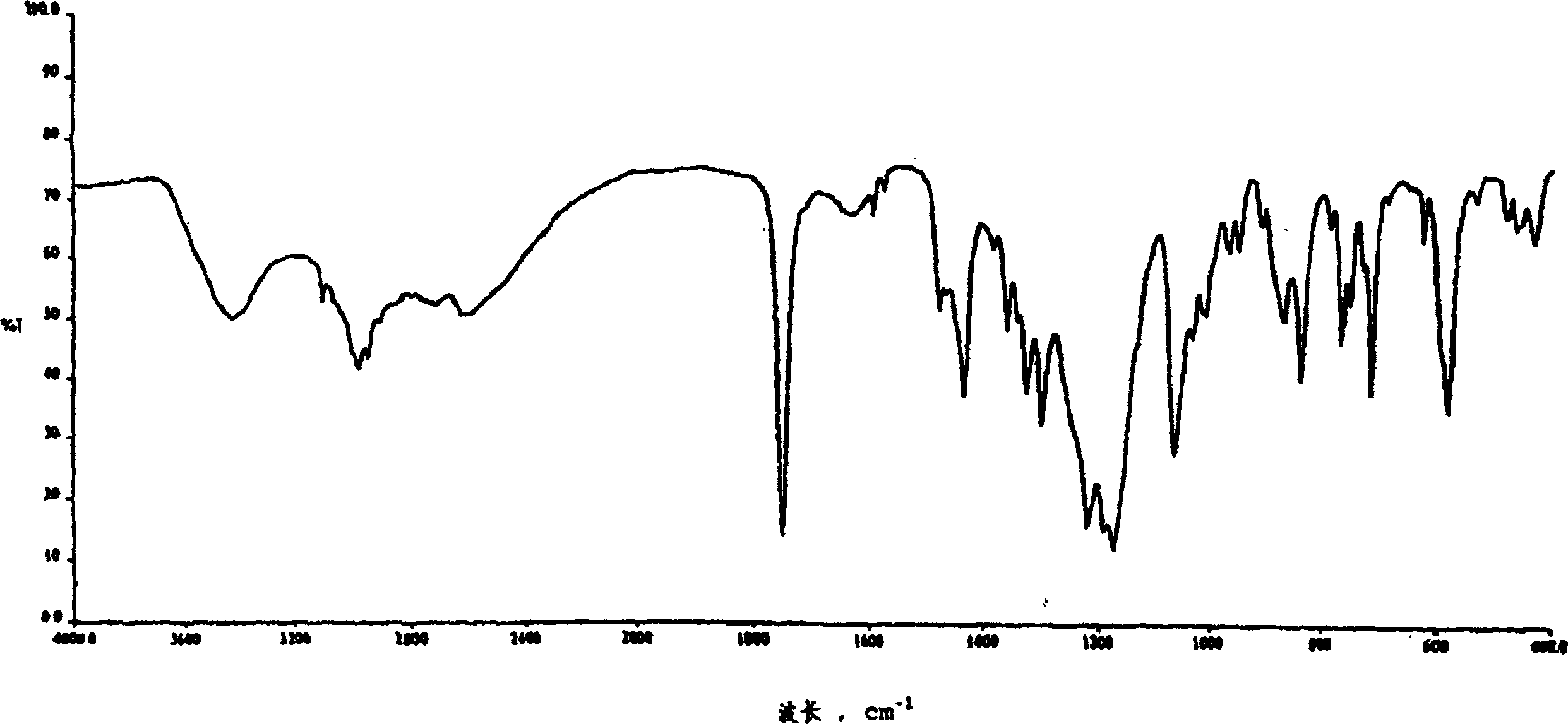
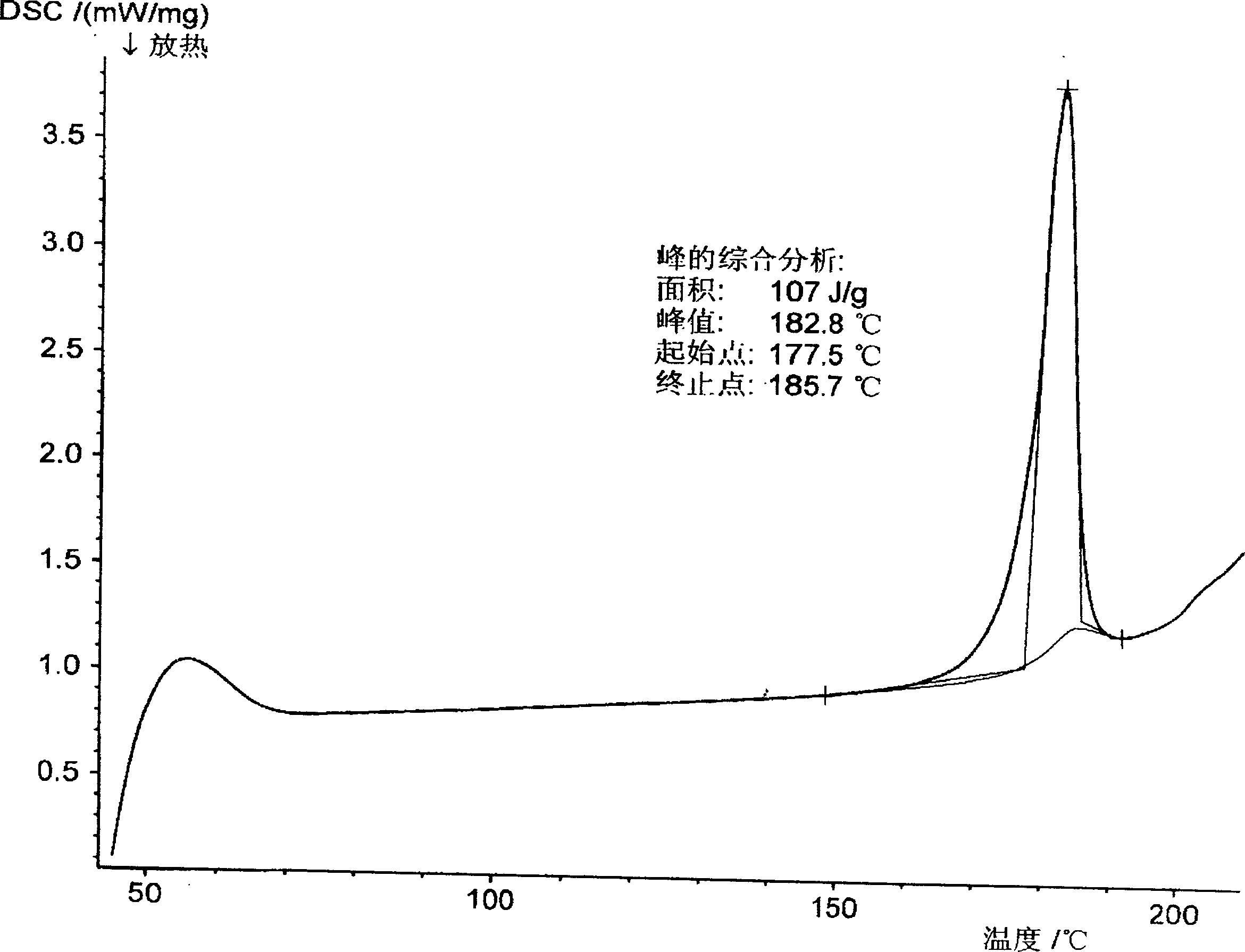
Method for preparing (+)-(s-)-clopiclogrel hydrogensulfate (I)
A technology for clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in the preparation of hydrogen sulfate type I crystals, and in the field of antithrombotic drugs-clopidogrel hydrogen sulfate, which can solve the flow of clopidogrel hydrogen sulfate. The performance and stability are not ideal, it is unfavorable for large-scale industrial production, and the preparation cycle is long.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of (+)-(S-)-clopidogrel free base
[0035]Inhalation of methylene chloride 200kg in the free still, add (+)-(S-)-clopidogrel hydrochloride 23kg, under stirring, dropwise add sodium bicarbonate aqueous solution (12kg sodium bicarbonate and 100kg water are made into), dropwise After adding, stir for another 30 minutes, let stand for 10 minutes, separate the lower organic phase, extract the aqueous layer twice with 100Kg×2 dichloromethane, combine the organic phases, wash the combined phase with 5% aqueous sodium bicarbonate solution to pH 7 ~8, washed once with 100kg of water, dried by adding 10kg of anhydrous magnesium sulfate, filtered under pressure, and distilled to recover dichloromethane to obtain 20kg of oily (+)-(S-)-clopidogrel free base.
Embodiment 2
[0036] Example 2 Preparation of (+)-(S-)-clopidogrel free base
[0037] Inhalation of methylene chloride 200kg in the free still, add (+)-(S-)-clopidogrel hydrochloride 23kg, under stirring, drip an aqueous sodium carbonate solution (7kg sodium carbonate and 100kg water), dropwise complete , stir for another 30 minutes, stand for 10 minutes, separate the lower organic phase, extract the aqueous layer twice with 100Kg×2 dichloromethane, combine the organic phases, and wash the combined phase with a 5% aqueous sodium bicarbonate solution to pH 7-8 , washed once with 100kg of water, added 10kg of anhydrous sodium sulfate, dried, filtered under pressure, and distilled to recover dichloromethane to obtain 20kg of oily (+)-(S-)-clopidogrel free base.
Embodiment 3
[0038] Example 3 Preparation of (+)-(S-)-clopidogrel free base
[0039] Inhalation of methylene chloride 200kg in the free still, add (+)-(S-)-clopidogrel sulfate 26.9kg, under stirring, dropwise add sodium carbonate aqueous solution (formed by 7kg sodium carbonate and 100kg water), dropwise complete , stir for another 30 minutes, stand for 10 minutes, separate the lower organic phase, extract the aqueous layer twice with 100Kg×2 dichloromethane, combine the organic phases, and wash the combined phase with a 5% aqueous sodium bicarbonate solution to pH 7-8 , washed once with 100kg of water, added 10kg of anhydrous sodium sulfate, dried, filtered under pressure, and distilled to recover dichloromethane to obtain 20kg of oily (+)-(S-)-clopidogrel free base.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com