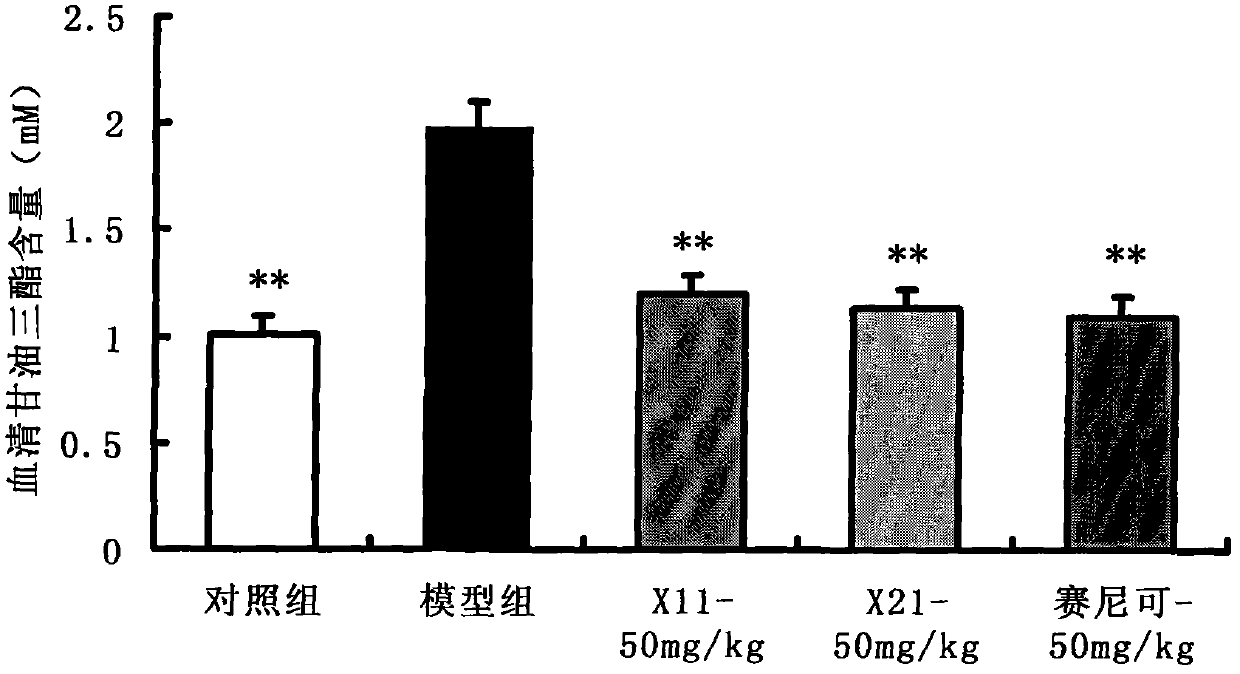
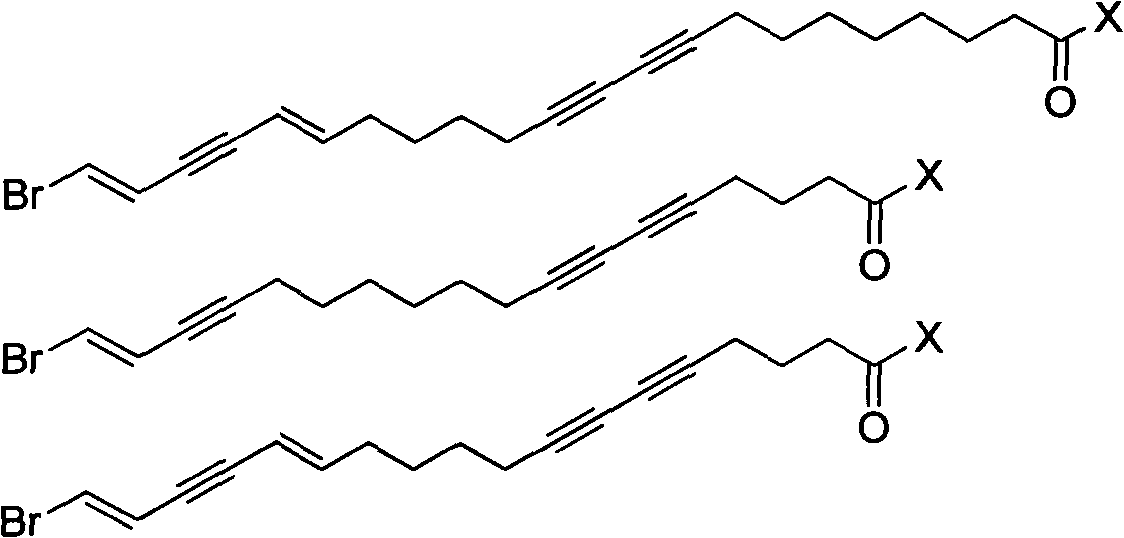
Enyne bromide compounds and preparation method and use thereof
A technology of acetylenic compounds and alkenyl bromide, which is applied in the field of medicine and can solve problems such as unseen biological activity research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
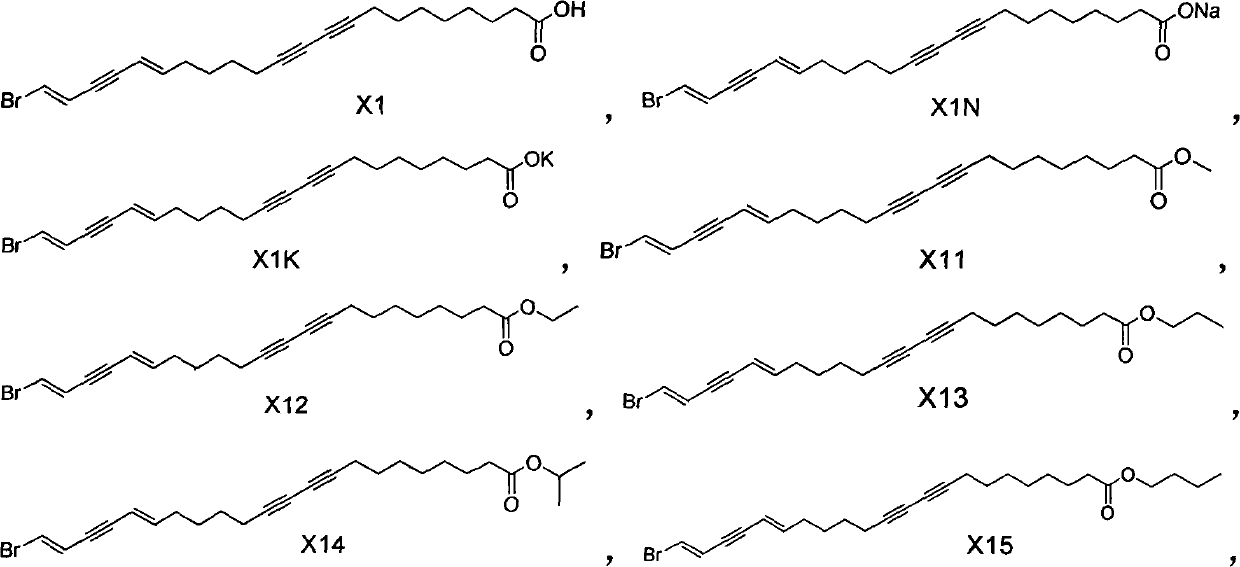
[0037](17E, 21E)-22-bromo-doco-17,21-diene-9,11,19-triynoic acid (compound X1), (E)-18-bromo-octadec-17- En-5,7,15-triynoic acid (compound X2) and (13E,17E)-18-bromo-octadeca-13,17-diene-5,7,15-triynoic acid (compound X3) preparation of
[0038] Step 1: Extraction: China Guangxi Weizhou Island sponge (Xestospongia testudinaria) dry weight 4kg, repeated ultrasonic extraction 3 times with acetone 3L, the extracts were combined and then concentrated under reduced pressure, the obtained crude extract was suspended in 1500ml 1N NaCl solution, and The suspension was repeatedly extracted 3 times with 1500 ml of ether, and the obtained extracts were combined and concentrated under reduced pressure to obtain 40 g of ether extract.
[0039] Step 2: Preparation of compounds X1, X2 and X3: The diethyl ether extract obtained in step 1 was subjected to silica gel column chromatography and eluted with petroleum ether / diethyl ether gradient (100:0→90:10→80:20→50: 50→20:80→10:90→0:100); amon...
Embodiment 2
[0044] (17E, 21E)-22-bromo-doco-17,21-diene-9,11,19-triynoic acid methyl ester (compound X11), (E)-18-bromo-octadecyl- 17-ene-5,7,15-triynoic acid methyl ester (compound X21) and (13E,17E)-18-bromo-octadec-13,17-diene-5,7,15-triynoic acid Preparation of methyl ester (compound X31)
[0045] Step 3: Preparation of compound X31: 40 g of ether extract was subjected to 200-300 mesh silica gel column chromatography, and petroleum ether / ether 100:0→90:10→80:20→50:50→20:80→10:90 → 0:100 gradient elution, the amount of each gradient is 5000ml; among them, petroleum ether / diethyl ether 50:50 eluent concentrate was obtained 2.0g, this part was methylated with diazomethane first, and then petroleum ether / chloroform / Methanol was eluted at 2:1:1, followed by silica gel column chromatography (petroleum ether / ether 100:0→90:10→80:20→70:30) to obtain (17E, 21E)-22-bromo-20 Dicarba-17,21-diene-9,11,19-triynoic acid methyl ester X11 11.3mg; (E)-18-bromo-octadec-17-ene-5,7,15-triynoic acid Me...
Embodiment 3
[0050] Preparation of sodium (17E,21E)-22-bromo-doco-17,21-diene-9,11,19-triynoate (compound X1N)
[0051] Step 4: Dissolving compound X1 obtained in Example 1 in tert-butanol and reacting with sodium tert-butoxide to obtain (17E, 21E)-22-bromo-docos-17,21-diene-9,11, Sodium 19-triynoate;
[0052] (17E,21E)-22-Bromo-doco-17,21-diene-9,11,19-triynoate sodium (compound X1N) has the following physical and chemical properties: colorless jelly, and the spectral data are as follows :C 22 h 26 BrO 2 Na; 1 H NMR (300MHz, CDCl 3 )δ H 6.63(d, J=14.0Hz, H-22), 6.30(dd, J=14.0, 2Hz, H-21), 6.17(dt, J=15.5, 7.0Hz, H-17), 5.55(dd, J =15.5, 2.0Hz, H-18), 2.35(t, J=7.0Hz, H-2), 2.25(t, J=7.0Hz, H-8, H-13), 2.14(m, H-16) , 1.63(m, H-3), 1.51(m, H-7, H-14, H-15), 1.45(m, H-4), 1.39(m, H-6), 1.35(m, H -5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com