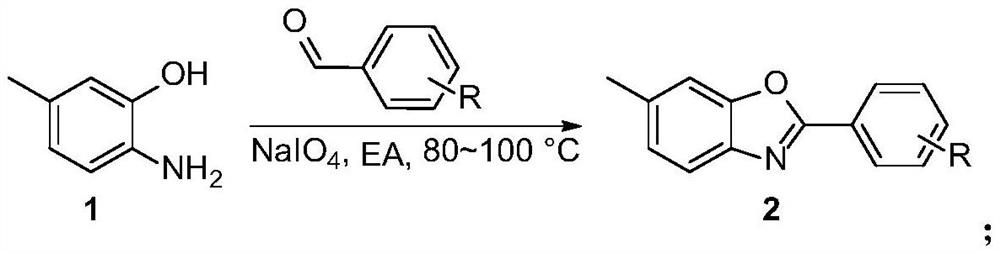
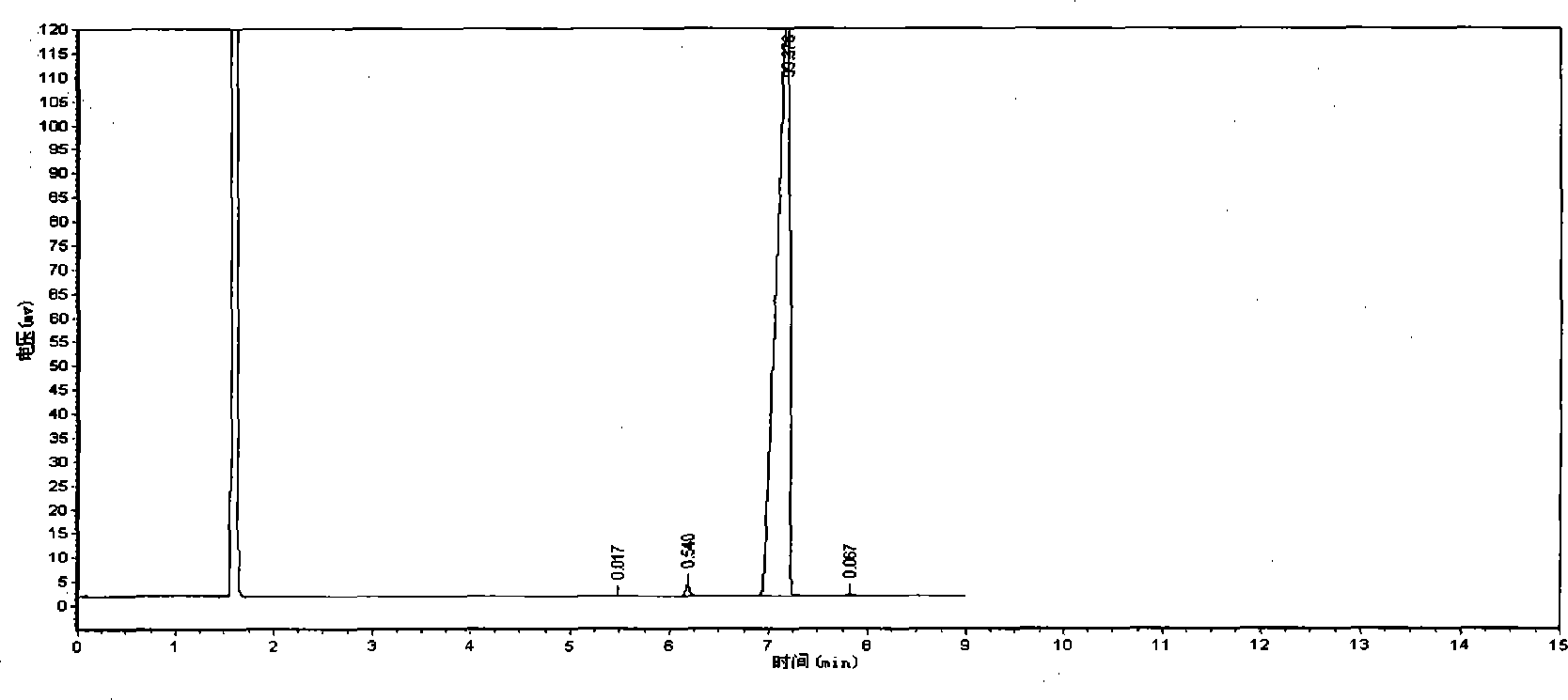
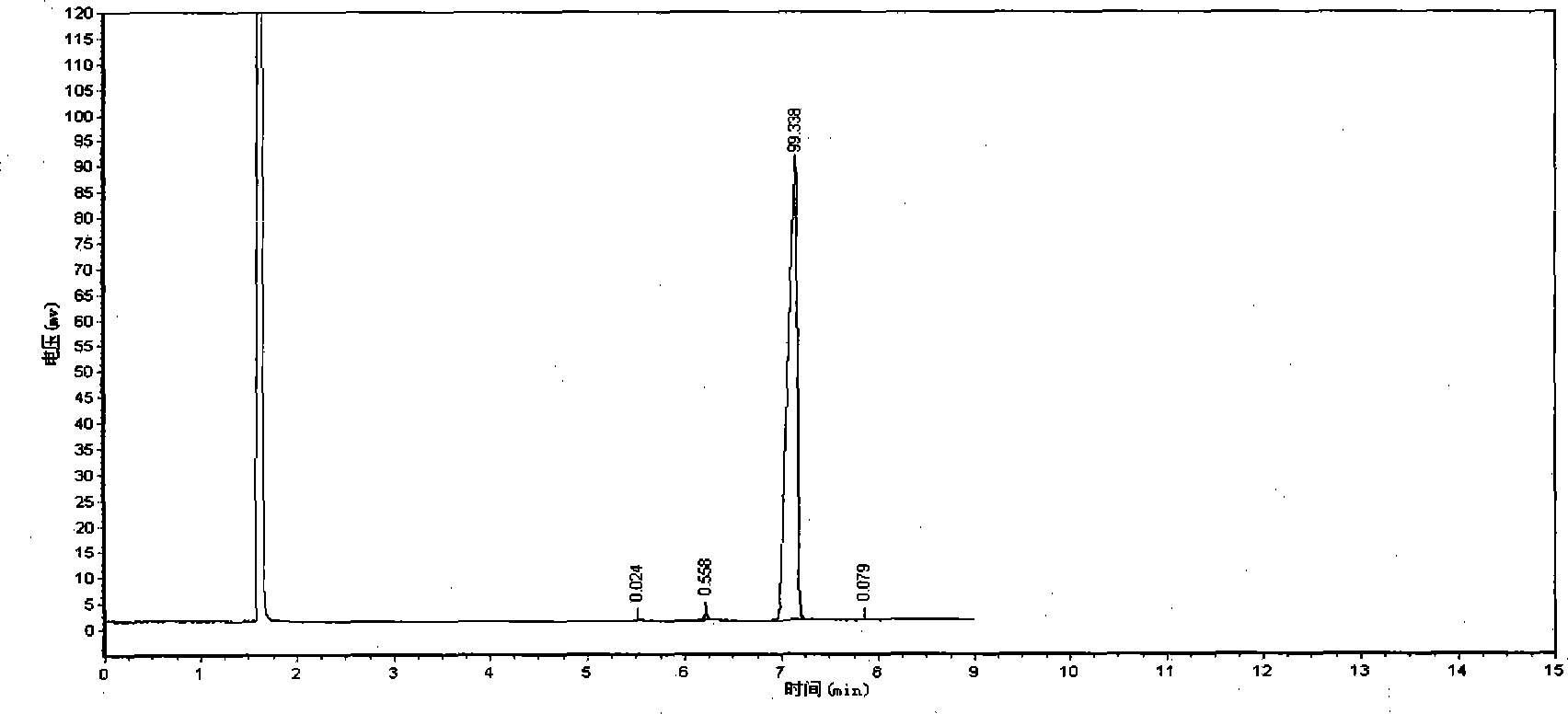
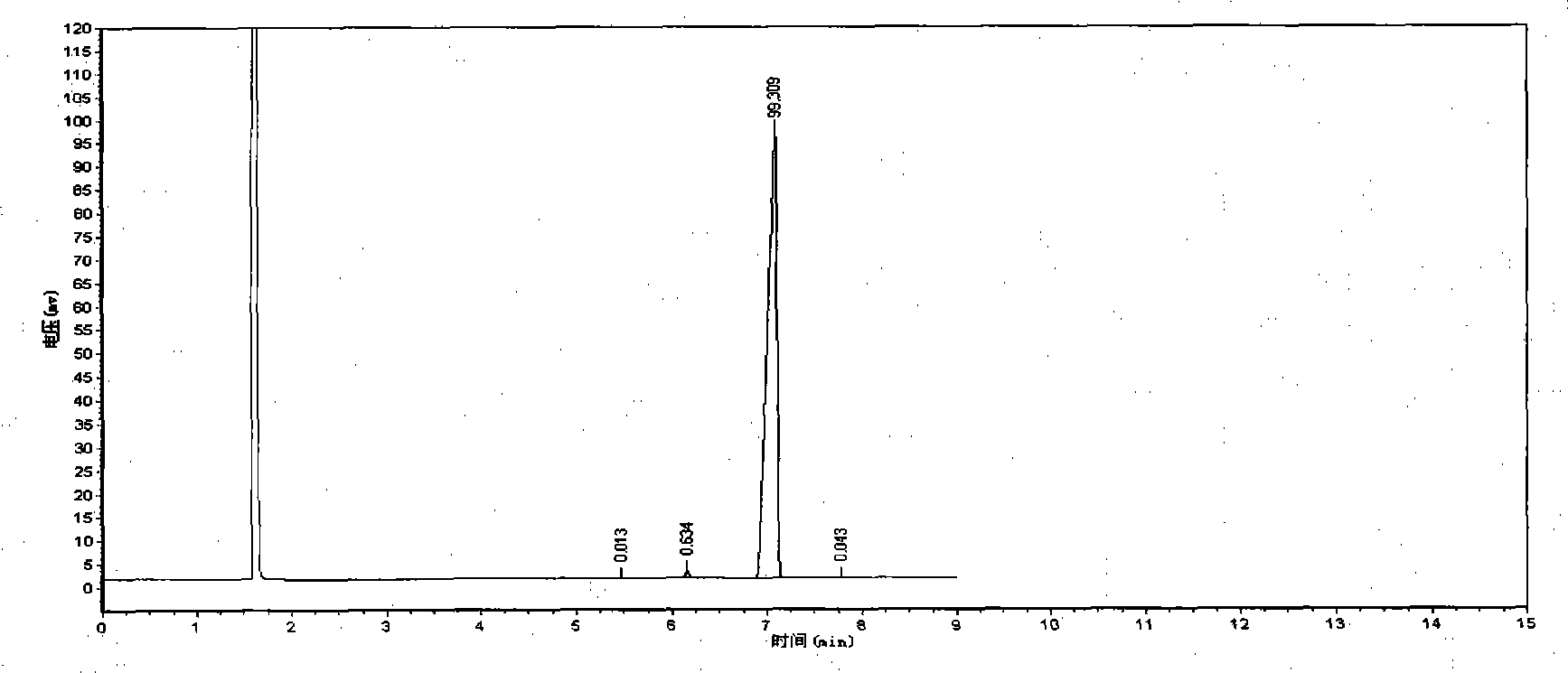
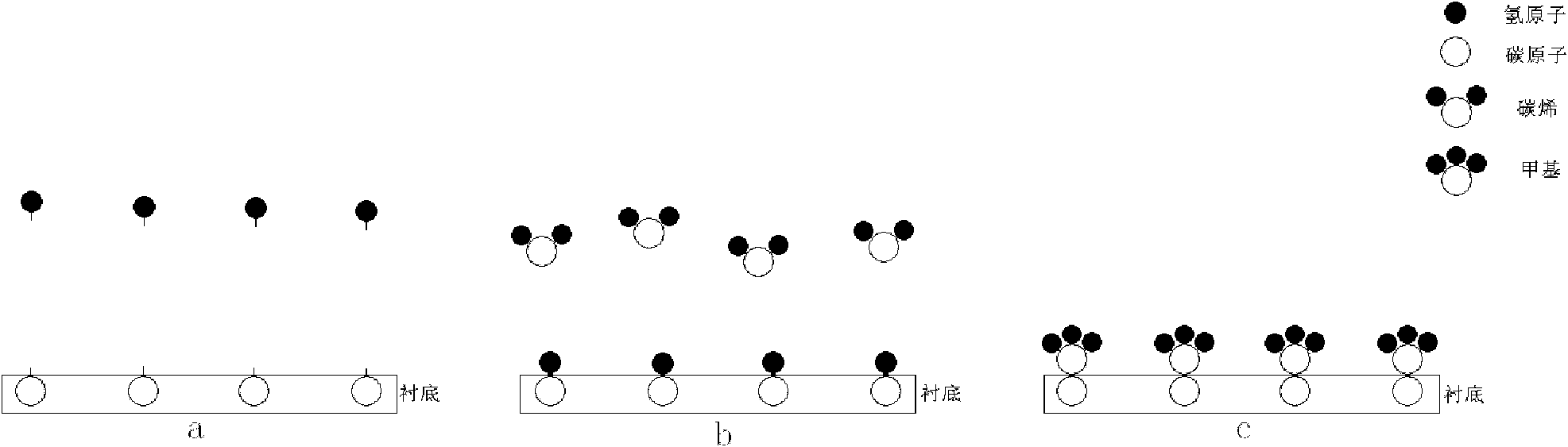Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
143 results about "Diazomethane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
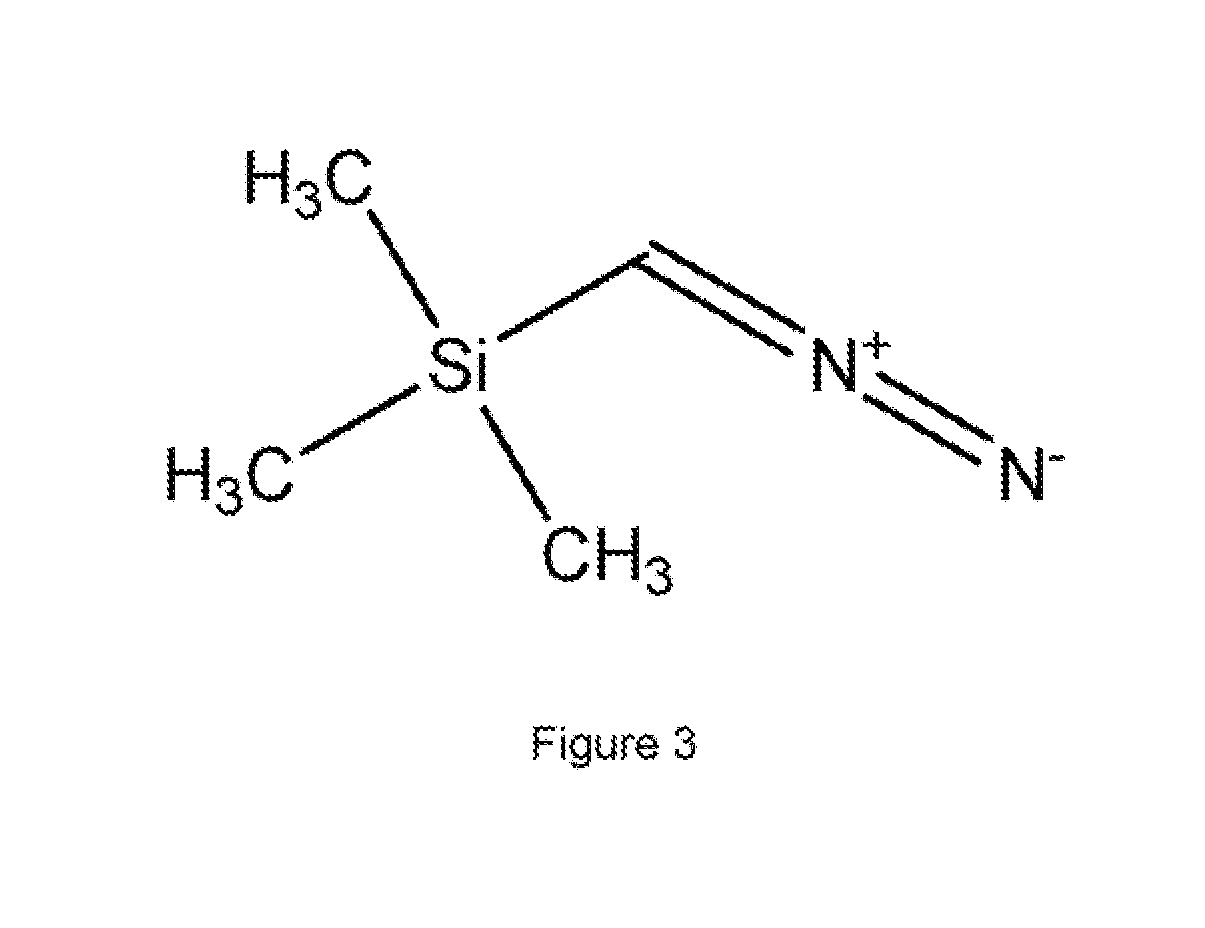
Diazomethane is the chemical compound CH₂N₂, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
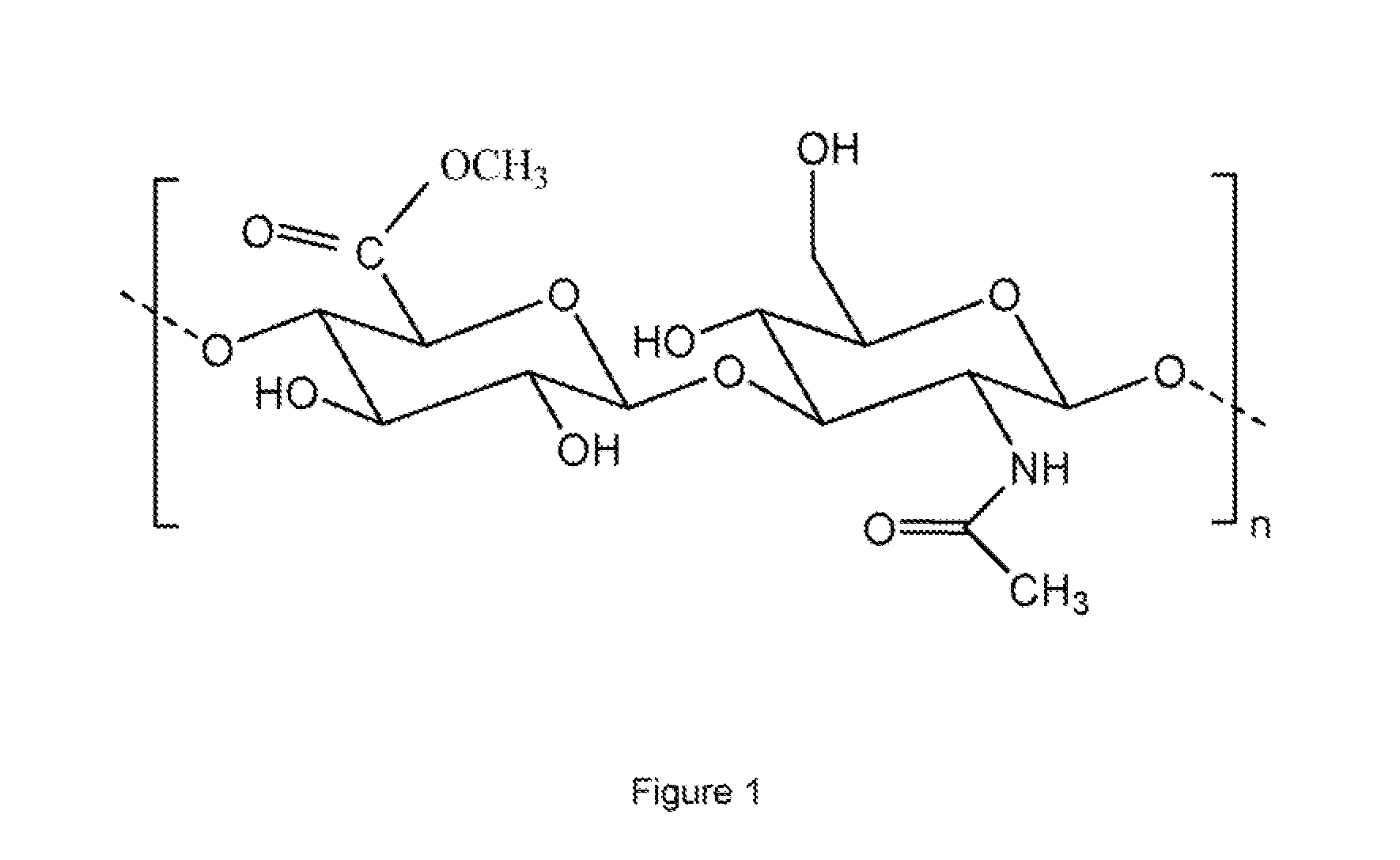
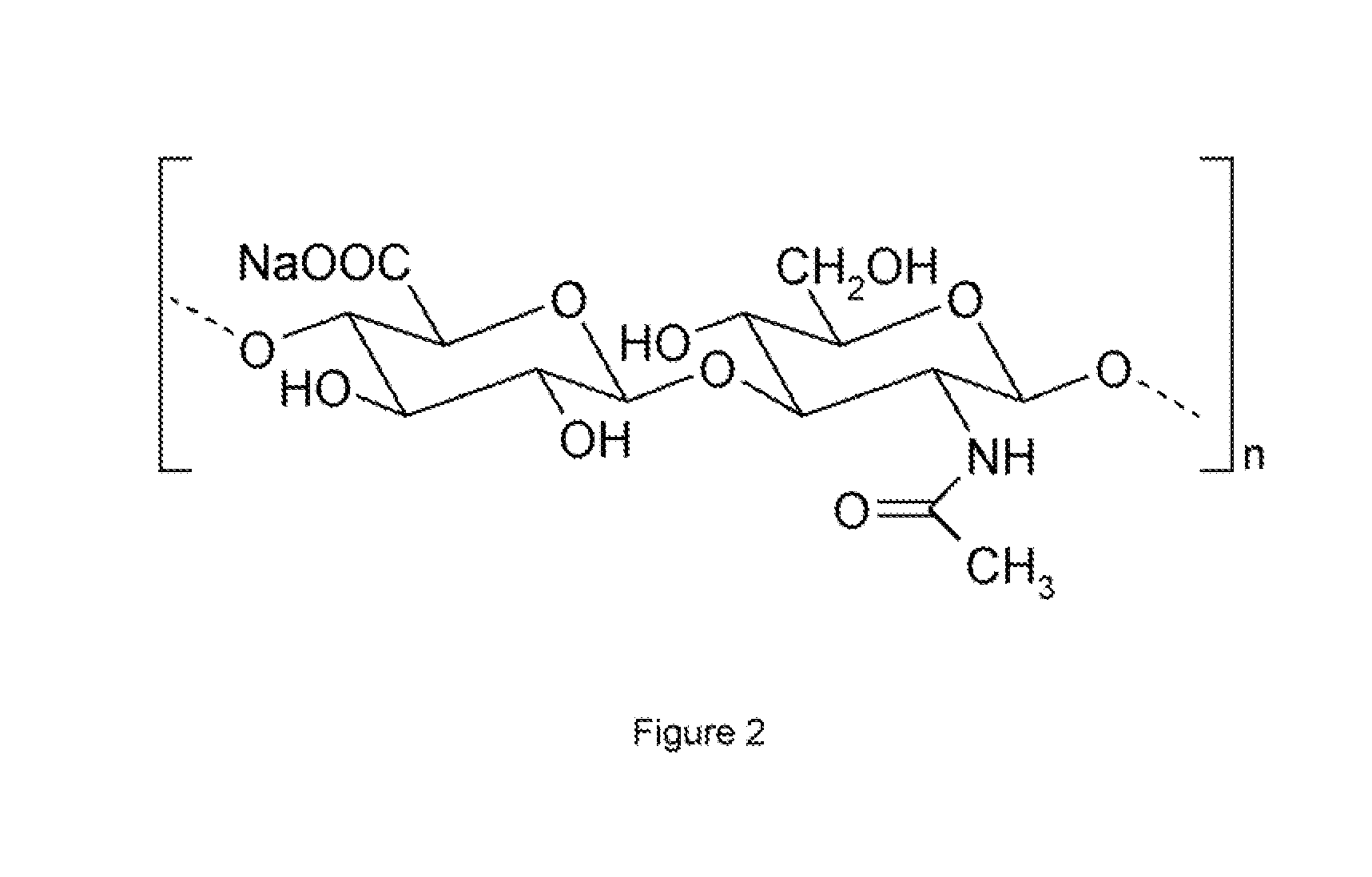
Polysaccharide derivatives and hydraulic compositions
InactiveUS6068697AHigh transparencyGood thickening effectSugar derivativesBacteriaHydrogen atomHydrogen
PCT No. PCT / JP97 / 04316 Sec. 371 Date Jul. 14, 1998 Sec. 102(e) Date Jul. 14, 1998 PCT Filed Nov. 26, 1997 PCT Pub. No. WO98 / 23647 PCT Pub. Date Jun. 4, 1998A polysaccharide derivative prepared by replacing all or part of the hydroxyl hydrogen atoms of a polysaccharide or polysaccharide derivative by (A) a hydrophobic substituent having a C8-C43 hydrocarbon chain as the partial structure and (B) an ionic hydrophilic substituent having at least one member selected from the group consisting of sulfonic, carboxyl phosphoric, and sulfate groups and salts thereof as the partial structure, wherein the average degree of replacement by the substituent (A) is 0.0001 or above but below 0.001 per constituent monosaccharide residue as determined by Zeisel's method or the diazomethane method and that by the substituent (B) is 0.01 to 2.0 per constituent monosaccharide residue as determined by colloidal titration. This polysaccharide derivative is useful as the admixture for hydraulic materials and can give stable hydraulic compositions excellent in dispersion.
Owner:KAO CORP
Synthetic method of 7-MAC intermediate
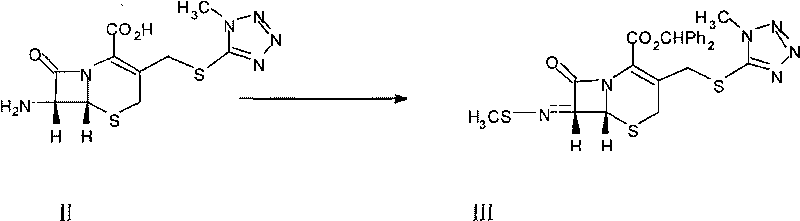
The invention discloses a synthetic method of a 7-MAC intermediate, which comprises the following steps: reacting 7-ACA as raw material with MMTZ to obtain 7-TMAC; enabling the 7-TMAC and methyl sulfur bromide to undergo imidization, and then, reacting the imidization product with diphenyl diazomethane to obtain the intermediate; and enabling the intermediate and a methoxylation reagent to undergo methoxylation reaction to obtain a finished product. In the synthetic method, the 7-ACA which can be obtained easily is used as original material to synthesize the 7-TMAC, thereby reducing the production cost. In the imidization reagent of the synthetic method, methyl sulfur chloride is replaced by the methyl sulfur bromide, thereby increasing the reaction activity, improving the yield, simplifying the operation and reducing the cost.
Owner:CANGZHOU SENARY CHEM SCI TEC
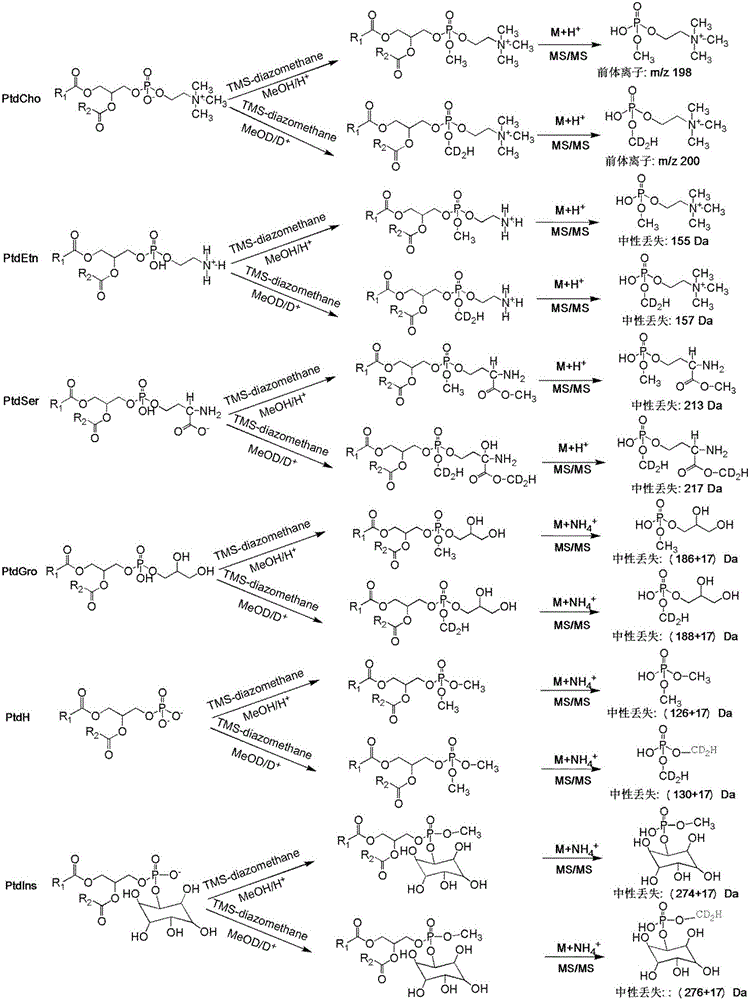
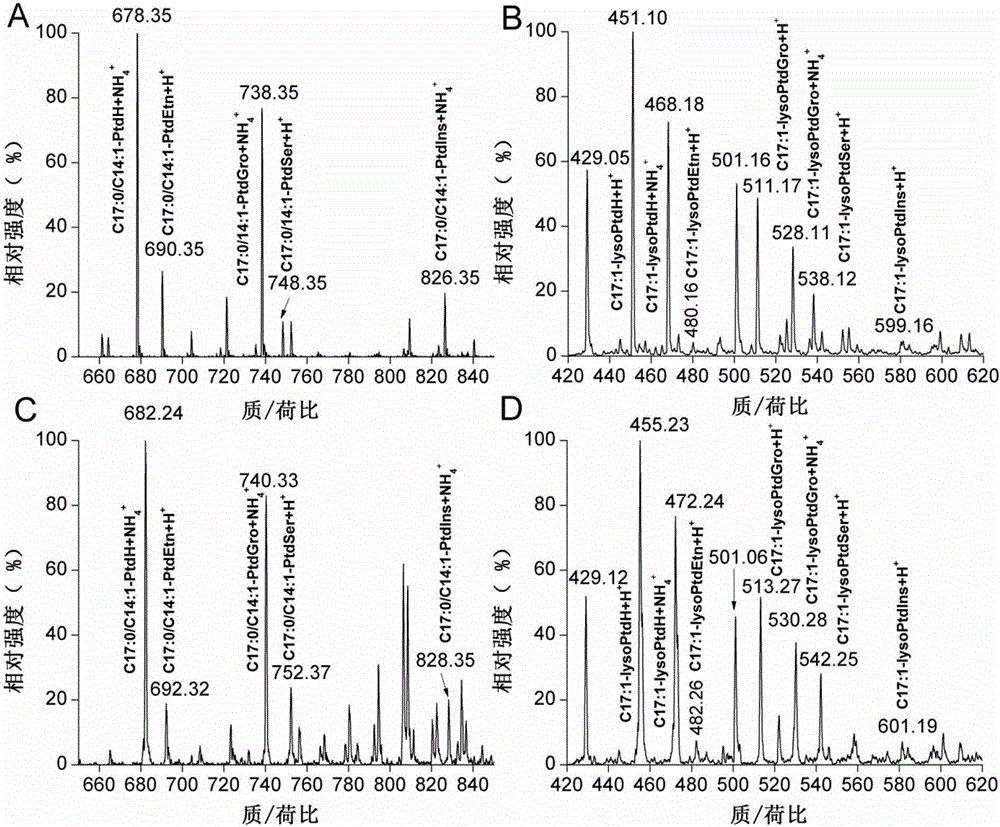
Phospholipid classification detection and quantification method based on stable isotope labeling
ActiveCN105067697AHigh sensitivityComponent separationMaterial analysis by electric/magnetic meansStable Isotope LabelingPhosphoric acid
The present invention discloses a phospholipid classification detection and quantification method based on stable isotope labeling, and belongs to the technical field of phospholipid quantification detection methods. According to the method, mainly trimethylsilyl diazomethane is used to respectively generate diazomethane and deuterium-labeled diazomethane in a methyl tert-butyl ether / methanol / 1N hydrochloric acid solution system and a methyl tert-butyl ether / deuteromethanol / 1N deuterium chloride solution system in an in-situ manner so as to further respectively carry out methyl esterification on the phosphoric acid group or carboxylic acid group in the phospholipid molecule to generate the light isotope labeled-phospholipid methyl esterification derivative and the heavy isotope labeled-phospholipid methyl esterification derivative, wherein the light isotope labeled-phospholipid methyl esterification derivative and the heavy isotope labeled-phospholipid methyl esterification derivative have the same physical and chemical properties and different molecular weights; and the relative intensity of the light isotope labeled-mass spectrum peak signal and the heavy isotope labeled-mass spectrum peak signal are compared to associate with the phospholipid molecule amount in the sample so as to achieve the relative quantification on the phospholipid. The method of the present invention has characteristics of enhanced sensitivity and completion within a shor time.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
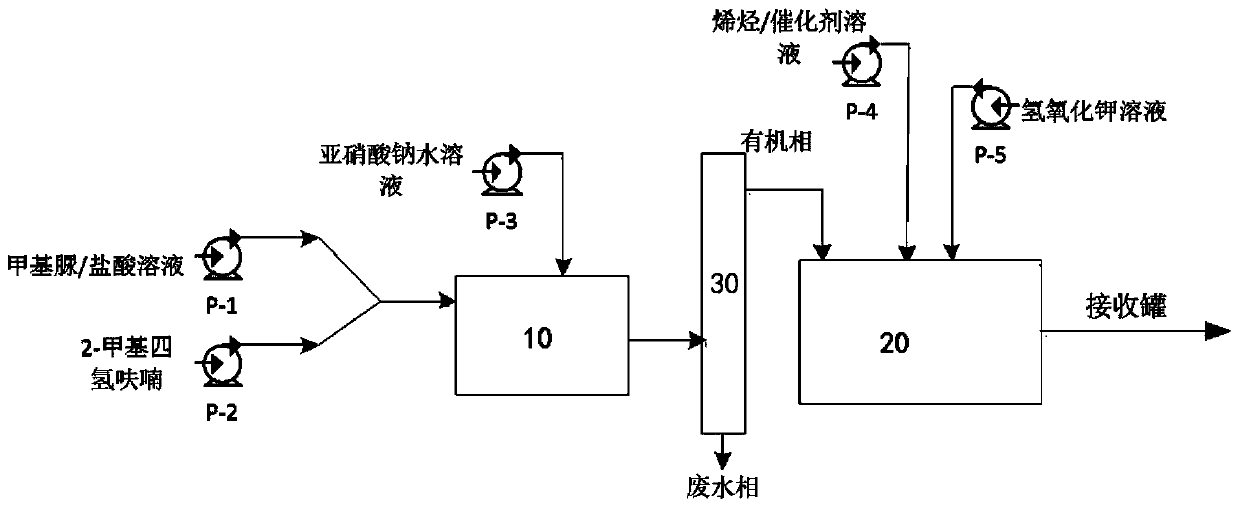
Method and device for continuously synthesizing cyclopropane compounds
PendingCN110577484AReduce transferAvoid the risk of pipeline diversionOrganic chemistryAutomatic controlCyclopropane
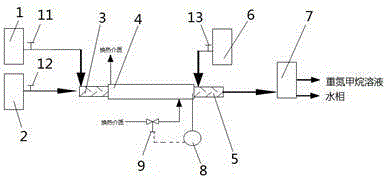
The invention discloses a method and a device for continuously synthesizing cyclopropane compounds. The method comprises the following steps of: continuously carrying out a synthesis reaction of a diazomethane precursor in a first reactor, flowing a reaction product of the first reactor into a separator for layering, overflowing an organic phase obtained by layering into a second reactor, continuously consuming the diazomethane precursor in the second reactor to prepare diazomethane, and carrying out an electron-rich mono-olefin cyclopropanation reaction in situ to obtain the cyclopropane compounds. The method can realize automatic control, reduce the transfer of high-risk materials, avoid the risk of pipeline transfer of a diazomethane solution, and effectively improve the production safety, also has simple equipment, can save equipment investment, and can safely and quantitatively realize simultaneous diazomethane generation and olefin cyclopropanation reaction.
Owner:ASYMCHEM LAB TIANJIN
Methyl esters of hyaluronic acid
InactiveUS20080182982A1Easy to disassembleInvention is rapidCosmetic preparationsSugar derivativesTrimethylsilylTrimethylsilyldiazomethane
Owner:NOVOZYMES AS
Preparation method of mild diazomethane derivative
ActiveCN106608788AHigh puritySynthetic asymmetrySilicon organic compoundsCatalystsSulfonyl chlorideOrganic solvent
The invention discloses a preparation method of a mild diazomethane derivative. The preparation method comprises that EWG-substituted benzene sulfonyl chloride and hydrazine hydrate undergo a reaction to produce EWG-substituted benzene sulfonyl chloride, the EWG-substituted benzene sulfonyl chloride and aldehyde or ketone undergo a reaction to produce EWG-substituted benzenesulfonylhydrazone, and the EWG-substituted benzenesulfonylhydrazone, a base and an organic solvent are mixed and undergo a replacement reaction to produce a diazomethane derivative. The diazomethane derivative is not separated and purified and is further used for a tension small ring synthesis reaction and an insertion reaction. The benzene ring of benzenesulfonylhydrazone is introduced with an electron-withdrawing group EWG, and through electron effects and steric hindrance effects, the benzenesulfonyl group on the benzenesulfonylhydrazone is easily separated so that a diazomethane derivative is produced under very mild conditions and especially at the room temperature.
Owner:NORTHEAST NORMAL UNIVERSITY
Diazomethane on-line deriving method
InactiveCN101704702AImprove securityEasy to operateOrganic compound preparationCarboxylic acid esters preparationMethaneChemistry
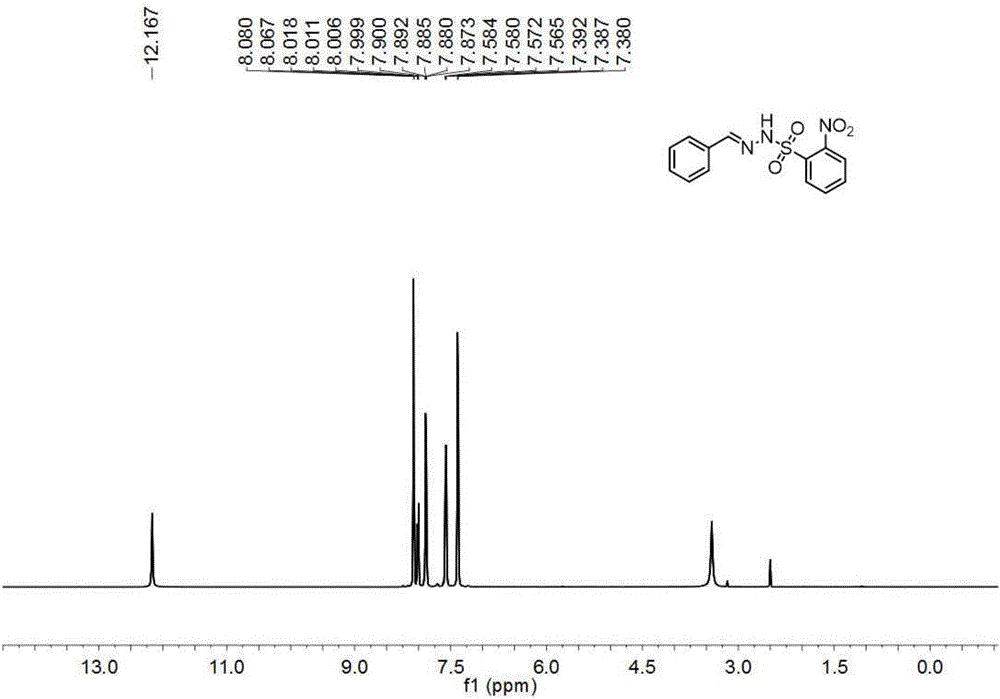
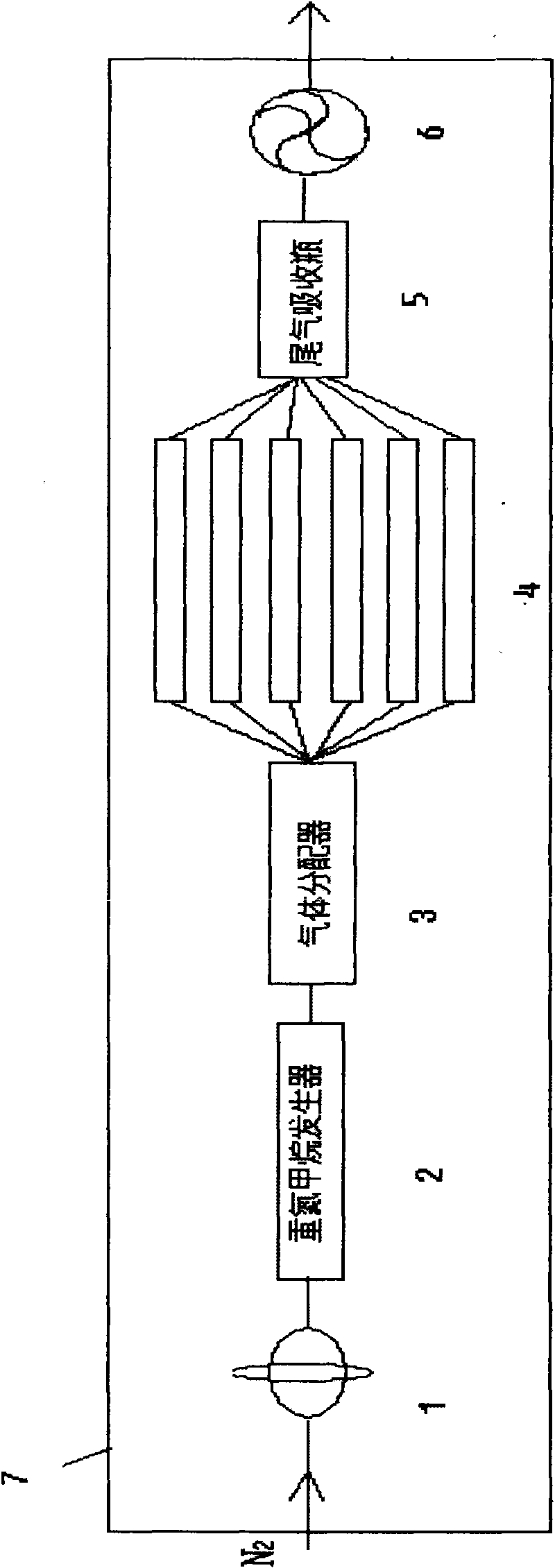
The invention relates to a diazomethane on-line deriving method, which comprises the following steps of: (1) a step of generating diazomethane; and (2) a step of deriving the diazomethane. The method can be realized under the action of N2. The method also comprises a tail gas absorption step after the step of deriving the diazomethane, wherein absorbent used in the tail gas absorption step for absorbing excessive diazomethane can be aether. The method also comprises a N2 flow rate control step before the step of generating the diazomethane and also comprises a diazomethane gas distribution step between (1) the step of generating the diazomethane and (2) the step of deriving the diazomethane. The method can be carried out in sealed environment. The diazomethane on-line deriving method can also comprise a step of exhausting the exhaust gas generated in the reaction from the sealed environment. The method organically integrates the preparation and the derivation of the diazomethane and has the characteristics of safety and high efficiency.
Owner:INST OF HYDROGEOLOGY & ENVIRONMENTAL GEOLOGY CHINESE ACAD OF GEOLOGICAL SCI
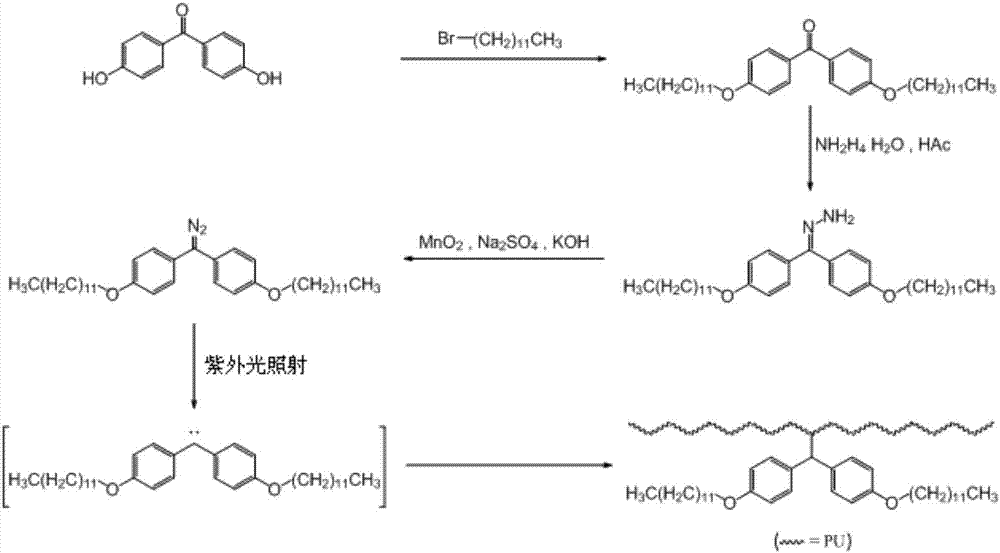
Method for preparing polyurethane-nanosilver long-acting antibacterial film by post-heat treatment modification method
The invention relates to a method for preparing a polyurethane-nanosilver long-acting antibacterial film by a post-heat treatment modification method, which includes the following steps: (1) diisocyanate and polytetrahydrofuran are mixed to react; a cross-linking agent and an acetone solution of a chain extender are added, dimethylolpropionic acid and a catalyst are added, reaction takes place, and thereby PU (polyurethane) is obtained; a dimethylformamide solution of silver nitrate is added into a polyurethane solution, a film is formed after uniform stirring, heat treatment is carried out, and thereby a polyurethane-nanosilver film is obtained; (2) 4,4'-dihydroxybenzophenone reacts with long-chain alkyl halide, so that 4,4'-bis-long-chain alkoxy benzophenone is obtained; the 4,4'-bis-long-chain alkoxy benzophenone reacts with hydrazine hydrate, so that 4,4'-bis-long-chain alkoxy benzophenone hydrazone is obtained; and the 4,4'-bis-long-chain alkoxy benzophenone hydrazone undergoes oxidation reaction, so that long-chain alkyl diazomethane is obtained; (3) the long-chain alkyl diazomethane is dissolved into a solvent, and is uniformly applied onto the surface of the polyurethane-nanosilver film, and after heat treatment, the polyurethane-nanosilver long-acting antibacterial film is obtained. The method can be used for realizing long-acting bacteria resistance.
Owner:QILU UNIV OF TECH
Positive photoresist composition and method of forming resist pattern
ActiveUS20060210916A1High resolutionHigh rectangularityPhotosensitive materialsRadiation applicationsResistMethacrylate
A positive resist composition that includes a resin component (A), which contains acid dissociable, dissolution inhibiting groups and exhibits increased alkali solubility under the action of acid, and an acid generator component (B) that generates acid on exposure, wherein the resin component (A) is a copolymer (A1) containing a first structural unit (a1) derived from hydroxystyrene and a second structural unit (a2) derived from a (meth)acrylate ester having an alcoholic hydroxyl group, in which a portion of the hydroxyl groups of the structural units (a1) and the alcoholic hydroxyl groups of the structural units (a2) have been protected with the acid dissociable, dissolution inhibiting groups; and either the acid generator component (B) includes a diazomethane-based acid generator and an onium salt-based acid generator; or the composition further contains a compound, which contains at least one acid dissociable, dissolution inhibiting group, and generates an organic carboxylic acid under the action of acid generated from the component (B).
Owner:TOKYO OHKA KOGYO CO LTD
Method for preparing 3-trifluoromethylisooxazole compound by one-pot
ActiveCN107963996AEasy to operateMild conditionsGroup 4/14 element organic compoundsCopperCoupling reaction
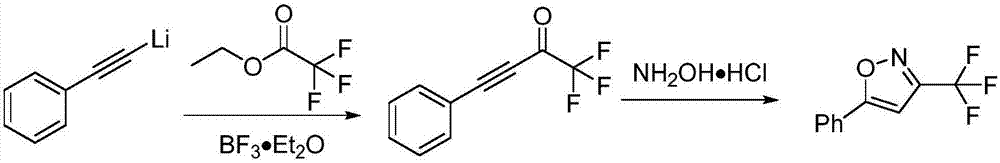
The invention discloses a novel method for preparing a 3-trifluoromethylisooxazole compound by one-pot. According to the method, trifluoroethylamine which can be purchased in markets is prepared intofluorinated diazomethane, and then the fluorinated diazomethane and an alkynes compound are in coupled reaction under catalysis of low-price copper. The method is simple to operate, reaction conditions are mild, the cost is low, by-products are fewer, the yield is high, tolerance of a functional group is high, and reaction can be amplified. Meanwhile, much deeper mechanism study is carried out, and a mechanism that a trifluoromethyl ketoximes compound intermediate may be produced in the reaction is proposed.
Owner:JIANGXI NORMAL UNIV
Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide
ActiveCN104387299ALow costImprove stabilitySulfonic acid amide preparationP-nitrobenzenesulfonyl chlorideNitro reduction
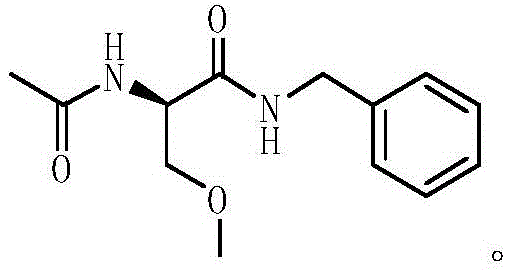
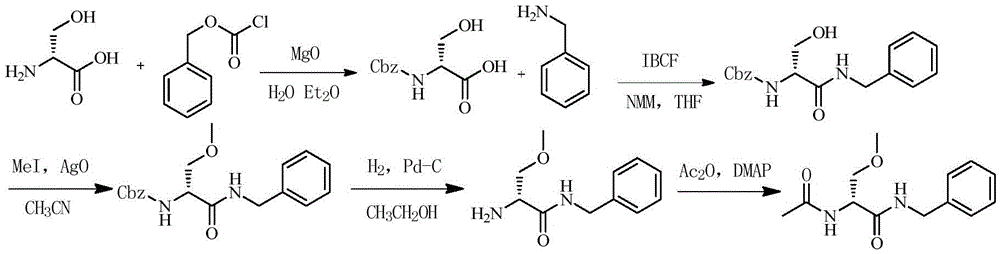
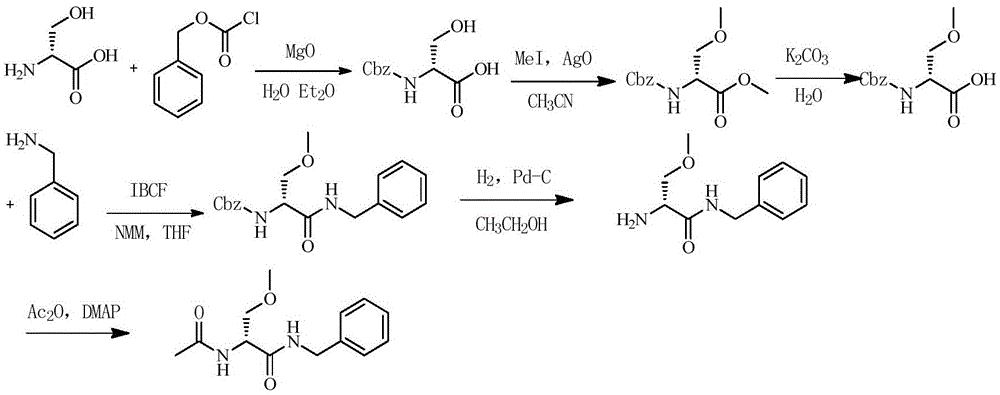
The invention discloses a method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide. The method comprises the following steps: S1: enabling L-phenylalanine and diazomethane to react to obtain a diazo methyl ketone intermediate product, and enabling the diazo methyl ketone intermediate product and haloid acid to react to obtain a compound A; S2, conducting carbonyl deoxidation on the compound A to obtain a compound B; S3, under the existence of iso-butylamine, conducting cyclization reaction and ring-opening reaction on the compound B in sequence to obtain a compound C; S4, enabling the compound C and nitrobenzenesulfonyl chloride to react to obtain a compound D; S5, conducting nitro reduction on the compound D to obtain the 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide. The method is simple in course, low in cost, mild in condition, and higher in intermediate product stability, and is beneficial for industrial application.
Owner:ASYMCHEM LAB TIANJIN +4
Preparation method and application of perfluoroalkyl diazomethane
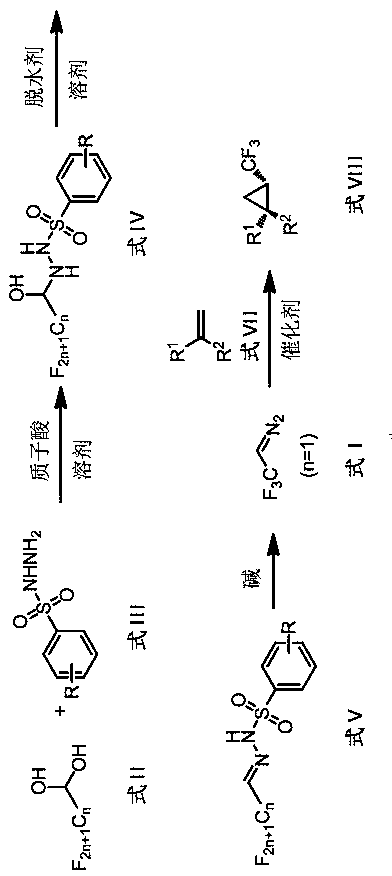
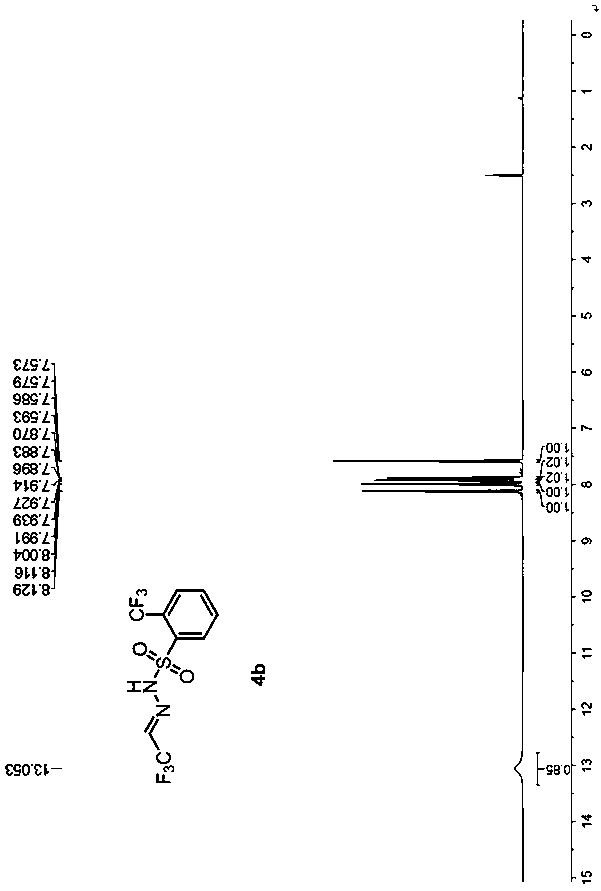
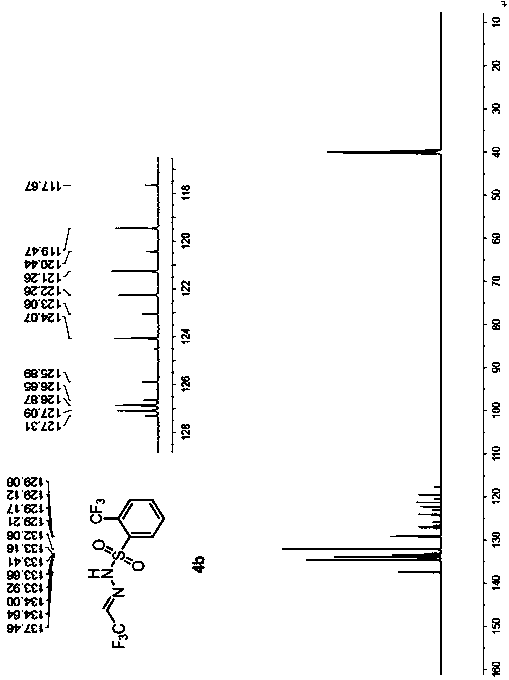
ActiveCN107739317AAvoid separationSafe and efficient separationSulfonic acid amide preparationHalogenated hydrocarbon preparationPhotochemistryChemistry
The invention discloses a method for mildly generating perfluoroalkyl diazomethane in situ. By utilizing the method, through starting with perfluoroalkyl aldehyde or a hydrate thereof, which is low inprice and is easily obtained, a substitution reaction is generated with a derivative of benzenesulfonyl hydrazide under the catalysis of protonic acid; the dehydration is carried out in the presenceof a dehydrating agent to obtain a perfluoroalkyl diazomethane precursor; the perfluoroalkyl diazomethane is slowly released in an alkaline condition. Further, by utilizing the preparation method of the perfluoroalkyl diazomethane, a novel method for synthesizing trifluoromethyl cyclopropane in a two-phase system through a one-pot method without carrying out separation is invented. By using the method, the separation of trifluoromethyl diazomethane which has toxicity and explosion danger is avoided; the method has the advantages of mild reaction condition, wide substrate range and good functional-group tolerance, and meanwhile, the method has the advantages of being simple and convenient to operate and needing no special equipment to slowly dropwise add, can be used for safely and efficiently synthesizing the trifluoromethyl cyclopropane, whose order of magnitude is 1 to 100 grams, in a laboratory and has further industrialized application potential.
Owner:NORTHEAST NORMAL UNIVERSITY
Method for preparing hydrophobic PU (polyurethane) film by ultraviolet radiation
The invention relates to a method for preparing a hydrophobic PU (polyurethane) film by ultraviolet radiation. The method comprises the following steps of (1) mixing diisocyanate and polytetrahydrofuran to react; adding a crosslinking agent and a chain expander, and then adding dimethylolpropionic acid and a catalyst to react, so as to obtain the PU; forming a film, and performing heat treatment, so as to obtain the PU film; (2) enabling 4,4'-dihydroxy benzophenone and 1-bromododecane to react, so as to obtain 4,4'-bis-dodecaoxy diphenyl ketone oxime; enabling the 4,4'-bis-dodecaoxy diphenyl ketone oxime and hydrazine hydrate to react, so as to obtain 4,4'-bis-dodecaoxy diphenyl ketone oxime hydrazone; oxidizing the 4,4'-bis-dodecaoxy diphenyl ketone oxime hydrazone, so as to obtain long-chain alkyl diazomethane; (3) dissolving the long-chain alkyl diazomethane into a solvent, uniformly coating to the surface of the PU film, and radiating by ultraviolet rays, so as to obtain the hydrophobic PU film. The method has the advantages that the reaction process is simplified, and the problem of difficulty in synthesizing of the hydrophobic PU film is solved.
Owner:QILU UNIV OF TECH
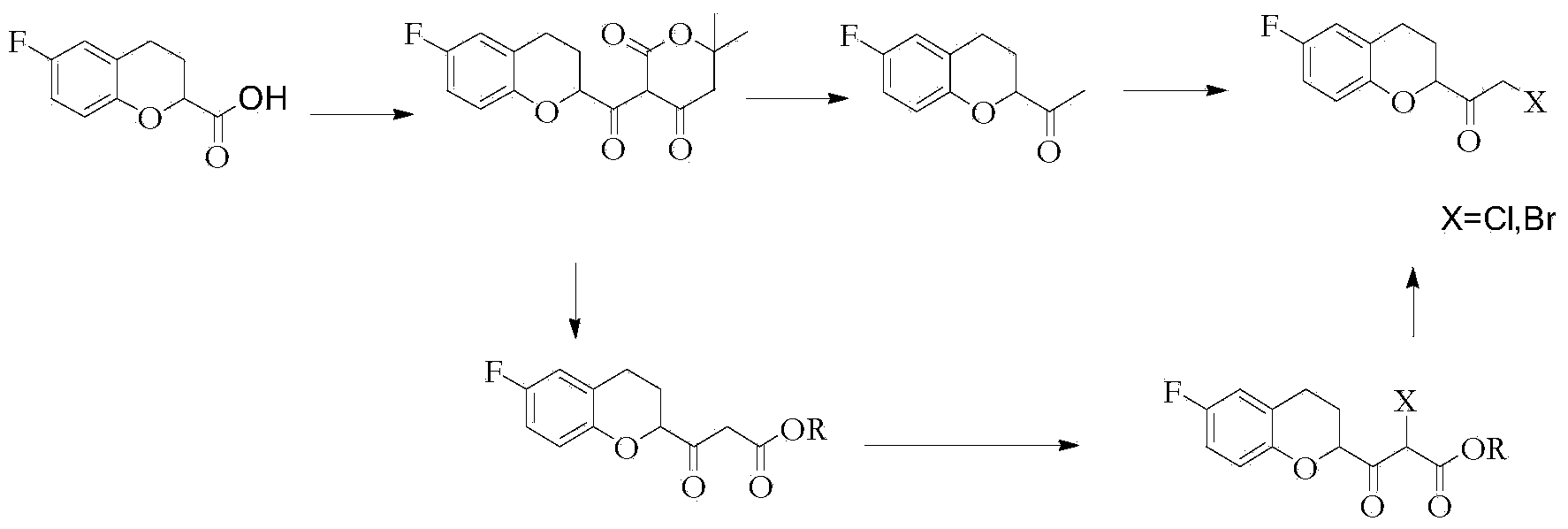
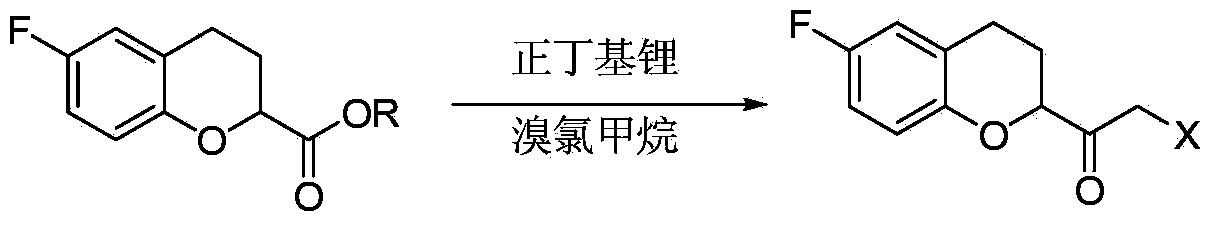
Method for preparing nebivolol midbody
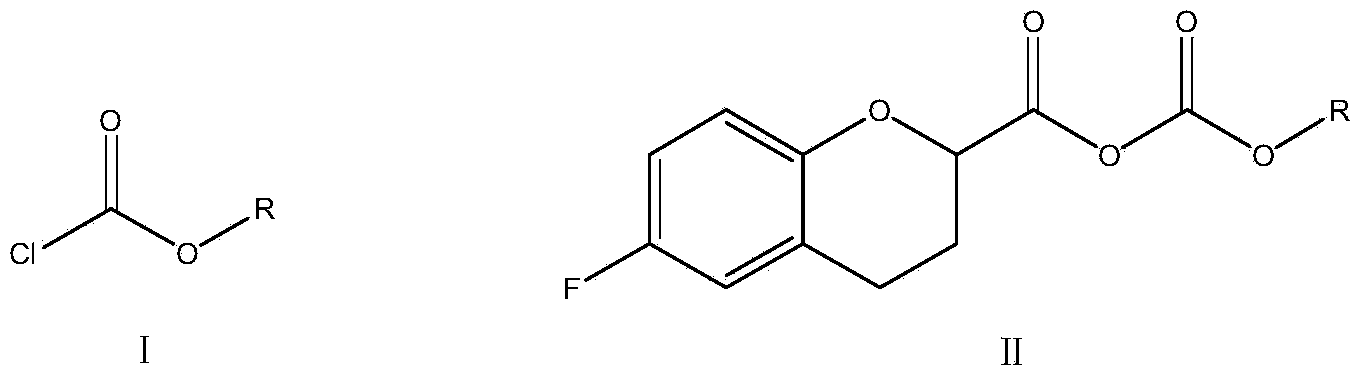
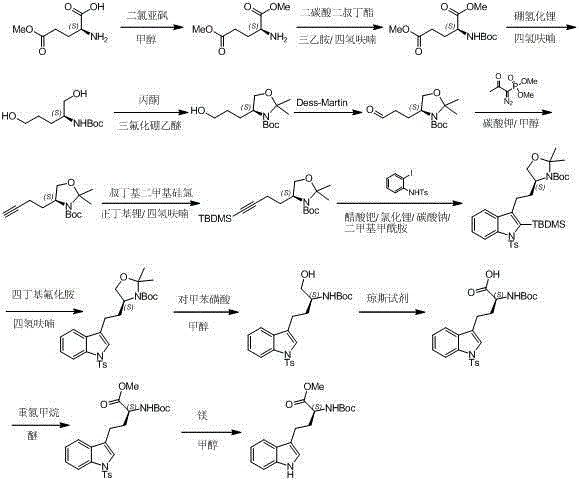
The invention relates to a method for preparing a nebivolol midbody, which belongs to the technical field of medicament synthesis, for solving the problems that the conventional raw material is high in cost, long in route and low in yield. The method comprises the following steps: enabling 6-fluorine chroman-2-formic acid as a raw material to react with chloro-carbonic ester with the presence of a deacid reagent, then adding diazomethane after the reaction is accomplished, so as to enable the midbody to react with the diazomethane to generate a reaction liquid of an intermediate product, and then adding a hydrogen halide gas or a hydrogen halide solution into the reaction liquid to perform halogenating reaction so as to obtain a compound of formula IV, namely, (6-(fluorine-3,4-dihydro-2H-benzopyran-2-methanol-2-yl) ethanone halogenate. The method provided by the invention has the advantages that the reaction route is short, the yield is high, and the used material is low in price and easy to purchase.
Owner:江苏八巨药业有限公司
Preparation method of L-N-Boc-high tryptophan methyl ester
InactiveCN102911106AEasy to operateSimple post-processingOrganic chemistryPtru catalystPyrrolidinones
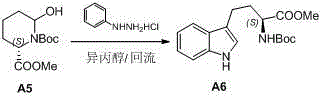
The invention discloses a preparation method of L-N-Boc-high tryptophan methyl ester, which can be mainly used for solving the problems that the reaction steps are more, the cost is low, the operation is difficult, and the single chirality of a final compound can not be ensured in an existing synthesizing method. The preparation method comprises the steps of conducting cyclization reaction to generate L-2-pyrrolidone-6-formic acid 1 by taking L-2-amino adipic acid as initial materials under the action of glacial acetic acid and water; 2. conducting an esterification reaction on the compound 1 and trimethyl silicon diazomethane to obtain L-2-pyrrolidone-6-methyl ester 2; 3. protecting N in the compound 2 by using Boc, then conducting the reduction reaction due to the action of a reducing agent, namely lithium triethylborohydride, and reducing carbonyl in the L-2-pyrrolidone-6-methyl ester protected by N-tert-butylcarbazate; and 4. finally synthesizing the high tryptophan methyl ester protected by the L-N-tert-butylcarbazate by two methods: synthesizing classical fisher benzazole in one method, and removing one molecular water by L-2-pyrrolidinol-6-methyl ester and iodoaniline, rearranging, and conducting Heck reaction under the action of a palladium catalyst to obtain the L-N-Boc-high tryptophan methyl ester in the other method.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Method for detecting ethrel
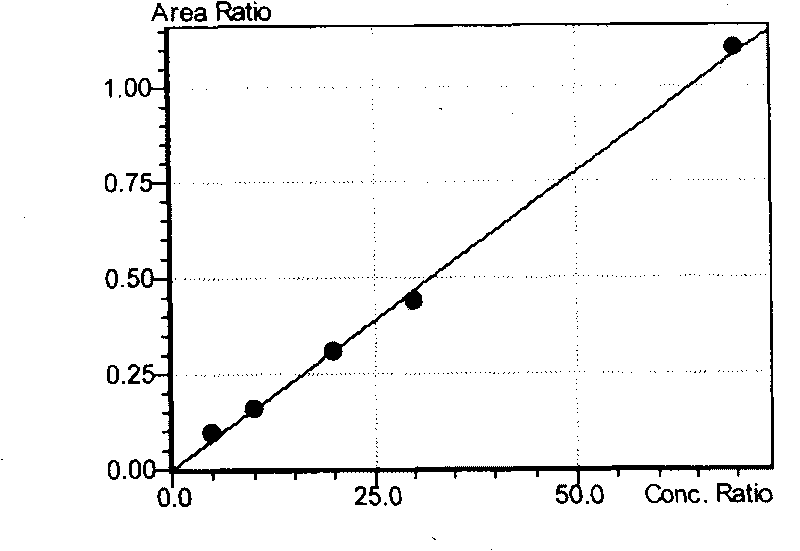
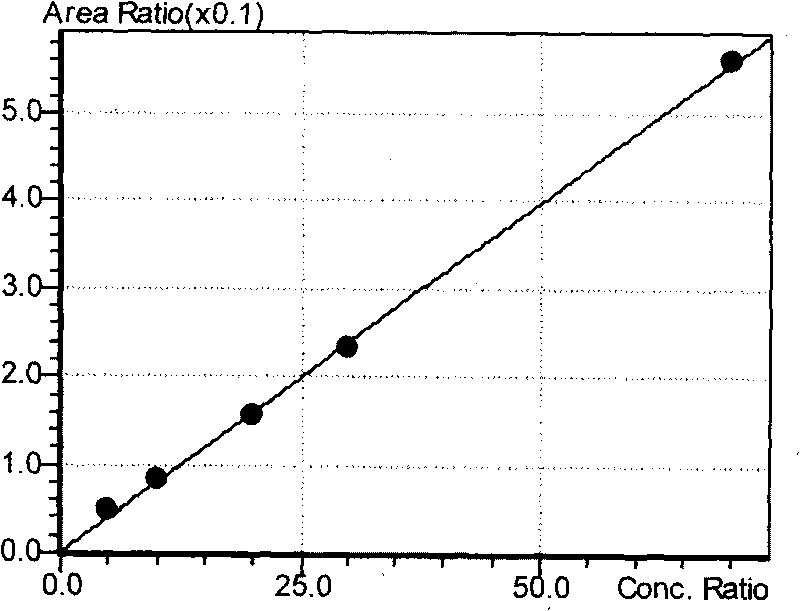
InactiveCN109725085AHigh detection sensitivity and responseLow detection limitComponent separationEsterification reactionPeak area
The invention discloses a method for detecting ethrel. The method includes the following steps: ethrel in a sample is extracted; a detection solution of ethrel derivatives is prepared, the sample is extracted through an analytical reagent, then sulfuric acid is used as a protective agent, heptafluorobutanol and heptafluorobutyric anhydride are used as derivatization reagents to make the ethrel inthe sample derive into the derivatives under the temperature condition of 60-120 DEG C, and then a chromatography reagent is added to obtain the detection solution; a derivative ethrel working solution is prepared; GC-MS / MS chromatographic analysis is conducted; an external standard method is used for quantification, the mass concentration of a target object is used as an abscissa, the corresponding peak area is used as an ordinate, and a standard curve is drawn. The ethrel is stable in the process of derivation, the speed of an esterification reaction is high, the speed of derivation is high,sensitivity is high, qualitative and quantitative detection is accurate, operation is easy, use of hypertoxic reagent diazomethane can be avoided, and the environment is protected.
Owner:HANGZHOU PROCESS DETECTION TECH CO LTD
Method for simultaneously determining residue of common herbicide in tobacco
InactiveCN107045023AReduce processReduce preparationComponent separationSolid phase extractionTandem mass spectrometry
The invention discloses a method for simultaneously determining the residue of a common herbicide in tobacco. The method comprises the following steps: using acidified acetonitrile for extracting a target object in a tobacco sample; using a florisil solid-phase extracting column for purifying; eluting with a mixed solution of acetone / cyclohexane at a volume ratio of 3:7; concentrating the eluent and then adopting trimethyl silanized diazomethane for derivatizing; and utilizing an online gel permeation chromatography-gas chromatography-tandem mass spectrum combined instrument for detecting. Compared with a standard method, the method has the advantages that the detection indexes of four standard methods are combined, dacthal is added, the detection efficiency is obviously increased, the sensitivity is higher, the anti-interference capacity is higher, and the high recovery rate of the sample and the excellent reproducibility are greatly guaranteed.
Owner:贵阳海关综合技术中心
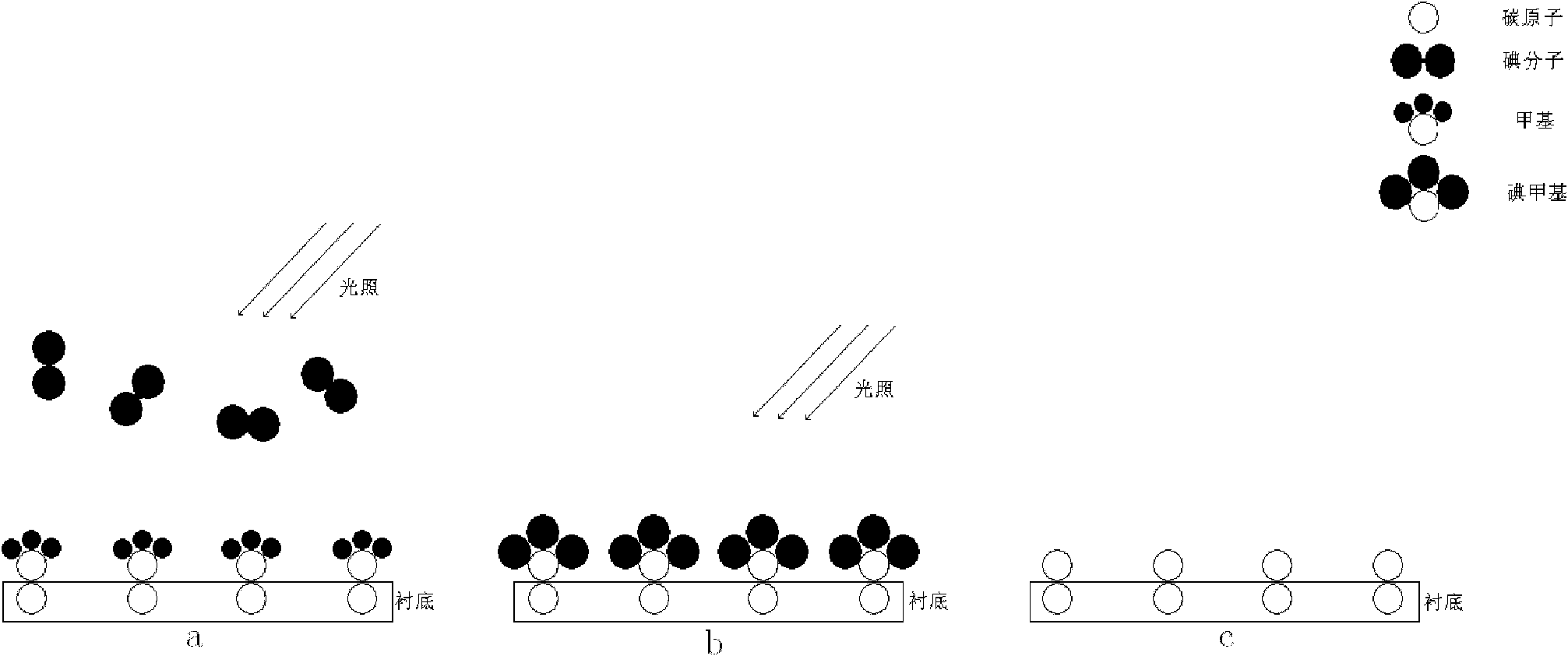
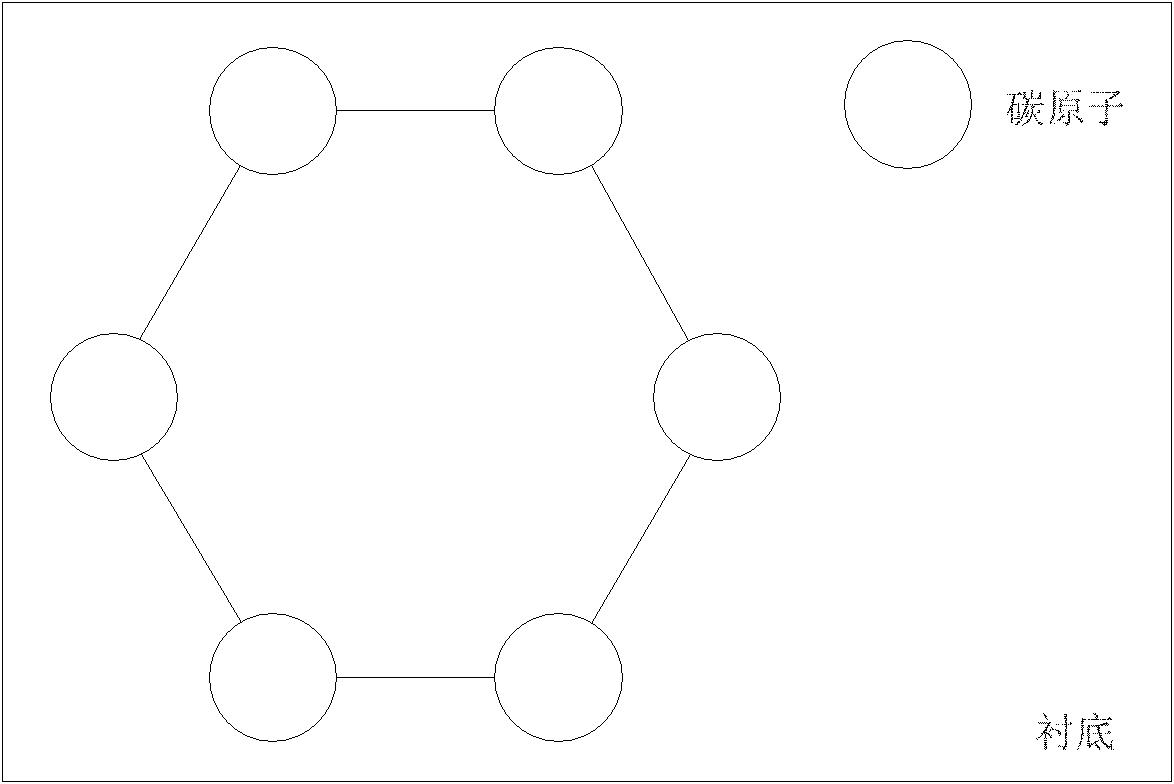
Method for preparing graphene film with single atomic layer
ActiveCN102051592AComplete structureImprove performanceChemical vapor deposition coatingIodineChemical adsorption
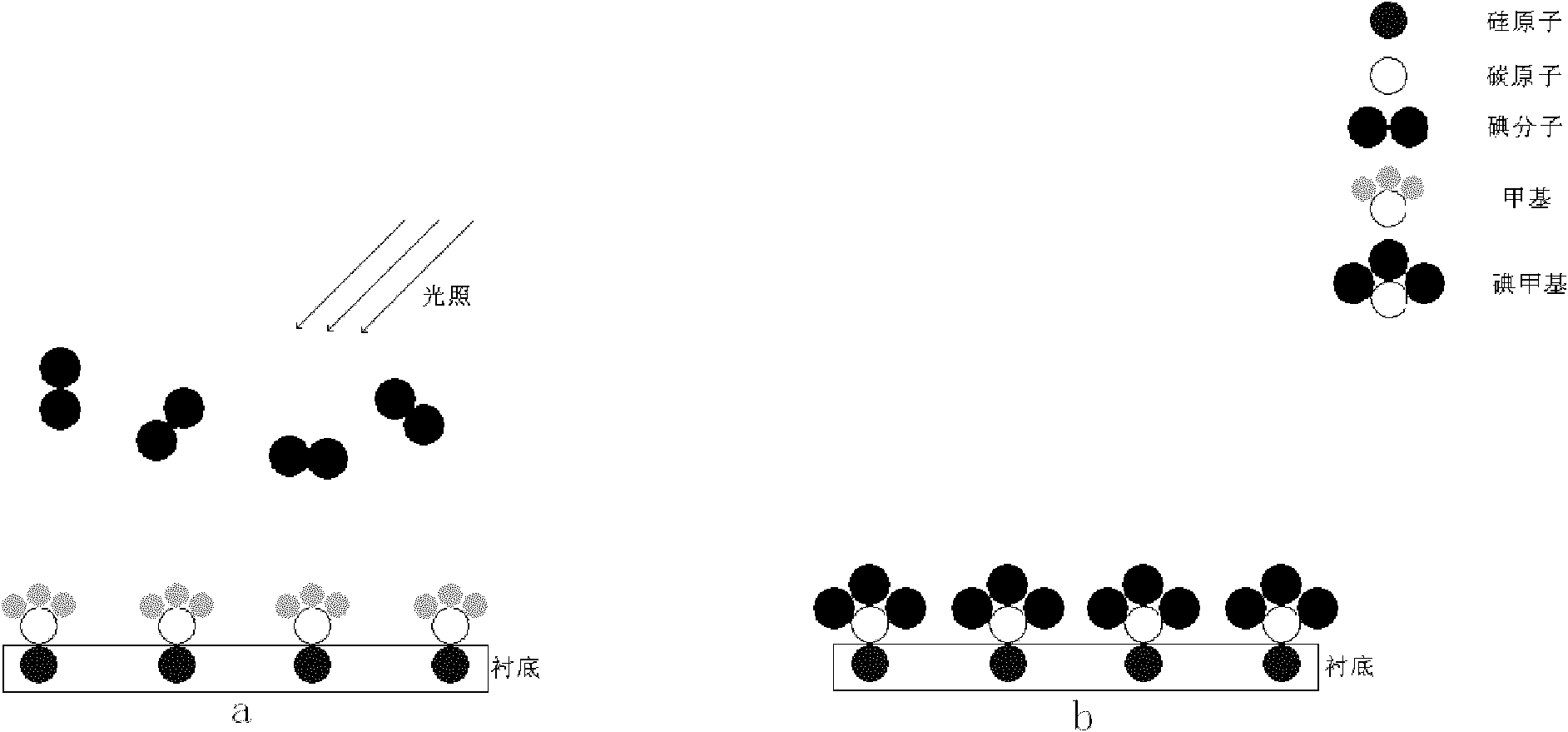
The invention relates to the technology of the preparation of graphene, in particular to a method for preparing a graphene film with a single atomic layer. The method comprises the following steps of: placing a silicon carbide substrate in a reaction chamber of atomic layer deposition equipment; introducing diazomethane into the reaction chamber of the atomic layer deposition equipment; performing chemical adsorption on the surface of the silicon carbide substrate by using the diazomethane; performing a halogenation reaction on the diazomethane and introduced gaseous iodine to form an instable carbon-iodine bond; and breaking the carbon-iodine bond by lighting to form the graphene film with the single atomic layer on the surface of the silicon carbide substrate. In the method, the graphene film with the single atomic layer is prepared by utilizing atomic layer deposition technology; and the preparation method contributes more to the deposition of carbon atoms and the growth of the graphene, namely the prepared graphene film has more uniform thickness, more complete structure and higher performance.
Owner:INST OF MICROELECTRONICS CHINESE ACAD OF SCI +1
Preparation method of modified graphene
ActiveCN105271192AImprove the degree of functional modificationSimple manufacturing processBenzophenoneBenzole
Owner:HARBIN INST OF TECH
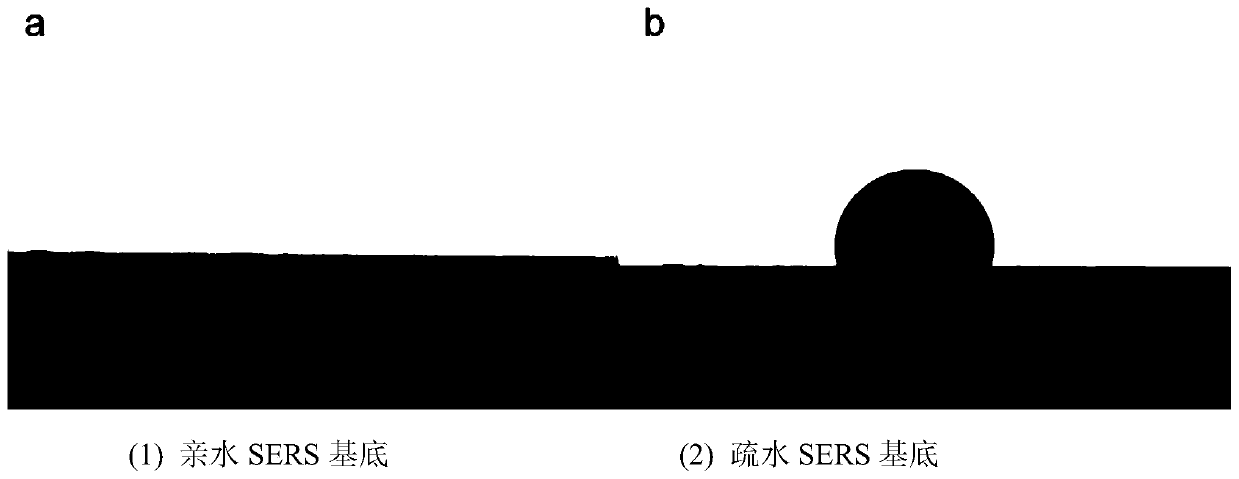
Hydrophobic paper surface-enhanced Raman substrate and application thereof
The invention relates to a hydrophobic paper surface-enhanced Raman substrate and an application thereof. The substrate comprises a paper substrate on which metal nanometer particles are covered; andN,N-long chain alkyl diazomethane hydrophobic layer connected with the paper substrate and / or metal nanometer particles through a chemical bond. The invention further discloses the application of thehydrophobic paper surface-enhanced Raman substrate in the analysis detection field. The hydrophobic SERS disclosed by the invention has good stability, the stability is strong since the hydrophobic function group is modified to the surface of the paper substrate through a chemical bonding way. After placing for 6 months, the R6G is used as a probe signal molecule to perform stability testing, andthe Raman enhancement signal strength change is less than 6%.
Owner:QILU UNIV OF TECH
Preparation method of hydrophobic paper surface enhanced Raman substrate
InactiveCN110186899AHydrophobicImprove stabilityRaman scatteringNano structuringIn situ polymerization
The invention relates to a preparation method of a hydrophobic paper surface enhanced Raman substrate. The method comprises the following steps: providing a paper substrate, and covering a metal nanolayer on the paper substrate to obtain a paper surface enhanced Raman substrate; and modifying N,N-long-chain alkyl diazomethane to the surface of the paper surface enhanced Raman substrate through achemical bond to obtain a hydrophobic SERS substrate. According to the preparation method, the paper substrate is used as a framework, the PDA is formed on the surface of the paper substrate by utilizing in-situ polymerization of DA, and then metal particles are reduced and grown to form a nano structure by utilizing the reducibility of phenolic hydroxyl in PDA molecules, so that a paper SERS substrate is prepared. Due to the adhesion of the PDA, the paper substrate and the metal nano particles can be tightly bridged together, and the stability of the SERS substrate is maintained; and meanwhile, the metal nano particles on the paper substrate are formed by in-situ reduction, the metal nanostructures are uniformly distributed, the SERS substrate with good repeatability can be obtained, andthe obtained Raman signal has strong repeatability.
Owner:QILU UNIV OF TECH
Preparation method of cefuroxime axetil
InactiveCN105131016ALow impurity contentGood colorOrganic chemistryBulk chemical productionCephalosporanic AcidsTrichloroacetyl isocyanate
The invention relates to a preparation method of cefuroxime axetil. The preparation method comprises the following steps: (1) reacting de-ammoniated formyl cephalosporanic acid with diphenyl diazomethane to generate acetyl-diphenyl methyl cephalosporanate; (2) reacting diphenyl methyl de-ammoniated formyl cephalosporanate with trichloroacetic isocyanate to generate diphenyl methyl cefuroxime ester; (3) hydrolyzing diphenyl methyl cefuroxime ester to obtain cefuroxime acid; (4) reacting cefuroxime acid with 1-acetoxyl-bromoethane to generate cefuroxime axetil. During the preparation process, the carboxyl group is protected by diphenyl diazomethane, the generation of impurities is reduced, and the product quality is improved.
Owner:JIANGSU QINGJIANG PHARMA
Preparation method of single crystal cubic carbon nitride thin film
ActiveCN102115878AComplete structureFacilitate depositionPolycrystalline material growthFrom chemically reactive gasesChemical adsorptionSingle crystal
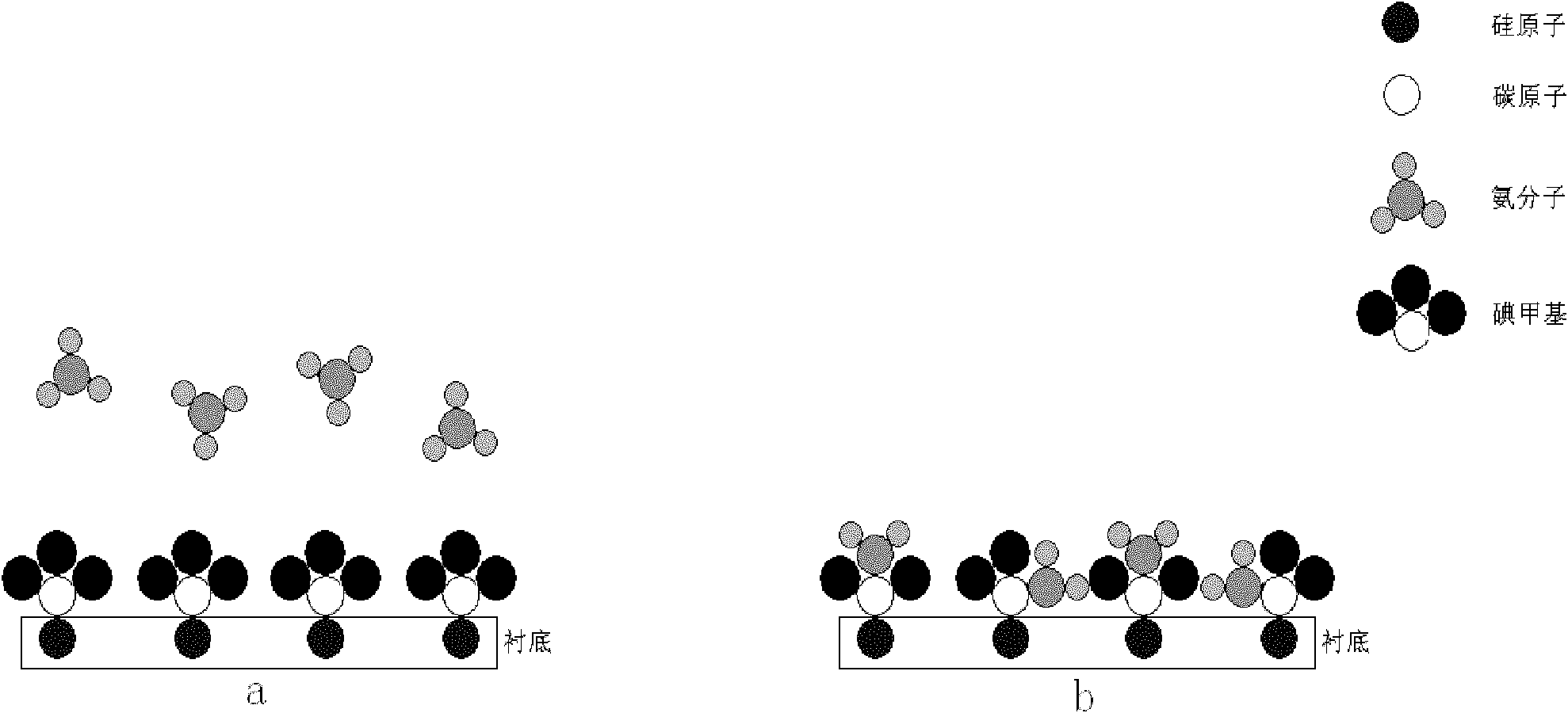
The invention relates to a preparation technology of carbon nitride, in particular to a preparation method of a single crystal cubic carbon nitride thin film. The preparation method comprises the following steps: a silicon substrate is placed in an atomic layer deposition equipment reaction cavity; diazomethane is led to the atomic layer deposition equipment reaction cavity to conduct chemical adsorption on the surface of the silicon substrate; the diazomethane is further reacted with gaseous iodine led to the atomic layer deposition equipment reaction cavity for halogenation, so as to form an unsteady carbon iodine bond; after the halogenation reaction is stopped, ammonia gas is further led to the atomic layer deposition equipment reaction cavity for amination reaction, and iodine atoms in the carbon iodine bond are replaced by nitrogen atoms in the ammonia gas; and after the amination reaction is completed, a substance formed on the surface of the silicon substrate is the single crystal cubic carbon nitride thin film. The preparation method utilizes the atomic layer deposition technology to prepare the single crystal cubic carbon nitride thin film, is simple to operate, achieves a high conversion rate, consumes little energy, and is more beneficial for the deposition of carbon atoms and the nitrogen atoms and the growth of the carbon nitride thin film; and the manufactured carbon nitride thin film has a complete structure.
Owner:INST OF MICROELECTRONICS CHINESE ACAD OF SCI +1
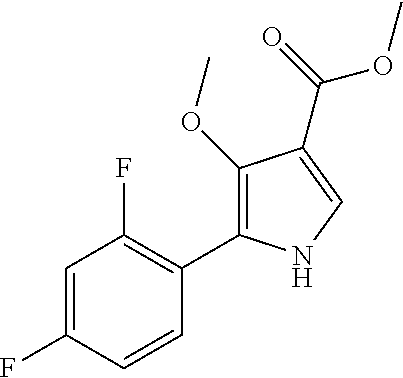
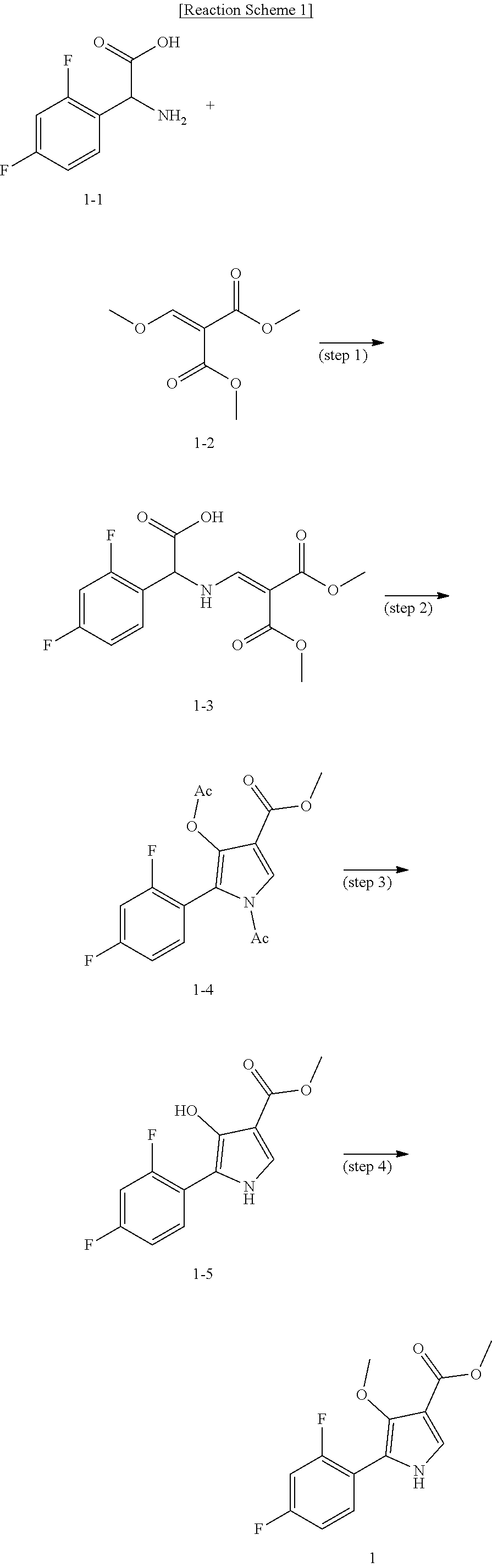
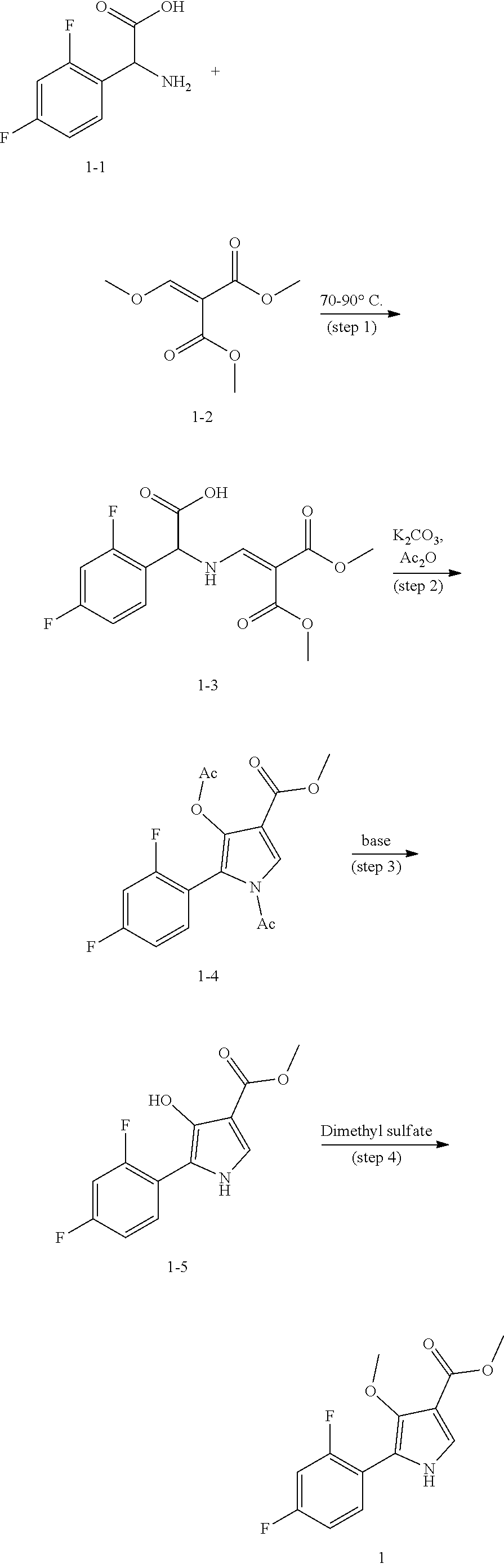
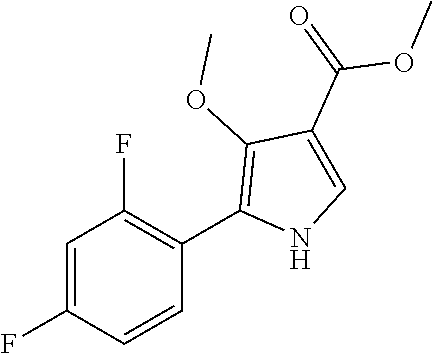
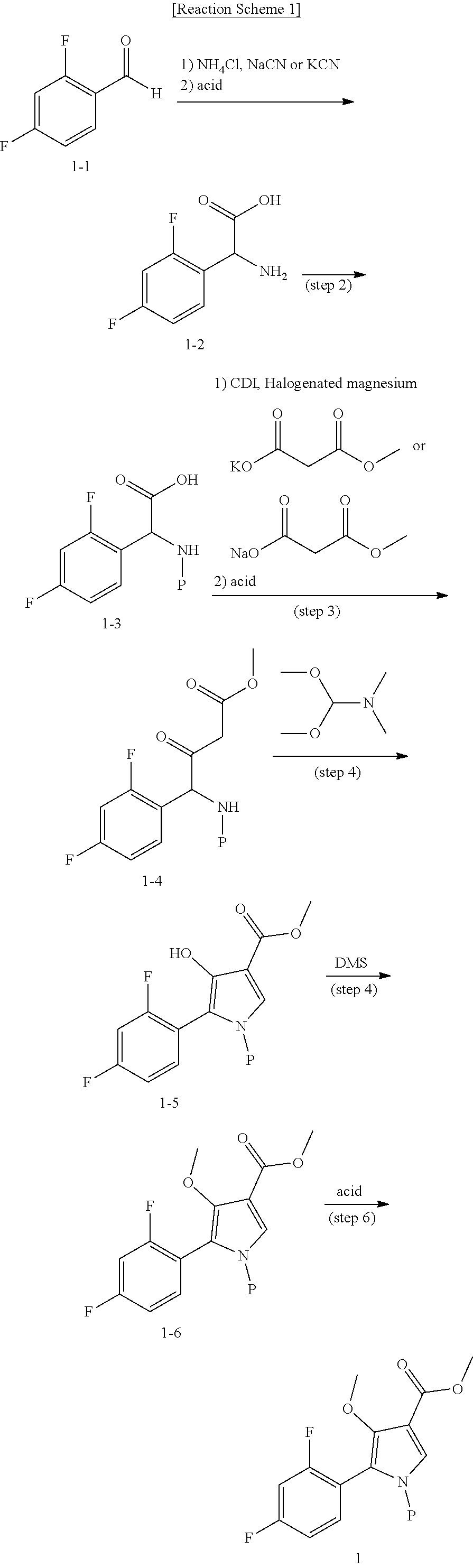
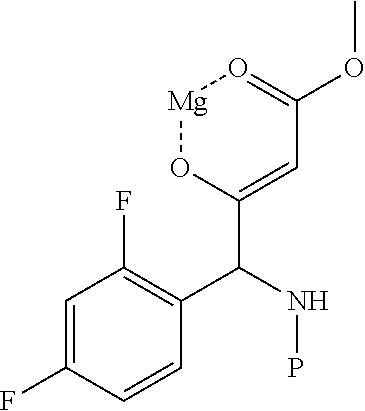
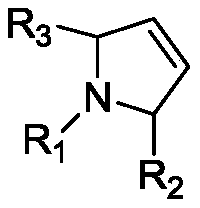
Method for preparing intermediate of 4-methoxypyrrole derivative
ActiveUS20200181079A1High yieldOrganic active ingredientsOrganic chemistryTrimethylsilylCombinatorial chemistry
The present invention relates to a method for preparing intermediates of 4-methoxypyrrole derivatives. The preparation method according to the present invention has advantages that a high-temperature reaction is not required as a whole, inexpensive and non-explosive reagents are used instead of (trimethylsilyl)diazomethane, and further an intermediate of 4-methoxy pyrrole derivatives can be prepared as a whole at a high yield.
Owner:DAEWOONG PHARM CO LTD
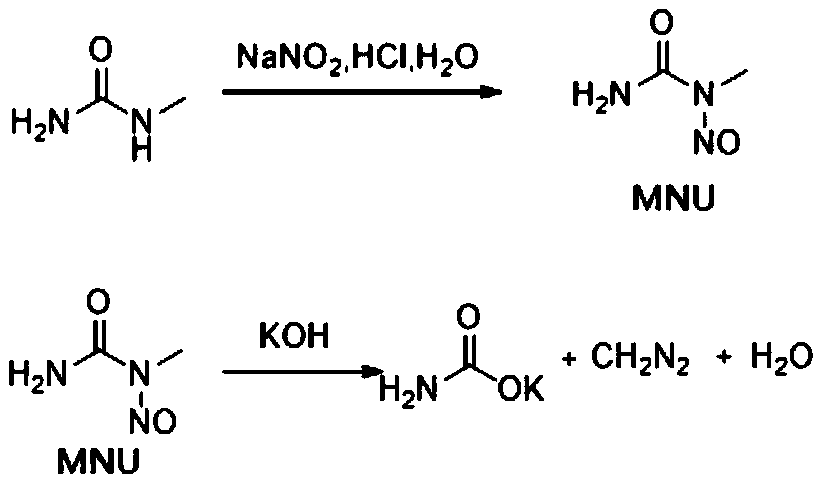
Method and equipment for preparing diazomethane
InactiveCN105418452AReduce processing costsPrecisely adjust the ratioOrganic chemistryNitrosoOrganic synthesis
The invention relates to a method and equipment for preparing diazomethane, and belongs to the field of organic synthesis. The method and the equipment have the advantages that the method and the equipment aim to overcome shortcomings of existing methods for preparing diazomethane and are short in process route, low in raw material cost and high in yield, preparation procedures are safe and environmental friendly, and effects of industrial continuous production can be realized; the method for preparing the diazomethane is implemented by the aid of the equipment, the diazomethane can be generated from a N-methyl-N-nitroso material and alkali metal hydroxide by means of completely closed reaction, stable organic solution can be formed by means of timely extraction by the aid of extraction agents, accordingly, the retention time of the diazomethane in water can be shortened, the decomposition rate of the diazomethane which can generate methyl alcohol and nitrogen when in water can be reduced, the diazomethane yield can be increased, and safety risks of operator poisoning and explosion due to leakage of gaseous diazomethane can be prevented.
Owner:YIBIN JIULING CHEM
Mass continuous safe production diazomethane reactor and working method thereof
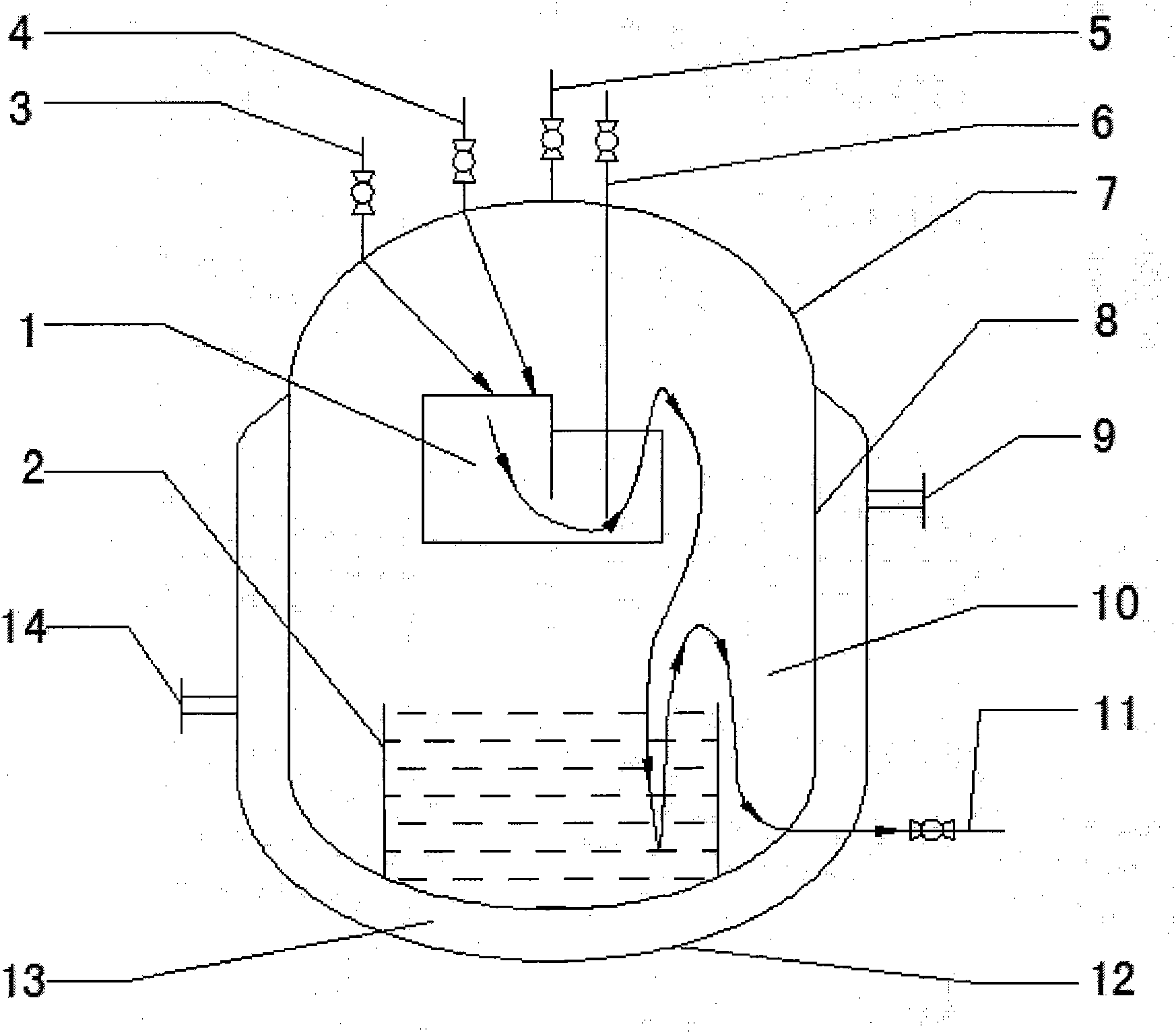
ActiveCN101844063BReduce the risk of explosionSimple structureTransportation and packagingMixersEngineeringReaction centre
The invention relates to a mass continuous safe production diazomethane reactor, which consists of a cavity body, a reaction center I, a reaction center II, a raw material A dripping port, a raw material B dripping port, a N2 air suction bubbling tube, a diazomethane discharging tube, a cooling jacket, a cooling medium inlet, a cooling medium outlet and a waste liquid discharging tube, wherein the cavity body consists of an upper seal end a reactor wall and a lower seal end. Working method comprises the following steps that: (1) diazald and NaOH enter the reaction center I inside the cavity body of the reactor respectively through the raw material A dripping port and the raw material B dripping port to be contacted and reacted with each other to generate diazomethane; (2) reaction liquid inside the reaction center I slowly flows into the reaction center II from an overflow port above the reaction center I, the raw materials which are not completely reacted are continuously reacted with each other to generate the diazomethane gas during the process. The reactor has the advantages that: under the condition that the explosion danger is reduced, the device has simple structure, mass and continuous production of the diazomethane can be realized, and the diazomethane is free from being stored and can be directly used.
Owner:ASYMCHEM LAB TIANJIN
Method for preparing intermediate of 4-methoxypyrrole derivative
ActiveUS20200181080A1Low production costHigh yieldOrganic chemistryBulk chemical productionMeth-Trimethylsilyl
The present invention relates to a method for preparing intermediates of 4-methoxypyrrole derivatives. The preparation method according to the present invention has advantages that the production cost can be lowered by using inexpensive starting materials, a high-temperature reaction is not required as a whole, inexpensive and non-explosive reagents are used instead of (trimethylsilyl)diazomethane, and further an intermediate of 4-methoxypyrrole derivatives can be prepared as a whole at a high yield.
Owner:DAEWOONG PHARM CO LTD
Synthesis method of Tafamidis and derivative thereof
The invention provides a synthesis method for preparing Tafamidis and a derivative thereof from 6-amino-m-cresol and an aldehyde compound under the action of sodium periodate and potassium permanganate. In the method, the use of an acylation reagent dichlorobenzoyl chloride and an esterification reagent (trimethylsilyl) diazomethane is avoided, and the problem that acyl chloride, a diazonium reagent, a strong acid and strong alkali additive and the like are harmful to the environment is solved; the reaction steps are few, and the total yield is high; methoxy, halogen, trifluoromethyl and the like can be well tolerated; and the iodate and the manganese dioxide after the reaction are insoluble in the reaction solution and can be well recycled, so that the pollution caused by the reaction system is greatly reduced, a more economical and environment-friendly path is provided for synthesis of Tafamidis and the derivative thereof, and the method has a huge application value.
Owner:HUNAN FIRST NORMAL UNIV
Method for testing pyromellitic acid anhydride purity and organic impurity
ActiveCN101251515AHigh-precision detectionSimple and fast operationComponent separationPreparing sample for investigationDiethyl etherPyromellitic dianhydride
The invention provides a test method for the purity of pyromellitic dianhydride and organic impurities, which is used to test the purity of PMDA products obtained after crude products of pyromellitic dianhydride are refined and organic impurities contained in the PMDA products; the method includes the preparation of color spectrum injection samples and the analysis of the chromatograph, wherein the preparation of color spectrum injection samples includes the following steps of an ethyl esterifying reaction step and a methyl esterifying reaction step; the ethyl esterifying reaction step is that pyromellitic dianhydride samples are weighed in a container, and ethanol is added for the ethyl esterifying reaction under a counterflow condition, after the reaction is ended, products together with the redundant ethanol are transferred in a watch glass, and the watch glass is transferred in a vacuum drying oven for removing the redundant ethanol, in the end, the ethyl esterifying crystals are obtained; the methyl esterifying reaction step is that the ethyl esterifying crystals are put in an esterifying container, and diazomethane ether saturated solution is added for the methyl esterifying reaction, the redundant ether is removed by steaming after the methyl esterifying reaction is ended, and the samples for the gas chromatographic analysis are obtained, and then samples for the gas chromatographic analysis are diluted by a thinning agent, here the color spectrum injection samples are obtained, and the color spectrum injection samples are sent to the chromatograph for analyzing the purity and organic impurities. The test method has the advantages of simple operation and quick and accurate detection of the purity and organic impurities of the pyromellitic dianhydride (PMDA).
Owner:常熟华虞环境科技有限公司
Lacosamide synthesis method
ActiveCN105646284AAvoid reuseReduce usageCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsAcetylation
The invention discloses a lacosamide synthesis method. According to the method, D-serine, tertiary amine and alkyl chloroformate react with benzylamine to generate R-2-isobutoxy carbonyl-N-benzyl-3-hydroxy propanamide; then R-2-isobutoxy carbonyl-N-benzyl-3-hydroxy propionamide reacts with diazomethane to generate R-2-isobutoxy carbonyl-N-benzyl-3-methoxyl propanamide, and then reduction and acetylization are conducted, so that lacosamide is obtained. The purity of lacosamide prepared through the method reaches 99.95% and above, and the chiral purity reaches 99.90%.
Owner:山东安信制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000011.PNG)
![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000021.PNG)
![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000031.PNG)