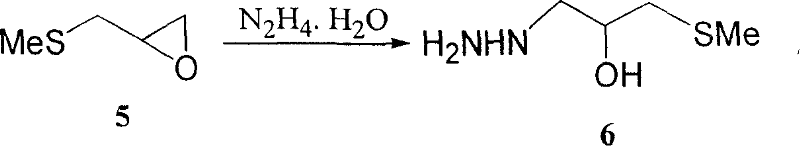Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Nifuratel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
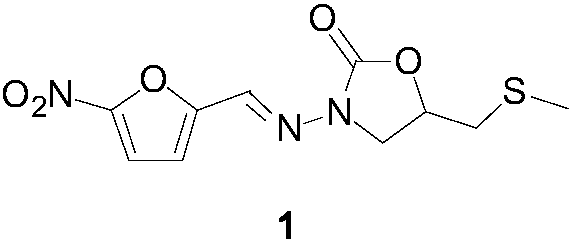
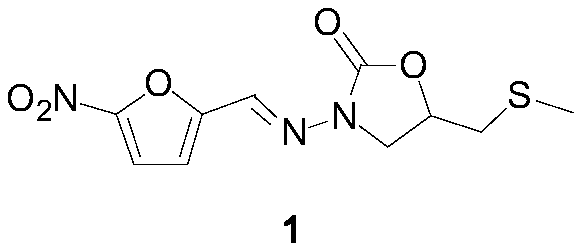
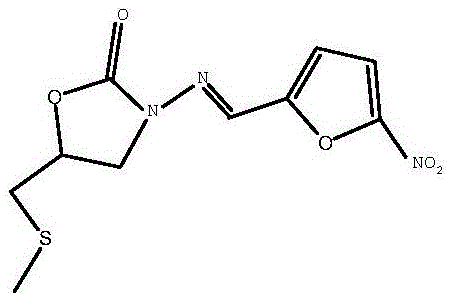
Nifuratel (brand name Macmiror, or — in combination with nystatin, — Macmiror Complex) is a drug used in gynecology. It is a local antiprotozoal and antifungal agent that may also be given orally. Nifuratel is not approved for use in the United States.
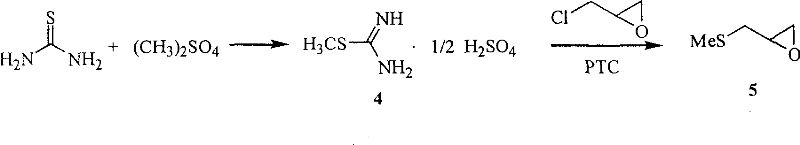
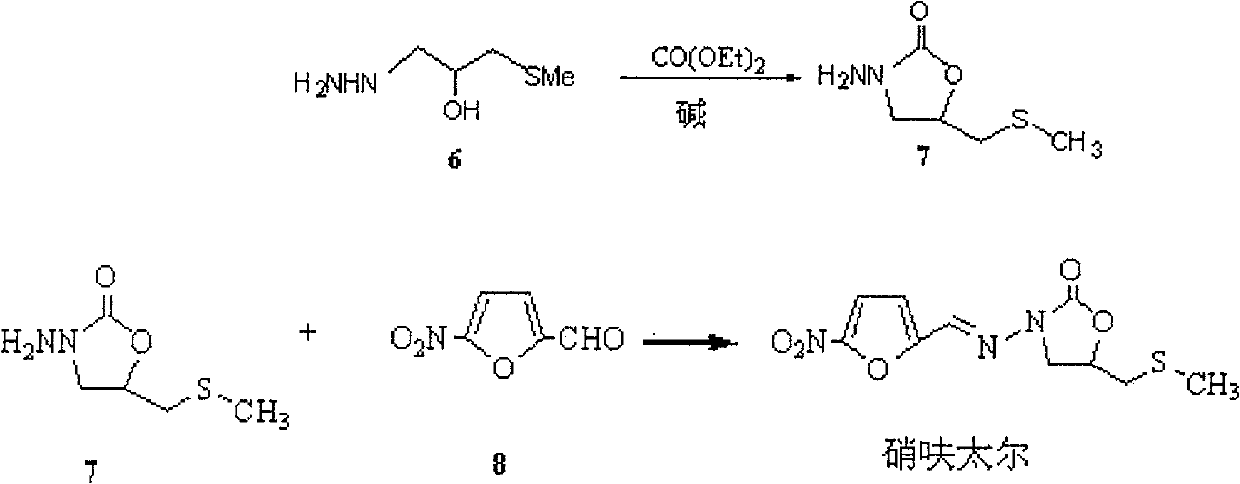
Production method of nifuratel
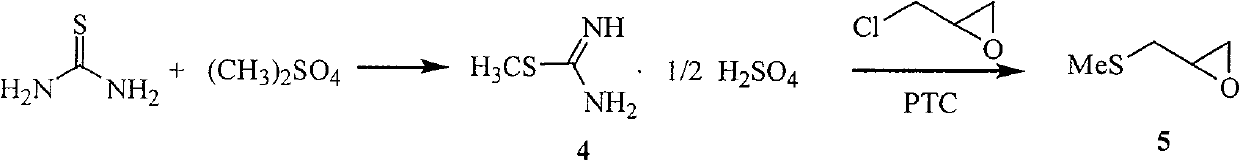
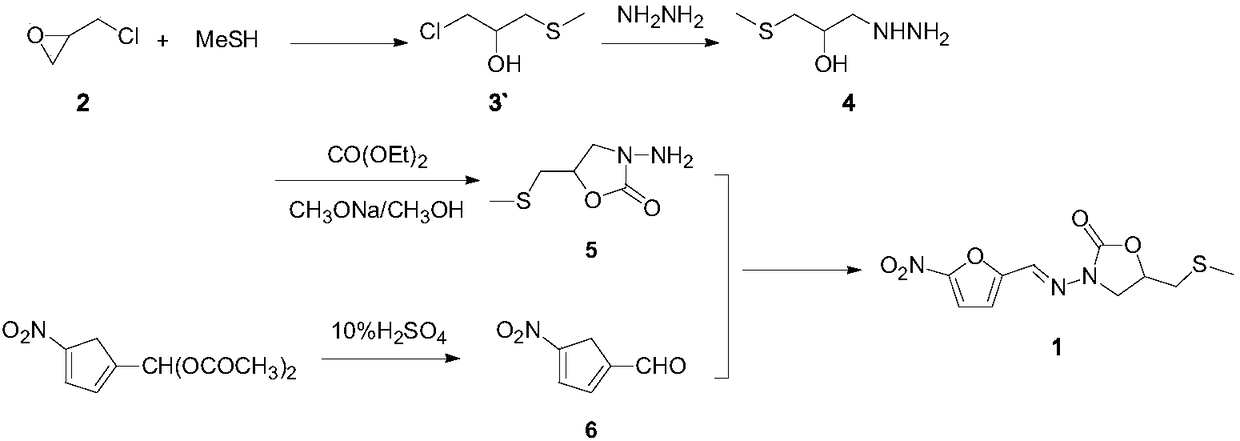
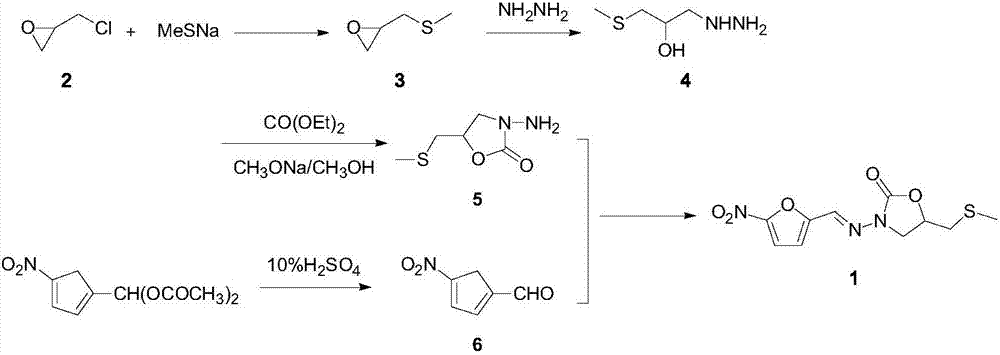
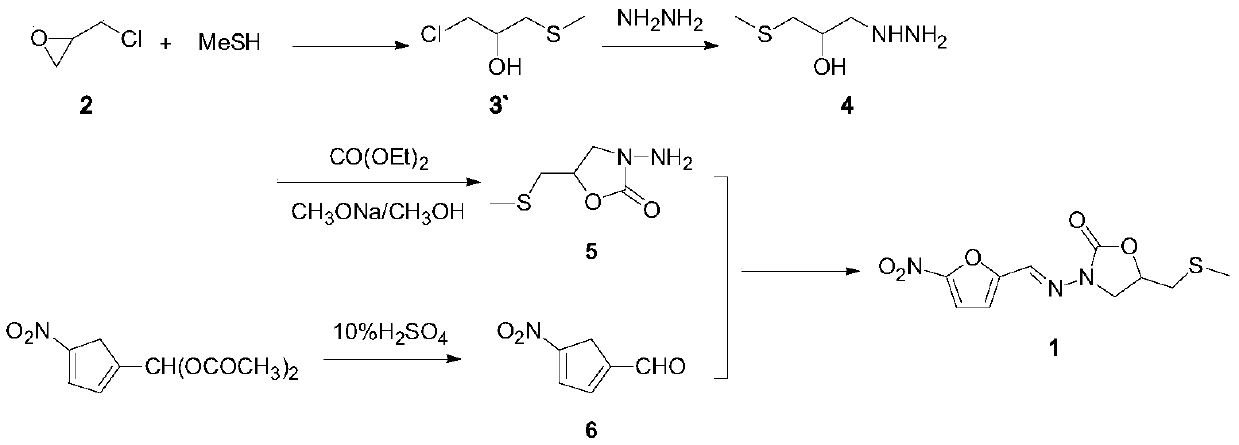
The invention relates to a medicine producing field, specifically, to an improved producing method of antibacterial medicine nifuratel. The method makes use of thiourea as initial material to produce nifuratel. The improvement is that it is no longer to use methyl mercaptan or methomyl in the known method while producing the intermediate 2-(methylthiomethyl)hydropropane. Furthermore, the invention is no longer to use metal natrium in the known method when the 3-methylthio-2-hydroxy-propylhydrazine and diethyl carbonate are heated to produce N-amido-5-methylthiomethyl-2-oxazolidone in the existance of alkali. All of the improvements largely improve the preparation of the nifuratel, especially for the production condition in a large scale, reduce the environment pollution and benefit to ensure the production safety.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
Nifuratel-nystatin vaginal gel and method for preparing same
InactiveCN1927215AGood dispersionFully contactedOrganic active ingredientsAntimycoticsGel basedMedicine
The invention discloses a vaginal gel which is prepared from the following raw materials: Nifuratel and Nysfungin 300-600 parts, glycerin 200-400 parts, gel base material 100-500 parts. The preparing process consists of steps of abrading homogeneously, charging charging base materials into turbid liquor and stirring homogeneously, and adjusting pH to be 6-7.
Owner:张彤丽
Nifuratel-nysfungin vaginal expandable suppository and its preparation method and detection method
ActiveCN103536612AGuaranteed effective concentrationPrevent outflowAntibacterial agentsOrganic active ingredientsSecondary InfectionsStearate
The invention relates to a nifuratel-nysfungin vaginal expandable suppository and its preparation method and detection method. The nifuratel-nysfungin vaginal expandable suppository comprises a drug-containing matrix comprising nifuratel, nysfungin and a matrix, and also comprises an expandable carrier. Through use of colloidal silicon dioxide in the drug-containing matrix, nysfungin hygroscopicity is reduced and suppository stability is improved. Nifuratel, nysfungin, povidone and hydroxypropyl methylcellulose form a skeleton clathrate so that nysfungin and nifuratel stability are further improved and the time of action produced by the active components on the vagina is further prolonged. Propylene glycol monostearate and Tween 80 can promote absorption of the active components by the vagina and suppository bioavailability is improved. The nifuratel-nysfungin vaginal expandable suppository utilizes seven novel lead-edge technologies and has the advantages of drug liquid leakage prevention, high stability, lasting curative effects and secondary infection prevention.
Owner:哈尔滨田美药业股份有限公司
Method for preparing nifuratel
InactiveCN103232445AAvoid the problem of unstable responseHigh yieldOrganic chemistryDistillationMedicine
The invention belongs to the field of drug synthesis and provides a novel method for synthetizing nifuratel. The synthesis method of the nifuratel disclosed by the invention innovatively adopts a post-treatment method of 'ethanol with water' to obtain a midbody 3-methyl mercapto-2-hydroxy propyl hydrazine (A1); high-vacuum reduced pressure distillation of the midbody 3-methyl mercapto-2-hydroxy propyl hydrazine (A1) is avoided; the nifuratel is prepared by a 'one-pot' method; preparation of a midbody N-amino-5-methylthiomethyl-2-oxazolidinone (A2) and post-treatment operation of 5-nitro-furfural (B1) are simplified; the purity of the obtained nifuratel product is higher than 99.8%; and the yield is higher than 30%. The synthesis method of nifuratel disclosed by the invention is simple, convenient and easy to control; the post-treatment operation is simple and feasible; industrialized production is facilitated; the used material is available in China; the pollution on the environment is small; and the material is cheap and easily available.
Owner:NAT INST OF PHARMA R & D CO LTD
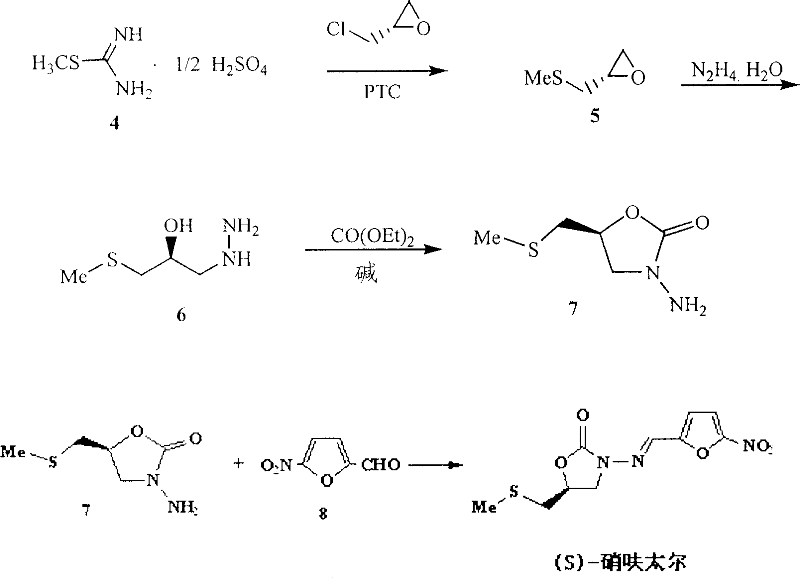
(S)-nifuratel, preparation method and application thereof
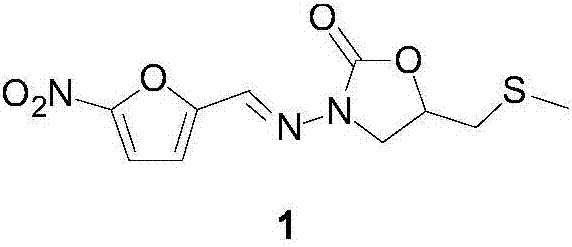
The invention discloses a nifuratel with a optical activity. Specifically, the invention provides a (S)-nifuratel, i.e. (S)-5-[(methylthio)methyl]-3-[(5-nitryl-2-furan)methylene]amido]-2-oxazolidone, the producing method and the combination including the optical activity compound. Compared to the racemic nifuratel, (S)-nifuratel has a better anti-inflammatory and anti-fungi activity. The invention also discloses the producing method for (S)-nifuratel which has the (S)-chloroepoxy propane as initial material, adopts the 3-dimension and selective synthesis method and produce a high optical purity (S)-nifuratel with a high yield.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
Preparation of nifuratel and nysfungin vaginal soft capsules
InactiveCN101332192AIncrease the effective concentrationMaintain physiological environmentOrganic active ingredientsPill deliveryBacteroidesDisease
The present invention relates to preparation of a new medicine Nifuratel nysfungin vagina tablet for gynecologic antisepsis and anti-inflammation. Nifuratel nysfungin can play a more complete role in curing mixed vagina infection (candida, trichomonas and bacteria) and pathogen which can not be or can not be timely and clearly diagnosed and preventing mildew secondary infection and recurrence after the treatment of other medicines. The present invention can be used for curing bacterial vaginopathy, trichomonas vaginitis, monilial vulvovaginitis, and vagina mixed infection. The advantage of the present invention lies in that the added pH buffer system ensures pH of the medicine is within 3.5 to 4.0 close to the physiological pH of vagina, thus effectively protecting the physiological environment of vagina, providing the appropriate living conditions beneficial to flora in vagina and promoting the recovery of diseases.
Owner:李国栋
Nifuratel nysfungin vaginal soft capsule and preparation process thereof
InactiveCN104434942ARestore balanceImprove stabilityAntibacterial agentsOrganic active ingredientsPlasticizerMedical prescription
The invention discloses a nifuratel nysfungin vaginal soft capsule and a preparation process thereof. The nifuratel nysfungin vaginal soft capsule comprises nifuratel, nysfungin, matrix and a moisturizer, wherein a soft capsule shell comprises gelatin, a plasticizer, water, a bacteriostatic agent, a pigment and an opaquing agent; lactobacillus is effectively protected when bacteria, mycete, trichomonad and the like are killed; restoration of balance of vaginal beneficial flora is facilitated; and through improvement of the prescription and the prepration process, the medicine stability and the medicinal effectiveness are greatly improved.
Owner:JIANGSU YUEXING PHARMA
Preparation method of nifuratel with high purity
InactiveCN101676284AGood lookingHigh purityAntibacterial agentsOrganic chemistryMethyl groupNifuratel
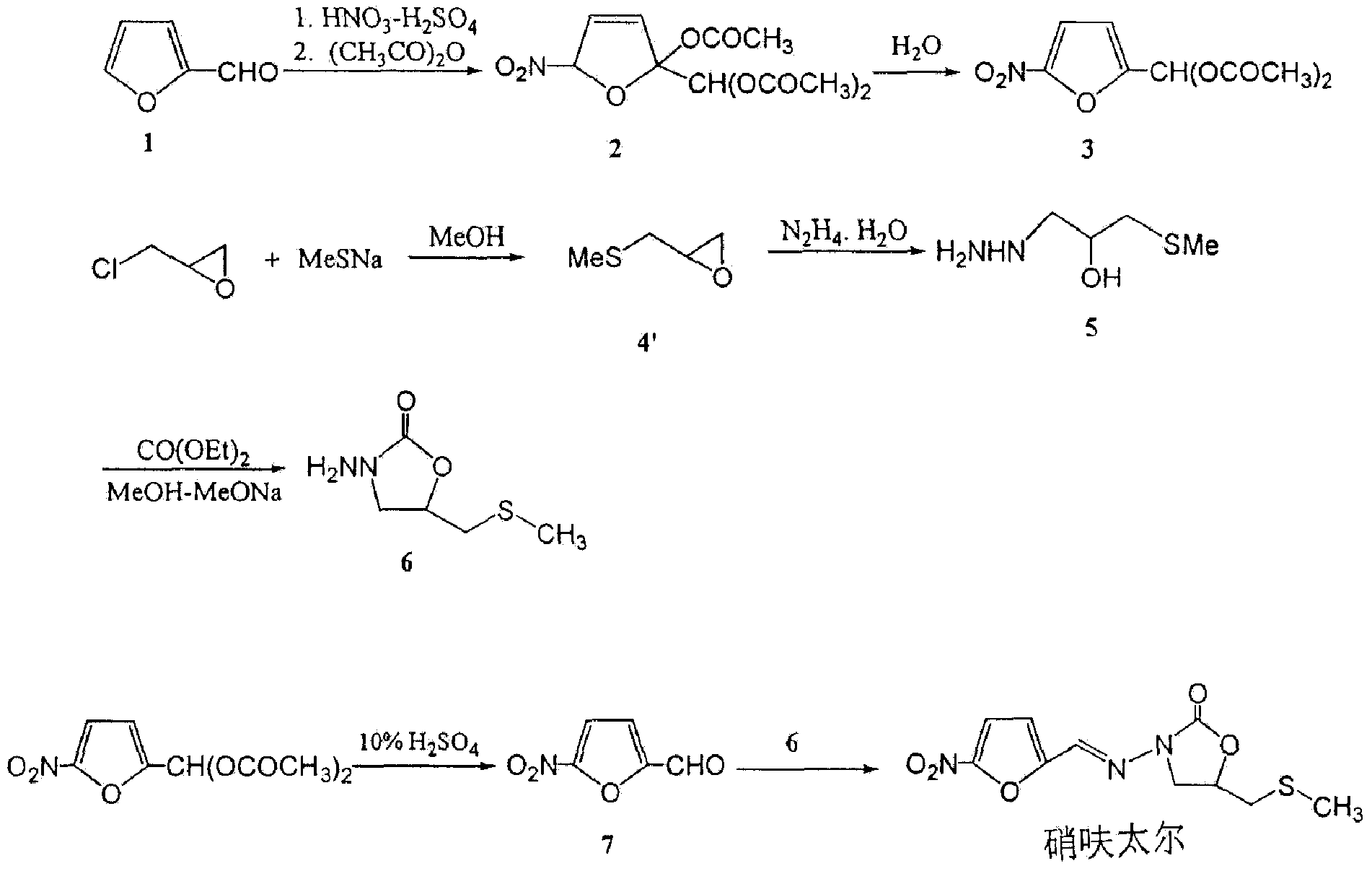
The invention discloses a preparation process of nifuratel, which is suitable for industrialization, and a purifying method, including: taking 3-ammo-5-[(methylthio)methyl]-2-oxadixyl and 5-nitrofurfuraldiacetate as the initial raw materials, acid-hydrolyzing and docking to generate nifuratel, post-processing and recrystallizating to obtain a highly finished product. The highly finished product obtained by the method has high purity, the operating method is simple, the production cost is low, the yield is high, the preparation process is more suitable for industrialization, and the preparationprocess has the competition in the same processes.
Owner:BEIJING D VENTUREPHARM TECH DEV
Nifuratel-nysfungin gel and preparation method thereof
ActiveCN102579473AFully contactedMaintain physiological environmentAntibacterial agentsOrganic active ingredientsDiseaseAdditive ingredient
The invention relates to a nifuratel-nysfungin gel and a preparation method of the nifuratel-nysfungin gel. The gel is prepared by nifuratel, nysfungin, hydrogel matrix and other auxiliary materials. According to the invention, the pharmaceutical ingredients are fully dissolved and evenly mixed in the gel matrix, and the pharmaceutical ingredients are fully contacted with the vaginal wall, so that the bacteriostasis of the medicine is fully exerted, and the nifuratel-nysfungin gel is safe and comfortable to use without foreign body sensation and greasy feeling. The nifuratel-nysfungin gel canbe used for treating vulva and vaginal infections and vaginal mixed infection caused by bacteria, trichomonas and candida, thus effectively maintaining the physiological environment of the vagina, providing the survival condition suitable for vaginal beneficial bacterium group and promoting disease recovery.
Owner:程雪翔
Preparation method for nifuratel
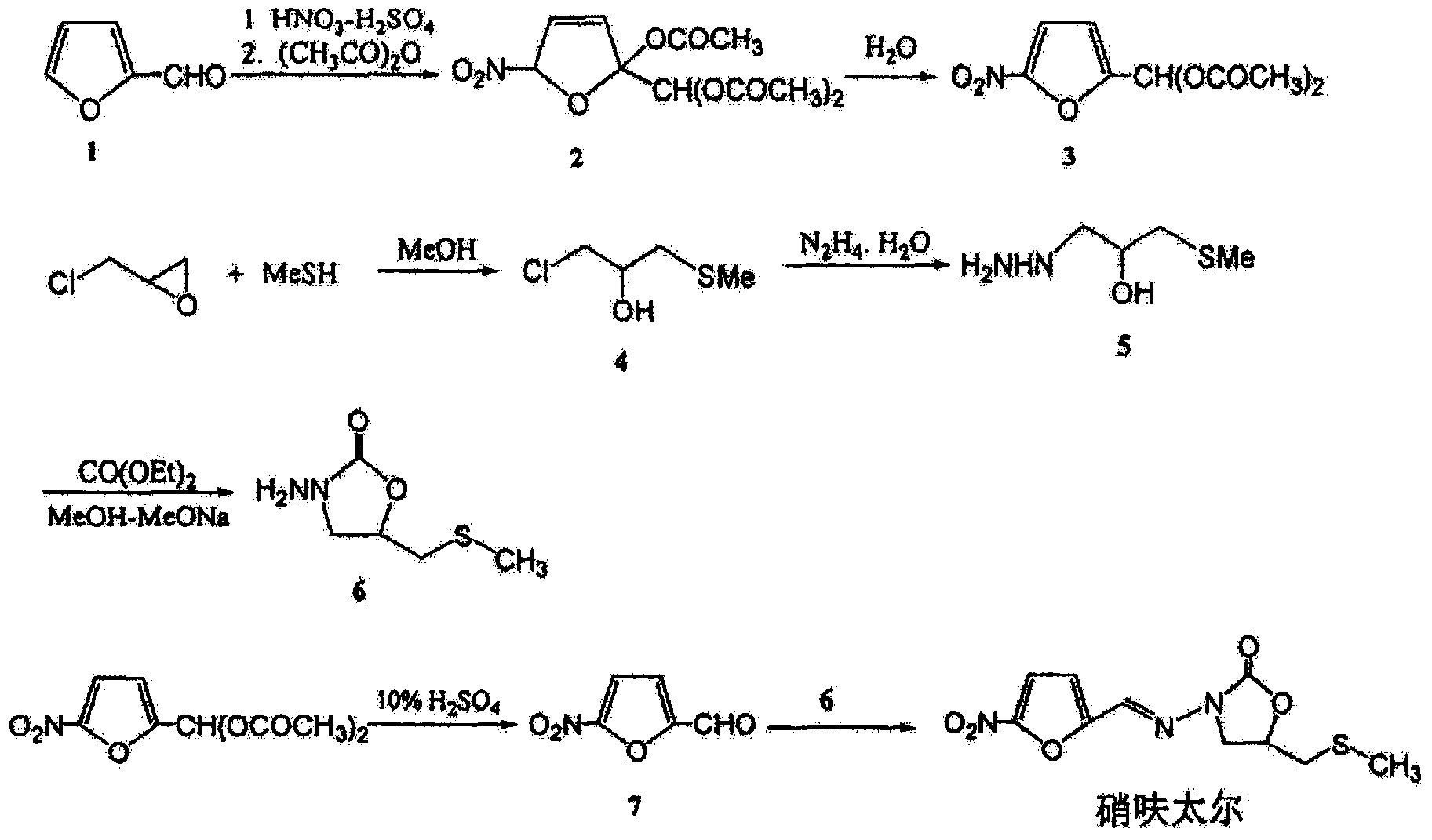
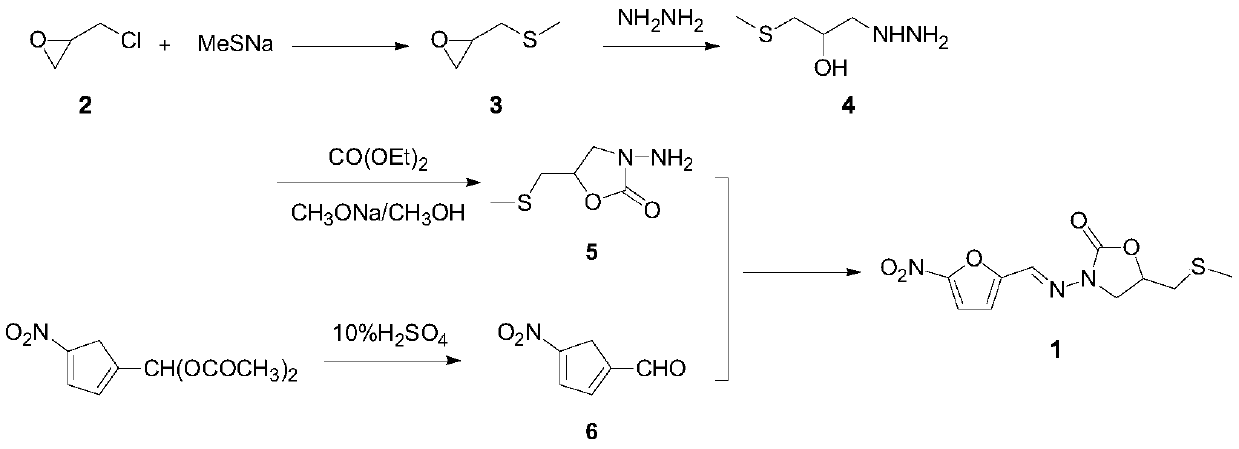
The invention relates to a method for preparing nifuratel. The method comprises the steps of (1) synthesizing 2-(methylmercapto-methyl)-oxacyclopropane through epoxy chloropropane and sodium methyl mercaptide; (2) generating reaction between hydrazine hydrate and the 2-(methylmercapto-methyl)-oxacyclopropane to synthesize 3-methylmercapto-2-hydroxy-propyl hydrazine; (3) generating reaction between diethyl carbonate and 3-methylmercapto-2-hydroxy-propyl hydrazine to prepare N-amino-5-methylmercapto-methyl-2-oxazolidinone; (4) hydrolyzing 5-nitrofuran formaldehyde diacetate ester under an acidic condition to prepare 5-nitro-2-furaldehyde; (5) generating reaction between the 5-nitro-2-furaldehyde and the N-amino-5-methylmercapto-methyl-2-oxazolidinone obtained in the step (3) to obtain the nifuratel, wherein in the step (1), 15-crown ether-5 is used as a catalyst, so that the conversion rate is high; no organic solvent is used during posttreatment of a product, and the posttreatment is simple.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Nifuratel-nystatin soft capsule-type suppository and preparation method thereof
InactiveCN108853003AImprove stabilityImprove sterilityAntibacterial agentsOrganic active ingredientsSterile environmentNifuratel
The invention relates to a nifuratel-nystatin soft capsule-type suppository and a preparation method thereof. Nifuratel-nystatin sol in the nifuratel-nystatin soft capsule-type suppository is preparedfrom the following components in parts by weight: 40-60 parts of nifuratel, 3-5 parts of nystatin, 2-3 parts of carbomer, 1-2 parts of ethylparaben, 50-70 parts of glycerinum, 70-100 parts of ethyl alcohol, 20-30 parts of pH buffer liquid and 700-800 parts of medical pure water. The preparation method comprises the following steps of 1, raw material preparation; 2, liquid medicine preparation, wherein the nifuratel and the nystatin are mixed evenly in a sterile environment, then through a sterile filtering system, sterile filtering is conducted, and then micro-pore filtering is conducted, sothat a liquid medicine is obtained for later use; 3, gel matrix preparation; 4, nifuratel-nystatin sol preparation; 5, capsule pressing; 6, soft capsule-type suppository preparation. The nifuratel-nystatin soft capsule-type suppository and the preparation method thereof have the advantages that body hormone secretion is not affected, the sterilization effect is good, and the preparation method issimple.
Owner:NANJING NANDA PHARMA
Production method of nifuratel
ActiveCN100516063CEasy to getHigh yieldAntibacterial agentsOrganic chemistryThioureaHydrazine compound
The invention relates to the field of medicine production, in particular, the invention discloses an improved production method of antibiotic nifuratel. The method uses thiourea as a starting material for the preparation of nifuratel, which is improved in that the intermediate 2-(methylthiomethyl)-oxirane is no longer used in the known method. Methyl mercaptan or sodium methyl mercaptide; In addition, 3-methylthio-2-hydroxyl-propylhydrazine and diethyl carbonate are heated to react in the presence of a base for ring closure to generate N-amino-5-methylthio In the technology of methyl-2-oxazolidinone, the production method of the present invention no longer uses metal sodium commonly used in known methods. All these improvements have greatly improved the preparation of nifuratel, especially the production conditions for large-scale industrial production, reduced environmental pollution, and are conducive to ensuring safe production.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
Synthetic method of nifuratel
The invention discloses a synthetic method of nifuratel. The synthetic method comprises the steps of carrying out a substitution reaction between epoxy chloropropane and sodium methyl mercaptide in the presence of a phase transfer catalyst to obtain epoxy propyl dimethyl sulfide, and then performing hydrazinolysis, cyclization and condensation on the obtained epoxy propyl dimethyl sulfide to obtain the nifuratel. The synthetic method is high in nifuratel yield, high in purity and low in impurity content; besides the method has the advantages that a ring-closure reaction is carried out under the alkaline condition of sodium methoxide, the use of metal sodium is avoided, production safety is ensured, and simultaneously, the reaction is easy to arouse, easy to control in process, the used raw materials are easy to get, basically no waste liquid is generated in the reaction of each step, and therefore, industrial pollution is greatly reduced; and as a result, the synthetic method of nifuratel is applicable to industrial production.
Owner:BEIJING CHENGYI INVESTMENT CO LTD
Nifuratel-nysfungin soft capsule suppository and preparation method thereof
ActiveCN103599120ARestore balanceTo achieve the purpose of "ecological treatment"Antibacterial agentsOrganic active ingredientsSuppositoryBiomedical engineering
The invention discloses a nifuratel-nysfungin soft capsule suppository. The nifuratel-nysfungin soft capsule suppository comprises 500mg of nifuratel, 200000IU of nysfungin, 8mg of promestriene, 25mg of carbomer, 700mg of glycerinum, 6mg of ethylparaben, 294mg of triethanolamine, 800mg of ethyl alcohol and 7.5g of water. The invention also discloses a preparation method of the nifuratel-nysfungin soft capsule suppository. The nifuratel-nysfungin soft capsule suppository can kill bacteria, mould and trichomonad, effectively protect lactobacillus and promote vaginal flora to restore balance, thus realizing the purpose of 'ecological treatment'; and the soft capsule suppository has both the dosage form characteristic of soft capsule (good stability of capsule core, capsule skin and drug) and local drug delivery characteristic of suppository.
Owner:四川摩尔生物制药有限公司
Nifuratel compound tablet and preparation method thereof
ActiveCN102885793AReduce frictionReduce stickinessOrganic active ingredientsAntisepticsPharmaceutical formulationNifuratel
The invention belongs to the field of medical preparations and discloses a nifuratel compound tablet and a preparation method thereof. The nifuratel compound tablet disclosed by the invention has the following advantages of high disintegration speed and dissolving rate, excellent stability, exact dosage, convenience to take and carry, easily-obtained raw materials and low cost. The preparation method of the nifuratel compound tablet is simple in operation, easy to control, time-saving, labor-saving and beneficial for large-scale industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Nifuratel gel and process for preparing same
InactiveCN101199474AGood treatment effectEasy to acceptAntibacterial agentsOrganic active ingredientsMedicineCurative effect
The invention provides a nifuratel gel and the related preparation method. The invention is made of nifuratel as the raw material, a gel substrate carbomer and other relative auxiliary materials and used to cure vulvar and vaginal infections and vaginal mixed infections caused by germs, trichomonads and candidas. The invention has the advantages of obvious curative effect, relative stable quality, and is convenient to carry and take, and is safe and sanitary. The invention is comprehensively improved in quality, and enriches the medication varieties after the formulation is changed, thus the invention has great significance for further meeting and guaranteeing the medication requirements of people.
Owner:程雪翔
Method for inspecting microbial limit of nifuratel and nysfungin vaginal soft capsules
InactiveCN108315384AAvoid false negativesImprove medication safetyMicrobiological testing/measurementMicroorganismYeast
The invention belongs to the technical field of drug detection, and particularly relates to a method for inspecting microbial limit of nifuratel and nysfungin vaginal soft capsules. The method comprises the following steps: preparing bacterial liquid; preparing a test solution; measuring a total quantity of aerobic bacteria, molds and yeasts; determining that a ratio of a value of the quantity ofbacteria in a test group minus the quantity of bacteria in a test sample group to the quantity of bacteria in a bacterial liquid group is qualified in a range of 0.5-2; inspecting control bacteria. The sample treatment adopted in the invention can better eliminate nifuratel, reduces bacteriostasis, has simple operation process and low pollution probability, prevents samples from false negative during inspection, and improves medication safety of patients.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Nifuratel-nystatin vaginal effervescent-tablets
InactiveCN1927213AEasy to storeNot easy to failOrganic active ingredientsAntimycoticsEffervescent tabletMedicine
The invention relates to a nifungin-canstat vagina effervescent tablet. Wherein, it uses nifungin and canstat, with acid-alkali adding system, and stuff or directly being compressed into tablet or particles then to be compressed into tablet, or uses macrogol to cover the alkali, then to be compressed into tablet. The invention uses the acid alkali system which will generate carbon dioxide gas when meeting water as slaking agent; therefore, the tablet will foam and slake when adsorbs water in vagina, to disperse the elements into vagina completely, as uterine cervix, etc.
Owner:张彤丽
Nifuratel preparation method
ActiveCN108084174AAvoid vacuum distillationEasy to useOrganic chemistryTert-butyl carbazateTrifluoroacetic acid
The invention belongs to the technical field of synthesis of medicines and particularly relates to a nifuratel preparation method. The nifuratel preparation method comprises the following steps: taking epoxy propyl dimethyl sulfide as the starting material, having a ring-opening reaction with tert-butyl carbazate to obtain N'-(2-hydroxyl-3-methylmercapto-propyl)-tert-butyl carbazate; having a ringclosing reaction with urea under the catalytic action of cuprous bromide, obtaining a key intermediate N-(Boc-amino)-5-methylmercapto-methyl-2-oxazolidinone; hydrolyzing 5-nitro furfural diacetate under the action of trifluoroacetic acid to obtain 5-nitrofurfural, condensing with N-(Boc-amino)-5-methylmercapto-methyl-2-oxazolidinone, and obtaining nifuratel. In the process route, the cheap easy-to-get agents are chose, the operation difficulty and the processing burden caused after the reaction are reduced, the environmental harm is reduced, the production safety is guaranteed, and the preparation method is an easy, green and economical process route for the preparation of the nifuratel.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Method for controlling impurities of nifuratel vaginal tablet
InactiveCN109839456AResolve interferenceSolve the separation problemComponent separationPotassium hydroxideGradient elution
The invention relates to the technical field of drug analysis, and in particular relates to a method for controlling impurities of a nifuratel vaginal tablet. The method is a gradient elution method with a detection wavelength of 260 nm. The mobile phase is phase A: a 9 to 11 mM KH2PO4 solution, and a potassium hydroxide aqueous solution is used to adjust pH. The volume ratio of phase B: acetonitrile: methanol is 18:82 to 22:78. The method provided by the invention can solve the problems of excipient interference and impurity separation at the same time, and can comprehensively and effectivelydetect the content of each impurity. An effective method is provided for impurity control of nifuratel in preparation. The method provides a basis for the formulation of preparation quality standards.
Owner:ANHUI PIOM PHARMA
Bacteriostatic tablet having vagina tightening effects
PendingCN111789937AFully nourishedEnergeticOrganic active ingredientsPeptide/protein ingredientsPharmacologyNifuratel
The invention relates to a vaginal repair technology, and specifically relates to a bacteriostatic tablet having vagina tightening effects. The bacteriostatic tablet consists of, by weight percentage,10-15% of AFGF freeze-dried powder, 10-15% of BFGF freeze-dried powder, 10-15% of diguanosine tetraphosphate, 5-10% of nifuratel, 5-15% of an adhesive, 30-50% of a filling agent, 2-10% of a disintegrating agent and 2-10% of a lubricating agent. The advantages of the bacteriostatic tablet are as follows: required nutrients can be provided; cell tissue of vagina can be fully nourished to keep the vagina lively; female vaginal mucosa and vagina can be repaired, inflammation diminishing, sterilization and peculiar smell removing can be realized, and functions of female self cleaning systems can be improved; broken fiber layers and damaged muscle layers can be rapidly repaired; the secretion of hormones can be stimulated, and the synthesis and secretion of collagen and elastic fiber can be promoted; and as the tablet is rich in sexual nerve repair factors, the sensitivity, pleasant sensation and happiness sensitivity can be effectively enhanced.
Owner:江苏艾特美科技有限公司
Nifuratel solid preparation and preparation method thereof
ActiveCN105560191AHigh dissolution rateImprove bioavailabilityAntibacterial agentsPowder deliveryTime rangeMANNITOL/SORBITOL
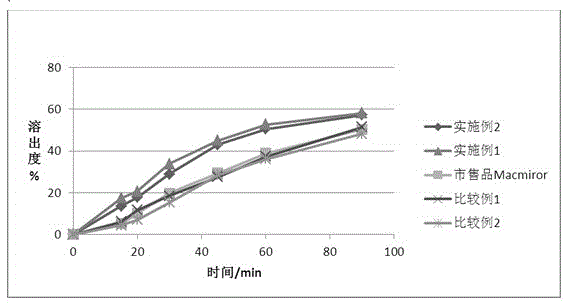
The invention provides a nifuratel solid preparation and a preparation method thereof. A nifuratel solid dispersion is contained. The solid dispersion comprises a nifuratel crude drug and an auxiliary material A. The auxiliary material A is selected from one or more of lactose, polyethylene glycol and mannitol. A preparation method of the nifuratel solid dispersion includes the steps that the nifuratel crude drug and the auxiliary material A are taken and mixed in a ball-milling mode in proportion, wherein the ball-milling mixing time ranges from 10 min to 360 min every kg of the nifuratel crude drug. The nifuratel solid dispersion and a nifuratel tablet containing the nifuratel solid dispersion have the high dissolution rate and bioavailability.
Owner:南京卓康医药科技有限公司
Nifuratel nystatin soft capsule and preparation method thereof
The invention relates to a nifuratel nystatin soft capsule and a preparation method thereof. The content of the soft capsule consists of nifuratel, nystatin, olive oil and medicinal vaseline. The capsule shell of the soft capsule comprises gelatin, glycerol, water and epsilon-polylysine. According to the nifuratel nystatin soft capsule, the formula of the nifuratel nystatin soft capsule is optimized, the olive oil is used for replacing the conventional medicinal soybean oil, and the epsilon-polylysine is used as the preservative so that the irritation of the nifuratel nystatin soft capsule to the vagina is reduced.
Owner:哈尔滨海涵丰生物科技开发有限公司
Nifuratel-containing pharmaceutical composition and preparation method thereof
ActiveCN111557897AFast releaseOvercoming the inability to disperseOrganic active ingredientsAntimycoticsDrugs preparationsPharmaceutical drug
The invention provides a pharmaceutical composition containing nifuratel and a preparation method of the pharmaceutical composition, which belongs to the technical field of pharmaceutical preparations, and is prepared from the following components in parts by weight: 1 part of nifuratel, 0.07 part of nystatin, 0.2-0.4 part of gelatin for capsules, 1.6-1.8 parts of dimethicone AK 1000 and 0.26-0.36part of purified water. The nifuratel-containing pharmaceutical composition provided by the invention overcomes the phenomenon that nifuratel nystatin vaginal soft capsules in the prior art cannot bedispersed during melting time limit detection. Experimental results show that when the pharmaceutical composition is subjected to melting time limit detection, the sustainable dispersion hydrophilicity is good, and the melting time limit is obviously superior to that of other similar products.
Owner:HAIHE PHARMA WENZHOU
Preparation process of anti-infective drug nifuratel
ActiveCN107987069AReduce usageReduce pollutionOrganic chemistryAntiinfectivesEpoxy2-Furancarboxaldehyde
The invention belongs to the technical field of drug synthesis and in particular relates to a preparation process of an anti-infective drug nifuratel. The preparation process comprises the following steps: taking iodomethane, sodium sulfide and chlorocyclopropane as initial raw materials to obtain epoxy propyl methyl sulfide, carrying out ring-opening reaction with hydrazine hydrate to obtain 3-methylmercapto-2-hydroxyl-propylhydrazine, carrying out a ring-closure reaction to obtain N-amino-5-methylthiomethyl-2-oxazolidinone, hydrolyzing 5-nitro furfural diacetate in the presence of trifluoroacetic acid to obtain 5-nitro-2-furancarboxaldehyde, and performing condensation with N-amino-5-methylthiomethyl-2-oxazolidinone, thereby obtaining the nifuratel. Safe and cheap reagents are selected in the process route, and environment hazards are reduced. Meanwhile, the operating difficulty and reaction after-treatment burdens are reduced, the production safety is ensured, the process is a simple, green and economic process route for preparing the nifuratel, and the obtained product is high in yield, excellent in purity and suitable for large-scale industrial production of the nifuratel.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
The preparation method of nifuratel
ActiveCN108084174BAvoid vacuum distillationEasy to useOrganic chemistryFuraldehydePharmaceutical Substances
The invention belongs to the technical field of synthesis of medicines and particularly relates to a nifuratel preparation method. The nifuratel preparation method comprises the following steps: taking epoxy propyl dimethyl sulfide as the starting material, having a ring-opening reaction with tert-butyl carbazate to obtain N'-(2-hydroxyl-3-methylmercapto-propyl)-tert-butyl carbazate; having a ringclosing reaction with urea under the catalytic action of cuprous bromide, obtaining a key intermediate N-(Boc-amino)-5-methylmercapto-methyl-2-oxazolidinone; hydrolyzing 5-nitro furfural diacetate under the action of trifluoroacetic acid to obtain 5-nitrofurfural, condensing with N-(Boc-amino)-5-methylmercapto-methyl-2-oxazolidinone, and obtaining nifuratel. In the process route, the cheap easy-to-get agents are chose, the operation difficulty and the processing burden caused after the reaction are reduced, the environmental harm is reduced, the production safety is guaranteed, and the preparation method is an easy, green and economical process route for the preparation of the nifuratel.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Synthetic method of nifuratel
The invention belongs to the field of medicine synthesis, and discloses a synthetic method of nifuratel. The synthetic method of nifuratel is to carry out ring closing reaction under the alkaline condition of sodium methoxide, therefore, the metallic sodium can be avoided being used, and the safety in production can be ensured; meanwhile, the reaction is easy to generate, the process is easy to control, high yield of the synthesized nifuratel is achieved, few impurities are generated, and the quality is stable; and moreover, the synthetic method of nifuratel is simple in operation, and the raw material is easy to obtain; the used solvent and the raw material out of the reaction can be recycled along with low cost; no waste liquid is generated in each step of the reaction; the pollution is greatly reduced; and the synthetic method is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Nifuratel tablet and preparation method thereof
InactiveCN107595797ALarge specific surface areaHigh mechanical strengthAntibacterial agentsOrganic active ingredientsCellulose acetateAdhesive
The invention discloses a nifuratel tablet. The nifuratel tablet comprises the following components in parts by weight: 100-120 parts of nifuratel, 5-10 parts of a disintegrating agent, 3-6 parts of alubricant, 30-50 parts of a filler, 3-5 parts of a flow aid, 20-30 parts of an adhesive and 5-10 parts of a coating material, wherein the coating material comprises the following components in partsby weight: 5-8 parts of sodium chloride, 1-3 parts of maltose, 8-10 parts of cellulose acetate and 2-3 parts of sorbitol. The preparation method comprises the following steps: mixing nifuratel with the adhesive; adding water, and performing stirring to be a paste; adding the disintegrating agent, the lubricant, the filler and the flow aid; performing mixing; performing pressing to produce a tabletcore; coating the tablet core with the coating material to obtain the nifuratel tablet. The nifuratel tablet is reasonable and scientific in compounding, and the nifuratel tablet prepared from the specific components is quick in disintegration, high in dissolution rate and good in stability.
Owner:四川摩尔生物制药有限公司
The preparation method of nifuratel
The invention relates to a method for preparing nifuratel. The method comprises the steps of (1) synthesizing 2-(methylmercapto-methyl)-oxacyclopropane through epoxy chloropropane and sodium methyl mercaptide; (2) generating reaction between hydrazine hydrate and the 2-(methylmercapto-methyl)-oxacyclopropane to synthesize 3-methylmercapto-2-hydroxy-propyl hydrazine; (3) generating reaction between diethyl carbonate and 3-methylmercapto-2-hydroxy-propyl hydrazine to prepare N-amino-5-methylmercapto-methyl-2-oxazolidinone; (4) hydrolyzing 5-nitrofuran formaldehyde diacetate ester under an acidic condition to prepare 5-nitro-2-furaldehyde; (5) generating reaction between the 5-nitro-2-furaldehyde and the N-amino-5-methylmercapto-methyl-2-oxazolidinone obtained in the step (3) to obtain the nifuratel, wherein in the step (1), 15-crown ether-5 is used as a catalyst, so that the conversion rate is high; no organic solvent is used during posttreatment of a product, and the posttreatment is simple.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Nifuratel film-coated tablet and preparation method thereof
The invention relates to a solid preparation of nifuratel, particularly relates to a nifuratel film-coated tablet and a preparation method thereof and belongs to the field of pharmaceutic preparations. The tablet core is composed of the following components in parts by weight: 200 parts of nifuratel, 35-95 parts of a thinner, 6-8 parts of a disintegrating agent, 0.9-1.5 parts of a flow agent, 0.9-1.5 parts of a lubricating agent and a proper amount of an adhesion agent, wherein the optimal nifuratel film-coated tablet is composed of the following components in parts by weight: 200 parts of nifuratel, 40-50 parts of lactose, 35-45 parts of microcrystalline cellulose, proper amount of 5 percent of a HPMC (Hydroxy propyl methyl cellulose) aqueous solution, 6-8 parts of crosslinking hydroxyl methyl cellulose sodium, 0.9-1.5 parts of silicon dioxide and 0.9-1.5 parts of magnesium stearate. According to the film-coated tablet, an LE yellow coating agent is used, and a coating solution is prepared by using 75 percent of ethanol and water. The nifuratel film-coated tablet has good in-vitro solubleness and the surface of the coating is glossy.
Owner:BEIJING SUNHO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com