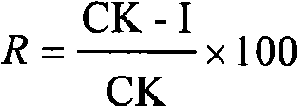Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
659 results about "Ethyl formate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethyl formate is an ester formed when ethanol (an alcohol) reacts with formic acid (a carboxylic acid). Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries. It occurs naturally in the body of ants and in the stingers of bees.
Vulcanization-oxidization mixing copper ore floatation method
InactiveCN101190426AHigh recovery rateImprove concentrate qualityFlotationVulcanizationHydroxamic acid
The invention discloses a sulfurization-oxidation mixed copper mine flotation method, comprising the steps of ore grinding and copper flotation: 20 to 50g / t of pentyl xanthic acid ethyl formate raw ore is added in the grinding process; meanwhile, a pH adjusting agent is added to lead the pH of the mine slurry to keep between 9.5 and 10.5; the invention comprises one time of fast selection, one time of coarse selection, one time of sweeping selection and three times of precise selection; in the fast selection process, 20 to 30g / t of nonyl hydroxamic acid raw ore is added and 20 to 30g / t of frother raw ore is added; in the coarse selection process, 10 to 30g / t of the pentyl xanthic acid ethyl formate raw ore and 10 to 20g / t of the nonyl hydroxamic acid raw ore as well as 10 to 20g / t of the frother raw ore are added; in the sweeping selection process, 10 to 20g / t of the nonyl hydroxamic acid raw ore is added; the pH of the flotation mine pulp is kept between 9.5 and 10.5 and the copper ore is recovered. Compared with the traditional flotation methods of copper sulphide ore and copper oxide ore, the invention improves the flotation efficiency, reduces flotation equipment and energy loss and increases the copper recovery for more than 10%.
Owner:CENT SOUTH UNIV
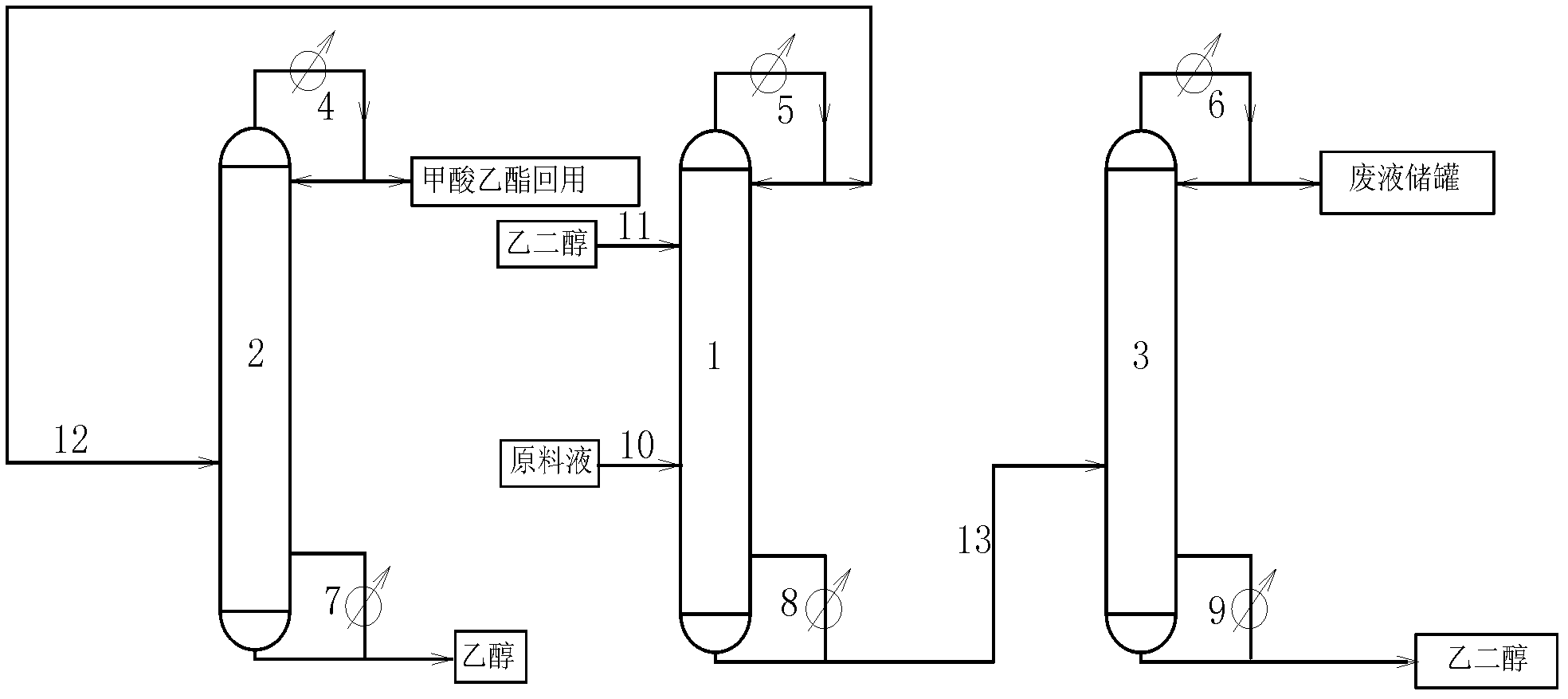
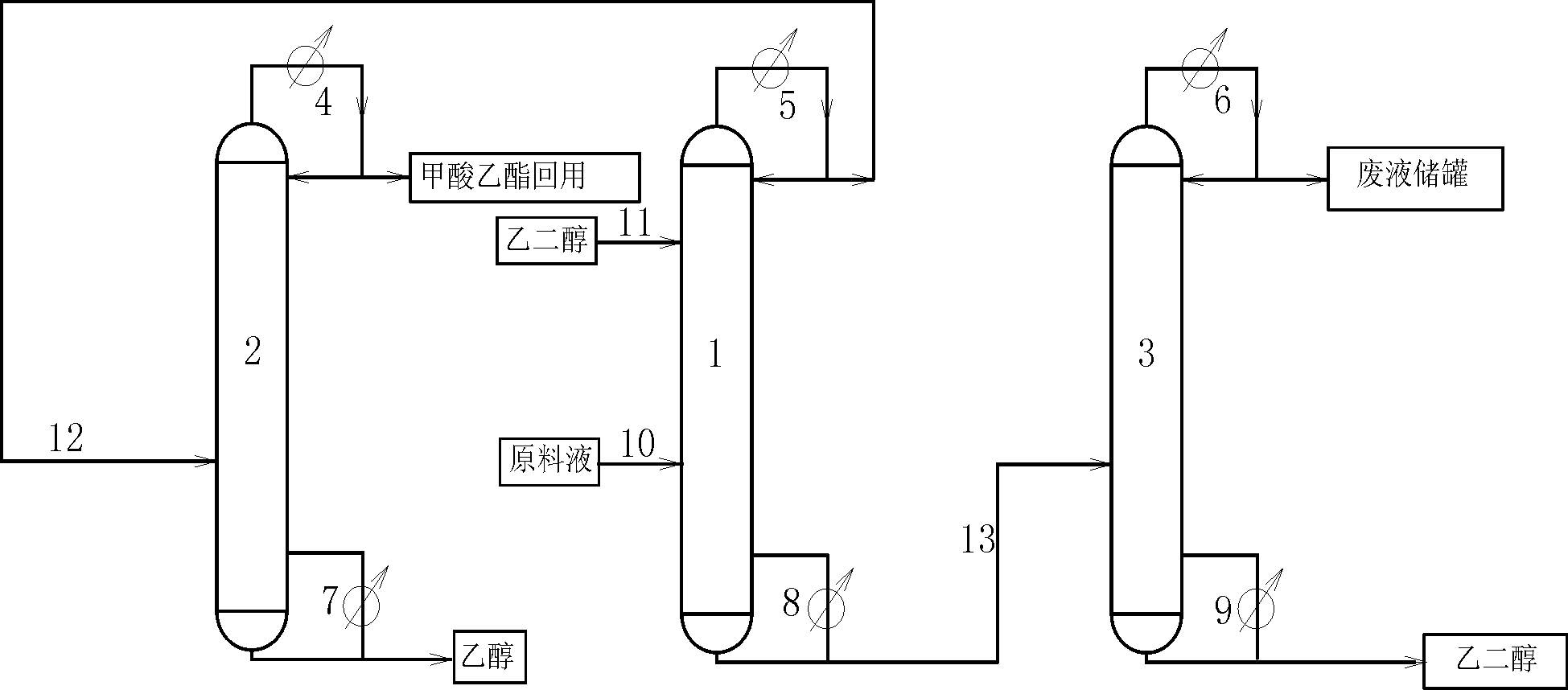
Technology of extractive distillation separation of ethyl acetate-ethanol-water
ActiveCN102627556AOrganic compound preparationCarboxylic acid esters preparationExtractive distillationEconomic benefits
The invention belongs to the extractive distillation process, in particular to a technology of extractive distillation separation of ethyl acetate-ethanol-water. The technology employs glycol as an extractant and joint operation of three tower model including a dehydration tower, a ethyl formate recovery tower and a glycol recovery tower to separate the three substances. The invention can ensure recycling of the extractant. A tower top 2 of the ethyl formate recovery tower obtains 90.7% of ethyl formate, a yield near 100%, and 99.6% of ethanol. Both ethyl formate and ethanol are able to be recycled, s as to save resources, reduce environmental pollution and produce enormous economic benefits.
Owner:HEBEI UNIV OF TECH
Flotation method of brass ore-containing complex lead-zinc sulphide ore
The invention discloses a flotation method of a brass ore-containing complex lead-zinc sulphide ore, which comprises the steps of: adjusting the pH value to be 10-11 in the process of ore grinding; adding ore pulp electric potential regulating agent-sodium pyrosulfite to adjust the electric potential of the ore pulp to be at 220-260mV (relative to hydrogen standard potential); adding zinc sulfate, ethoxy-dithioformicacid ethyl formate and ethyl thio carbamate grinding ore; performing one roughing, one scavenging and two selecting to enrich copper-lead mineral by means of mixing and floating and to form into copper-lead mixed concentrate; performing reagent desorption to the copper-lead mixed concentrate by adding active carbon; adding potassium peroxydisulfate and carboxymethyl starch to restrain lead-containing mineral in the copper-lead mixed concentrate such as galena and the like; performing one roughing, one scavenging and three selecting to obtain copper concentrate; adjusting the pH value of tailings after recovering copper mineral by floating the copper-lead mixed concentrate to be 9.0; adding sodium pyrosulfite, ethyl thio carbamate and butyl ether alcohol; and performing one roughing, one scavenging and two selecting to obtain lead concentrate. The method guarantees the grade of the copper and the lead concentrate, and reduces the environmental pollution.
Owner:CENT SOUTH UNIV +1
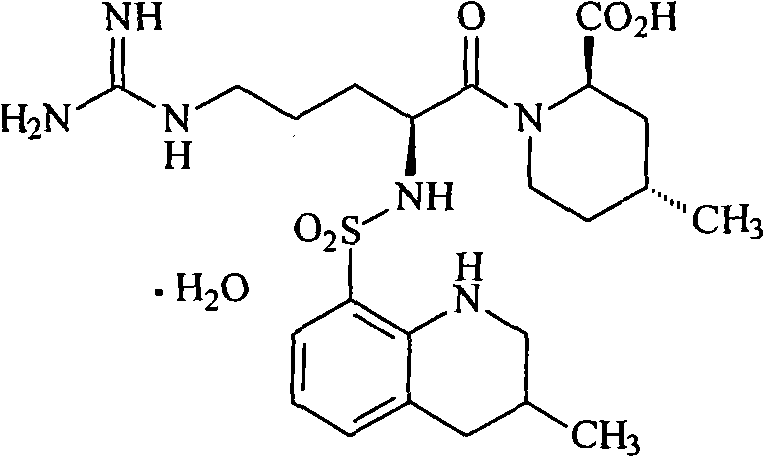
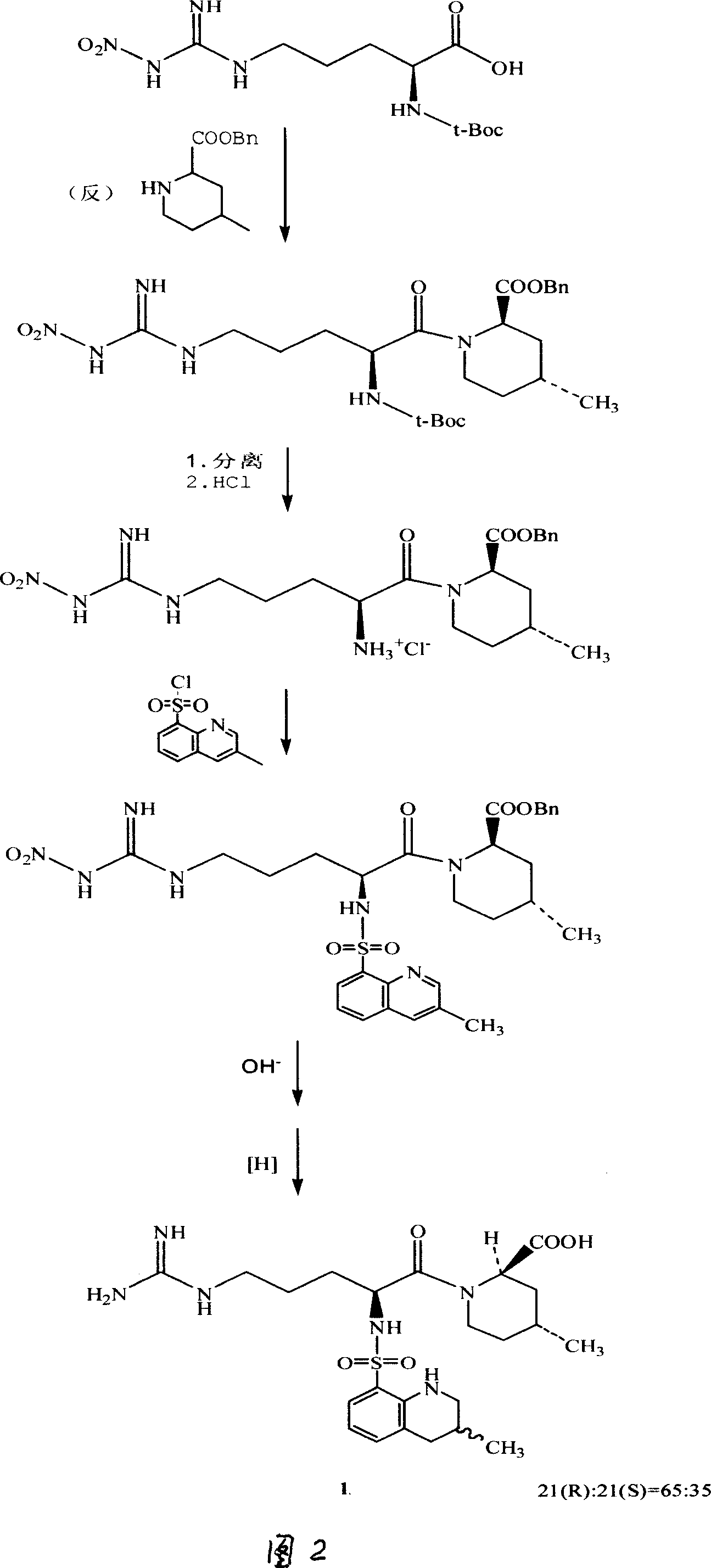
Argatroban and preparation thereof
InactiveCN101348481AThe synthesis process is simpleEasy to operateOrganic chemistryDiethyl phosphateOrganic solvent
The invention relates to a method for synthesizing argatroban. The method comprises the following steps that nitryl L-arginie and quinoline sulfonchloride are condensed, and undergo amidation with piperidine ethyl formate, followed by hydrolysis and hydrogenation to obtain argatroban; the amidation is to make carboxylate (c-v) and (2R, 4R) 4MPE (Z-VII) react in an organic solvent in the presence of condensing agent, or the presence of both condensing agent and dehydration promoter, in which the molecular ratio of carboxylate (c-v): (2R, 4R) 4MPE (Z-VII): condensing agent: dehydration promoter is 1: 0.8-1.2: 0.8-1.2: 0-1.2. The condensing agent adopted by the invention is diphenylphosphoryl azide, diethylthiophosphoryl, chlorophosphoric acid diethyl or bromophosphoric acid diethyl. The invention has simplified operation, lowered cost, decreased pollution, increased yielding rate, and is suitable for large-scale industrialized production of argatroban.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV +1
Azeotropic compositions comprising methyl perfluoropentene ethers for cleaning applications
The present disclosure provides azeotropic and azeotrope-like compositions comprised of methylperfluoropentene ethers and at least one of methanol, ethanol, 2-propanol, hexane, heptane, trans-1,2-dichloroethylene, ethyl formate, methyl formate, HFE-7100, HFE-7200 and 1-bromopropane or combinations thereof. The present disclosure also provides for methods of use for the azeotropic and azeotrope-like compositions.
Owner:EI DU PONT DE NEMOURS & CO
Method for detecting ethyl carbamate in yellow wine
InactiveCN101620206AAchieve separationEasy to detectComponent separationPreparing sample for investigationDistillationSolid phase extraction
The invention discloses a method for detecting ethyl carbamate in yellow wine, comprising the following steps: (1) pre-processing the yellow wine by using reduced pressure distillation so as to reduce the volume of the yellow wine to 1 / 5-1 / 10 of the initial volume; (2) mixing the pre-processed yellow wine and hydrochloric acid as well as 9-hydroxyl solutions by tons to carry out derivatizing reaction to obtain the derivatized yellow wine; and (3) carrying out component separation on the derivatized yellow wine by using an effective liquid chromatograph and calculating the content of the ethyl carbamate by using an external standard method. The method has simple operation and low detection cost, and has simpler needed apparatus, easier sample pre-processing, more convenient needed detection condition and shorter spent detection time when compared with an analysis method for solid phase extraction-gas chromatography / mass spectrometry (SPE-GC / MS).
Owner:ZHEJIANG UNIV
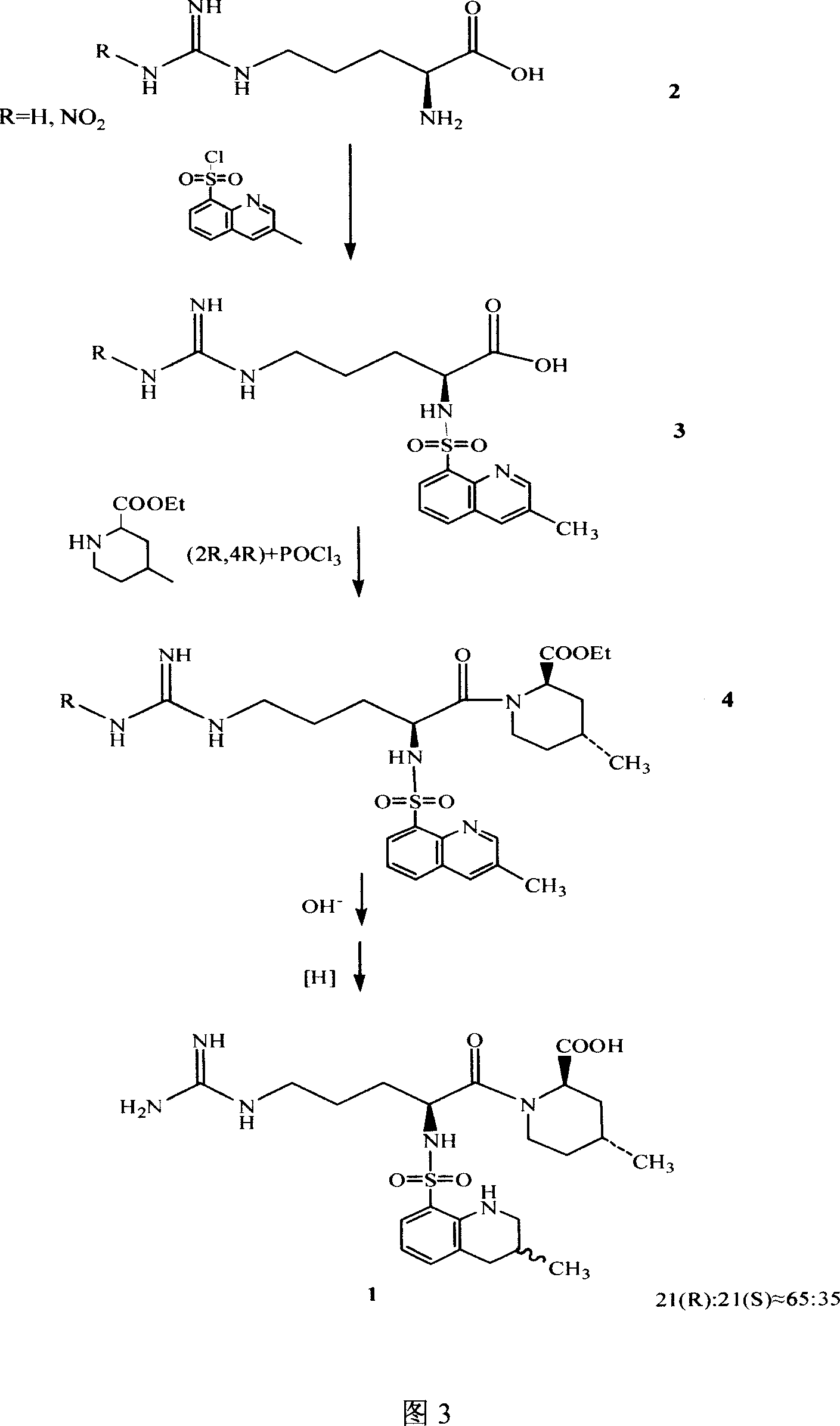
Process for preparing argatroban intermediates
The invention discloses a preparing method of Argatroban intermediate, which is characterized by the following: adopting NG-nitro-N2-tBoc -L-arginine and (2R,4R)-4-methyl-2-piperidine ethyl formate to condensate; making DCC as dehydrant; reacting under -5-35deg.c for 1-5h and stirring; cooling reacting liquid; filtering to remove solid; stratifying filtrate; removing water layer; washing organic layer through sodium hydroxide solution, citrate solution and saturated salt water; drying; evaporating dichloromethane; obtaining yellow solid product.
Owner:TIANJIN WEIJIE TECH
In-situ synthetic method of geolyte containing copper wires
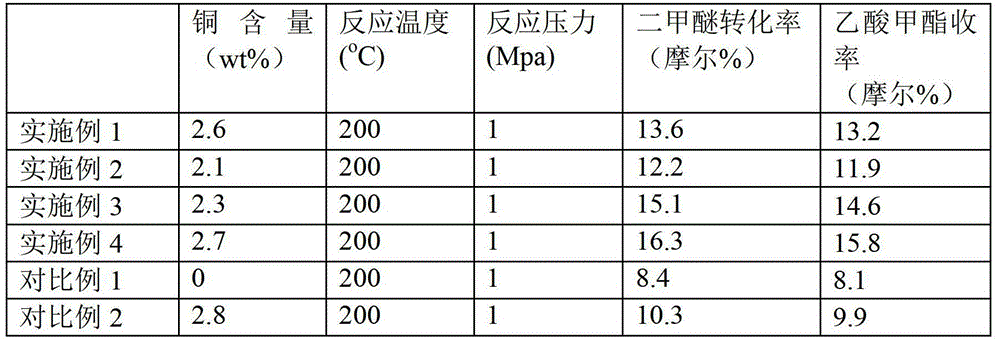
The invention provides an in-situ synthetic method of geolyte containing copper wires. The method comprises the steps of mixing silicon sources, aluminum sources, organic template agents, copper sources and water to obtain raw material solutions; aging the raw material solutions and crystallizing under a hydrothermal condition; washing, drying and roasting to prepare the geolyte containing copper wires. The invention also provides the geolyte containing copper wires prepared by using the method and a method for synthesizing ethyl formate by means of dimethyl ether carbonylation by using the geolyte containing copper wires as a catalyst. The geolyte containing copper wires represents high activity and high product selectivity in reaction of dimethyl ether carbonylation synthesized methyl acetate.
Owner:SHANGHAI BI KE CLEAN ENERGY TECH
Aromatic nitrile-base thiazole derivatives for inhibiting xanthine oxidase activity, preparation method and application
ActiveCN101386604AEasy to operateHigh yieldOrganic active ingredientsOrganic chemistryCyclobutaneLithium formate
The invention disclosed an aryl nitrile group thiazole derivative for inhibiting the activity of xanthine oxidase, a method for preparing the same and application thereof. In the aryl nitrile group thiazole, R1 is methyl, ethyl, propyl, isopropyl, isobutyl, methyl cyclopropane, methyl cyclobutane, isoamyl, methyl cyclopentane, methyl cyclohexane or aromatic ring methyl, R2 is methyl or trifluoromethyl, and R3 is formic acid, sodium formate, potassium formate, lithium formate, methyl formate, or ethyl formate. Simultaneously, the invention discloses a method for synthesizing the aryl nitrile group thiazole derivative by using inexpensive raw materials, which has the advantages of simple operation, high yield, easy purification of products, application to industrial production and the like, and can obtain an efficient compound with low toxicity through screening; besides, the effective compound is expected to be widely applied to inhibit the activity of the xanthine oxidase required on animals and humans, and to become a new generation of antigout drugs and hyperuricemia drugs with special effect.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
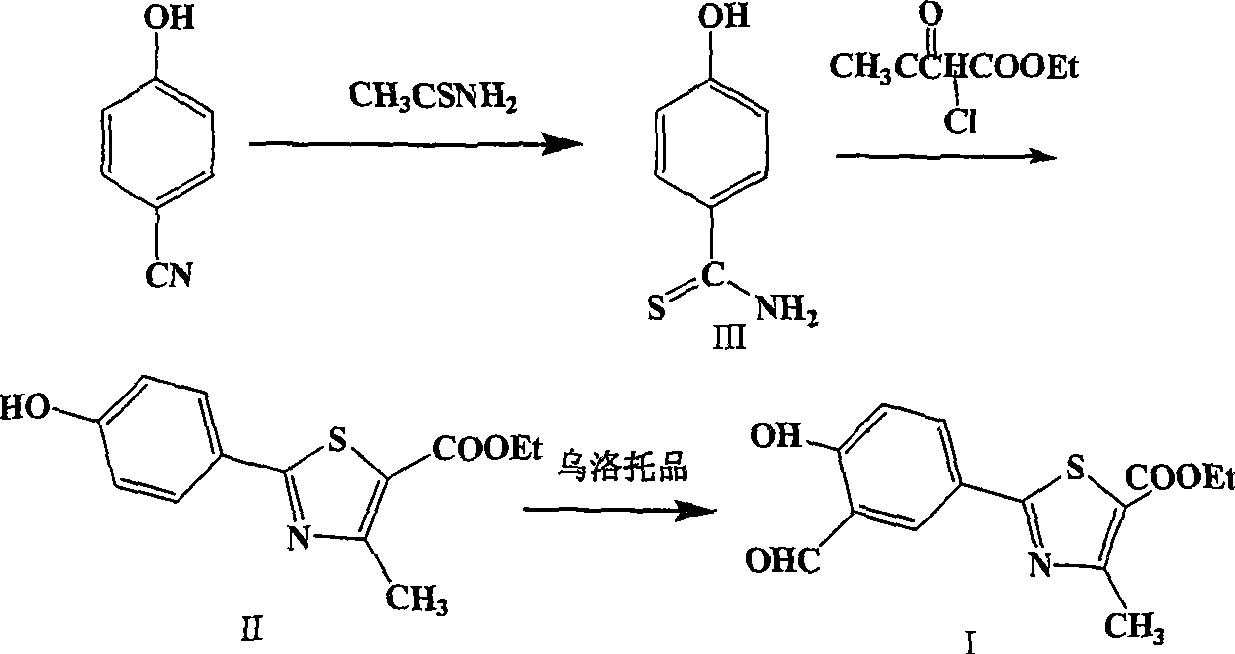
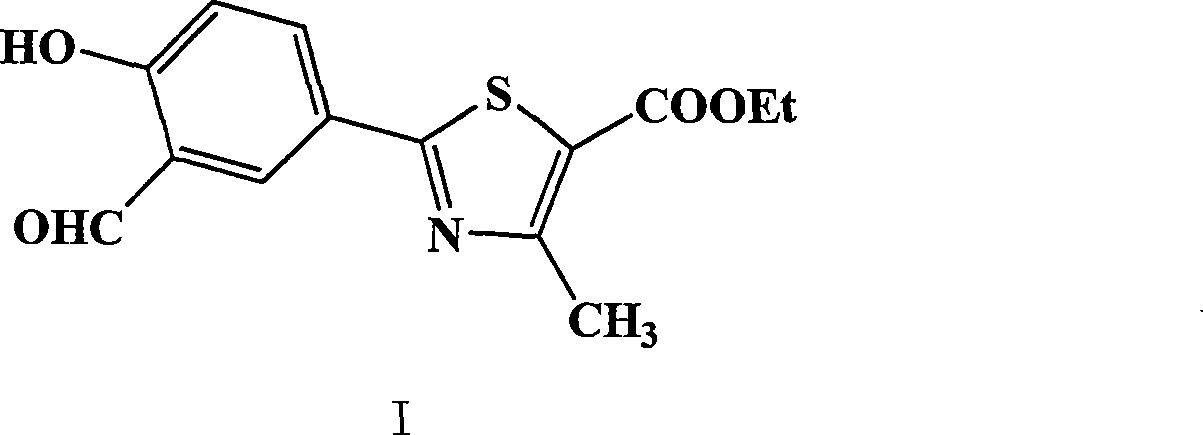
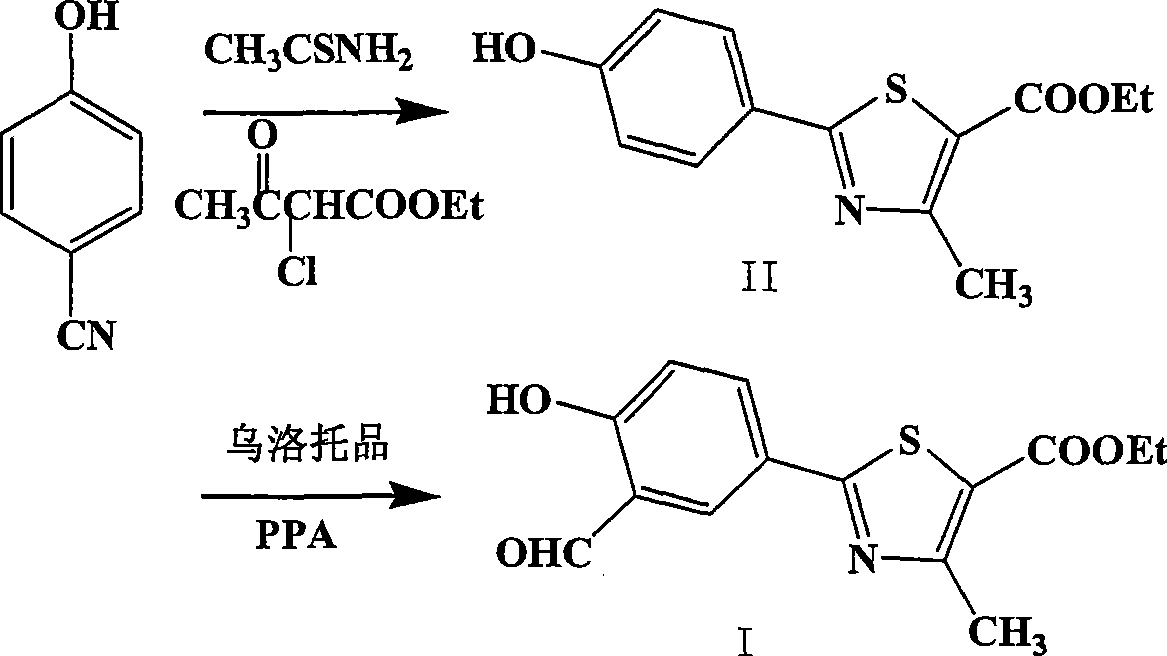
Preparation of 2-(3-carboxaldehyde-4-hydroxy phenyl)-4-methyl-5-thiazole ethyl formate
InactiveCN101412699AEmission reductionHigh yieldOrganic chemistryHexamethylenetetramineEthyl acetate
The invention provides a method for preparing 2-(3-formaldehyde base-4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate. The method uses para-cyano-phenol and thioacetamide as raw materials which are reacted with each other to obtain 4-hydroxy-thiobenzamide, and the obtained product is reacted with 2-chloro acetylacetic ether to obtain 2-(4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate, and then is reacted with urotropine to obtain the 2-(3-formaldehyde base-4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate.
Owner:SHANGHAI INST OF PHARMA IND +1
Methanol diesel fuel complex additive and preparation method thereof
ActiveCN101709234AGood compatibility stabilityStable in natureLiquid carbonaceous fuelsCyclohexanoneN-Butylamine
The invention discloses a methanol diesel fuel complex additive and a preparation method thereof. The complex additive is prepared from the following raw materials in parts by volume: 1-4 parts of diethyl phthalate, 1-8 parts of n-butylamine, 1-9 parts of isooctanol, 1-6 parts of cyclohexanone, 13-42 parts of lauryl methacrylate, 7-30 parts of mixed fatty glyceride, 1-5 parts of phenyl propargyl ether, 1-8 parts of dihexadecyl dimethyl ammonium chloride, 2-12 parts of ethyl formate, 2-14 parts of isooctyl nitrate and 1-6 parts of polyoxyethylene hexadeeylalkyl ether selies. The methanol diesel fuel complex additive can enable methanol diesel fuel to be evenly mixed and to be stably stored for more than 6 months, which effectively improves the fire behaviour of the methanol diesel fuel and inhibits the volatilization when the methanol content is higher; moreover, the production process is simple and the use is convenient, thereby being beneficial to the popularization and application of the methanol diesel fuel.
Owner:临沂星火知识产权服务有限公司
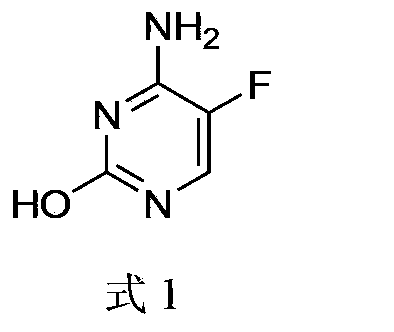
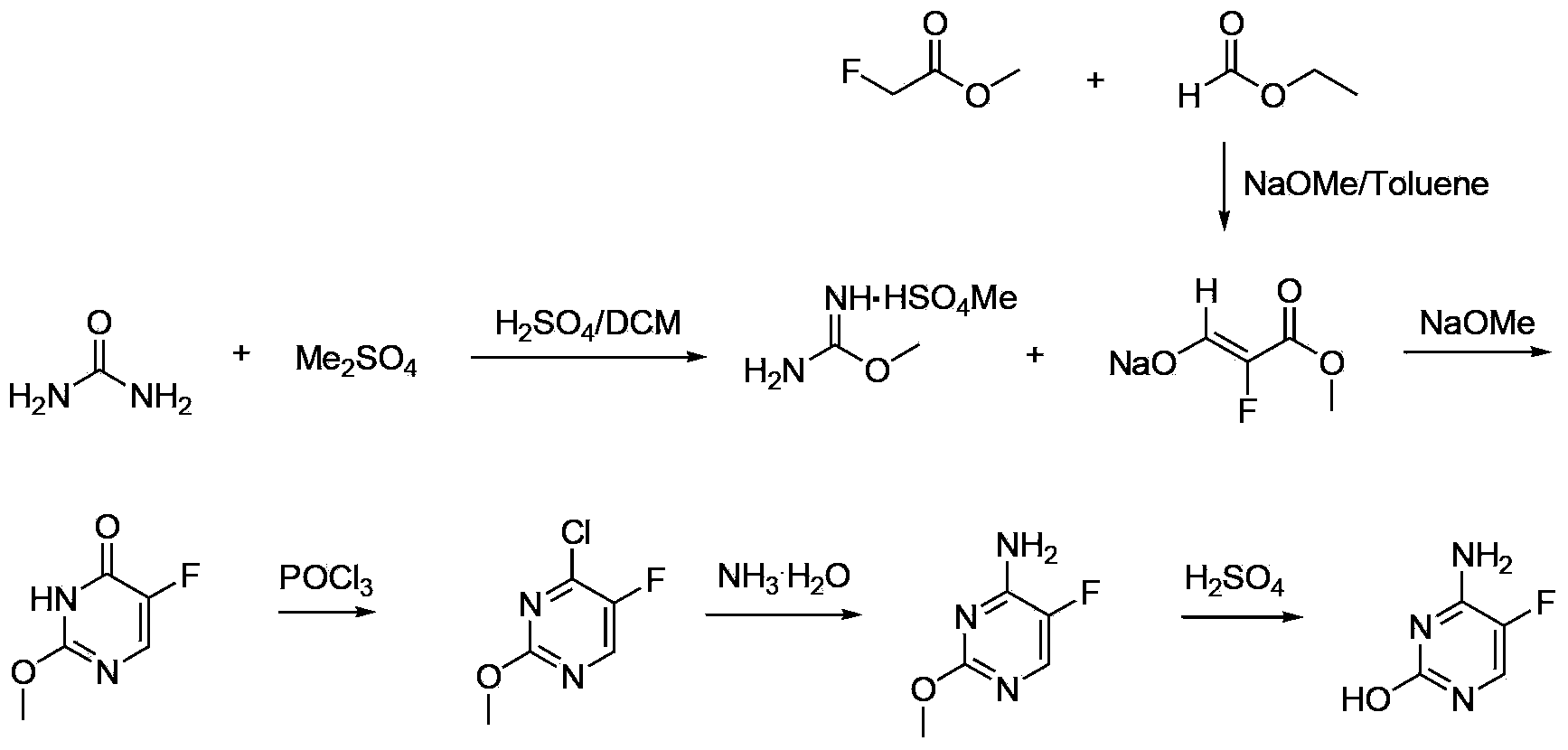
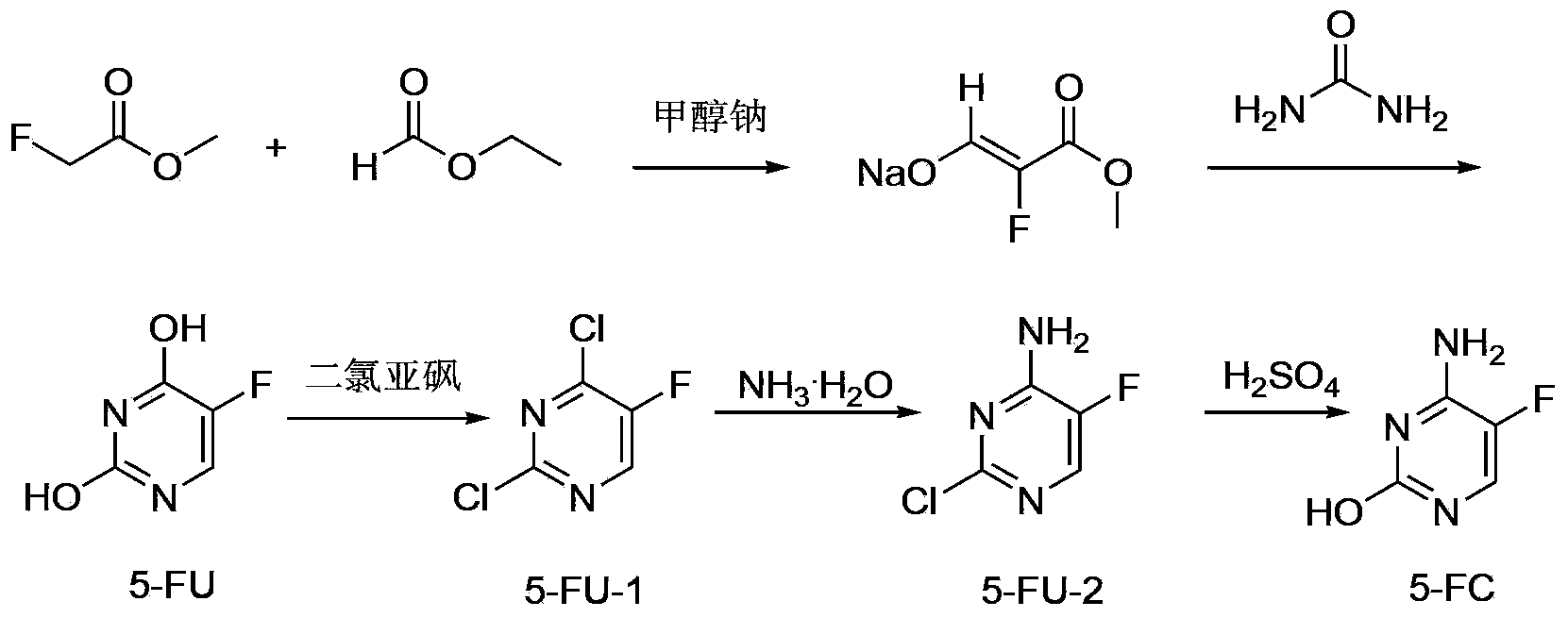
5-fluorocytosine preparation method
ActiveCN103435557AHigh yieldShorten the production cycleOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the field of organic chemical synthesis, particularly relates to a 5-fluorocytosine preparation method, and aims to solve the technical problems about use of highly toxic chemicals, complex equipment, tedious post-processing, large pollution and high cost in a traditional synthesis method. The technical scheme to solve the technical problems by the invention is to provide the 5-fluorocytosine preparation method which comprises the following steps: enabling methyl fluoroacetate to conduct a condensation reaction with ethyl formate and then conduct a reaction with urea, a chlorination reaction with thionyl chloride, then an ammonolysis reaction with ammonium hydroxide and finally a hydrolysis reaction with sulfuric acid to obtain 5-fluorocytosine. The invention provides the novel method with high yield, low pollution and low cost for the industrial production of 5-fluorocytosine.
Owner:ASTATECH CHENGDU BIOPHARM CORP
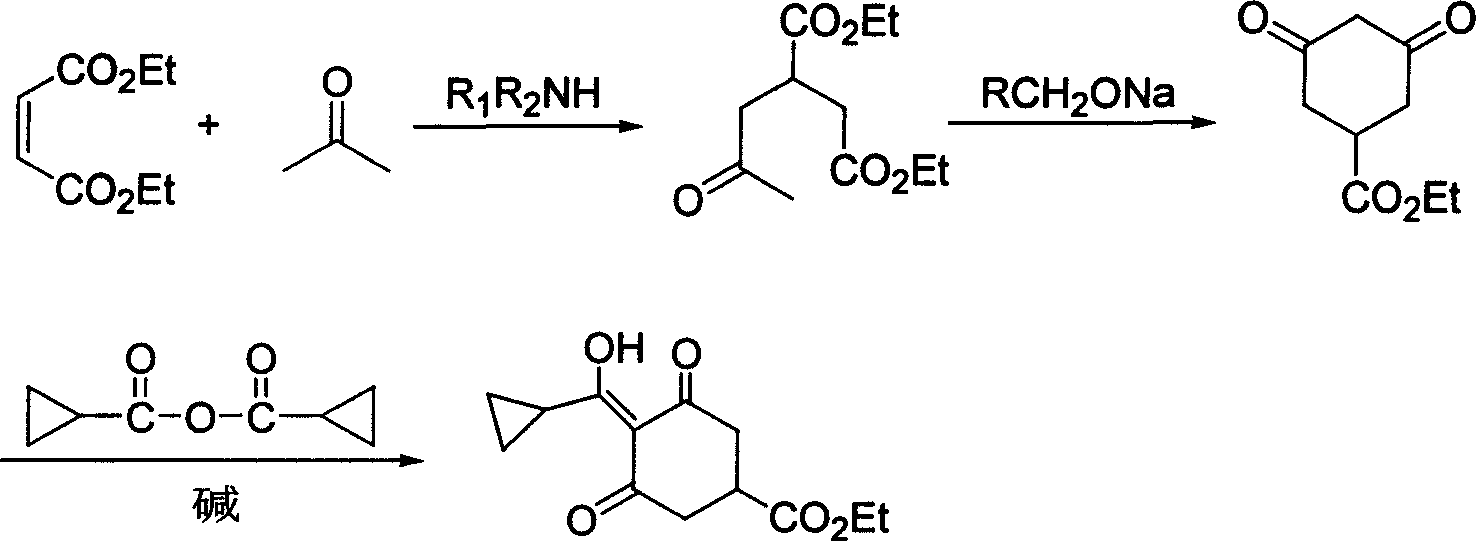
Method for preparing trinexapac-ethyl
InactiveCN1850776AEasy to operateMild responseOrganic compound preparationCarboxylic acid esters preparationHigh pressureMedicinal chemistry
This invention discloses anti inverse ester preparation method. 2-acetonyl-1, 4 diethyl succiante is high pressure condensed by diethyl maleate and acetone under organic amine condition. Then it cycles to get 3, 5-dioxygen hexahydrobenzoic acid ethyl esterunder sodium alcoholate condition, finally anti inverse ester is got by reacting with ring third formic anhydride under alkali condition. The operation is simple, reaction is wild, material is easy to get, yield is high, product purity is high, and generating cost is reduced, so it is propitious to industrial manufacure.
Owner:ZHEJIANG UNIV
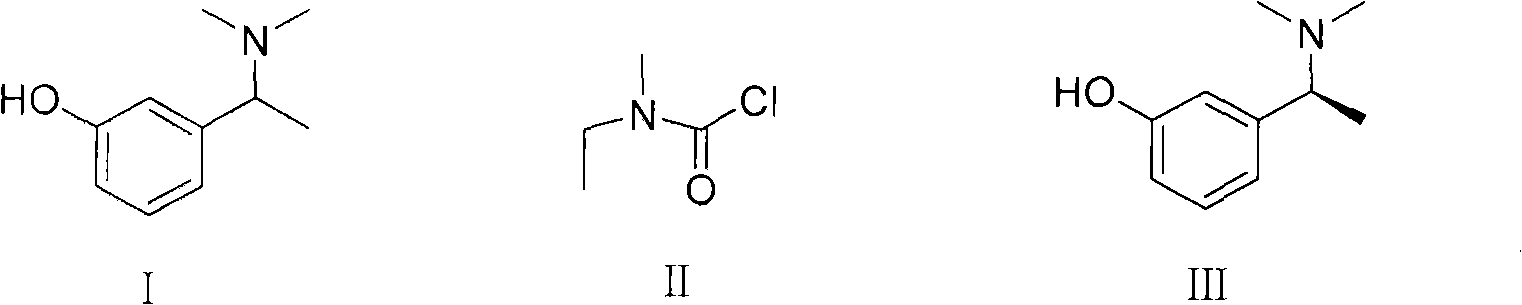
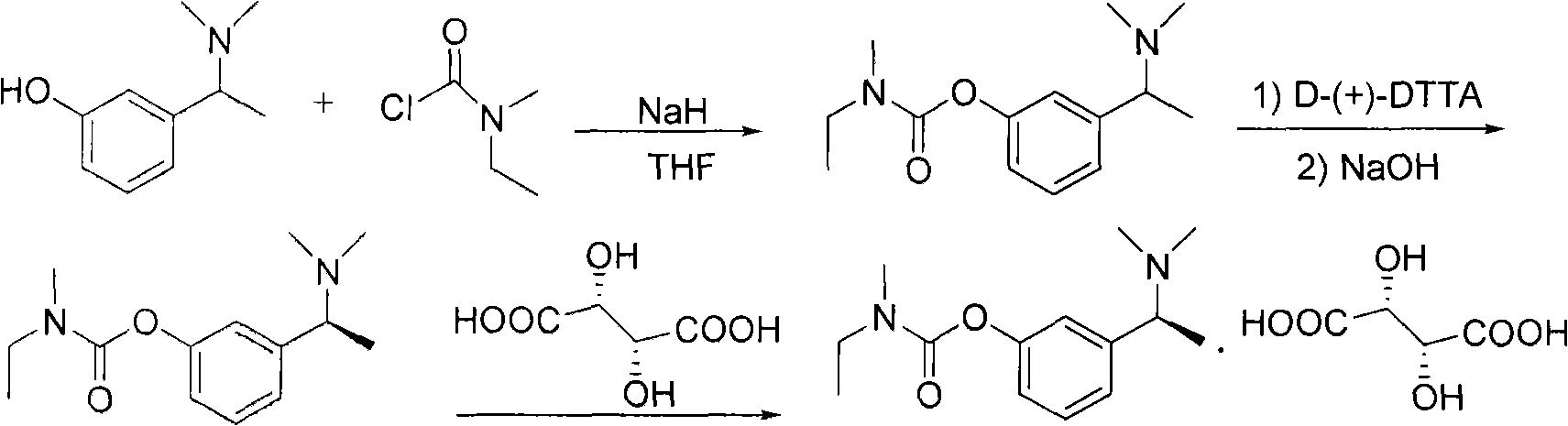
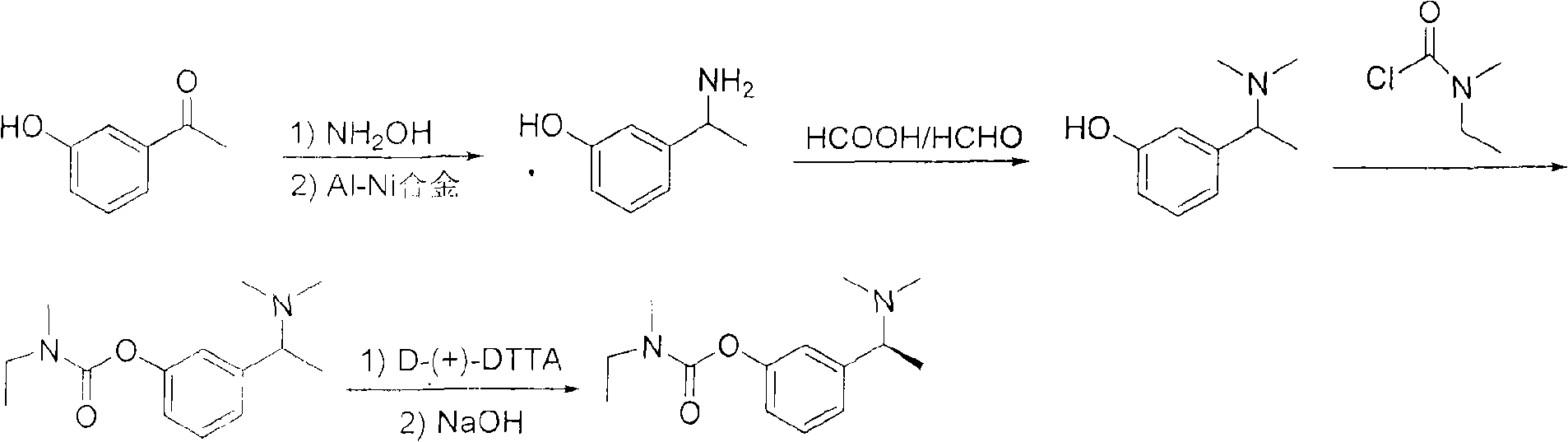
Method for preparing rivastigmine hydrogen tartrate and application thereof
InactiveCN101580482AEasy to operateLow costNervous disorderCarbamic acid derivatives preparationPhosgeneTriphosgene
The invention relates to a method for preparing rivastigmine hydrogen and tartrate thereof, which comprises the following steps: taking metamethoxyacetophenone as an initial raw material, and obtaining 1-(3-methoxyphenyl)ethanol by the reduction; then performing the chlorination to obtain 1-(chloroethyl)-3-methoxyphenyl; then reacting the1-(chloroethyl)-3-methoxyphenyl with dimethylamine hydrochloride to obtain 1-(3-methoxyphenyl)-N, N-dimethylethanamine; demethylating the reaction product to obtain 3-[1-(dimethylamino)ethyl]phenol; then performing salt formation resolution with (s)-(+)-camphor-10-sulfonic acid, recrystallizing, and dissociating to obtain (s)-3-[1-(dimethylamino)ethyl]phenol; then taking ethylamine as a raw material to react with ethyl formate to obtain formylethylamine; then reacting the formylethylamine with phosphorus oxychloride to obtain an imine intermediate; reducing the imine intermediate by sodium borohydride to obtain ethyl methyl amine; then reacting the ethyl methyl amine with triphosgene to obtain N-methyl-N-ethylformyl chloride; and finally using (s)-3-[1-dimethylamino)ethyl]phenol to condensate with the N-methyl-N-ethylformyl chloride, and then performing salt formation with levotartaric acid to obtain the rivastigmine hydrogen tartrate. The method has the advantages of easily-obtained raw materials, simple and convenient operation, low cost, high yield and small pollution, and is a brandnew synthesis route at present.
Owner:SHENYANG PHARMA UNIVERSITY
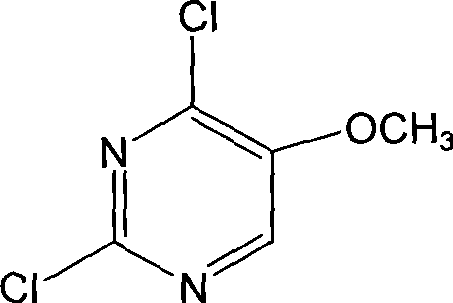
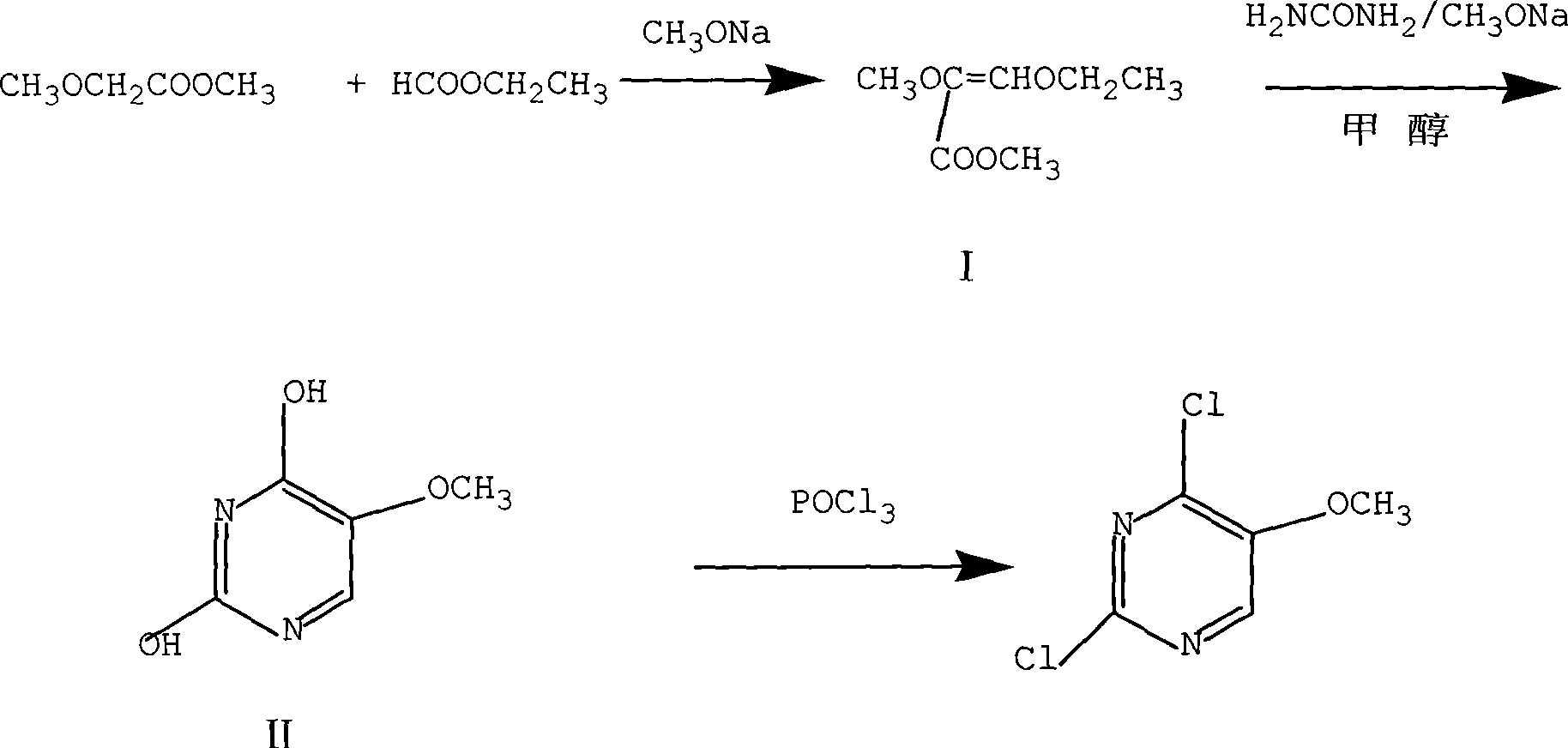
Preparation of 2,4-dichloro-5-methoxy pyrimidine
InactiveCN101486684ARaw materials are easy to getEasy to synthesizeOrganic chemistrySodium methoxideMethyl methoxyacetate
The invention discloses a preparation method of 2, 4-dichloro-5-methoxypyrimidine, pertaining to the technical field of pesticide intermediate preparation. The method includes steps as follows: 2, 4-dihydroxy-5-methoxypyrimidine is prepared, and ethyl formate and solid sodium methoxide are added into a reaction device and stirred. After the temperature is lowered, methyl methoxyacetate is added for carrying out a condensation reaction to obtain a compound I, and then methanol and carbamide are added into the compound I and a refluxing reaction is carried out. A compound II is obtained after condensation, dissolution with water, cooling, neutralization, filtration and drying; the 2, 4-dichloro-5-methoxypyrimidine is prepared, and a chlorinating agent and an acid-binding agent are added into the compound II; and then a temperature reaction, dilution and filtration are carried out in sequence to obtain a crude product of the 2, 4-dichloro-5-methoxypyrimidine. The crude product is refined to obtain a pure product of the 2, 4-dichloro-5-methoxypyrimidine. The method has the advantages of easy availability of all raw materials, convenient synthesis, not exacting technological conditions, overall yield up to 57 percent to 67 percent, purity over 99.6 percent and applicability to industrialized production.
Owner:JIANGSU HUAYI TECH
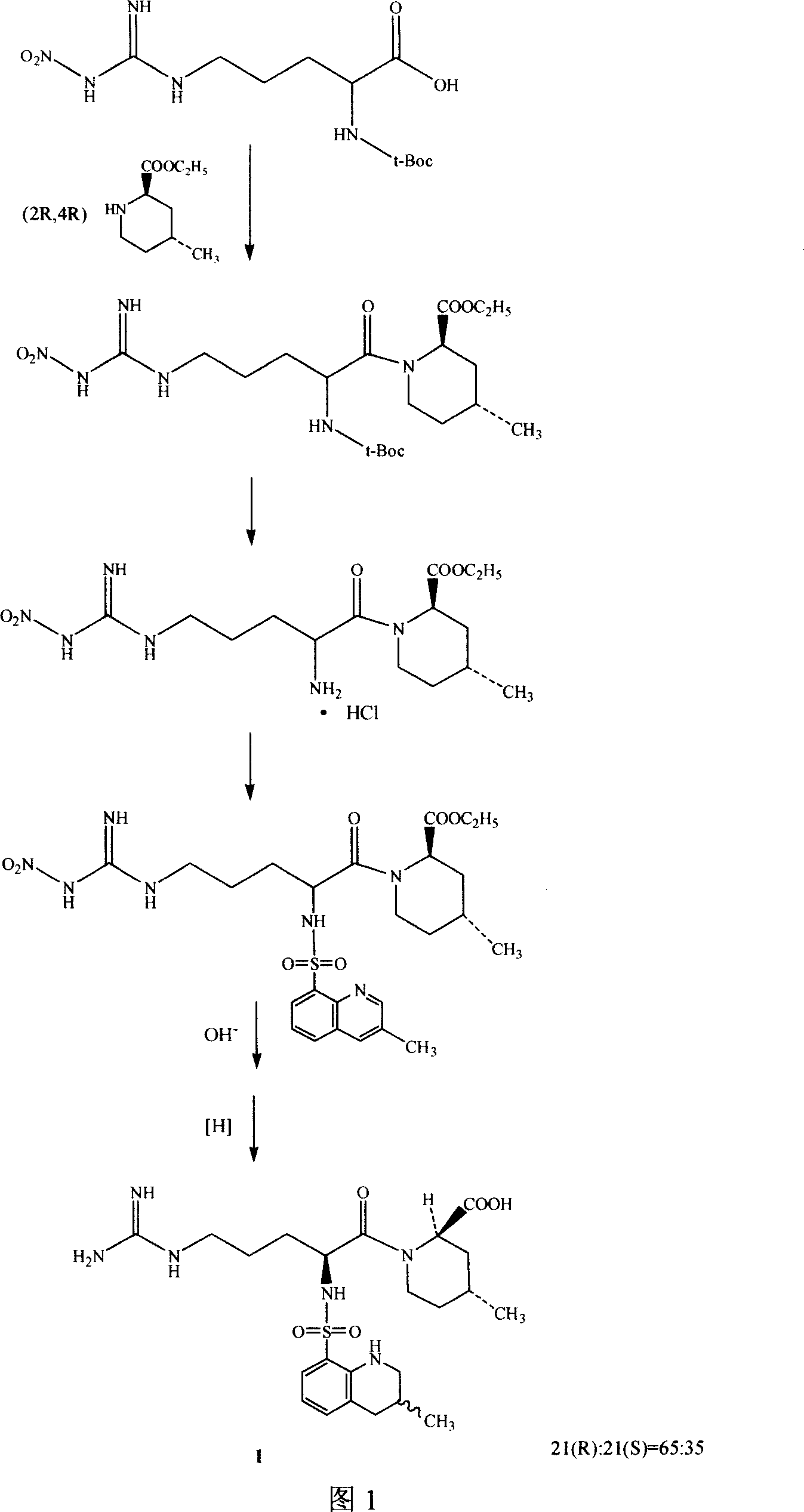
Directional synthesis method for 21(S) argatroban
The invention discloses a directional synthesis method for 21(S) argatroban. The directional synthesis method comprises the following steps of: reacting (2R,4R)-1-[NG-nitro-L-arginyl]-4-methyl-2-piperidine ethyl formate hydrochloride serving as a raw material and (3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl chloride to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic ether, then hydrolyzing and acidifying the intermediate in aqueous solution of sodium hydroxide to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic acid, and finally hydrogenating the intermediate to obtain single diastereoisomer 21(S) argatroban by catalysis of palladium carbon.
Owner:TIANJIN WEIJIE TECH
Method for preparing (+)-(s-)-clopiclogrel hydrogensulfate (I)
This invention relates to anti thrombus medicine (+)-(S-)-chlorine pyrrole rayl bisulfate preparation method. (+)-(S-)-chlorine pyrrole rayl free alkali shown as formula (I) is added in organic solvent, and weight concentration 10-100 perent sulphuric acid solution is dropwised at 6-20 degree centigrade, then the reaction is kept for 10 minute to 1.5 hours at 50-65 degree centigrade and the temperature is protected, finally formula (II) compound is got after filtration. The sulphuric acid solution is made by sulfuric acid dissolved in organic solvent, the organic solvent is one or more of the following, ethyl formate, methyl acetate, ethyl acetate, butyl acetate, aether, isopropyl ether, tertiary butyl methyl ether or methylene chloride. Mol ratio of compound (I) and sulfuric acid is 1:0.95-1.05. The synthesis in this invention is safe and high efficiency, it is propitious to industrial manufacture, product purity is high, yield is high, and crystal form fluidity is good.
Owner:ZHEJIANG HISOAR PHARMA
Process for preparing amlodipine benzenesulphonate
InactiveCN1927837ALow costShort reaction pathOrganic chemistryBulk chemical productionBenzaldehydeAmlodipine
The present invention discloses preparation process of Amlodipine benzene sulfonate. The preparation process includes the following steps: 1. the reaction between 4-[2-(tritylamindo)ethoxy] ethyl acetoacetate and amine compound to produce 3-amino-4-[2-( tritylamindo)ethoxy] ethylcrotonate; 2. the reaction between o-chluoro benzaldehyde and methyl acetoacetate under the catalysis of alkali to produce 2-(2-o-chluorobenzal)-ethyl acetoacetate; 3. the reaction of the products in the foregoing steps to obtain 4-(2-chlorophenyl)-6-methyl-2-((2-(tritylamido) ethoxy) methyl)-1, 4-dihydropyridyl-3-ethyl formate-5-methyl formate; and 4. further direct reaction with benzene sulfonic acid to eliminate protecting group and form Amlodipine benzene sulfonate.
Owner:上海开特生物科技有限公司
Synthesis of plant growth hormone 3-indolebutyric acid
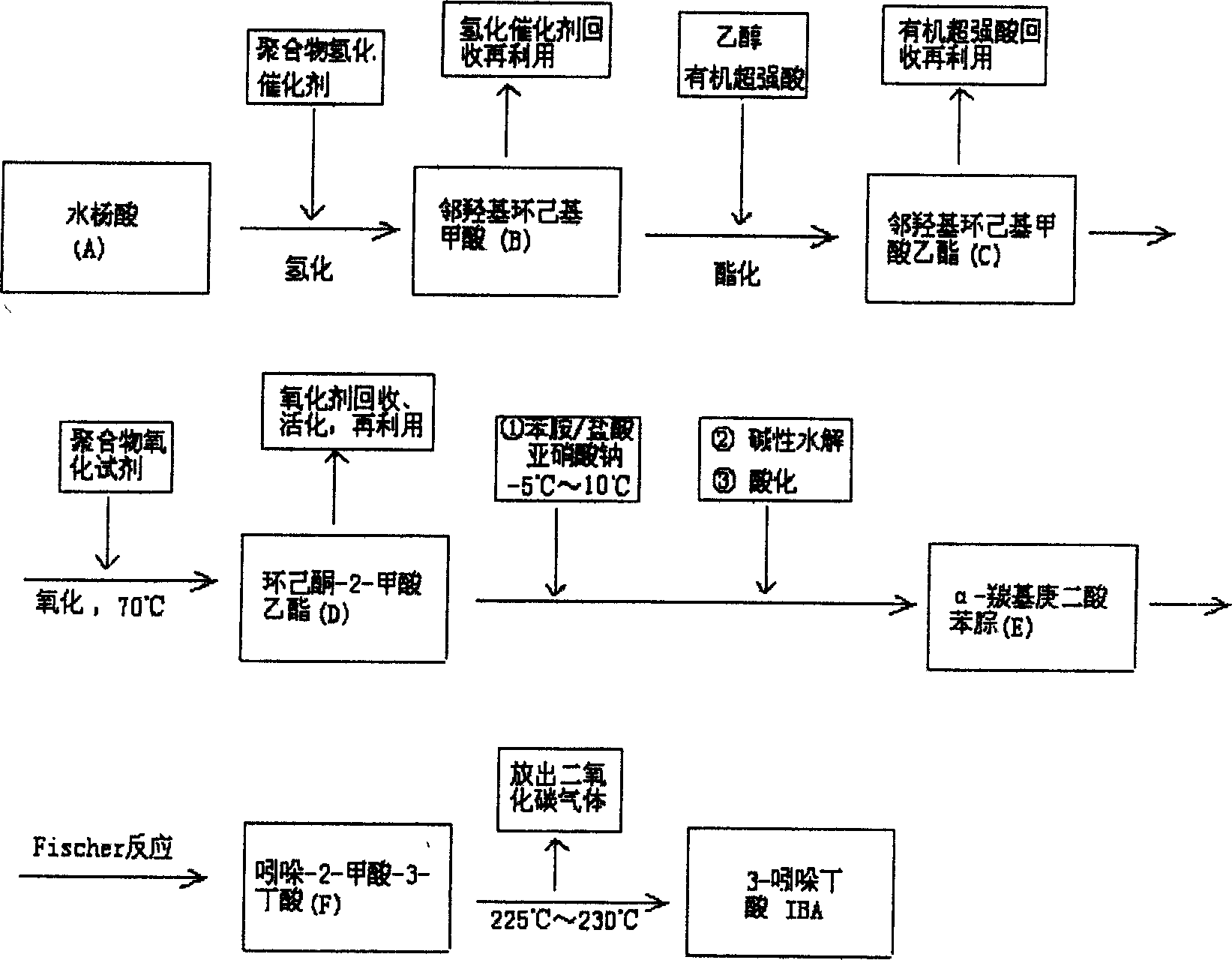
Synthesis of auxin 3-indolebutyric acid is carried out by catalytic hydrogenating for salicylic acid from polymer hydrogenation catalyst at normal temperature and pressure, esterifying with organic super acidic as catalyst with salicylic acid and alcohol=1: 20-1: 80, oxidation reacting from polymer oxidant at 70 Deg C. with saliclyl cyclomenthyl formate: polymer oxidant=1: 1.5-1: 2, condensation reacting at -5-10 Deg C., basic hydrolyzing under water bath condition, acidifying at room temperature with dimethylketone-2-ethyl formate: aniline=1: 1, re-arranging from Fischer, heating to 225-230 Deg C. and decarboxylation reacting. It costs low, has no environmental pollution and gentle reaction condition.
Owner:TIANJIN NORMAL UNIVERSITY
Synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate
ActiveCN102911058ANo purification requiredShort reaction timeOrganic compound preparationCarboxylic acid esters preparationKetoneSodium salt
The invention provides a synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate. The synthetic method comprises the following steps: (1) carrying out annulation reaction on 2-acetonyl-1,4-diethyl succinate and organic alkaline at the temperature of 20-120 DEG C for 0.5-5 hours in a non-polarity organic solvent to obtain 3,5-cyclohexanedione-1-ethyl formate; and (2) adding organic amine and cyclopropanecarboxylic acid chloride, and carrying out acylation reaction at the temperature of minus 5-50 DEG C, so as to obtain 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate in the presence of micromolecular alcohol, ether, ketone and nitrile the carbon atoms of which are below C8 and are used as additives. According to the method, special additives are added before acylation so that cyclopropanecarboxylic acid chloride can directly react with intermediate-state 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol sodium salt to obtain the trinexapac-ethyl precursor 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate, thereby shortening reaction time, simplifying synthesis process and improving yield; and the product is directly rearranged without purification so as to obtain the final product trinexapac-ethyl.
Owner:JIANGSU YOUJIA CHEM +1
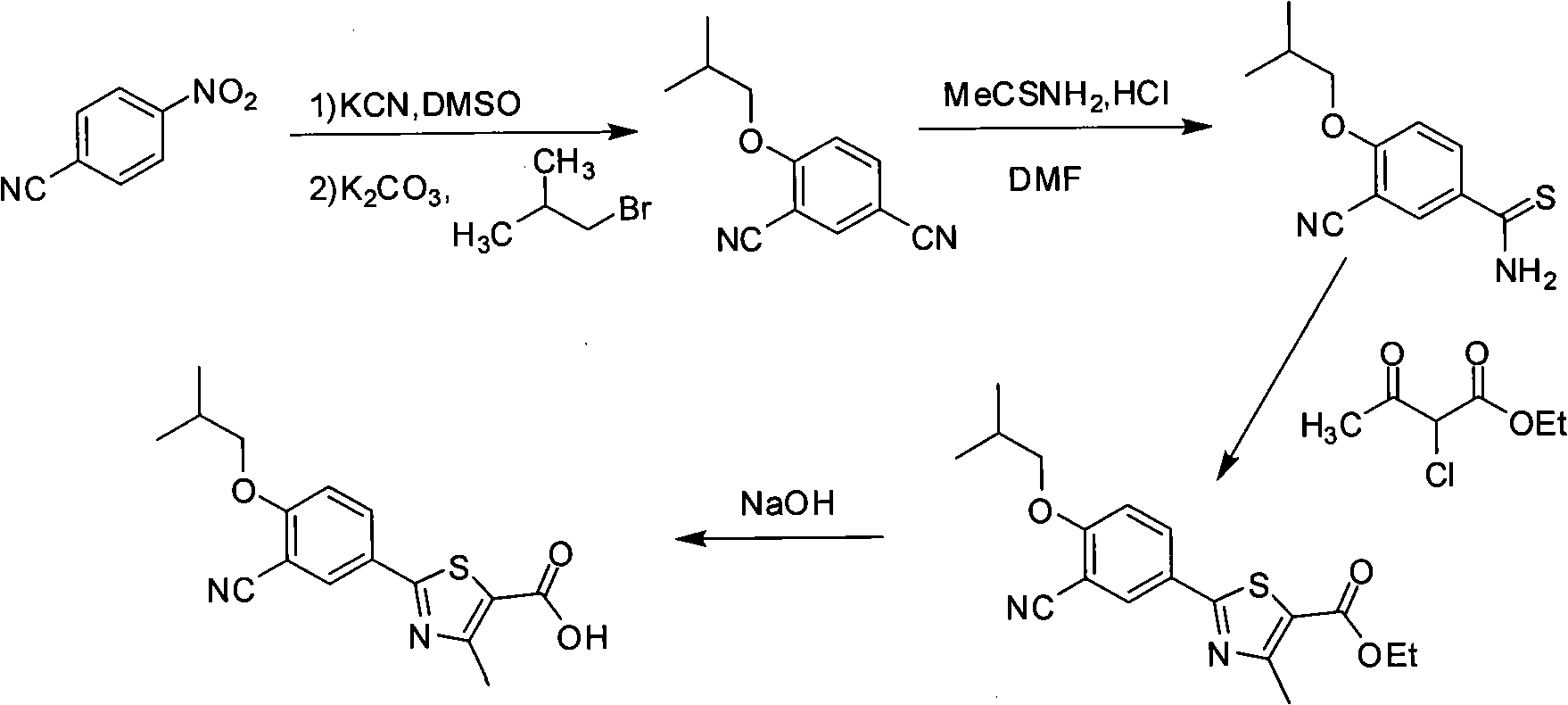
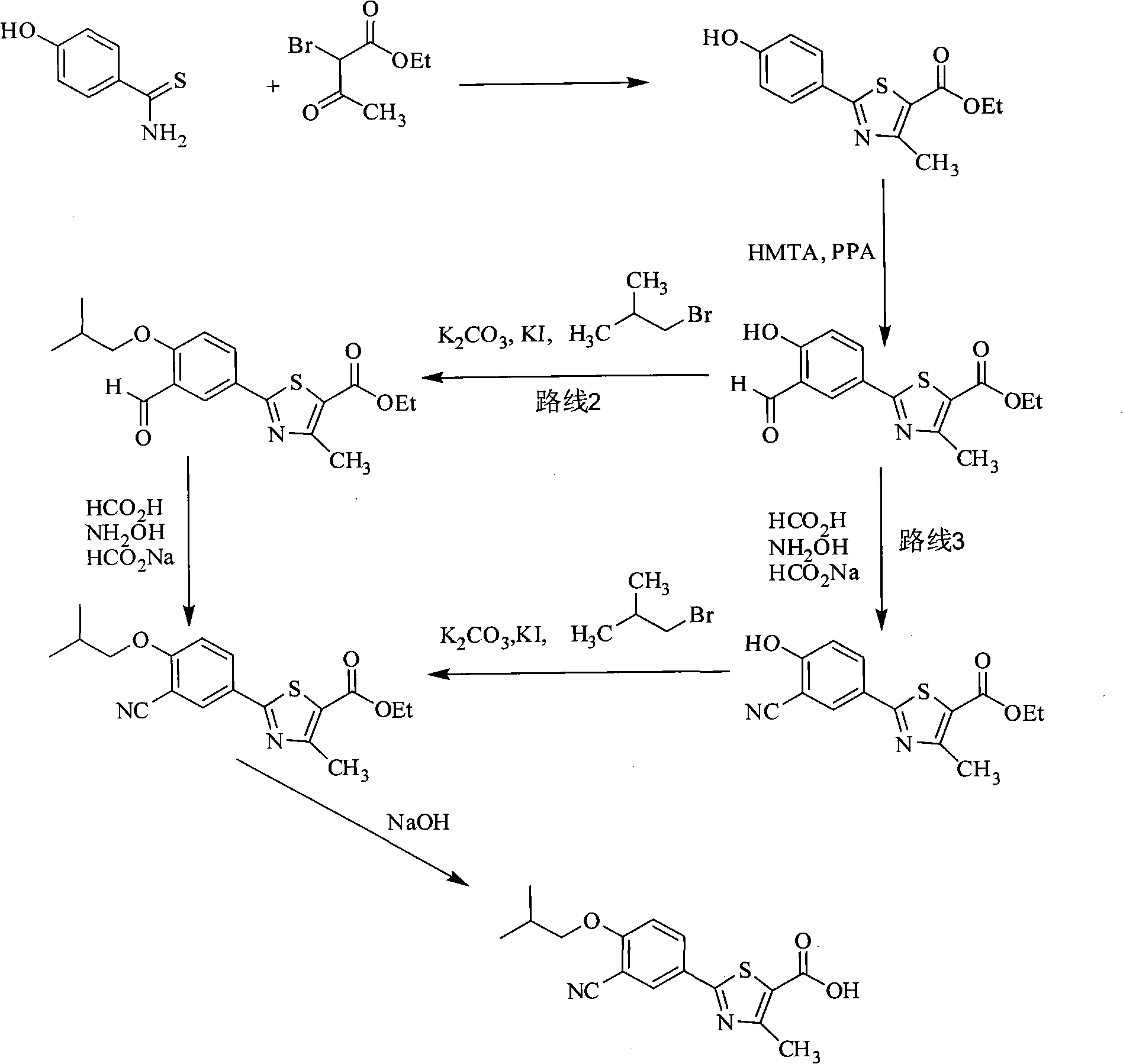
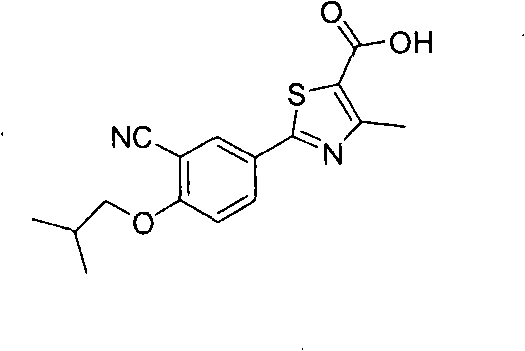
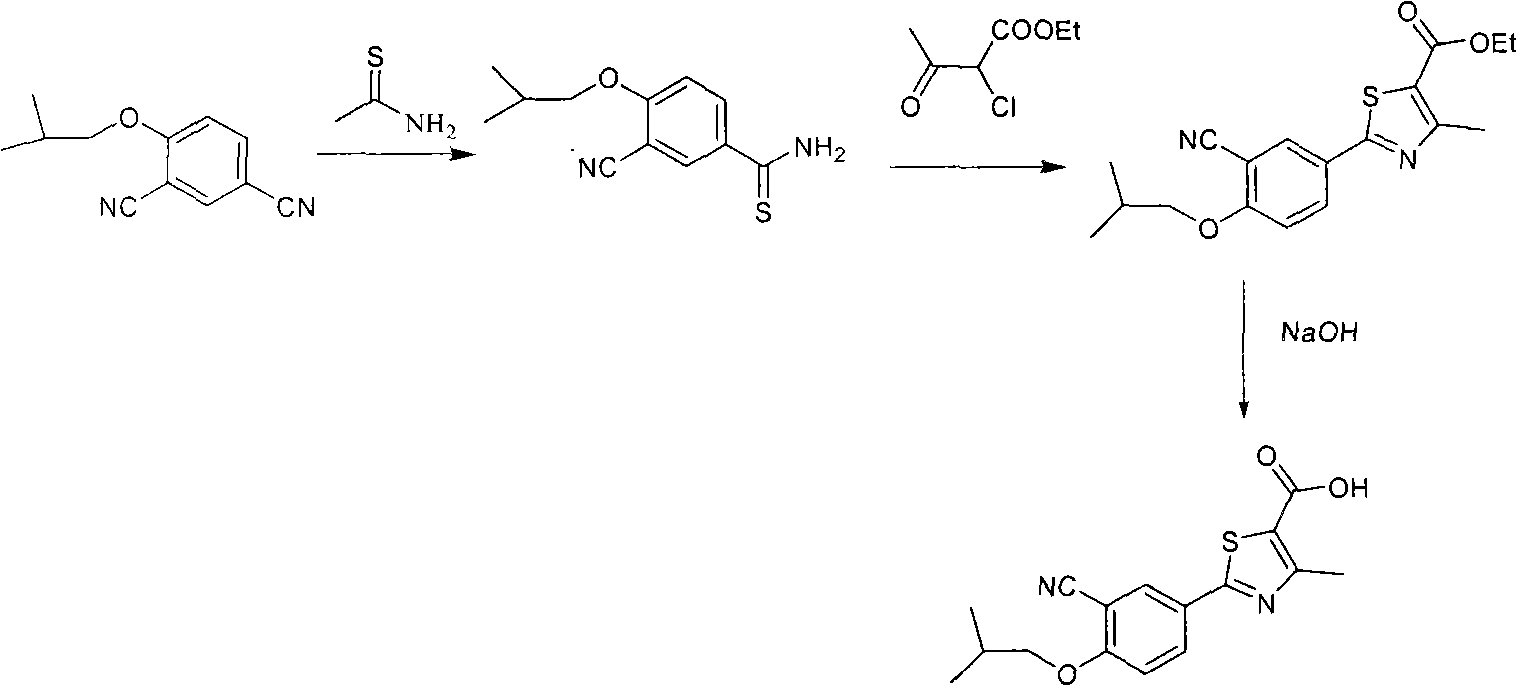
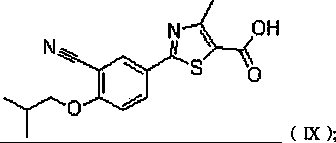
Synthesis method of 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole formic acid
InactiveCN101863854ARaw materials are easy to getSimple and fast operationOrganic chemistryFormylation reactionSynthesis methods
The invention belongs to the technical field of medicine, relating to a synthesis method of 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole formic acid. The synthesis method is characterized by comprising the following steps of: brominating to obtain 3-bromine-4-hydroxyl cyanobenzene by using 4-hydroxyl cyanobenzene as a raw material, alkylating by using bromo-isobutane to obtain 3-bromine-4-isobutoxy cyanobenzene, and cyaniding with cuprous cyanide to obtain 4-isobutoxy-1,3-benzene dinitrile; carrying out a formylation reaction with sodium bisulfide to prepare 3-cyan-4-isobutoxyphenylthioformamide in the presence of anhydrous magnesium chloride, and cyclizing with 2-chloroacetoacetic acid ethyl ester to prepare 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole ethyl formate; and finally, hydrolyzing to prepare the 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole formic acid. The method has the characteristics of easy raw material obtaining, simple and convenient operation, higher yield, lower cost and higher final product purity (the HPLC, High Performance Liquid Chromatography purity is not lower than 99.9 percent) and is suitable for industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
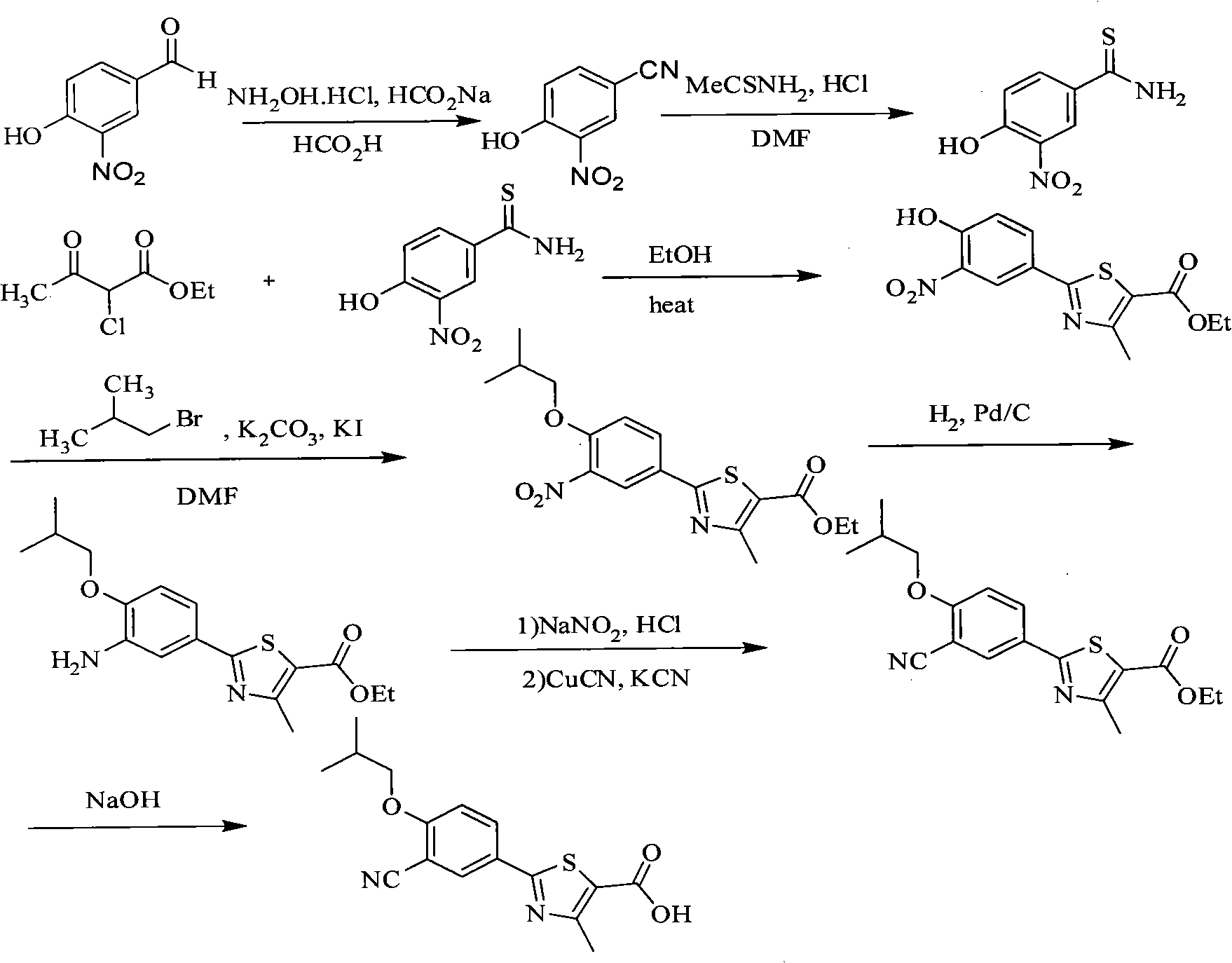
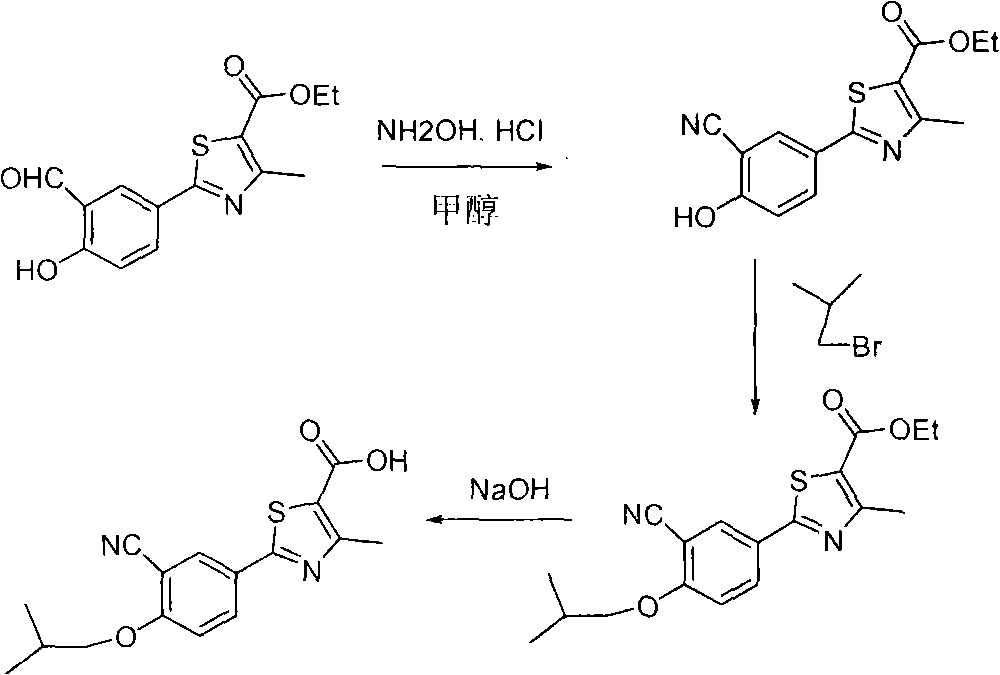
Method for preparing febuxostat intermediate
The invention relates to a method for preparing a febuxostat intermediate, which comprises the following steps of: dissolving 2-[4-hydroxyphenyl]-4-methylthiazol-5-ethyl formate in a mixed acid reaction solvent, adding a certain amount of urotropine, heating to react for 1-36 h at certain temperature, and treating the reaction liquid to obtain corresponding heterocyclic aldehyde.
Owner:CHINA RESOURCES SAIKE PHARMA
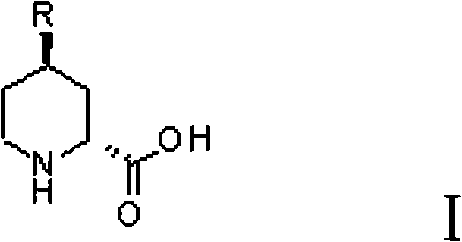
Preparation method for (2R, 4R)-4-substituted-2-piperidine carboxylic acid compound and intermediate thereof
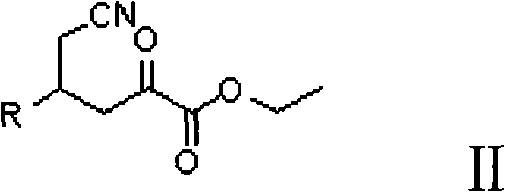
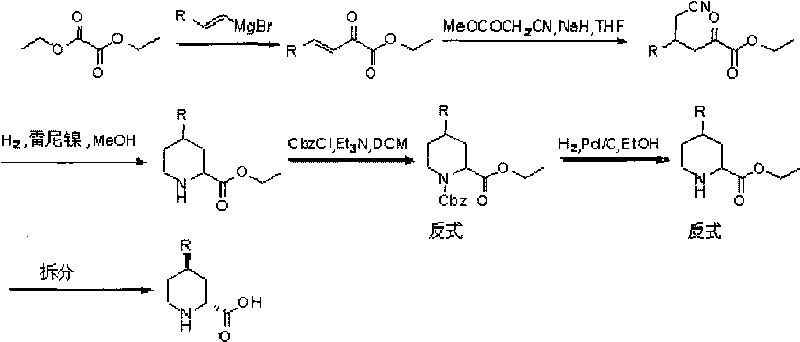
InactiveCN101712645ARaw materials are easy to getSimple processAsymmetric synthesesSynthesis methodsOrganic synthesis
The invention relates to a synthesis method for preparing a (2R,4R)-4-methyl-2 -piperidine carboxylic acid compound taking diethyl oxalate as starting materials and an intermediate thereof, and belongs to the field of organic synthesis. The synthesis method comprises the following steps of: taking the diethyl oxalate and 1-bromo-substituted-propylene as the starting materials, performing a Grignard reaction and an addition reaction on the diethyl oxalate and 1-bromo-3-substituted-propylene to obtain intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate; and then performing a cyclization reaction, a benzyl ester protection reaction and a deprotection reaction on the intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate to obtain trans-4-substituted-2-piperidine ethyl formate; and finally, splitting the trans-4-substituted-2-piperidine carboxylic acid ethyl ester to obtain a chiral target product (2R,4R)-4-methyl-2-piperidine formic acid compound. The preparation method has the advantages of readily available raw materials, simple process, and mild reaction condition.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
Corrosion inhibitor
ActiveUS20060186380A1Growth inhibitionAvoid corrosionOther chemical processesDrilling compositionMannich reactionActive agent
The corrosion inhibitor blend of at least one corrosion inhibitor base (which may be a Mannich reaction product), a solvent selected from the group consisting of C1 acids and ester and salt derivatives thereof, and optionally a surfactant, has been found to be effective as a corrosion inhibitor for metals in acid media, particularly fluids containing halogen acids. The corrosion inhibitor has improved performance over similar or identical corrosion inhibitor compositions where an alcohol such as methanol is used as a solvent. Suitable, non-limiting possibilities for the solvent include, but are not necessarily limited to formic acid, formate salts, methyl formate, ethyl formate, benzyl formate, formate salts of amines, inorganic formate, and mixtures thereof.
Owner:BAKER HUGHES INC
Preparation method of high-purity febuxostat
The invention relates to a preparation method of high-purity febuxostat. The method comprises the steps: carrying out etherification reaction on ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate and refined bromo-isobutane, then conducting cyaniding and hydrolysis to obtain other crude febuxostat, and recrystalizing to obtain high-purity febuxostat. In febuxostat prepared by using the method, the content of impurity 2-(3-cyano-4-n-propoxy phenyl)-4-methylthiazole-5-formic acid is smaller than 0.10%.
Owner:CHINA RESOURCES SAIKE PHARMA
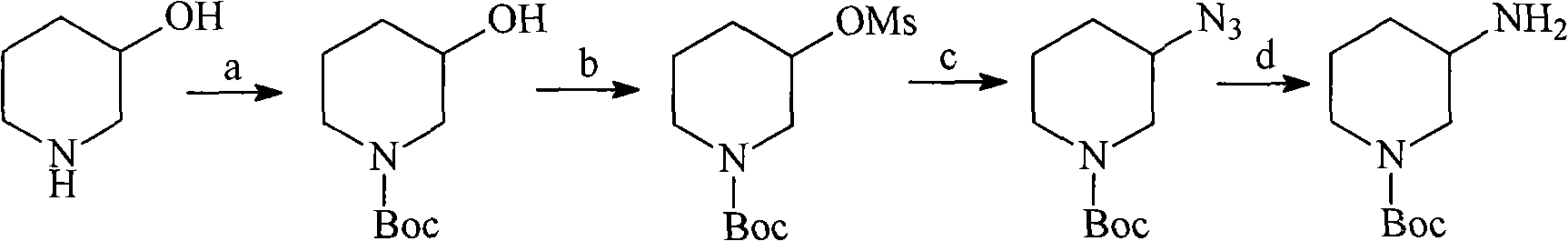
N-Boc-3-aminopiperidine and synthesizing method of optical isomer thereof
The invention discloses an intermediate N-Boc-3-aminopiperidine and a synthesizing method of optical isomer thereof. The prior synthesizing route has the disadvantages of high requirements on reaction conditions, high possibility of racemization, a great number of byproducts, as well as difficult post-treatment and difficult industrialization. The synthesizing method comprises the following steps: taking halogenated hydrocarbon as a solvent and organic alkali as an acid-binding agent, 3-piperidine ethyl formate is added with di-tert-butyl dicarbonate by dripping at temperature of 0-10 DEG C to obtain N-Boc-3-piperidine ethyl formate; 1,4-dioxane is used as a solvent, the N-Boc-3-piperidine ethyl formate undergoes ammonolysis reaction to obtain N-Boc-3-piperidine formamide; and the N-Boc-3-piperidine formamide is added by dripping in a solution with sodium hypochlorite and sodium hydroxide to obtain N-Boc-3-aminopiperidine. The invention has the advantages of no racemization during the reaction, high optical purity of the product, relatively moderate reaction conditions, simple operation, low total production cost, and easy large-scale industrial production.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
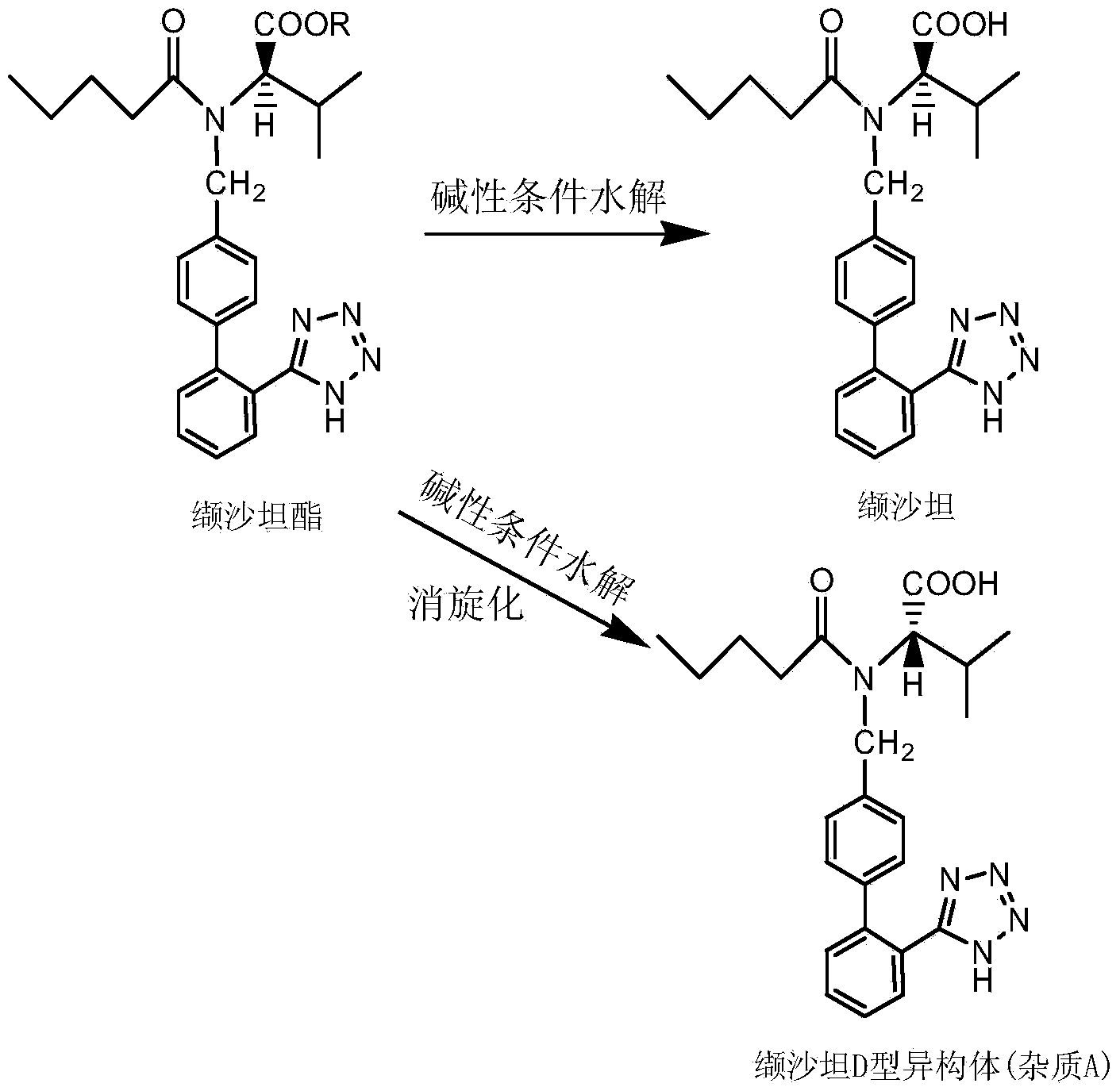
Valsartan refining method
The invention discloses a valsartan refining method, belonging to the technical field of medical chemistry. The method comprises the following steps: dissolving a valsartan crude product with isomer content of 1.0-10.0% in an alcohol solvent-ester solvent system; heating to 30-70 DEG C so as to completely dissolve the valsartan crude product; and slowly cooling, separating out crystals and filtering to obtain a valsartan refined product. The alcohol solvent is one of methanol, ethanol and isopropyl alcohol or the mixture of two or three of methanol, ethanol or isopropyl alcohol in any proportion; and the ester solvent is one of ethyl formate, ethyl acetate and methyl acetate or the mixture of two or three of ethyl formate, ethyl acetate and methyl acetate in any proportion. The mass ratio of the alcohol solvent to the ester solvent to the valsartan crude product is (0.05-1.0):(1.0-10.0):1. By adopting the method disclosed by the invention, the isomer content in the valsartan crude product is reduced to be hardly detected from 1.0-10.0%, and the quality of the finished product of valsartan is improved; the particle size of the finished product of valsartan is improved, thereby facilitating the product charge, centrifugation and drying; and the method is simple to operate, low in cost and suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for preventing and curing plant disease by increasing plant immunity and use thereof
The invention provides a plant protective method and application of inducing immune system of plant by 4-methyl-1,2,3-thiadiazolyl-5-formic acid (NK-F001), sodium 4-methyl-1,2,3-thiadiazolyl-5-formate (NK-F002), 4-methyl-1,2,3-thiadiazolyl-5-ethyl formate (NK-F003) and methenaimde (SZG-7) to generate resistance against the plant virus. The invention also provides an application for improving the plant immunity by combination of two or three of 4-methyl-1,2,3-thiadiazolyl-5-formic acid (NK-F001), sodium 4-methyl-1,2,3-thiadiazolyl-5-formate (NK-F002), 4-methyl-1,2,3-thiadiazolyl-5-ethyl formate (NK-F003), methenaimde (SZG-7), benzthiadiazole (activated ester, BTH), tiadinil (TDL), DL-Beta-aminobutyric acid, virazole, ningnanmycin, antofine, Bingduxing No.1, Bingduxing No.2, XY-13 and XY-30 so as to induce the plant to generate antiviral activity, processing dosage forms of single and mixture preparations, and compositions and contents of effective components in the dosage forms. The invention discloses a plant inducing range and plant antiviral inducing range of compounds and compositions thereof, and application of plant protection in the fields of agriculture and horticulture thereof.
Owner:LIER CHEM CO LTD
Preparation methods of compound 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate and febuxostat
The invention provides a preparation method of 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate which is obtained by using 4-isobutoxy cyanophenyl as an initial raw material and through a series of reactions. The invention also provides a preparation method of febuxostat, which comprises the following steps: reacting 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate with hydroxylamine hydrochloride under the action of a catalyst to obtain a compound with a structure as shown in formula (VIII); hydrolyzing the compound with the structure as shown in formula (VIII) under an alkaline condition, and performing acidification to obtain febuxostat. The preparation method of the invention prepares febuxostat without using cyanides, and is high in safety. The preparation methods of the invention are simple in operation and high in yield. Experiment results show that the yield of step (A) is up to 90%, the yield of step (B) is up to 85%, the yield of step (C) is up to 90%, the yield of step (D) is up to 90%, and the yield of step (E) is up to 97%.
Owner:ZHEJIANG AUSUN PHARMA
Preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine
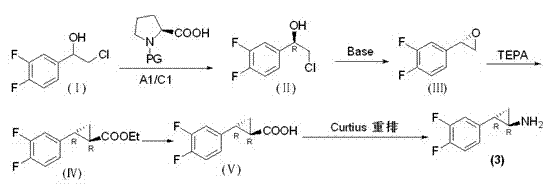
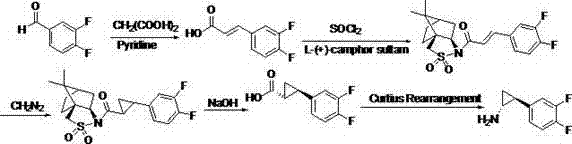
InactiveCN102775314AFew stepsEasy to operateHydroxy compound separation/purificationPreparation by rearrangement reactionsEpoxyPtru catalyst
The invention provides a preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine. The preparation method comprises the following steps: enabling racemic chloro phenethyl alcohol (I) and N-protection proline to undergo a reaction under the effects of a condensing agent A1 and a catalyst C1, and obtaining chiral chlorohydrin (II); enabling the chiral chlorohydrin (II) to generate an epoxy compound (III) under the conditions of alkalinity; enabling the epoxy compound (III) and TEPA to react and generate cyclopropyl ethyl formate (IV) under the conditions of alkalinity; removing ester from cyclopropyl ethyl formate (IV) under the conditions of alkalinity, and generating cyclopropanecarboxylic acid (V); and enabling cyclopropanecarboxylic acid (V) and azide to generate a target compound (3) by Curtius rearrangement. The preparation method has the advantages that the steps of a used synthetic process route are few, the operation is simple, and industrial production is achieved easily; a kinetic resolution method is utilized to synthesize chiral chlorohydrin (II) and is simple in reaction conditions and easy to operate; and a TEPA method is utilized synthesize the cyclopropyl ethyl formate, the product yield is high, cis-trans selectivity is good, and the purity is over 99%.
Owner:江苏富泽药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com