Method and device for continuously synthesizing cyclopropane compounds
A compound, the technology of cyclopropane, applied in the field of continuous synthesis of cyclopropane compounds, can solve the problems of continuous reaction equipment chain length diazomethane solution pipeline transfer, high cost of permeable membrane manufacturing, affecting catalytic activity, etc., to avoid pipeline Transfer risks, improve production safety, and realize the effect of automatic control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Olefin compounds:
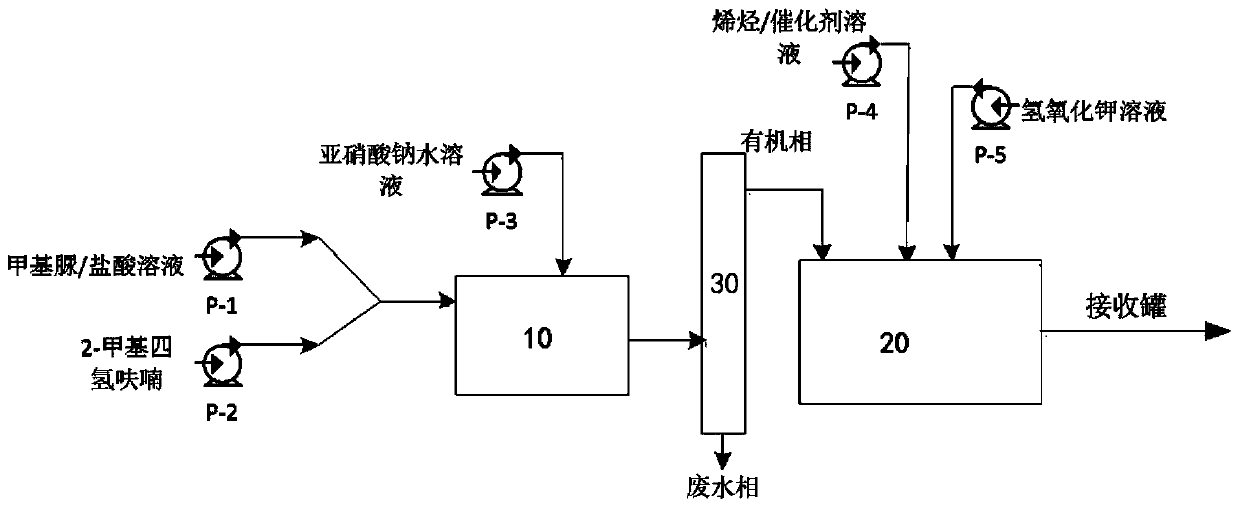
[0041] Continuous reaction: See Table 1 for the feed pump speed and reaction parameters, and the schematic diagram of the reaction process is shown in figure 1 shown.
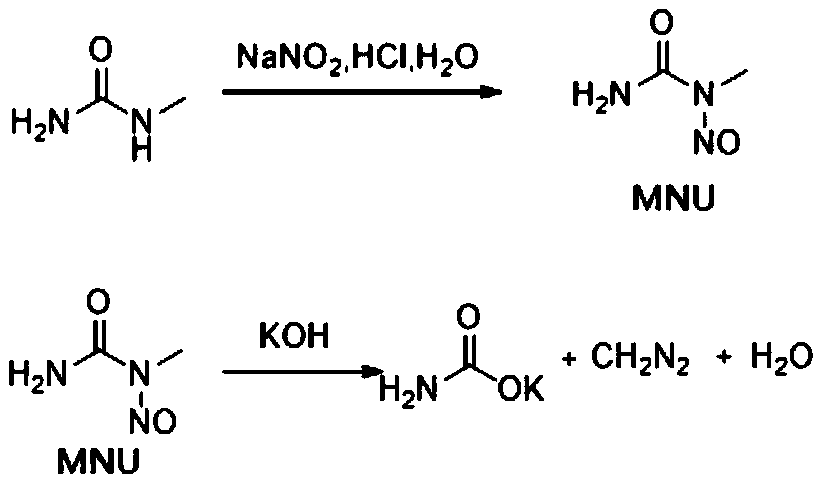
[0042] (1) Prepare the solution: add 370g of purified water and 82.5g of N-methylurea to a 2L Erlenmeyer flask, stir until fully dissolved, then slowly add 190g of concentrated hydrochloric acid (mass fraction: 36%), stir evenly and set aside (methylurea urea / hydrochloric acid solution). Add 370g of purified water into the 1L Erlenmeyer flask, add 130g of solid sodium nitrite, stir until fully dissolved (sodium nitrite aqueous solution), and set aside. Add 250ml 2-methyltetrahydrofuran, 50g olefin compound (20.38mmol), and 0.4g palladium acetate to a 1L conical flask, stir until completely dissolved, and set aside (olefin / catalyst solution). Add 600g purified water in 1L Erlenmeyer flask, add 210g potassium hydroxide in batches, stir until fully dissolved, stand-by (potassium hyd...
Embodiment 2
[0052] The difference from Example 1 is: the temperature of the first reactor is controlled at 30-35°C. Typical sample: olefin feedstock, 18.8% cyclopropane product, 65.2%; yield 63%.
Embodiment 3
[0054] The difference with Example 1 is: the residence time of the first reactor is 5min. Typical sample: olefin feedstock, 8.2% cyclopropane product, 75.2%; yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com