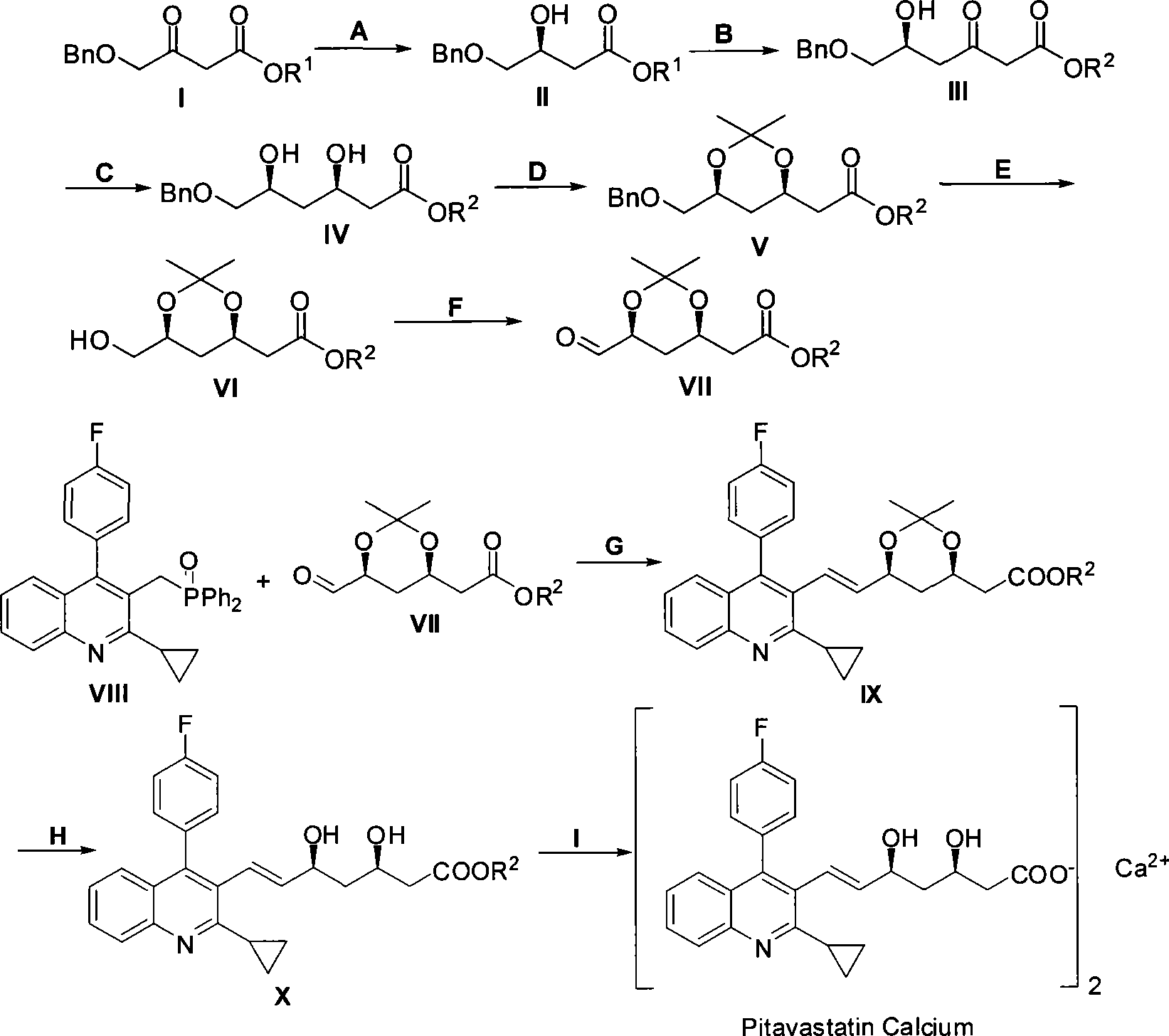
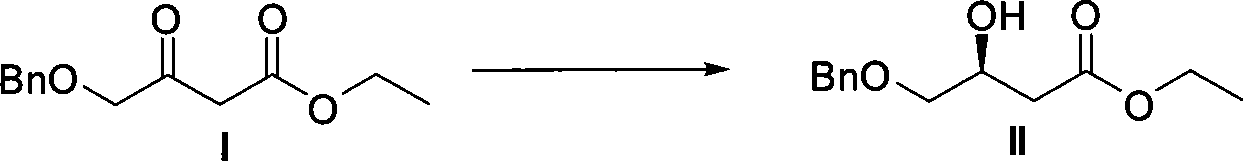Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
A pitavastatin calcium, asymmetric technology, applied in the field of cholesterol-lowering drugs in the field of medicinal chemistry technology, can solve problems such as the source of chiral side chains, and achieve the effect of solving expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The embodiments of the present invention are described in detail below: the present embodiment is implemented under the premise of the technical solution of the present invention, and detailed implementation and specific operation process are provided, but the protection scope of the present invention is not limited to the following implementation example.
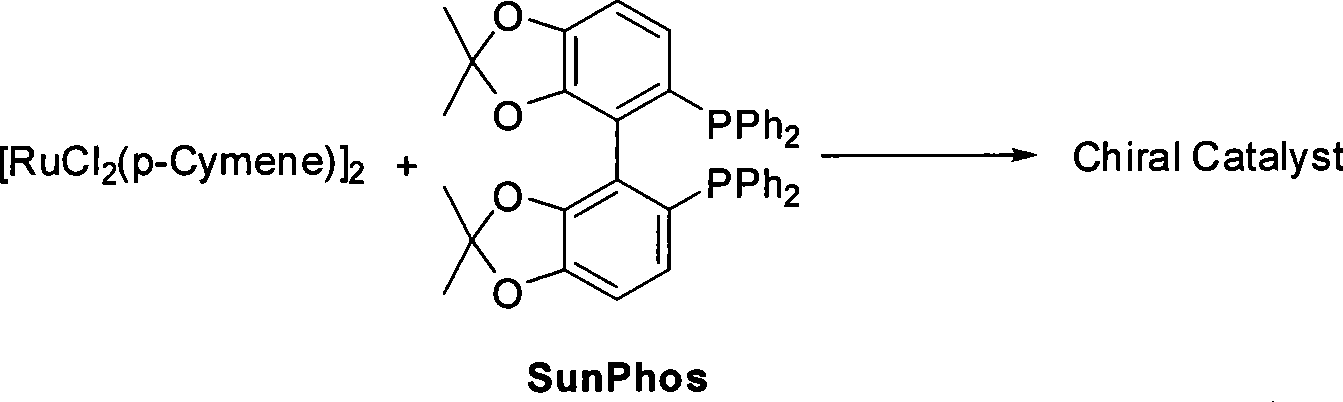
[0031] 1. Preparation of (S)-4-benzyloxy-3-hydroxybutyric acid ethyl ester and chiral catalyst (using Sunphos
[0032] For example, others can be prepared in a similar way). Reaction formula:
[0033]
[0034] Steps
[0035] Schlenk reaction tube, pumped and baked three times, added [RuCl 2 (p-Cymene)] 2 (21.6mg, 35.3μmol, 1eq), Sunphos (56.1mg, 77.0μmol, 2.2eq), replace nitrogen three times, add EtOH / CH 2 Cl 2 (9mL / 3mL), heated to 40°C for 3.5h, and dried to obtain an orange powdery solid catalyst.
[0036]
[0037] In a 150mL small beaker, add I (50.0g, 211.6mmol, 1eq), EtOH (80mL), chiral catalyst (S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com