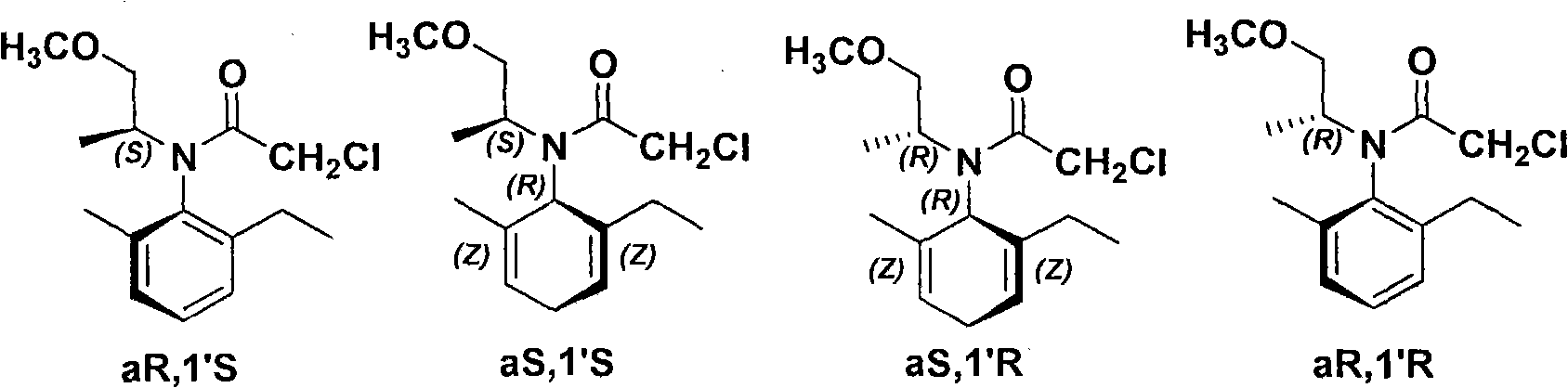
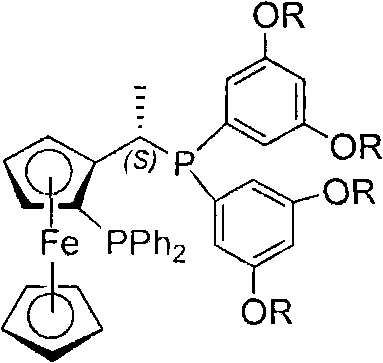
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
A technology of chiral bisphosphine ligands and composite catalysts, applied in asymmetric synthesis, organic compound/hydride/coordination complex catalysts, organic chemistry methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Example 1 Ligand Ia (General Structural Formula I, R=methyl)
[0033]Under the protection of nitrogen, add (R)-(S)-1-dimethylaminoethylferrocene (III, 0.1mol) and absolute tetrahydrofuran (100mL) to the reaction flask successively, and control the reaction flask with a dry ice-acetone bath. The temperature does not exceed -45~-35°C, add butyllithium n-hexane solution (0.11mol) dropwise, keep warm at -35°C for 30min, slowly rise to 40°C, keep warm for 1h, add diphenyl chloride dropwise Phosphine tetrahydrofuran solution, after the dropwise addition, keep warm for 6h. The reaction system was lowered to 0°C, and saturated aqueous sodium bicarbonate solution was added dropwise, the organic layer was separated with a separatory funnel, the aqueous layer was extracted with ether (3×30mL), the organic layers were combined, dried over anhydrous sodium sulfate, and filtered , precipitation, the obtained reddish-brown oily crude product is done column chromatograp...
Embodiment 2
[0035] Preparation of Example 2 Ligand Ib (such as general structural formula I, R=tert-butyl)
[0036] Use phosphonane IVb (IV, R = tert-butyl, 0.11mol) to replace phosphonane IVa, and (R)-N, N-dimethyl-1-[(S)-2-(diphenylphosphino) Ferrocenyl]ethylamine was prepared according to the same operation steps in Example 1 to obtain bisphosphine ligand Ib with a yield of 72% and a melting point of 50-52°C.
Embodiment 3
[0037] Preparation of Example Three Ligand Ic (such as general structural formula I, R=ethyl)
[0038] Use phosphonane IVc (IV, R = ethyl, 0.11mol) instead of phosphonane IVa, and (R)-N, N-dimethyl-1-[(S)-2-(diphenylphosphino) di Ferrocenyl]ethylamine was prepared according to the same operation steps in Example 1 to obtain bisphosphine ligand Ic with a yield of 72% and a melting point of 63-66°C.
[0039] The preparation of embodiment three composite catalysts Ia-Ir and the hydrogenation reaction of EMA-imine
[0040] Diphosphine ligand Ia (0.01mmol), [Ir(cod)Cl] 2 (0.005mmol), tetrabutylammonium iodide (0.005mmol), and glacial acetic acid (5mL) were mixed, and stirred at room temperature for 5min to obtain in-situ catalysts Ia-Ir.
[0041] Freshly distilled EMA-imine (1 mol), glacial acetic acid (80 mL) were added to a 500 mL autoclave.
[0042] Catalysts Ia-Ir were added into the reactor, and after gas exchange, the pressure was adjusted to 90 kg and the hydrogenation re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com