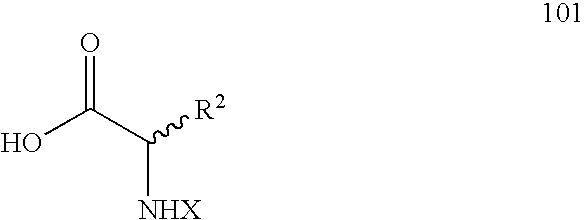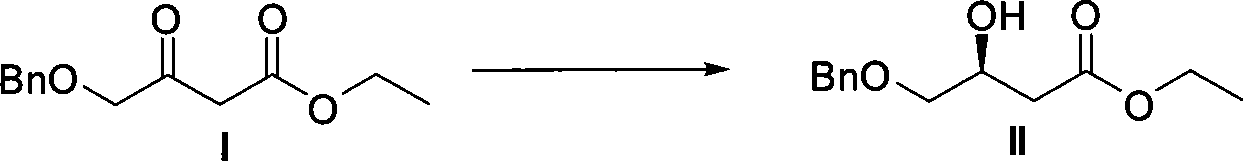Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1893results about "Asymmetric syntheses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
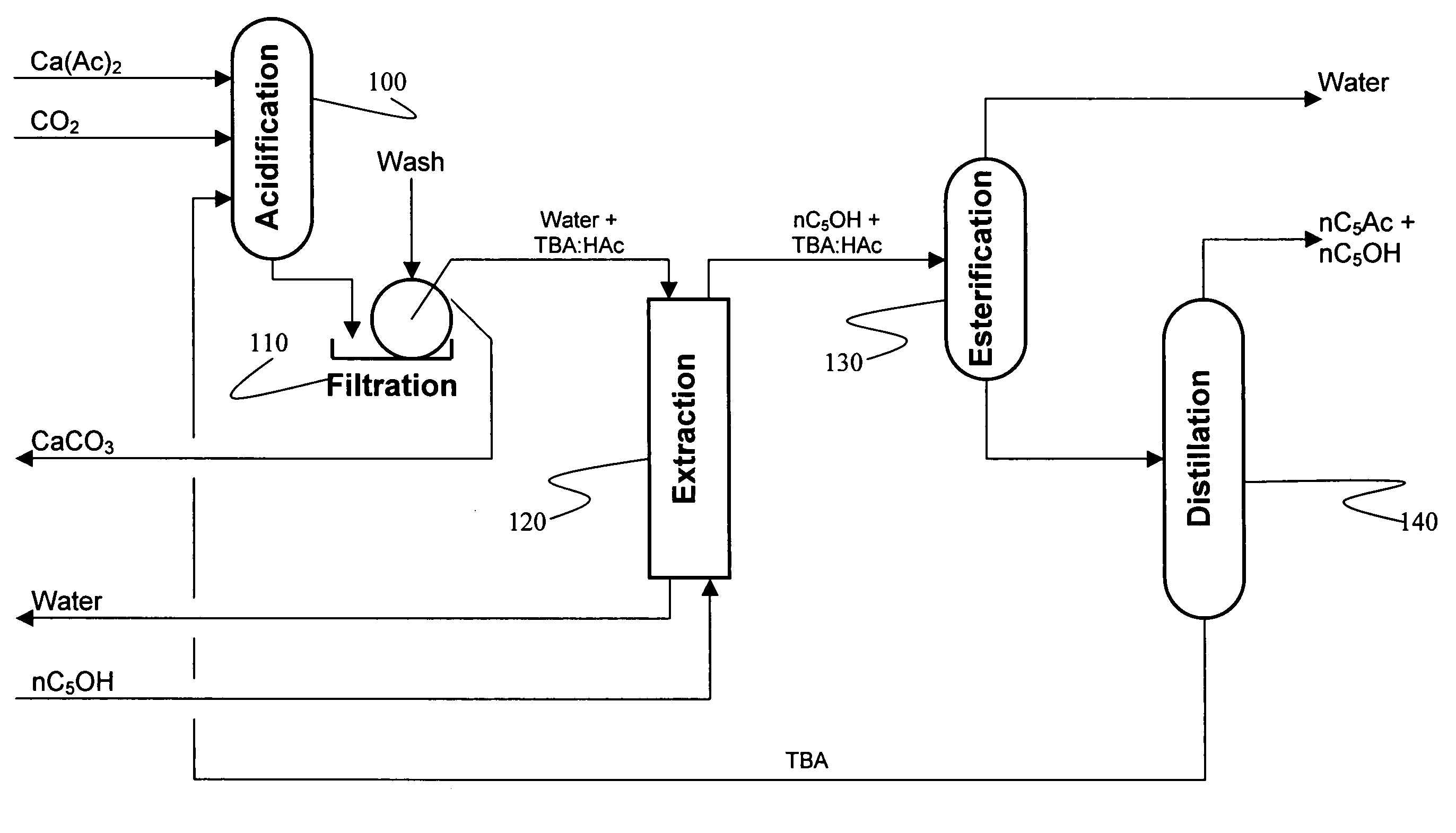
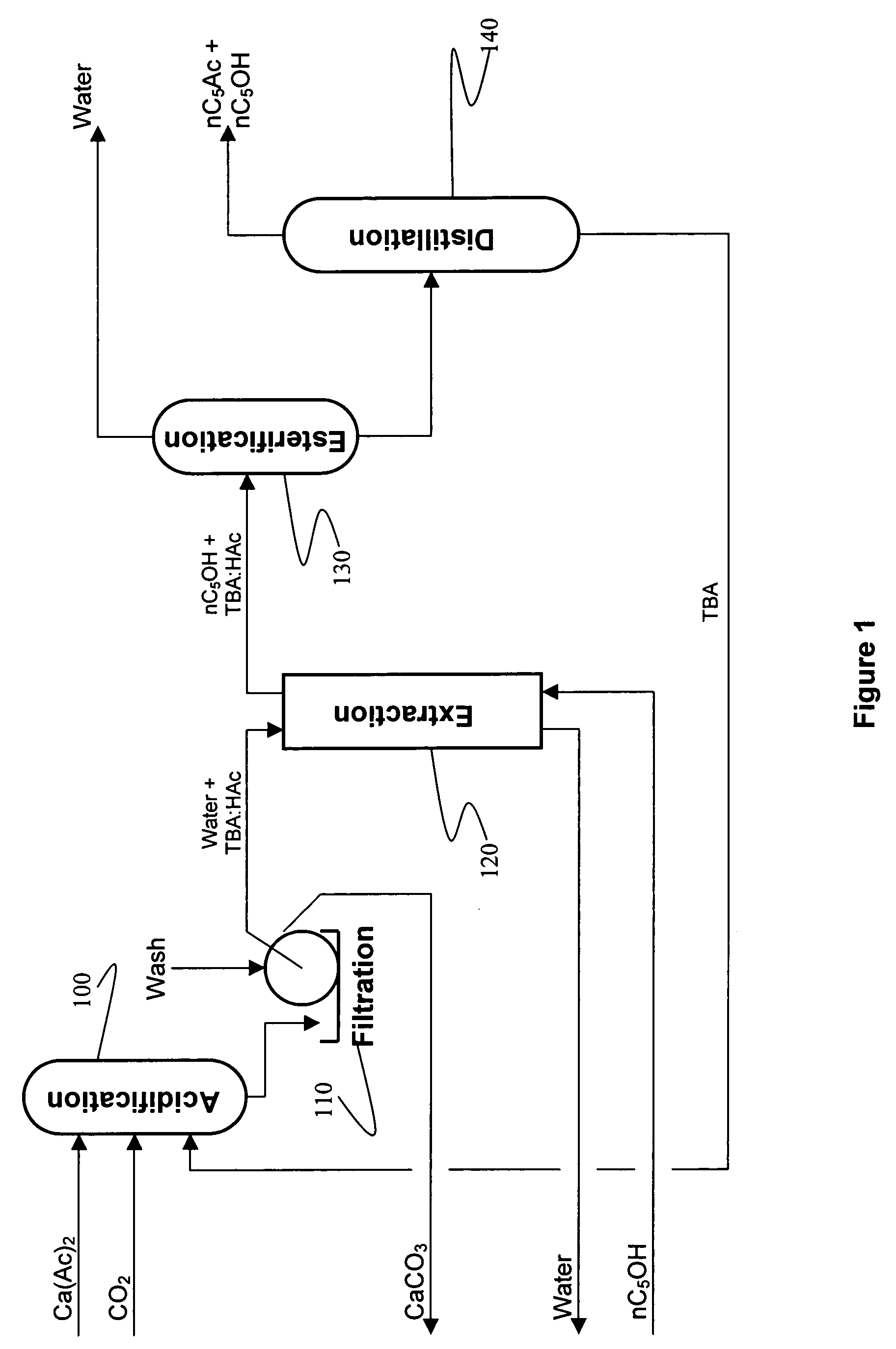
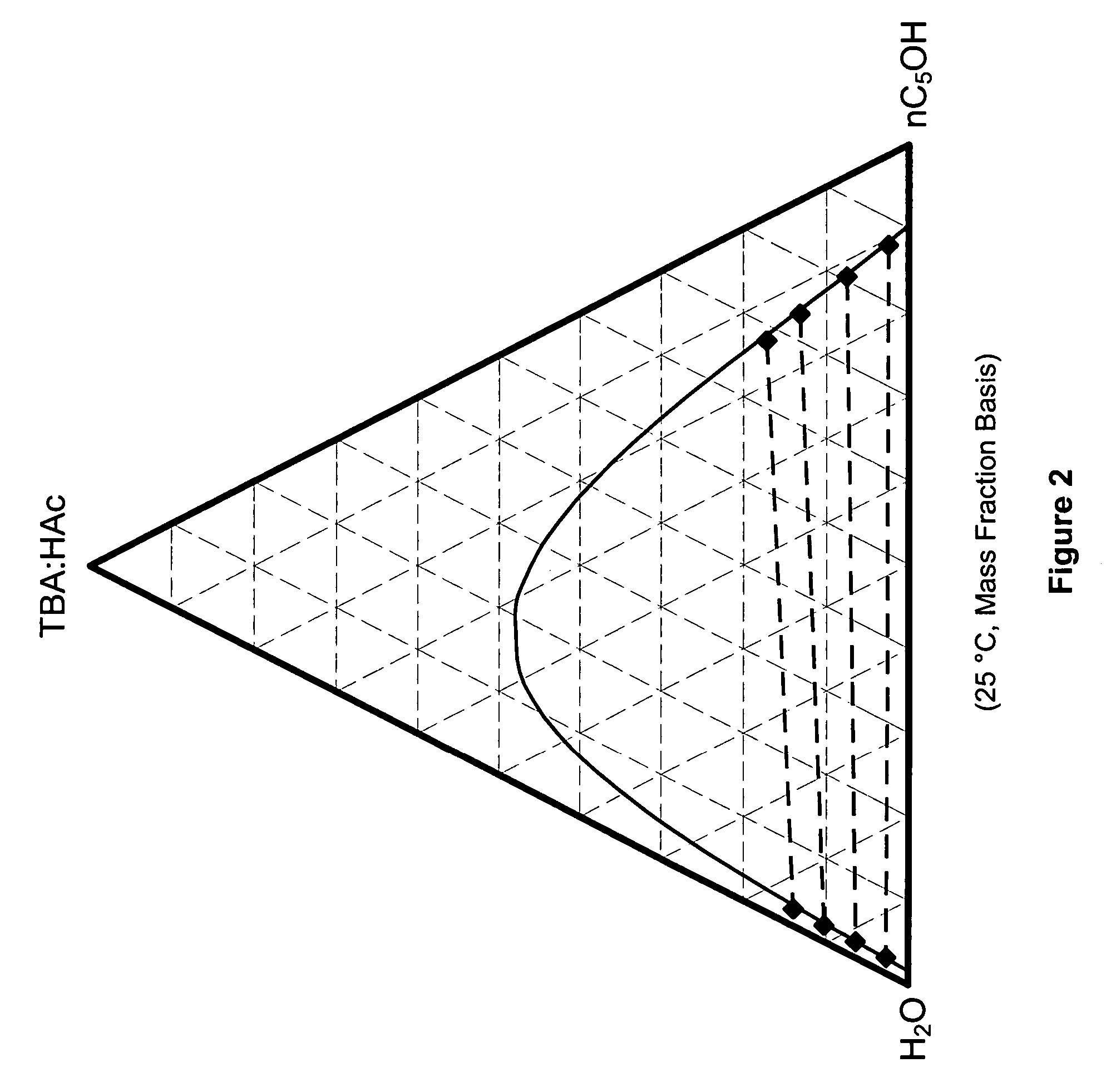
Recovery of organic acids
InactiveUS7601865B2Promote recoveryOvercome consumptionPreparation from carboxylic acid saltsOrganic compound preparationOrganic acidAlcohol
Owner:ZEACHEM
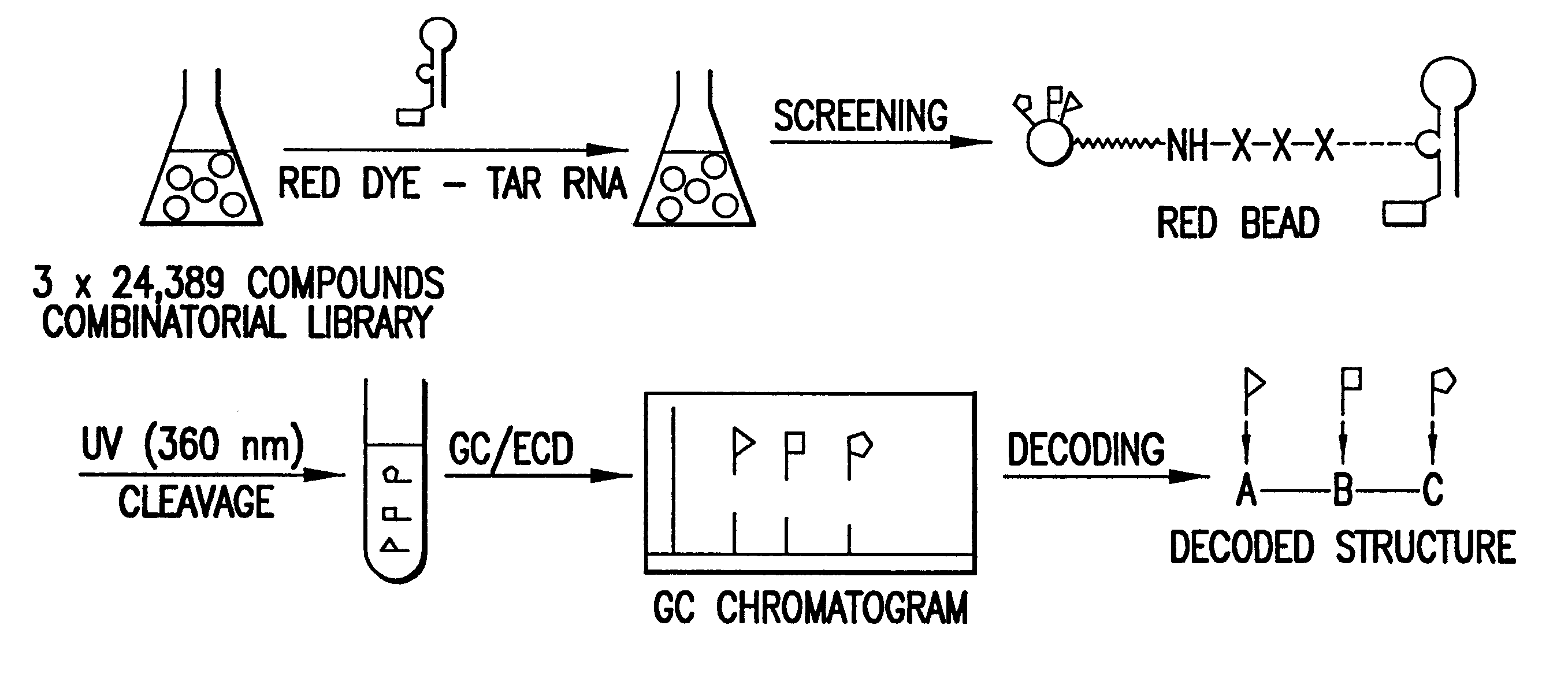
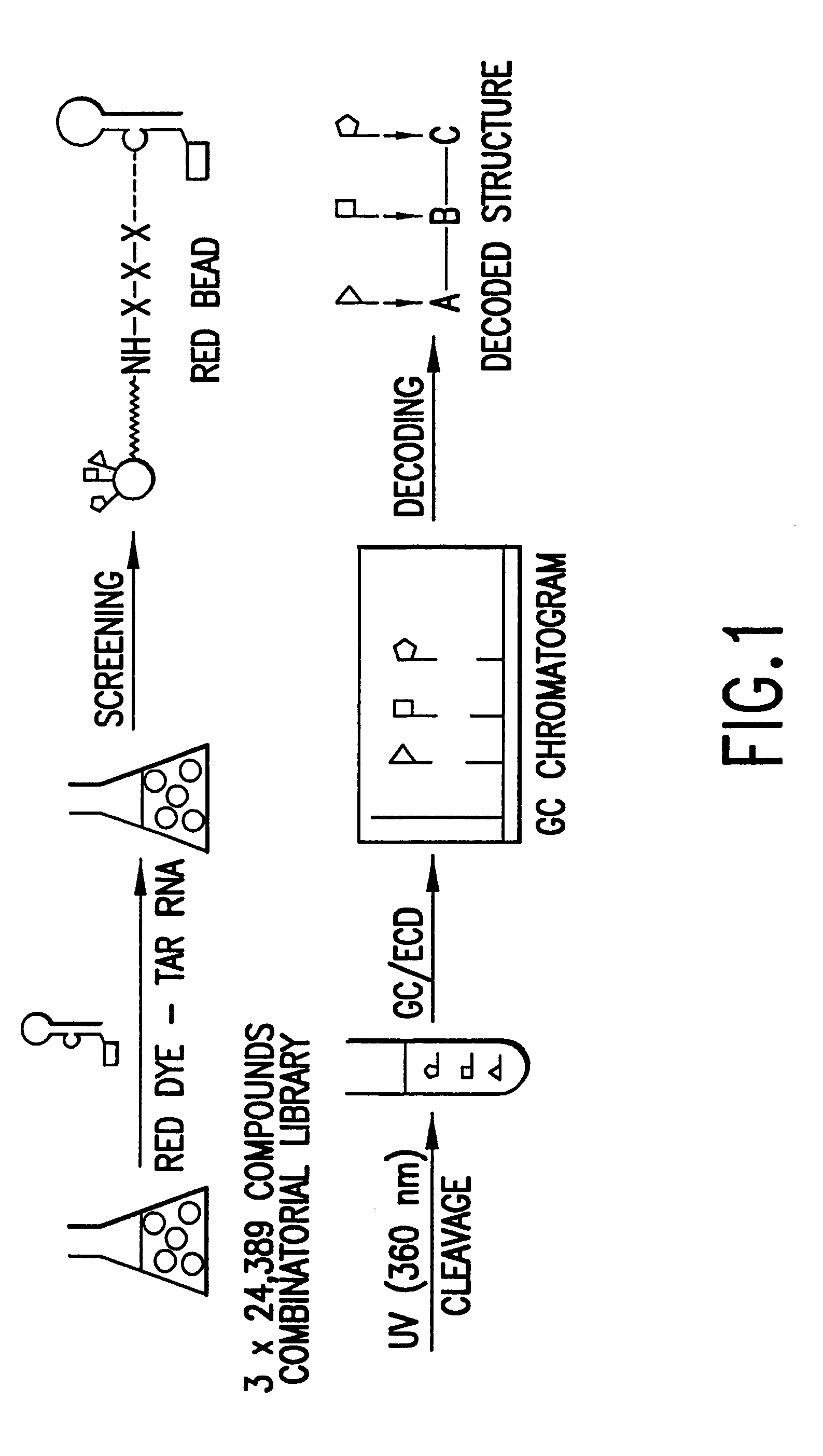
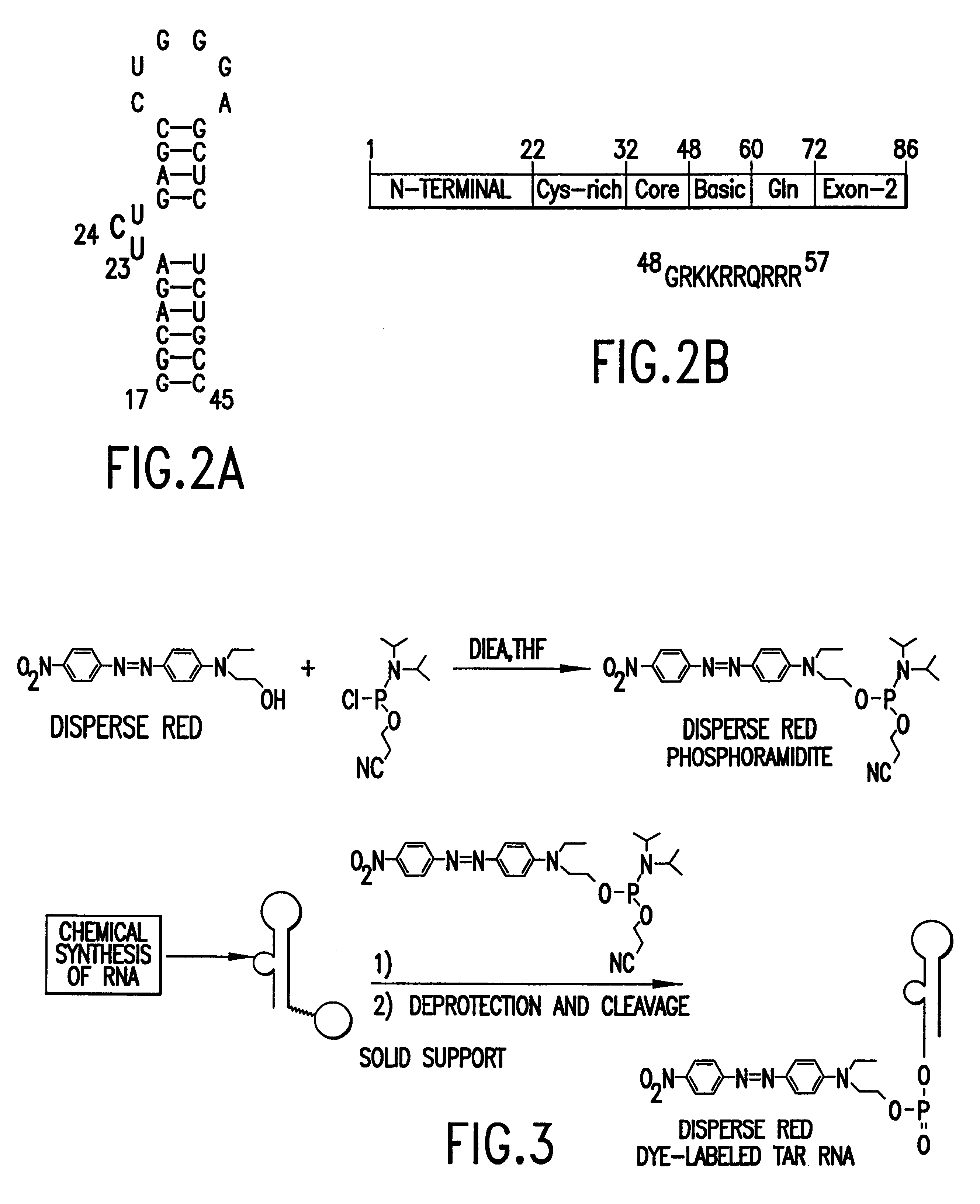
Methods for identifying RNA binding compounds
Owner:RUTGERS THE STATE UNIV
Process for the oxidative purification of terephthalic acid
InactiveUS20040110980A1Organic compound preparationCarboxylic preparation by oxidationSlurryCarboxylic acid
Disclosed is a process to produce a purified carboxylic acid slurry. The process comprises removing impurities from a crystallized product in a solid liquid displacement zone to form the purified carboxylic acid slurry. The process produces purified carboxylic acid slurry having good color and low impurity levels without the use of purification steps like hydrogenation or an impurity removal process.
Owner:ALPEK POLYESTER SA DE CV
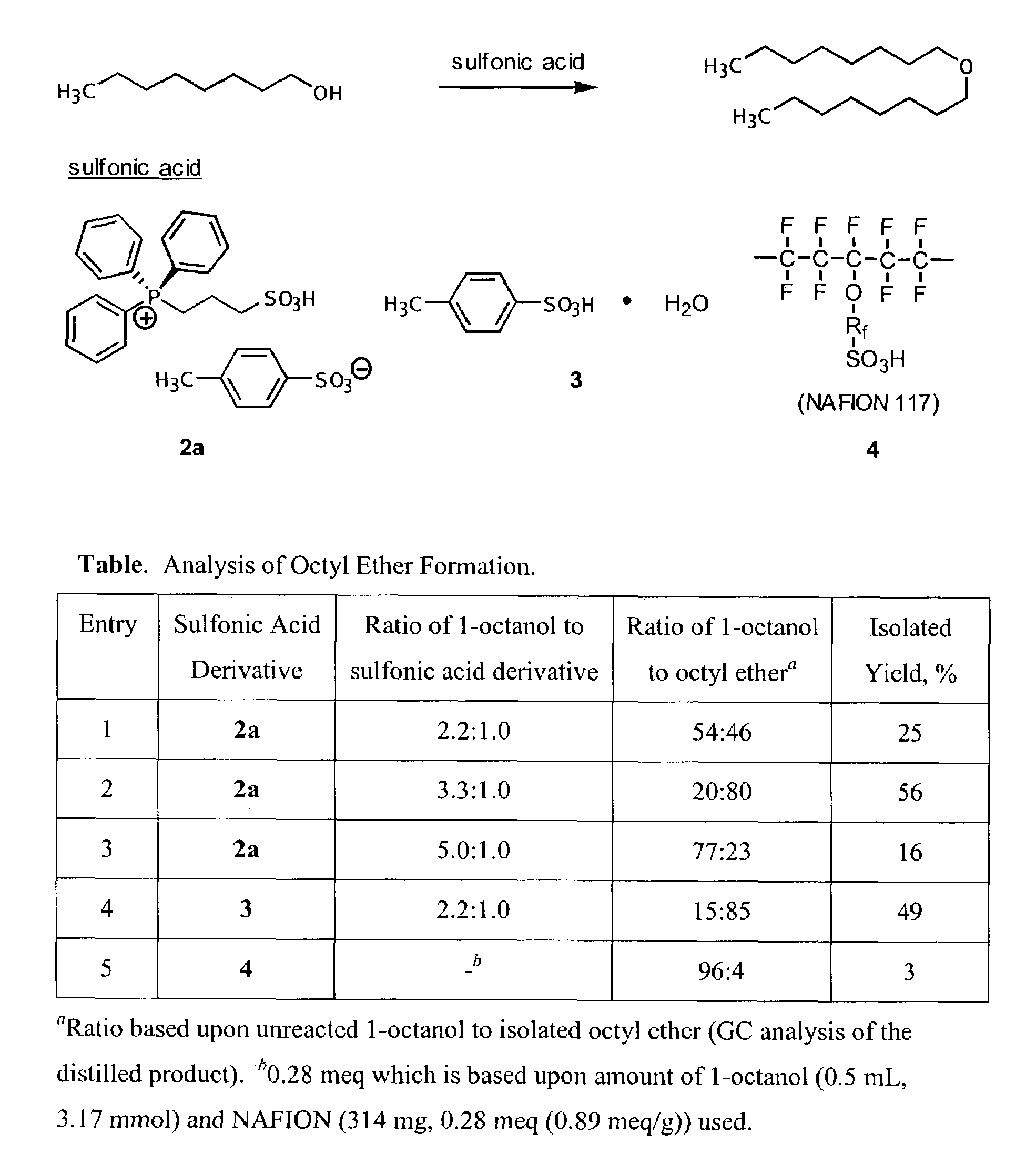
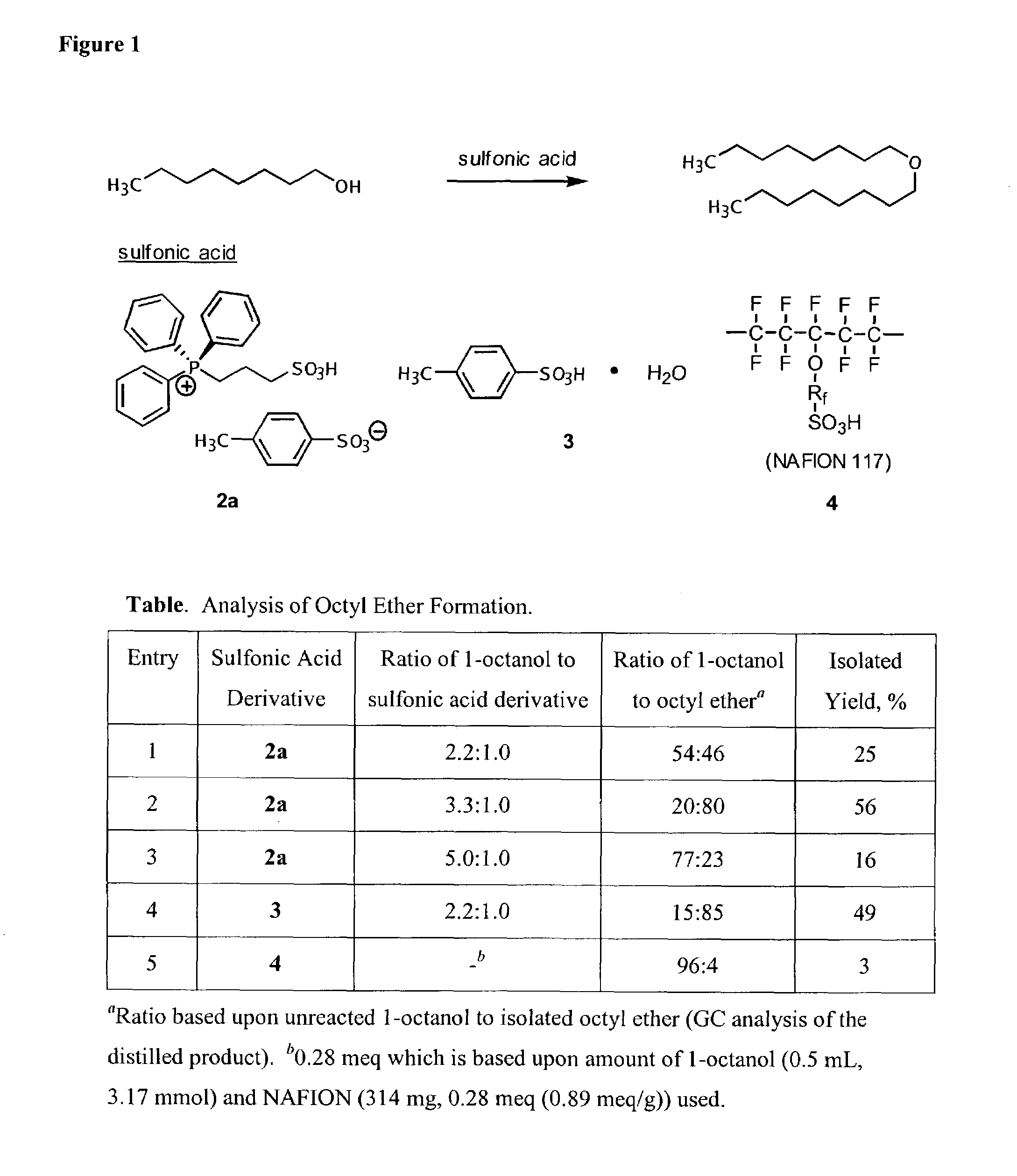
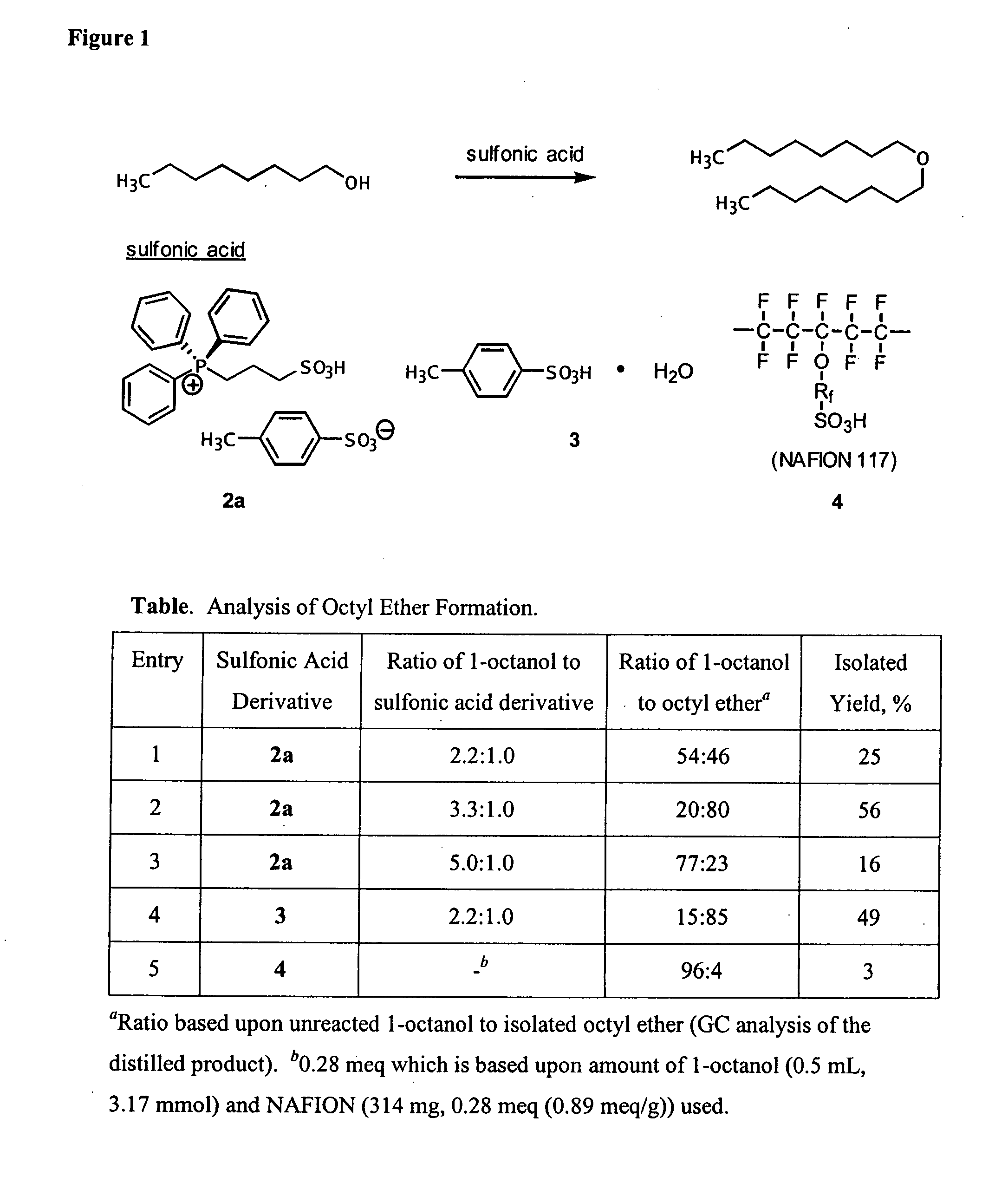
Functionalized ionic liquids, and methods of use thereof
One aspect of the present invention relates to ionic liquids comprising a pendant Bronsted-acidic group, e.g., a sulfonic acid group. Another aspect of the present invention relates to the use of an ionic liquid comprising a pendant Bronsted-acidic group to catalyze a Bronsted-acid-catalyzed chemical reaction. A third aspect of the present invention relates to ionic liquids comprising a pendant nucleophilic group, e.g., an amine. Still another aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to catalyze a nucleophile-assisted chemical reaction. A fifth aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to remove a gaseous impurity, e.g., carbon dioxide, from a gas, e.g., sour natural gas.
Owner:UNIV OF SOUTH ALABAMA
Synthesis of UDP-glucose: N-acylsphingosine glucosyltransferase inhibitors
Disclosed is a novel enantiomeric synthesis cermamide-like inhibitors of UDP-glucose: N-acylsphingosine glucosyltransferase. Also disclosed are novel intermediates formed during the synthesis.
Owner:GENZYME CORP
Amine-containing lipids and uses thereof
Nitrogen-containing lipids prepared from the conjugate addition of amines to acrylates, acrylamides, or other carbon-carbon double bonds conjugated to electron-withdrawing groups are described. Methods of preparing these lipids from commercially available starting materials are also provided. These amine-containing lipids or salts forms of these lipids are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems. Given the amino moiety of these lipids, they are particularly suited for the delivery of polynucleotides. Complexes or nanoparticles containing the inventive lipid and polynucleotide have been prepared. The inventive lipids may also be used to in preparing microparticle for drug delivery. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
Synthesis of epothilones and related analogs
InactiveUS6989450B2Easy to understandOrganic active ingredientsGroup 4/14 element organic compoundsEpothiloneStereoisomerism
The present invention relates to methods for use in producing epothilones and analogs and derivatives thereof. A general method according to the present invention broadly comprises performing an aldol condensation of a first compound with a second compound thereby to form a third compound selected from the formulas: and stereoisomers thereof, and performing a macrolactonization of the third compound. The present invention also provides chemical compounds, and methods for producing such chemical compounds, that are useful in producing epothilones and analogs and derivatives thereof.
Owner:UNIVERSITY OF MISSISSIPPI
Compositions comprising a solvated metal
InactiveUS7332627B2Increase the amount of metalImprove stabilityBiocideGroup 3/13 organic compounds without C-metal linkagesOrganic acidOrganic fluid
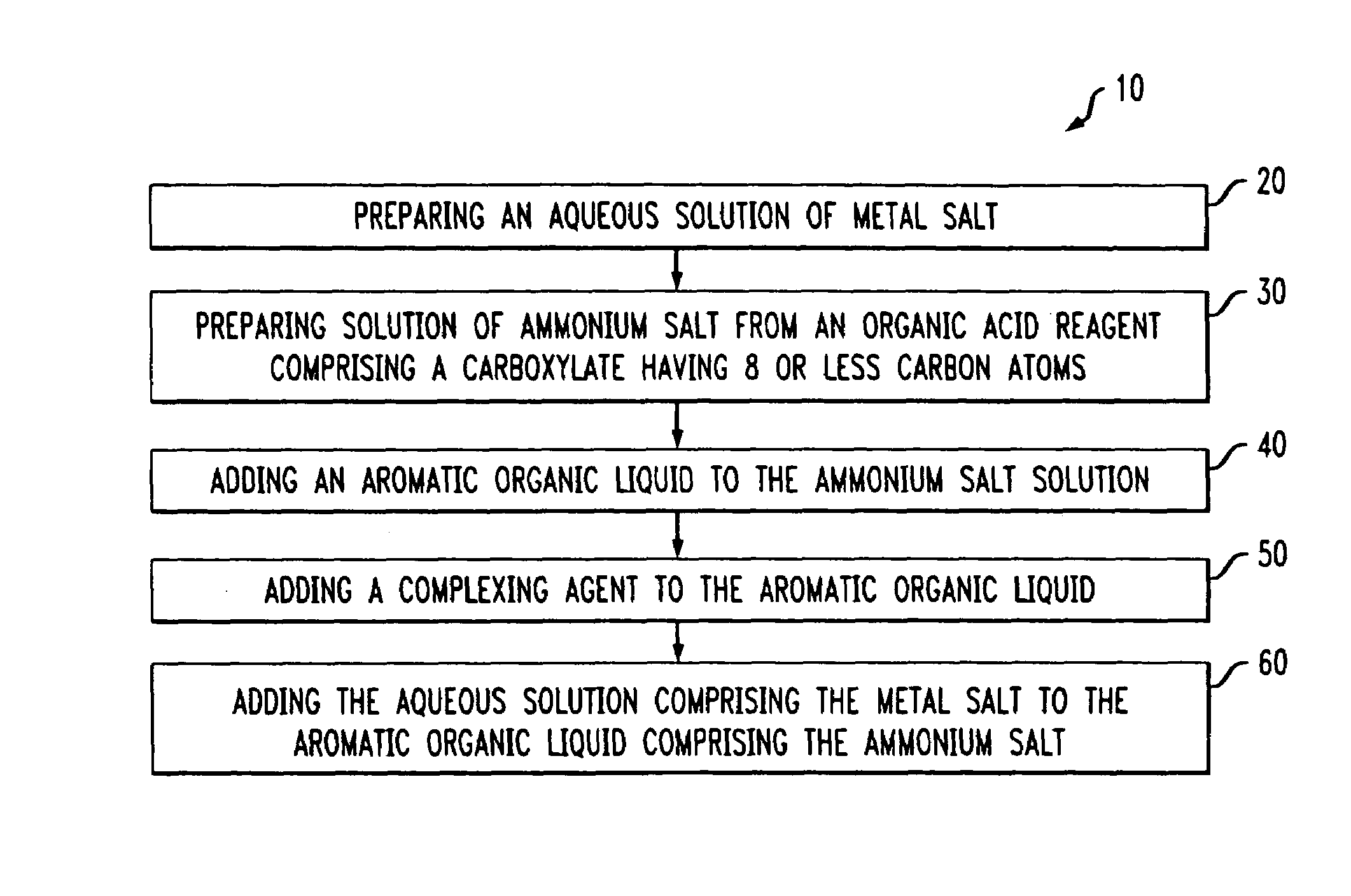
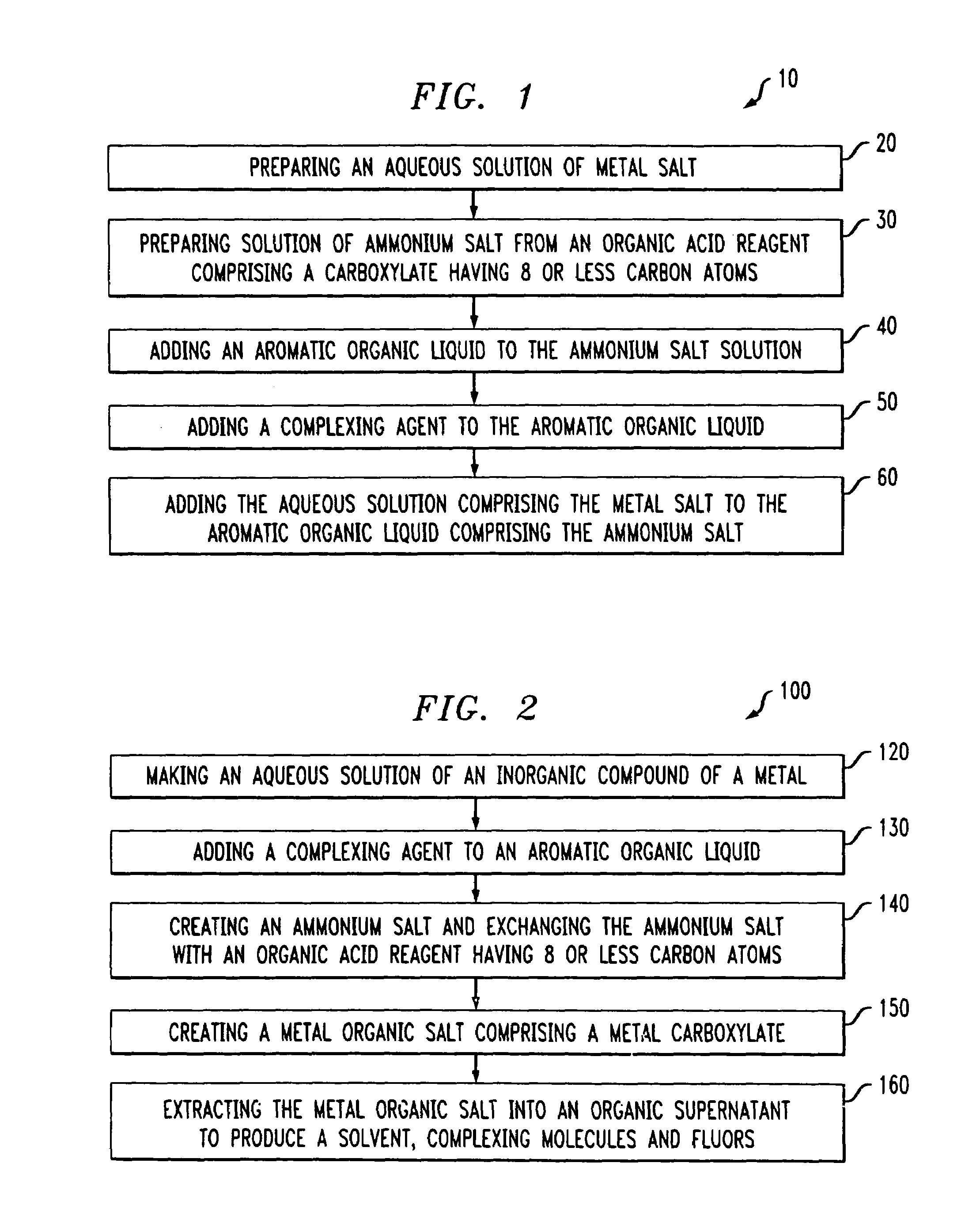
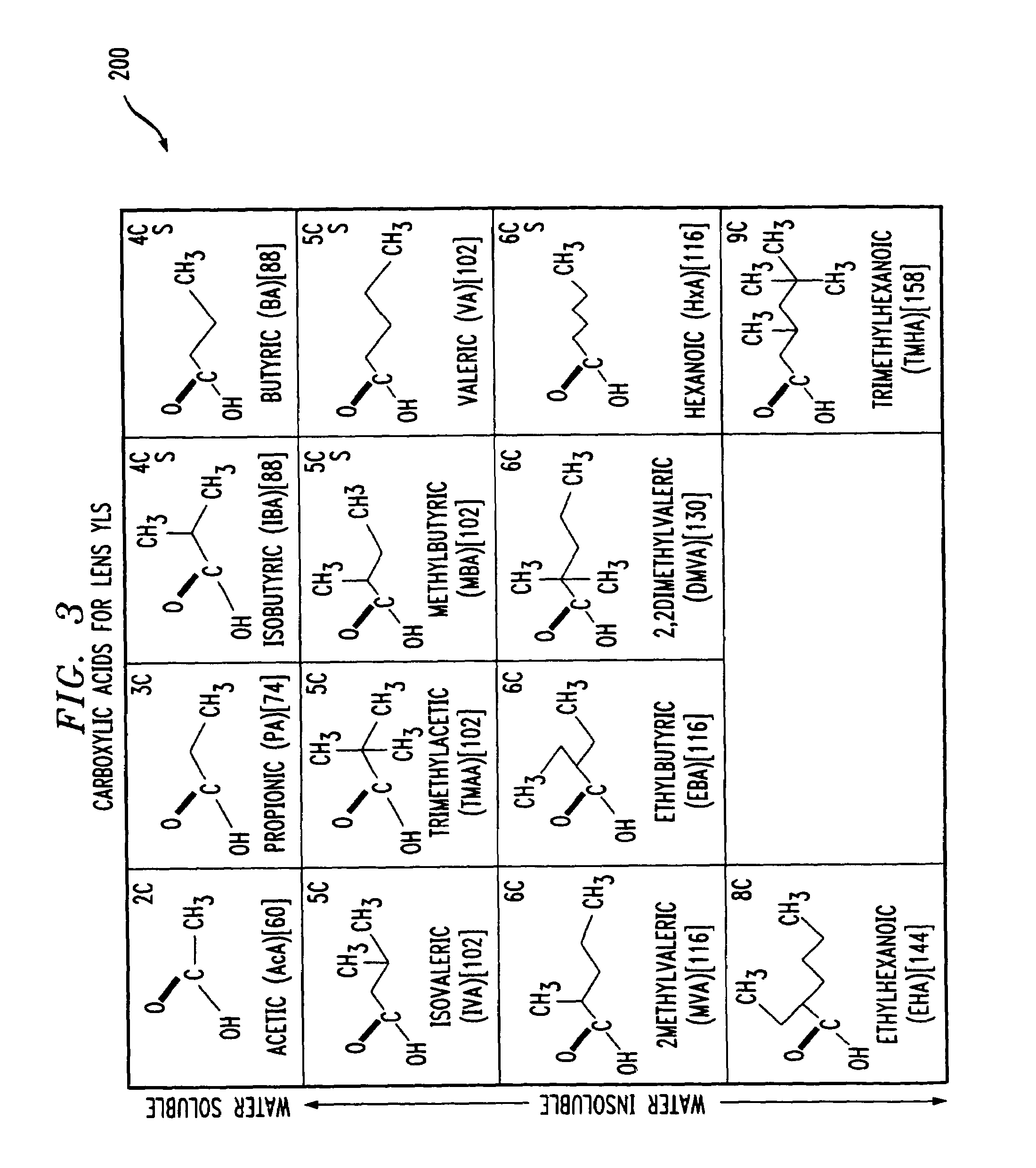
A method of solvating metal ions in an aromatic organic liquid. The method includes adding an ammonium salt having an organic acid reagent to the aromatic organic liquid. The organic acid reagent comprises a carboxylate having eight (8) or less carbon atoms. Thereafter, an aqueous solution comprising a metal salt is added to the aromatic organic liquid comprising the ammonium salt and the organic acid reagent. As a result, at least 10% by weight of metal ions is solvated in the aromatic organic liquid.
Owner:LUCENT TECH INC
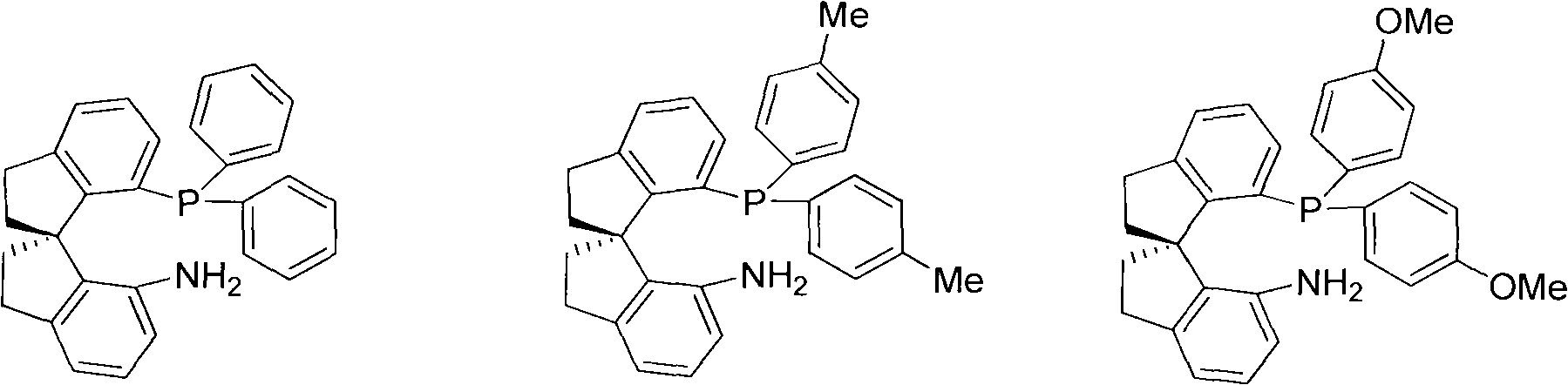
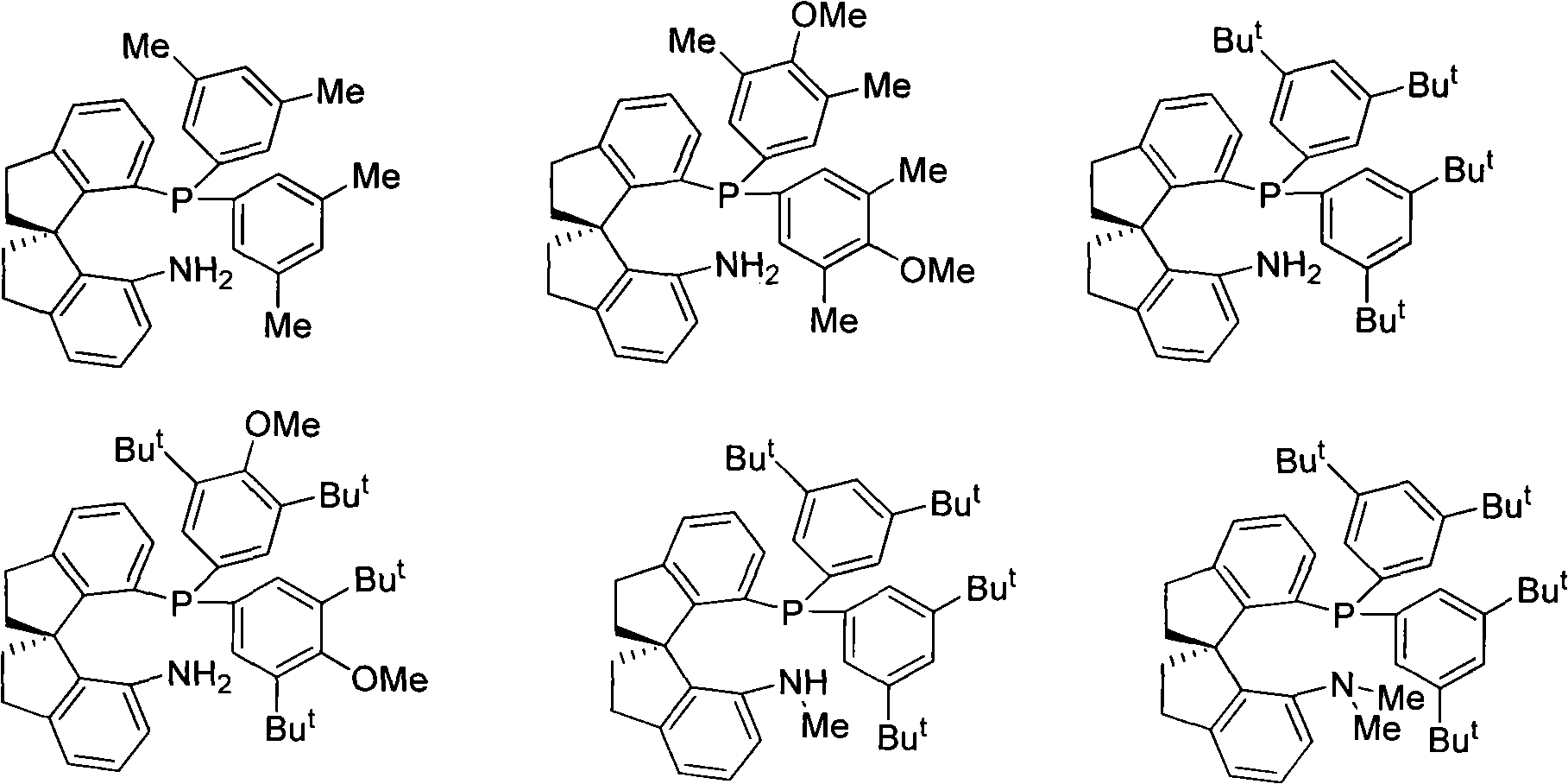
Chiral spiro aminophosphine ligand compound and synthesis method as well as application thereof
InactiveCN101671365AHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsIridiumSynthesis methods
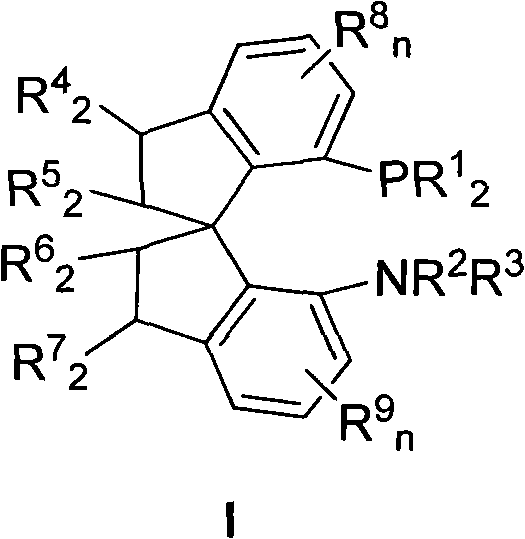
The invention relates to a chiral spiro aminophosphine ligand compound and a synthesis method as well as an application thereof. The chiral spiro aminophosphine compound contains a compound with a structure shown in I, or a racemic body or an optical isomer thereof, or a catalyzed acceptable salt thereof, wherein the main structure characteristic is with a chiral spirobiindane matrix. The chiral spiro aminophosphine compound can be synthesized by taking 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane or substituted 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane as chiral starting materials, which has optical activity and spiro matrix. The chiral spiro aminophosphine compound can be used as a chiral ligand for an asymmetrically catalyzed hydrogenation of an iridium catalyzed carboxy compound and achieves high yield and enantioselectivity (97%ee). The reactive activity is also high and the consumption of the catalyst can be reduced to 0.01% molar.
Owner:NANKAI UNIV
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
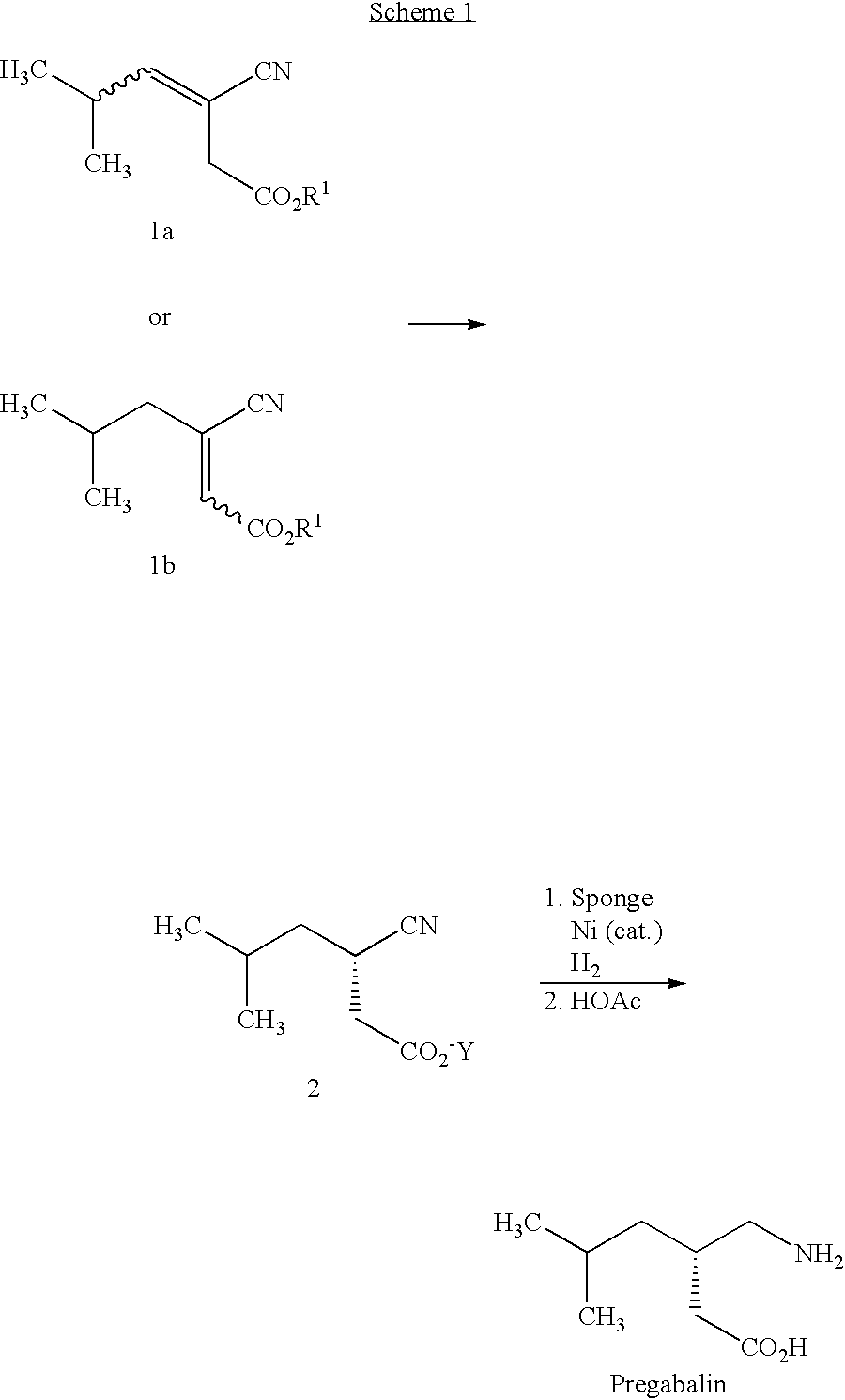
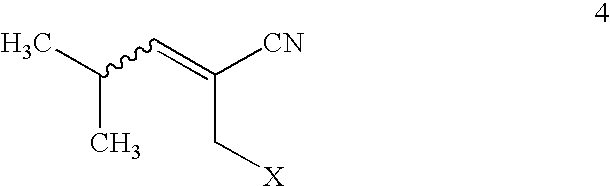
Asymmetric synthesis of pregabalin
InactiveUS6891059B2Effective approachDisposal of minimizedNervous disorderCarboxylic acid nitrile preparationPregabalinAsymmetric hydrogenation
This invention provides a method of making (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid (pregabalin) or a salt thereof via an asymmetric hydrogenation synthesis. Pregabalin is useful for the treatment and prevention of seizure disorders, pain, and psychotic disorders. The invention also provides intermediates useful in the production of pregabalin.
Owner:WARNER-LAMBERT CO
Asymmetric auxiliary group
To provide a chiral reagent or a salt thereof. The chiral reagent has following chemical formula (I). In the formula (I), G1 and G2 are independently a hydrogen atom, a nitro group (—NO2), a halogen atom, a cyano group (—CN), a group of formula (II) or (III), or both G1 and G2 taken together to form a group of formula (IV).
Owner:WAVE LIFE SCI LTD
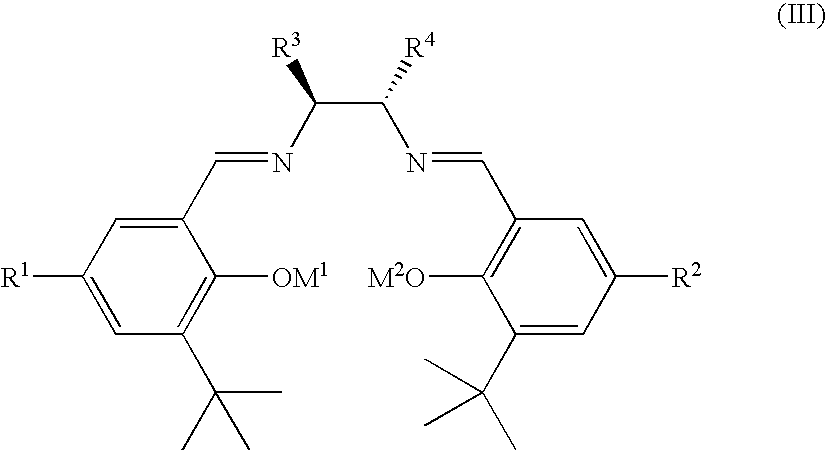
Chiral salen catalyst and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst
InactiveUS6884750B2High optical purityHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsFood additiveEpoxide
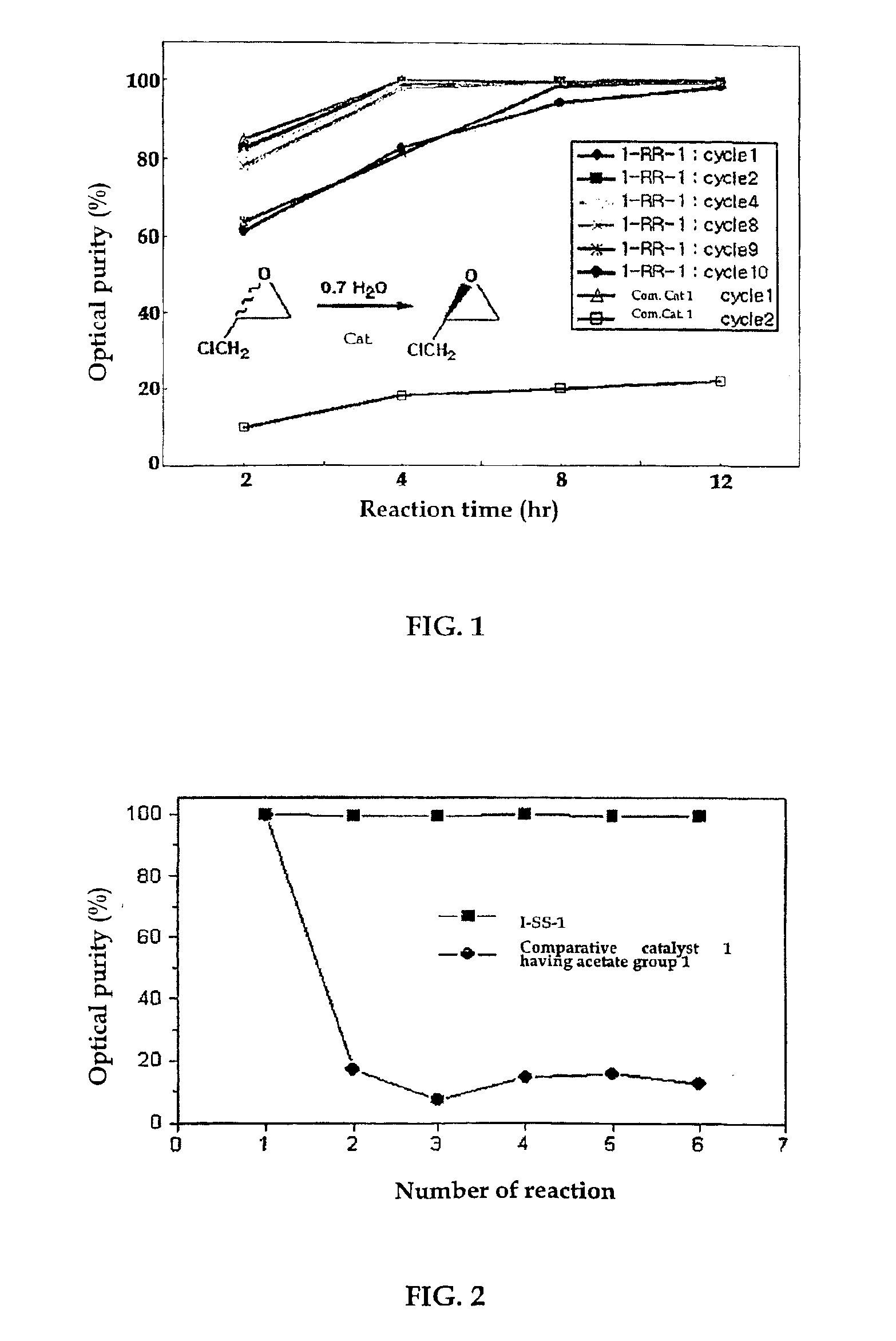
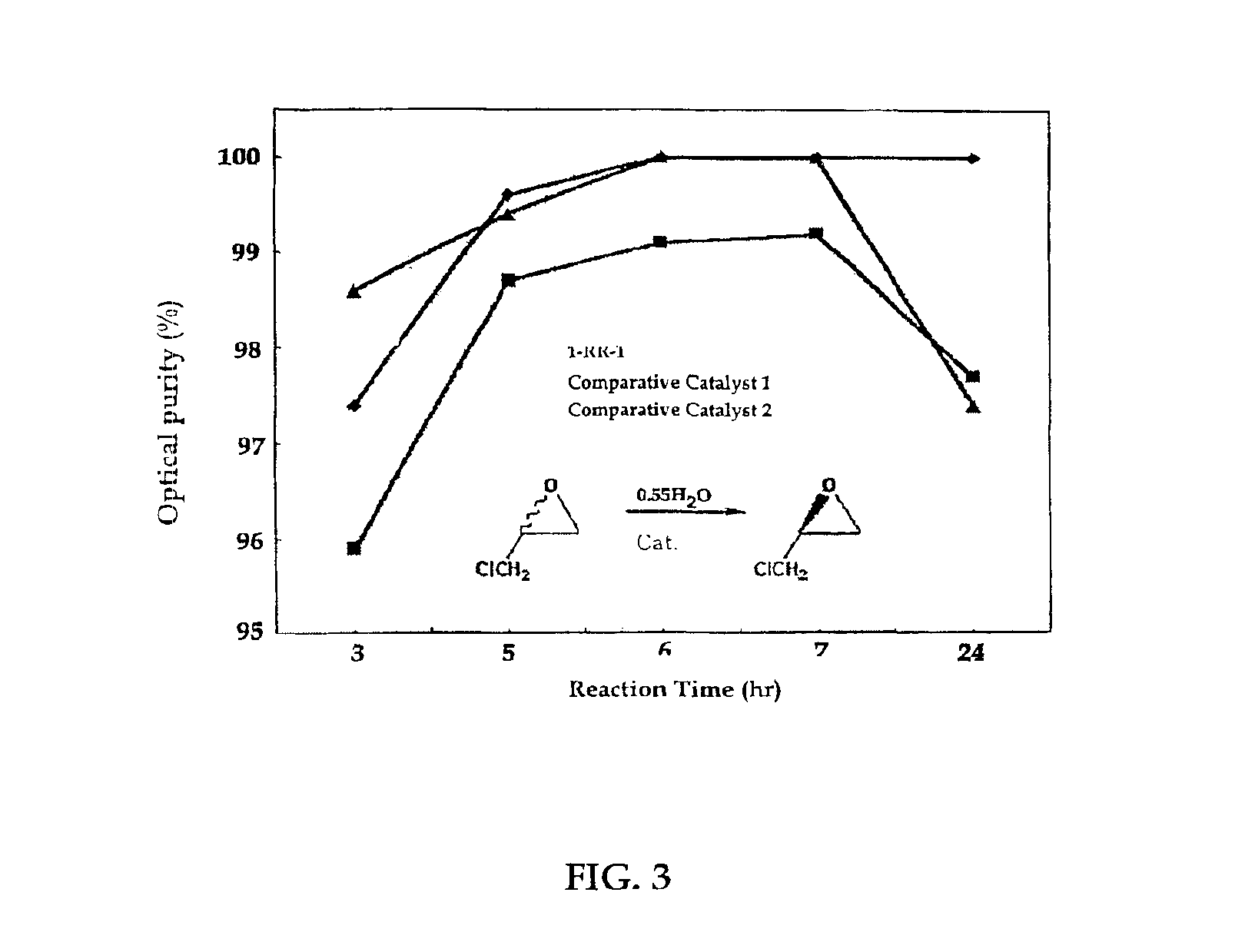
The present invention relates to new chiral salen catalysts and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst. More particularly, the present invention is to provide novel chiral salen catalysts and their uses for producing chiral compounds having high optical purity to be used as raw materials for preparing chiral medicines or food additives in a large scale economically, wherein the chirl salen catalyst having a particular molecules structure can be reused continuously without any activating process of used catalysts and cause no or little racemization after the reaction is completed because it maintains its catalytic activity after the reaction process.
Owner:RS TECH CORP
Novel ruthenium carbonyl complex having tridentate ligand, its production method and use
ActiveUS20110237814A1Improve stabilityEasy to operateRuthenium organic compoundsSilicon organic compoundsAlcoholRuthenium
The present invention relates to a ruthenium carbonyl complex that is represented by the following Formula (1):RuXY(CO)(L) (1)(in the Formula (1), X and Y, which may be the same or different from each other, represent an anionic ligand and L represents a tridentate aminodiphosphine ligand which has two phosphino groups and a —NH— group), its production method, and a method for production of alcohols by hydrogenation-reduction of ketones, esters, and lactones using the complex as a catalyst.The ruthenium carbonyl complex of the invention has a high catalytic activity and it can be easily prepared and handled.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Main-group metal-based asymmetric catalysts and applications thereof
InactiveUS6844448B2Lactams preparationCarbamic acid derivatives preparationTetradentate ligandNucleophile
The present invention relates to a method and catalysts for the stereoselective addition of a nucleophile to a reactive π-bond of a substrate. The chiral, non-racemic catalysts of the present invention constitute the first examples of catalysts for nucleophilic additions that comprise a main-group metal and a tri- or tetra-dentate ligand.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Platinum/carbon nanotube catalyst and preparation method and application thereof
InactiveCN102039121AMild conditionsEasy to manufactureOrganic reductionMaterial nanotechnologyCarbon nanotubeRoom temperature
The invention relates to a platinum / carbon nanotube catalyst suitable for multiphase asymmetric hydrogenation reaction. Platinum is loaded on a carbon nanotube carrier. A preparation method comprises the following steps of: heating a purified carbon nanotube in nitric acid, washing, filtering, washing by using water until the pH value of filtrate is neutral, drying to obtain the carbon nanotube carrier, soaking in aqueous solution of chloroplatinic acid, and performing ultrasonic treatment at room temperature; and stirring and impregnating a mixture of the carbon nanotube and the aqueous solution of chloroplatinic acid, raising the temperature to 110 DEG C from room temperature, drying at the temperature of 110 DEG C, grinding into fine powder, reducing by using aqueous solution of sodiumformate with heating, filtering, washing by using deionized water and drying. The invention also provides the preparation method of the catalyst and application of the catalyst to the multiphase asymmetric hydrogenation reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for converting an alcohol to the corresponding fluoride
InactiveUS6248889B1Good choiceLow costOrganic compound preparationOrganic halogenationAlcoholOrganic base
A process for preparing a fluoride from its corresponding alcohol comprises the steps of (a) forming a mixture comprising (i) at least one fluorinated, saturated aliphatic or alicyclic sulfonyl fluoride (for example, perfluorobutanesulfonyl fluoride) and (ii) at least one primary or secondary alcohol; and (b) adding a molar excess of at least one strong, aprotic, non-nucleophilic, hindered, double bond-containing, organic base (for example, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)) to the mixture.
Owner:3M INNOVATIVE PROPERTIES CO
Chiral polymeric salen catalyst, and a process for preparing chiral compounds from racemic epoxides by using them
InactiveUS6903043B2Avoid deactivationEasy to produceRuthenium organic compoundsOrganic compound preparationDiolHydrolysis
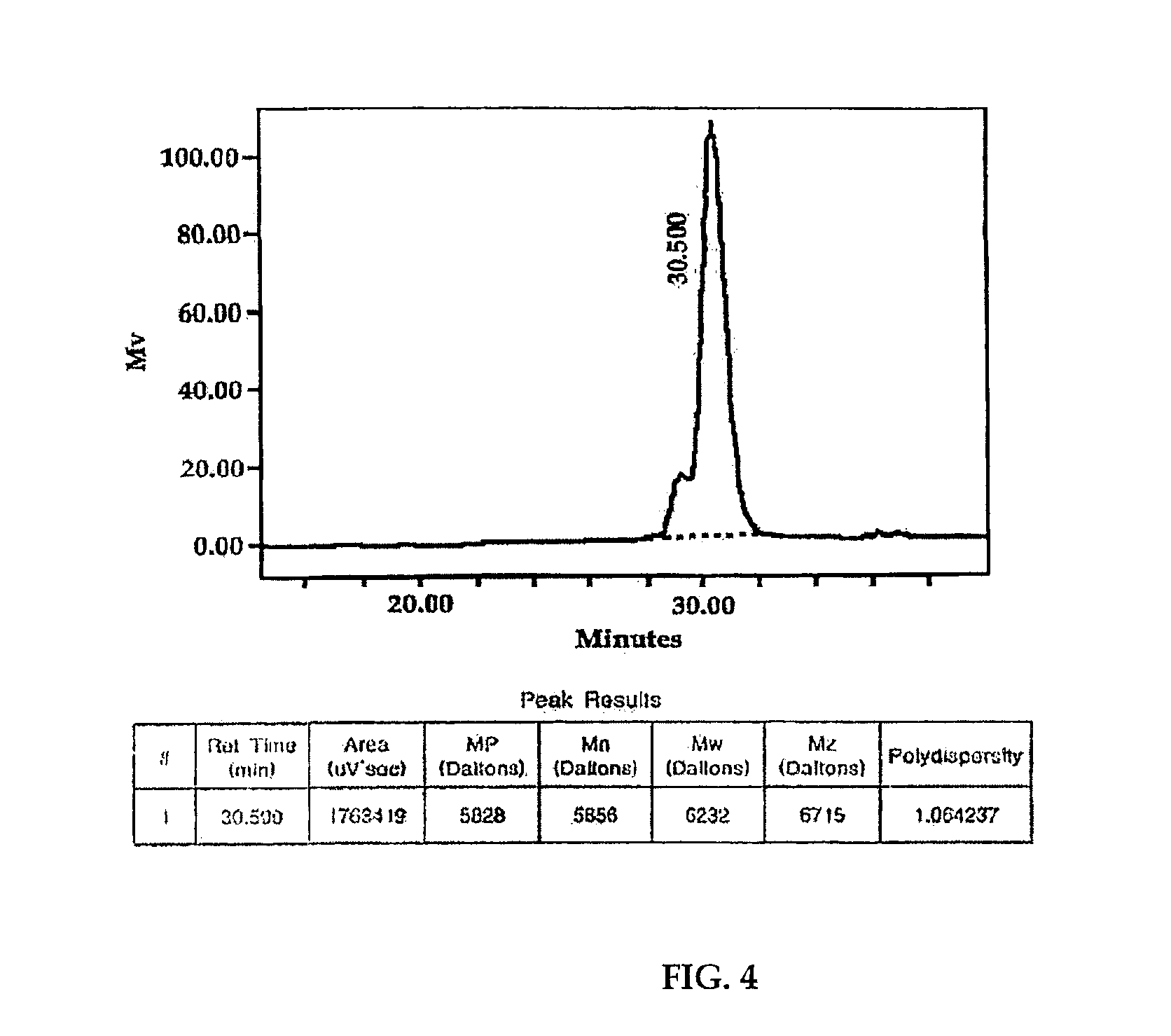
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide a chiral polymeric salen catalyst and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis or racemic epoxides.
Owner:RSTECH CO LTD
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
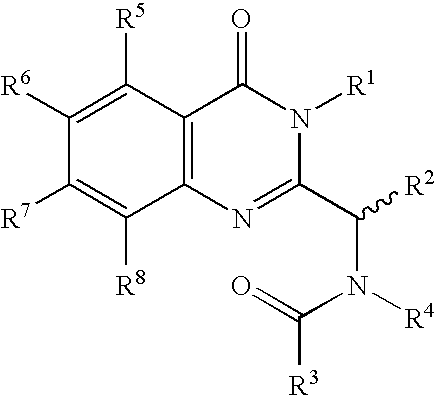
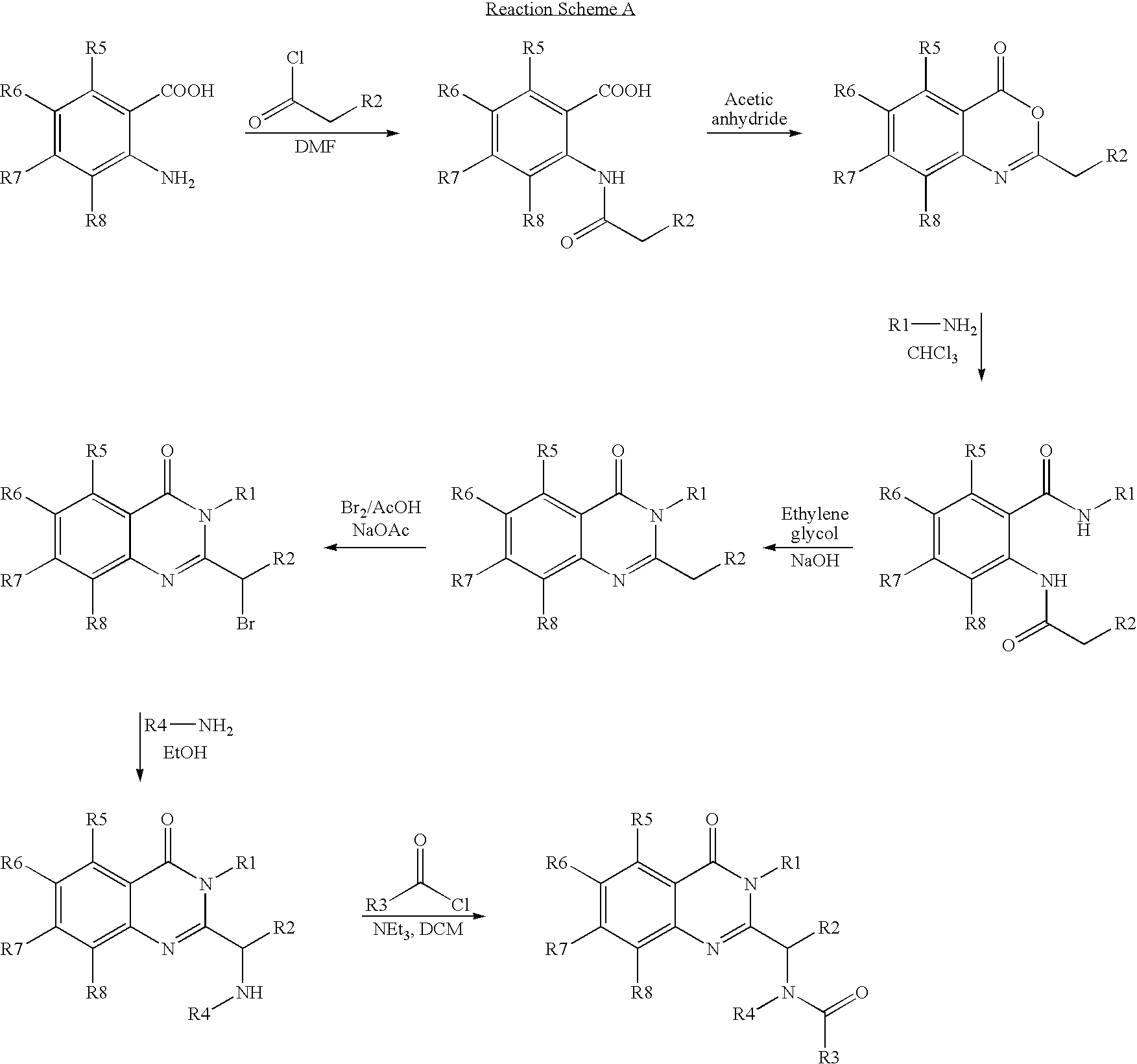
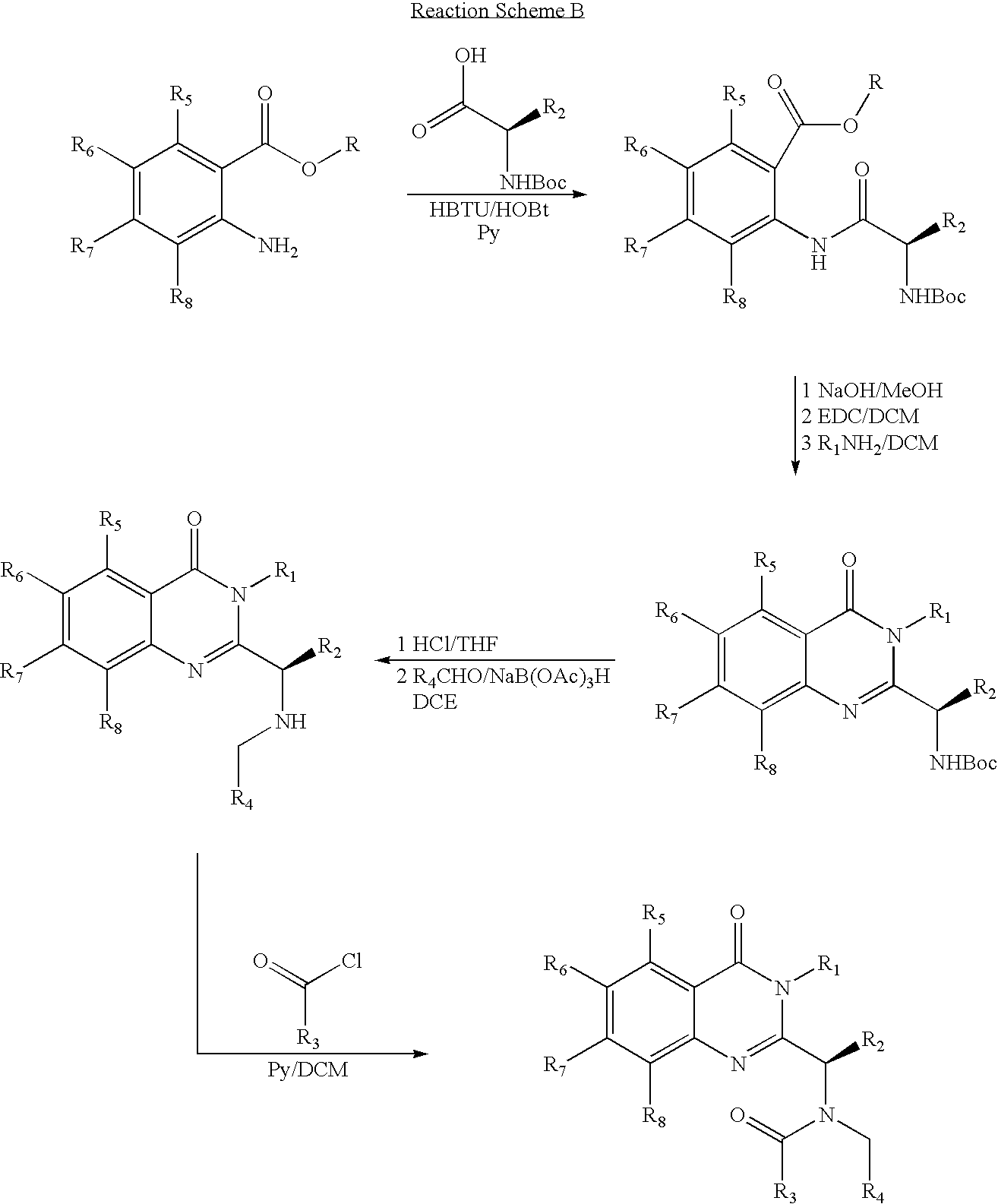
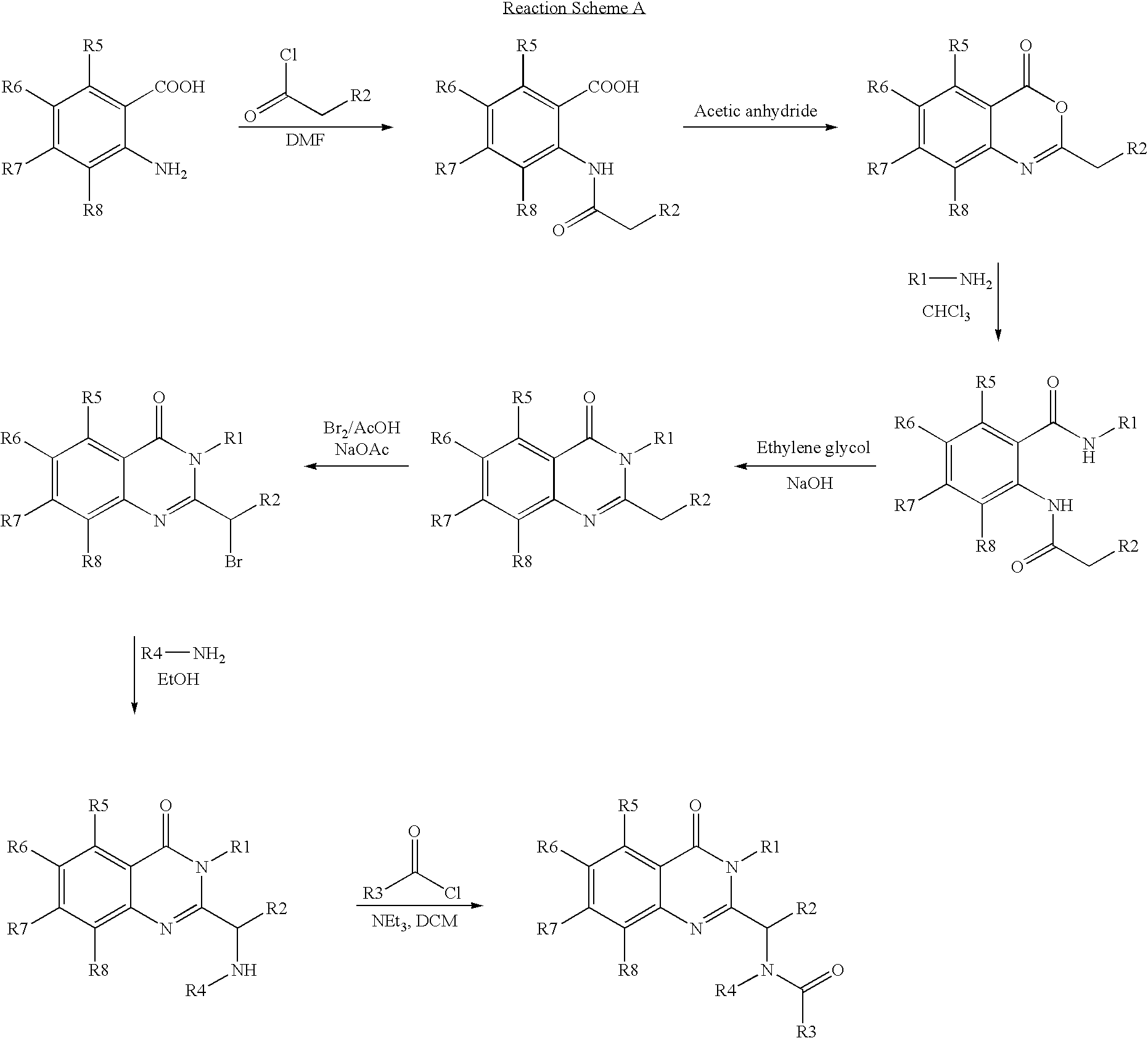
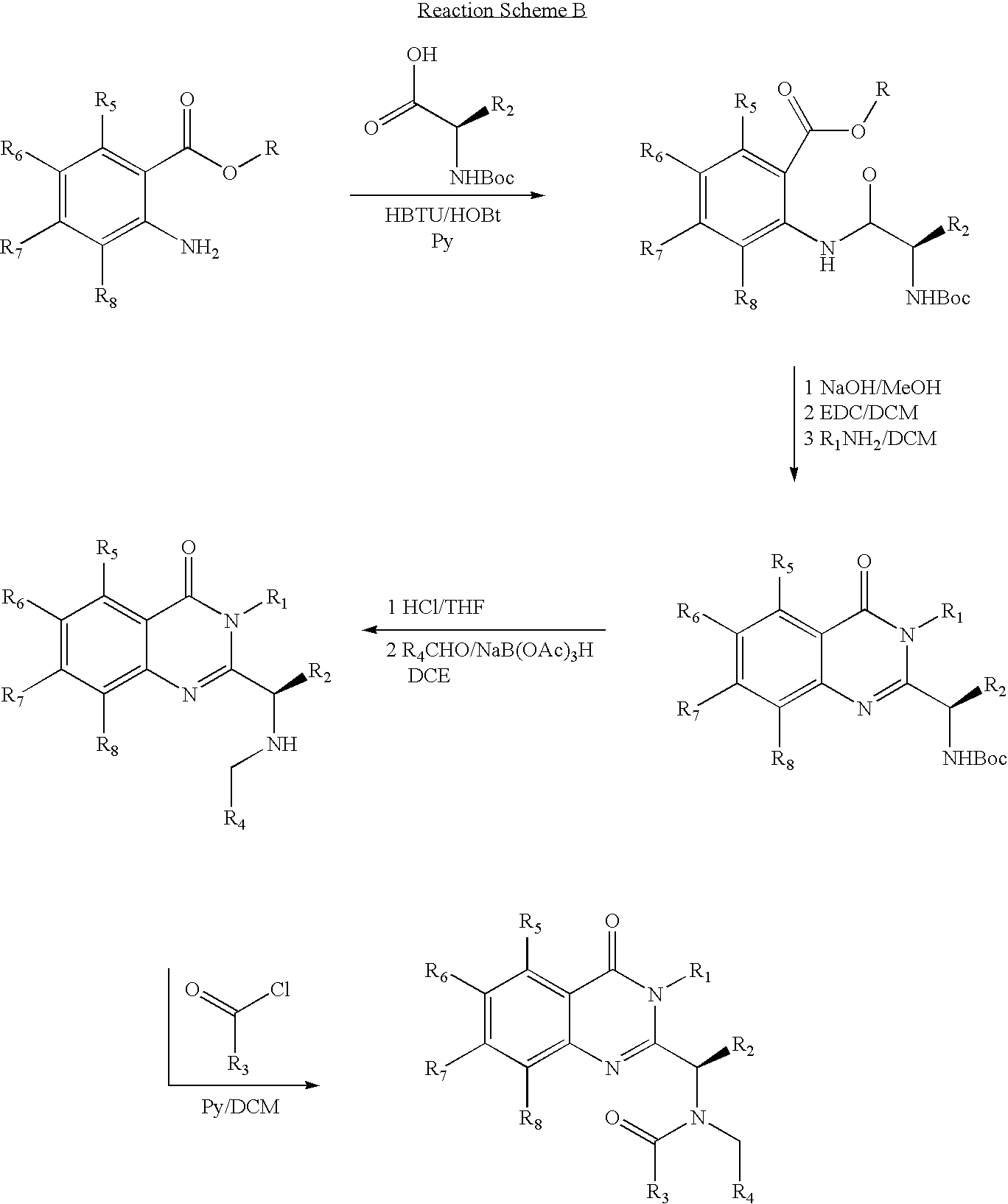
Syntheses of quinazolinones
The present invention provides novel quinazolinone compositions of matter comprising enantiomerically pure compound represented by Formula I:or a pharmaceutically acceptable salt thereof, having a detectable amount of one or more starting material and / or reagent used in the synthesis thereof.
Owner:CYTOKINETICS INC
Syntheses of quinazolinones
Owner:CYTOKINETICS INC
Functionalized ionic liquids, and methods of use thereof
InactiveUS20080112866A1Group 5/15 element organic compoundsCarboxylic acid esters preparationChemical reactionSide chain
One aspect of the present invention relates to ionic liquids comprising a pendant Bronsted-acidic group, e.g., a sulfonic acid group. Another aspect of the present invention relates to the use of an ionic liquid comprising a pendant Bronsted-acidic group to catalyze a Bronsted-acid-catalyzed chemical reaction. A third aspect of the present invention relates to ionic liquids comprising a pendant nucleophilic group, e.g., an amine. Still another aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to catalyze a nucleophile-assisted chemical reaction. A fifth aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to remove a gaseous impurity, e.g., carbon dioxide, from a gas, e.g., sour natural gas.
Owner:UNIV OF SOUTH ALABAMA
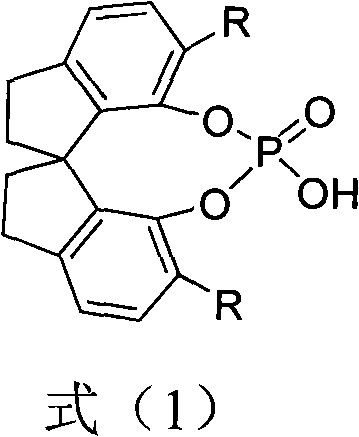
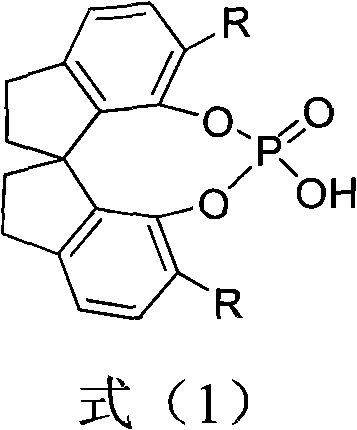
Chiral spiro-phosphate and preparation method and application thereof
ActiveCN102030780ACatalyzes a wide range of organic reactionsHigh activityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAlkyl transferPhosphate
The invention relates to a chiral spiro-phosphate and a preparation method and application thereof. The chiral spiro-phosphate compound has a structure of a formula (1) and has the main structural characteristic of chiral spiral dihydroindene skeleton. The chiral spiro-phosphate compound can be synthesized by using 1,1'-spiro-dihydroindene-7,7'-diphenol with spiral skeleton and optical activity as a chiral initial raw material. The chiral spiro-phosphate is a novel protonic acid organic small molecular catalyst, can be widely applied in various catalytic asymmetric organic reactions, particularly can be applied in asymmetrical catalytic reaction of indole alkylation, and has and high enantiomer selectivity, the reaction condition is mild , and the yield is good.
Owner:ZHEJIANG UNIV
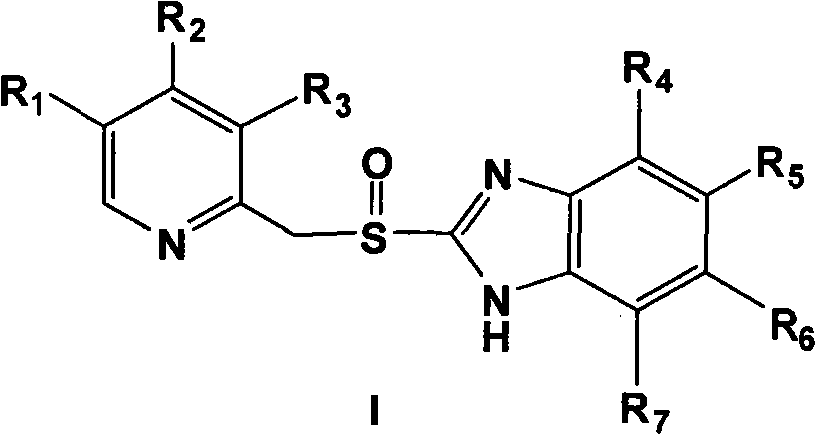
Novel method for preparing chiral sulphoxide compound
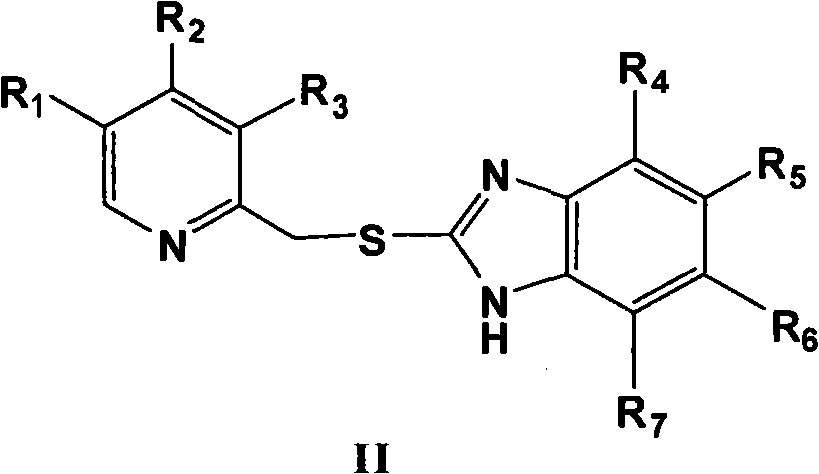
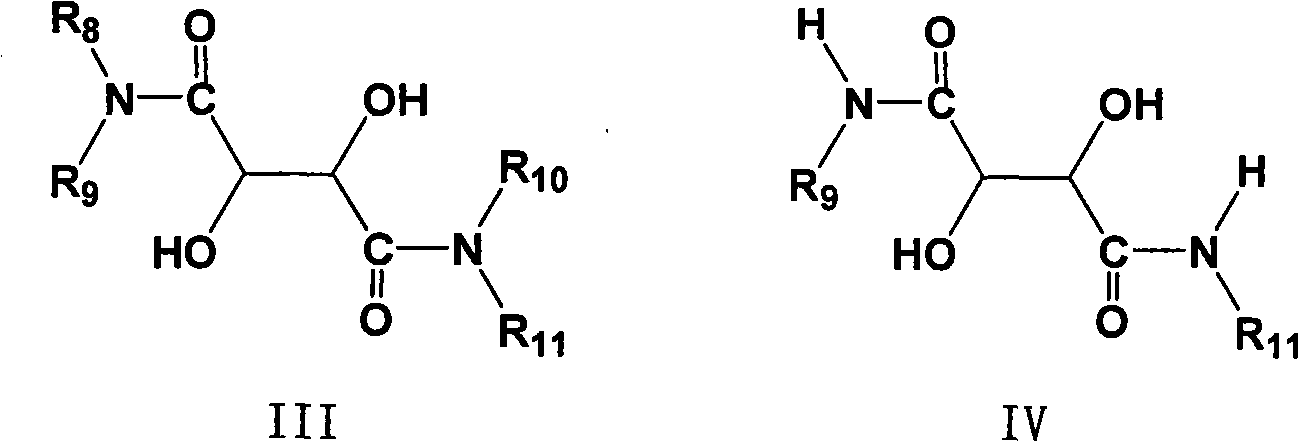
The invention provides a novel method for preparing optically pure substituted [(pyridyl methylene) sulfinyl]-1H-benzimidazole sulphoxide compound by enantioselective synthesis. The method requiring protection is to directly and asymmetrically oxidize prochiral thioether into a corresponding optically pure sulphoxide compound or a sulphoxide compound rich in single enantiomer by a mild and cheap oxidizing agent in the presence of a complex compound catalyst formed by an accessible and stable (+)- or (-)- tartaric acid diamide ligand shown in a general formula and titanium. Therefore, optically pure omeprazole, lansoprazole and pantoprazole can be obtained, wherein R8, R9, R10 and R11 are the same or different, and are selected from hydrogen, alkyl, aralkyl, aryl, organic polymers or a silica loading body.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
Waste water treatment in a method for producing formic acid which is free of water
InactiveUS6867329B2Save energyLow investment costOxygen-containing compound preparationOrganic compound preparationFractionating columnWaste treatment
The invention relates to a process for obtaining anhydrous or substantially anhydrous formic acid, in which firstly aqueous formic acid is prepared by hydrolysis of methyl formate and is freed from water in the subsequent work-up. The process has the special feature that steam, which is employed for the hydrolysis of methyl formate and for heating a distillation column serving for work-up, is also used as stripping steam for waste-water stripping. The stripped waste water is produced during work-up.
Owner:BASF AG
Hydrodeoxygenation catalyst
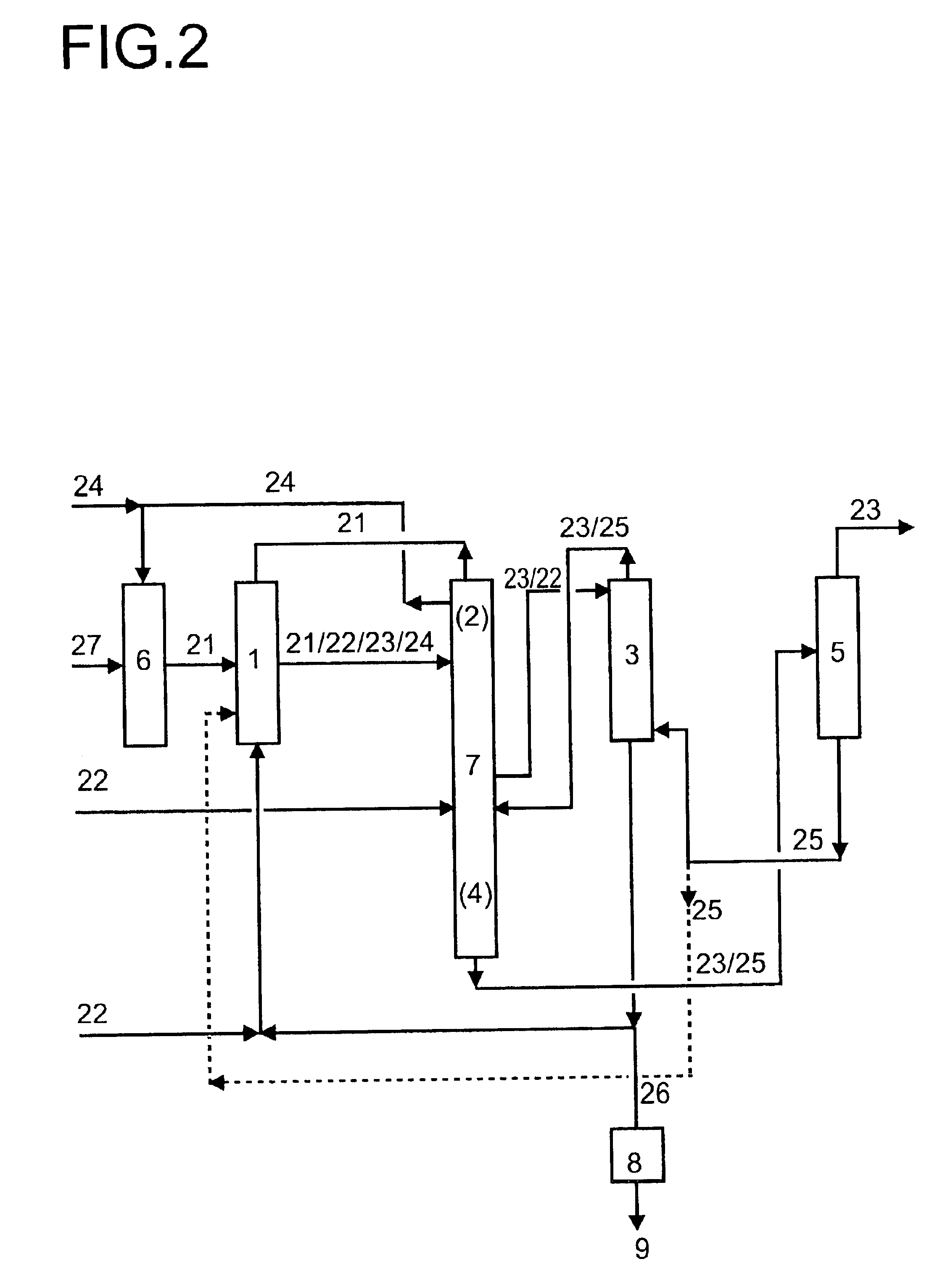
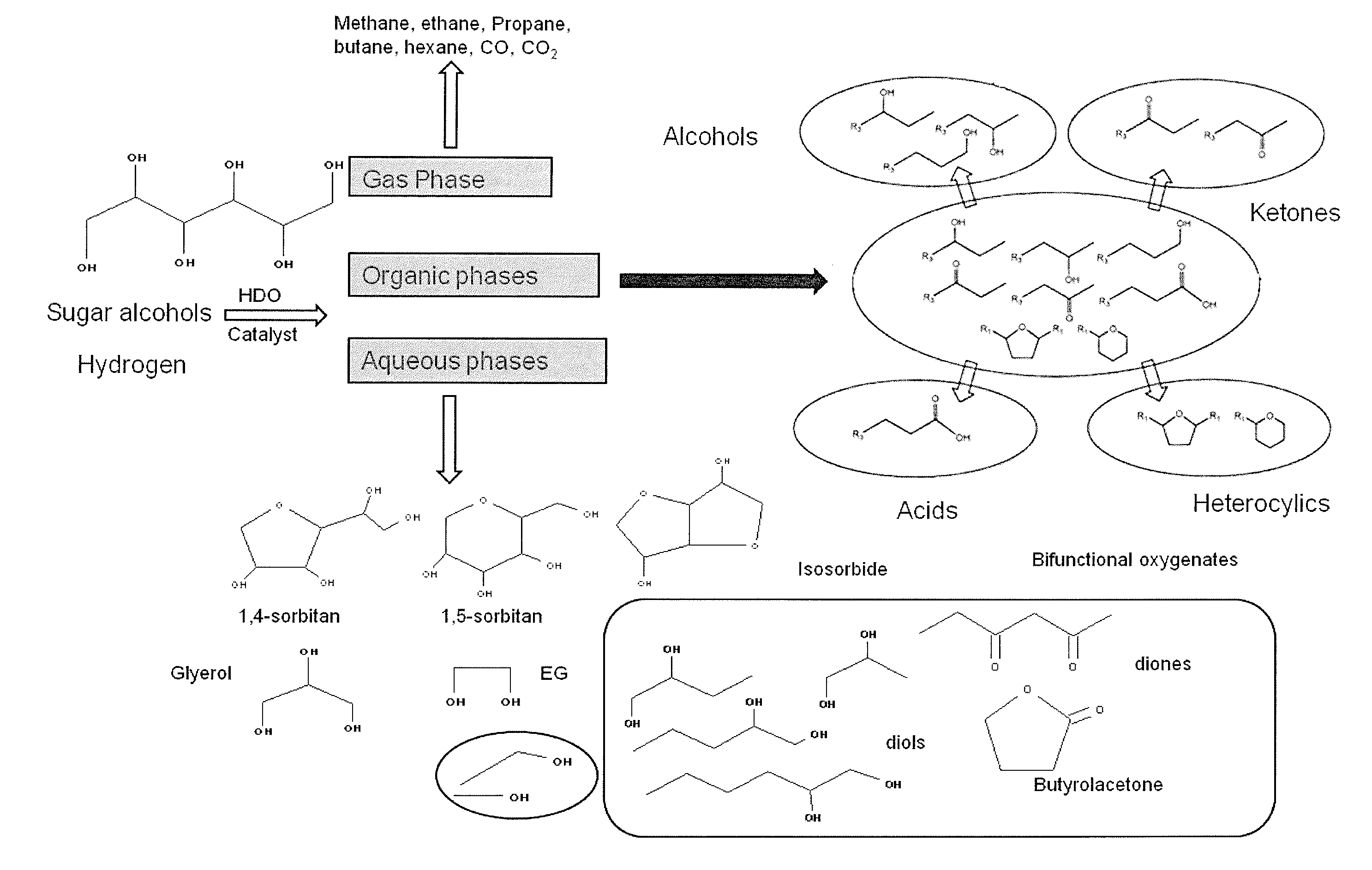
InactiveUS20140031546A1Hydrocarbon by hydrogenationLiquid hydrocarbon mixture productionRheniumIridium
A hydrodeoxygenation catalyst comprises a metal catalyst, an acid promoter, and a support. The metal catalyst is selected from platinum, palladium, ruthenium, rhenium rhodium, osmium, iridium, nickel, cobalt, molybdenum, copper, tin, or mixtures thereof. The support is a promoted-zirconium material including texture promoters and acid promoters. The hydrodeoxygenation catalyst may be used for hydrodeoxygenation (HDO) of sugar or sugar alcohol in an aqueous solution. In one embodiment the HDO catalyst may be used for HDO of fatty acids such as fatty acid methyl esters (FAME), triglycerols (in plant oil and animal fat), pyrolysis oil, or lignin. The hydrodeoxygenation catalyst for fatty acid process does not require the use of an acid promoter, it is optional.
Owner:CLARIANT CORP
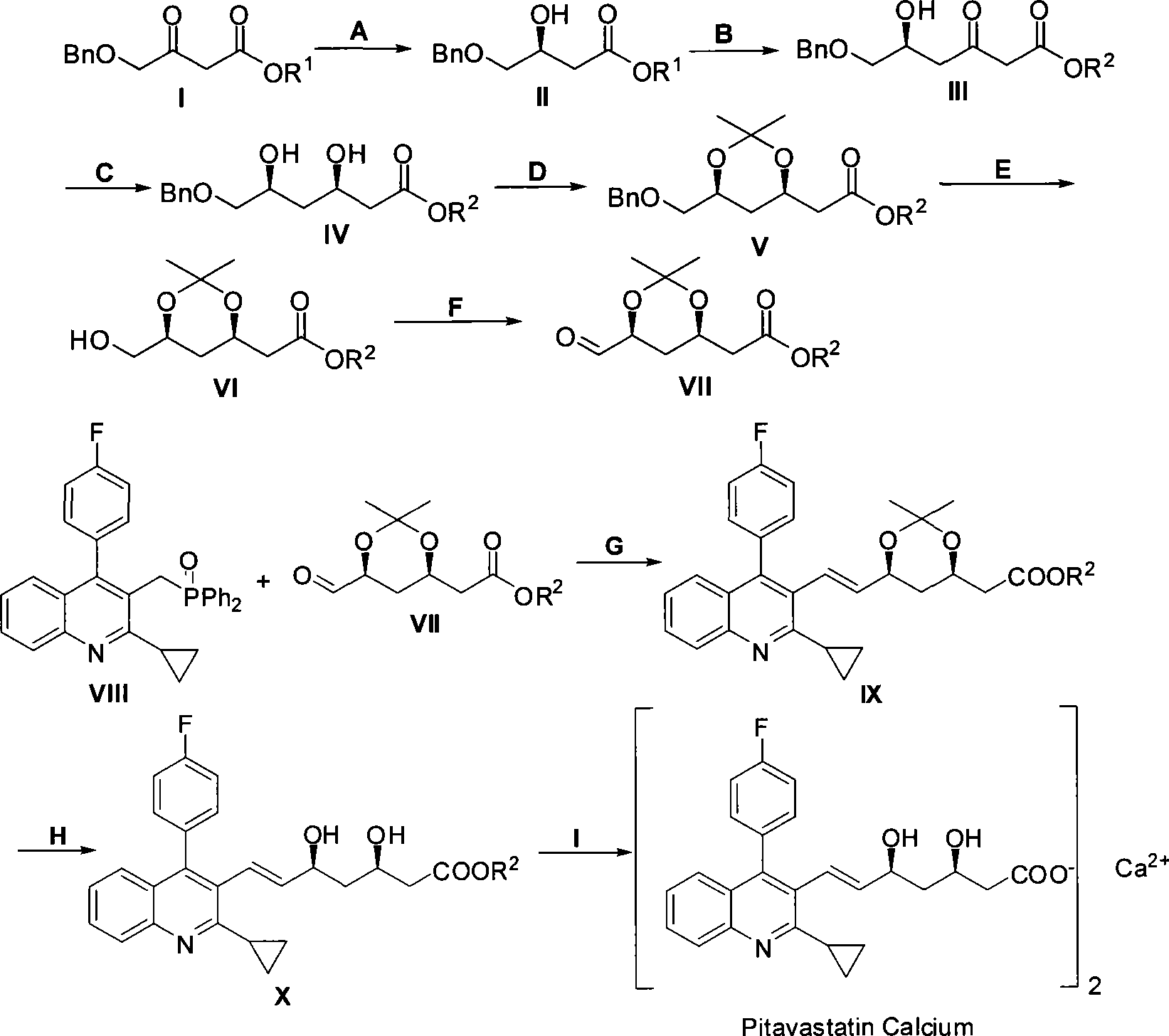
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Liquid crystal compounds, preparation thereof, and composition containing the same
ActiveUS7022259B2Increase powerShorten the timeLiquid crystal compositionsOrganic compound preparationAlcoholChain structure
A liquid crystal compound with high helical twisting power, a method for preparing the same, and a liquid crystal composition containing the same. The liquid crystal compounds include cyclic group chain structures similar to the liquid crystal to serve as the core structure, and multi-ring structures of natural alcohol with optical activity, such as alcoholates of terpenol, borneol, cinchonidine, quinine, or derivatives thereof. As a result, the liquid crystal composition containing the liquid crystal compounds can filter out light of specific wavelengths from incident light due to optical activity and high helical twisting power thereof.
Owner:IND TECH RES INST
Ionic liquids
InactiveUS20070129568A1Sufficient solubilityReduce volatilityGroup 5/15 element organic compoundsTransportation and packagingPhosphoniumChemical reaction
Low melting point organic liquid compounds with high boiling points are prepared by a preferred process having the J+xQy(R—COO−)x-y where x is 1 to 8, preferably 1-3, y is 0 to x−1, where R—COO− is an anion selected from the group consisting of 2-ethyl hexanoate, pivalate, neodecanoate, and mixtures thereof, Q is another anion or mixture of other anions, and J+x is a cation selected from cations of Groups IA, IIA, IB, IIB, IIIB, IVB, VB, VIB, VIIB, VIII and lanthanide metals, cations selected from cations of B, Si, Ge, As, Sb, Te and Po metalloids, an ammonium cation derived from ammonia or an organic amine, an organic phosphonium cation, and mixtures thereof the organic liquid compounds being substantially free of volatile organic compounds. These compounds, as liquids, are useful as low volatile organic solvents, e.g., solvents in which a variety of chemical reactions may be carried out.
Owner:NGIMAT CO
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com