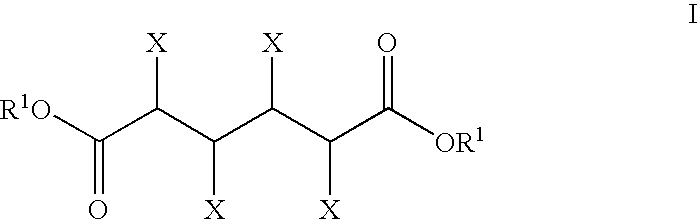
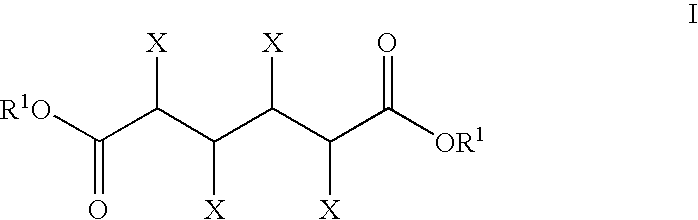
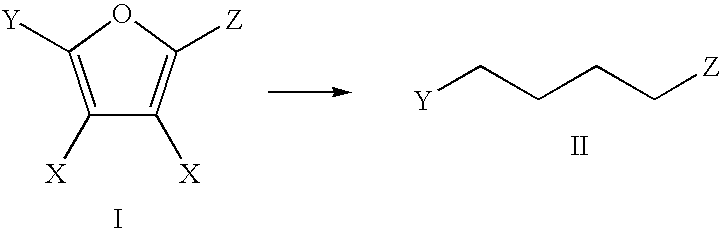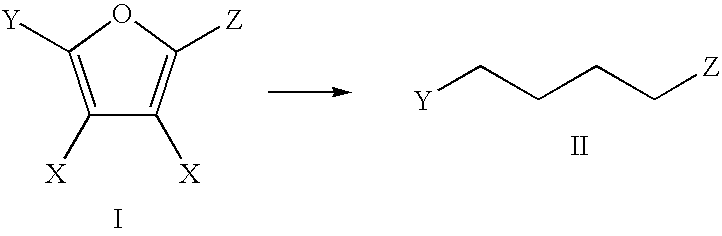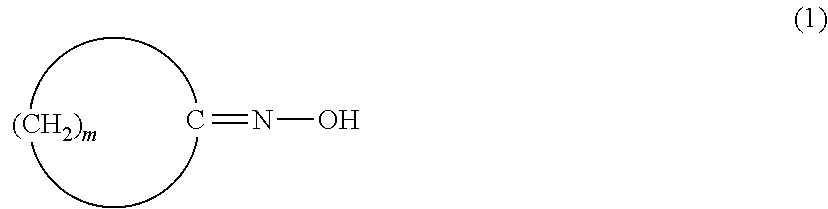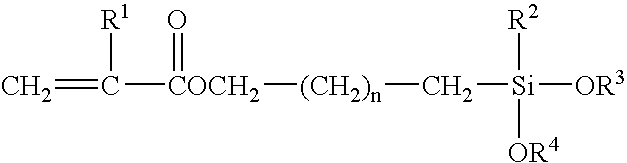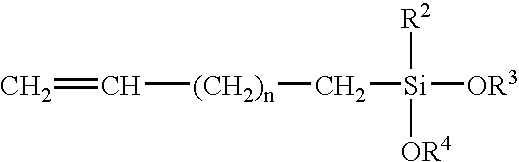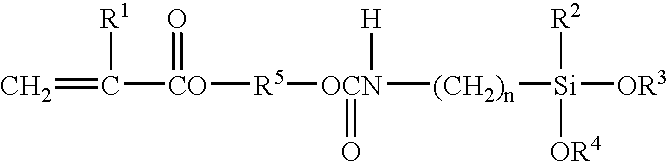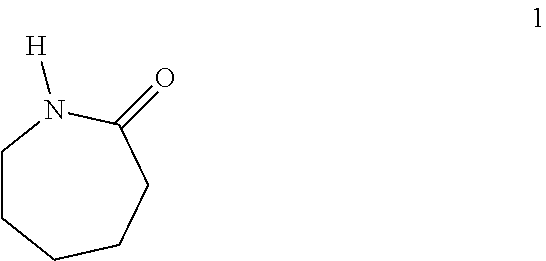Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
521results about "Lactams preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Production of adipic acid and derivatives from carbohydrate-containing materials
ActiveUS8669397B2Low costLactams preparationCarboxylic acid nitrile preparationCatalytic oxidationHydrodeoxygenation
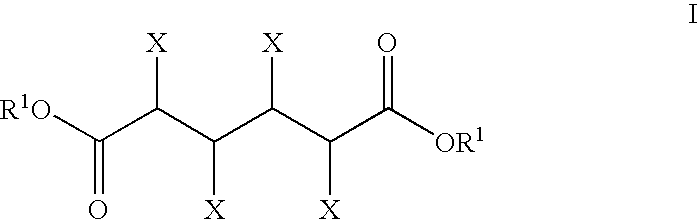
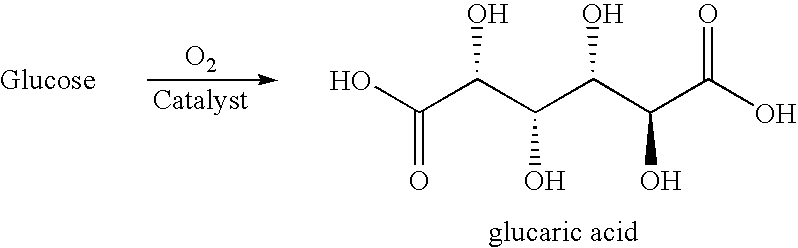
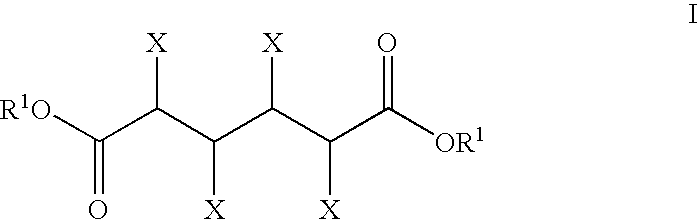
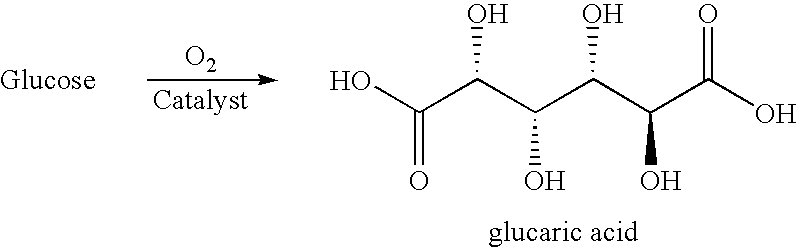
The present invention generally relates to processes for the chemocatalytic conversion of a glucose source to an adipic acid product. The present invention includes processes for the conversion of glucose to an adipic acid product via glucaric acid or derivatives thereof. The present invention also includes processes comprising catalytic oxidation of glucose to glucaric acid or derivative thereof and processes comprising the catalytic hydrodeoxygenation of glucaric acid or derivatives thereof to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Process for producing cyclic compounds
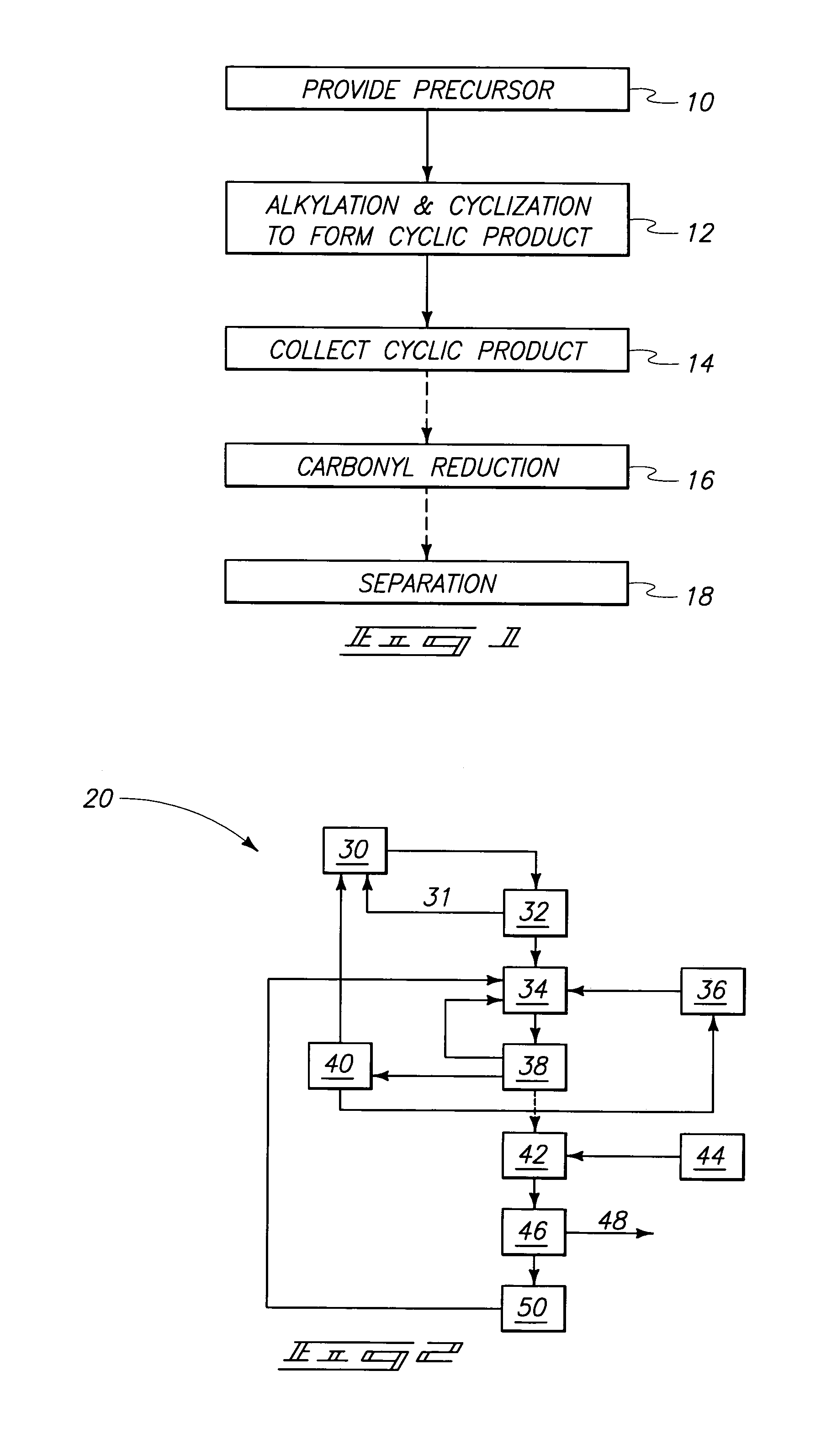
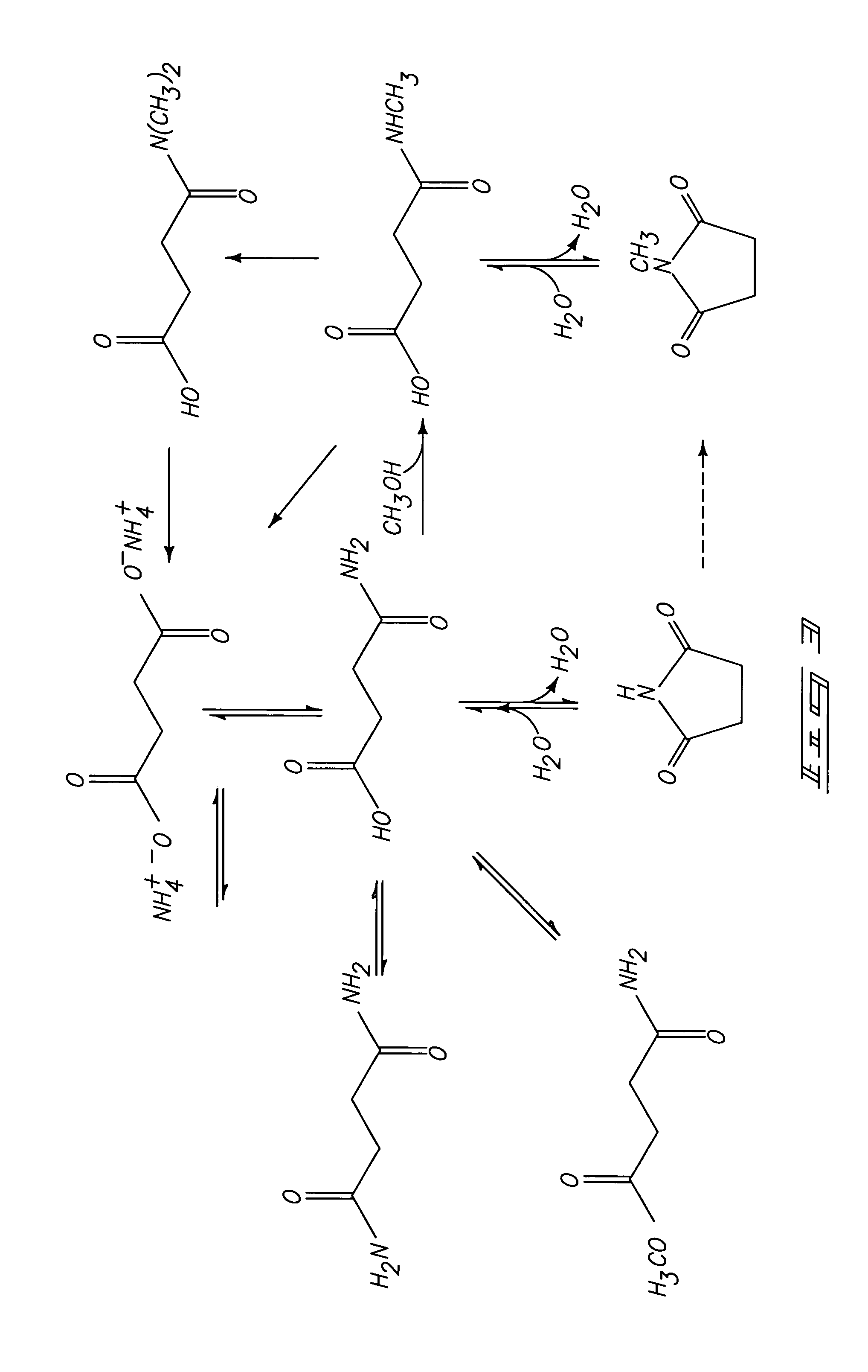
The invention includes methods of processing an initial di-carbonyl compound by conversion to a cyclic compound. The cyclic compound is reacted with an alkylating agent to form a derivative having an alkylated ring nitrogen. The invention encompasses a method of producing an N-alkyl product. Ammonia content of a solution is adjusted to produce a ratio of ammonia to di-carboxylate compound of from about 1:1 to about 1.5:1. An alkylating agent is added and the initial compound is alkylated and cyclized. The invention includes methods of making N-methyl pyrrolidinone (NMP). Aqueous ammonia and succinate is introduced into a vessel and ammonia is adjusted to provide a ratio of ammonia to succinate of less than 2:1. A methylating agent is reacted with succinate at a temperature of from greater than 100° C. to about 400° C. to produce N-methyl succinimide which is purified and hydrogenated to form NMP.
Owner:BATTELLE MEMORIAL INST
Production of Adipic Acid and Derivatives from Carbohydrate-Containing Materials
The present invention generally relates to processes for the chemocatalytic conversion of a carbohydrate source to an adipic acid product. The present invention includes processes for the conversion of a carbohydrate source to an adipic acid product via a furanic substrate, such as 2,5-furandicarboxylic acid or derivatives thereof. The present invention also includes processes for producing an adipic acid product comprising the catalytic hydrogenation of a furanic substrate to produce a tetrahydrofuranic substrate and the catalytic hydrodeoxygenation of at least a portion of the tetrahydrofuranic substrate to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Method for preparing catalyst of containing MFI structured molecular sieve
ActiveCN1600428APromote regenerationImprove conversion rateLactams preparationMolecular sieve catalystsBeckmann rearrangementMolecular sieve
A MFI-molecular sieve catalyst for preparing caprolactam from cyclohexanone oxime by gas-phase Beckmann rearrangement is prepared through proportionally mixing MFI-molecular sieve with alkaline silica gel, shaping, drying and calcining. It has high conversion rate and selectivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for purifying and refining epsi caprolactam
ActiveCN101070299AMeet the requirements of industrial productsLactams preparationLactams separation/purificationPotassium permanganatePhotochemistry
This invention relates to a refine means of epsilon - caprolactam by separation and purification. The feature of the invention lay in that it includes the step of crystallize epsilon - caprolactam from ethereal solution containing crude epsilon - caprolactam.The product purity can reach 99.98%upwards, absorption value of potassium permanganate is greater than 10000 or more, the extinction value of epsilon - caprolactam at 290nm wavelength is 0.05 or smaller. The invention wholly meet the require of industrial product.
Owner:CHINA PETROLEUM & CHEM CORP +1
Production of Adipic Acid and Derivatives from Carbohydrate-Containing Materials
ActiveUS20100317823A1Increase costLow costCarboxylic acid nitrile preparationOrganic chemistry methodsCatalytic transformationCatalytic oxidation
The present invention generally relates to processes for the chemocatalytic conversion of a glucose source to an adipic acid product. The present invention includes processes for the conversion of glucose to an adipic acid product via glucaric acid or derivatives thereof. The present invention also includes processes comprising catalytic oxidation of glucose to glucaric acid or derivative thereof and processes comprising the catalytic hydrodeoxygenation of glucaric acid or derivatives thereof to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Method for purifying and refining epsi-caprolactam
ActiveCN101070298AMeet the requirements of industrial productsLactams preparationLactams separation/purificationEtherLength wave
This invention relates to a refine means of epsilon - caprolactam by separation and purification. The feature of the invention is that it includes the step of crystallize epsilon - caprolactam from ethereal solution containing crude epsilon - caprolactam. The product purity can reach 99.98%upwards, absorption value of potassium permanganate is greater than 10000 or more, the extinction value of epsilon - caprolactam at 290nm wavelength is 0.05 or smaller. The invention wholly meets the require of industrial product.
Owner:CHINA PETROLEUM & CHEM CORP +1
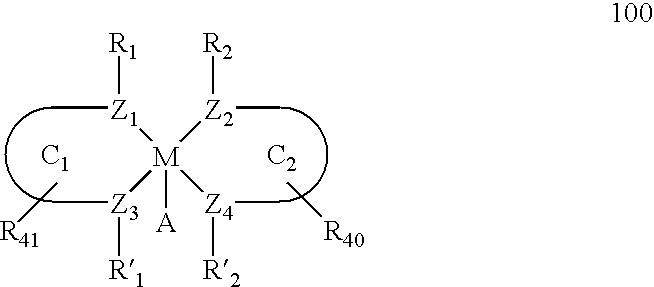
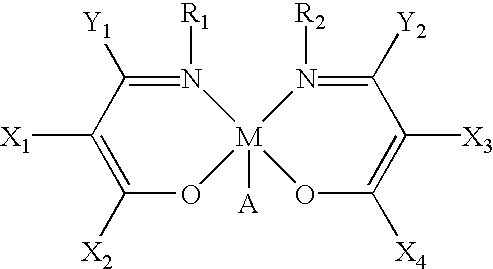
Main-group metal-based asymmetric catalysts and applications thereof
InactiveUS6844448B2Lactams preparationCarbamic acid derivatives preparationTetradentate ligandNucleophile
The present invention relates to a method and catalysts for the stereoselective addition of a nucleophile to a reactive π-bond of a substrate. The chiral, non-racemic catalysts of the present invention constitute the first examples of catalysts for nucleophilic additions that comprise a main-group metal and a tri- or tetra-dentate ligand.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Production of adipic acid and derivatives from carbohydrate-containing materials
ActiveUS8501989B2Lactams preparationCarboxylic acid nitrile preparationHydrodeoxygenationAdipic acid
Owner:ARCHER DANIELS MIDLAND CO
Process for producing epsi-caprolactam
InactiveUS6265574B1High yieldSteadily producedLactams preparationMolecular sieve catalystsBeckmann rearrangementFluidized bed
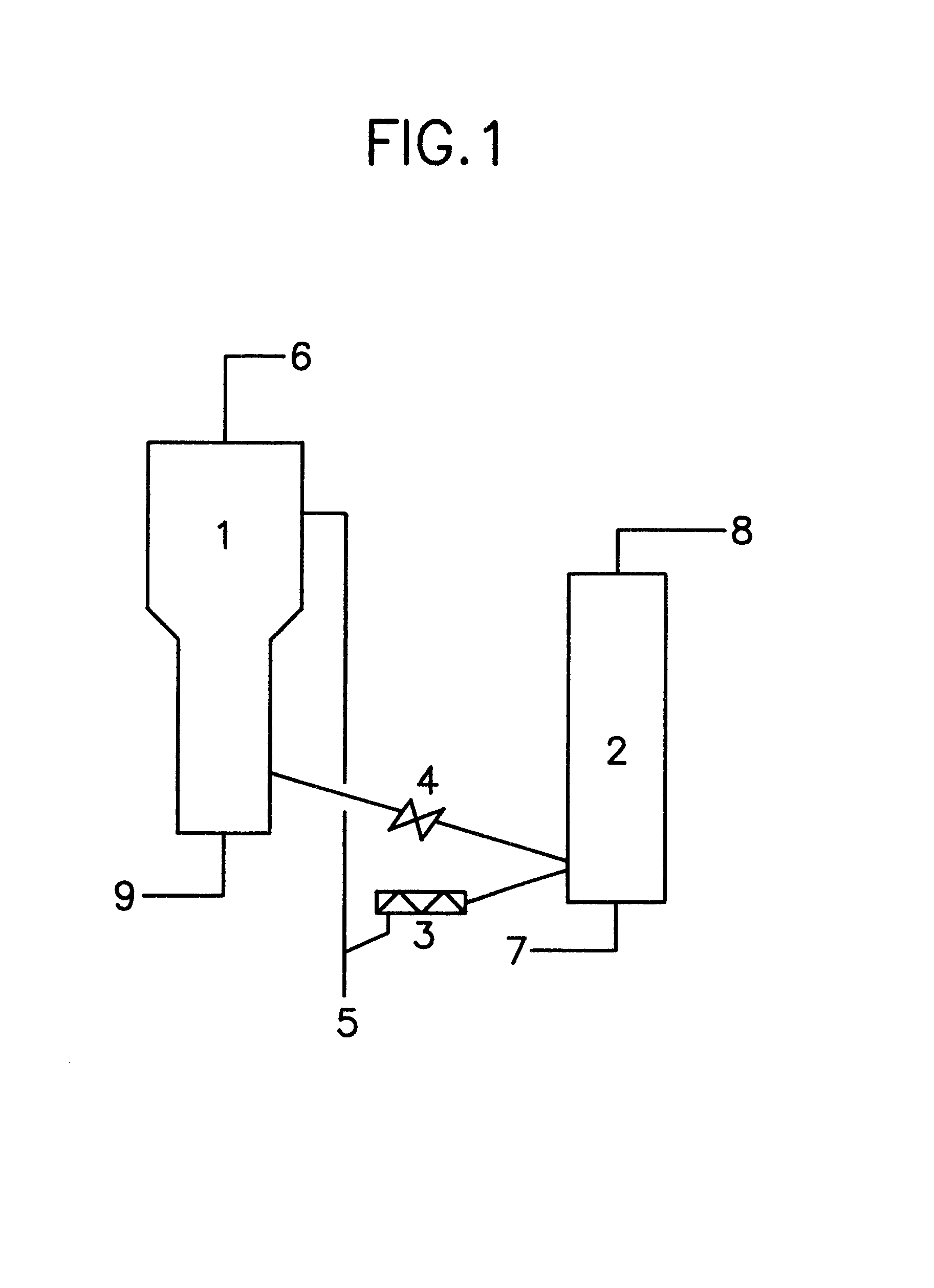
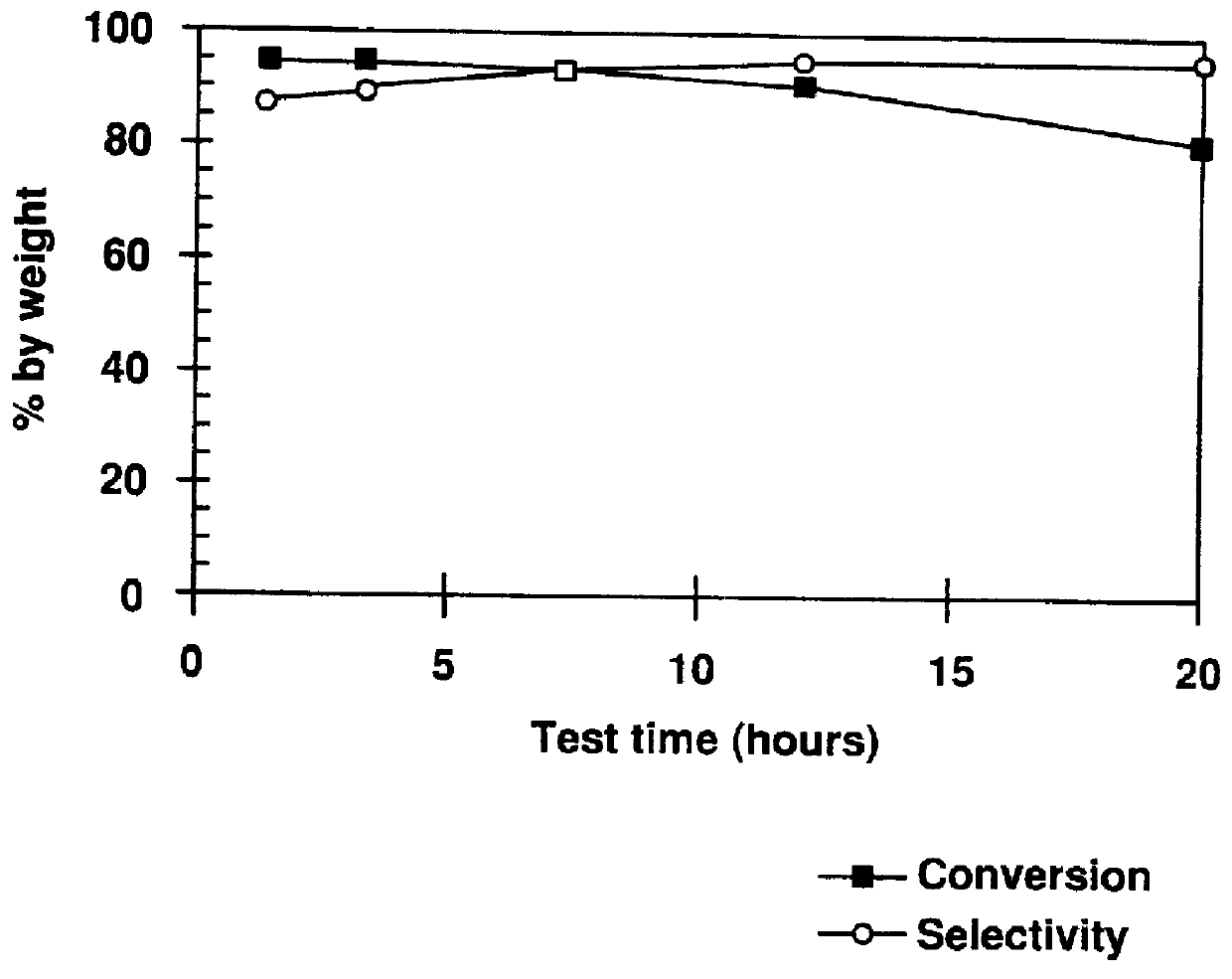
A process for producing epsi-caprolactam is provided which comprises the steps of subjecting cyclohexanone oxime to a gaseous phase Beckmann rearrangement reaction in a fluidized bed system using a solid catalyst and re-generating the catalyst, wherein said process comprises a step of treating the catalyst with an oxygen-containing gas at an elevated temperature in a re-generation step so that the nitrogen content of the catalyst falls within a range of 10 ppm to 2,500 ppm on its way to the reaction step from the re-generating step. According to the present invention, epsi-caprolactam is produced with a high conversion or a high selectivity without interrupting the rearrangement reaction or the re-generation step.
Owner:SUMITOMO CHEM CO LTD
Method for producing caprolactam by taking high-purity benzene as raw material
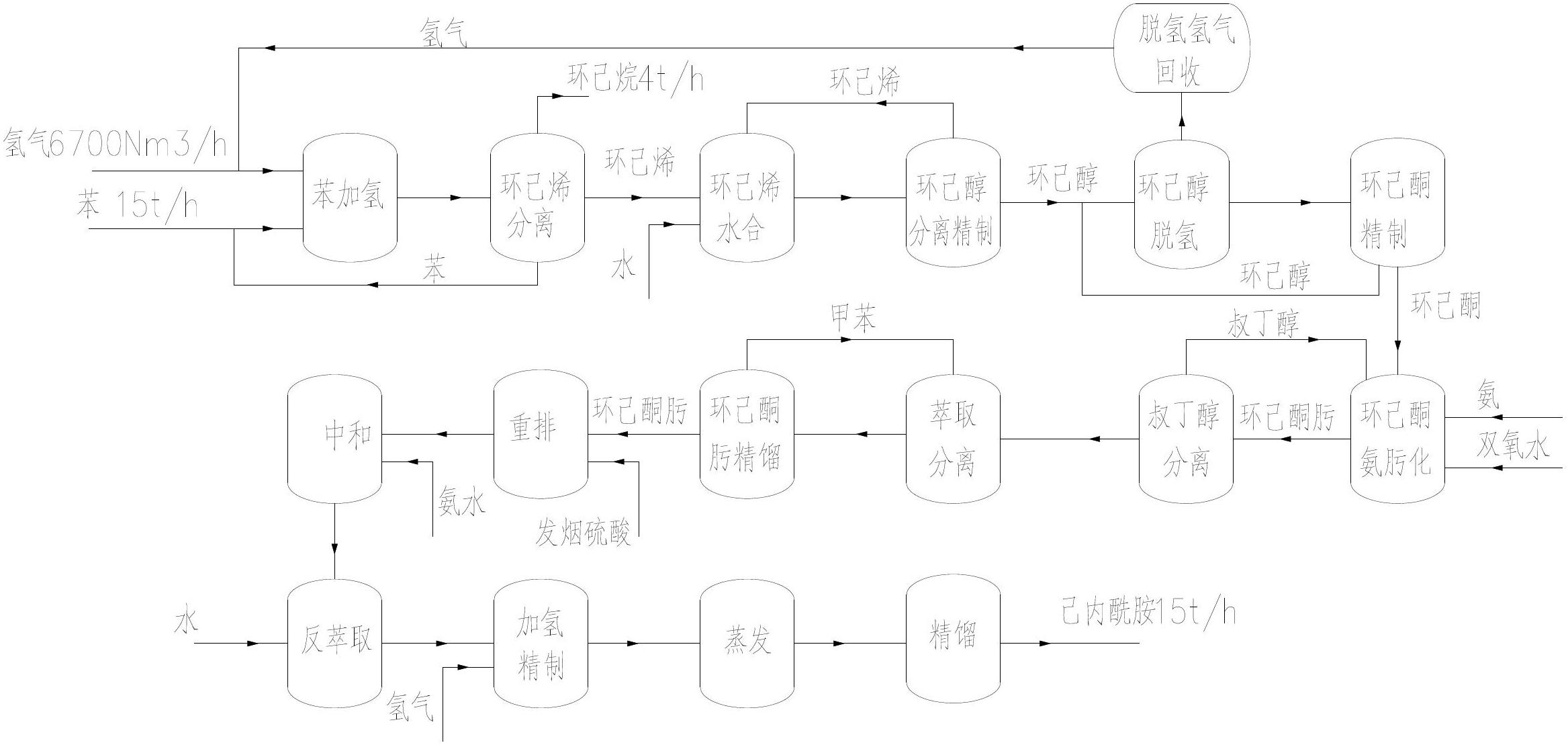
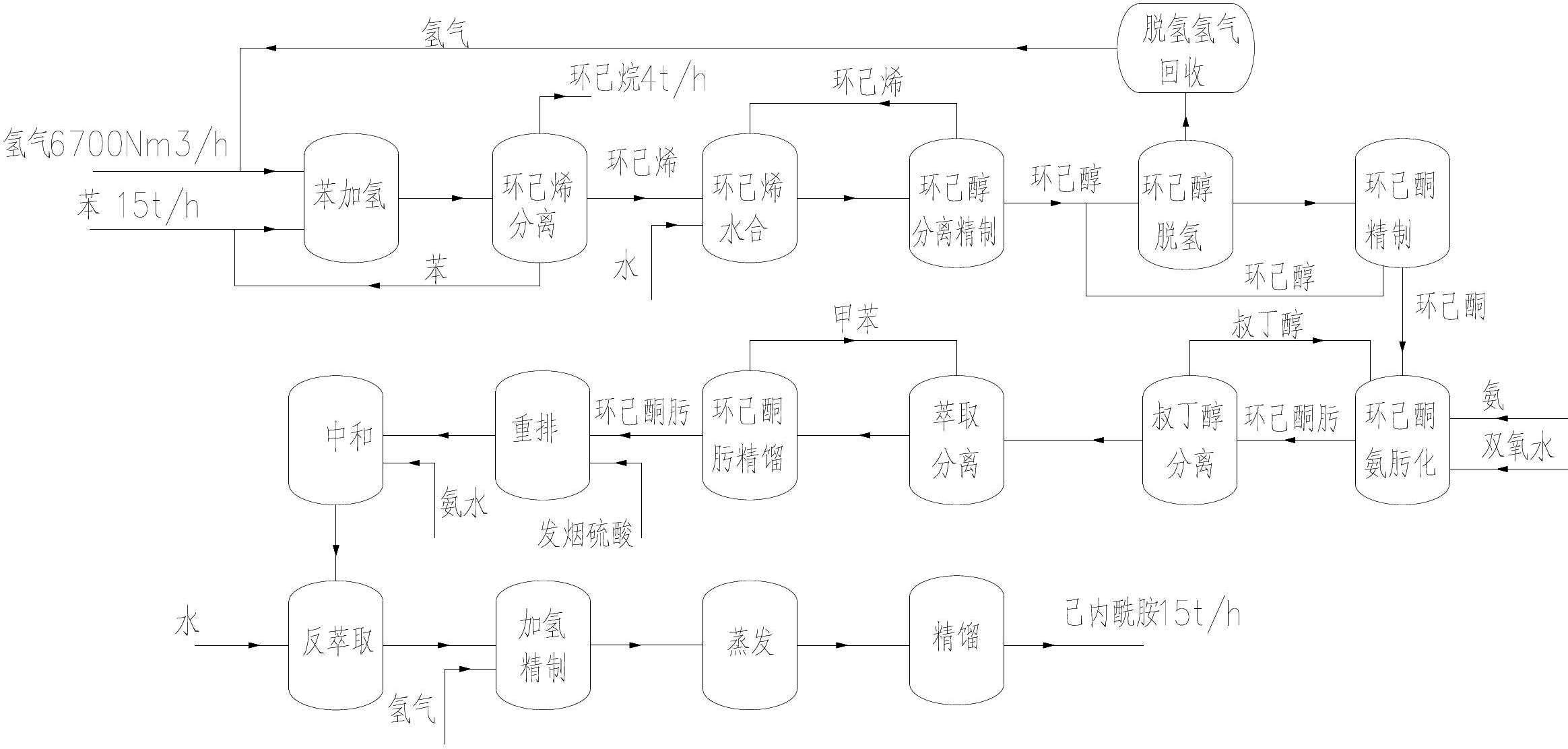
ActiveCN102675176AAvoid it happening againQuality improvementLactams preparationHydration reactionCyclohexene
The invention provides a method for producing caprolactam by taking high-purity benzene as a raw material, and the method comprises the following steps of: A. preparing cyclohexene from the raw material benzene through hydrogenation; B. separating and purifying cyclohexene; C. carrying out hydration on the cyclohexene for preparing cyclohexanol; D. separating and purifying cyclohexanol; E. carrying out dehydrogenation on cyclohexanol for preparaing cyclohexanone; F. refining cyclohexanone; G. carrying out oximation on cyclohexanone so as to prepare cyclohexanone-oxime; H. refining cyclohexanone-oxime; I. carrying out rearrangement on refined cyclohexanone-oxime so as to prepare caprolactam; and J. refining caprolactam, wherein the high-purity benzene is adopted as a raw material, so that the purity of benzene is more than 99.95%, the sulphur content is less than 5ppm, and the methylbenzene is not more than 100ppm. The method has the beneficial effects that the high-purity benzene is adopted as a raw material, so that the impurity is less, and the product quality is high; the raw material is high in comprehensive utilization rate and low in hydrogen consumption; and the mass of the raw material and intermediate products generated in all the steps of reaction can be strictly controlled, so that the direct commercial value of the intermediate products is fully exerted, and the optimal quality of the product caprolactam can be reached.
Owner:CHINA TIANCHEN ENG +3
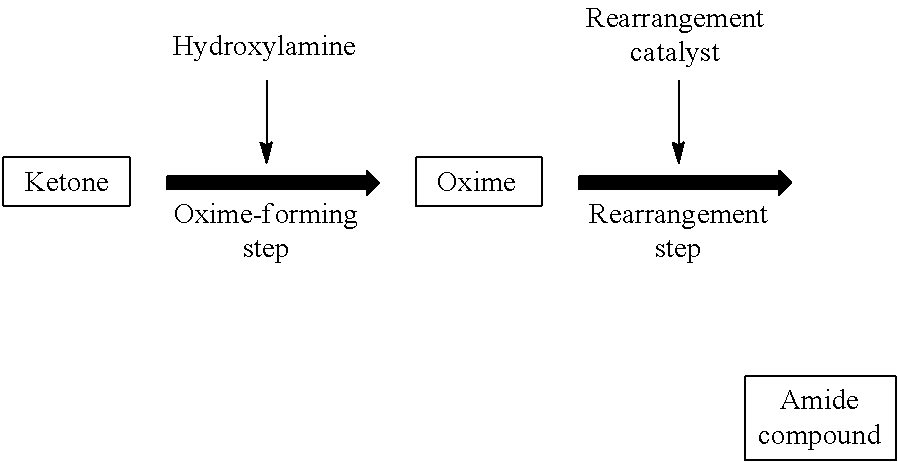
Method for producing amide compound
A method for producing a high purity, high quality amide compound, particularly a lactam. An amount of each of a halide, an aldehyde compound, an alcohol compound and a nitrile compound contained in a solution recycled into an oxime-forming step is controlled to an amount of 0.4 mol % or less based on the ketone as a starting material. One or more of a ketone, an oxime and an amide compound are purified by hydrogenation and / or crystallization for eliminating impurities containing a double bond. A content of impurities having a cyclic bridge structure is controlled using a cycloalkanone purified by recrystallization.
Owner:UBE IND LTD
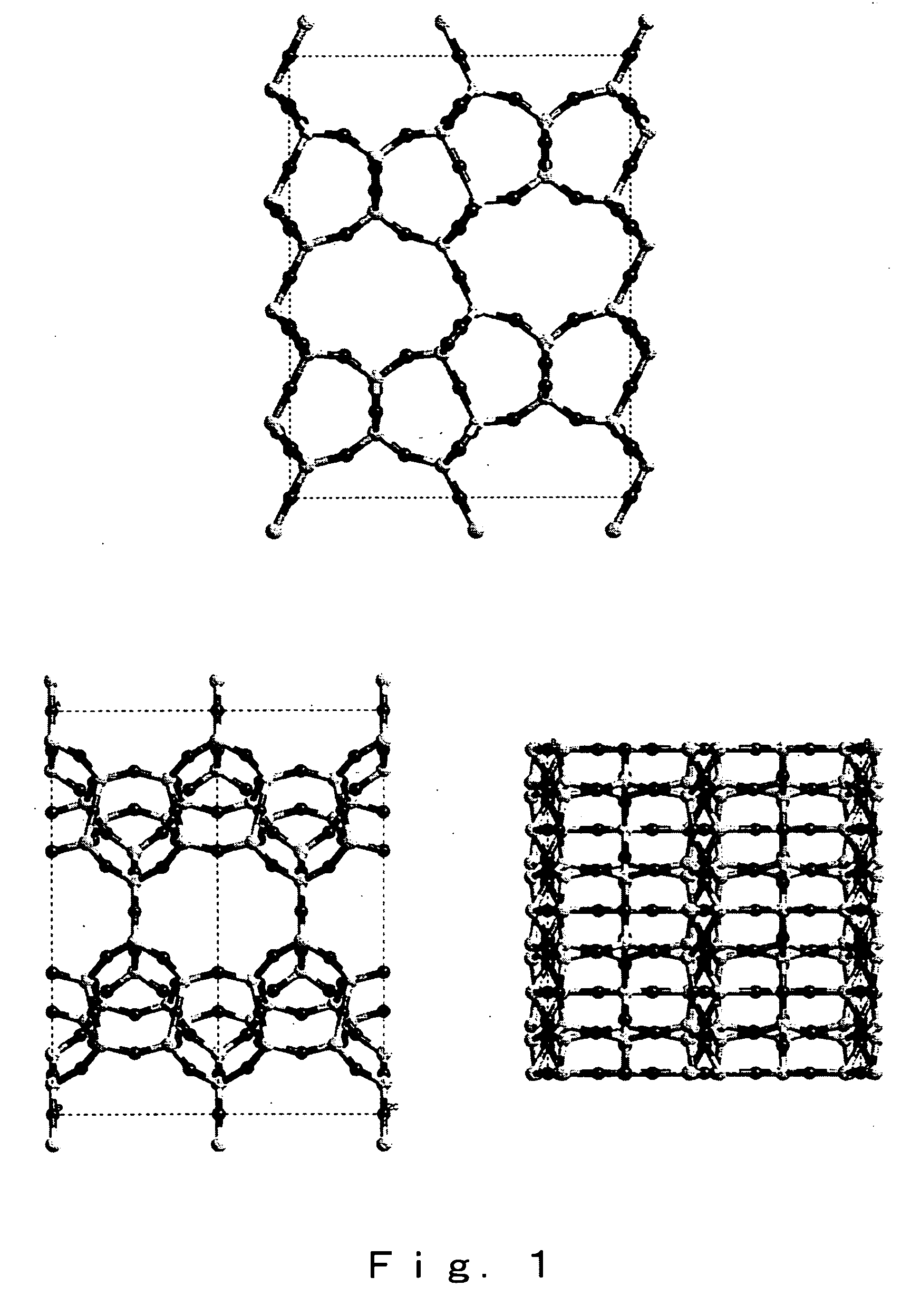
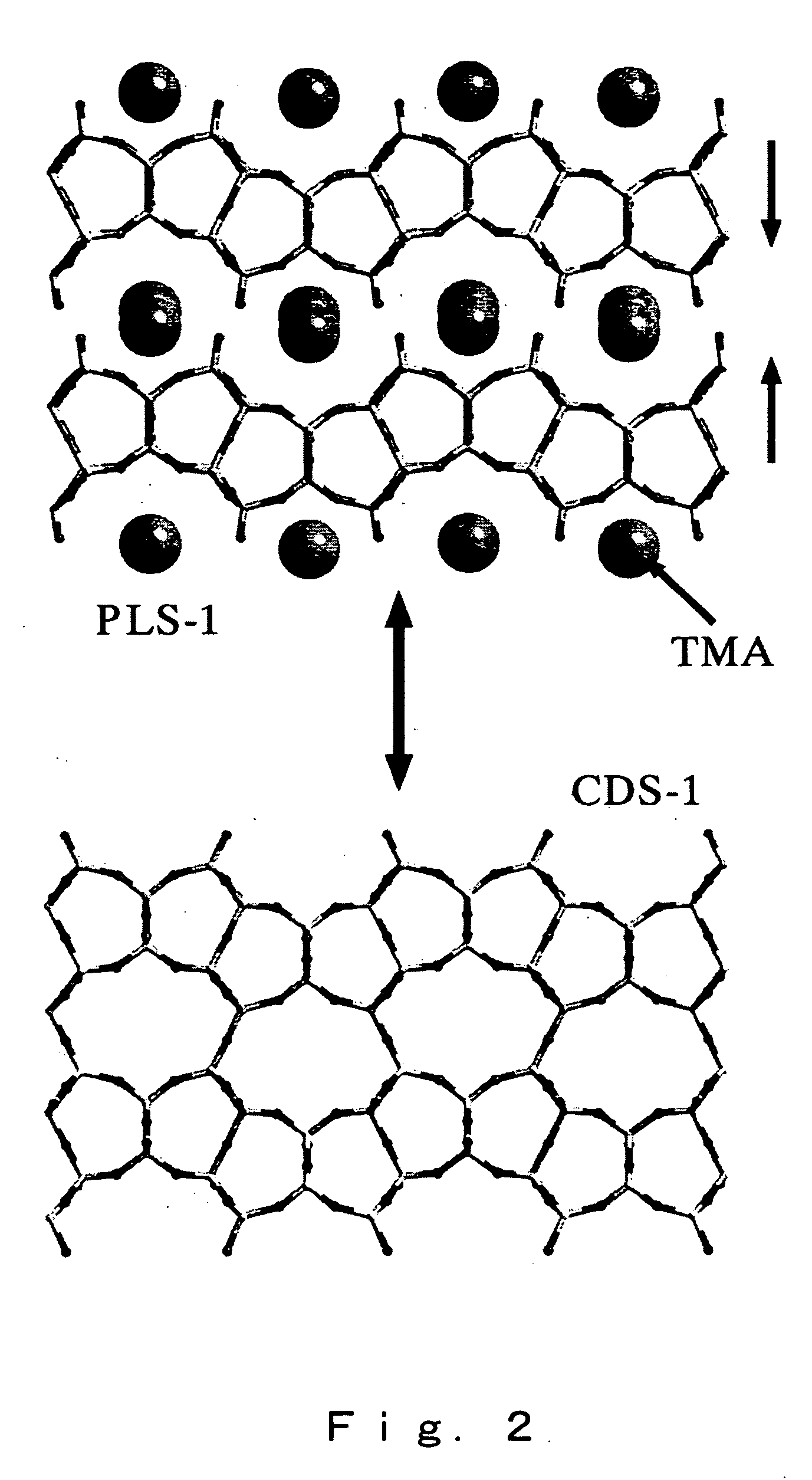
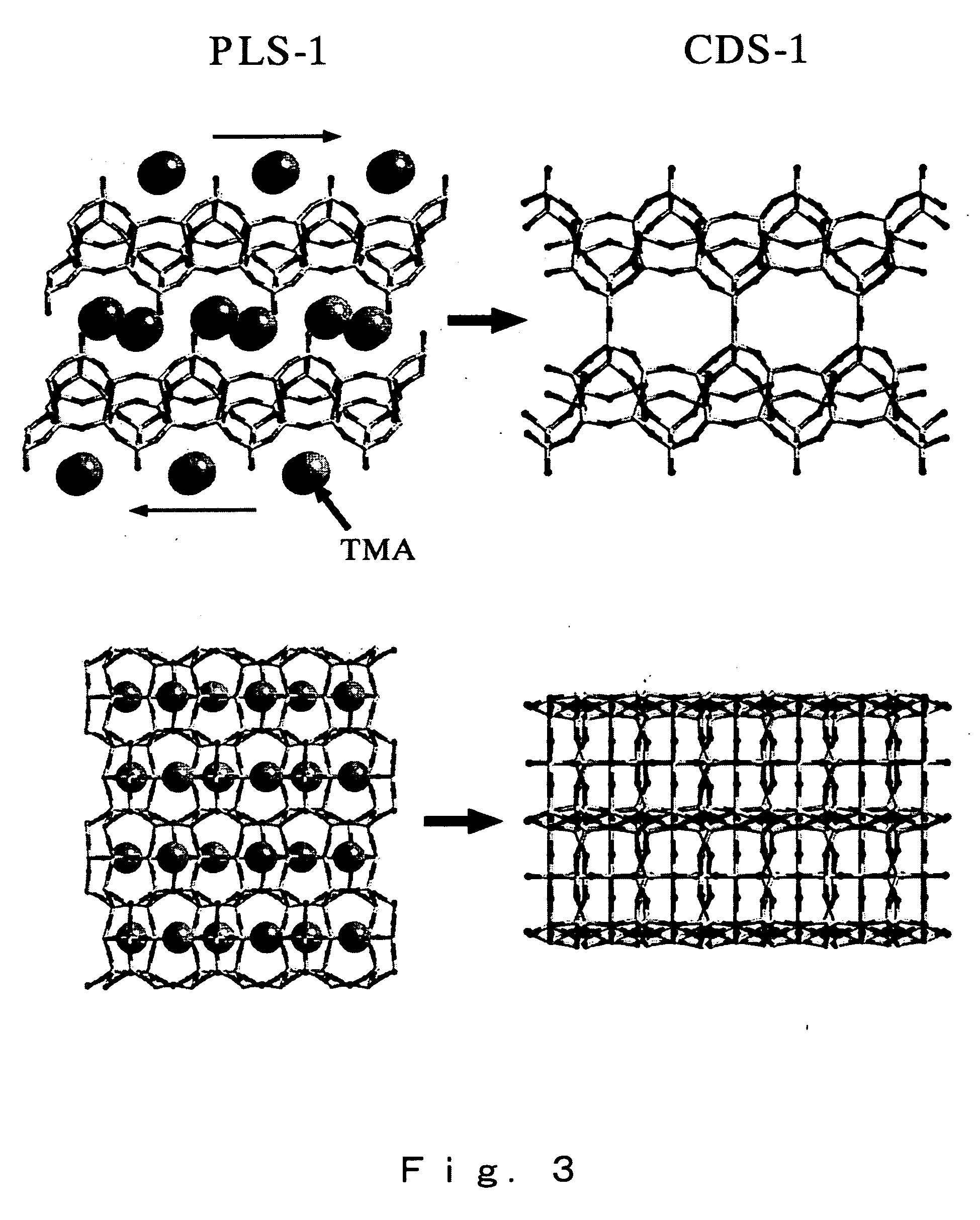
High silica cds-1 zeolite
InactiveUS20070112189A1Novel crystal structureHigh in silicaSemi-permeable membranesMembranesChemical compositionPhysical chemistry
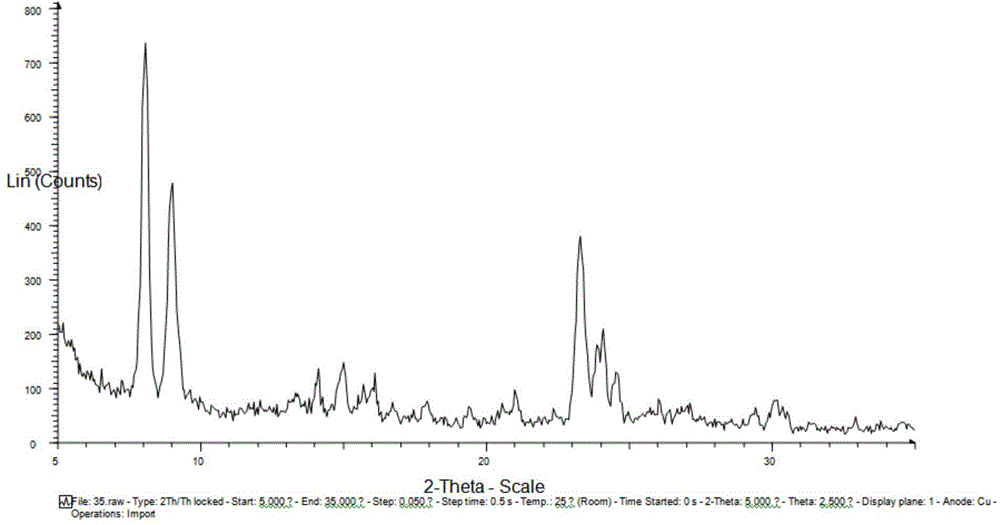
A high-silica content zeolite having a novel crystal structure, a zeolite membrane and manufacturing methods for these are provided, and the present invention relates to a zeolite having the chemical composition represented by [(Si36-xTy.O72).Mz] (wherein M is a cation of an alkali metal such as Li, Na, K or Rb, T represents Al, Ga, Fe and Ce as skeleton substituting elements, x satisfies 0≦x≦3.0, y satisfies 0≦y≦1.0 and z satisfies 0≦z≦3.0), and having a micropore formed of covalent bonds between Si and O atoms, with a specific diffraction peak at 2θ in powder x-ray diffraction, together with a zeolite membrane and methods for manufacturing these.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Process for producing pentacyl-type crystalline zeolites and a process for producing epsi-caprolactam using the same
InactiveUS6303099B1High activityGood reproducibilityAluminium compoundsLactams preparationPotassiumAmmonium hydroxide
A process for producing a pentacyl-type crystalline zeolite is provided which comprises the steps of (i) preparing a mixture of a silicon compound, water and tetrapropyl ammonium hydroxide, (ii) conducting a hydrothermal reaction of the mixture to obtain a reaction mixture containing zeolite crystals, (iii) separating zeolite crystals from the reaction mixture to collect a remaining solution, (iv) calcinating the resulting zeolite crystals, (v) treating the calcinated zeolite crystals with a solution such as ammonia water and (vi) recycling the solution collected in step (iii) to step (i), wherein in the mixture prepared in step (i) a molar ratio of hydroxide ion to silicon is from about 0.1 to about 0.4 and a molar ratio of potassium to silicon is about 0.1 or less. With this process, a zeolite catalyst having good performance can be produced with good reproducibility.
Owner:SUMITOMO CHEM CO LTD
Process for preparing caprolactam by cyclohexanone-oxime gas phase rearrangement
ActiveCN1621405AHigh yieldReduce consumptionLactams preparationBeckmann rearrangementMolecular sieve
The process of preparing caprolactam with cyclohexanone oxime includes the vapor Beckmann rearrangement reaction of cyclohexanone oxime inside one first fixed bed reactor in the presence of MFI structure molecular sieve catalyst; the decomposition and conversion of the reaction side product O-alkyl-epsilon-caprolactim into caprolactam inside one second fixed bed reactor in the presence of MFI structure molecular sieve catalyst and water; and the separation and purification of the reaction effluent. Compared with single vapor Beckmann rearrangement reaction process of cyclohexanone oxime, the present invention has 1-3 % raised caprolactam yield.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nanometer all-silicon molecular sieve and its preparation method and use
InactiveCN102432032AHigh crystallinityRegular shapeLactams preparationMolecular sieve catalystsBeckmann rearrangementMass number
The invention provides a nanometer all-silicon molecular sieve. The nanometer all-silicon molecular sieve is prepared from tetrapropylammonium hydroxide, a silicon source, water and amino acids. A mole ratio of the silicon source to tetrapropylammonium hydroxide to water is 1: [0.05 to 0.50]: [15 to 65]. The mass of the used amino acids is 0.5 to 5% of that of the silicon source. A mole number and a mass number of silica represent a mole number and a mass number of the silicon source. The invention also provides a preparation method of the nanometer all-silicon molecular sieve, and a use of the nanometer all-silicon molecular sieve in a cyclohexanone-oxime gas phase beckmann rearrangement reaction. The nanometer all-silicon molecular sieve has a high degree of crystallization, regular morphology and adjustable particle sizes of 40 to 160 nanometers. In a cyclohexanone-oxime gas phase beckmann rearrangement reaction, the nanometer all-silicon molecular sieve shows excellent catalytic activity, selectivity and stability.
Owner:HUNAN UNIV
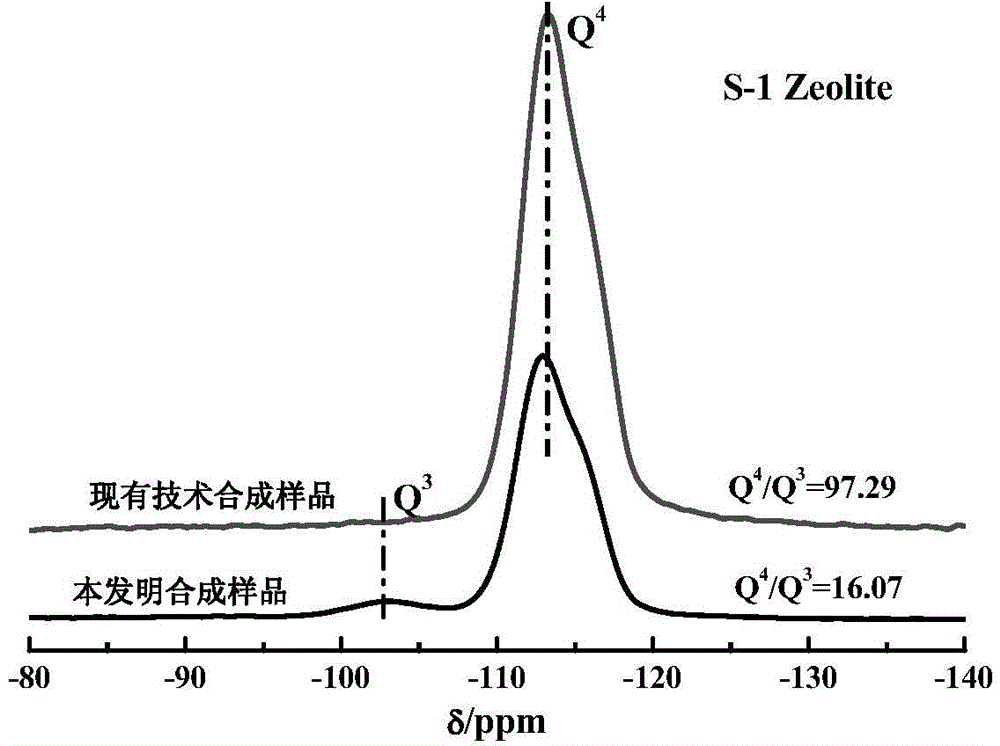
All-silicon molecular sieve and synthetic method thereof
ActiveCN104556087AHigh catalytic activityFlexible adjustment of particle sizeLactams preparationSilicaMolecular sieveBeckmann rearrangement
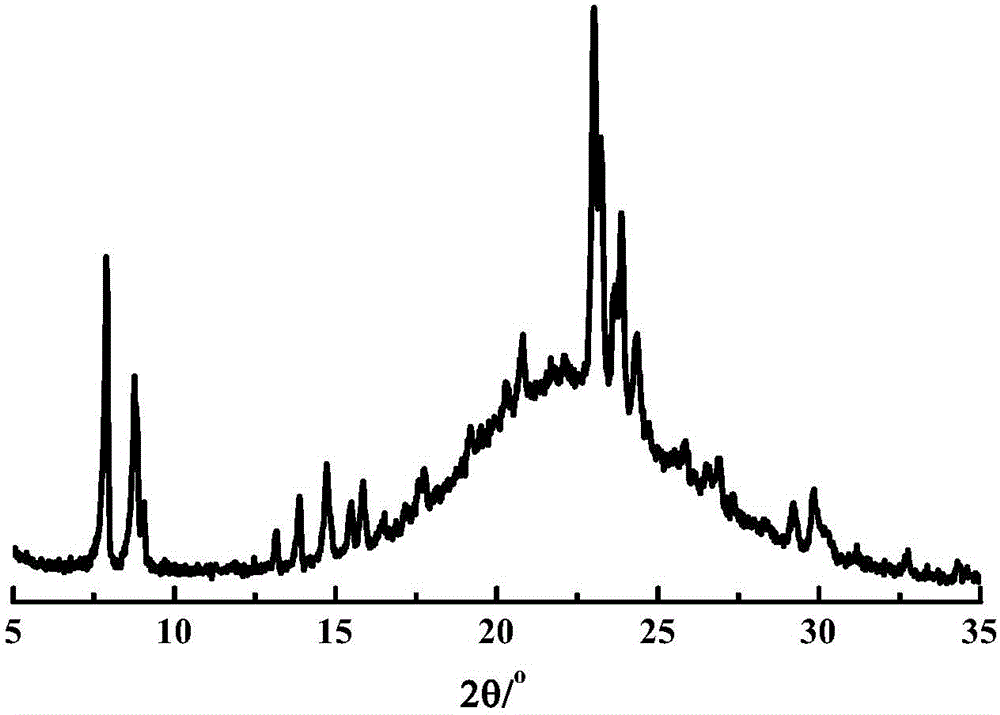
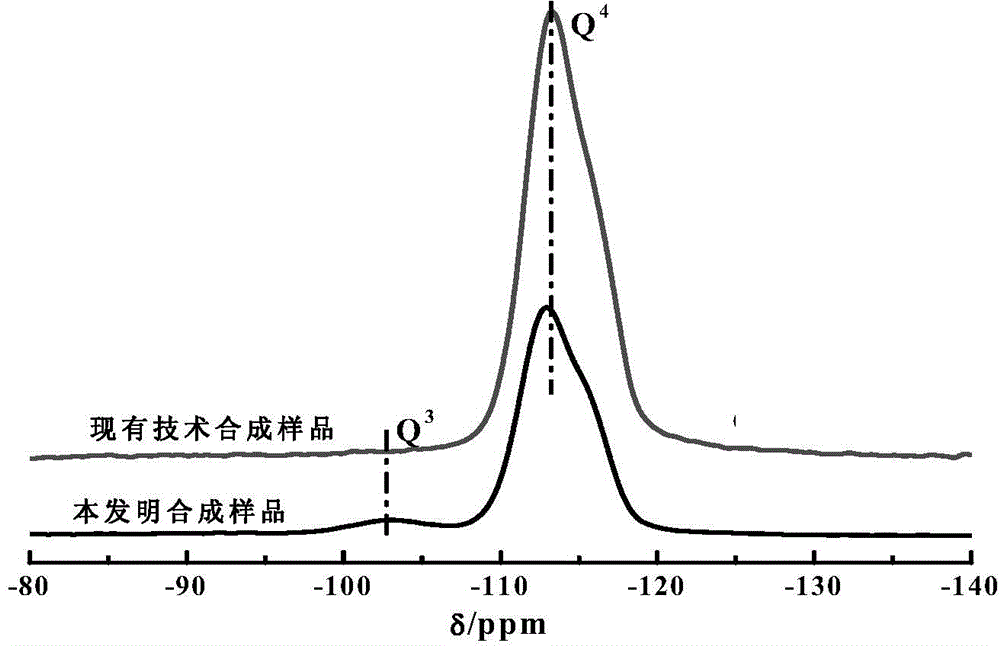
The invention provides an all-silicon molecular sieve and a synthetic method thereof. Q4 / Q3 of the all-silicon molecular sieve is (10-90):1, wherein Q4 is the peak intensity expressed by the peak height at the chemical shift of -112+ / -2ppm in a 29SiNMR spectrogram of the all-silicon molecular sieve, and Q3 is the peak intensity expressed by the peak height at chemical shift of -103+ / -2ppm in the 29SiNMR spectrogram of the all-silicon molecular sieve. The synthetic method of the all-silicon molecular sieve comprises the following steps: mixing a template agent, an organic silicon source, an inorganic ammonium source and water to be subjected to hydrolytic alcohol removal, aging the obtained product, mixing the aging product with a solid silicon source, crystallizing the mixture in a closed reaction kettle, and recovering the all-silicon molecular sieve. The all-silicon molecular sieve provided by the invention has higher activity in cyclohexanone-oxime beckmann rearrangement.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for the carbonylation of pentenenitrile
Processes to prepare 5-cyanovaleric acid or its ester are provided, by carbonylation of a pentenenitrile, wherein pentenenitrile is reacted with carbon monoxide and water and / or an alcohol in the presence of a catalyst system. The catalyst system contains:(a) a metal of Group VIII or a compound thereof and(b) a bidentate phosphine, arsine and / or stibine ligand, wherein the bidentate ligand has the general formula (I):R1R2-M1-R-M2-R3R4 (I) wherein M1 and M2 are independently P, As or Sb, R is a divalent organic bridging group, which bridging group comprises a chain of 3 to 5 atoms directly connecting the 2 phosphorus atoms, which chain consists of carbon atoms and optionally a nitrogen, oxygen or sulphur atom or a silano or dialkylsilicon group, which alkyl groups independently comprise from 1 to 4 carbon atoms, and R1–R4 represent the same or different optionally substituted tertiary alkyl groups,(c) an acid having a pKa less than 3, as measured at 18° C. in an aqueous solution.ε-caprolactam is also prepared by reduction of 5-cyanovaleric acid or ester obtained above to 6-aminocaproic acid or ester, and then cyclisation of the 6-aminocaproic acid or ester to ε-caprolactam.
Owner:SHELL OIL CO
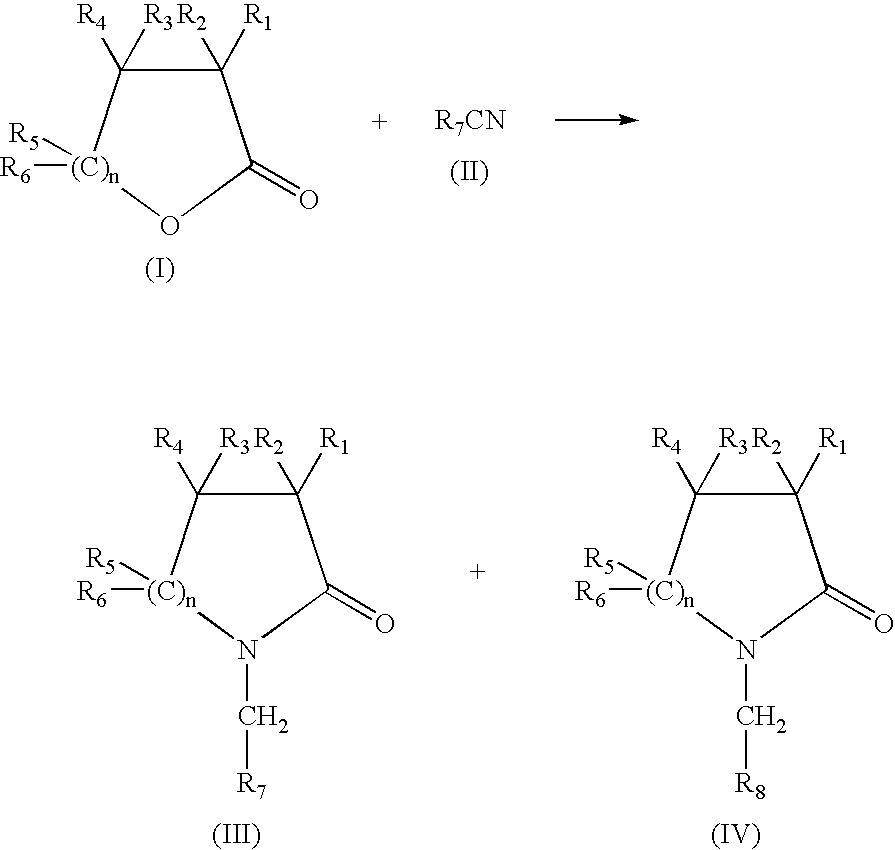
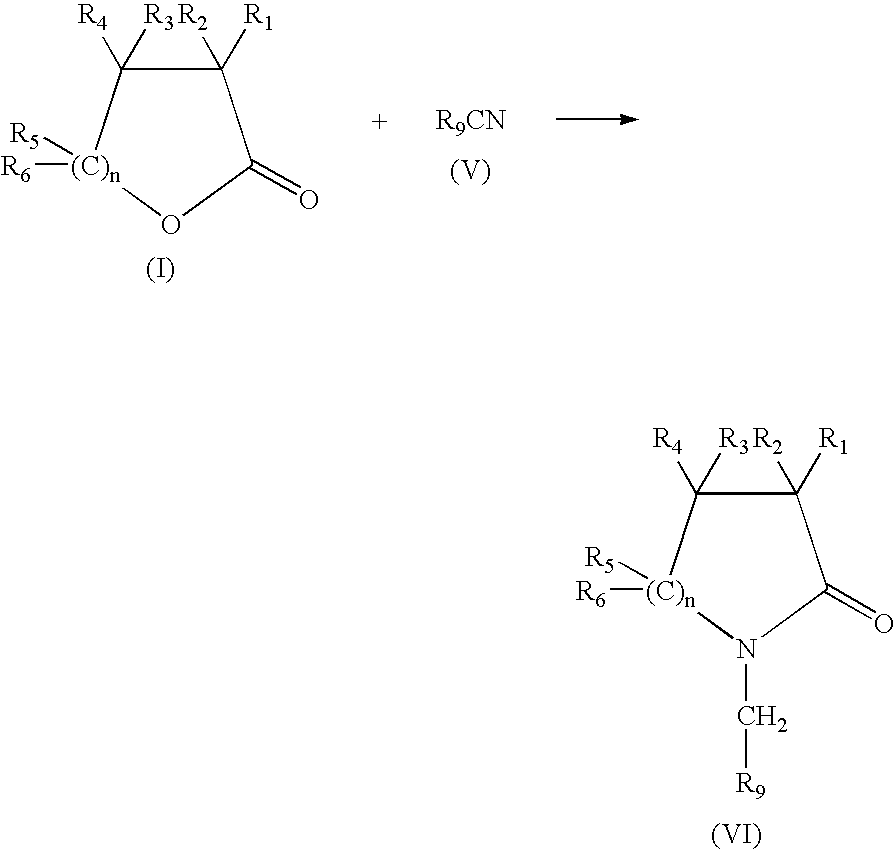
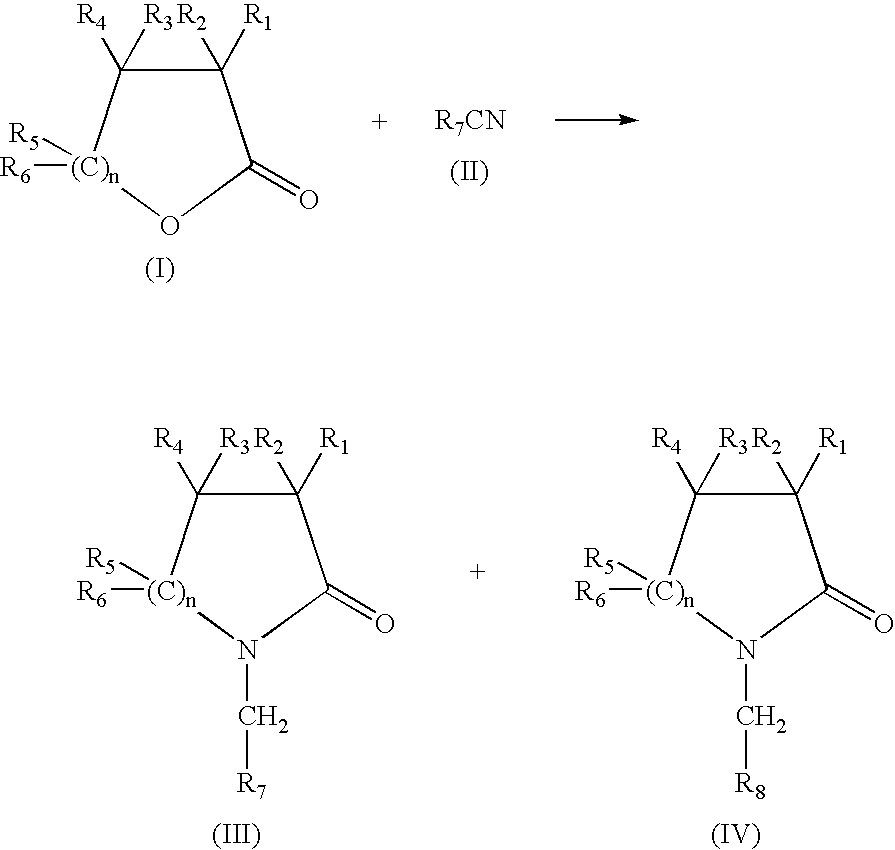
Production of N-(methyl aryl)-2-lactam, N-(methyl cycloalkyl)-2-lactam and N-alkyl-2-lactam by reductive amination of lactones with aryl and alkyl cyano compounds
Owner:EI DU PONT DE NEMOURS & CO
Layered silicate catalysts pillared with metal oxide
InactiveUS6703501B1Speed up the conversion processHigh selectivityLactams preparationMolecular sieve catalystsPorous catalystCyclohexanone oxime
The present invention relates to porous catalysts comprising layered silicate and metal oxides, and a method of preparing epsilon-caprolactam from cyclohexanone oxime using the catalyst. This new catalyst can resolve the environmental and safety problems arising from conventional liquid acid process. Also the present catalyst solves the problem of short lifetime of current solid acid catalysts. Moreover, the present catalyst provides higher selectivity and yield.
Owner:KOREA INST OF SCI & TECH
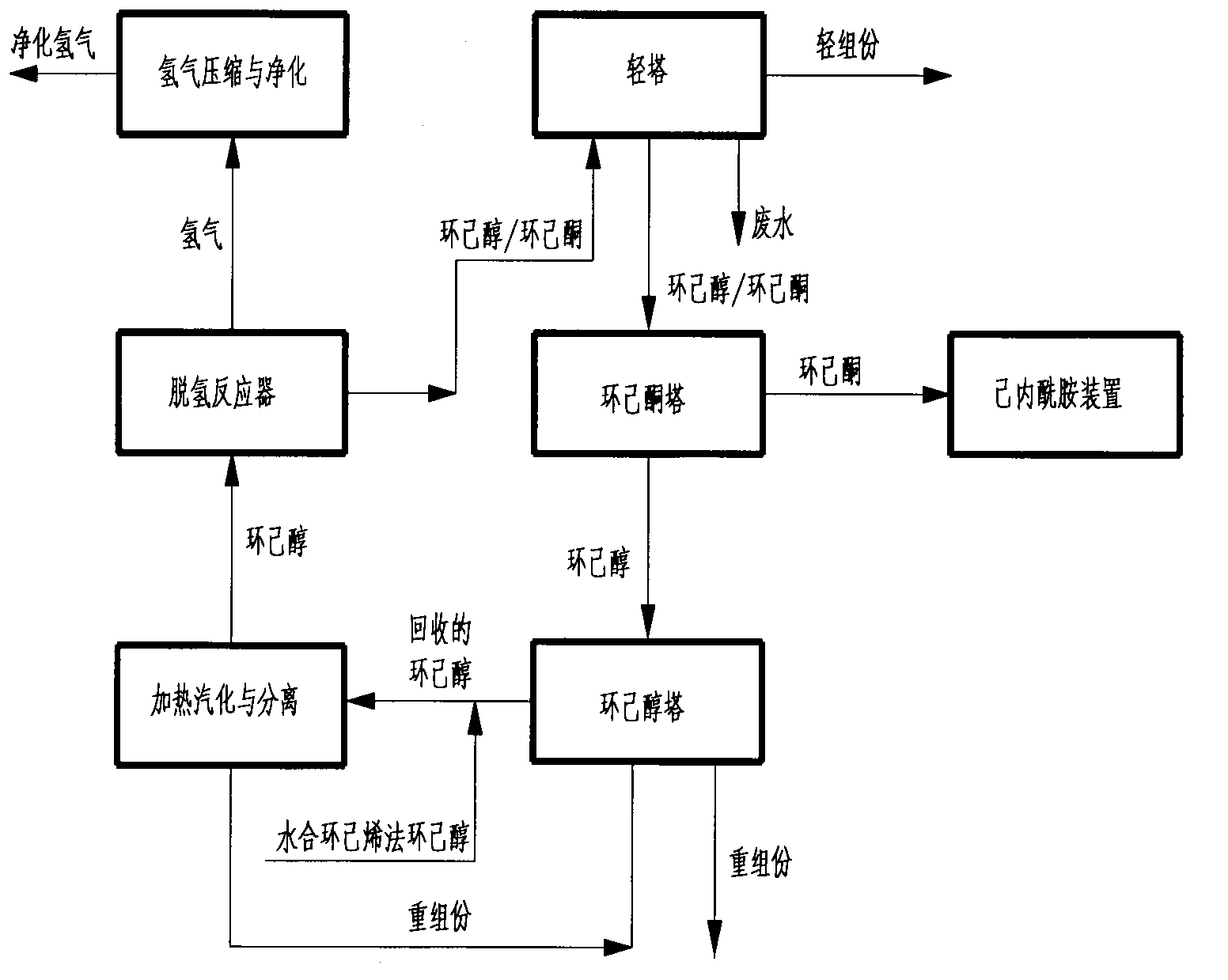
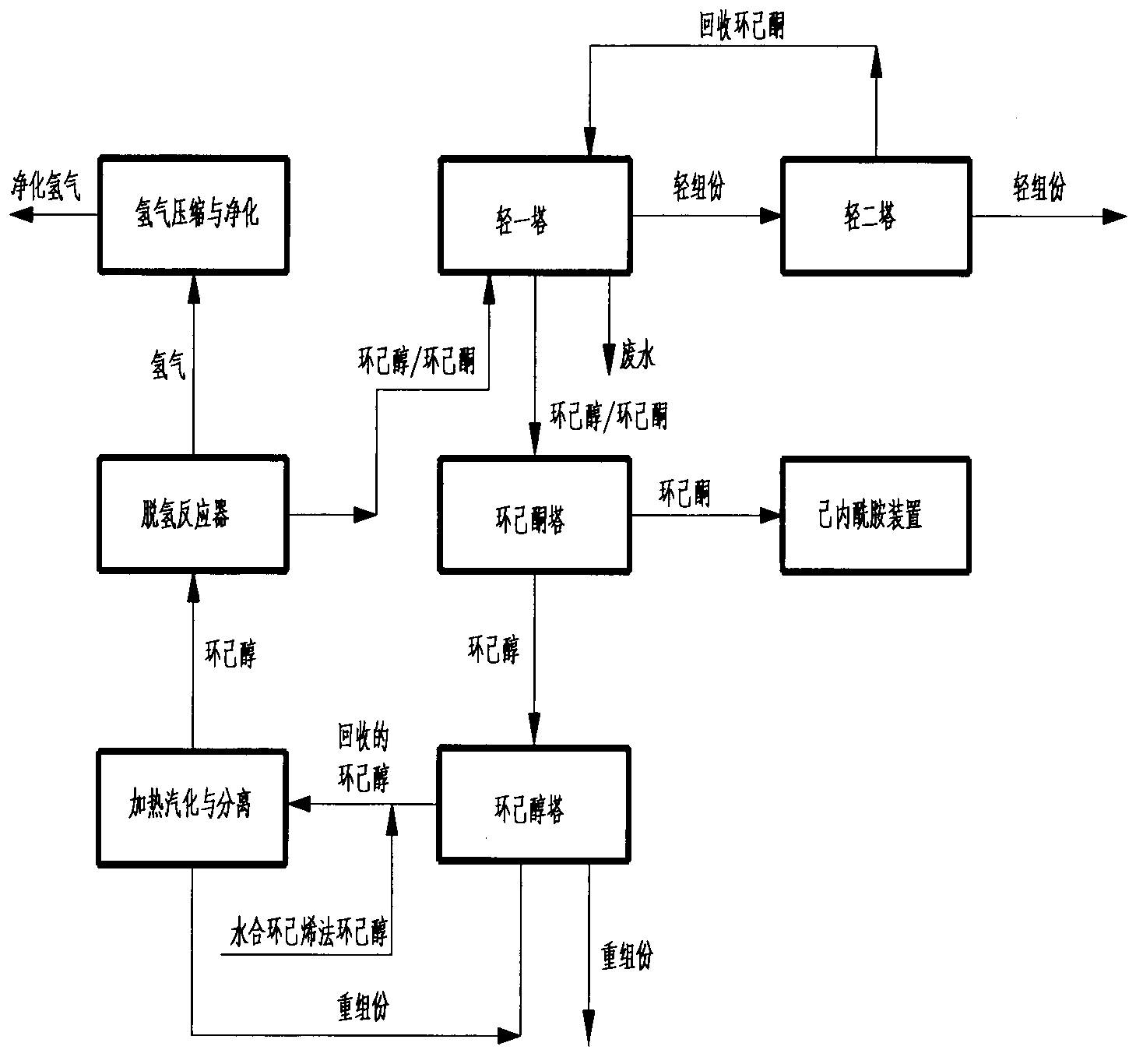
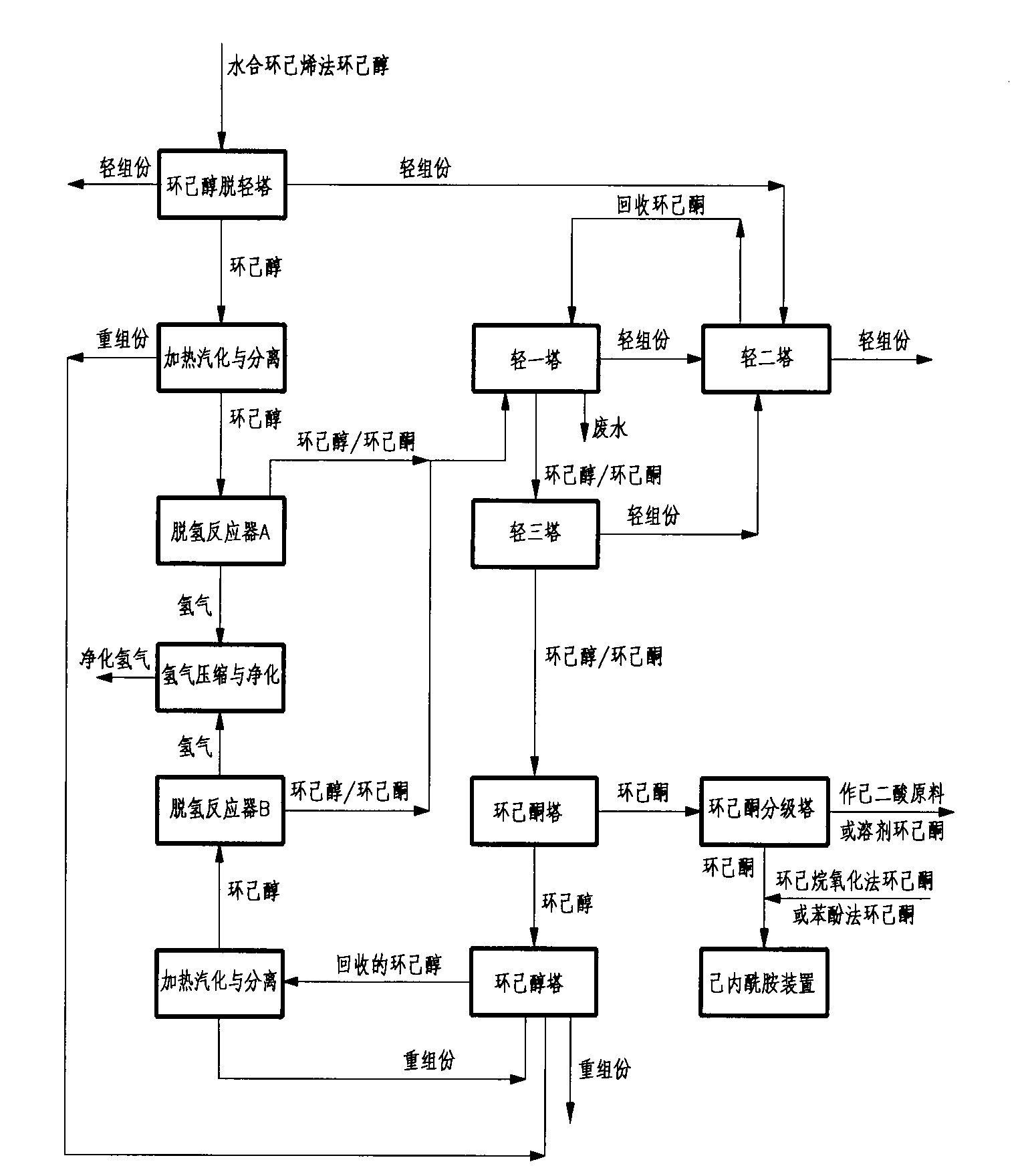
Method for preparing cyclohexanone serving as raw material of caprolactam from cyclohexene-hydration cyclohexanol
InactiveCN103265418AHigh purityReduce cycle lossLactams preparationOrganic compound preparationCyclohexanoneCyclohexene
The invention relates to a method for preparing cyclohexanone serving as a raw material of caprolactam from cyclohexene-hydration cyclohexanol. The method comprises the following steps of: (A) removing light components from the cyclohexene-hydration cyclohexanol in a cyclohexanol light component removal tower; (B) heating to vaporize the cyclohexene-hydration cyclohexanol so as to separate and remove heavy components; (C) carrying out dehydrogenation reaction on the cyclohexene-hydration cyclohexanol so as to convert the cyclohexene-hydration cyclohexanol into the cyclohexanone; (D) removing light components by a light component removal tower; (E) removing cyclohexanol and heavy components by a cyclohexanone tower; (F) grading cyclohexanone by a cyclohexanone grading tower; (G) removing heavy components by a cyclohexanol tower; (H) heating to vaporize the recovered cyclohexanol so as to separate and remove heavy components; (I) carrying out dehydrogenation reaction on the recovered cyclohexanol so as to convert the recovered cyclohexanol into the cyclohexanone; and (J) carrying out compressing and purifying treatment on byproduct hydrogen gas of the cyclohexanol dehydrogenation reaction. The method further comprises a process for mixing the cyclohexanone with qualified oxidation-method or phenol-method cyclohexanone. The method has the advantages that the energy consumption and the loop loss of the cyclohexanol are reduced, light-component impurities are removed effectively, and the content of harmful impurities in cyclohexene-hydration cyclohexanone is reduced to meet the requirements for the production of high-quality caprolactam.
Owner:NANJING DELIFEI TECH CONSULTING
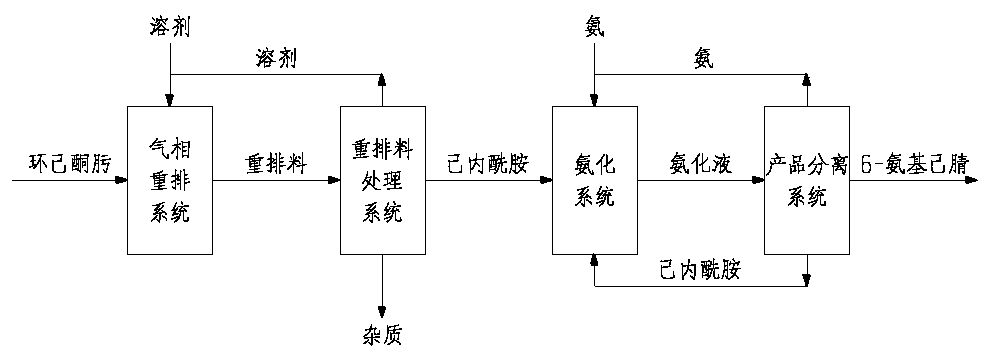
Method for preparing 6-aminocapronitrile from cyclohexanone-oxime
InactiveCN110835311AReduce consumptionReduce processing complexityLactams preparationLactams separation/purificationDistillationIon exchange
The invention discloses a method for preparing 6-aminocapronitrile from cyclohexanone-oxime. The method comprises the following steps: carrying out a rearrangement reaction on cyclohexanone-oxime to obtain a rearranged reaction material; and treating the rearranged reaction material to obtain caprolactam, carrying out an ammoniation dehydration reaction on the caprolactam to obtain an ammoniated dehydrated reaction material, and performing separation to obtain the 6-aminocapronitrile. Compared with the prior art, the method of the invention reduces complex separation and refining processes such as back extraction, ion exchange, hydrogenation, evaporation and distillation required after liquid phase rearrangement of the cyclohexanone-oxime, reduces the processes of crystallization, solventrefining and recovery needed after gas phase rearrangement, and organically combines the caproamide evaporation and rearrangement treatment processes in the ammoniation dehydration reaction process. The steam consumption can be reduced by 2.0-3.0 t / t 6-aminocapronitrile, and the cyclohexanone-oxime consumption is reduced by 20-50 kg / t 6-aminocapronitrile.
Owner:HUNAN BAILI ENGINEERING SCIENCE AND TECHNOLOGY CO LTD
Method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas
ActiveCN103896839AImprove conversion rateHigh selectivityLactams preparationMolecular sieve catalystsBeckmann rearrangementMolecular sieve
The invention provides a method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas. According to the method, under the condition of rearrangement reaction, cyclohexanone-oxime is in contact with a catalyst, and the catalyst is obtained by the steps of forming a molecular screen with an aluminum-free MFI (Melt Flow Index) structure, and enabling the molecular screen to be in contact with an alkaline buffer solution of a nitrogen-containing compound. The method is high in cyclohexanone-oxime conversion rate, and is capable of realizing long-period and continuous production; the selectivity of caprolactam is capable of reaching 95.8 percent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method for catalyst containing MFI structure zeolite
InactiveCN102233277APromote regenerationGood choiceLactams preparationMolecular sieve catalystsNitrogenSilica gel
The invention relates to a preparation method for a catalyst containing a MFI structure zeolite. The method is characterized in that: raw materials comprising the MFI structure zeolite and alkaline silica sol are subjected to mixing, forming, drying and baking to obtain a baked product, then the baked product contacts a alkaline buffer solution of a nitrogen compound, wherein a pH value of the alkaline silica sol is 9-11, a weight ratio of the MFI structure zeolite to the alkaline silica sol (according to a SiO2 content) is 1-50:1.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing all-silicon micro-mesoporous composite material
ActiveCN104556085AFlexible adjustment of particle sizeLower synthesis costLactams preparationSilicaMolecular sieveAmmonium compounds
The invention relates to a method for synthesizing an all-silicon micro-mesoporous composite material. The method for synthesizing the all-silicon micro-mesoporous composite material comprises the following steps: mixing a template agent, an organic silicon source, an inorganic ammonium source and water, ageing, uniformly mixing with a solid silicon source, crystallizing, and recycling an all-silicon micro-mesoporous composite molecular sieve, wherein the template agent is an organic quaternary ammonium compound, a long-chain alkylammonium compound and optional organic amine. The synthesized all-silicon micro-mesoporous composite material has a high defect site and has high activity when used for converting of cyclohexanone-oxime.
Owner:CHINA PETROLEUM & CHEM CORP +1
Scratch and mar resistant low VOC coating composition
A curable coating composition, and process of application thereof, particularly useful as a clearcoating applied over a pigmented basecoat that has significantly decreased VOC and improved scratch, etch, and mar resistance, is provided in accordance with the present invention, which is contains a silane functional oligomeric or polymeric material comprising carbamate groups and a crosslinking component comprising groups that are reactive with the carbamate groups.
Owner:LOPER SCOTT W +1
Method for synthesizing cyclohexanone-oxime and caprolactam
ActiveCN104341318ALow yieldImprove conversion rateLactams preparationOximes preparationGas phaseCyclohexanone oxime
The invention relates to a method for synthesizing cyclohexanone-oxime and caprolactam, particularly a method for synthesizing cyclohexanone-oxime and caprolactam by performing catalytic hydrogenation on nitrocyclohexane used as a raw gas phase. The method has the advantages of high controllability, low cost, environmental protection, higher conversion ratio of raw materials and higher yield, and has industrialization application prospects. The reaction process is simple, no coproduct ammonium sulfate is generated in the reaction process, and no wastewater or waste gas is generated to pollute the environment, and thus, the method is an environment-friendly production technique.
Owner:XIANGTAN UNIV
Method of producing epsilon -Caprolactam, MFI zeolite catalysts on whose surface symmetrically arranged OH groups are present and to a method of producing them
A method of producing epsilon -caprolactam from cyclohexanone in the gaseous phase using MFI catalysts on whose surface symmetrically arranged OH groups are present.
Owner:SUMITOMO CHEM CO LTD
Process for Preparing Caprolactam and Polyamides Therefrom
InactiveUS20130085255A1Reduce dependenceEasy to useLactams preparationOrganic compound preparationMuconic acidPolymer science
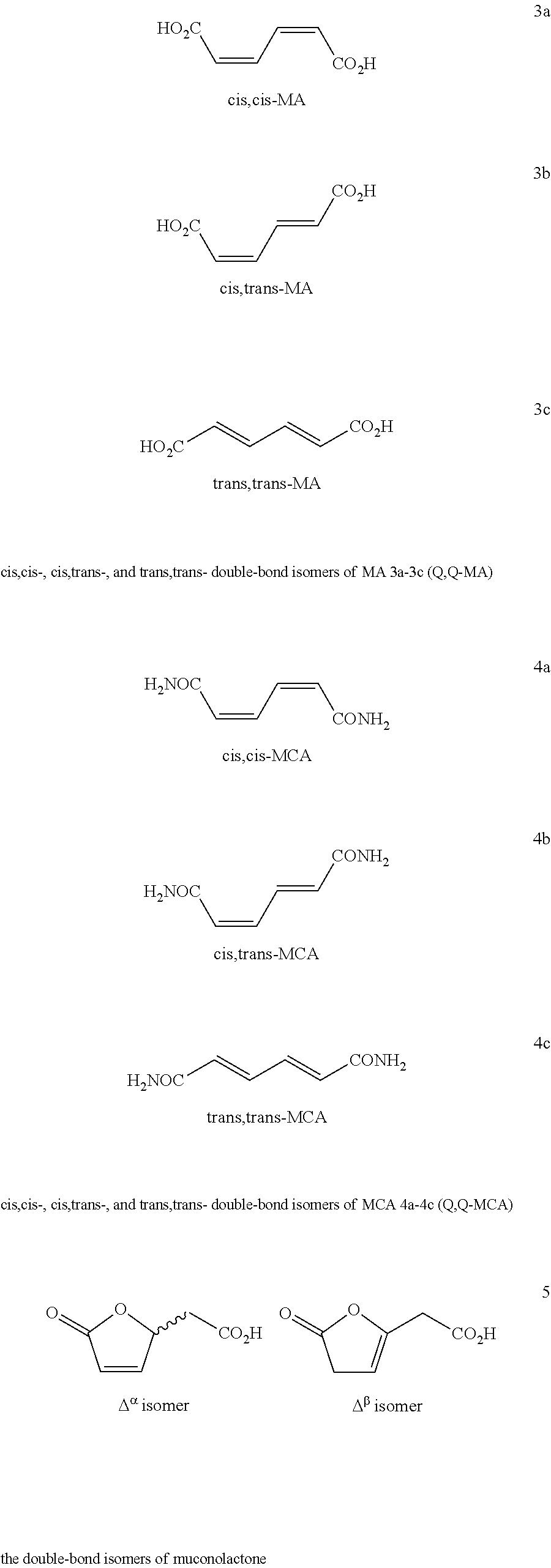
Provided herein are processes for preparing caprolactam from a starting material such as one or more of the cis,cis-, cis,trans- and trans,trans-double-bond isomers of muconamide, muconic acid ester, or muconic acid. The starting material, intermediates, and caprolactam prepared therefrom can contain carbon atoms derived from biomass containing detectable 14C content determined according to ASTM D6866 and optionally containing a 14C content up to 0.0000000001% (one part per trillion). Caprolactam so prepared can be used to make various polyamides.
Owner:AMYRIS INC
Catalyst for producing caprolactam as well as preparation method and application thereof
InactiveCN104307556AImprove hydrophobicityHigh reactivityLactams preparationMolecular sieve catalystsMolecular sieveNitrogen
The invention discloses a catalyst for producing caprolactam as well as a preparation method and application of the catalyst. The catalyst for producing the caprolactam is prepared by the following steps: synthesizing an MFI type zeolite molecular sieve; putting the MFI type zeolite molecular sieve into an nitrogen-containing organic alkali and organic silicon mixed water solution, and then carrying out a reaction at the temperature of 120-190 DEG C for 24-72 hours for modification treatment; mixing the zeolite molecular sieve treated by modification with a bonding agent, feeding a nitric acid solution into the mixture, kneading, molding, drying, and roasting to obtain the molecular sieve-based catalysts. After the zeolite molecular sieve is treated by the nitrogen-containing organic alkali combined with organosilane, the hydroxyl active center on the surface of the zeolite molecular sieve is effectively inhibited, so that the hydrophobicity of the catalyst is improved, and the reaction product caprolactam is rapidly diffused outside from pores of the catalyst; therefore, the side reactions of the caprolactam on the surface of the catalyst can be reduced, and the reaction activity, stability and product selectivity of the catalyst are improved.
Owner:江苏黄马化工有限公司
Popular searches
Catalyst activation/preparation Carboxylic preparation by ozone oxidation Chemical recycling Amino compound preparation Carboxylic preparation by oxidation Metal/metal-oxides/metal-hydroxide catalysts Nitro compound preparation Oxygen compounds preparation by reduction Hydroxy compound preparation Carboxylic acid amides preparation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com