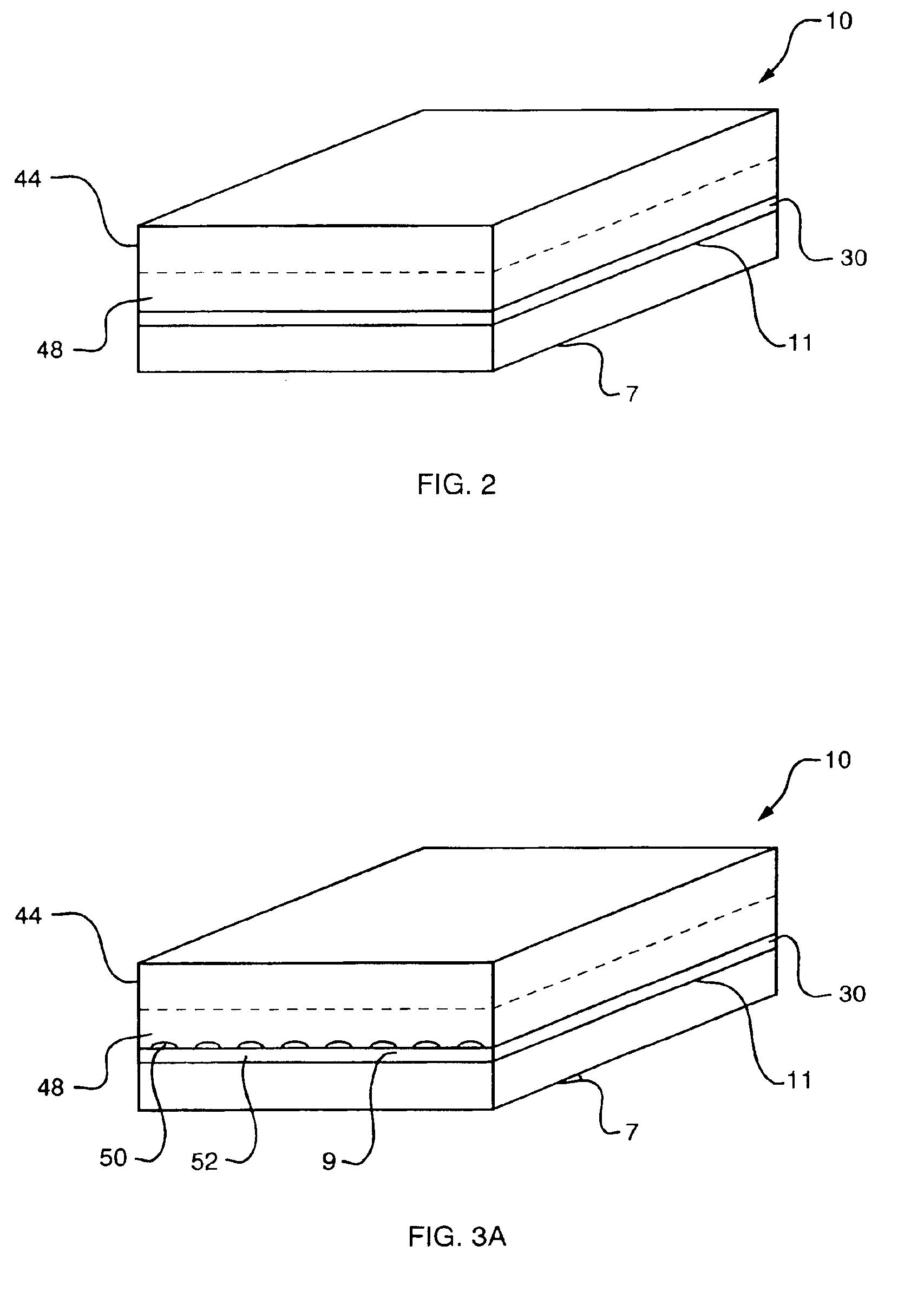
Modified diffusion layer for use in a fuel cell system
InactiveUS6890680B2Improve fuel efficiencyEasy to operateCell electrodesFuel cell auxillariesPreferential flowFuel cells
A fuel cell diffusion layer providing a preferential path by which liquid reactants or byproducts may be supplied to or removed from a direct oxidation fuel cell is described. The modified diffusion layer will be typically on the cathode side of the fuel cell and its use is to eliminate or minimize flooding of the cathode diffusion layer area, which is a performance limiting condition in direct methanol fuel cells. In accordance with one embodiment of the invention, the diffusion layer includes a substrate that is coated with a microporous layer. A pattern may be embossed into the diffusion layer, to create preferential flow paths by which water will travel and thereby be removed from the cathode catalyst area. This avoids cathode flooding and avoids build up of potentially destructive pressure by possible cathodic water accumulation. This also provides a means for collecting cathode water for redirection In accordance with another aspect of the invention, the preferential path is established by applying a thicker microporous layer to the carbon cloth or carbon paper and drying it in such a fashion so that when it dries, the surface of the microporous layer cracks to provide the pathways.
Owner:MTI MICROFUEL CELLS
Electrochemical catalysts
InactiveUS20080280190A1Improve performanceReduce the amount of solutionMaterial nanotechnologyFuel and primary cellsElectrochemical responseHydrogen fuel cell
A composition useful in electrodes provides higher power capability through the use of nanoparticle catalysts present in the composition. Nanoparticles of transition metals are preferred such as manganese, nickel, cobalt, iron, palladium, ruthenium, gold, silver, and lead, as well as alloys thereof, and respective oxides. These nanoparticle catalysts can substantially replace or eliminate platinum as a catalyst for certain electrochemical reactions. Electrodes, used as anodes, cathodes, or both, using such catalysts have applications relating to metal-air batteries, hydrogen fuel cells (PEMFCs), direct methanol fuel cells (DMFCs), direct oxidation fuel cells (DOFCs), and other air or oxygen breathing electrochemical systems as well as some liquid diffusion electrodes.
Owner:BRICOLEUR PARTNERS LP
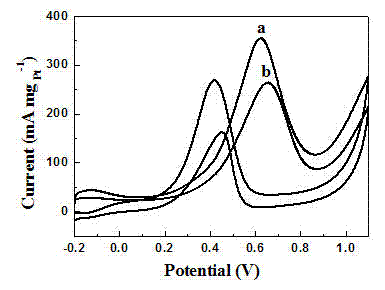
Oxygen reduction catalyst prepared from grapheme modified by macrocyclic compound, and preparation method thereof
InactiveCN101773855AEasy to operateEase of conditionsCell electrodesOrganic-compounds/hydrides/coordination-complexes catalystsOxygenUltrasonic dispersion
The invention discloses an oxygen reduction catalyst prepared from grapheme modified by a macrocyclic compound, and a preparation method thereof. The grapheme modified by a macrocyclic compound is used as the catalyst and is used for catalyzing the oxygen reduction of batteries under acidic, neutral and alkaline conditions. The catalyst can efficiently reduce oxygen in a solution, the direct 4e process can be realized, the spike potential of the oxygen reduction under alkaline condition can reach 0.1 V (vs. Ag / AgCl), and the stability is high. The catalyst has simple preparation process, low cost and good catalytic activity. The preparation method of the catalyst comprises the following steps of: firstly, conducting ultrasonic dispersion after proportionally mixing grapheme and the macrocyclic compound; then, filtering; and finally, drying to obtain the catalyst. In addition, the catalyst has great anti-poisoning effect on carbon monoxide, methanol, formaldehyde, glucose and the like which can poison a platinum catalyst. The oxygen reduction catalyst can be applied to the fields of proton exchange membrane fuel batteries, direct methanol fuel batteries, metal-air battery cathode materials and the like.
Owner:SOUTH CHINA UNIV OF TECH
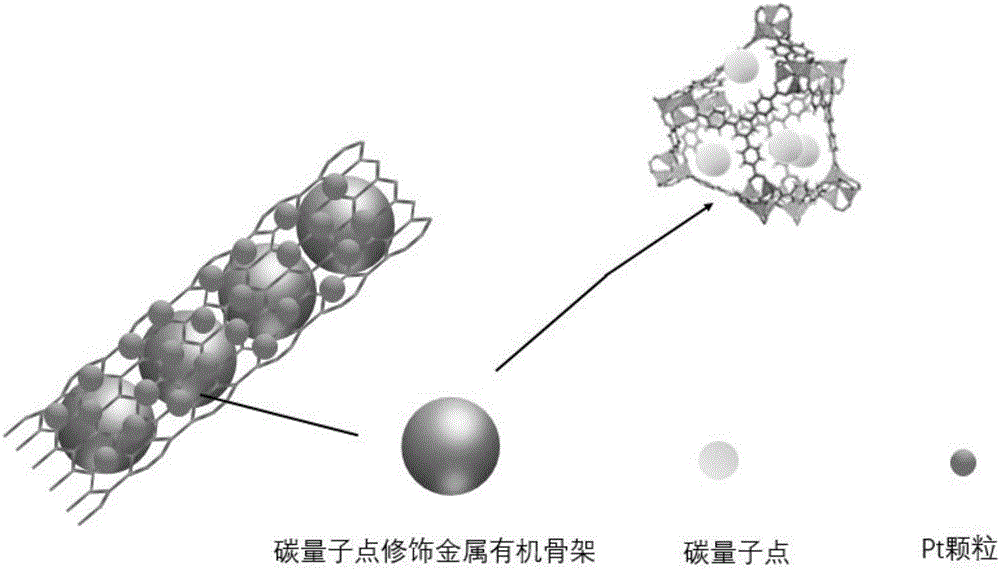
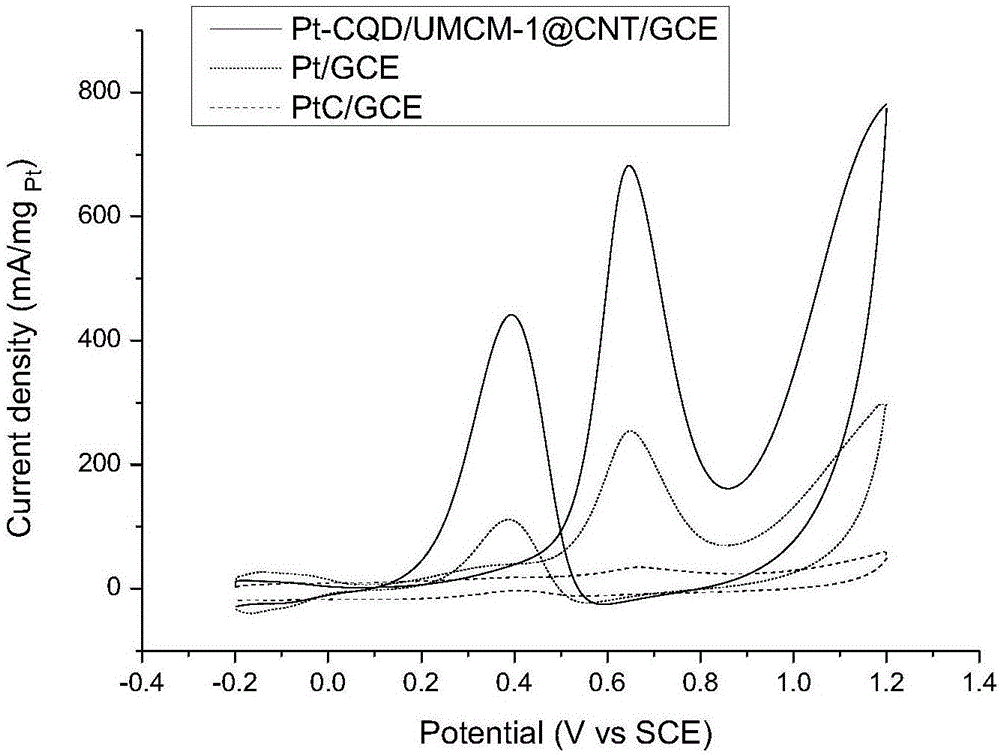
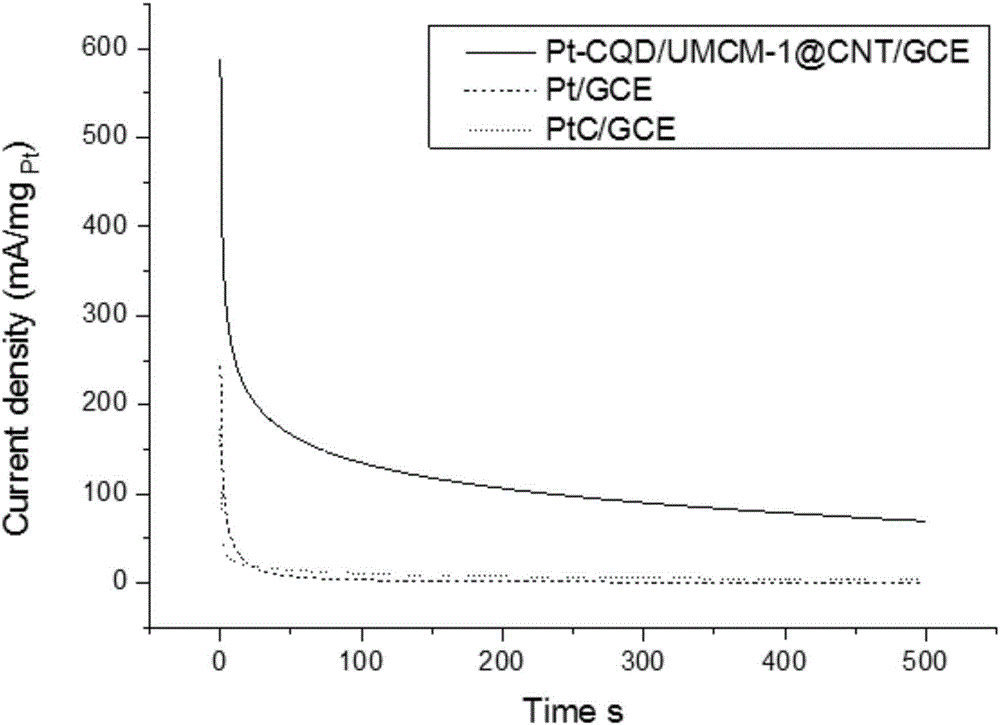
Preparation of carbon nanotube material embedded with quantum-dot modified metal organic framework
The invention provides preparation of a carbon nanotube material embedded with a quantum-dot modified metal organic framework. The preparation comprises the following steps of firstly, adding a certain amount of powder quantum dots to a formed metal organic framework precursor solvent so that the quantum dots of which the diameters are matched with the sizes of pore passages are embedded into the pore passages of the formed metal organic framework; secondly, uniformly mixing the obtained quantum-dot modified metal organic framework and melamine powder, and performing high-temperature processing to obtain a composite material (QD / UMCM-1@CNT) in a carbon nanotube embedded with the quantum-dot modified metal organic framework; and finally, enabling Pt to be loaded on a surface of the composite material carbon nanotube (Pt / QD / UMCM-1@CNT) by microwave radiation heating to be used as a positive electrode catalyst of a methanol fuel cell. Compared with a traditional hydrothermal method for synthesis of the carbon nanotube, the quantum-dot modified metal organic framework obtained by high-temperature processing is embedded into the composite material in the carbon nanotube, the structural aspects such as the length and the diameter of the carbon nanotube are more consistent, and a catalyst substrate with a unified structure is easier to form.
Owner:QINGDAO UNIV
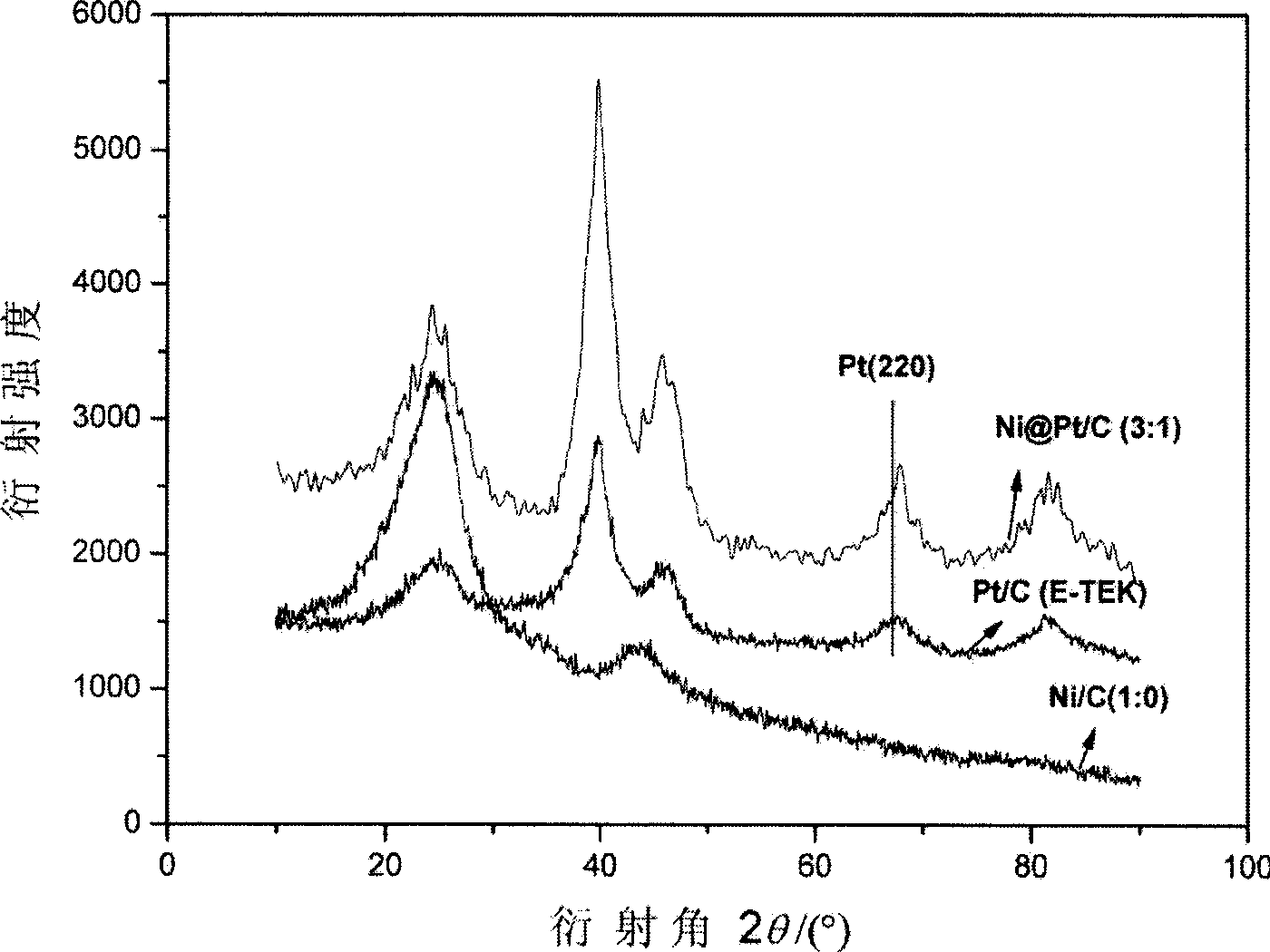
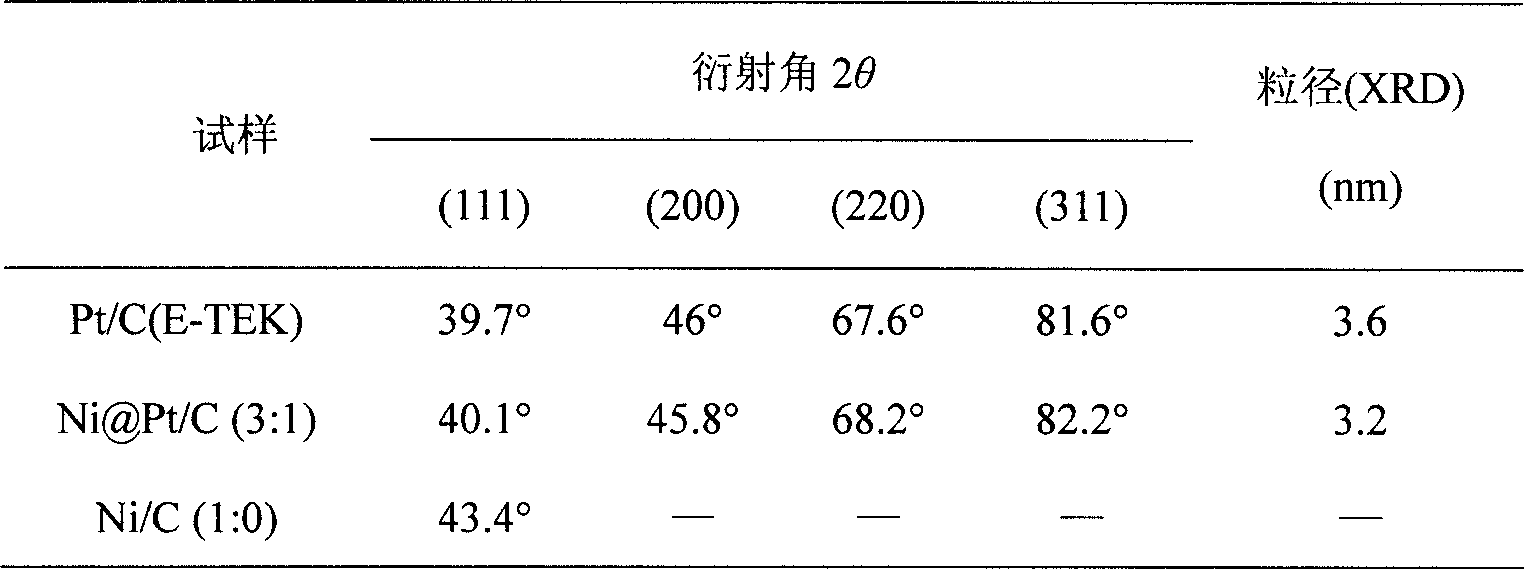
Preparation method of carbon supported core-shell Ni-Pt particles for direct methanol fuel cells
ActiveCN101455970AImprove catalytic performanceLow priceCell electrodesMetal/metal-oxides/metal-hydroxide catalystsChemistryInorganic chemistry
The invention provides a method for preparing carbon-supported nucleocapsid type Ni-Pt particles for direct methanol fuel cell catalysts, which belongs to a preparation process of direct methanol fuel cell catalysts. The method comprises the steps of adopting sodium citrate as a stabilizer, adopting cationic surfactant CTAB as dispersant, using sodium hypophosphite to reduce nickel acetate, generating a Ni kernel on the surface of Vulcan XC-72 or mesoporous carbon treated with sodium borohydride, washing superfluous sodium hypophosphite and generating a Pt shell on the surface of the Ni kernel through chemical replacement. The catalyst has a structure with the Ni kernel and the Pt shell, and has the advantages of low Pt support amount and high catalytic activity.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
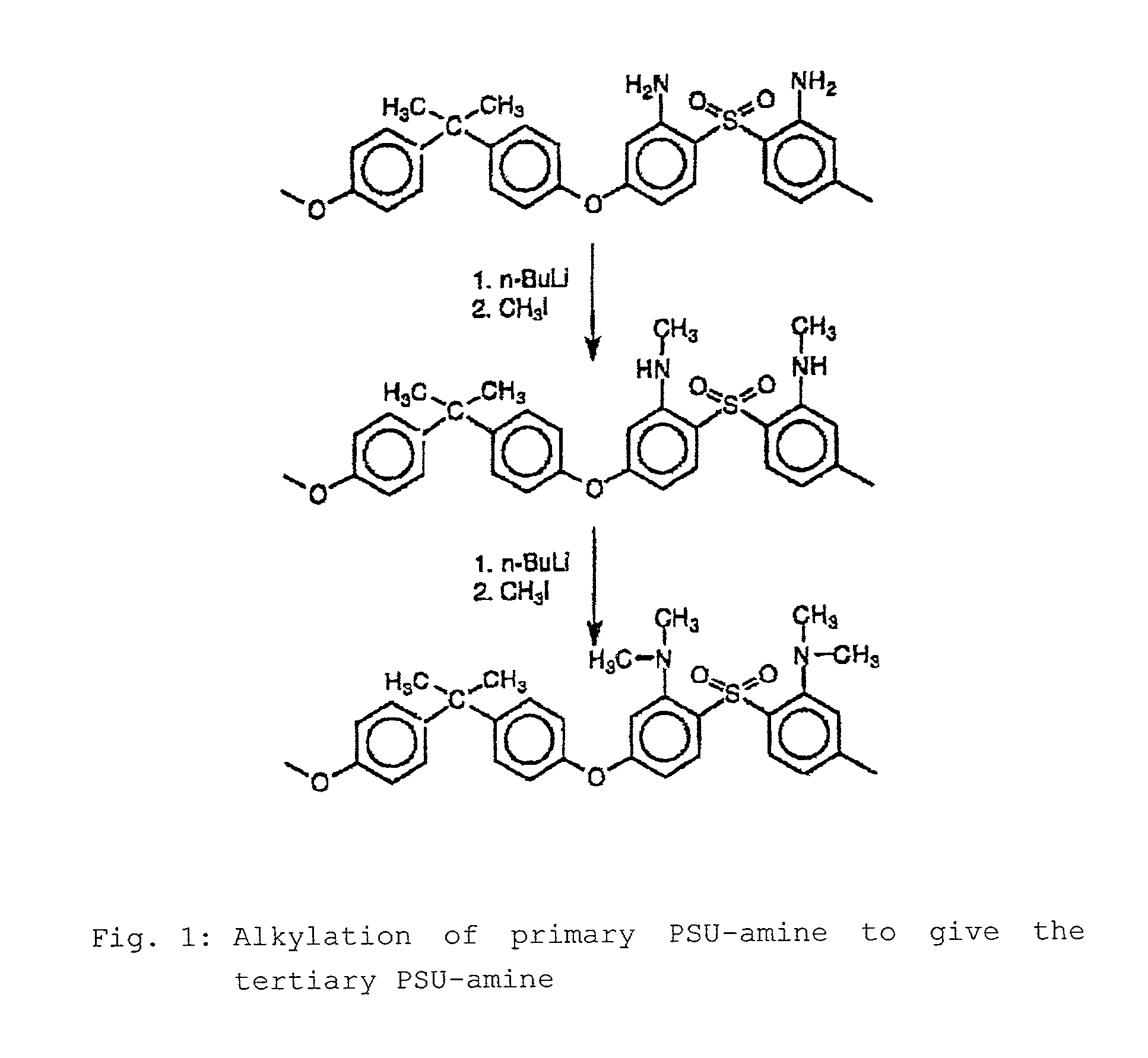
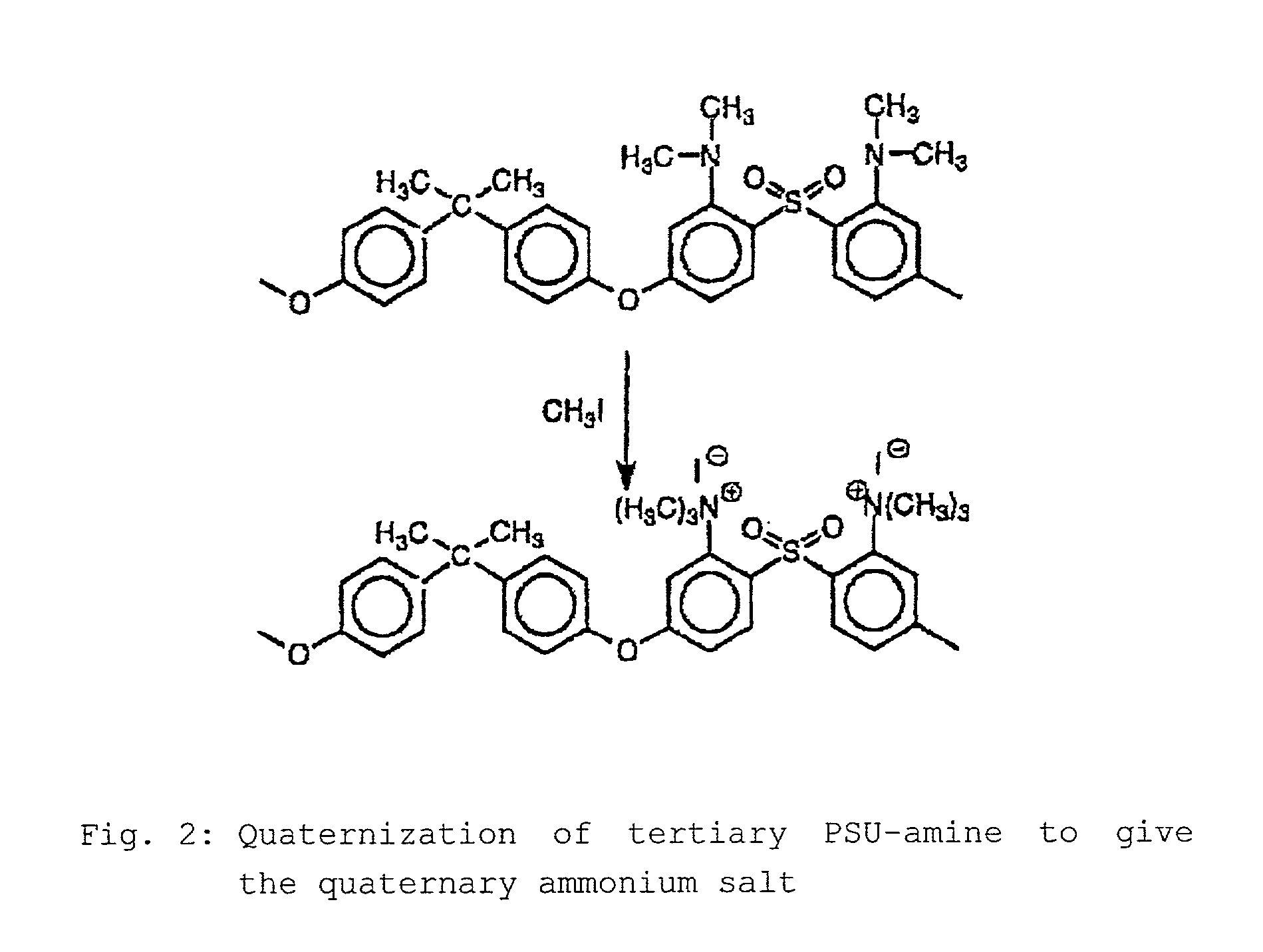
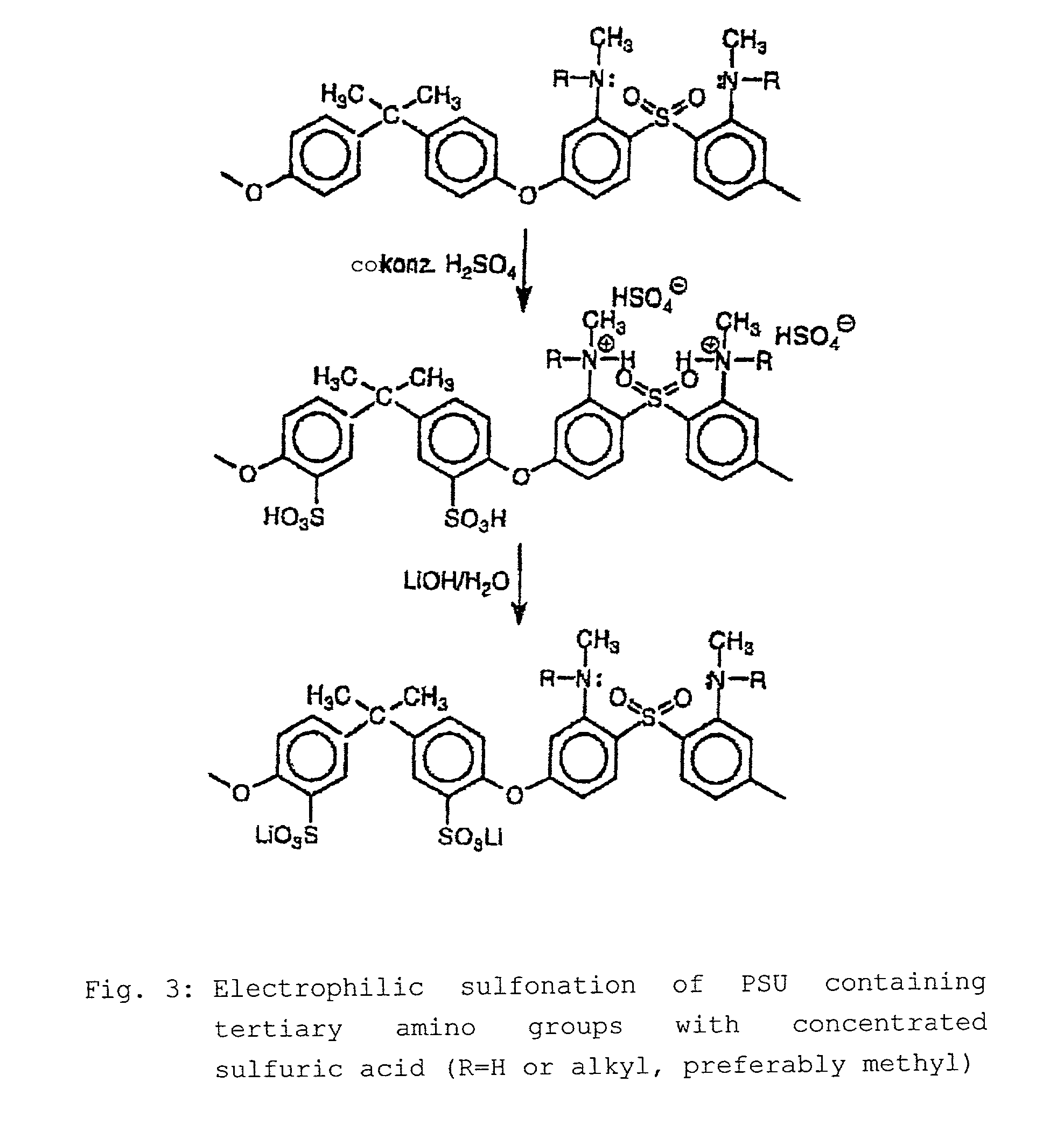
Step-by-step alkylation of polymeric amines
InactiveUS7132496B2Ion-exchange process apparatusNon-metal conductorsHydrophilic polymersOrganic base
The invention relates to the following: a method for step-by-step alkylation of primary polymeric amines by step-by-step deprotonation with a metallo-organic base and a subsequent reaction with an alkyl halide; a method for modifying tertiary polymeric amines with other functional groups; polymers with secondary / tertiary amino groups and with quaternary ammonium groups; polymers with secondary / tertiary amino groups and other functional groups, especially cation exchanger groupings; membranes consisting of the above polymers, either non-crosslinked or ionically or covalently cross-linked; acid-base-blends / membranes, and a method for producing same, consisting of basic polymers with polymers containing sulphonic acid, phosphonic acid or carboxyl groups; the use of ion exchanger polymers as membranes in membrane processes, e.g., polymer electrolyte membrane fuel cells, direct methanol fuel cells, redox batteries, or electrodialysis; the use of the inventive hydrophilic polymers as membranes in dialysis and reverse osmosis, nanofiltration, diffusion dialysis, gas permeation, pervaporation and perstraction.
Owner:HARING THOMAS
Proton exchange composite membranes, composite solutions, and method for manufacturing and fuel cell using the same
InactiveUS7029559B2Effective blockingImprove proton conductivityIon-exchange process apparatusNon-metal conductorsFuel cellsOrganoclay
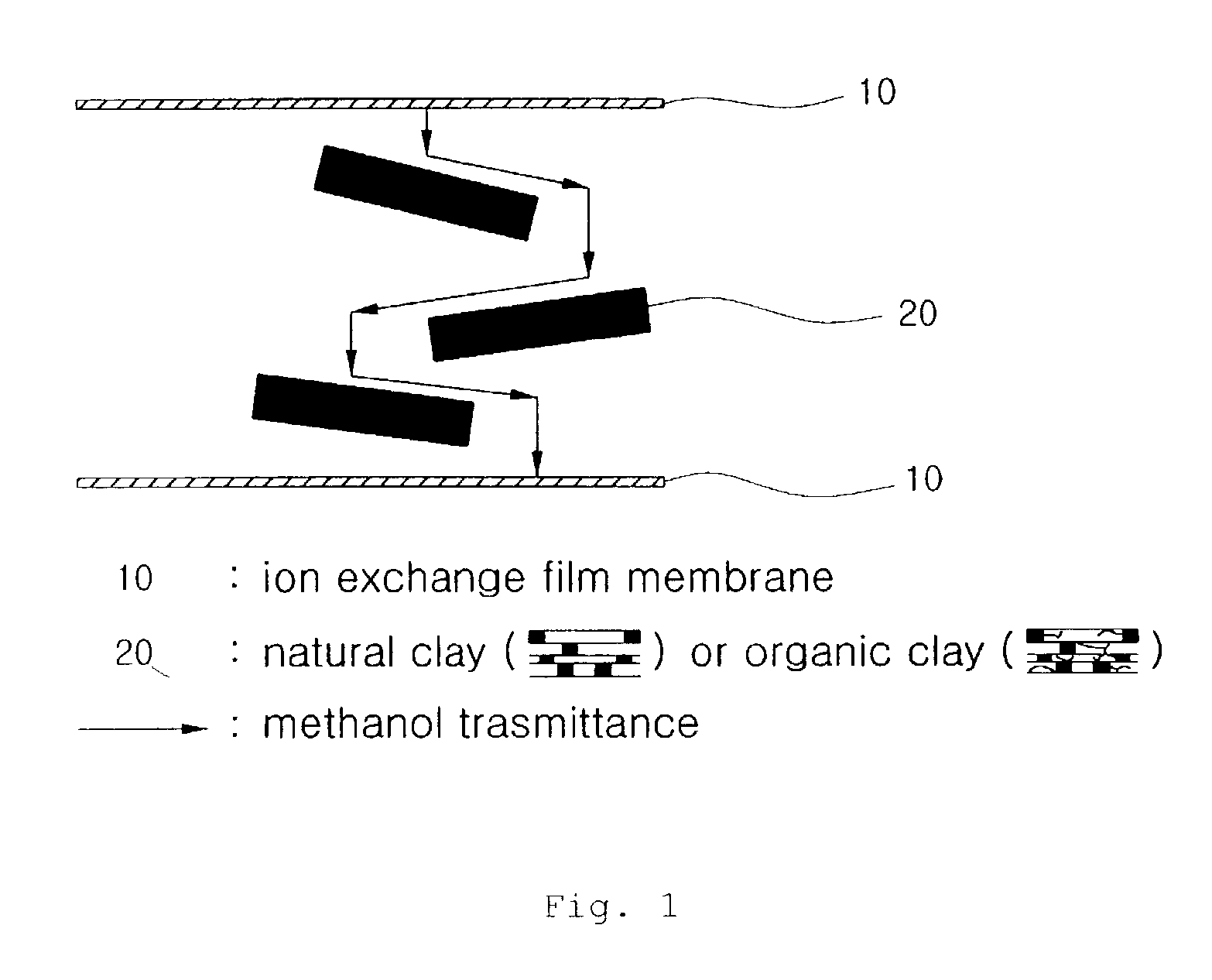
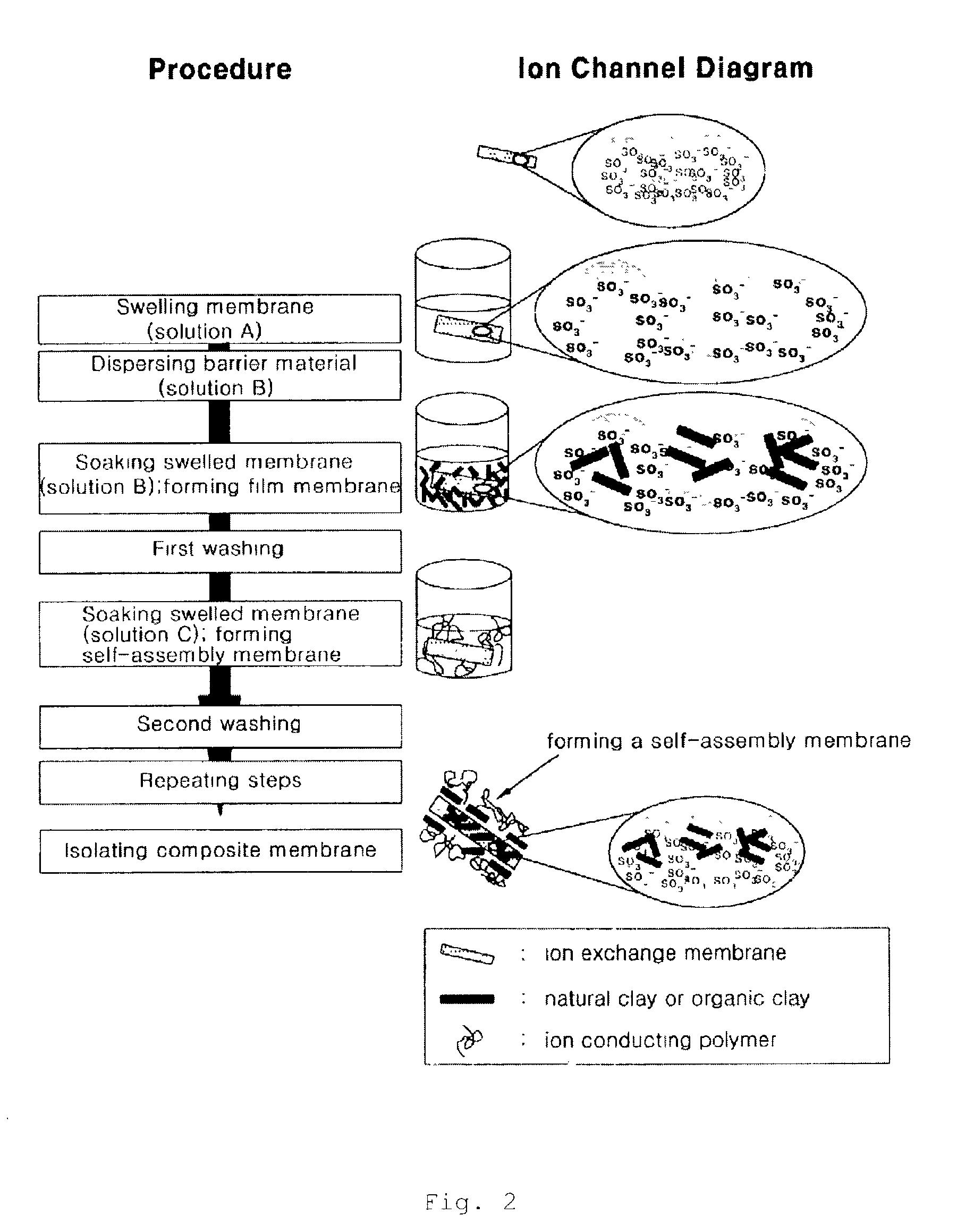
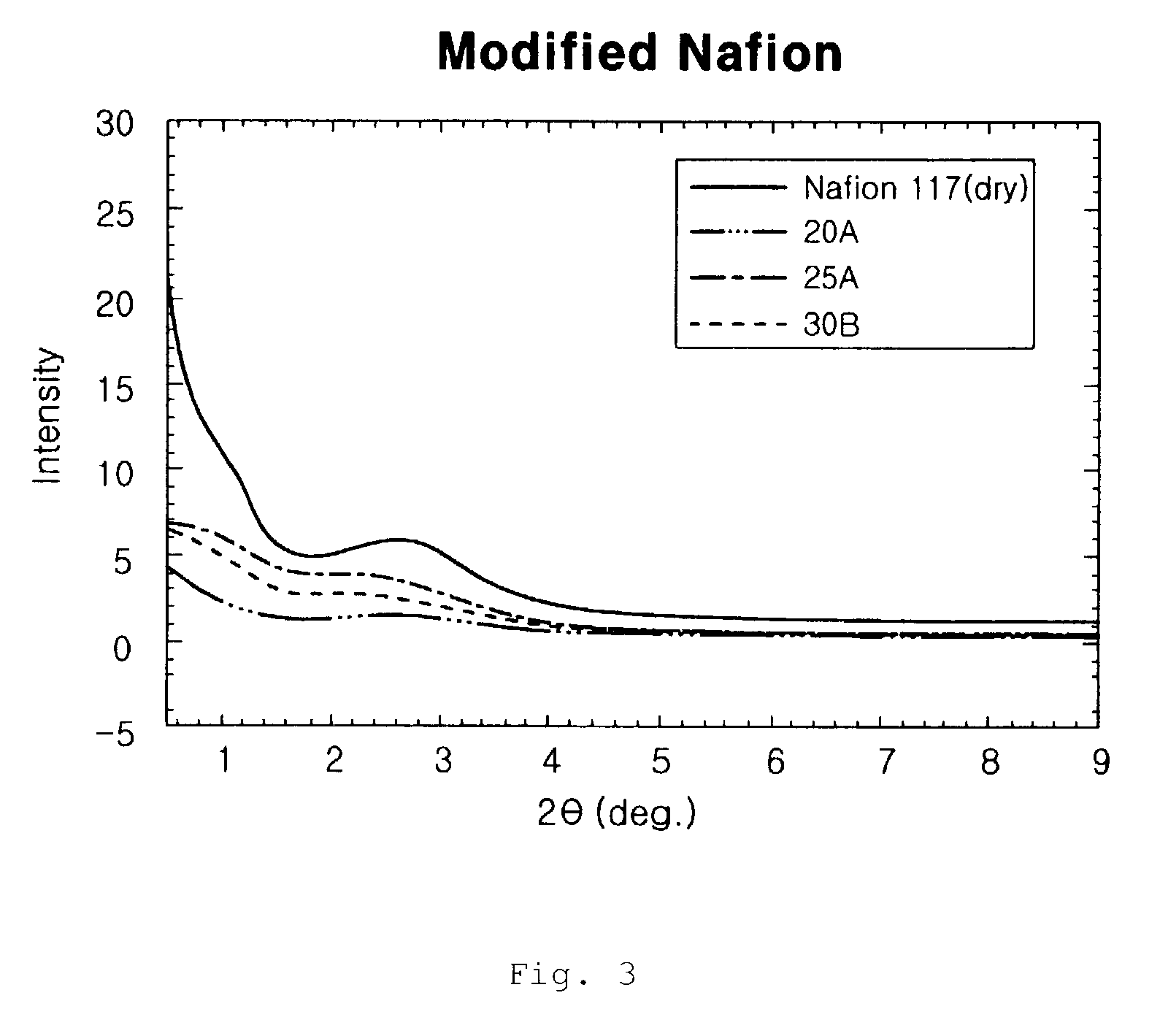
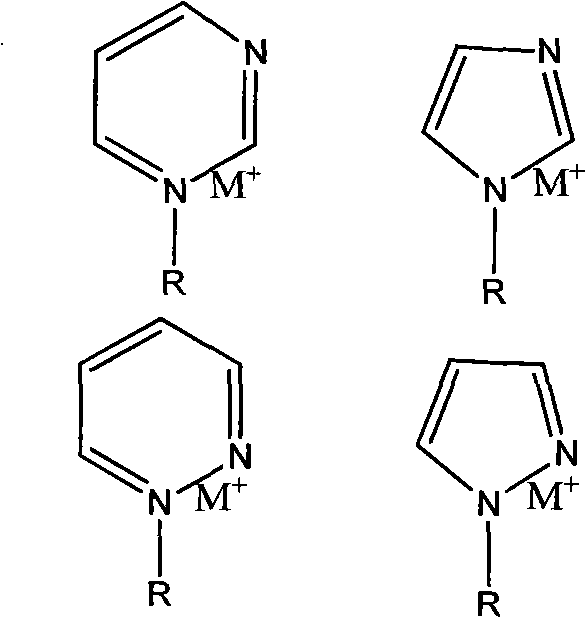
An ion exchange composition membrane is made up of dispersed natural clay and / or organized clay in ion conducting polymeric film as a methanol barrier material. The membrane exhibits low methanol crossover, high proton conductivity and is thus suitable for use in direct methanol fuel cells with low costal advantage.
Owner:KOREA INST OF SCI & TECH
High-electric conductivity aromatic polymer ionic liquid diaphragm material and preparation method thereof
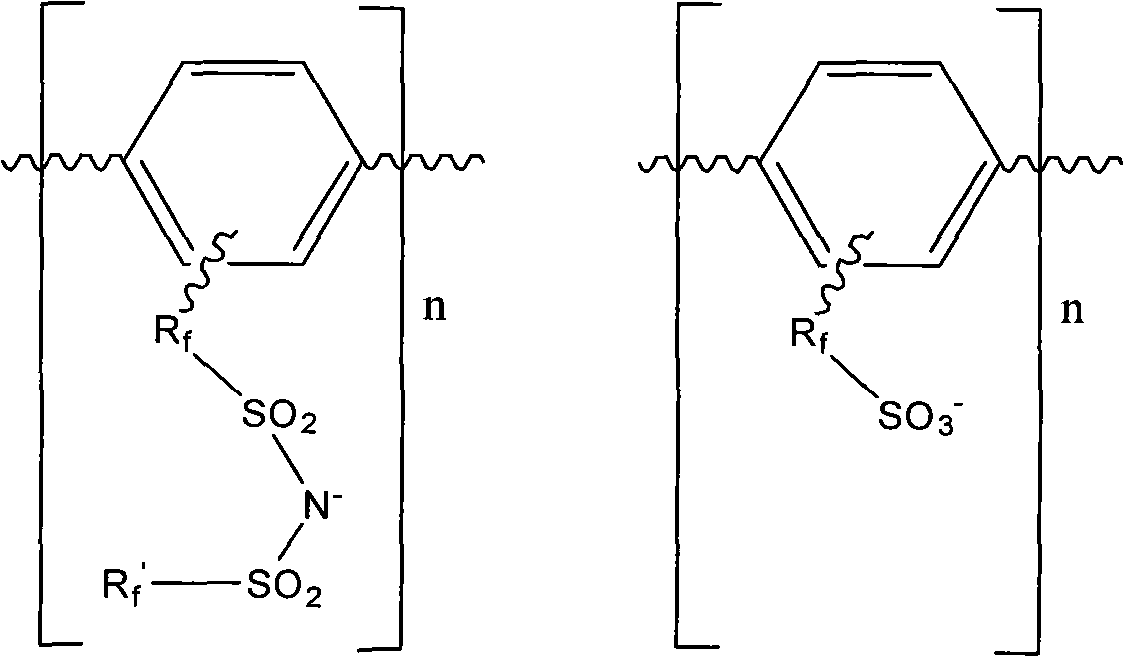
ActiveCN101935398ANot easy to loseImprove conductivitySemi-permeable membranesOrganic diaphragmsSide chainSolvent
The invention discloses a high-electric conductivity aromatic polymer ionic liquid diaphragm material and a preparation method thereof. The high-conductivity aromatic polymer ionic liquid diaphragm material comprises an cation and an anion, wherein the cation is a composite cation formed by combining at least one ion of hydrogen ions or metal ions with organic molecules; and the anion is a fluorine-contained sulfonic acid anion or a fluorine-contained sulfimide anion which is connected to an aromatic benzene ring-contained polymer side chain. Compared with the prior art, the polymer ionic liquid diaphragm material of the invention has the advantages of difficult loss of ionic liquid, electric conductivity without depending on water, penetration resistance of OH-anion, methanol or vanadium ions, and the like, can form a stable ion channel which has high ionic activity, high chemical stability, low ion activating energy and easy adsorption of organic polar solvents and is an ideal diaphragm material which can be applied to various fields of lithium ion batteries, secondary lithium ion batteries, methanol fuel batteries, liquid flow batteries, electrodialysis water treatment, atomic energy industry and analysis, catalytic synthesis, chlor-alkali industry, and the like.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
PtRu/graphene nano electro-catalyst and preparation method thereof
InactiveCN101740786AReductiveGood dispersionCell electrodesMetal/metal-oxides/metal-hydroxide catalystsSodium acetrizoateSodium acetate
The invention discloses a preparation method of PtRu / graphene nano electro-catalyst, comprising the following steps of: ultrasonically dispersing oxidized nano graphite sheets into liquid polylol; then adding a chloroplatinic acid solution and a sodium acetate solution, sufficiently mixing, wherein the content of the oxidized nano graphite sheets contained in a mixture is 0.3-1.1 g / L, the concentration of chloroplatinic acid is 0.0004-0.002 mol / L, the concentration of ruthenium chloride is 0.0004-0.0013 mol / L, and the concentration of sodium acetate is 0.005-0.027 mol / L; transferring the mixture to a microwave hydro-thermal reaction kettle for microwave hydro-thermal reaction for 5-10 minutes; and filtering, washing and drying to obtain the PtRu / graphene nano electro-catalyst, wherein the mass fraction of a PtRu alloy contained in the PtRu / graphene nano electro-catalyst is 20-40 percent, the mass fraction of graphene is 80-60 percent, the atomic ratio of the PtRu alloy is Pt:Ru=1:2-1.5:1, and the liquid polylol is propanetriol or glycol. The preparation method has energy saving, fastness, simple process, and the like; and in addition, the prepared PtRu / graphene nano electro-catalyst has good electrocatalysis property for the oxidation of methanol and ethanol and is widely used as anode catalysts of direct methanol fuel cells.
Owner:ZHEJIANG UNIV
Gas diffusion cathode using nanometer sized particles of transition metals for catalysis
A gas diffusion cathode for electrochemical cells provides higher power capability through the use of nano-particle catalysts. The catalysts comprise nanometer-sized particles of transition metals such as nickel, cobalt, manganese, iron, palladium, ruthenium, gold, silver, and lead, as well as alloys thereof, and respective oxides. These catalysts can substantially replace or eliminate platinum as a catalyst for oxygen reduction. Cathodes using such catalysts have applications to metal-air batteries, hydrogen fuel cells (PEMFCs), direct methanol fuel cells (DMFCs), direct oxidation fuel cells (DOFCs), and other air breathing electrochemical systems.
Owner:BRICOLEUR PARTNERS LP
Proton conducting polymer film and method for production thereof
An object of the present invention is to provide a proton conducting polymer membrane that has excellent mechanical properties and high methanol barrier properties, in addition to high proton conductivity, and is useful as an electrolyte in polymer electrolyte fuel cells and direct methanol fuel cells. The present invention provides a proton conducting polymer membrane having a product of a proton conductivity at 23° C. and a methanol barrier coefficient at 25° C. in an aqueous methanol solution of a specified concentration being a specified value or more. The present invention also provides a proton conducting polymer membrane having an ion exchange capacity of 0.3 milli-equivalent / g or more, and having a crystalline phase.
Owner:KANEKA CORP
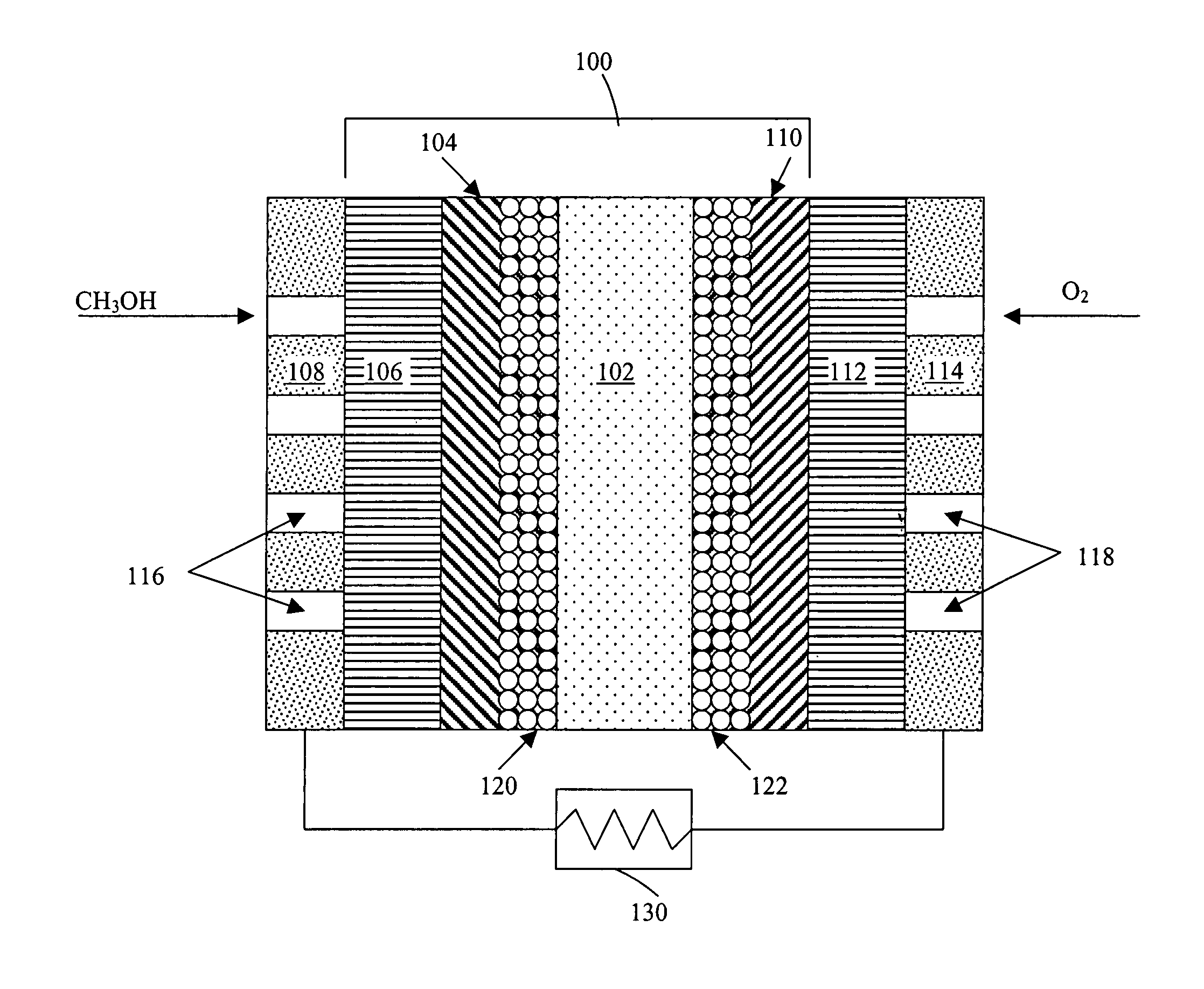
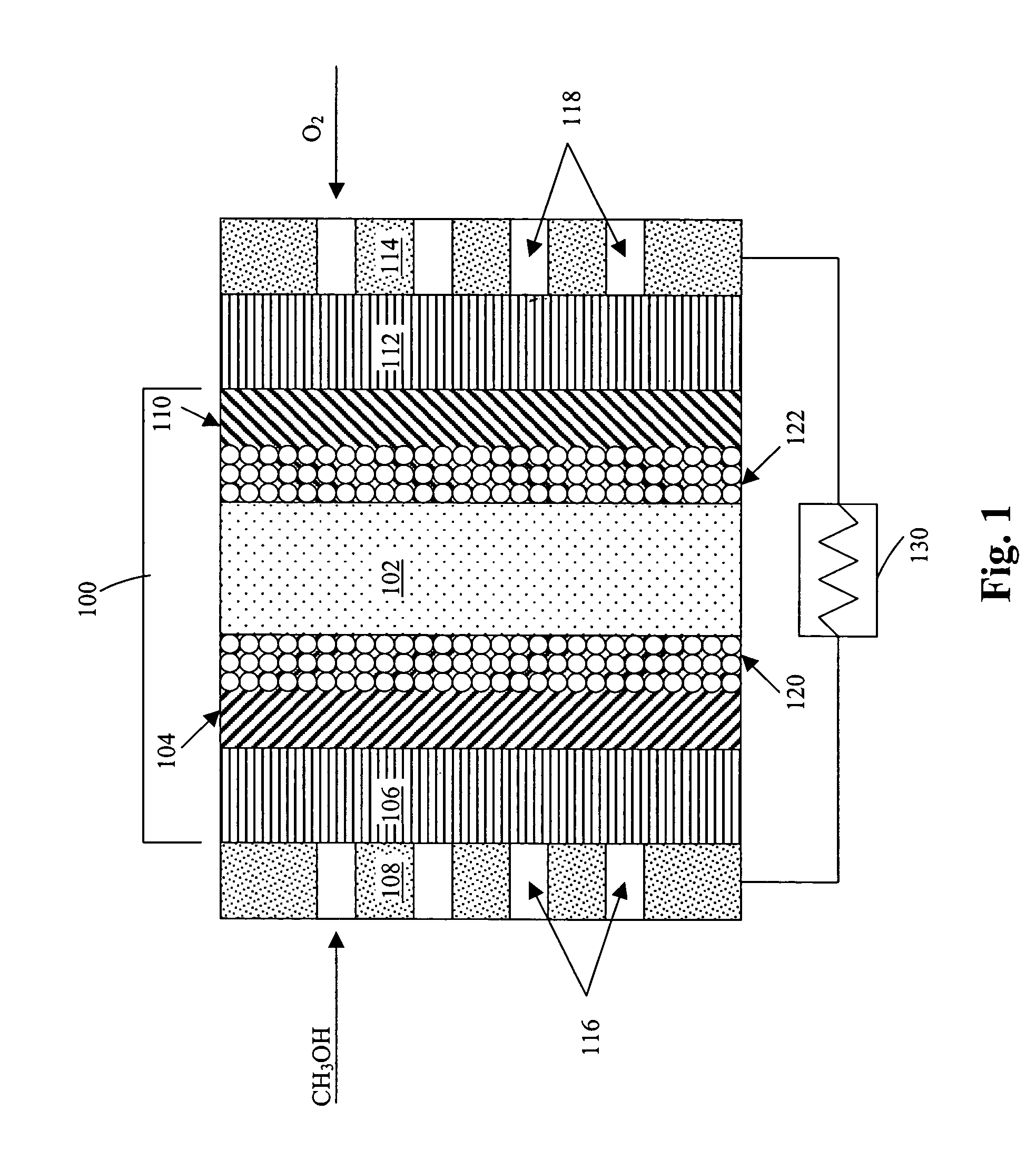
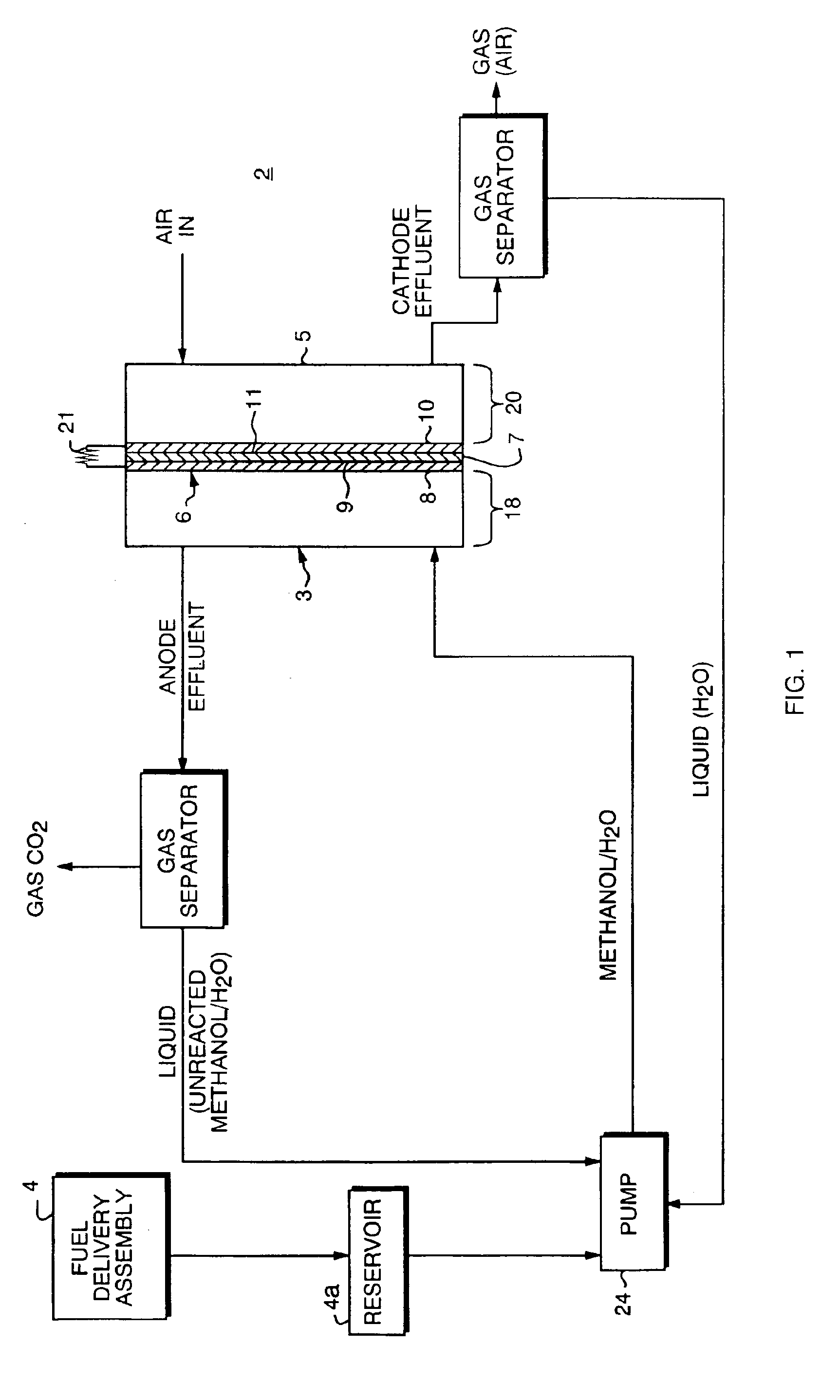
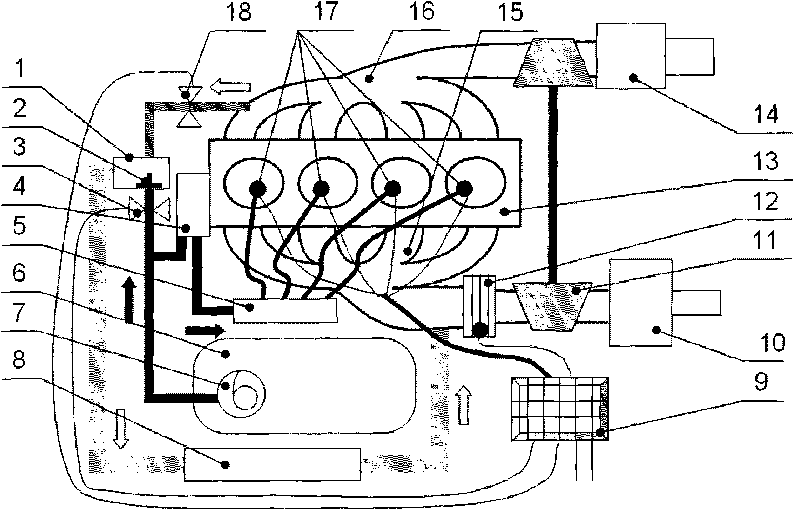
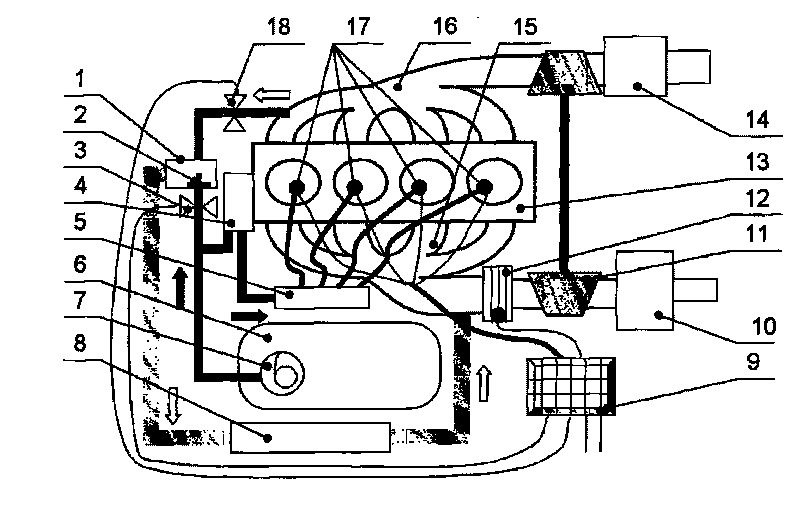
Thermal-fluids management system for direct methanol fuel cells
InactiveUS20060292412A1Well mixedEasy to moveReactant parameters controlFuel cells groupingFuel cellsMethanol fuel
The present invention provides a system and method for passive thermal-fluids management in a liquid feed fuel cell. In particular, the present invention provides a system and method for passive thermal-fluids management in a direct methanol fuel cell having a methanol storage medium and a methanol and water mixing medium. The fuel cell may also include a methanol distribution medium that facilitates uniform distribution of methanol to the mixing medium and the anode, wherein the methanol and water are used for fuel by the direct methanol fuel cell.
Owner:UNIV OF CONNECTICUT
Pt-CeO2/graphene electro-catalyst and preparation method thereof
InactiveCN101733094AReductiveGood dispersionCell electrodesCatalyst activation/preparationSodium acetateCerium
The invention discloses a Pt-CeO2 / graphene electro-catalyst which uses graphene as a carrier, platinum as an active component and CeO2 as an auxiliary component, wherein the mass fraction of the platinum contained in the Pt-CeO2 / graphene electro-catalyst is 20 percent; and the mole ratio of the platinum and cerium is 1:1-2.5:1. A preparation method of the Pt-CeO2 / graphene electro-catalyst comprises the following steps of: ultrasonically dispersing oxidized nano graphite sheets into glycol; then adding a chloroplatinic acid solution, an aqueous ammonium ceric nitrate solution and an aqueous sodium acetate solution, and sufficiently mixing; transferring a mixture to a microwave hydro-thermal reaction kettle; and after microwave hydro-thermal reaction, filtering, washing and drying to obtain the Pt-CeO2 / graphene electro-catalyst. The preparation method has energy saving, fastness, simple process, and the like; and in addition, the prepared Pt-CeO2 / graphene electro-catalyst has high electrocatalysis activity for the electrochemical oxidation of methanol and is widely used for direct methanol fuel cells.
Owner:ZHEJIANG UNIV
Three-dimensional nitrogen-doped graphene platinoid-loaded composite electro-catalyst and preparation method thereof
InactiveCN104353480ARaw materials are easy to getHigh yieldPhysical/chemical process catalystsCell electrodesNickel saltDoped graphene
The invention discloses a three-dimensional nitrogen-doped graphene platinoid-loaded composite electro-catalyst. A preparation method comprises the following steps of ultrasonically dispersing graphene oxide sheet in an aqueous solution, adding urea and soluble nickel salt for full and uniform mixing, transferring the mixture into a hydrothermal reaction kettle for reaction, performing freeze-drying to obtain three-dimensional nitrogen-doped graphene, dissolving the three-dimensional nitrogen-doped graphene in ethylene glycol, sequentially adding chloroplatinic acid, copper chloride dehydrate and glutamic acid, and performing microwave reaction to obtain the catalyst. The method has the characteristics of that energy is saved, the speed is high, and the operation is simple; the raw materials are easy to obtain, the yield is high, the capability of platinum in the direct electro-catalytic oxidation of methanol under an acidic condition can be remarkably improved, peak current is 3 to 4 times than that of a commercial carbon black platinum-loaded electro-catalyst and a commercial carbon black platinum-ruthenium-loaded electro-catalyst, and the prepared catalyst is widely applied to a methanol fuel cell.
Owner:GUANGXI NORMAL UNIV
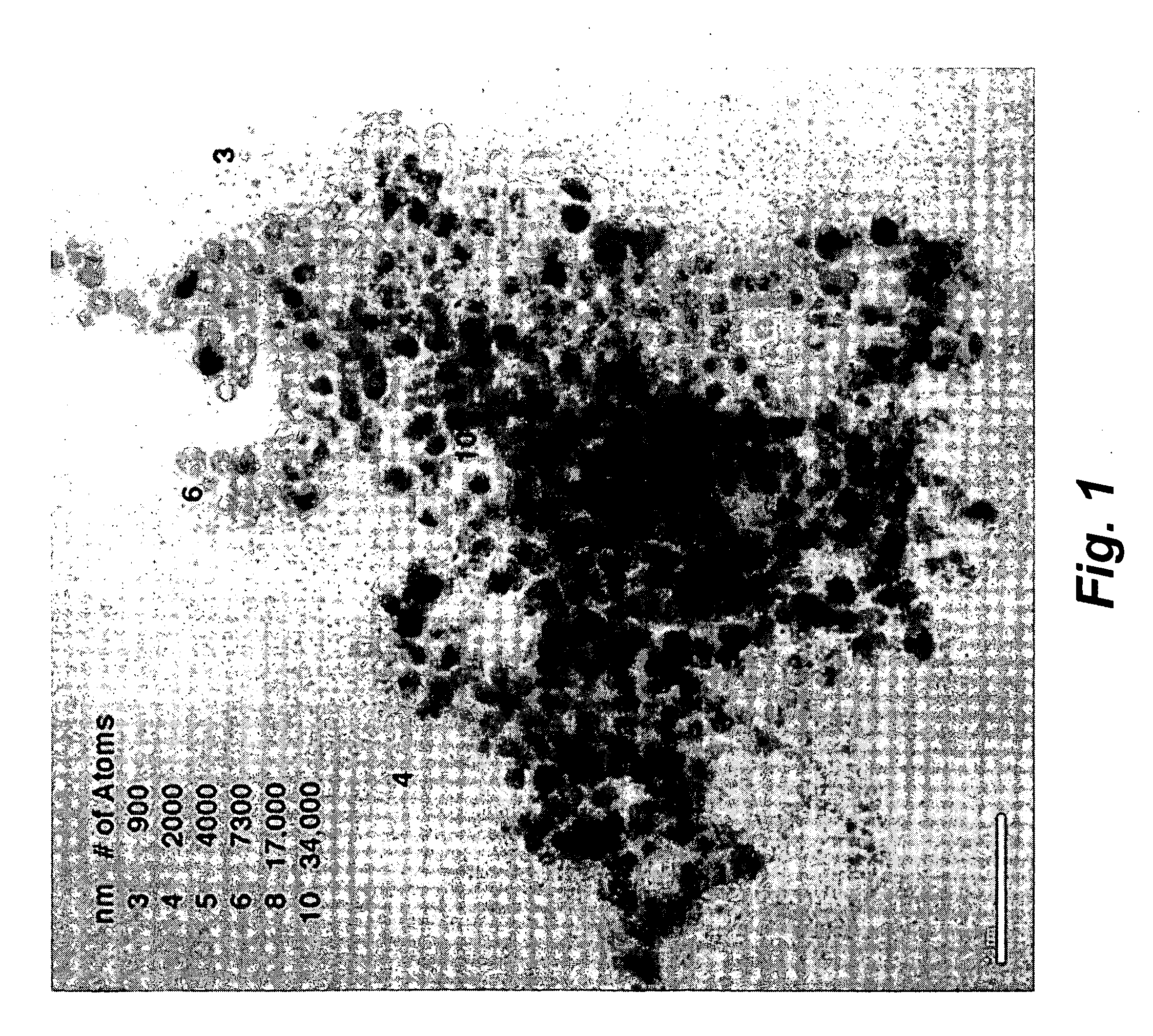
Method for manufacture of noble metal alloy catalysts and catalysts prepared therewith
ActiveUS20060094597A1High degreeEasy to manufactureMaterial nanotechnologyGas treatmentGas phaseReaction temperature
The present invention provides a method for manufacture of supported noble metal based alloy catalysts with a high degree of alloying and a small crystallite size. The method is based on the use of polyol solvents as reaction medium and comprises of a two-step reduction process in the presence of a support material. In the first step, the first metal (M1=transition metal; e.g. Co, Cr, Ru) is activated by increasing the reaction temperature to 80 to 160° C. In the second step, the second metal (M2=noble metal; e.g. Pt, Pd, Au and mixtures thereof) is added and the slurry is heated to the boiling point of the polyol solvent in a range of 160 to 300° C. Due to this two-step method, an uniform reduction occurs, resulting in noble metal based catalysts with a high degree of alloying and a small crystallite size of less than 3 nm. Due to the high degree of alloying, the lattice constants are lowered. The catalysts manufactured according to the method are used as electrocatalysts for polymer electrolyte membrane fuel cells (PEMFC), direct-methanol fuel cells (DMFC) or as gas phase catalysts for CO oxidation or exhaust gas purification.
Owner:UMICORE AG & CO KG
Preparation method of membrane electrode of direct methanol fuel cell
The invention relates to a preparation method of the membrane electrode of a direct methanol fuel cell. The method comprises the following steps: an electrostatic spinning technology is adopted to construct a nano-fiber network structure thin membrane mixed by active carbon powder and Nafion resin; a precious metal nano-catalyst is deposited on the surface of the manufactured nano-fiber network structure thin membrane, so that a cathode catalyst layer thin membrane and an anode catalyst layer thin membrane are manufactured respectively; or the mixture of the precious metal nano-catalyst and the Nafion resin is taken as raw materials to directly construct the cathode catalyst layer thin membrane and the anode catalyst layer thin membrane through the electrostatic spinning technology; a cathode gas diffusion layer, the anode catalyst layer thin membrane, a Nafion membrane, the cathode catalyst layer thin membrane and a cathode gas diffusion layer are hot-pressed finally, so that the aggregation of the membrane electrode of the direct methanol fuel cell is manufactured; the membrane electrode with a nano-fiber three-dimensional network structure is constructed through the electrostatic spinning technology, so that the maximization of the three-phase reaction interface of the membrane electrode is achieved, and the improvement of electrocatalytic activity, mass-transfer efficiency and utilization efficiency of the catalyst is achieved.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
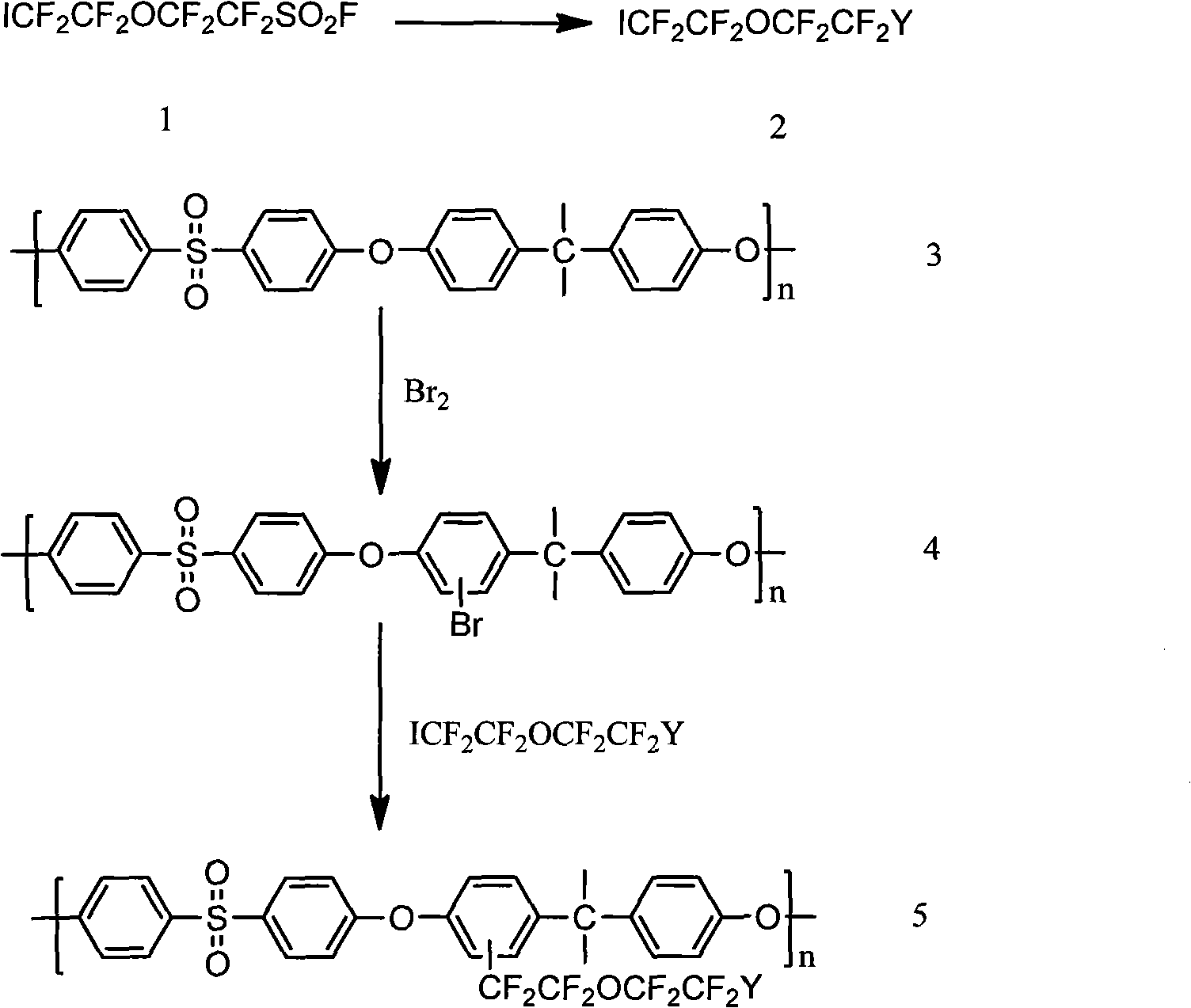
Processes, Framed Membranes and Masks for Forming Catalyst Coated Membranes and Membrane Electrode Assemblies
InactiveUS20080075842A1Easy and economical and efficient to manufactureImprove efficiencyIon-exchanger regenerationCell electrodesCoated membraneFuel cells
A process for preparing catalyst coated membranes and membrane electrode assemblies for use in direct methanol fuel cells is provided. Cathode and anode layers are formed by spraying catalyst-containing inks onto a novel framed electrolytic membrane to form a catalyst coated membrane. The spraying process optionally employs one or more masks, which carefully control where the catalyst-containing ink is deposited. Following application of the cathode and anode layers, diffusion layers are prepared and inserted onto the catalyst coated membranes, and pressed to form membrane electrode assemblies.
Owner:CABOT CORP
Methods to control water flow and distribution in direct methanol fuel cells
InactiveUS20060134487A1Good cell performanceGood fuel efficiency performanceWater management in fuel cellsSolid electrolyte fuel cellsFuel efficiencyAir cathode
A direct methanol fuel cell unit is provided with a fuel cell including an anode, a cathode with a hydrophobic microporous layer, an electrolyte membrane put in-between, and a fuel supply path supplying fuel to the anode. The fuel supply path is provided with an upwind water barrier preventing back-diffusion of water and a gas flow path channeling gas generated at the anode and disposed between the barrier and the anode. A water-rich zone is formed between the water barrier and the cathode microporous layer. Water loss from either side of this zone is eliminated or minimized, thereby permitting direct use of highly concentrated methanol in the fuel flow path with good fuel efficiency and power performance. The cell unit can be applied equally well to both an active circulating air cathode and an air-breathing cathode.
Owner:EC POWER LLC +1
Carbon-supported CoN fuel-cell catalyst as well as preparation method and application thereof
InactiveCN102324531ALow costHigh catalytic activityPhysical/chemical process catalystsCell electrodesNon platinumSolvent
The invention relates to a carbon-supported CoN fuel-cell catalyst as well as a preparation method and application thereof. The catalyst comprises the following components in mass percentage: 40-99 percent of carbon material and 1-60 percent of active component. The catalyst is prepared through the following steps of: dispersing the components into a mortar loaded with a solvent, fully grinding until the solvent is completely volatilized, and obtaining a catalyst precursor after vacuum drying; and roasting for 2-4 hours by increasing the temperature for 200-900 DEG C at the speed of 20 DEG C / minute under the protection of an inert-gas atmosphere. The catalyst is applied to alkaline fuel cells, direct methanol fuel cells, direct ethanol fuel cells or low-temperature fuel cells. The catalyst is a non-platinum catalyst, so that the cost of the fuel cells can be markedly lowered; and the preparation method disclosed by the invention is simple and easy to operate, has low cost, is suitable for industrialized production and has good application prospects.
Owner:DONGHUA UNIV
Proton exchange membrane materials for the advancement of direct methanol fuel-cell technology
A new class of hybrid organic-inorganic materials, and methods of synthesis, that can be used as a proton exchange membrane in a direct methanol fuel cell. In contrast with Nafion® PEM materials, which have random sulfonation, the new class of materials have ordered sulfonation achieved through self-assembly of alternating polyimide segments of different molecular weights comprising, for example, highly sulfonated hydrophilic PDA-DASA polyimide segment alternating with an unsulfonated hydrophobic 6FDA-DAS polyimide segment. An inorganic phase, e.g., 0.5–5 wt % TEOS, can be incorporated in the sulfonated polyimide copolymer to further improve its properties. The new materials exhibit reduced swelling when exposed to water, increased thermal stability, and decreased O2 and H2 gas permeability, while retaining proton conductivities similar to Nafion®. These improved properties may allow direct methanol fuel cells to operate at higher temperatures and with higher efficiencies due to reduced methanol crossover.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Compression-ignition methanol engine and control method thereof
ActiveCN101718224AHigh thermal efficiencyEmission reductionElectrical controlInternal combustion piston enginesMethanol fuelMixed gas
The invention relates to a compression-ignition methanol engine and a control method thereof. The compression-ignition methanol engine comprises an engine main body, an EGR system, a methanol fuel supply system and a DME synthesis system, wherein the methanol fuel supply system is connected with the engine main body and used for directly supplying methanol fuel for the engine main body; the methanol fuel supply system is also connected with the DME synthesis system and used for supplying the methanol fuel for the DME synthesis system; the EGR system is connected with the DME synthesis system and used for supplying EGR gas for the DME synthesis system; the DME synthesis system is connected with the methanol fuel supply system and the EGR system and treats the methanol fuel and the EGR gas which are supplied by the two systems; and the DME synthesis system is connected with the engine main body and supplies mixed gas for the engine main body.
Owner:CHERY AUTOMOBILE CO LTD
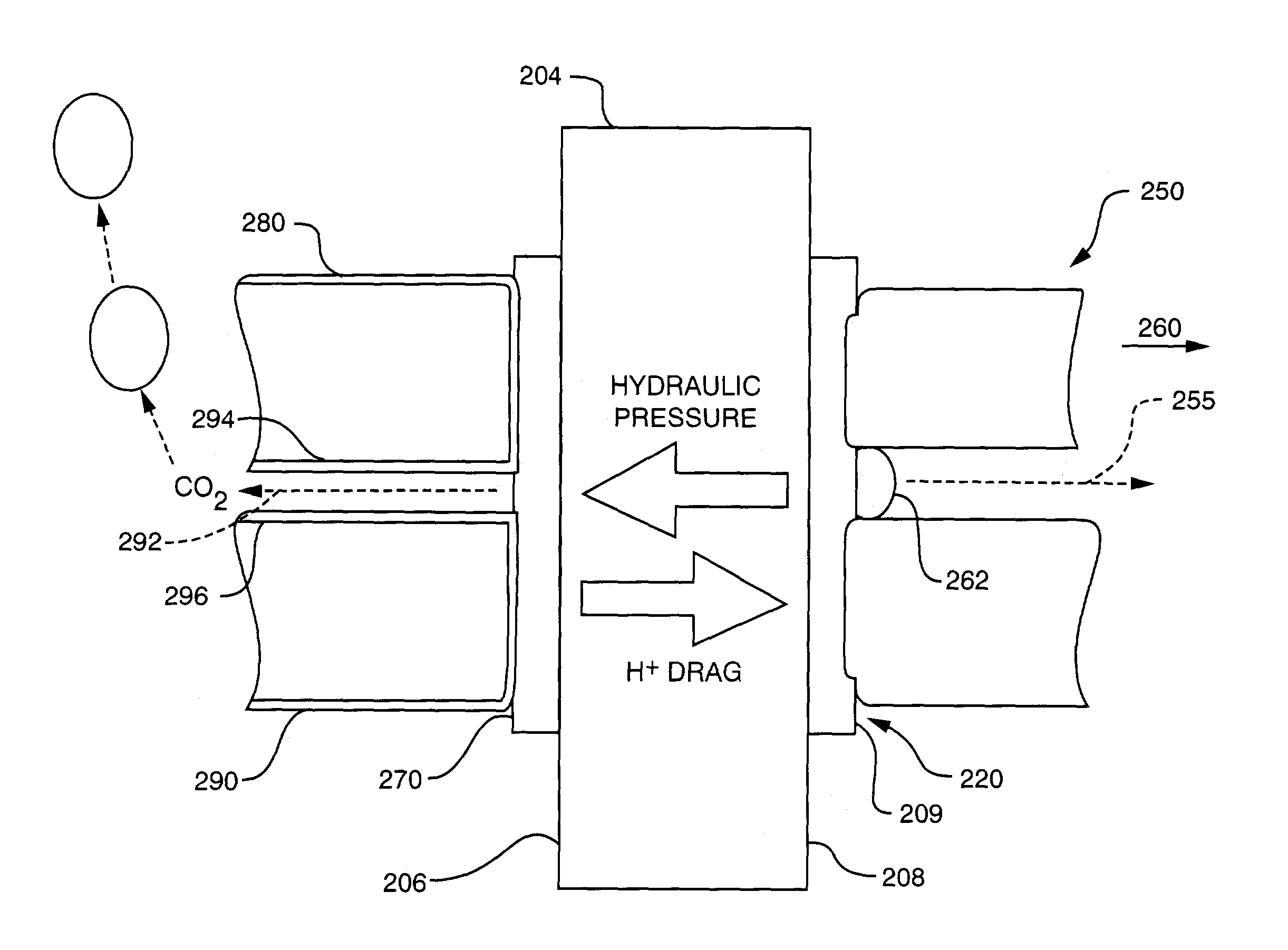
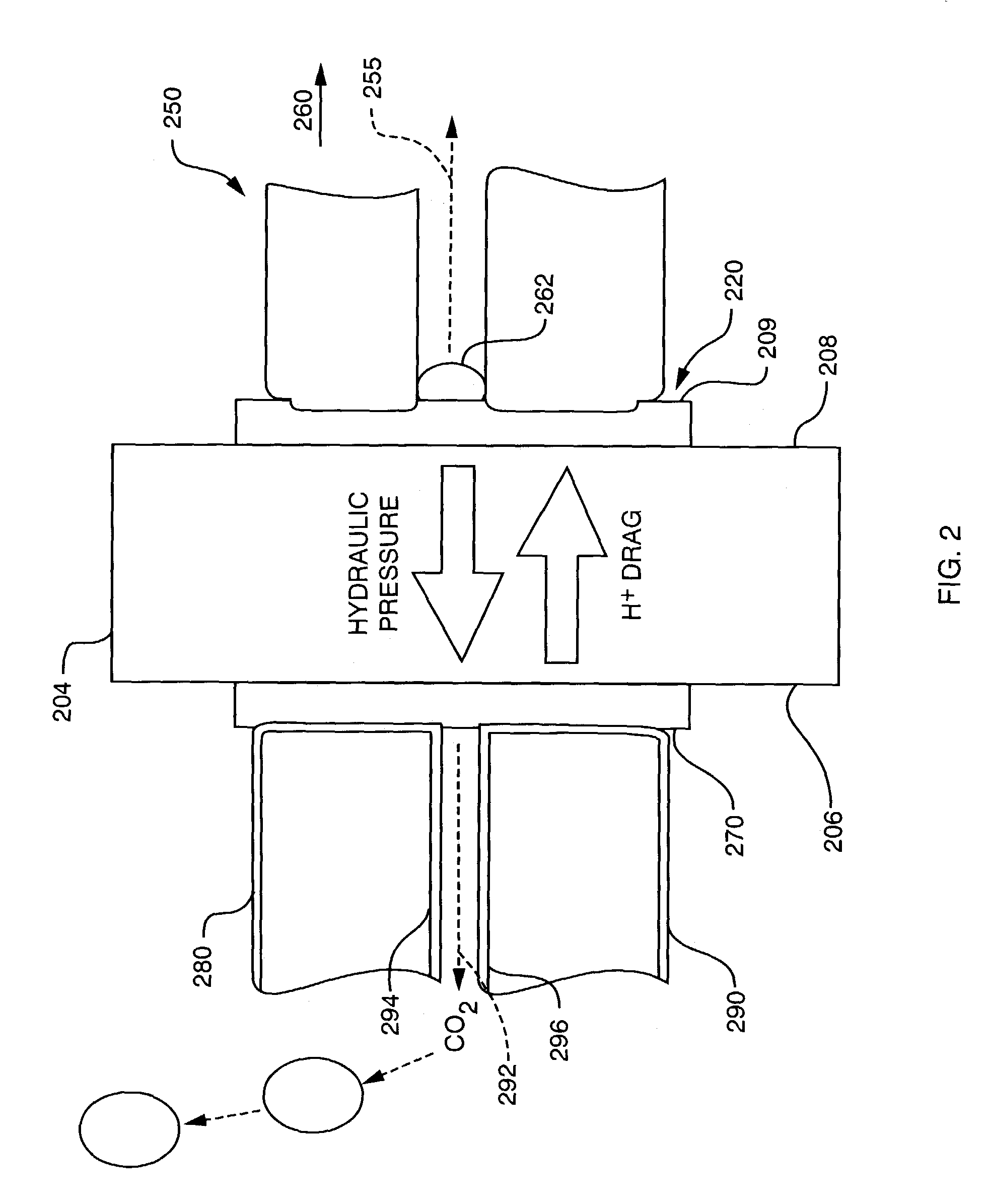
Passive water management techniques in direct methanol fuel cells
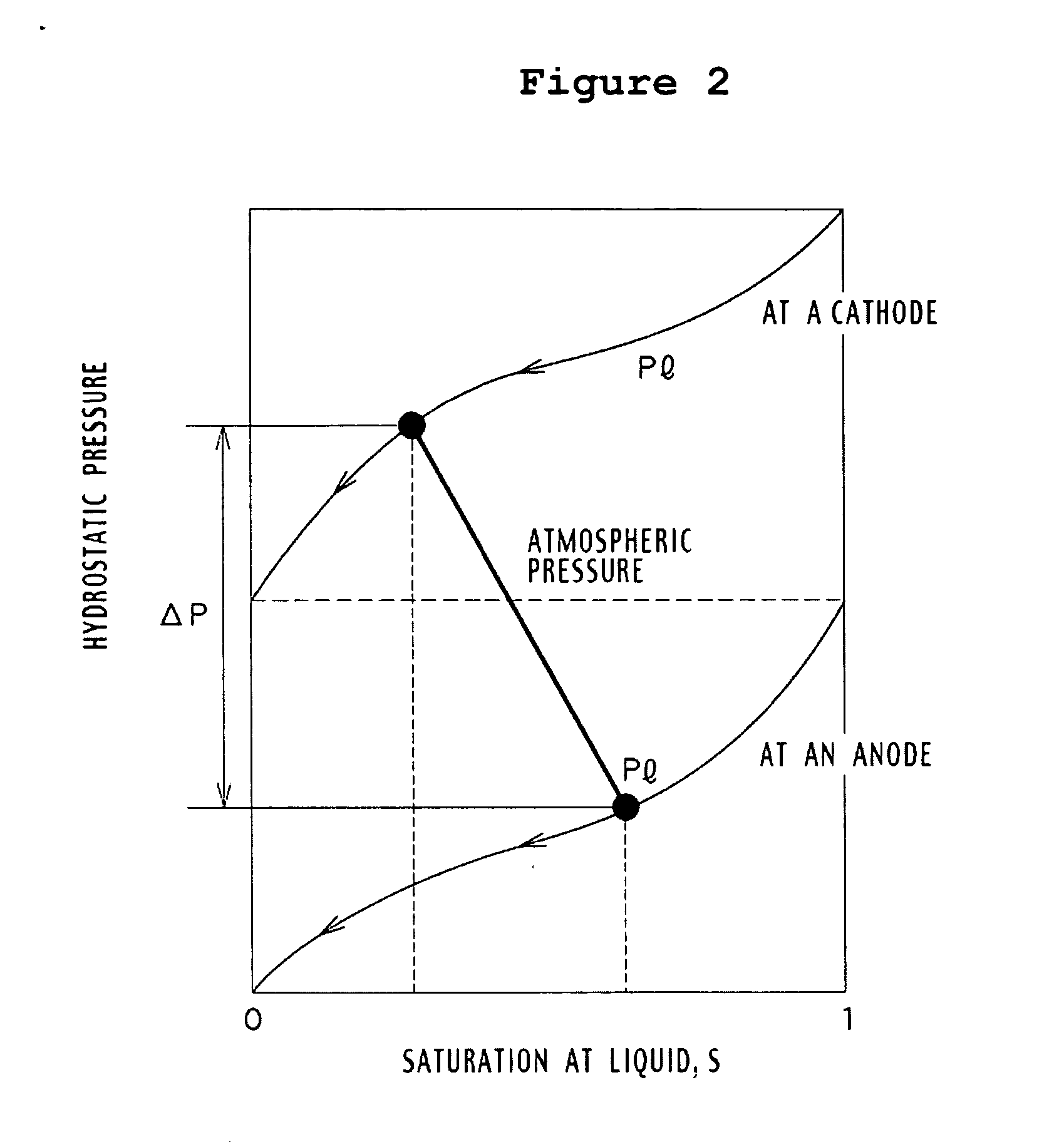
InactiveUS7282293B2Increase water flowConvenient paymentWater management in fuel cellsActive material electrodesWater vaporLiquid water
Passive water management techniques are provided in an air-breathing direct oxidation fuel cell system. A highly hydrophobic component with sub-micrometer wide pores is laminated to the catalyzed membrane electrolyte on the cathode side. This component blocks liquid water from traveling out of the cathode and instead causes the water to be driven through the polymer membrane electrolyte to the cell anode. The air-breathing direct oxidation fuel cell also includes a layer of cathode backing and additional cathode filter components on an exterior aspect of the cell cathode which lessen the water vapor escape rate from the cell cathode. The combination of the well laminated hydrophobic microporous layer, the thicker backing and the added filter layer, together defines a cathode structure of unique water management capacity, that enables to operate a DMFC with direct, controlled rate supply of neat (100%) methanol, without the need for any external supply or pumping of water. The cell anode is provided with a hydrophilic backing layer. When the water is driven through the polymer membrane electrolyte from the cell cathode to the cell anode chamber, it is available for the anodic reaction, and any excess water is carried out along CO2 ventilation channels to the outside environment.
Owner:MTI MICROFUEL CELLS
Non noble metal catalyst for cathode of direct methanol fuel cell, and preparation method
Existing catalysts are noble metals generally, which are costly and easy poisoning. Active component of the disclosed catalyst is transition metal nitride. Carrier is activated carbon powder Vulcan XC-72. Percentage content of mass of transition metal nitride in active component is 1-6%. The preparation method includes steps: dissolving macrocyclic compound of transition metal, Vulcan XC-72 into organic solvent, and carrying out ultrasonic action for 30-60 minutes; stirring up and drying out the said solution under normal temperature, and obtaining powder after drying; loading the powder to closed container and letting ammonia into the container; controlling temperature of heat treatment at 600-1000 deg.C, and time as 0.5-10h so as to obtain the catalyst after natural cooling. Area ratio activity of carbon carried platinum catalyst is 1.5mA / cm2, and the disclosed carbon carried nitride catalyst reaches to 3.1 mA / cm2. Features are: simple flow and easy controlled procedure.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com