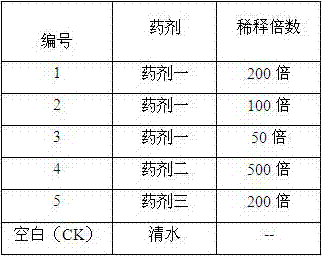
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
207 results about "Cyclopropanecarboxylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
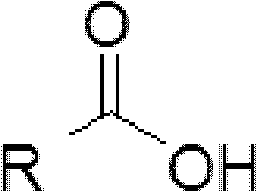
Cyclopropanecarboxylic acid is a monocarboxylic acid and a member of cyclopropanes. It is a conjugate acid of a cyclopropanecarboxylate . Ontology Summary from ChEBI
Preparation method of Ticagrelor intermediate
ActiveCN102796007AEasy to prepareEasy to operatePreparation by rearrangement reactionsChemical synthesisTicagrelor
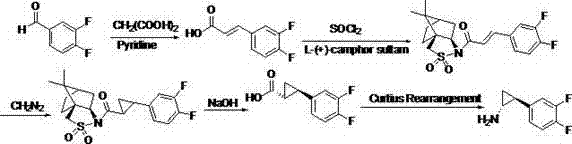
The invention relates to the medicine chemical synthesis field, and especially discloses a preparation method of a Ticagrelor intermediate. The preparation method comprises the following steps: 1) taking 3,4-difluorobenzaldehyde (I) as an initial raw material, reacting with a phosphorus ylide material liquid to obtain (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II); 2) performing a Simons-Smith asymmetric cyproteronethe reaction on the (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II) to obtain trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester (III); 3) performing aminolysis on the trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV); and 4) performing a Huffman rearrangement reaction on the trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV) to obatain the Ticagrelor intermediate (V). The method of the invention has the advantages of simple process, convenient operation, mild reaction condition and easy control, low cost and easy acquisition of raw material, high product yield and product purity, and is adapted to large scale industrial production.
Owner:JINAN RUIFENG PHARMA +2
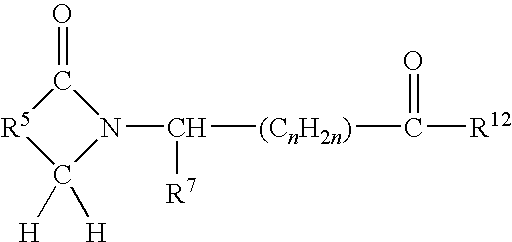
Preparation and activity of aryl formyl amino cyclopropanecarboxylic acid
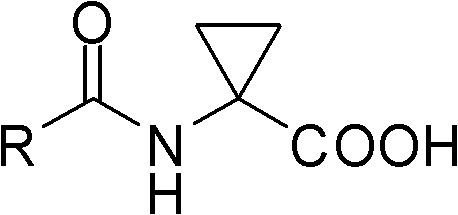
The invention discloses aryl formyl amino cyclopropanecarboxylic acid serving as a novel plant growth regulator. The compound provided by the invention is shown as a general formula I, wherein in the formula I, R may be R1 or R2; R1 may be phenyl or a benzene ring containing a substituent group; R2 may be pyridyl or a pyridine ring containing a substituent group; and the substituent groups in the benzene ring containing the substituent group or the pyridine ring containing the substituent group may be one or any two of halogen, NO2, alkyl and alkoxy. Experiments prove that the aryl formyl amino cyclopropanecarboxylic acid compound synthesized by the invention has the plant growth regulation activity of inhibiting seed germination by the measurement on the germination activity of wheat seeds.
Owner:CHINA AGRI UNIV
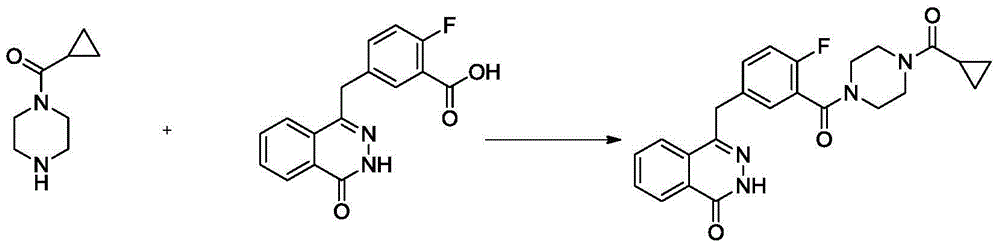
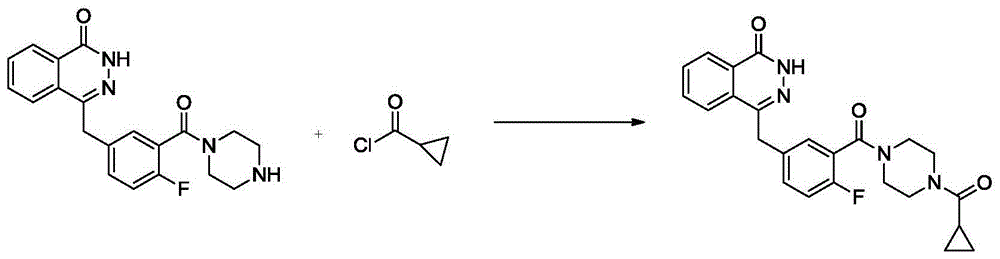
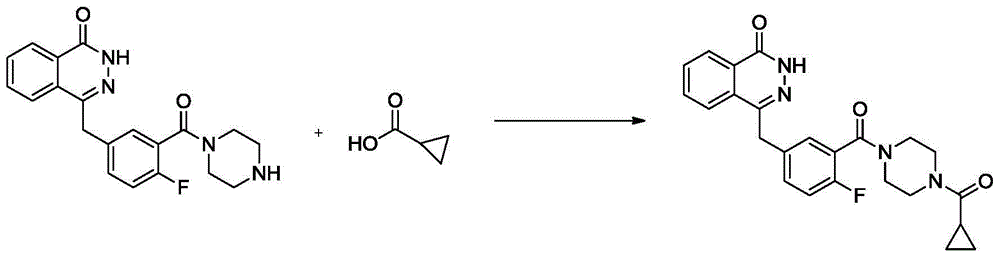
Preparation method for olaparib
The invention discloses a preparation method for olaparib. The preparation method comprises the following steps: subjecting 4-(4-fluoro-3-(piperazine-1-carbonyl)benzyl)phthalazin-1(2H)-one, a condensing agent, cyclopropanecarboxylic acid, alkali and a polar organic solvent to a reaction at 0 to 120 DEG C for 2 to 8 h; adding water; allowing a solid to be precipitated; and carrying out pumping filtration, washing and drying so as to obtain olaparib. According to the invention, cyclopropanecarboxylic acid is used as a raw material; reaction conditions are mild; operational safety is good; organic solvents like dichloromethane is not needed for aftertreatment; separation and purification steps are simple; the yield of olaparib is high, as high as 92% or above; raw materials are easily available; the method is simple; side reactions are few; the chromatographic purity of olaparib is high; industrial scale-up production can be easily implemented; and the method has good industrial application prospects.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
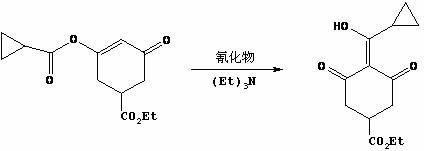
Synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate
ActiveCN102911058ANo purification requiredShort reaction timeOrganic compound preparationCarboxylic acid esters preparationKetoneSodium salt
The invention provides a synthetic method of plant growth regulator trinexapac-ethyl intermediate 3-carbethoxy-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate. The synthetic method comprises the following steps: (1) carrying out annulation reaction on 2-acetonyl-1,4-diethyl succinate and organic alkaline at the temperature of 20-120 DEG C for 0.5-5 hours in a non-polarity organic solvent to obtain 3,5-cyclohexanedione-1-ethyl formate; and (2) adding organic amine and cyclopropanecarboxylic acid chloride, and carrying out acylation reaction at the temperature of minus 5-50 DEG C, so as to obtain 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate in the presence of micromolecular alcohol, ether, ketone and nitrile the carbon atoms of which are below C8 and are used as additives. According to the method, special additives are added before acylation so that cyclopropanecarboxylic acid chloride can directly react with intermediate-state 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol sodium salt to obtain the trinexapac-ethyl precursor 3-ethoxycarbonyl-5-oxo-cyclohexane-1-enol cyclopropanecarboxylate, thereby shortening reaction time, simplifying synthesis process and improving yield; and the product is directly rearranged without purification so as to obtain the final product trinexapac-ethyl.
Owner:JIANGSU YOUJIA CHEM +1
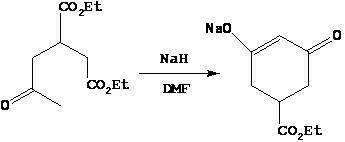
Method for preparing trinexapac-ethyl
InactiveCN102101830AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationSuccinic acidHigh pressure
The invention discloses a method for preparing trinexapac-ethyl, which comprises the following steps of: performing high pressure condensation on diethyl maleate and acetone serving as raw materials in the presence of diethylamine to obtain 2-acetone-1,4-succinic acid diethyl ester; forming a ring in the presence of sodium hydride to obtain 3-ethoxycarbonyl-5-oxocyclohexyl-1-ene-1-sodium alkoxide; reacting with cyclopropanecarboxylic acid chloride to obtain 3-ethoxycarbonyl-5-oxocyclohexyl-1-ene-1-cyclopropanecarboxylic acid ester alkoxide; and rearranging in the presence of cyanide and triethylamine to obtain the trinexapac-ethyl. The method has the advantages of high yield, mild reaction and high product purity, and is suitable for small-scale laboratorial preparation and industrial production.
Owner:张家港田由新材料科技有限公司
Tubular continuous method for preparing cyclopropanecarboxylic acid
InactiveCN101693660AIncrease production capacityHigh yieldOxygen-containing compound preparationOrganic compound preparationContinuous reactorAutomatic control
The invention discloses a tubular continuous method for preparing cyclopropanecarboxylic acid. In the method, gamma-butyrolactone used as a raw material reacts with thionyl chloride and alcohol to produce 4-chlorobutyrate; after the reaction liquid is processed in an acid gas removing way and an alcohol recycling way, the reaction liquid and liquid sodium alkoxide are synthesized to obtain cyclopropanecarboxylate in a tubular reactor; the rectified cyclopropanecarboxylate is firstly hydrolyzed in alkali liquor and then neutralized with acid to obtain crude cyclopropanecarboxylic acid; and the crude cyclopropanecarboxylic acid is rectified to obtain the pure cyclopropanecarboxylic acid. The synthetic technique of the cyclopropanecarboxylic acid adopts the tubular continuous reactor, thereby effectively reducing the equipment investment and enhancing the investment efficiency; the continuous reaction can conveniently realize automatic control, thereby ensuring the continuity and the stability of the production, saving the manual cost, realizing the safe production operation and enhancing the purity and the yield of the product; and the invention adopts a technique of adding solid caustic soda again in the second aqueous phase for recycling, thereby fully and comprehensively utilizing the resources, enhancing the yield for hydrolysis and greatly reducing the cost.
Owner:ZHEJIANG UNIV +1
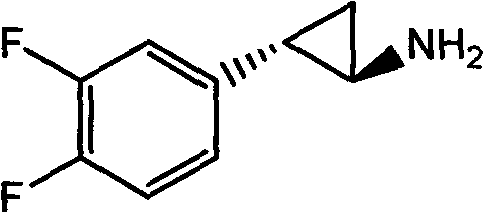
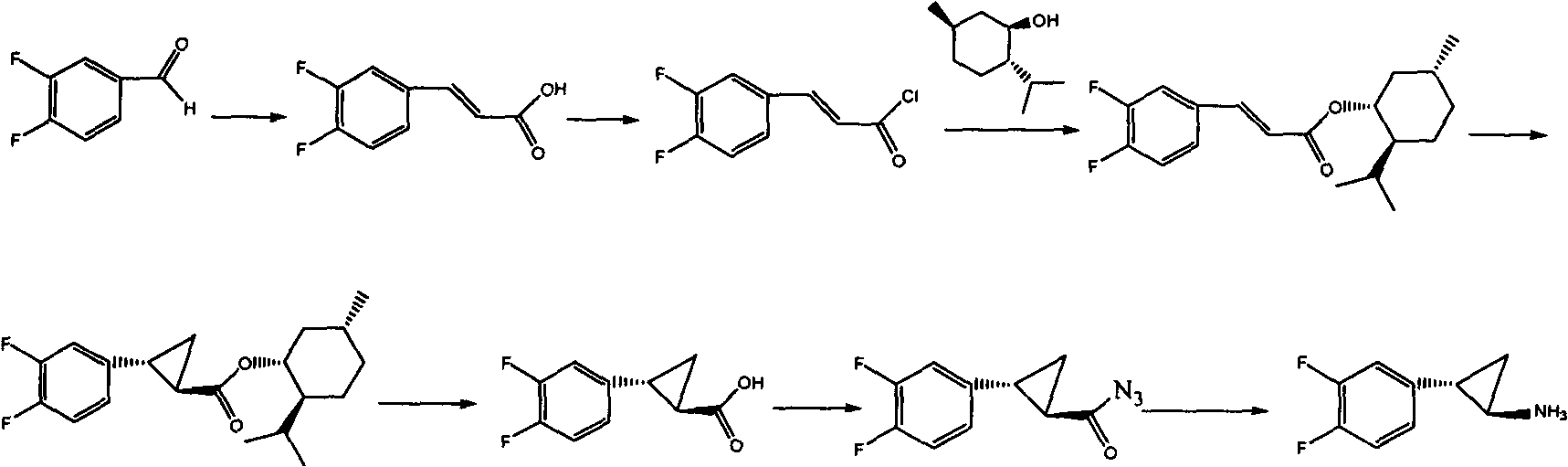
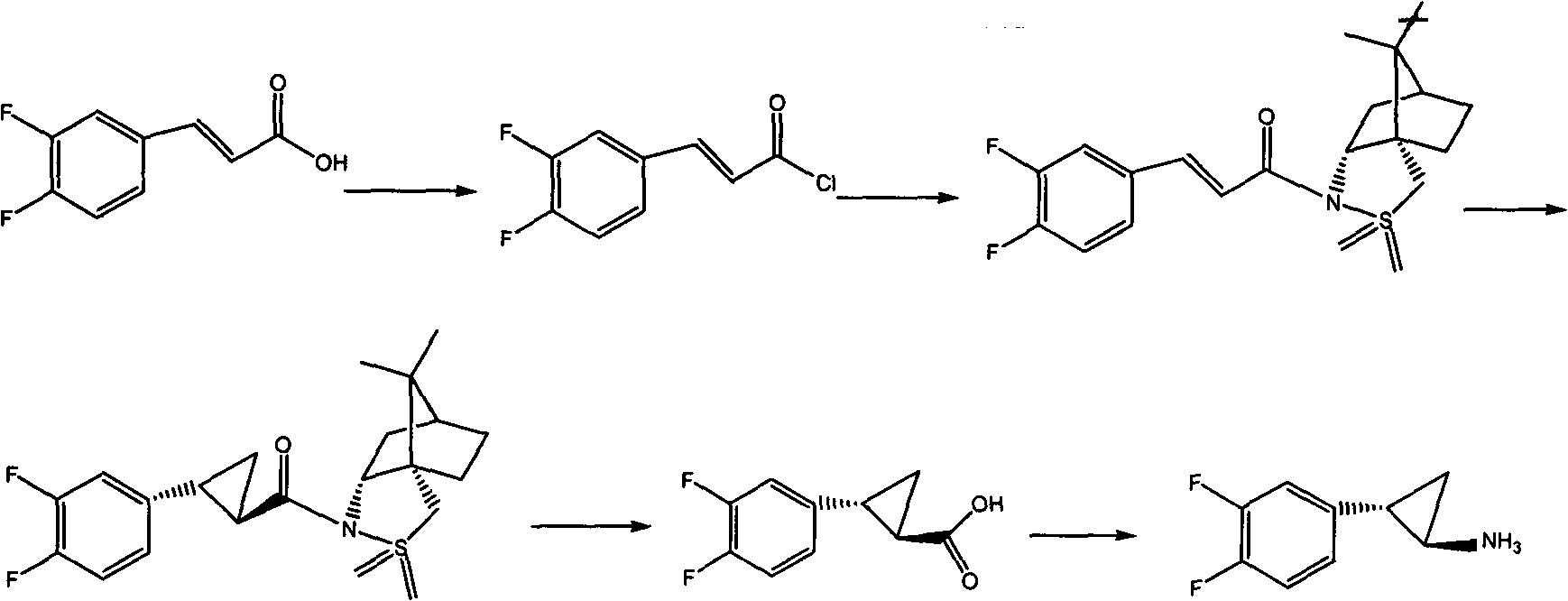
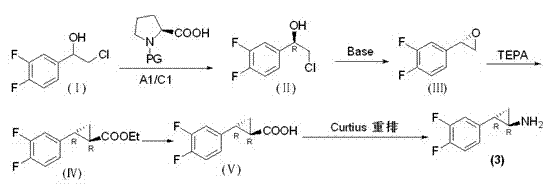
Preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine
InactiveCN102775314AFew stepsEasy to operateHydroxy compound separation/purificationPreparation by rearrangement reactionsEpoxyPtru catalyst
The invention provides a preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine. The preparation method comprises the following steps: enabling racemic chloro phenethyl alcohol (I) and N-protection proline to undergo a reaction under the effects of a condensing agent A1 and a catalyst C1, and obtaining chiral chlorohydrin (II); enabling the chiral chlorohydrin (II) to generate an epoxy compound (III) under the conditions of alkalinity; enabling the epoxy compound (III) and TEPA to react and generate cyclopropyl ethyl formate (IV) under the conditions of alkalinity; removing ester from cyclopropyl ethyl formate (IV) under the conditions of alkalinity, and generating cyclopropanecarboxylic acid (V); and enabling cyclopropanecarboxylic acid (V) and azide to generate a target compound (3) by Curtius rearrangement. The preparation method has the advantages that the steps of a used synthetic process route are few, the operation is simple, and industrial production is achieved easily; a kinetic resolution method is utilized to synthesize chiral chlorohydrin (II) and is simple in reaction conditions and easy to operate; and a TEPA method is utilized synthesize the cyclopropyl ethyl formate, the product yield is high, cis-trans selectivity is good, and the purity is over 99%.
Owner:江苏富泽药业有限公司
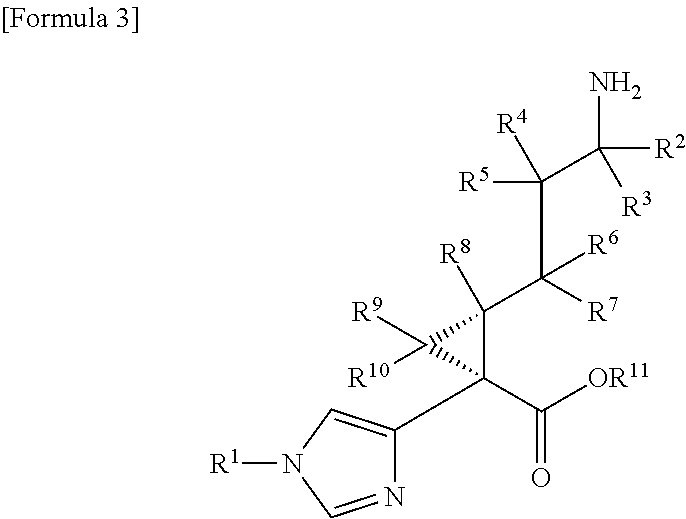
Cyclopropanecarboxylic acid derivative
InactiveUS20130012532A1High activityGood characterAntibacterial agentsBiocidePulmonary artery embolismHydrogen atom
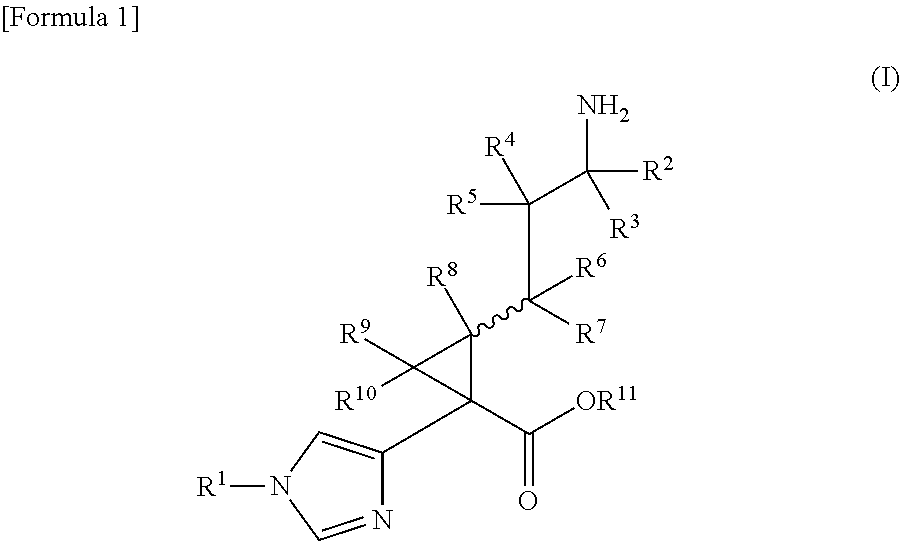
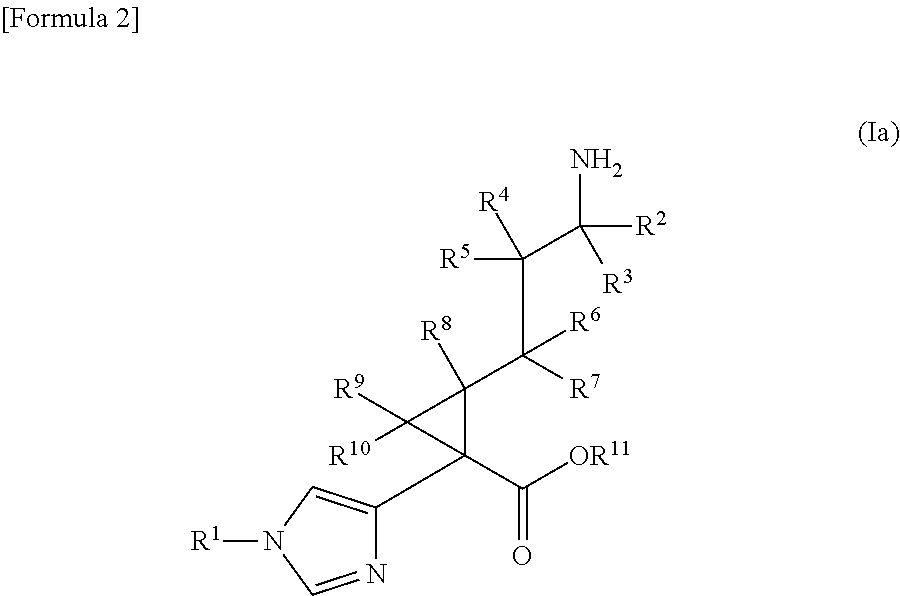
A compound represented by the following general formula (I) or a pharmacologically acceptable salt thereof, wherein R1 represents a C1 to C6 alkyl group which may be substituted by one to three groups selected from substituent group A, or the like (substituent group A: a hydroxy group, a halogeno group, a cyano group, a nitro group, an amino group, a carboxy group, a C1 to C3 alkyl group, etc.); R2, R3, and R8 each independently represent a hydrogen atom or a C1 to C3 alkyl group; R4, R5, R6, R7, R9, and R10 each independently represent a hydrogen atom or the like; and R11 represents a hydrogen atom or the like, has TAFIa enzyme inhibitory activity and is useful as a therapeutic drug for myocardial infarction, angina pectoris, acute coronary syndrome, cerebral infarction, deep vein thrombosis, pulmonary embolism, or the like.
Owner:DAIICHI SANKYO CO LTD
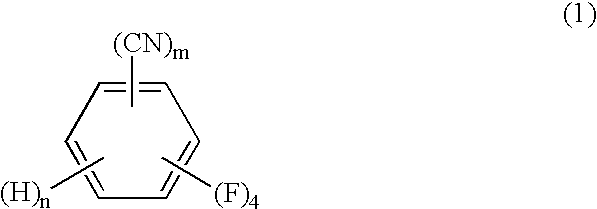
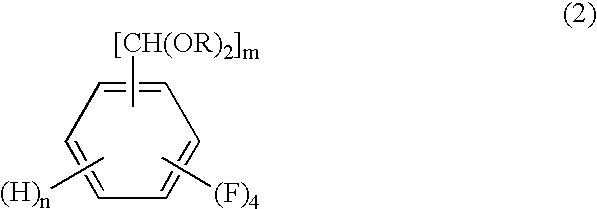
Process for producing tetrafluorobenzenemethanols
InactiveUS6624336B1High purityHigh yieldOrganic compound preparationHydroxy compound preparationOrganic chemistryCyclopropanecarboxylic acid
The present invention relates to a process by a series of reactions using tetrafluorocyanobenzens as material for producing tetrafluorobenzenemethanols, tetrafluorobenzenecarbaldehyde dialkylacetals and tetrafluorobenzenecarbaldehydes in a high purity and a high yield which are useful as intermediates in the production of cyclopropanecarboxylic acid esters having insecticidal action, and also relates to a novel tetrafluorobenzenecarbaldehyde dimethylacetal.
Owner:SHOWA DENKO KK
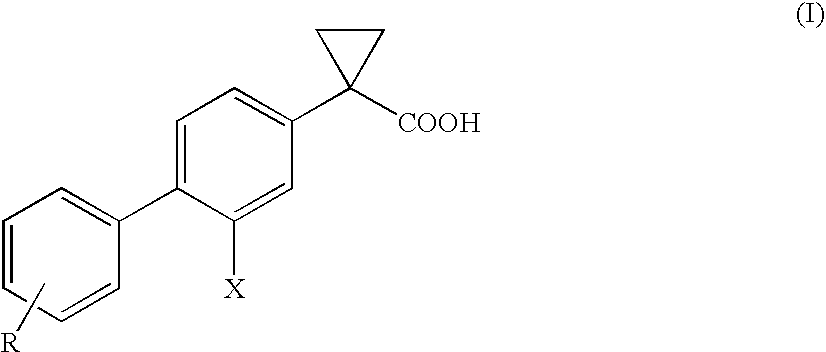
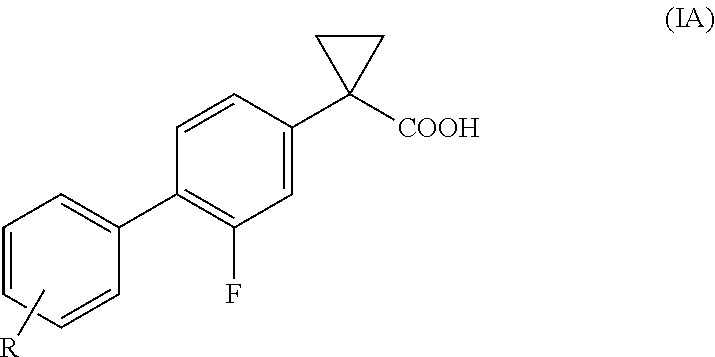
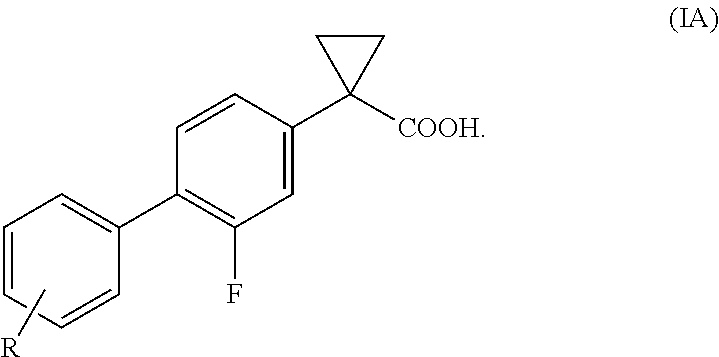
Process of preparing derivatives of 1-(2-halobiphenyl-4-yl)-cyclopropanecarboxylic acid
ActiveUS20090312426A1Efficient preparationHigh yieldBiocideNervous disorderSuzuki reactionMedicinal chemistry
Compounds represented by formula (I):may be conveniently prepared by a process in which a Suzuki reaction is performed as an early step.
Owner:CHIESI FARM SPA
Preparing method of cis, trans-ethyl 2, 2-dimethyl-3-(1-isobutenyl)cyclopropane-1-carboxylate
PendingCN106316845AEasy to operateHigh yieldOrganic compound preparationCarboxylic acid esters separation/purificationSolventSodium nitrite
The invention discloses a method of preparing cis, trans-ethyl 2,2-dimethyl-3-(1-isobutenyl)cyclopropane-1-carboxylate, comprising of making the ethyl glycinate hydro and sodium nitrite in the solvent diazo-react without additional addition of organic acid or mineral acid to get ethyl diazoacetate which is to react with the 2,5-Dimethyl-2,4-hexadiene under the catalyst effect and then be desolvated and rectificated to get ethyl chrysanthemumate.The invention is easy to use with high production yield and high contents.
Owner:江苏优普生物化学科技股份有限公司
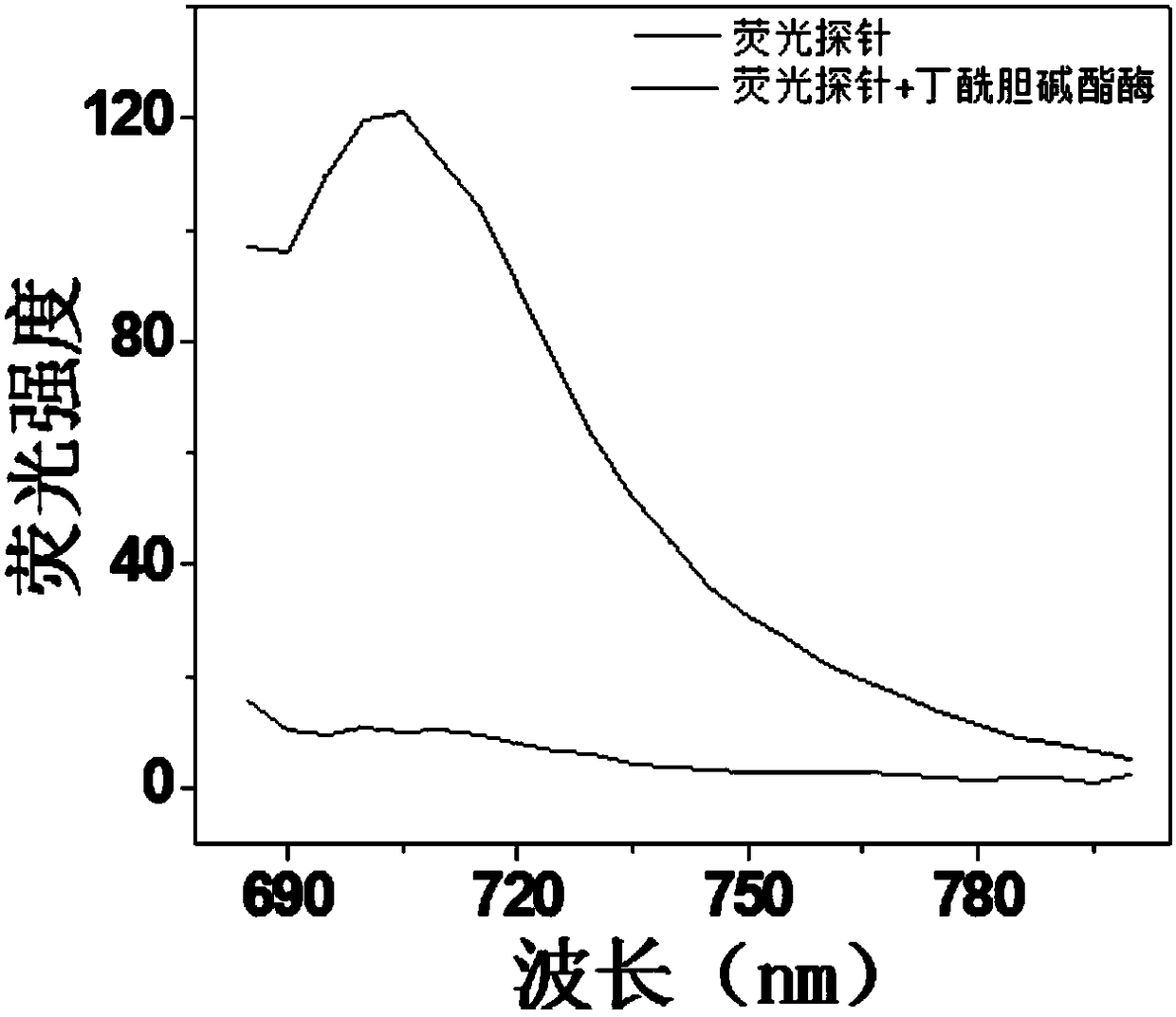
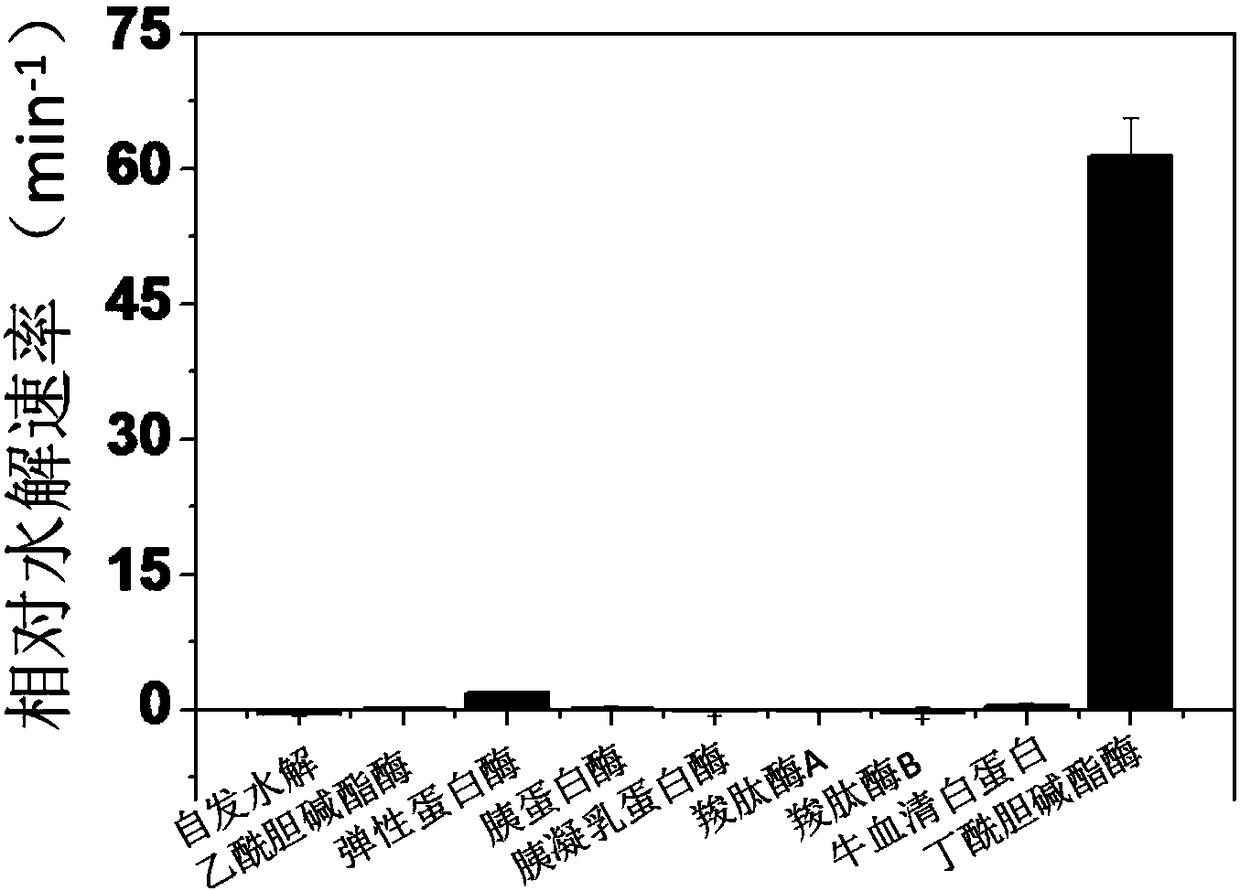
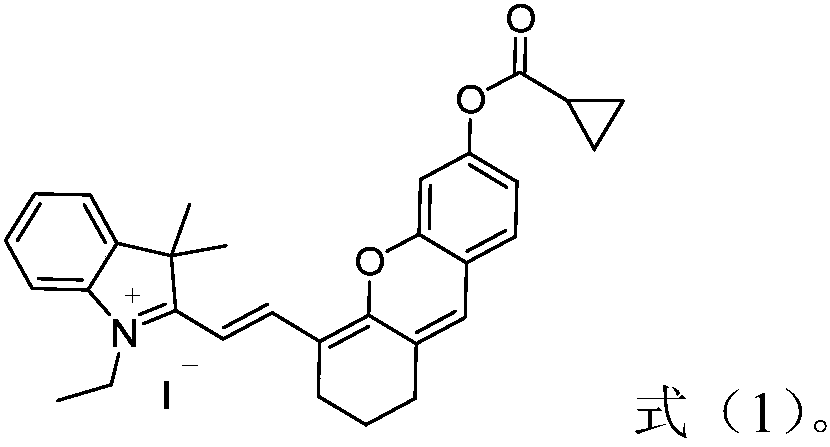
Near-infrared hemicyanine-based fluorescent probe for detecting butyrylcholinesterase, preparation method and applications thereof
ActiveCN108192597AHigh sensitivityHigh selectivityOrganic chemistryFluorescence/phosphorescenceOrganic solventCholinesterase
The invention relates to the field of detection of butyrylcholinesterase, and discloses a near-infrared hemicyanine-based fluorescent probe for detecting butyrylcholinesterase, a preparation method and applications thereof, wherein specifically the near-infrared hemicyanine-based fluorescent probe has a structure represented by a formula (1). The method comprises that a compound represented by a formula (2) contacts cyclopropanecarboxylic acid chloride in an organic solvent under a nucleophilic reaction condition in the presence of an acid binding agent. The applications comprise: applicationsin the detection of the activity of butyrylcholinesterase, and a detection method and a detection kit for the activity of butyrylcholinesterase. The near-infrared hemicyanine-based fluorescent probeof the present invention has advantages of high sensitivity, good selectivity, high fluorescence intensity change, easy detection, high stability, convenient use and low cost in the application related to the detection of the activity of carboxypeptidase Y. The formulas (1) and (2) are defined in the specification.
Owner:HUAZHONG NORMAL UNIV
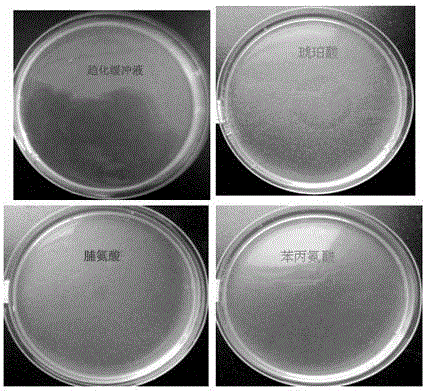
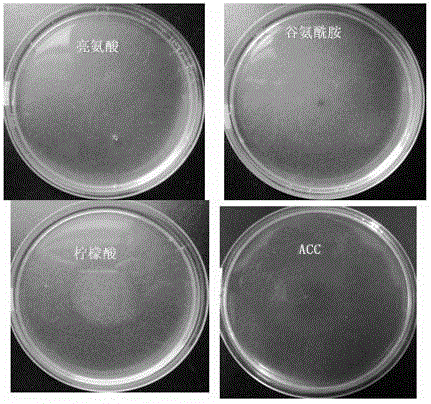
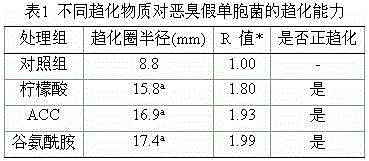
Application of 1-aminocyclopropane-1-carboxylic acid as bacterial chemotactic substance
The invention provides an application of ACC (1-aminocyclopropane-1-carboxylic acid) as a bacterial chemotactic substance. Through the plate chemotaxis assay, swam plate assay and capillary chemotaxis assay, a new use of ACC is found, namely, ACC has positive chemotaxis effect on bacteria, in particular, Pseudomonas putida, so that ACC can be used as the bacterial chemotactic substance.
Owner:HENAN AGRICULTURAL UNIVERSITY
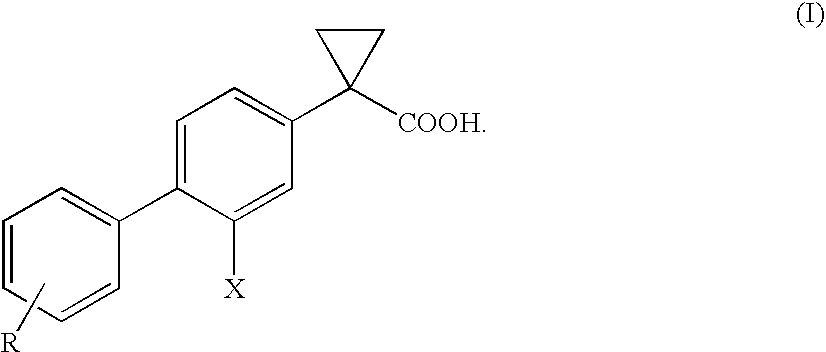
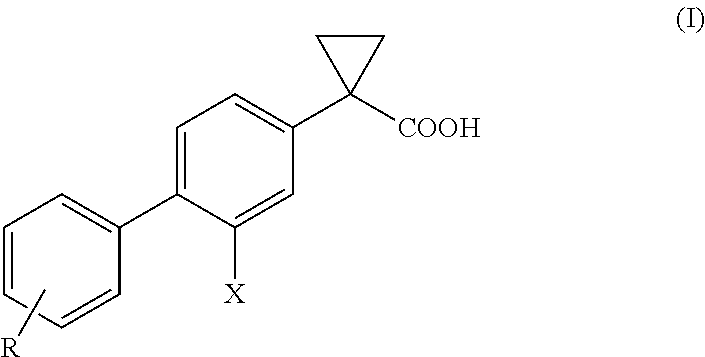
Process for the preparation of derivatives of 1-(2- halobiphenyl-4-yl)-cyclopropanecarboxylic acid
ActiveUS20110039934A1Improve processing efficiencyIncrease productionBiocideNervous disorderMedicinal chemistryCyclopropanecarboxylic acid
Owner:CHIESI FARM SPA
Process for production of formylcyclopropanecarboxylic ester
InactiveUS20050240050A1Organic compound preparationCarboxylic acid esters preparationArylHydrogen atom
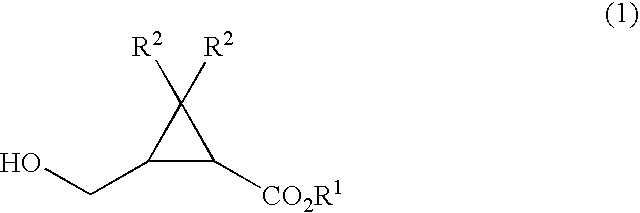
There is provided a production method of formylcyclopropanecarboxylate compound of formula (2): wherein R1 and R2 are as defined below, which comprises reacting a cyclopropanecarboxylate compound of formula (1): wherein and R1 represent a linear, branched or cyclic alkyl group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aralkyl group, R2 represents a hydrogen atom or a methyl group, with at least one oxidizer selected from the group consisting of hypohalite, N-halosuccinimide, a trichloroisocyanuric acid, and iodine, in the presence of a nitroxy radical compound.
Owner:SUMITOMO CHEM CO LTD
Bacillus cercus and chiral 2,2-dimethyl cyclopropanecarboxylic acid/cyclopropancarboxamid prepared from the same
The invention provides a new strain which is bacillus cereus ZJB-07112 and the application of the bacillus cereus in chiral catalysts preparation of (S)-(+)-2, 2- dimethyl cyclopropyl carboxylic acid and R-(-)-2, 2-dimethyl formamide. The invention has the advantages that a microbiological bacterial strain which contains amidase and the process for the chiral catalysts preparation of (S)-(+)-2, 2-dimethyl cyclopropyl carboxylic acid and R-(-)-2, 2-dimethyl formamide by using the microbiological bacterial strain, which has a perfect application prospect.
Owner:ZHEJIANG UNIV OF TECH +1
1-amino-1-cyclopropanecarboxylic acid hydrochloride formulations
The present invention relates to 1-amino-1-cyclopropanecarboxylic acid hydrochloride salt formulations and methods of their use.
Owner:VALENT BIOSCIENCES CORP
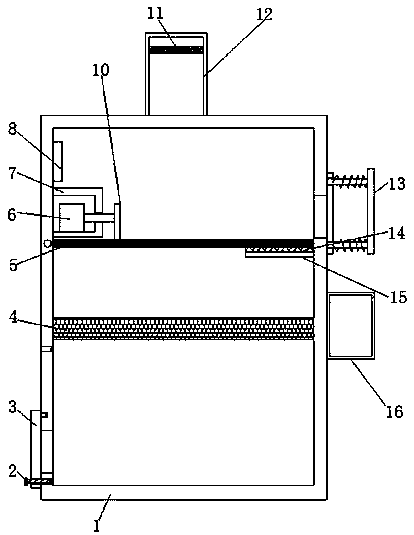
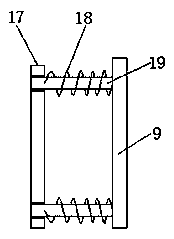
Raw material stirring device for cyclopropanecarboxylic acid synthesis
PendingCN108855380ASimple structureEasy to operateRotary stirring mixersTransportation and packagingCouplingEngineering
The invention discloses a raw material stirring device for cyclopropanecarboxylic acid synthesis. The raw material stirring device for cyclopropanecarboxylic acid synthesis comprises a stirring box. The upper part of the stirring box is connected with a screening pipe. The upper part of the screening pipe is connected with a feed hopper. The screening pipe is internally provided with a screening plate. One side of the screening pipe is connected with a material guide pipe. The stirring box is internally connected with a fixing plate. The fixing plate is provided with a grinding device. The bottom of the screening plate is vertically connected with a connection rod. One end of the connection rod is connected with a push block. The stirring box is internally and vertically provided with a guide rod. The guide rod is sleeved with the push block. The exterior of one side wall of the stirring box is fixedly provided with a stirring motor through screws. An output shaft of the stirring motoris connected with a rotating rod through a coupling. The raw material stirring device for cyclopropanecarboxylic acid synthesis is simple in structure and reasonable in design, raw materials can be screened before the cyclopropanecarboxylic acid raw materials are mixed, relatively large screened raw material particles can be automatically processed, it does not need to additionally exert power inthe process, resources are saved, and operation is easy.
Owner:兰州鸿瑄科技有限公司
1-amino-1-cyclopropanecarboxylic acid formulations
ActiveUS20180279622A1Enhancing grape colorationBiocideAnimal repellantsCarboxylic acidMedicinal chemistry
The present invention relates to stable 1-amino-1-cyclopropanecarboxylic acid formulations and methods of their use.
Owner:VALENT BIOSCIENCES CORP
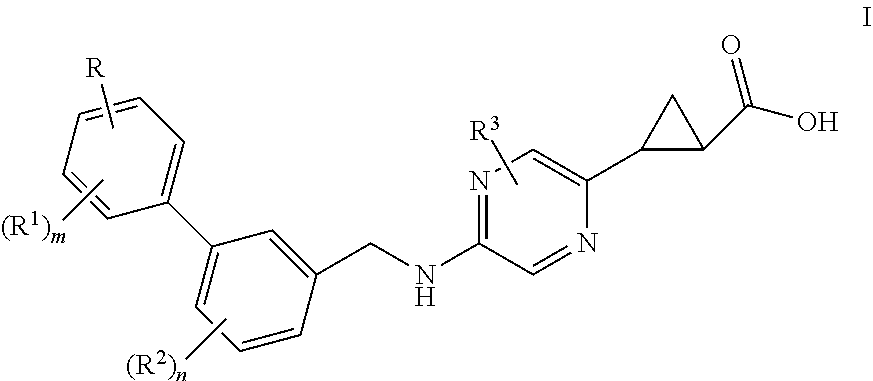
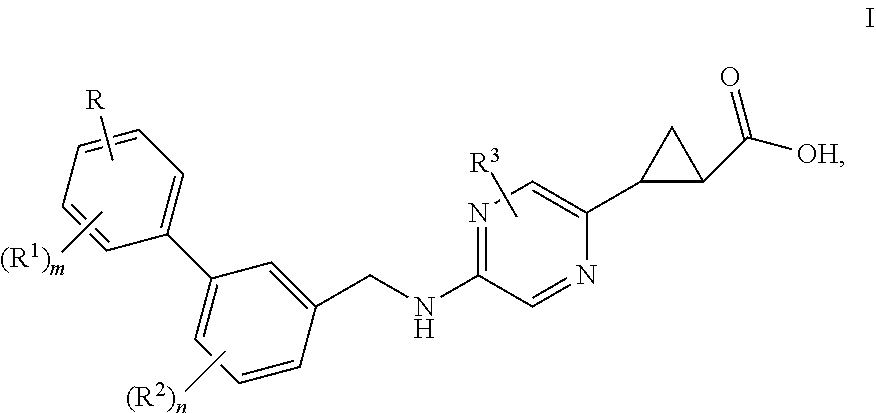
Benzylaminopyridylcyclopropanecarboxylic acids, pharmaceutical compositions and uses thereof
ActiveUS10538490B2Improve effectivenessImprove stabilityOrganic chemistryMetabolism disorderDiseaseDiabetes mellitus
Owner:BOEHRINGER INGELHEIM INT GMBH
Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate
The invention belongs to the field of chemical synthesis of drugs, particularly relates to a synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and an intermediate and aims to provide a synthesis method of a compound (1). The method comprises steps as follows: a, a compound (4) and boron tribromide react, and a compound (3) is synthesized; b, the compound (3) and difluorodibromomethane have a ring closing reaction in the presence of a phase-transfer catalyst, and a compound (2) is synthesized; c, the compound (2) is hydrolyzed, and the compound (1) is synthesized. According to the synthetic method and the intermediate, one novel compound (4) is provided, one novel preparation method of the compound (1) is obtained, with the adoption of the method, not only can the overall yield of the reaction be increased, but also expensive reaction raw materials can be avoided, and the reaction cost is reduced to the great extent; besides, according to the method, utilization of a palladium catalyst and a dangerous sodium cyanide reagent can further be avoided, the safety of pharmaceutical production is guaranteed, and the method is applicable to industrial production.
Owner:ZHEJIANG YONGNING PHARMA
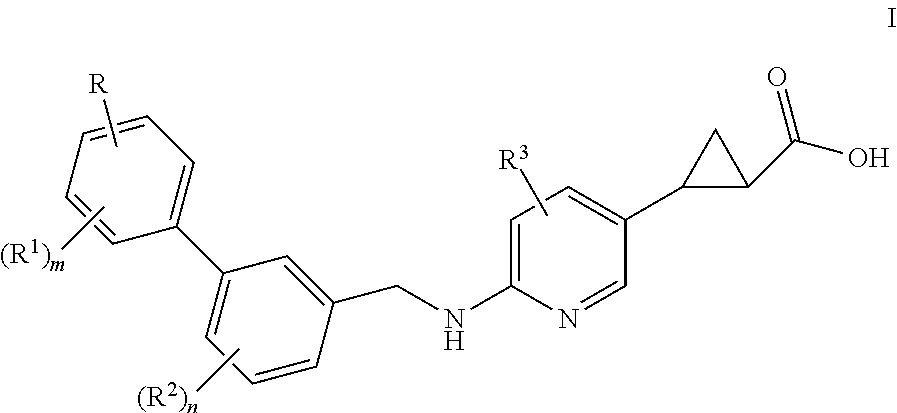
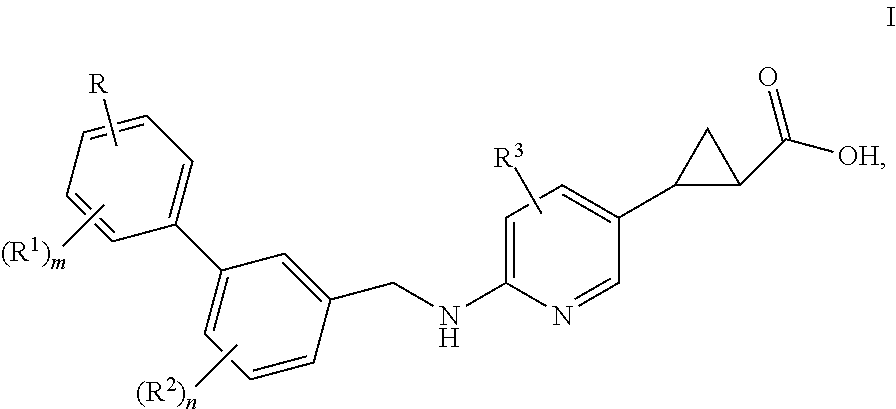
Benzylaminopyrazinylcyclopropanecarboxylic acids, pharmaceutical compositions and uses thereof
ActiveUS20190389815A1Improve effectivenessImprove stabilityOrganic active ingredientsOrganic chemistryDiseaseDiabetes types
The present invention relates to compounds of general formula I,wherein the groups R, R1, R2, R3, m and n are defined herein, which have valuable pharmacological properties, in particular bind to the GPR40 receptor and modulate its activity. The compounds are suitable for treatment and prevention of diseases which can be influenced by this receptor, such as metabolic diseases, in particular diabetes type 2.
Owner:BOEHRINGER INGELHEIM INT GMBH
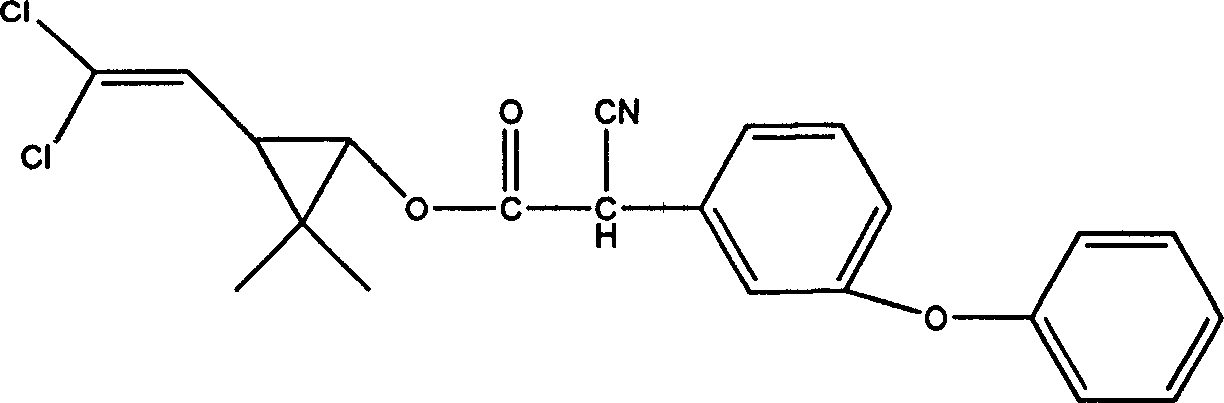
Alpha-cypermethrin anti-fouling composition
InactiveCN1820603AInhibition of attachmentNo toxicityBiocideAntifouling/underwater paintsCypermethrinPyrethrum extract
The alpha-cypermethrin anti-fouling preparation is one kind of efficient environment friendly marine use anti-fouling preparation prepared with artificially synthesized derivative of pyrethrin as the pyrethrum extract and has low cost, high efficiency and environment friendship. Alpha-cypermethrin has the molecular expression of C22H19Cl2NO3, molecular weight of 416.35 and molecular structure as shown.
Owner:XIAMEN UNIV
Process for prepn. of cyclopropane formic ether
The invention discloses a method for preparing cyclopropane carboxylate of formula (1): the method comprises making cyclopropane carboxylate of formula (2) and formula ( 3) The monohydric compound reaction.
Owner:SUMITOMO CHEM CO LTD
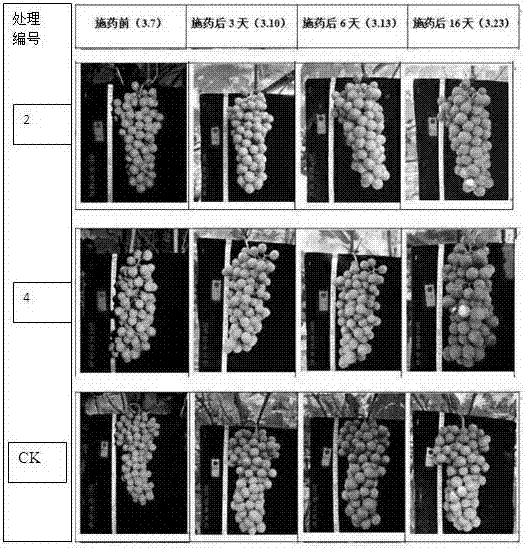
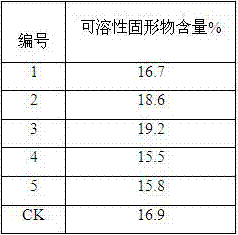
Grape regulating chemical
ActiveCN107410351AGood coloring effectGood colorPlant growth regulatorsBiocideSide effectWater dispersible
The invention discloses a grape regulating chemical which is a solution, a suspension, wettable powder or water dispersible granules prepared from a compounded coloring agent and auxiliary materials, wherein the compounded coloring agent is prepared from 2,4-dichlorobenzoyl amino cyclopropanecarboxylic acid and an active aid through uniform mixing; and the active aid is brassinolide, diethylaminoethyl hexanoate or guayule. The grape regulating chemical disclosed by the invention is safe and efficient, is good in grape coloring effect, high in coloring speed, small in side effect and capable of improving the quality of grapes, improving the commodity of grapes and increasing the economic benefits.
Owner:李怡舒 +1
Sewage treatment device for cyclopropanecarboxylic acid synthesis
PendingCN108675475ARemove in timeGuaranteed processing efficiencyTreatment involving filtrationMultistage water/sewage treatmentMicrocontrollerMicrocomputer
The invention discloses a sewage treatment device for cyclopropanecarboxylic acid synthesis. The sewage treatment device comprises a case, wherein the top end of the case is provided with a water inlet; the top end of the case is fixedly provided with a feeding pipe in the water inlet position; a first filtering screen is fixedly arranged inside the feeding pipe; a single chip microcomputer and afixing box are respectively and fixedly arranged on the top end of the inner wall of one side of the case; a through hole is formed in one side of the fixing box; an air cylinder is arranged inside the fixing box; a piston rod of the air cylinder is glidingly connected with the fixing box in a through hole position; the piston rod of the air cylinder is fixedly provided with a push plate; a secondfiltering screen is arranged under the fixing box; one end of the second filtering screen is rotationally connected with the inner wall of the case; one side, far away from the fixing box, of the case is provided with an opening; a plugging device is arranged in the opening position at the outer side of the case. Compared with the existing device, the sewage treatment device has the advantages that the sewage treatment efficiency can be well ensured; in addition, contaminants accumulated onto the surface of the filtering screen can be timely discharged; the sewage treatment work proceeding can be further ensured.
Owner:兰州鸿瑄科技有限公司
METHODS FOR TREATMENT OF MULTIPLE MYELOMA USING CYCLOPROPANE CARBOXYLIC ACID {2-(Is)-(3-eTHOXY-4METHOXY-PHENYL)-2-METHANESULFONYL-ETHYL}-3-OXO-2.3-DIHYDRO-1H-ISOINDOL-4-YL}-AMIDE
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of a selective cytokine inhibitory drug alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of a selective cytokine inhibitory drug. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Method for manufacturing 2-(hydroxymethyl) cyclopropane carboxylic acid compound
InactiveCN1681767AOrganic compound preparationCarboxylic acid esters preparationPalladium catalystCarboxylic acid
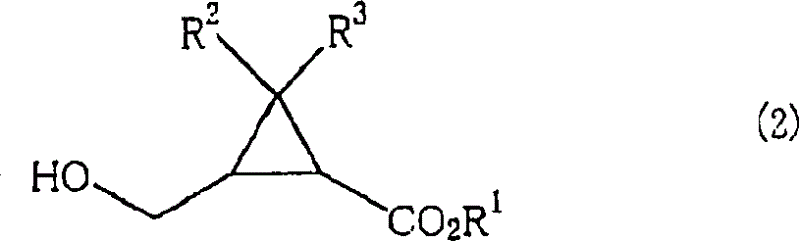
A process for production of 2-(hydroxymethyl)cyclo- propanecarboxylic acids represented by the general formula (2): (2) [wherein R<1> is hydrogen, linear, branched, or cyclic alkyl, or substituted or unsubstituted aryl; and R<2> and R<3> are each independently hydrogen or methyl], characterized by reacting a compound represented by the general formula (1): (1) [wherein R<1>, R<2>, and R<3> are each as defined above; and R<4> is C1-2 alkyl substituted with at least one member selected from among substituted aryl groups and unsubstituted aryl groups] with a hydrogen donor in the presence of a catalyst selected from the group consisting of ruthenium catalysts, cobalt catalysts, rhodium catalysts, nickel catalysts, palladium catalysts, and platinum catalysts.
Owner:SUMITOMO CHEM CO LTD
Process for producing cyclopropanecarboxylates
InactiveUS6909013B2Conveniently producedEasy to makeOrganic compound preparationCarboxylic acid esters preparationOrganic chemistryCyclopropanecarboxylic acid
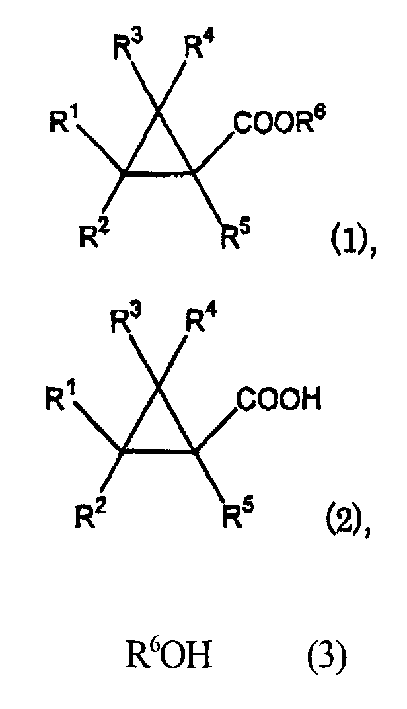
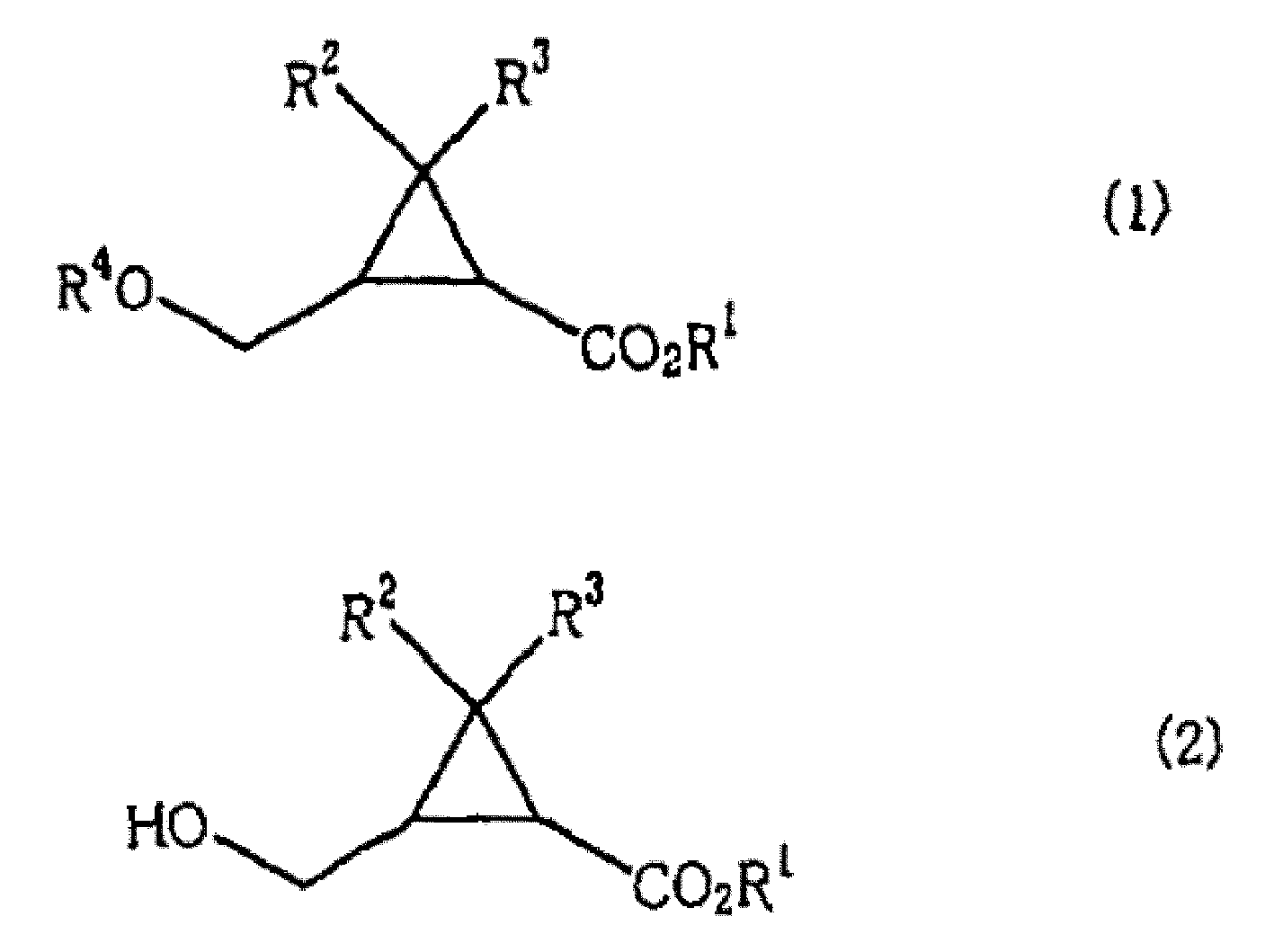
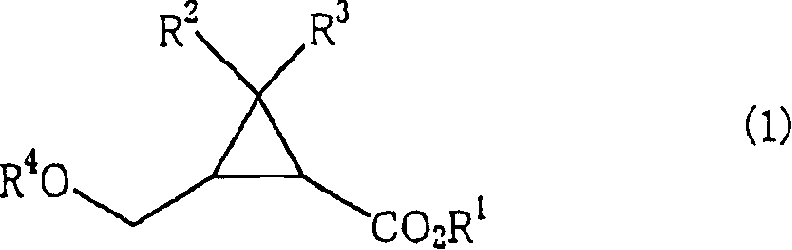
There is disclosed a process process for producing a cyclopropanecarboxylate of formula (1): which process comprises reacting cyclopropanecarboxylic acid of formula (2): with a monohydroxy compound of formula (3):R6OH (3),in the presence of a catalyst compound comprising an element of to Group 4 of the Periodic Table of Elements.
Owner:SUMITOMO CHEM CO LTD
Process for reductive dehaloganation
A method of producing 2-fluorocyclopropane-1-carboxylic acid ester, which comprise by allowing a compound represented by the following formula (1): wherein X represents a chlorine atom, a bromine atom or an iodine atom; and R1 represents an alkyl group having 1 to 8 carbon atoms, an aryl group having 6 to 12 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, or an aralkyl group composed of an aryl group having 6 to 12 carbon atoms and an alkylene group having 1 to 6 carbon atoms; to react with a reducing agent in the presence of a phase transfer catalyst. According to the production method of the present invention, the reaction time of dehalogenation can be greatly shortened.
Owner:DAIICHI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

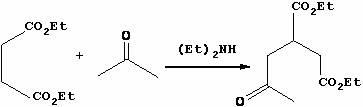

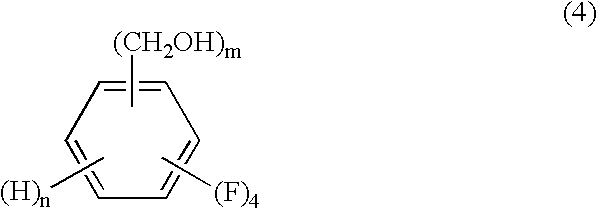
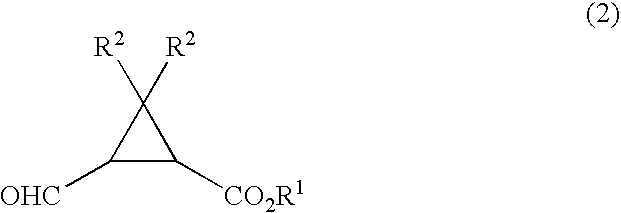
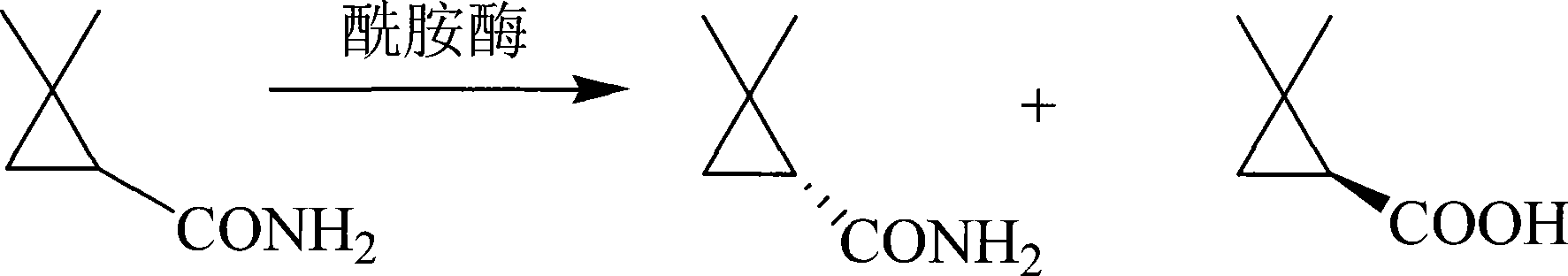



![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000011.PNG)
![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000021.PNG)
![Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate Synthetic method of 1-(2,2-difluoro-benzo[d][1,3]) dioxole-5-yl) cyclopropanecarboxylic acid and intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a697c075-5f54-4317-bd82-eb08d7e49b7e/BDA0000782155620000022.PNG)