Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
722 results about "Cyclopropane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclopropane is the cycloalkane molecule with the molecular formula C₃H₆, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms resulting in D₃ₕ molecular symmetry. The small size of the ring creates substantial ring strain in the structure.
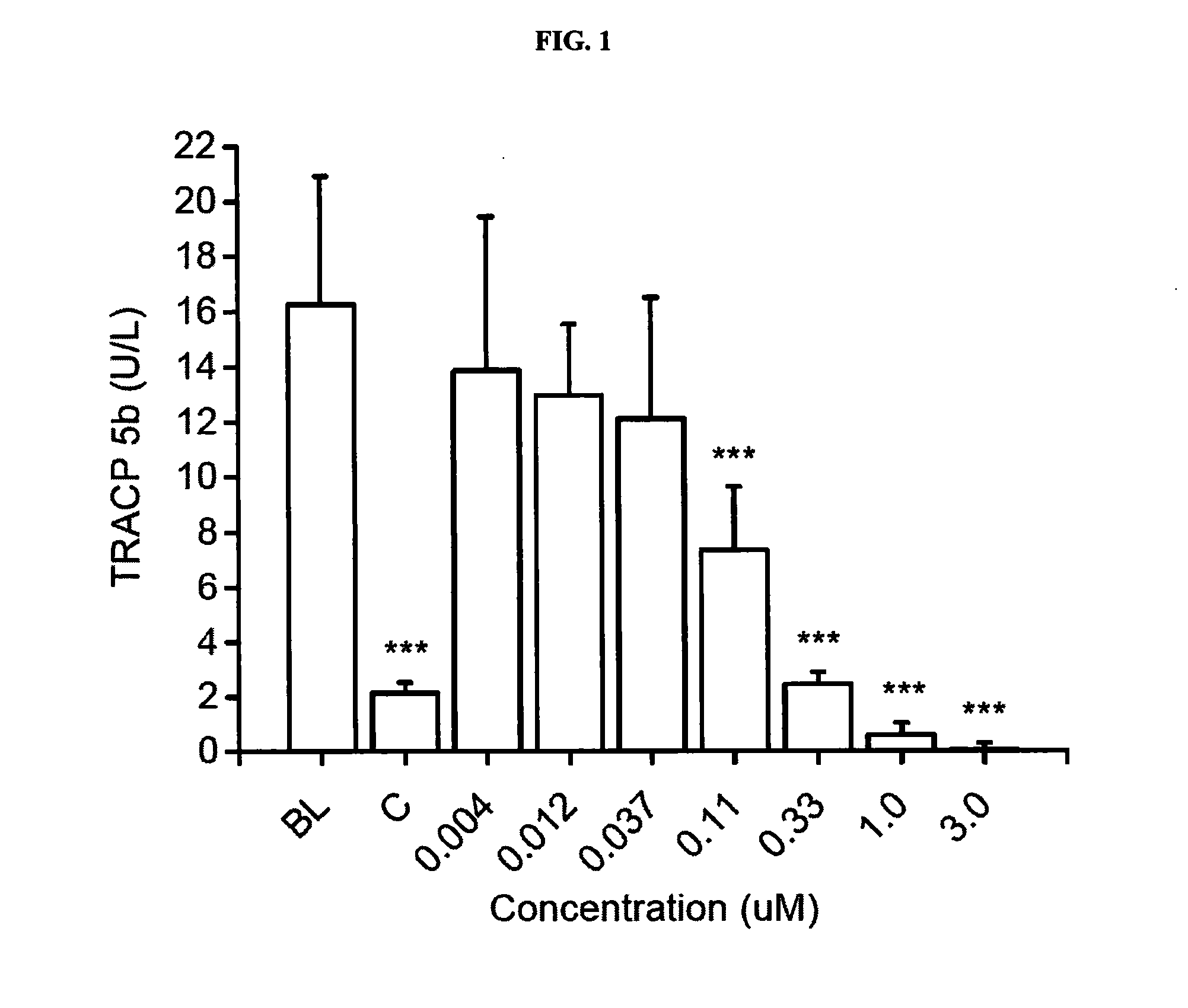
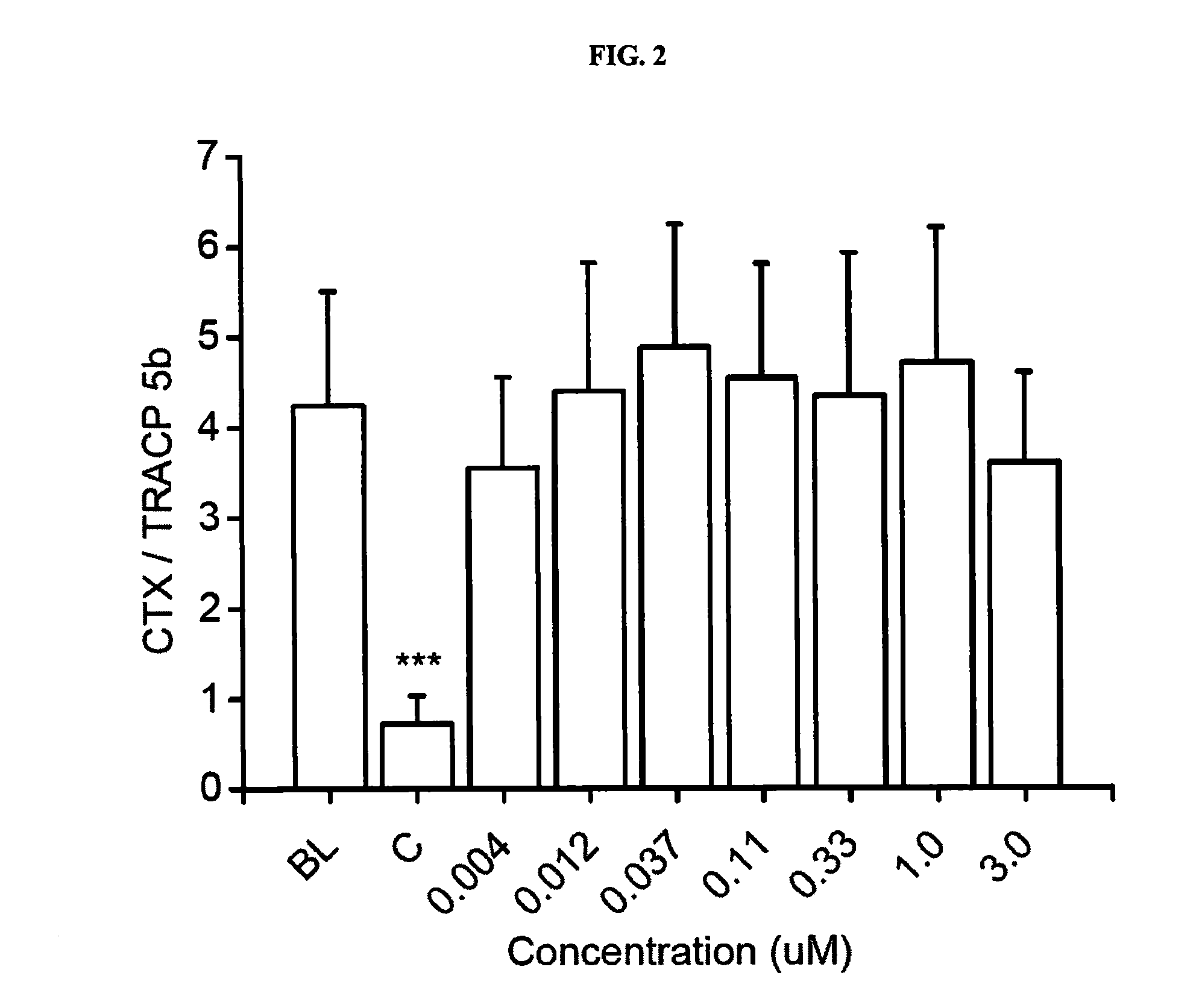
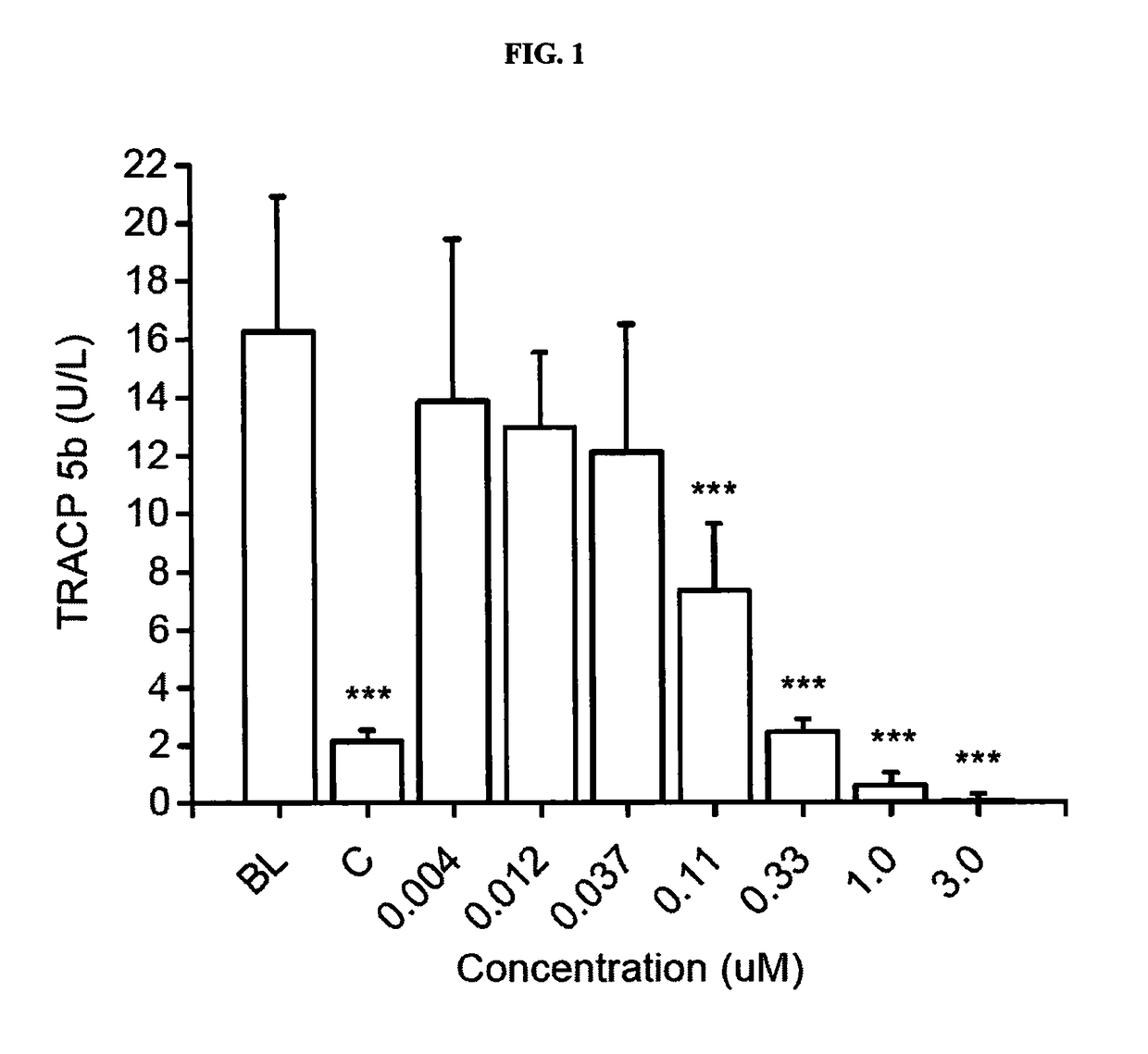
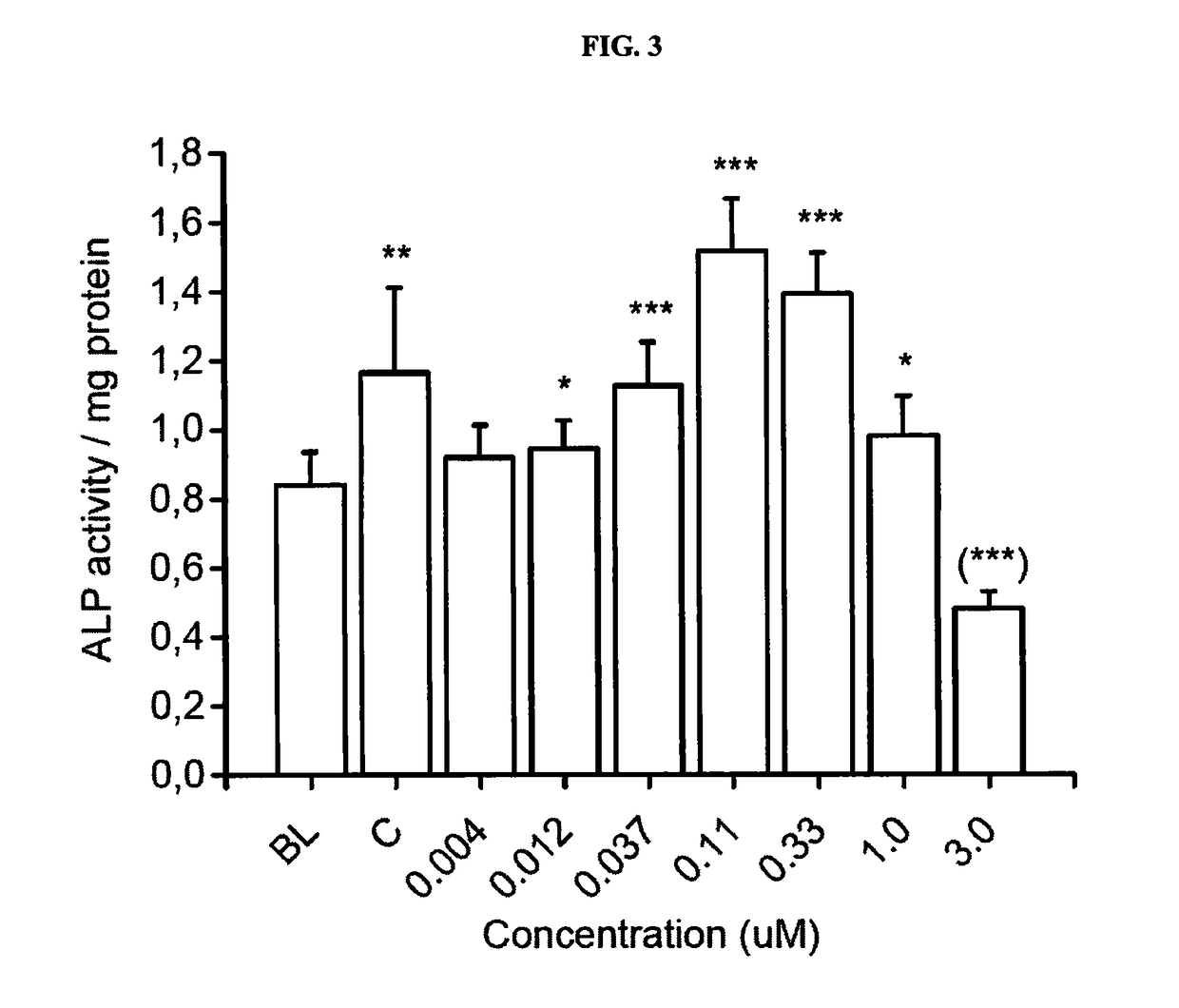
Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a diluent, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
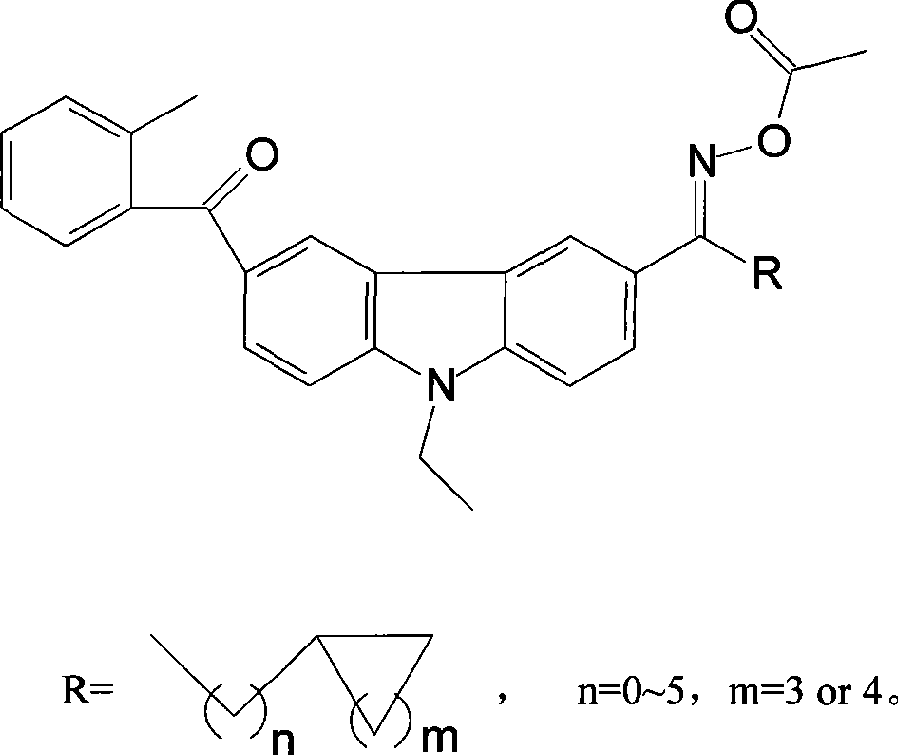
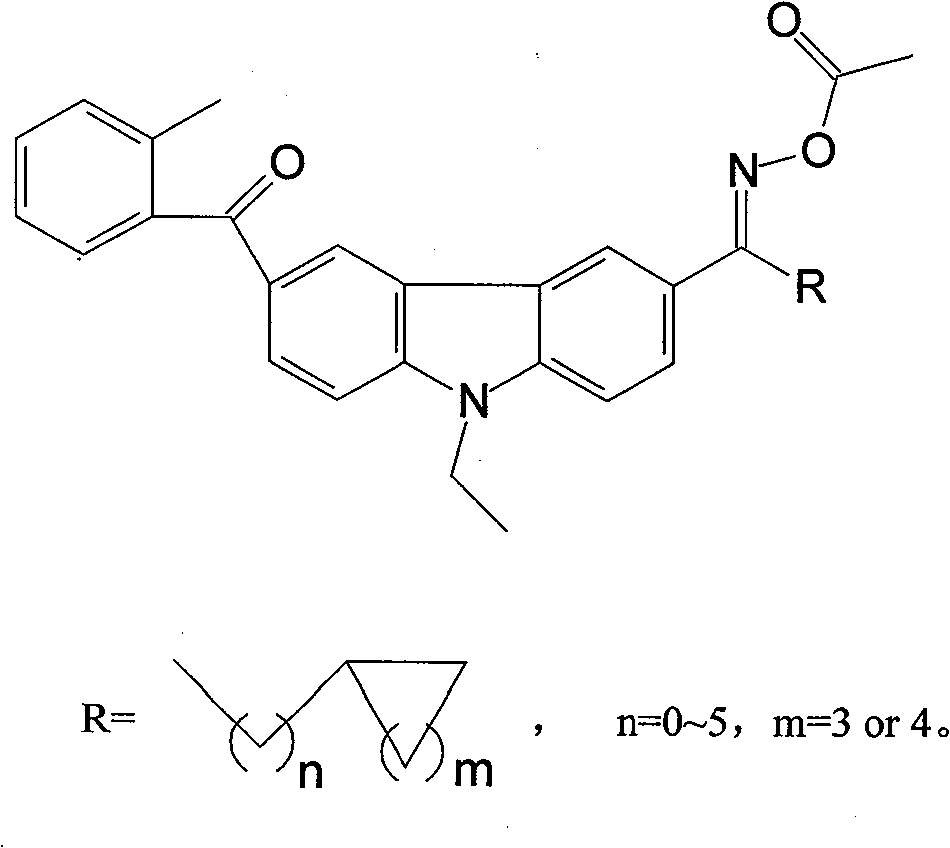
Carbazole oxime ester lightlike initiating agent
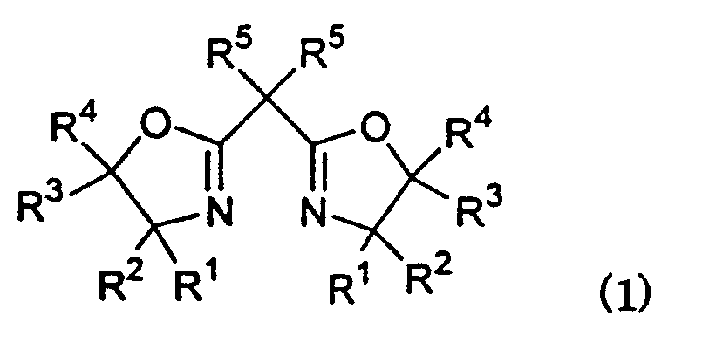
The invention relates to the photoinitiator technical field, in particular to an oxime ester photoinitiator and a preparation method thereof. A carbazole oxime ester photoinitiator has a structural general formula as the right formula, R=formula (1), n=0-5, m=3 or 4, R radical is aliphatic ketone with cyclane, the cyclane is cycloaliphatic ring from cyclopropane to cyclooctane, branched-chain aliphatic hydrocarbon connects the cyclane and the ketone, and the chain usually has 0-6 carbon atoms. The carbazole oxime ester photoinitiator with the structure is a brand-new compound with good photoinitiator performance, and solves the problem of poor sensitivity, thermal stability and solubility of the existing carbazole oxime ester photoinitiators.
Owner:CHANGZHOU TRONLY NEW ELECTRONICS MATERIALS
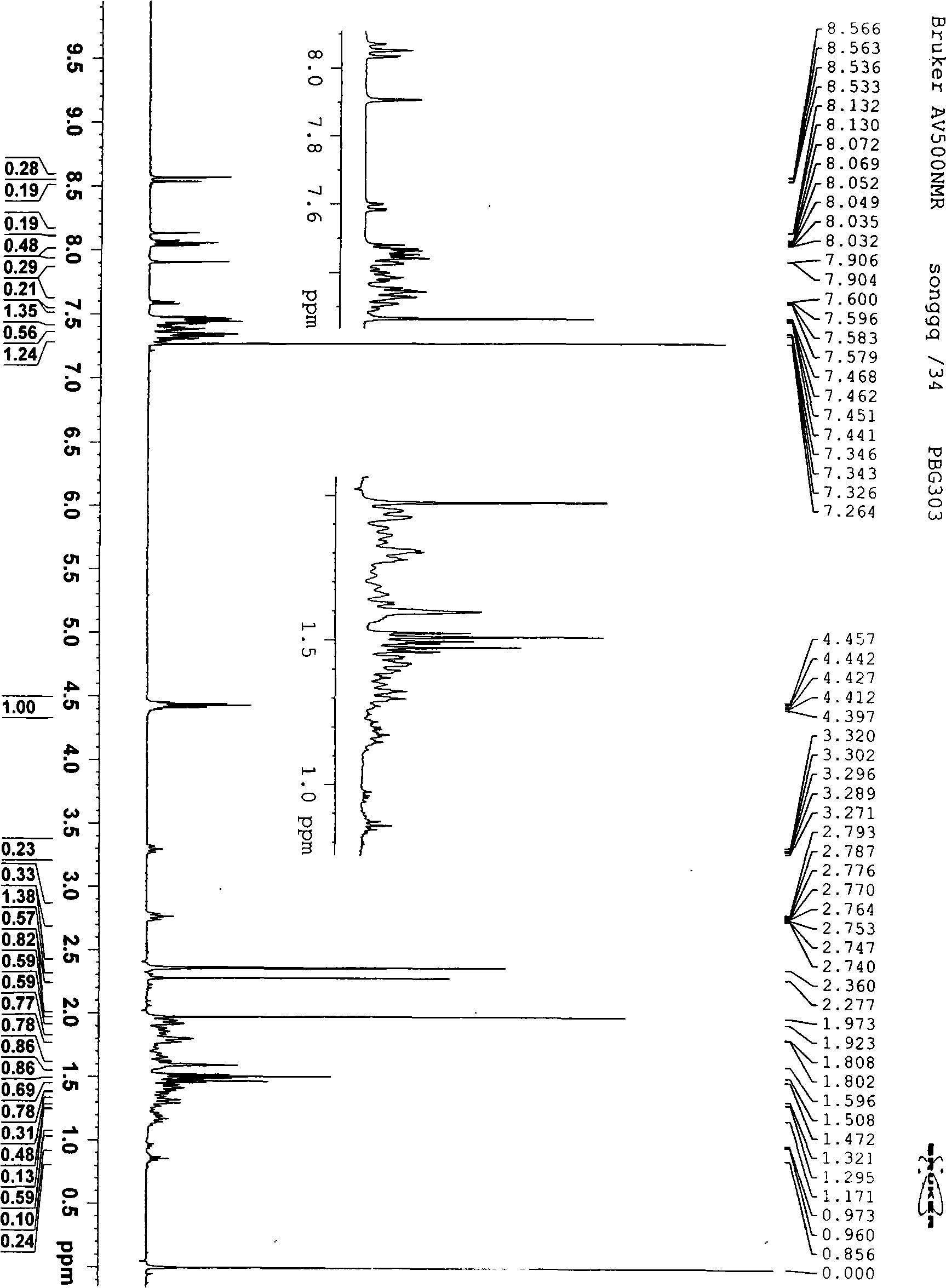
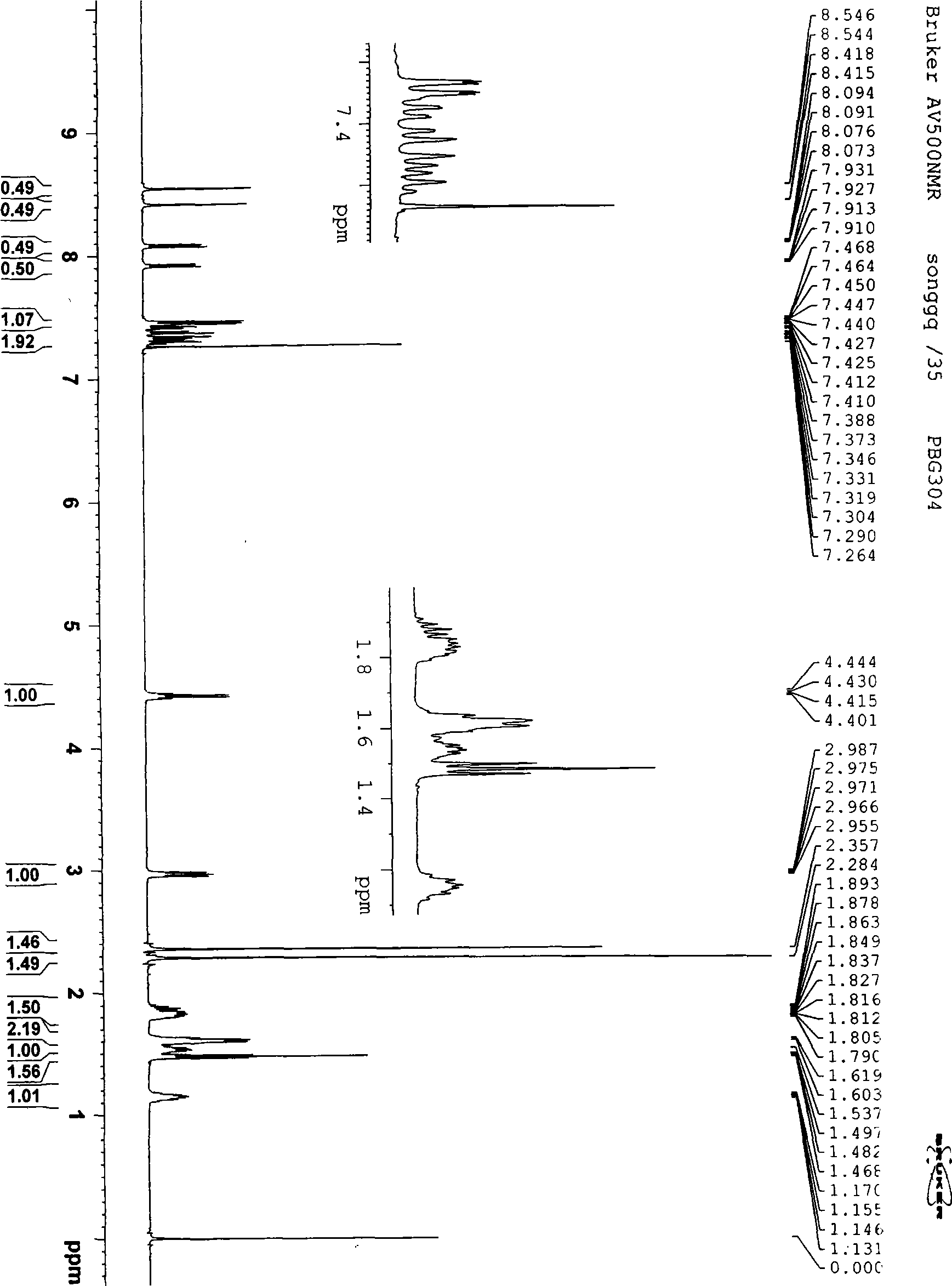
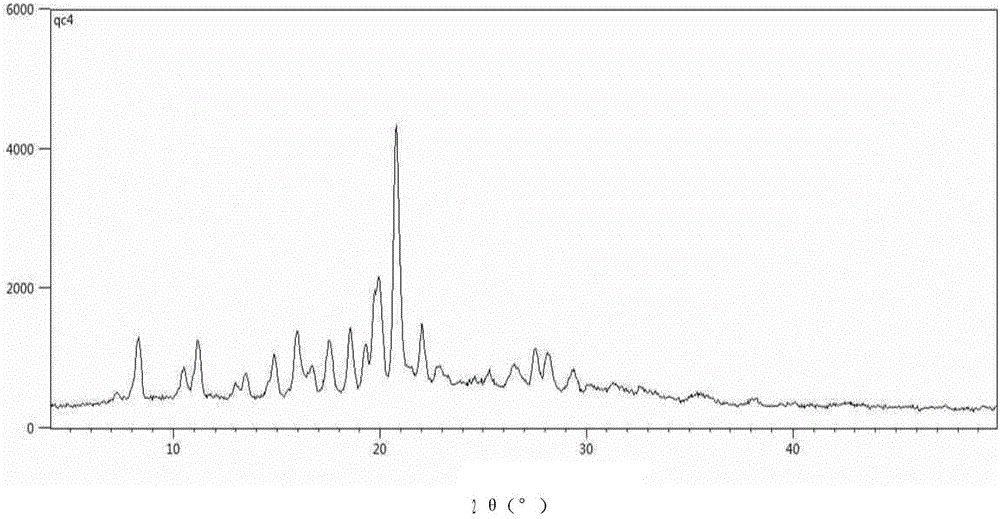
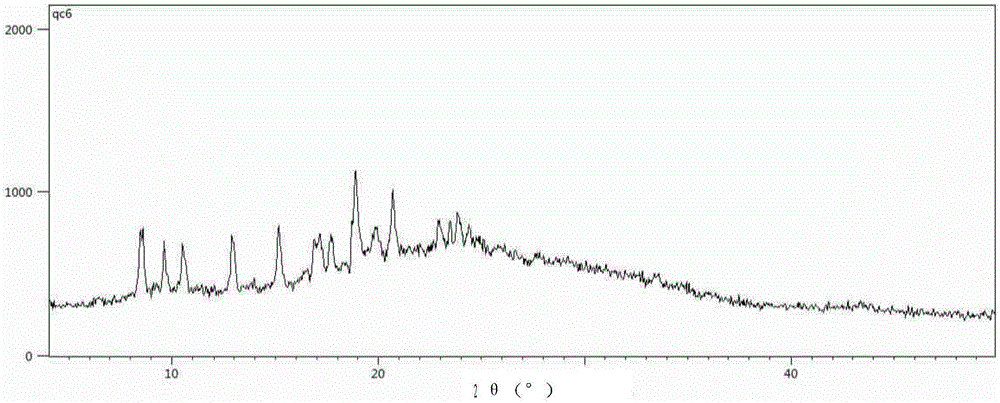
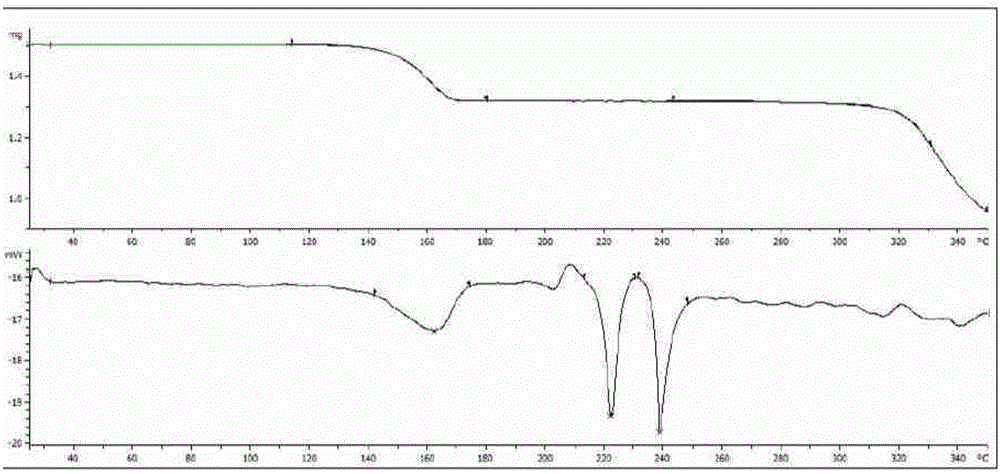
Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide
The present invention relates to solid forms of (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide (Compound 1) in substantially crystalline form (Form A) or amorphous form, pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
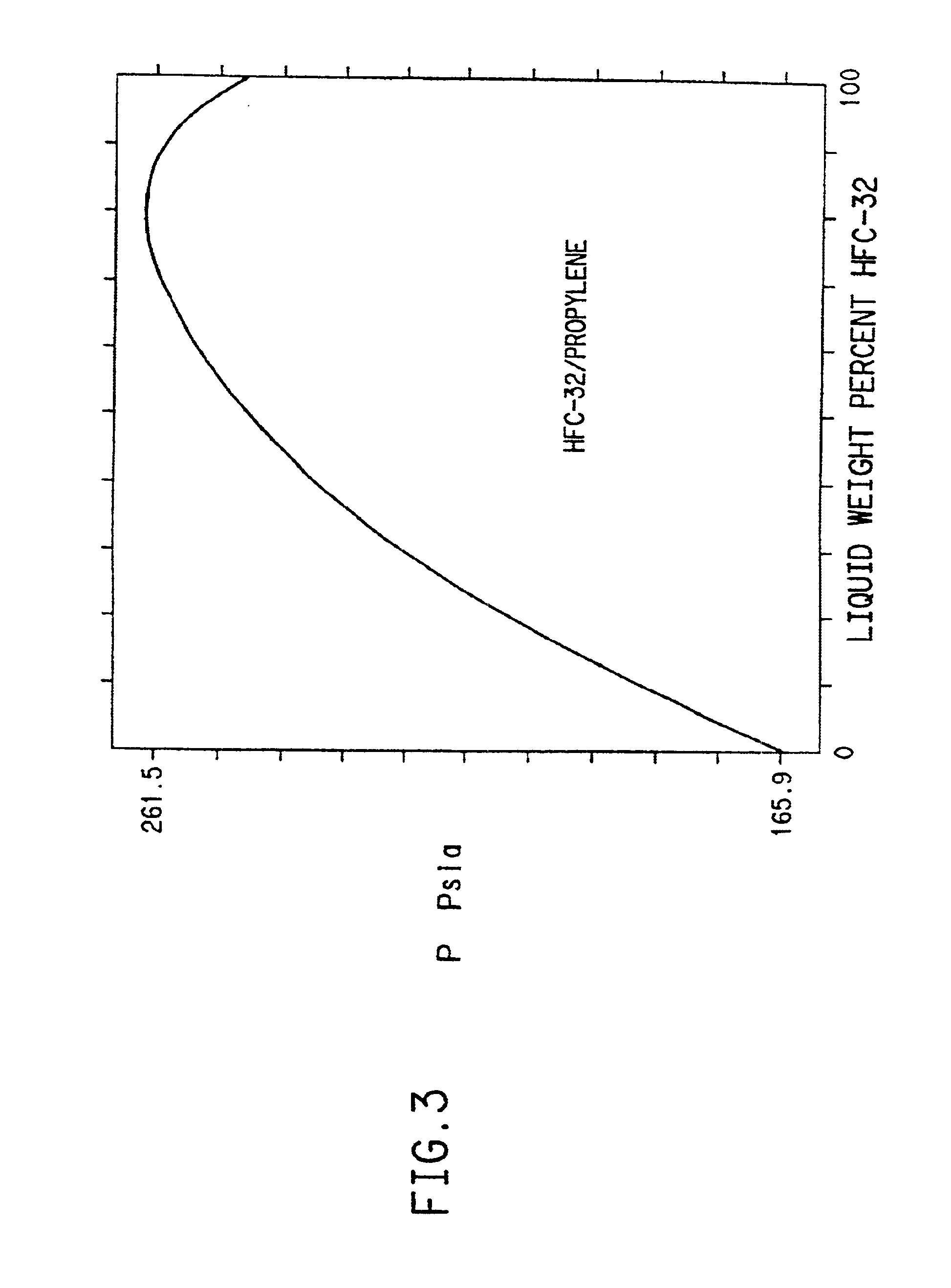
Multi-element mixed working substance adapted to double temperature preparation of single-unit vapor compression type refrigerator
The invention relates the multicomponent mixed working substance used for preparing dual-temperature by single-machine vapor compression refrigerator. The low temperature is between -60- -40Deg.C and the high temperature is between -25- 10Deg.C. The multicomponent mixed working substance comprises lower boiling working substance and high boiling working substance. The lower boiling working substance comprises tetrafluoromethane, ethylene, ethane, fluoroethylene, trifluoromethane, fluoromethane, hydrofluoeic ether, carbon dioxide, difluoromethane and penfluoroethane; the high boiling working substance comprises trifluoro-thane, hydrofluoeic ether, propylene, propane, perfluoropropylamine, propadiene, cyclopropane, difluo-monochloromethane, penta-monochloroethane, perfluoroethane, difluoroethane, isobutene, butane, butylenes, isobutylene, sevofluoropropane, hexafluoropropane, penfluoropropane and tetrachloromonofluoroethane.
Owner:XI AN JIAOTONG UNIV
Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxo-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
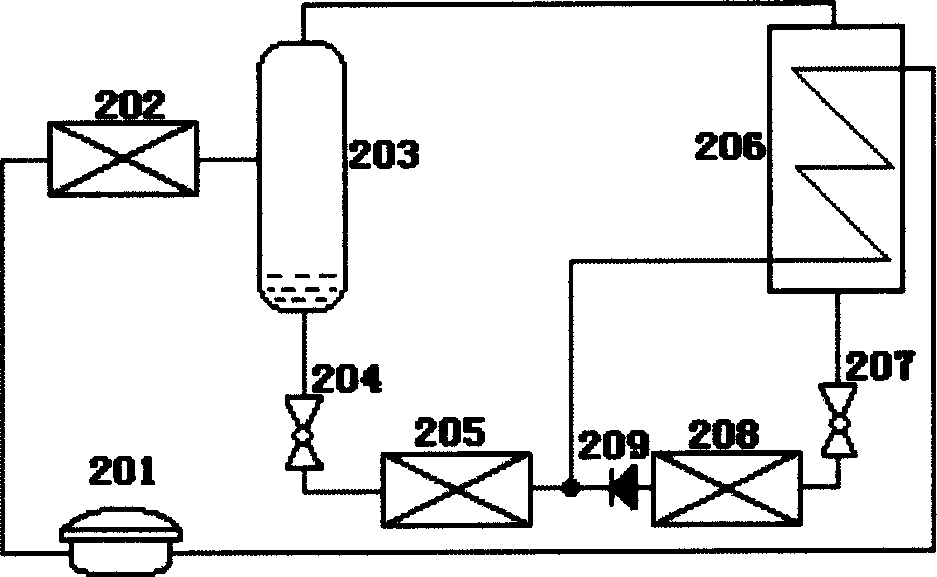
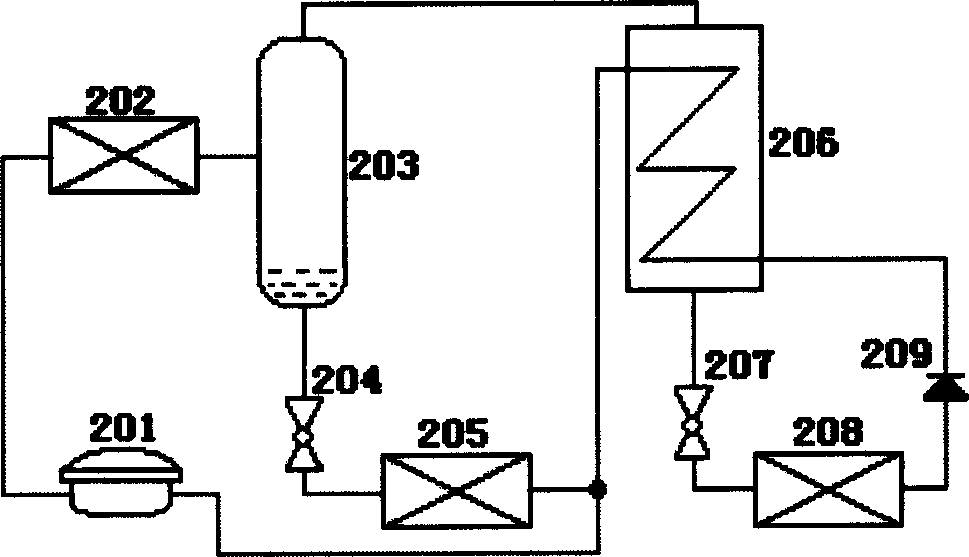
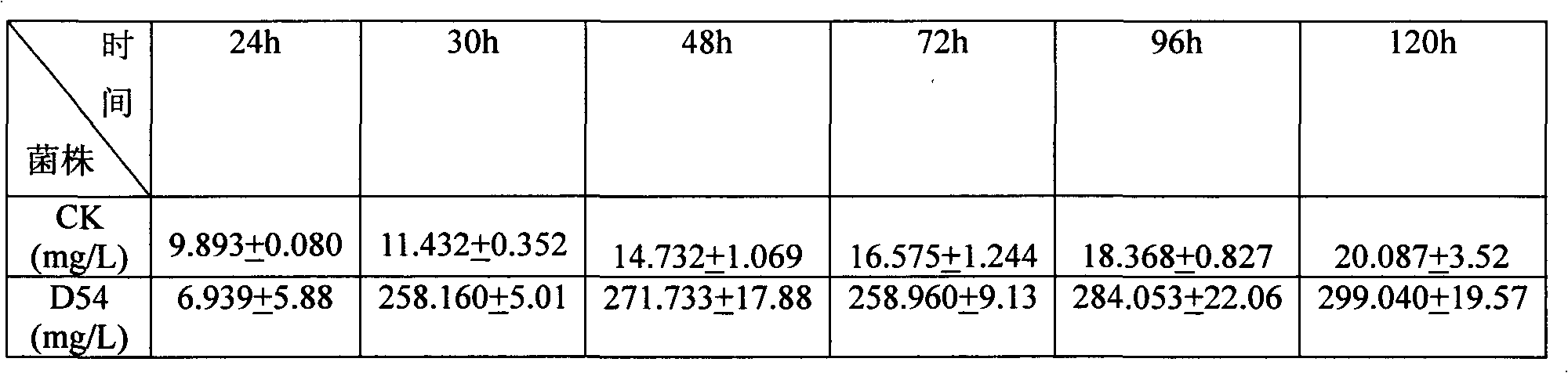
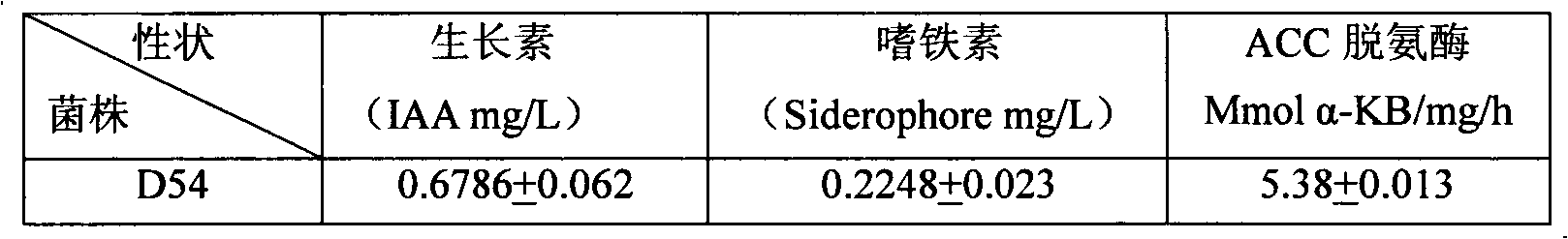
Heavy metal resistance plant growth-promoting bacteria preparation and applying method thereof
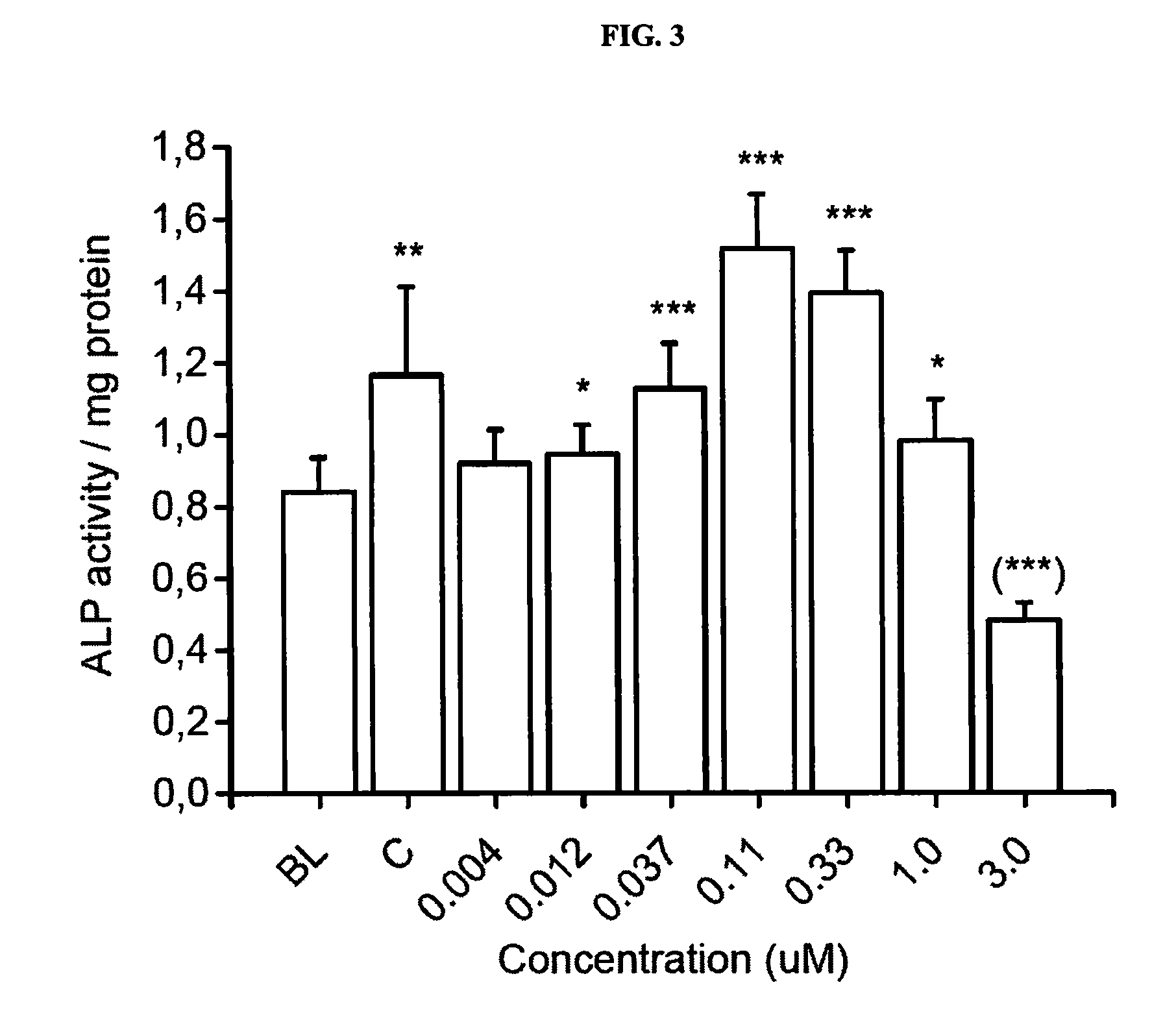
InactiveCN101671636APromote absorptionPromote growthBiocidePlant growth regulatorsHigh resistanceDisease
The invention relates to heavy metal resistance plant growth-promoting bacteria and a preparation applying method thereof, which belongs to the bioremediation field of heavy metal polluted environment. A bacteria strain D54 with a preservation number of CGMCC No. 3223 belongs to the Burkholderia sp. and has higher resistance to a plurality of heavy metals, wherein the resistances to Pb <2+>, Cd <2+>, Cu <2+>, Zn <2+> respectively reach 800 mg / L, 1500 mg / L, 150 mg / L and 2500 mg / L. In addition, the bacteria strain D54 has the plant growth-promoting functions of producing plant growth hormone (IAA), producing 1-amino-1-carboxyl cyclopropane (ACC) deaminase, secreting siderophore, dissolving inorganic phosphate, fixing nitrogen and the like, has the biological prevention functions of antagonizing plant pathogenic bacteria inbreak and the like, and can obviously improve the biomass of the plants applied the invention and improve the resistance to diseases and stresses. The number of the effective viable bacteria in liquid preparation reaches 1-2 billion / ml and the number of the effective viable bacteria in solid preparation reaches 1 billion / g. Soaking seeds for 1-2 hours in the liquidpreparation which is diluted 100 times and irrigating the diluted liquid preparation 1-2 times (10ml / kg) after 2-3 weeks of the sprouting of the seeds can effectively improve plant viable bacteria infection probability.
Owner:AGRO ENVIRONMENTAL PROTECTION INST OF MIN OF AGRI
Carboxylic acid compound cyclopropane ring
InactiveUS20050075393A1Lower metabolismSmooth transmissionBiocideNervous disorderSynapseMemory disorder
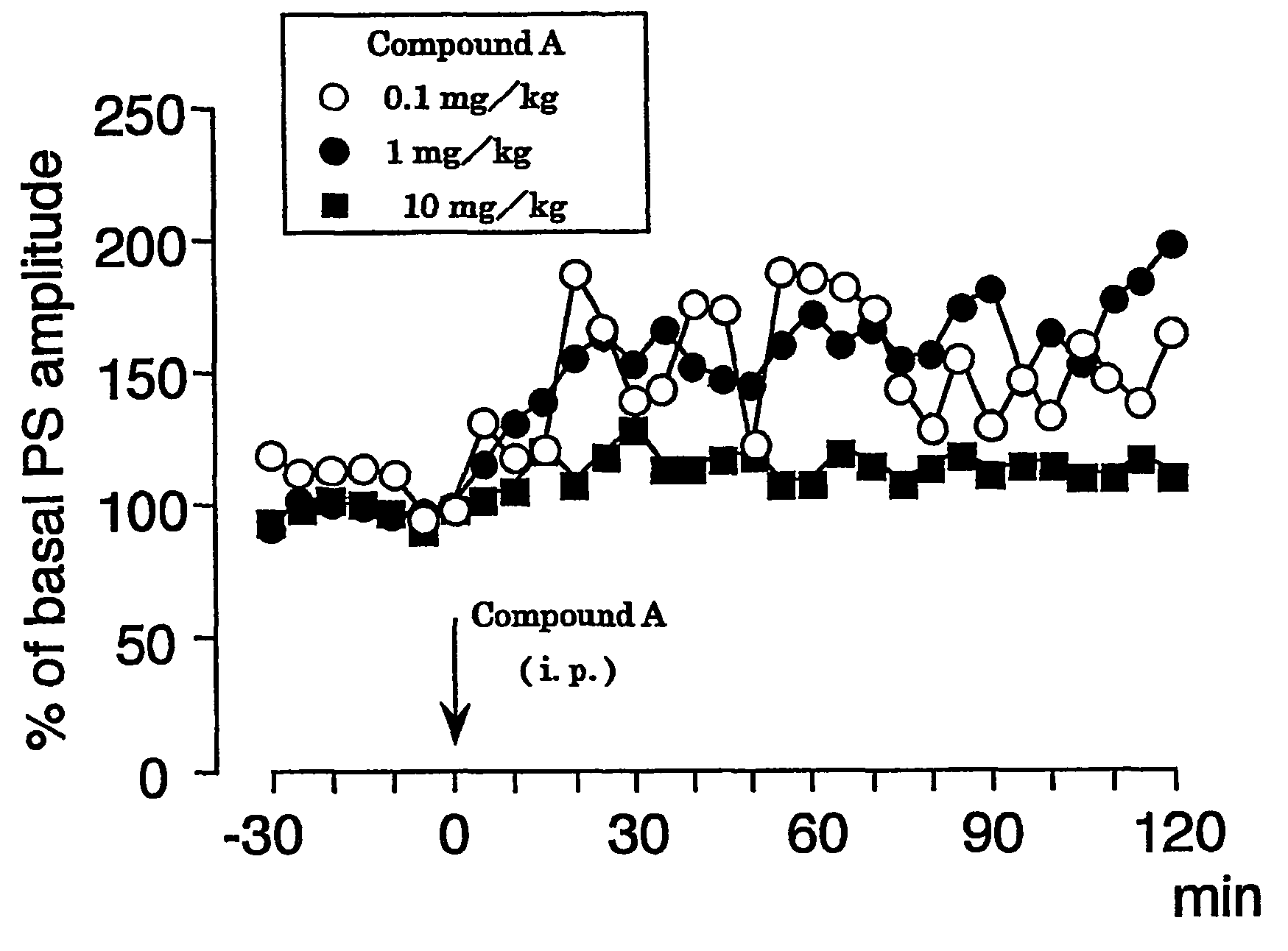
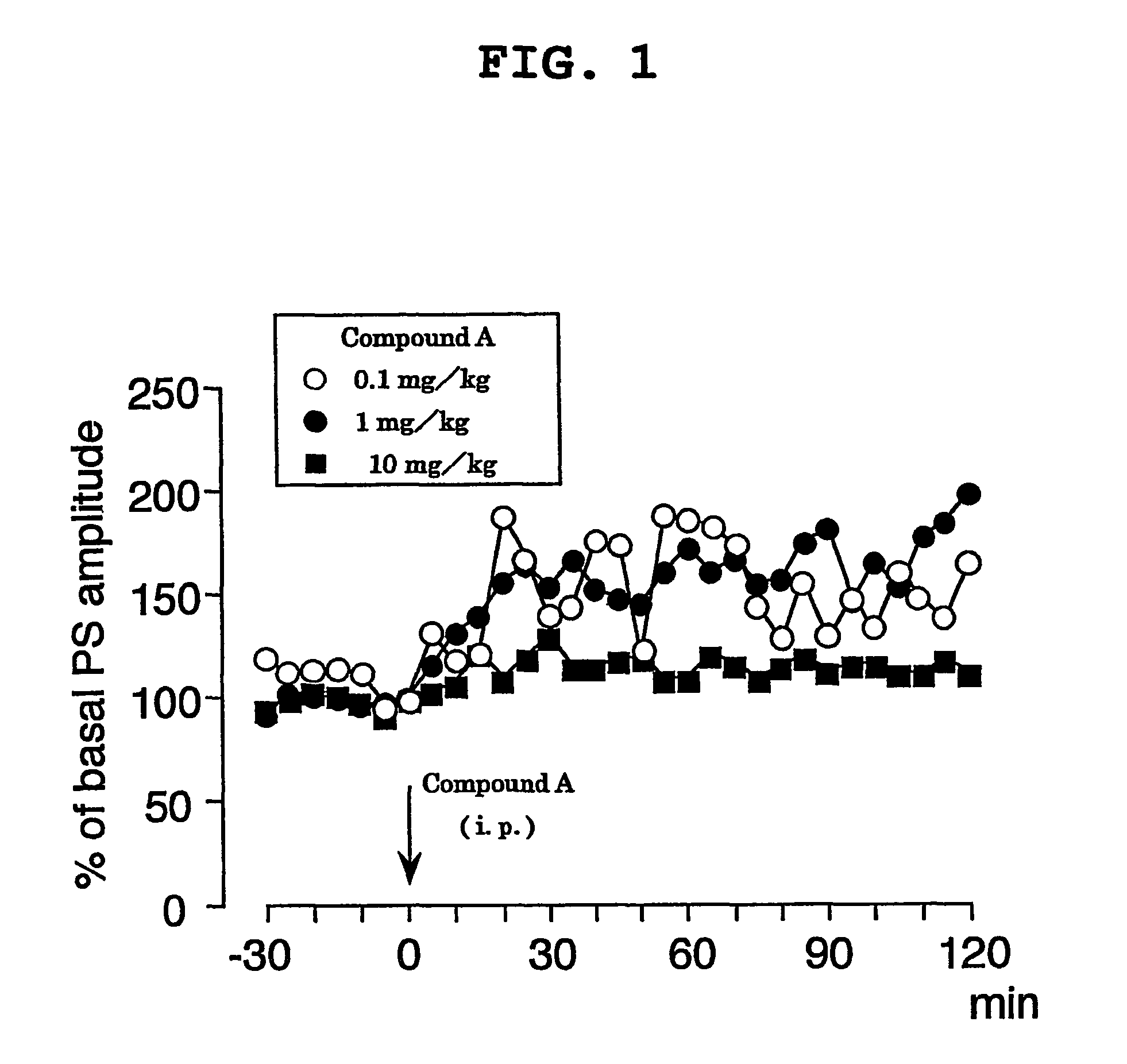
A carboxylic acid compound having cyclopropane ring(s) of the formula (I): wherein R is alkyl or alkenyl optionally having one or more 1,2-cyclopropylene in a carbon chain and / or optionally having cyclopropyl at the end of a chain, X is a single bond or alkylene, wherein the total number of carbon less the number of cyclopropane ring is 10-25, and a pharmaceutically acceptable salt thereof are provided. The compound (I) shows an LTP-like potentiation of synaptic transmission, allows slow metabolism in the living body, shows a stable LTP-like potentiation of synaptic transmission, and is useful as an agent for LTP-like potentiation of synaptic transmission, a cognition-enhancing drug or an agent for the prophylaxis and treatment of dementia, a learning and memory disorder and a neurotransmitter release disorder.
Owner:NISHIZAKI BIOINFORMATION RES INST +1
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
The present invention relates to a substantially crystalline and free solid state form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Form I), pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
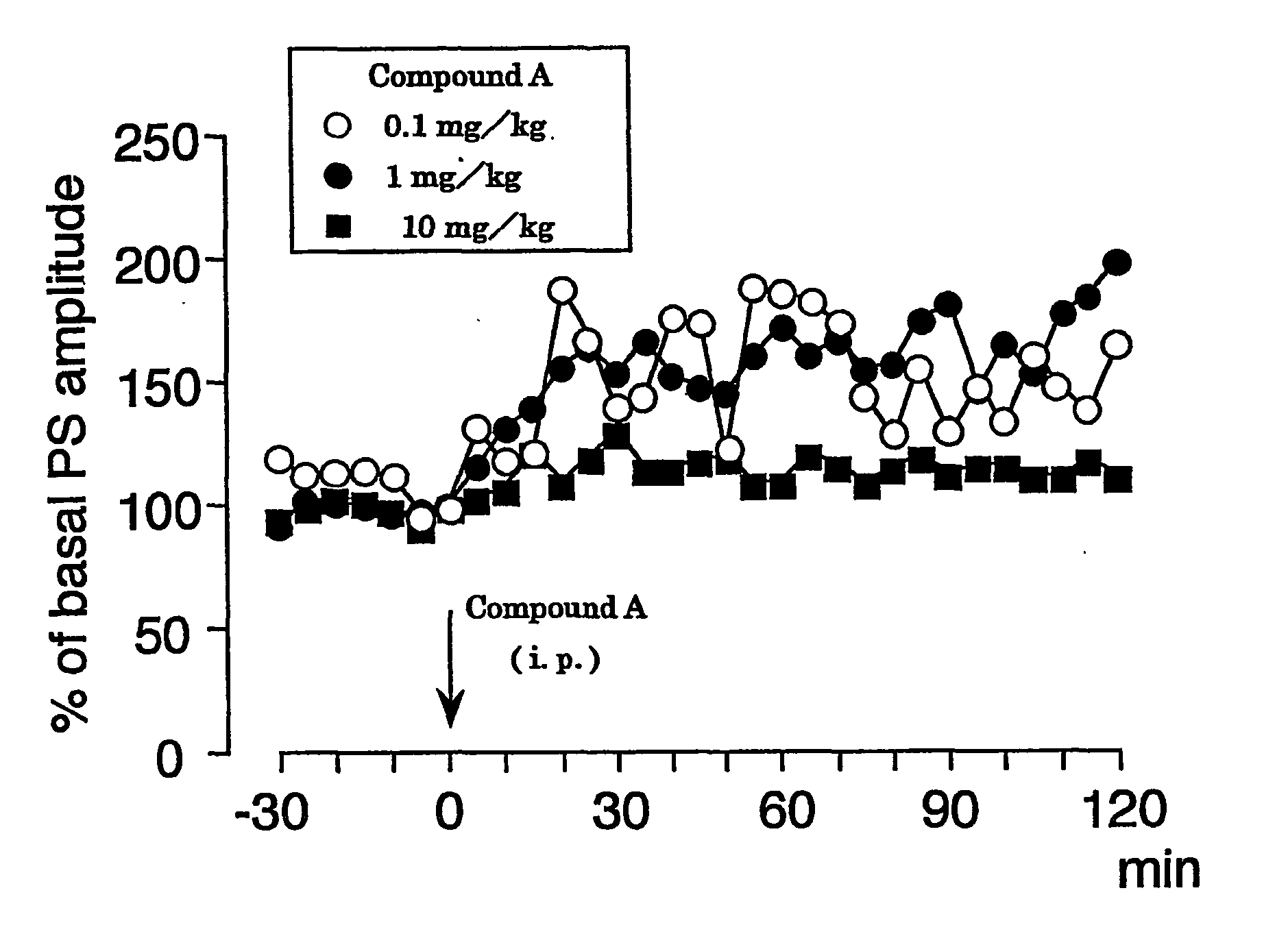
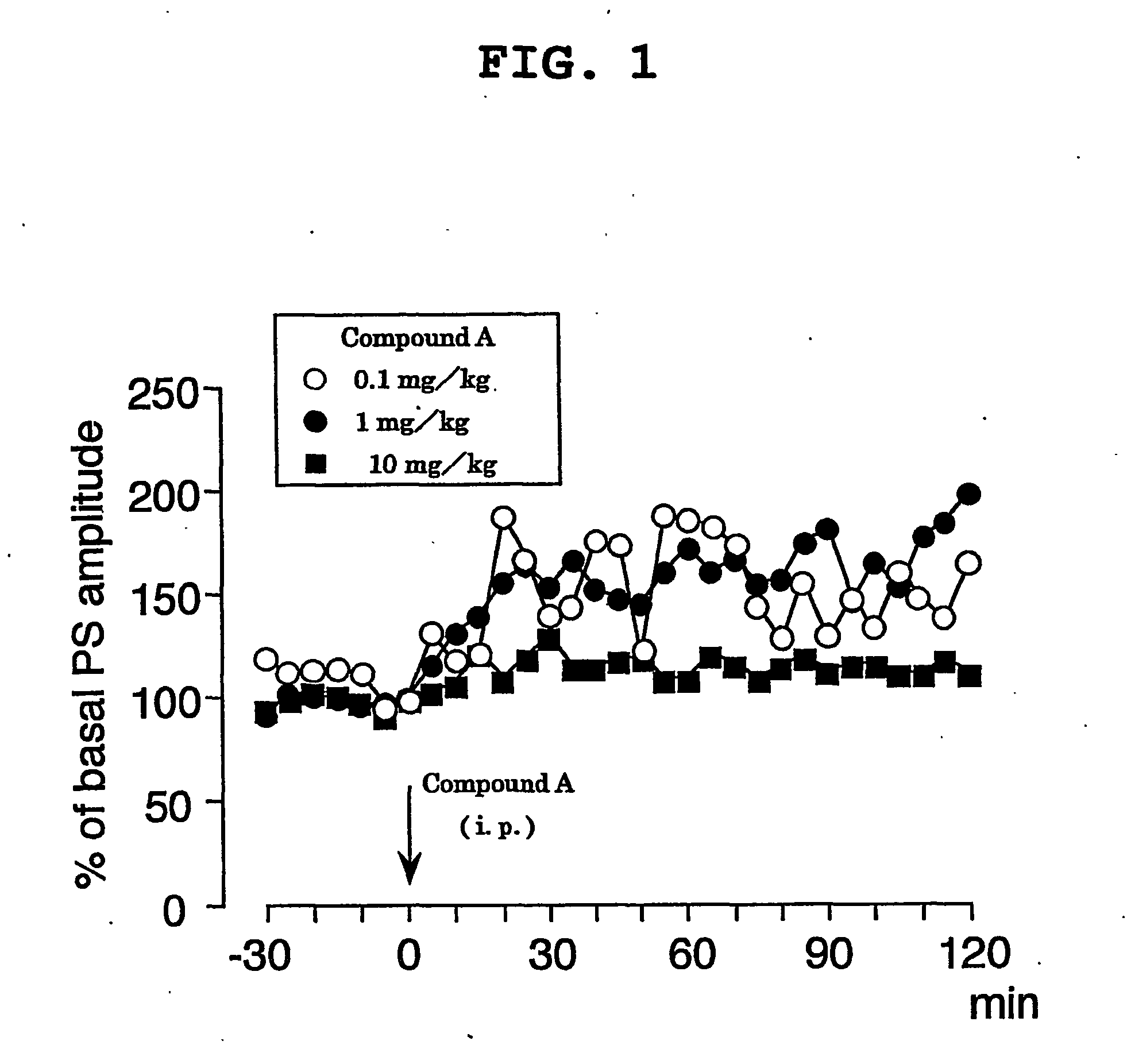
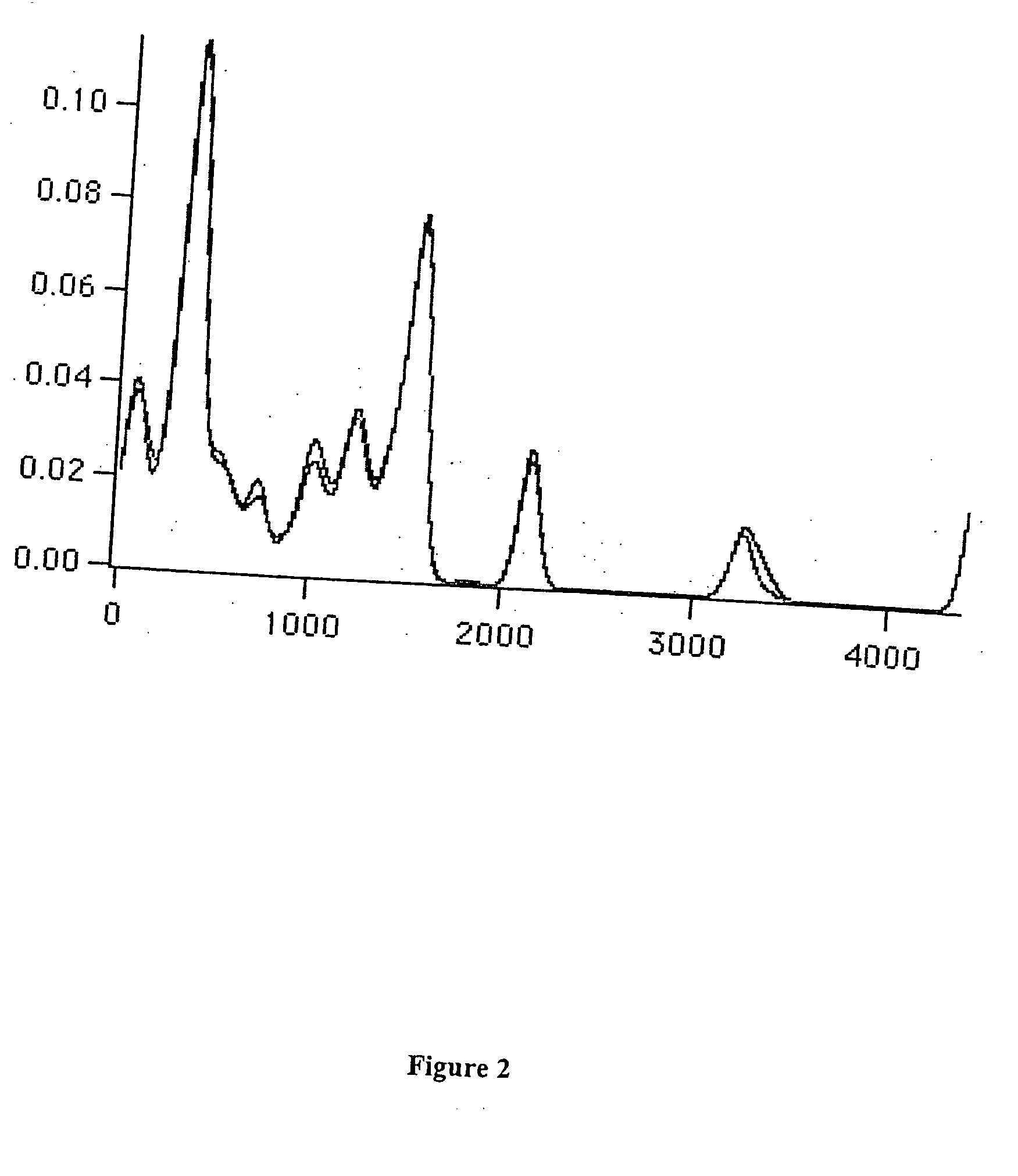
Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
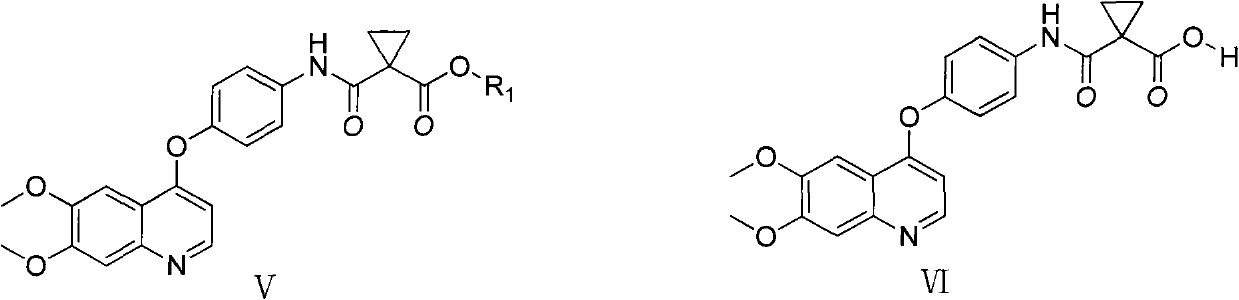
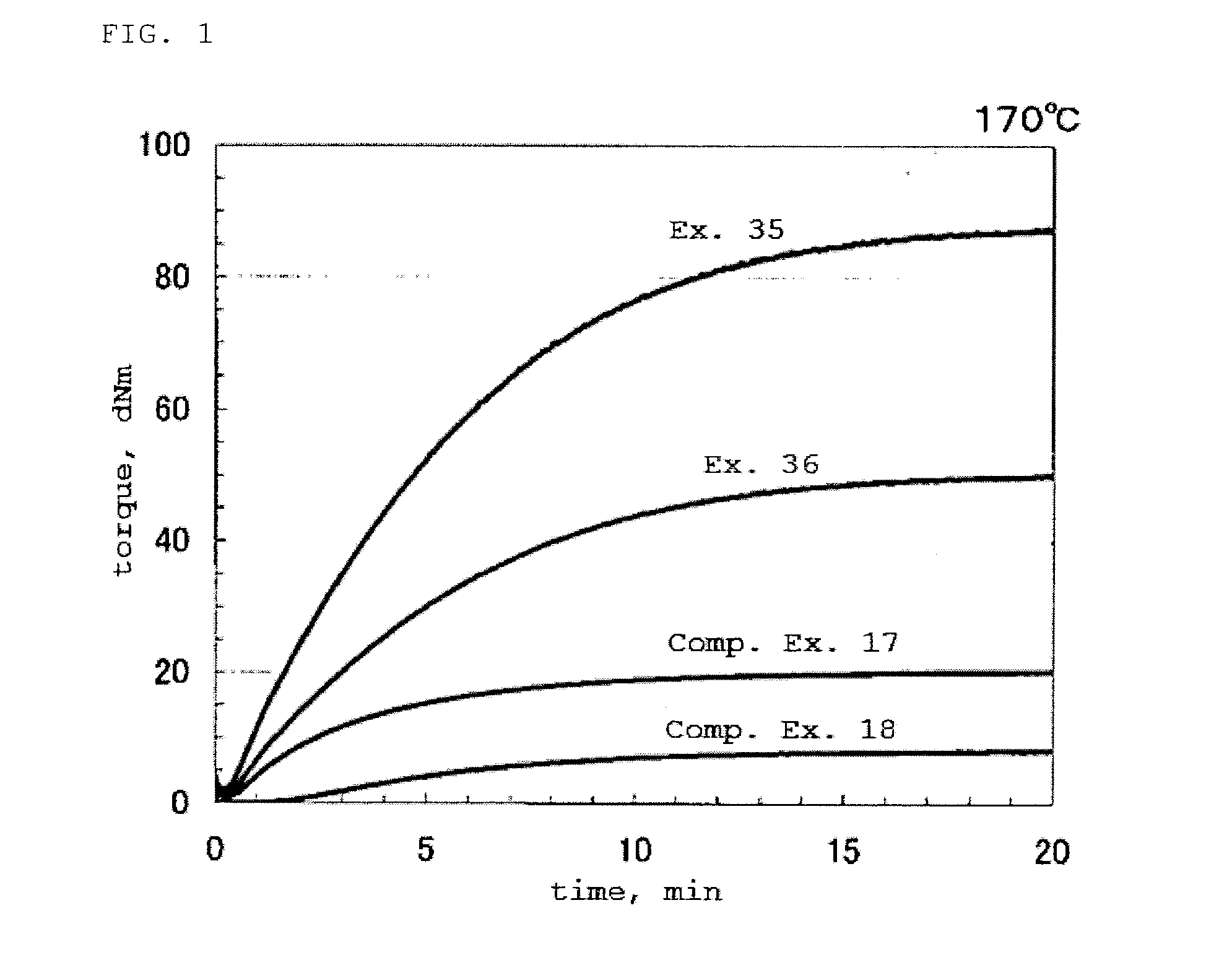
This invention relates crystalline hydrates of N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]quinolin-4-yl}oxy)phenyl]-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide, Compound (I). The invention provides methods for treatment of cancer by exploiting the modulation of protein kinase activity. The invention also provides pharmaceutical compositions containing a crystalline hydrate of Compound (I) and a pharmaceutically acceptable excipient.
Owner:EXELIXIS INC
Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
This invention relates to three crystalline forms of N-[3-fluoro-4-((6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]quinolin-4-yl|oxy)phenyl]-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide. Compound (I), designated as Form A, Form B, and Form C. The invention provides methods for treatment of cancer by exploiting the modulation of protein kinase activity. The invention also provides pharmaceutical compositions containing a crystalline form of Compound (I) and a pharmaceutically acceptable excipient.
Owner:EXELIXIS INC
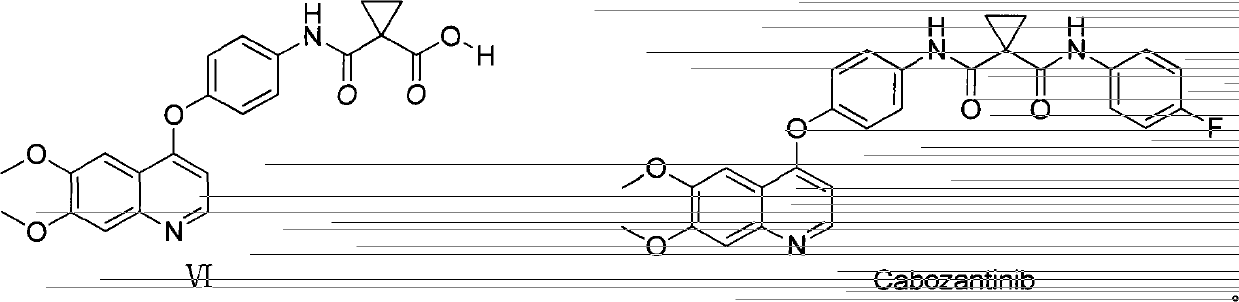
Preparation method for tyrosine kinase inhibitor and midbody thereof
ActiveCN103664776AMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationTyrosine-kinase inhibitorQuinoline
The invention relates to a preparation method for a tyrosine kinase inhibitor and a midbody thereof. According to the method, a compound 1,1-cyclopropane dicarboxylic acid diester is taken as a raw material, and 1-((4-((6,7-dimethoxy quinoline-4-yl) oxy) phenyl) carbamoyl) cyclopropane formic ether is prepared by two ways and reacts with p-fluoro aniline after being hydrolyzed so as to prepare Cabozantinib. The reaction conditions of the preparation method are mild, the synthesis cost is lowered, and the preparation method is simple and convenient to operate and is applicable to industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Copolymer of olefin and conjugated diene, and process for producing the same
ActiveUS20120059135A1Maintain good propertiesLower glass transition temperatureSide chainSA copolymer
An object of the invention is to provide copolymers which have a double bond in a side chain and are substantially free of unsaturated bonds in the main chain, copolymers which have a cyclic structure and are substantially free of unsaturated bonds in the main chain, and processes for economically synthesizing these copolymers.A copolymer of the invention is obtained by copolymerizing at least ethylene and a conjugated diene. In the copolymer, structural units derived from the conjugated diene represent 1 to 90 mol %. Structural units resulting from 1,2-addition of the conjugated diene and having a side-chain double bond represent 0 to 90 mol %, structural units resulting from 1,4-addition of the conjugated diene represent 0 to 3 mol %, structural units resulting from 1,3-addition of the conjugated diene represent 0 to 3 mol %, and the total of structural units resulting from 1,2-addition of the conjugated diene and having a 1,2-cyclopropane skeleton and structural units resulting from 1,2-addition of the conjugated diene and having a 1,2-cyclopentane skeleton represent 4 to 100 mol %.
Owner:MITSUI CHEM INC
Aromatic nitrile-base thiazole derivatives for inhibiting xanthine oxidase activity, preparation method and application
ActiveCN101386604AEasy to operateHigh yieldOrganic active ingredientsOrganic chemistryCyclobutaneLithium formate
The invention disclosed an aryl nitrile group thiazole derivative for inhibiting the activity of xanthine oxidase, a method for preparing the same and application thereof. In the aryl nitrile group thiazole, R1 is methyl, ethyl, propyl, isopropyl, isobutyl, methyl cyclopropane, methyl cyclobutane, isoamyl, methyl cyclopentane, methyl cyclohexane or aromatic ring methyl, R2 is methyl or trifluoromethyl, and R3 is formic acid, sodium formate, potassium formate, lithium formate, methyl formate, or ethyl formate. Simultaneously, the invention discloses a method for synthesizing the aryl nitrile group thiazole derivative by using inexpensive raw materials, which has the advantages of simple operation, high yield, easy purification of products, application to industrial production and the like, and can obtain an efficient compound with low toxicity through screening; besides, the effective compound is expected to be widely applied to inhibit the activity of the xanthine oxidase required on animals and humans, and to become a new generation of antigout drugs and hyperuricemia drugs with special effect.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
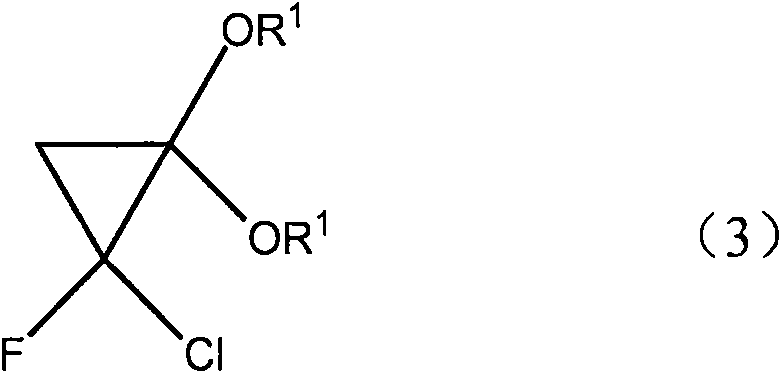
Novel synthetic method for F-acrylic acid and derivative thereof
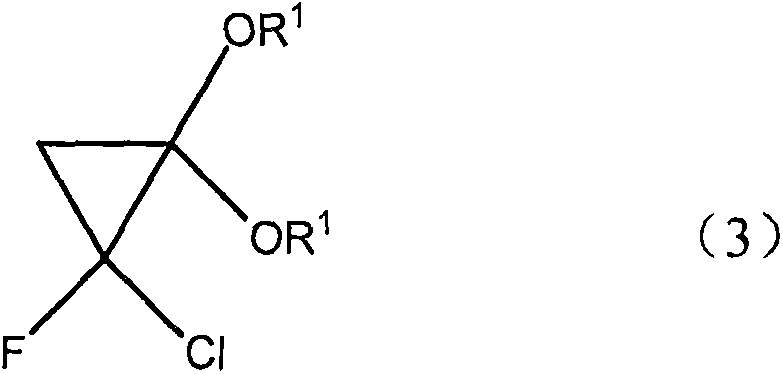
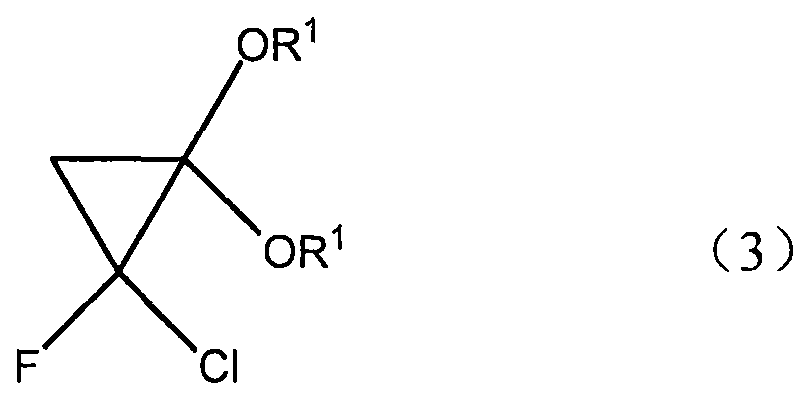
InactiveCN105130798ASimple processEasy to operateOrganic compound preparationCarboxylic acid esters preparationFluorideCyclopropane
The present invention discloses a novel synthetic method for F-acrylic acid and a derivative thereof. According to the process, ketene diethyl acetal with a molecular formula (2) CH2=C(OR1)2 is used for preparing 2-fluoride acrylic acid with a molecular formula (1) CH2=CF-CO2R and an ester thereof through a cyclopropane derivative with a molecular formula (3). According to the synthetic method disclosed by the invention, the synthetic method has the characteristics that the process is simple, the operability is high, raw materials used in preparation are cheap and the toxicity is low.
Owner:朱虹
Method for Treating Osteoporosis
This invention is directed to the treatment of osteoporosis using N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide.
Owner:EXELIXIS INC
Production method of nifuratel
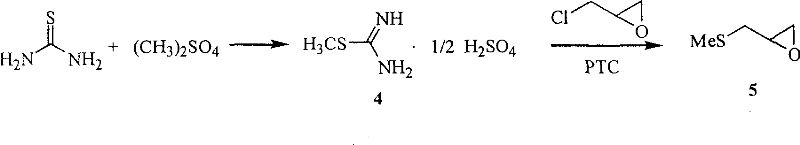
The invention relates to a medicine producing field, specifically, to an improved producing method of antibacterial medicine nifuratel. The method makes use of thiourea as initial material to produce nifuratel. The improvement is that it is no longer to use methyl mercaptan or methomyl in the known method while producing the intermediate 2-(methylthiomethyl)hydropropane. Furthermore, the invention is no longer to use metal natrium in the known method when the 3-methylthio-2-hydroxy-propylhydrazine and diethyl carbonate are heated to produce N-amido-5-methylthiomethyl-2-oxazolidone in the existance of alkali. All of the improvements largely improve the preparation of the nifuratel, especially for the production condition in a large scale, reduce the environment pollution and benefit to ensure the production safety.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
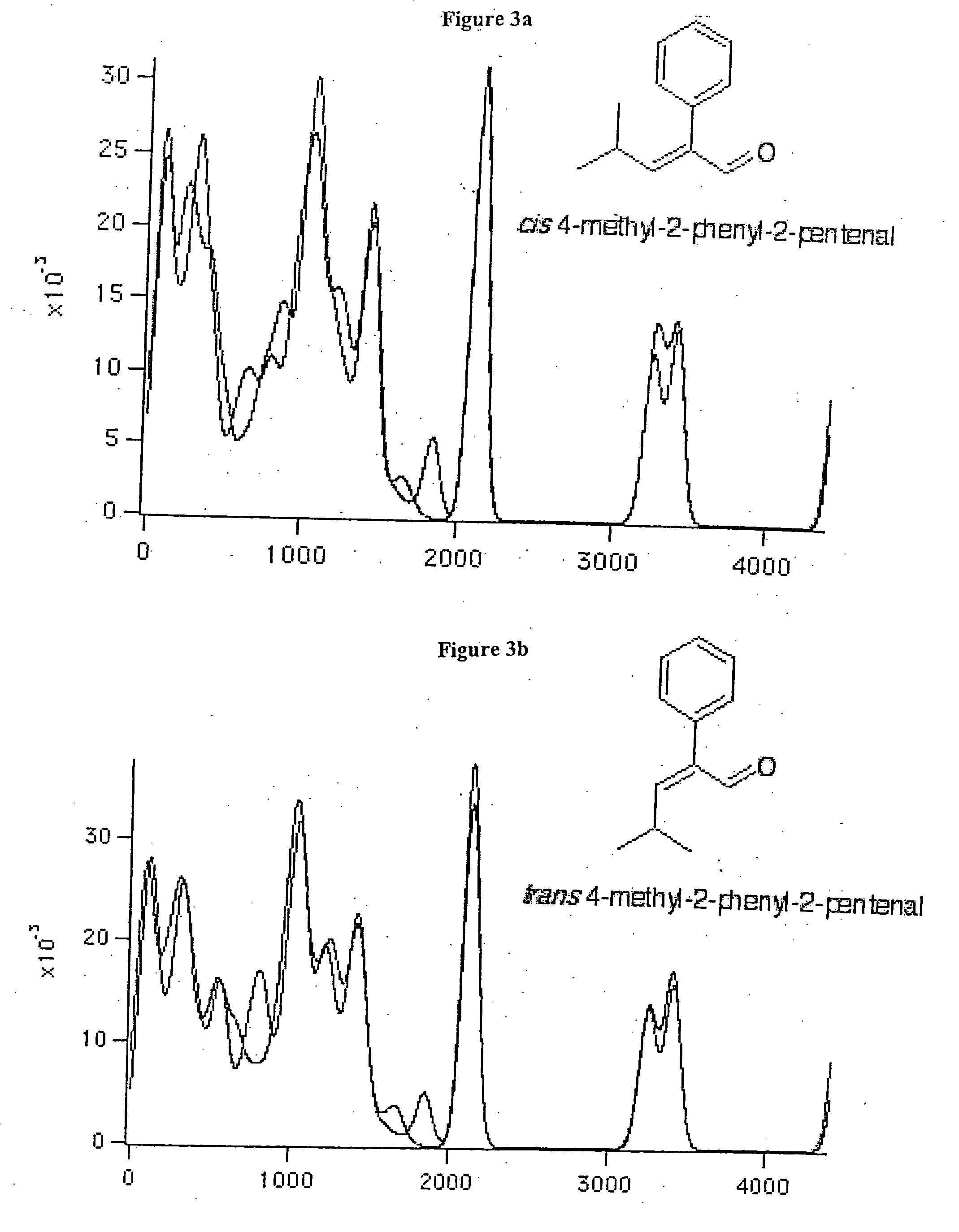
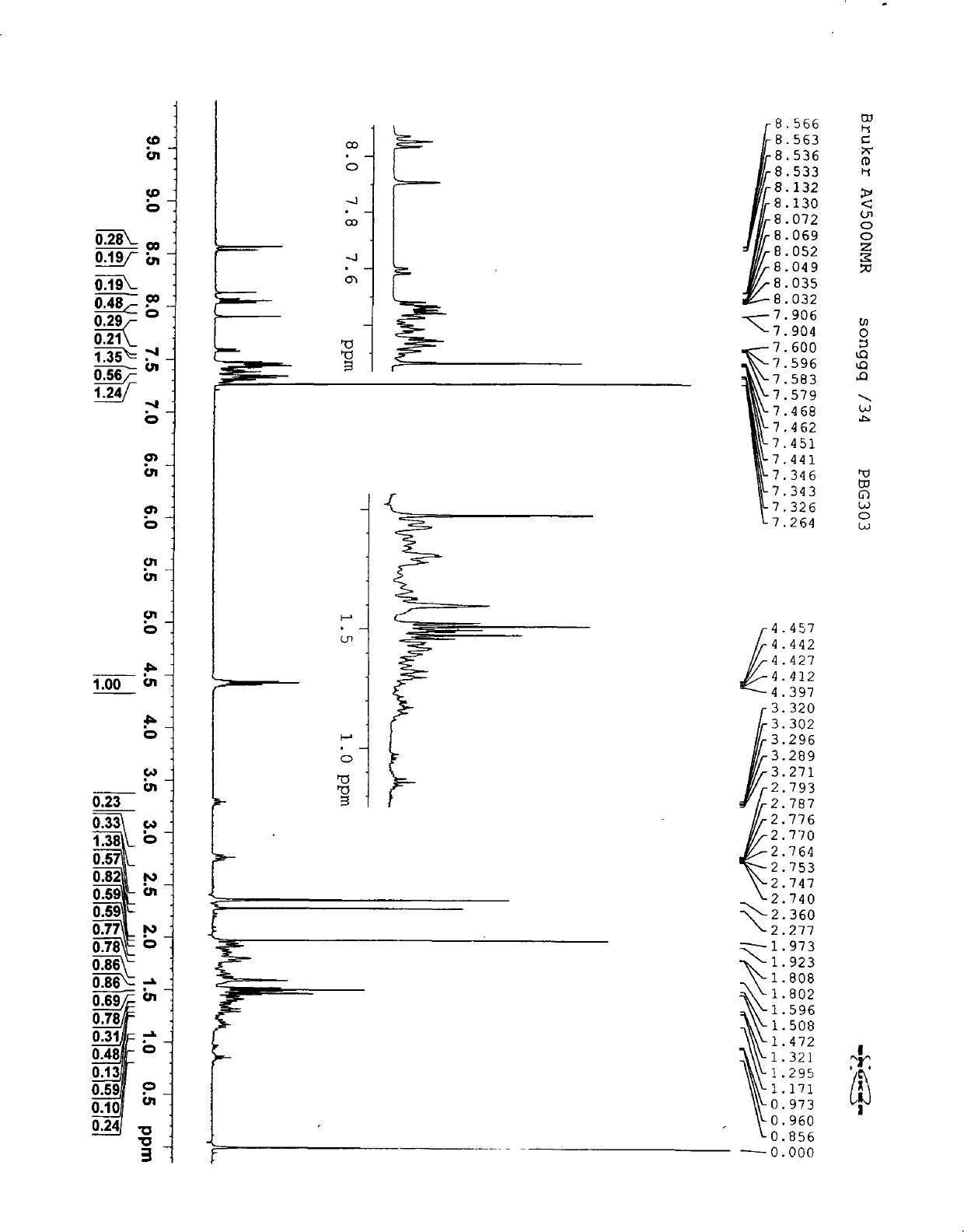
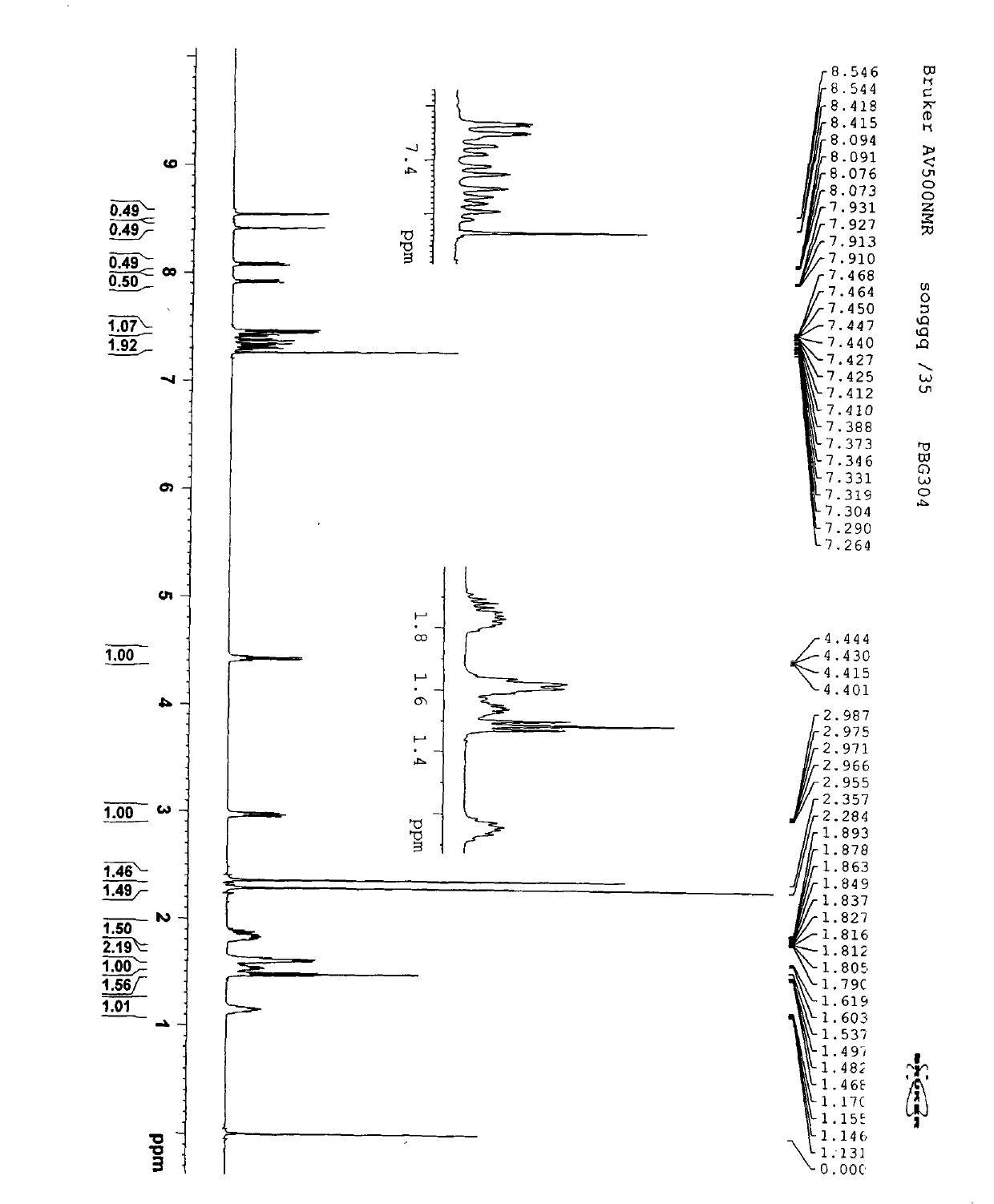
Aromachemicals
InactiveUS20050119156A1Longer useful shelf lifeImprove suppression propertiesCosmetic preparationsToilet preparationsHypochloriteBleach
Improved aromachemical derivatives, and fragrances and flavorings including the derivatives, that have a longer useful shelf life than the aromachemicals from which they can be derived, are disclosed. In particular, the derivatives maintain the fragrance characteristics of the aromachemicals, while lowering the allergic properties, increasing the stability, and / or increasing the odor intensity. Also disclosed are methods of making the derivatives, and articles of manufacture including the derivatives. In one embodiment, the derivatives are prepared by replacing one or more double bonds in citral with a thioether, cyclopropyl, oxirane, or thiirane group. The cyclopropane ring can be unsubstituted, or substituted with one or two lower alkyl, preferably methyl groups. The alkyl groups can optionally be substituted, for example, with electron donating groups, electron with drawing groups, groups which increase the hydrophilicity or hydrophobocity, and the like. In another embodiment, the derivatives are prepared by replacing the aldehyde group in the essential oil with a nitrile, methyl ether or acetal group. The acetal groups can provide the compounds with a long lasting flavor or fragrance, where the acetals slowly hydrolyze to provide the aldehyde group in the parent essential oil. In some embodiments, both the aldehyde and at least one of the double bond functional groups are both derivatized as described herein. Examples of suitable articles of manufacture include candles, air fresheners, perfumes, disinfectant compositions, hypochlorite (bleach) compositions, beverages such as beer and soda, denture cleanser tablets and flavored orally-delivered products such as lozenges, candies, and the like.
Owner:FLEXITRAL INC
Carbazole oxime ester lightlike initiating agent
The invention relates to the photoinitiator technical field, in particular to an oxime ester photoinitiator and a preparation method thereof. A carbazole oxime ester photoinitiator has a structural general formula as the right formula, R=formula (1), n=0-5, m=3 or 4, R radical is aliphatic ketone with cyclane, the cyclane is cycloaliphatic ring from cyclopropane to cyclooctane, branched-chain aliphatic hydrocarbon connects the cyclane and the ketone, and the chain usually has 0-6 carbon atoms. The carbazole oxime ester photoinitiator with the structure is a brand-new compound with good photoinitiator performance, and solves the problem of poor sensitivity, thermal stability and solubility of the existing carbazole oxime ester photoinitiators.
Owner:CHANGZHOU TRONLY NEW ELECTRONICS MATERIALS
Method for treating osteoporosis
This invention is directed to the treatment of osteoporosis using N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide.
Owner:EXELIXIS INC
JAK inhibitor crystal forms, preparation methods and applications thereof
InactiveCN105061420AGood physical and chemical propertiesImprove stabilityOrganic active ingredientsAntipyreticPharmaceutical formulationChemical property
The present invention discloses four crystal forms of a JAK inhibitor N-(5-(4-(1,1-dioxothiomorpholinyl)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide, and methods for preparing the four crystal forms, wherein the four crystal forms respectively are a crystal form H1, a crystal form H2, a crystal form H3 and a crystal form H4, the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.3 DEG, 11.2 DEG, 16.0 DEG, 17.5 DEG, 18.5 DEG, 19.3 DEG, 19.7 DEG, 20.0 DEG, 20.7 DEG, 22.0 DEG and the like, the crystal form H2 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.3 DEG, 12.8 DEG, 14.0 DEG, 16.4 DEG, 18.7 DEG, 20.5 DEG, 23.5 DEG, 29.4 DEG, 33.1 DEG, 33.4 DEG and the like, the crystal form H3 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.6 DEG, 9.8 DEG, 10.7 DEG, 15.1 DEG, 15.3 DEG, 16.8 DEG, 16.9 DEG, 19.8 DEG, 20.0 DEG, 24.9 DEG and the like, and the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.6 DEG C, 9.6 DEG, 10.5 DEG, 12.9 DEG, 15.1 DEG, 17.2 DEG, 18.9 DEG, 19.9 DEG, 20.7 DEG, 23.8 DEG and the like. According to the present invention, the four crystal forms have advantages of excellent physical and chemical properties, good stability, simple preparation operation and the like, are suitable for pharmaceutical formulation applications.
Owner:CHARM PHARMATECH NANJING
Copolymer of olefin and conjugated diene, and process for producing same
Disclosed are: a copolymer having a double bond in a side chain thereof and having substantially no unsaturated bond in the main chain thereof, or a copolymer having a cyclic structure and having substantially no unsaturated bond in the main chain thereof; and a process for synthesizing the copolymer in an economically advantageous manner. The copolymer is produced by copolymerizing at least ethylene and conjugated dienes, wherein the content of constituent units derived from the conjugated dienes is 1 to 90 mol%, the content of a constituent unit derived from the 1,2-addition of a conjugated diene having a double bond in a side chain thereof is 0 to 90 mol%, the content of constituent units derived from the 1,4-addition of the conjugated dienes is 0 to 3 mol%, the content of constituent units derived from the 1,3-addition of the conjugated dienes is 0 to 3 mol%, and the total content of a constituent unit derived from the 1,2-addition of a conjugated diene having a 1,2-cyclopropane skeleton and a constituent unit derived from the 1,2-addition of a conjugated diene having a 1,2-cyclopentane skeleton is 4 to 100 mol%.
Owner:MITSUI CHEM INC
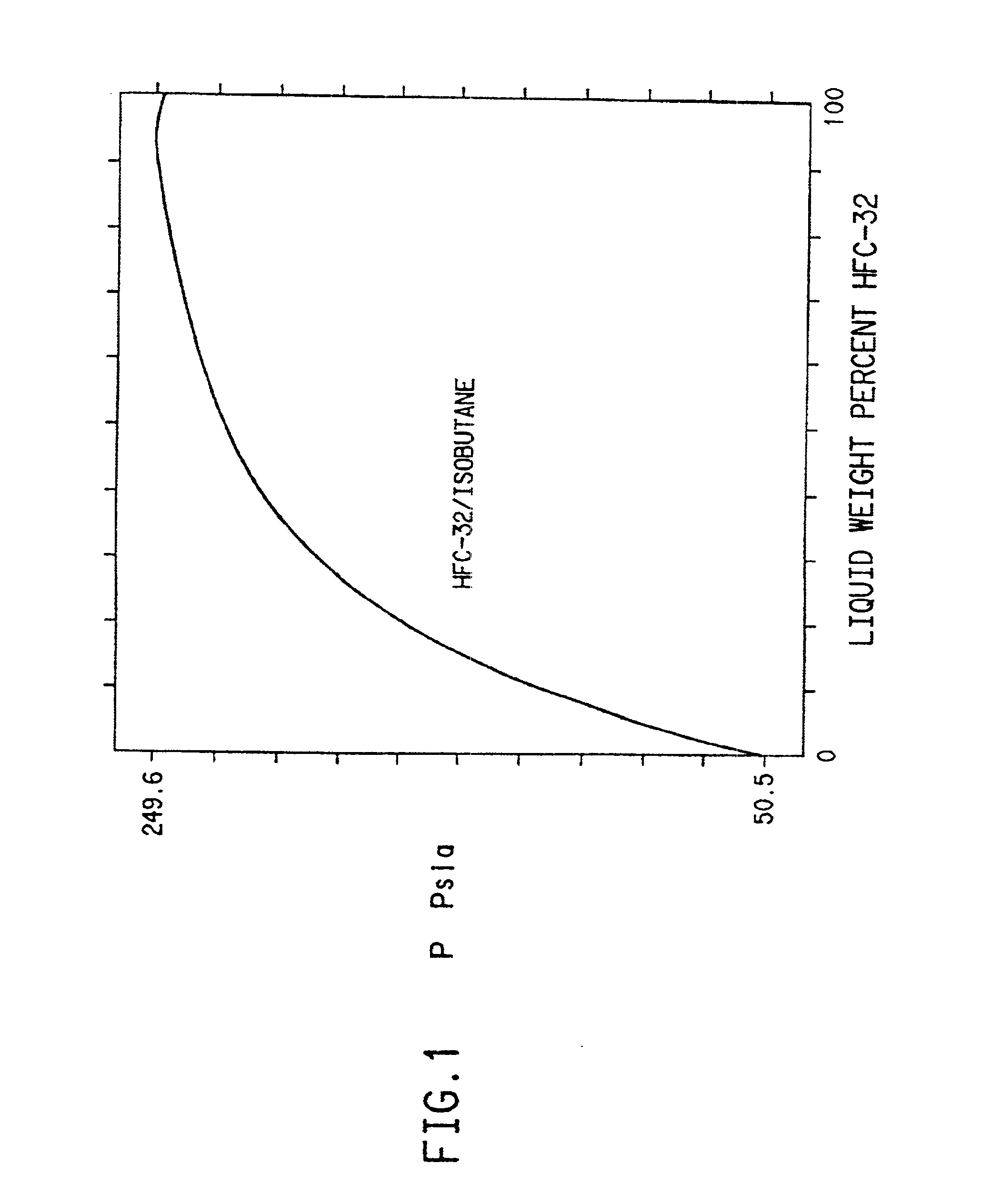
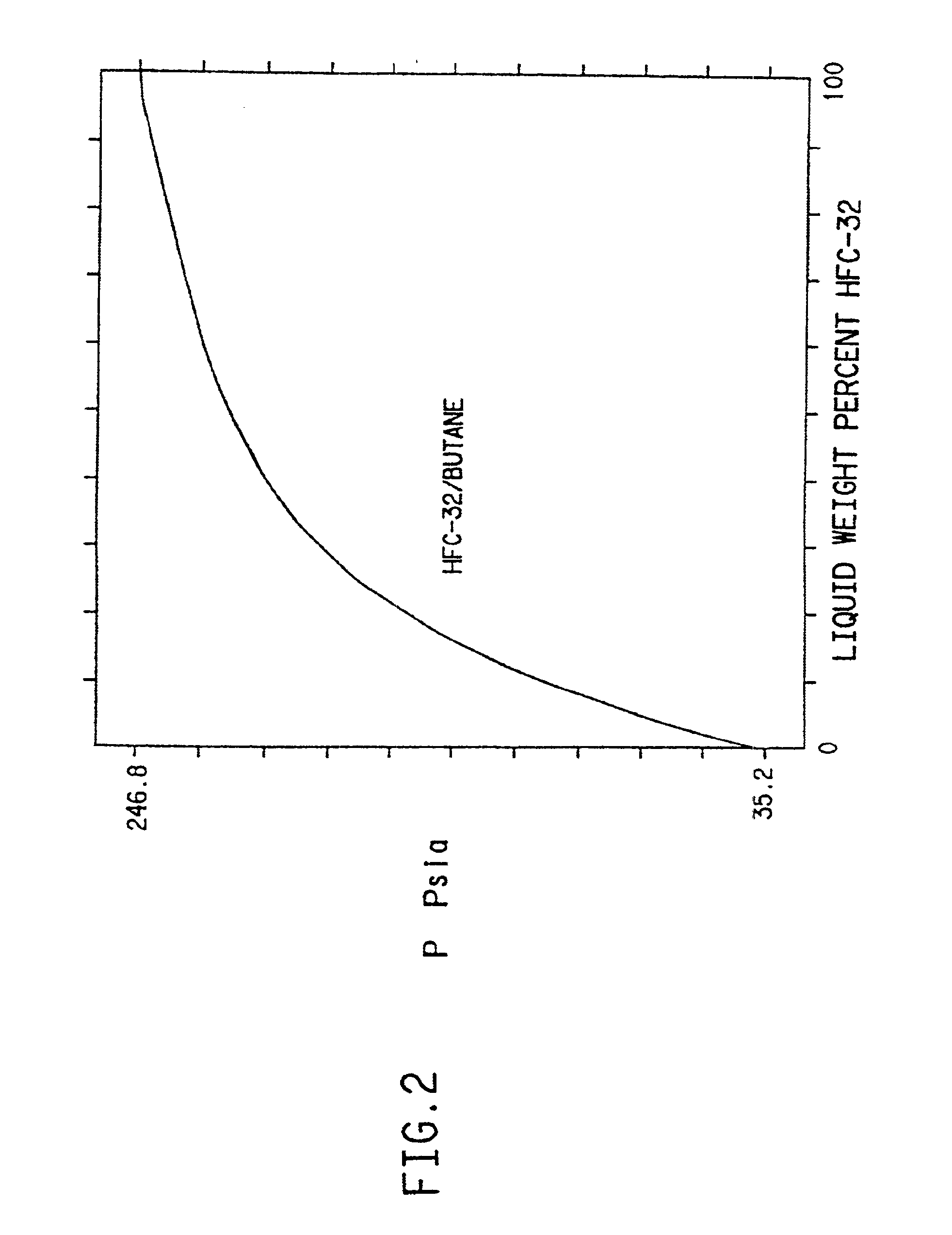
Hydrofluorocarbon compositions
Refrigerant compositions include mixtures of difluoromethane and isobutane, butane, propylene or cyclopropane; pentafluoroethane and propylene or cyclopropane; 1,1,2,2-tetrafluoroethane and propane; 1,1,1,2-tetrafluoroethane and cyclopropane; 1,1,1-trifluoroethane and DME or propylene; 1,1-difluoroethane and propane, isobutane, butane or cyclopropane; fluoroethane and propane or cyclopropane; 1,1,1,2,2,3,3-heptafluoropropane and butane, cyclopropane, DME, isobutane or propane; or 1,1,1,2,3,3,3-heptafluoropropane and butane, cyclopropane, isobutane or propane.
Owner:EI DU PONT DE NEMOURS & CO
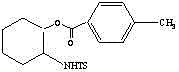
Method for performing ring-opening for cyclohexylaziridine by carboxylic acid
The invention discloses a method for performing ring-opening for cyclohexylaziridine by a carboxylic acid. The method includes that in a polar aprotic solvent system, alkali metal type inorganic base is utilized as a catalyst, a monocarboxylic acid is utilized as a nucleophilic reagent, and the cyclohexylaziridine which is activated by tosyl is subjected to a ring-opening reaction. The method has the advantages that the ring-opening reaction is simple in process, the reaction condition is mild, the solvent is environment-friendly, the carboxylic acid is utilized as the nucleophilic reagent, atom economical requirement of green chemistry is met, the catalytic agent is cheap, and the catalytic activity is high.
Owner:TAIYUAN UNIV OF TECH
Refrigerant mixture comprising difluromethane, pentafluroethane and 1,1,1-trifluoroethane
A novel refrigerant composition useful as a substitute for HCFC-22, comprising a first constituent of difluoromethane (CH2F2, HFC-32); a second constituent of pentafluoroethane (CHF2CF3, HFC-125); a third constituent of 1,1,1-trifluoroethane (CH3CF3, HFC-143a); a fourth constituent selected from the group consisting of cyclopropane (C3H6, RC-270), 1,1,1,2,3,3,3-heptafluoropropane (CF3CHFCF3, HFC-227ea), 1,1,1,2,2-pentafluoropropane (CH3CF2CF3, HFC-245cb), isobutane (CH(CH3)2CH3, R-600a), octafluorocyclobutane (C4F8, RC-318), 1,1,1,2,3,3-hexafluoropropane (CHF2CHFCF3, HFC-236ea), butane (C4H10, R-600), bis(difluoromethyl)ether (CHF2OCHF2, HFE-134) and pentafluoroethylmethylether (CF3CF2OCH3, HFE-245).
Owner:KOREA INST OF SCI & TECH
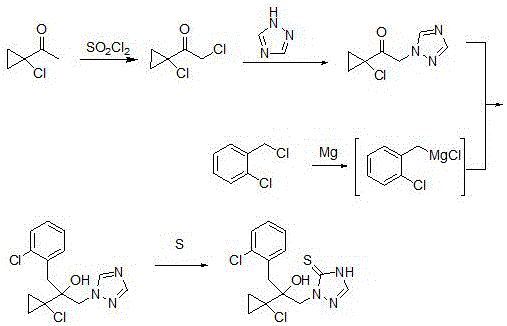
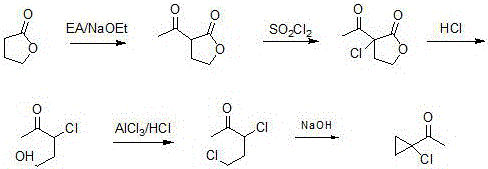
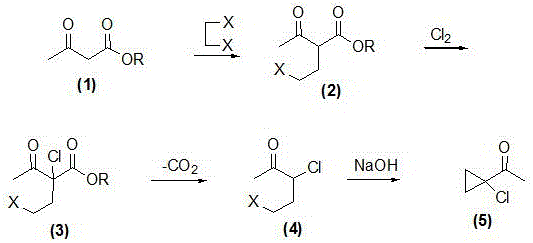
Synthetic method of prothioconazole midbody 1-chloro-1-acetyl cyclopropane
ActiveCN106278850AEasy to recycleHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationAlkyl transferQuaternary ammonium cation
The invention relates to a synthetic method of a prothioconazole midbody 1-chloro-1-acetyl cyclopropan, which relates to the technical field of chemical production. The synthetic method comprises the following steps: mixing alkyl acetoacetate and 1,2-dihaloalkane or ethylene glycol disulfonate, performing mono-alkylation reaction, after chloration reaction, mixing with a strong acid, performing hydrolysis, and obtaining 3,5-dichloropentane-2-ketone or 3-chloro-4- sulfonate pentane-2-ketone; and finally taking quaternary ammonium salt as a catalyst, mixing the 3,5-dichloropentane-2-ketone or 3-chloro-4-sulfonate pentane-2-keton and alkali liquor, performing ring closing reaction, and obtaining 1-chloro-1-acetyl cyclopropane. By adopting the synthetic method, the cost is reduced, three wastes (waste water, waste gas and waste residues) are reduced, safety and reliability are realized, and the reaction yield is high.
Owner:YANGZHOU TIANCHEN FINE CHEM
Asymmetric copper compound and cyclopropanation reaction with it
InactiveCN1384105AGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationStrong acidsCombinatorial chemistry
Owner:SUMITOMO CHEM CO LTD
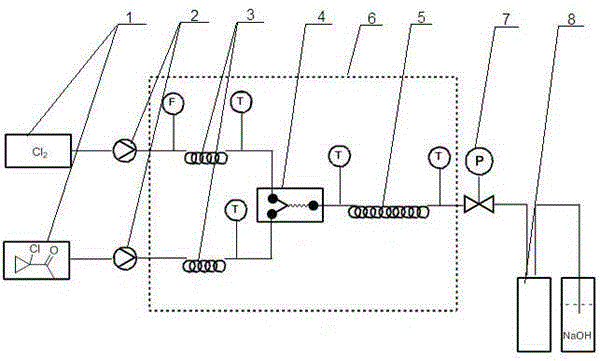
Method using micro reactor to prepare 1-chloro-1'-chloroacetyl cyclopropane
ActiveCN105384617AHigh selectivityEasy to operateOrganic compound preparationCarbonyl compound preparationWater chlorinationSolvent free
The invention discloses a method using a micro reactor to prepare 1-chloro-1'-chloroacetyl cyclopropane. The reactions can be carried out in the presence or absence of a solvent. According to the preparation method, 1-chloro-1'-acetyl cyclopropane and chlorine gas are pumped into a micro reaction channel to carry out reactions by a feed pump according to a certain ratio. The device comprises a raw material bottle, a feed pump, a pre-heating or pre-cooling pipe, a micro reactor, a time delay pipe, a temperature controlling device, a counterbalance valve, and a product receiver. A micro reactor, which can quickly mix the raw materials and has a good heat exchange effect, is used as the reaction device; so that 1-chloro-1'-acetyl cyclopropane and chlorine gas can carry out high selective(alpha-position hydrogen atom) chlorination reactions in a micro channel under high speed and efficient mixing to generate 1-chloro-1'-chloroacetyl cyclopropane. The provided method has the advantages of simple operation, mild conditions, high selectivity, and low energy consumption; compared with the conventional reactions, the using amount of solvent is reduced, and moreover, the method is environment-friendly and can be easily applied to industrialization.
Owner:大连科铎环境科技有限公司
Carboxylic acid compound having cyclopropane ring
A carboxylic acid compound having cyclopropane ring(s) of the formula (I): wherein R is alkyl or alkenyl optionally having one or more 1,2-cyclopropylene in a carbon chain and / or optionally having cyclopropyl at the end of a chain,X is a single bond or alkylene, wherein the total number of carbon less the number of cyclopropane ring is 10-25, and a pharmaceutically acceptable salt thereof are provided. The compound (I) shows an LTP-like potentiation of synaptic transmission, allows slow metabolism in the living body, show a stable LTP-like potentiation of synaptic transmission, and is useful as an agent for LTP-like potentiation of synaptic transmission, a cognition-enhancing drug or an agent for the prophylaxis and treatment of dementia, a learning and memory disorder and a neurotransmitter release disorder.
Owner:NISHIZAKI BIOINFORMATION RES INST +1
New synthetic method of Caronic anhydride
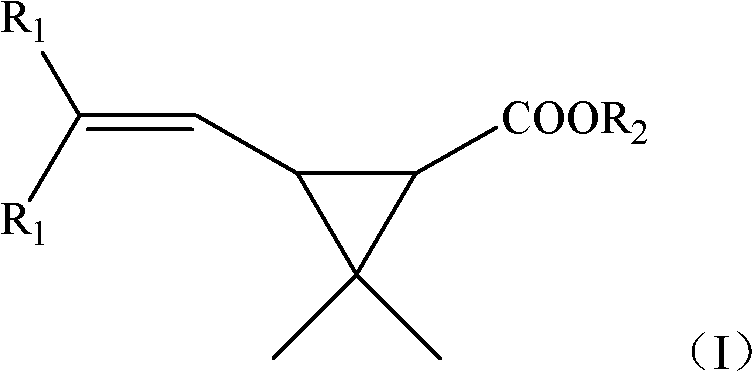
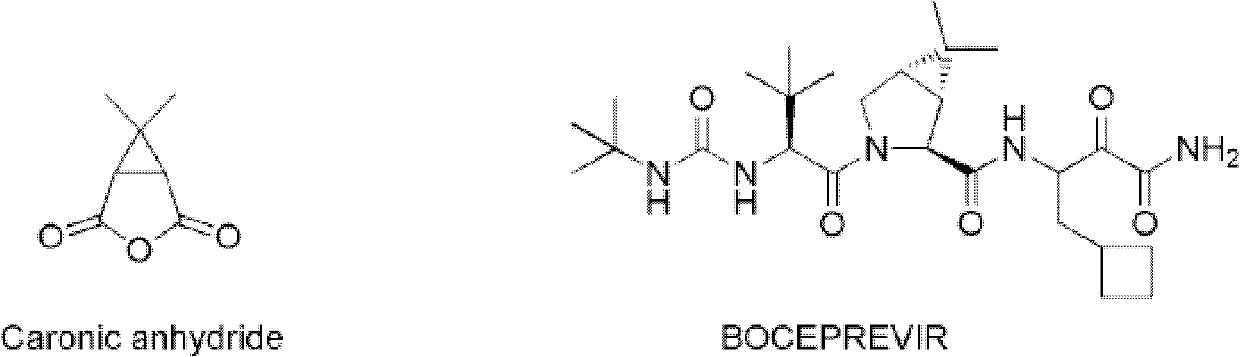
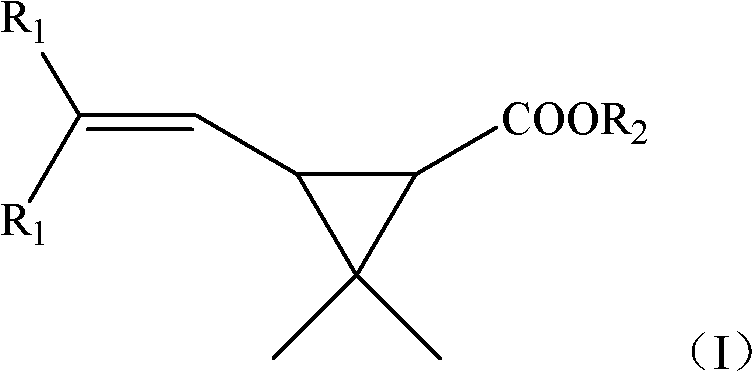
The invention provides a new synthetic method of 6,6-dimethyl-3-oxiabicyclo[3.1.0]hexane-2,4-dione (Caronic anhydride). According to the method, chrysanthemum monocarboxylate which has a structure represented by formula (I) is oxidized with a clean ozonization technology, and the obtained oxidation product is saponified and acidified to generate 3,3-dimethyl-1,2-cyclopropanedicarboxylic acid, R1 in the formula (I) is methyl or chlorine, and R2 in the formula (I) is methyl or ethyl; and 3,3-dimethyl-1,2-cyclopropanedicarboxylic acid is subjected to a dehydration ring-closure reaction in a system of acetic anhydride and sodium acetate to generate the Caronic anhydride. Compared with the prior art, the new method of the invention, which allows no waste acids to be generated, is a green production technology.
Owner:JIANGSU YANGNONG CHEM +1
Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound
ActiveCN106432052AHigh yieldRaw materials are easy to obtainOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsRare earth
The invention discloses a method for catalytically preparing a spiral [cyclopropane-1,3'- indole] compound. According to the method, silicon amino rare earth compounds of [(Me3Si)2N]3Ln(mu-Cl)Li(THF)3 is used as a catalyst for catalytically replacing isatin, phosphite ester and olefin to prepare a product through one-pot reaction; in the catalyst, (Me3Si)2N represents trimethyl silicon amino; Ln represents positive trivalent rare earth metal irons and are one kind of metal irons selected from lanthanum, samarium, gadolinium, erbium or ytterbium; mu- represents a bridge key; THF represents tetrahydrofuran. In the method, the synthesis method of the catalyst is simple; the reaction raw materials are simple and can be easily obtained; the application range of a substrate is wide; the efficiency of the one-pot reaction method is high; the reaction conditions are mild; the yield of most target products can reach 85 percent or higher.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00001.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00002.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00003.png)
![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00001.png)
![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00002.png)
![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00003.png)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00001.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00002.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00003.PNG)
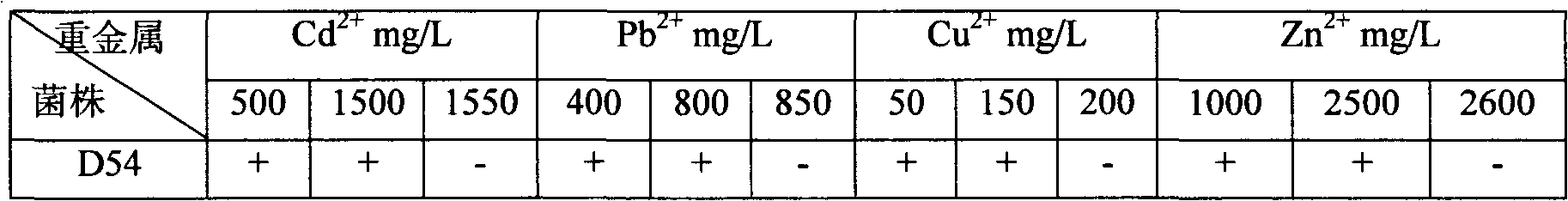

![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00002.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00003.png)
![Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/94937a71-e336-4b0a-abab-97ae6daa1636/US20130143881A1-20130606-D00001.png)
![Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/94937a71-e336-4b0a-abab-97ae6daa1636/US20130143881A1-20130606-D00002.png)
![Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Hydrated Crystalline Forms of N-[3-fluoro-4-(oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/94937a71-e336-4b0a-abab-97ae6daa1636/US20130143881A1-20130606-D00003.png)
![Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bbe067df-df92-48ad-b244-2fdb9128d04d/US08673912-20140318-D00001.png)
![Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bbe067df-df92-48ad-b244-2fdb9128d04d/US08673912-20140318-D00002.png)
![Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Crystalline Forms on N-[3-fluoro-4-({6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]-quinolin-4-yl}oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bbe067df-df92-48ad-b244-2fdb9128d04d/US08673912-20140318-D00003.png)






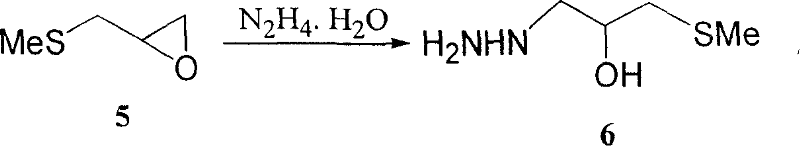






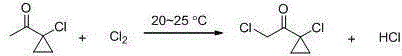
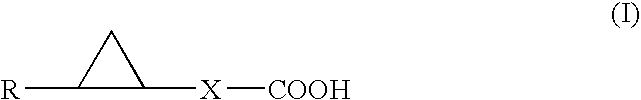
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/100537DEST_PATH_IMAGE014.png)
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/102811DEST_PATH_IMAGE021.png)
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/118281DEST_PATH_IMAGE005.png)