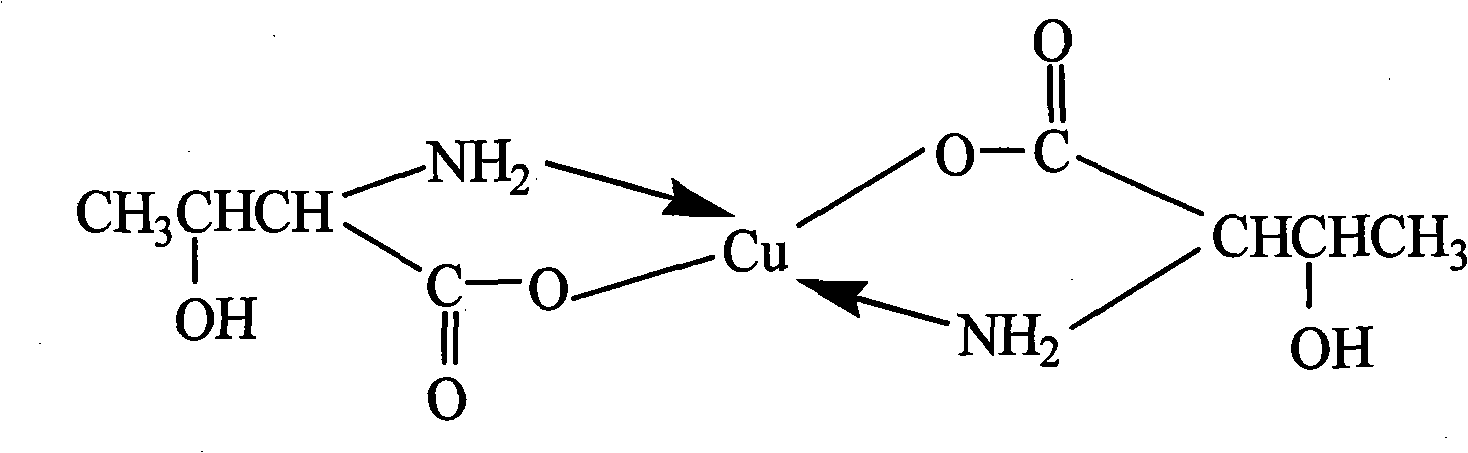
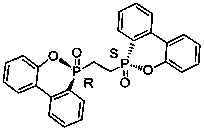
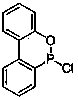
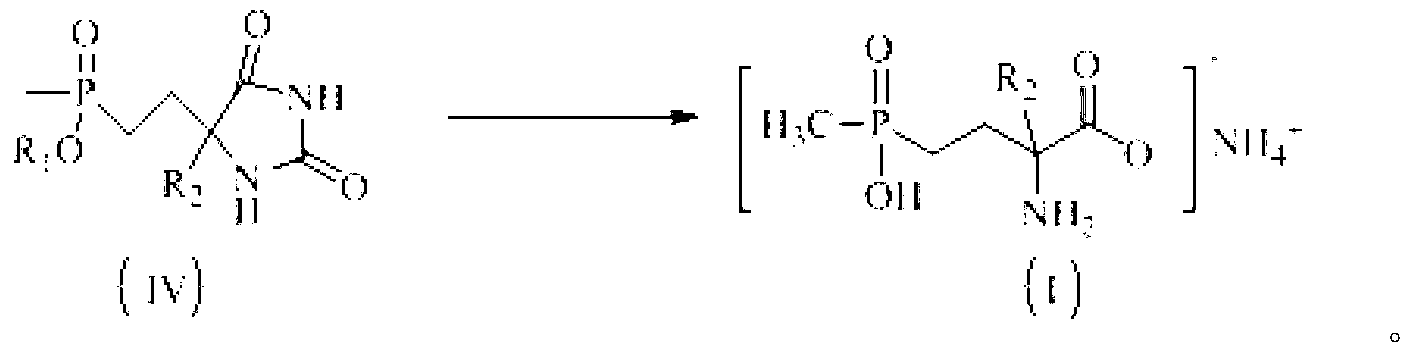
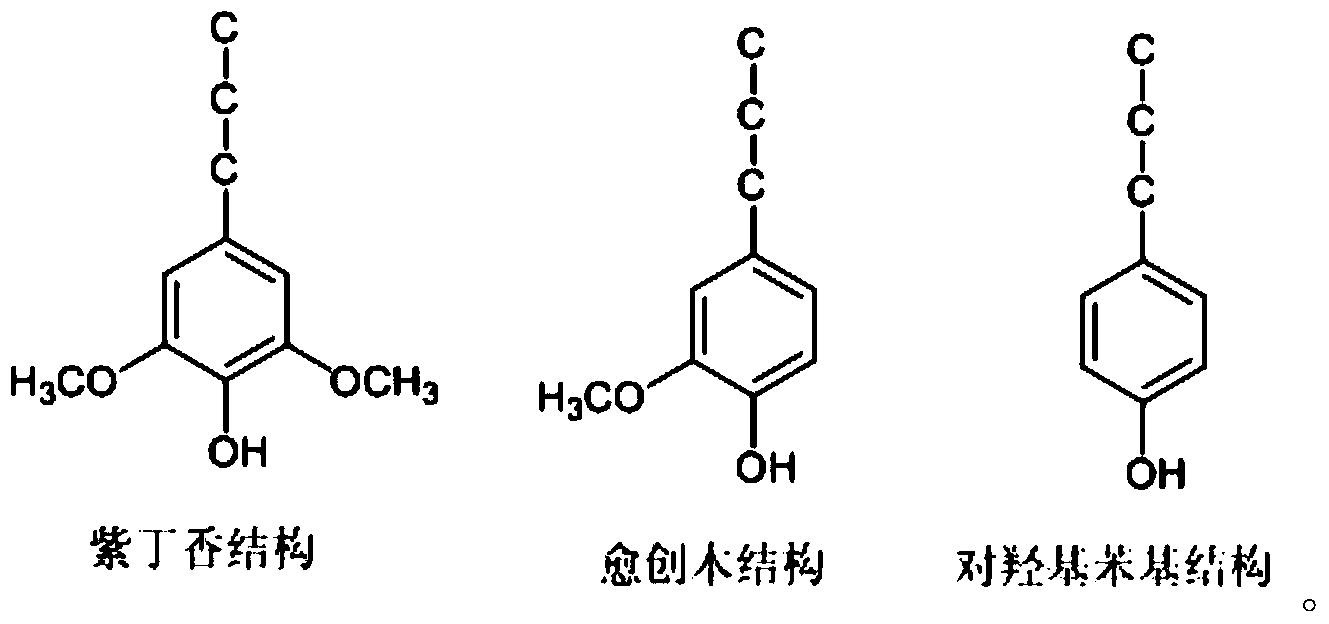
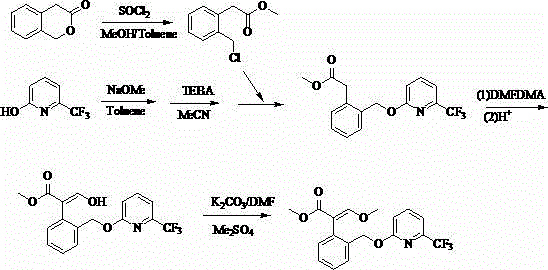
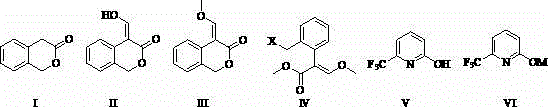
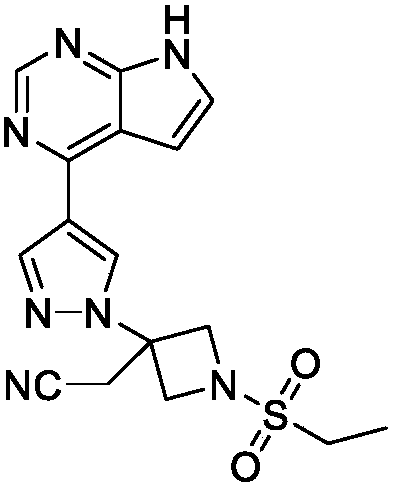
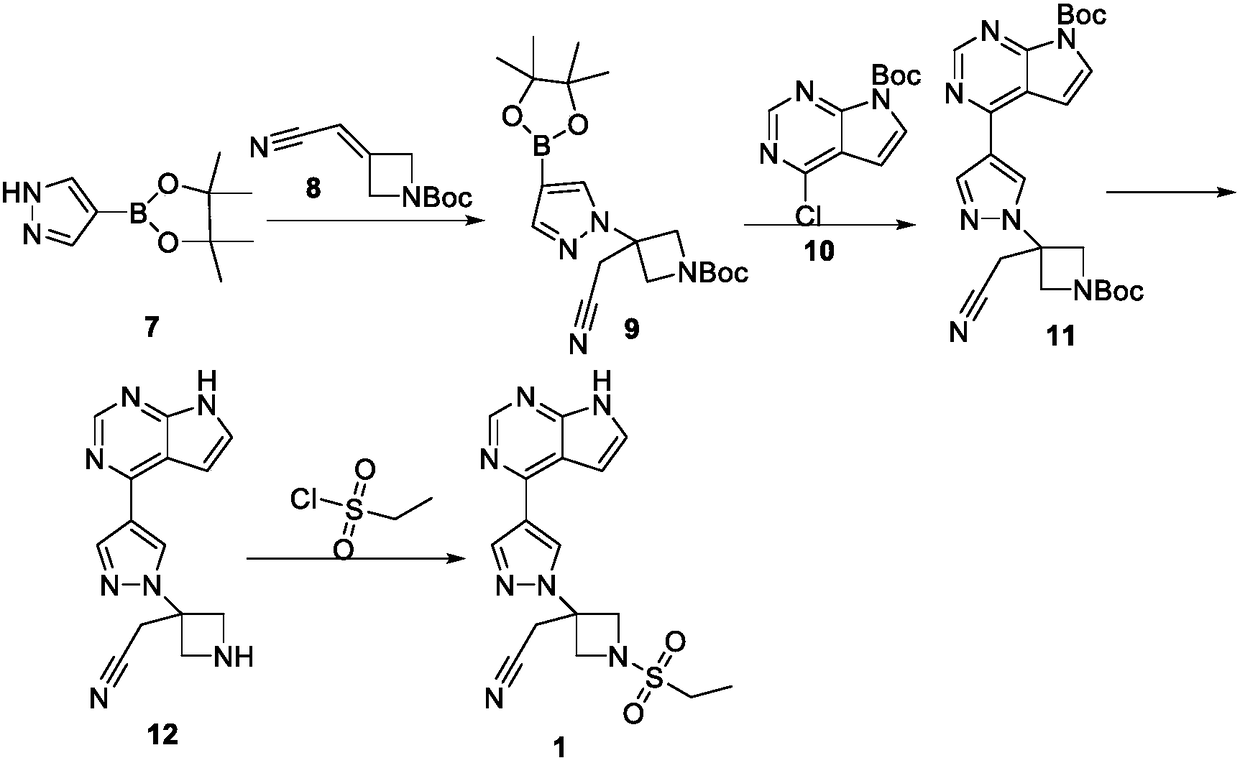
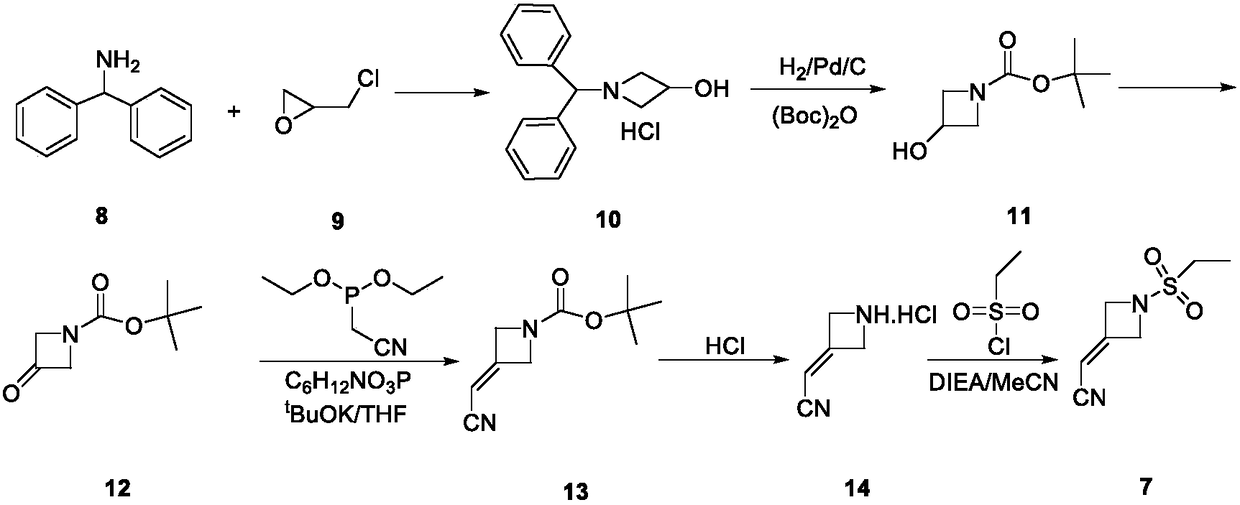
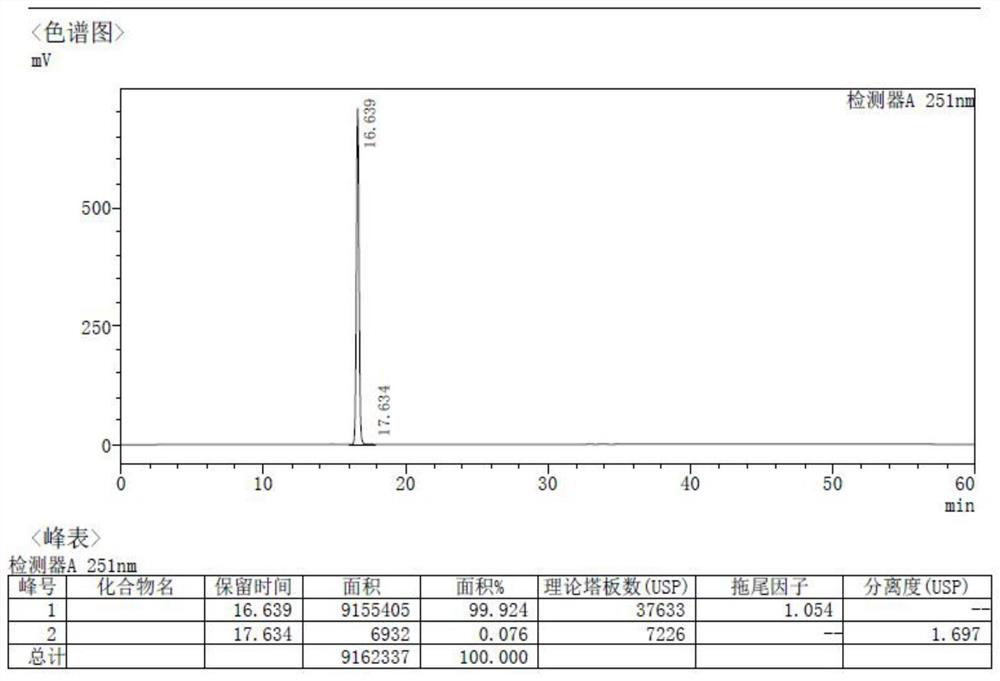
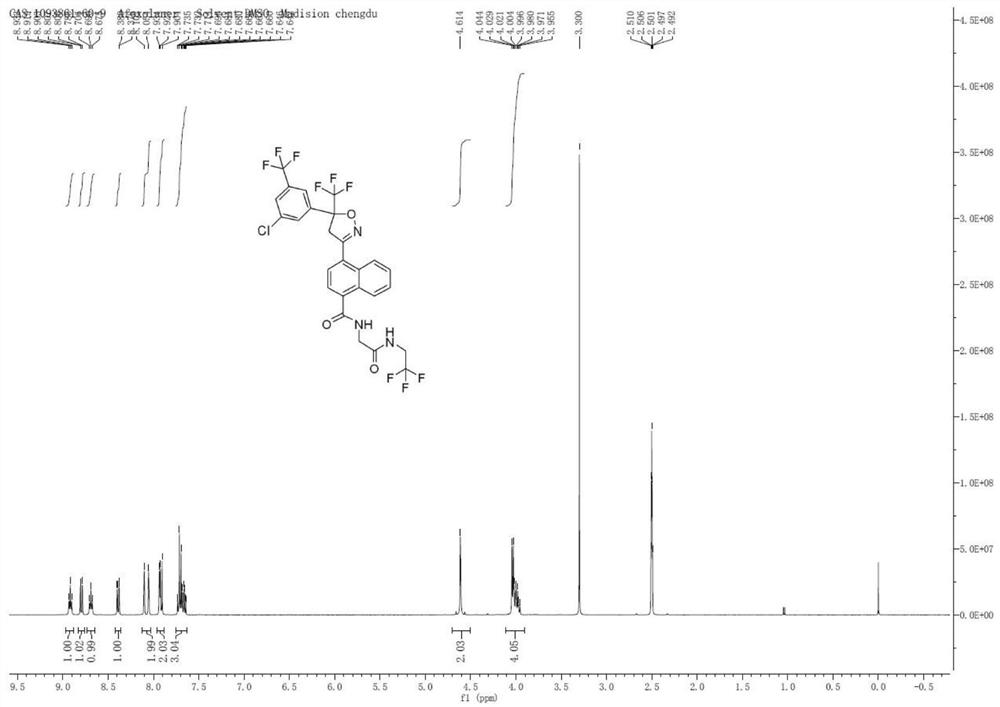
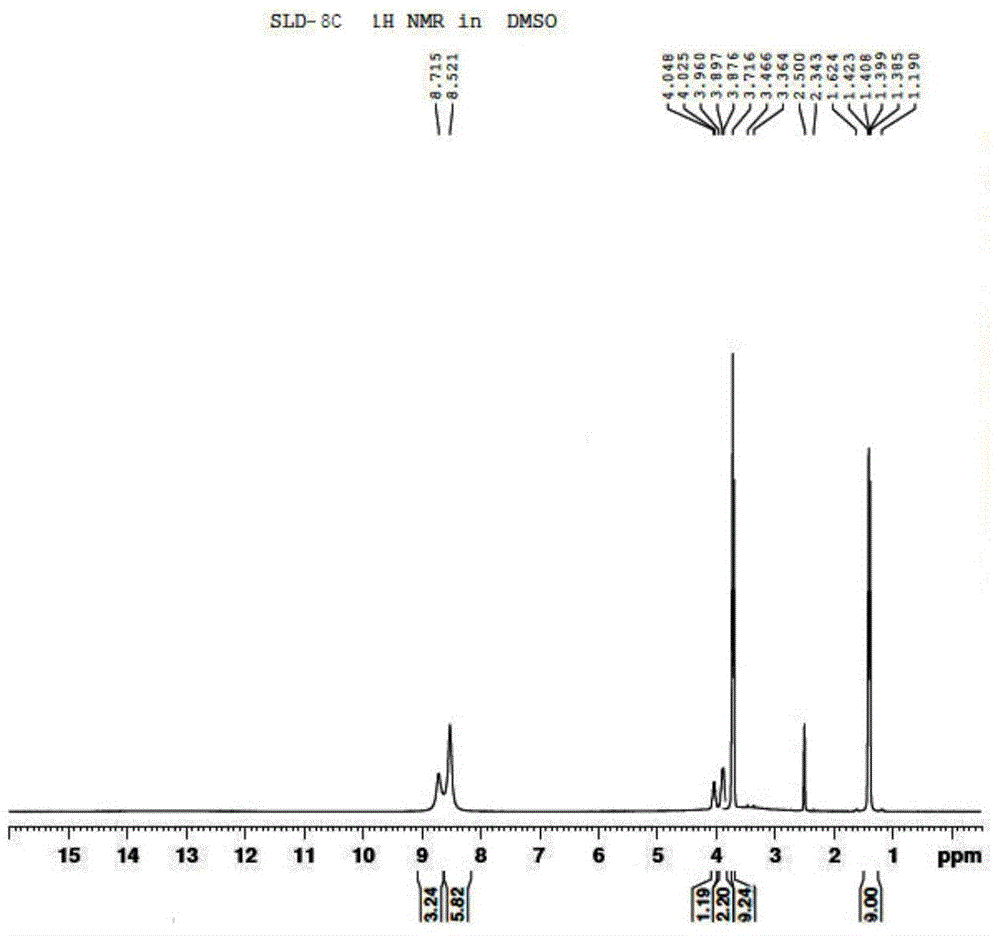
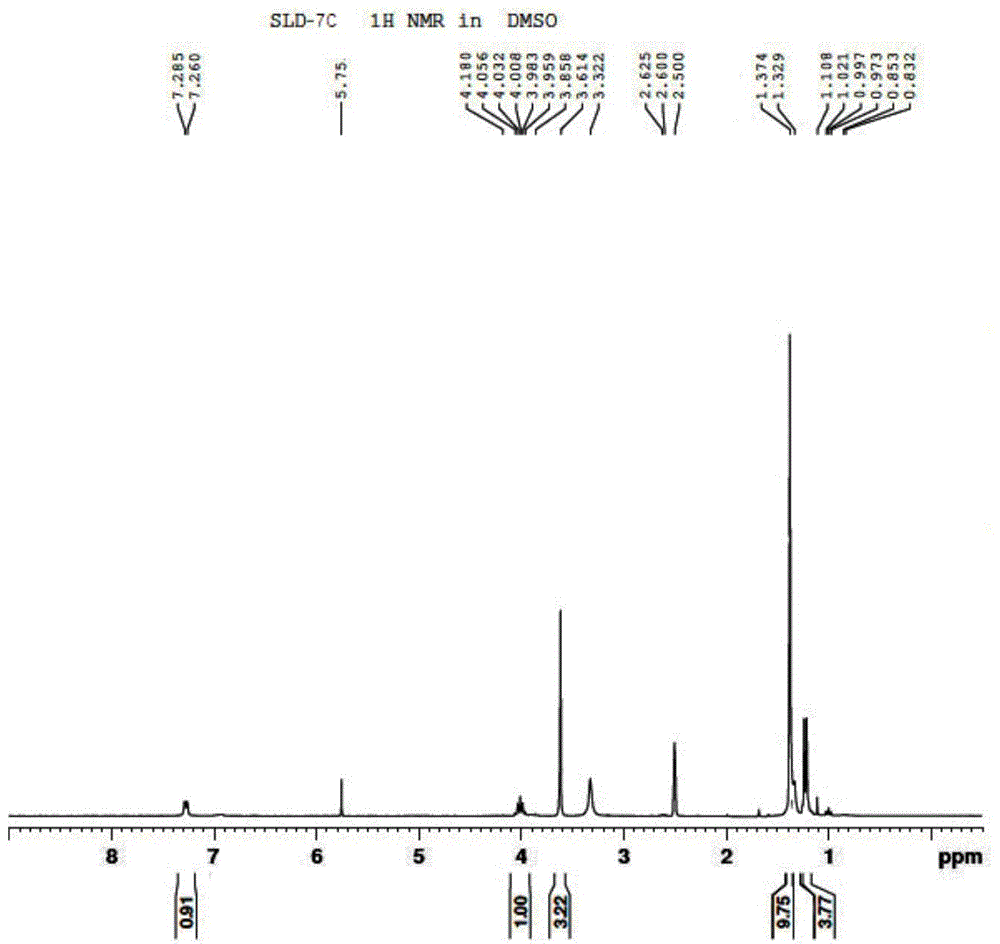
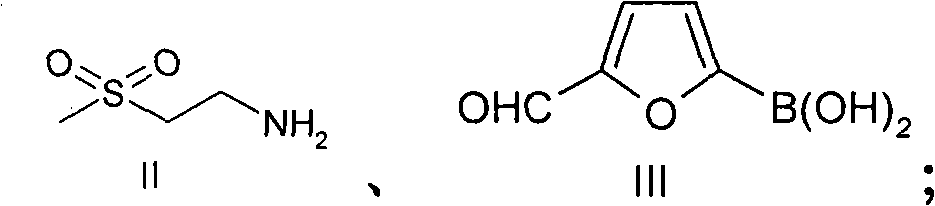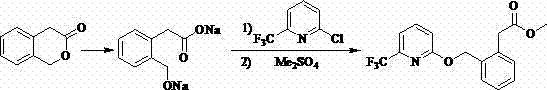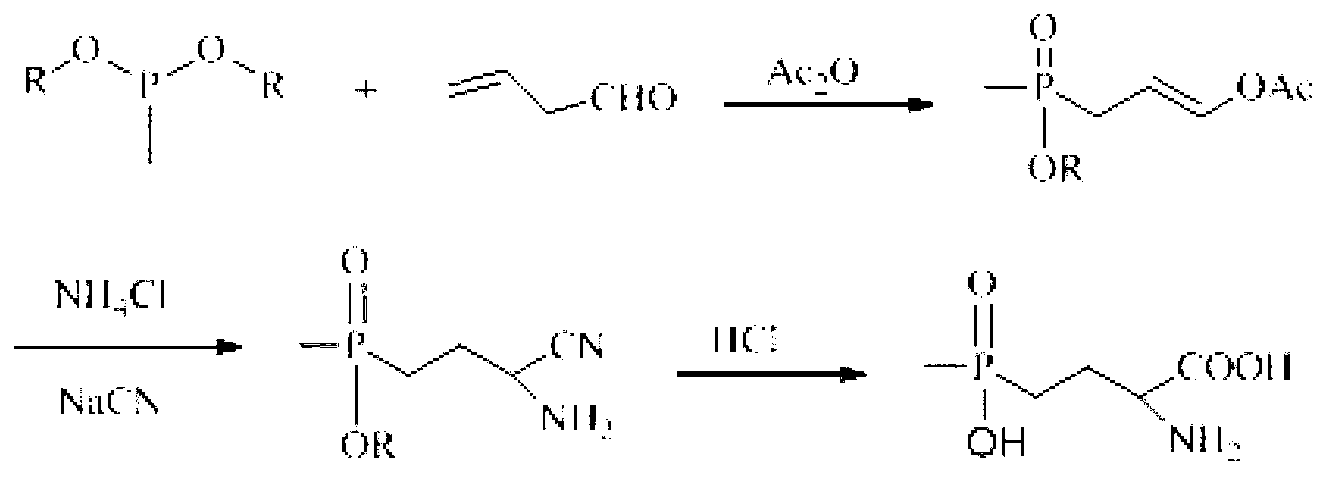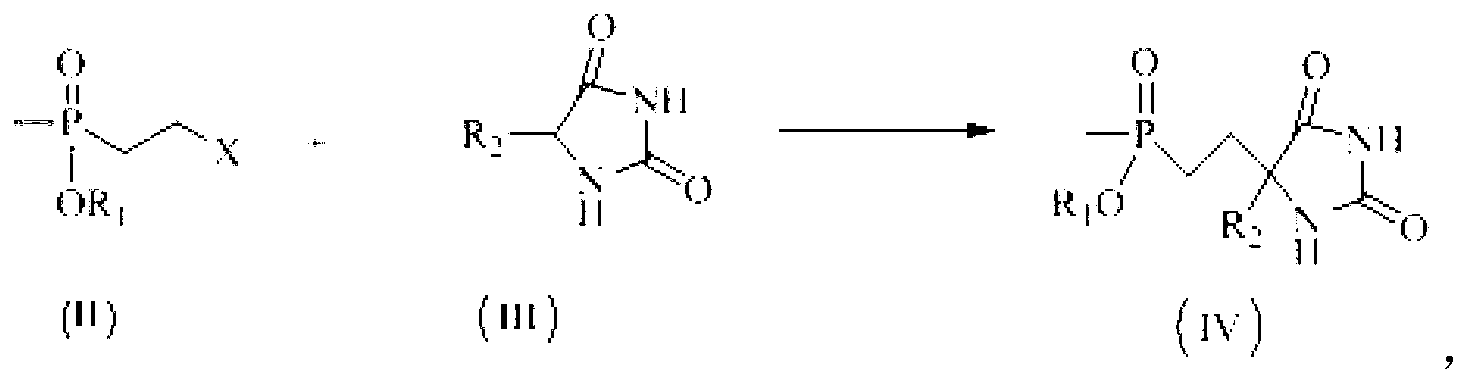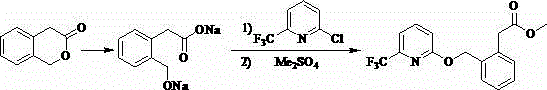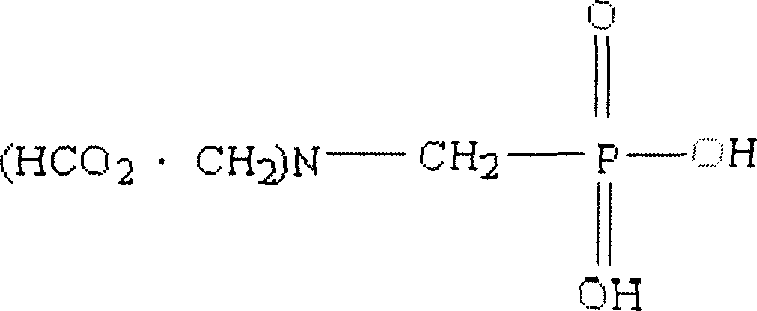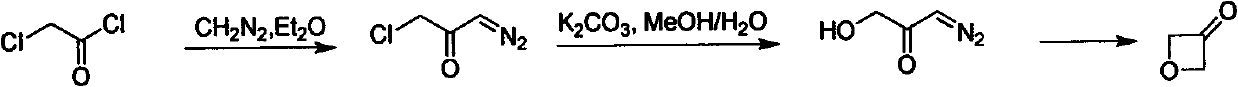Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
511results about How to "Short reaction path" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
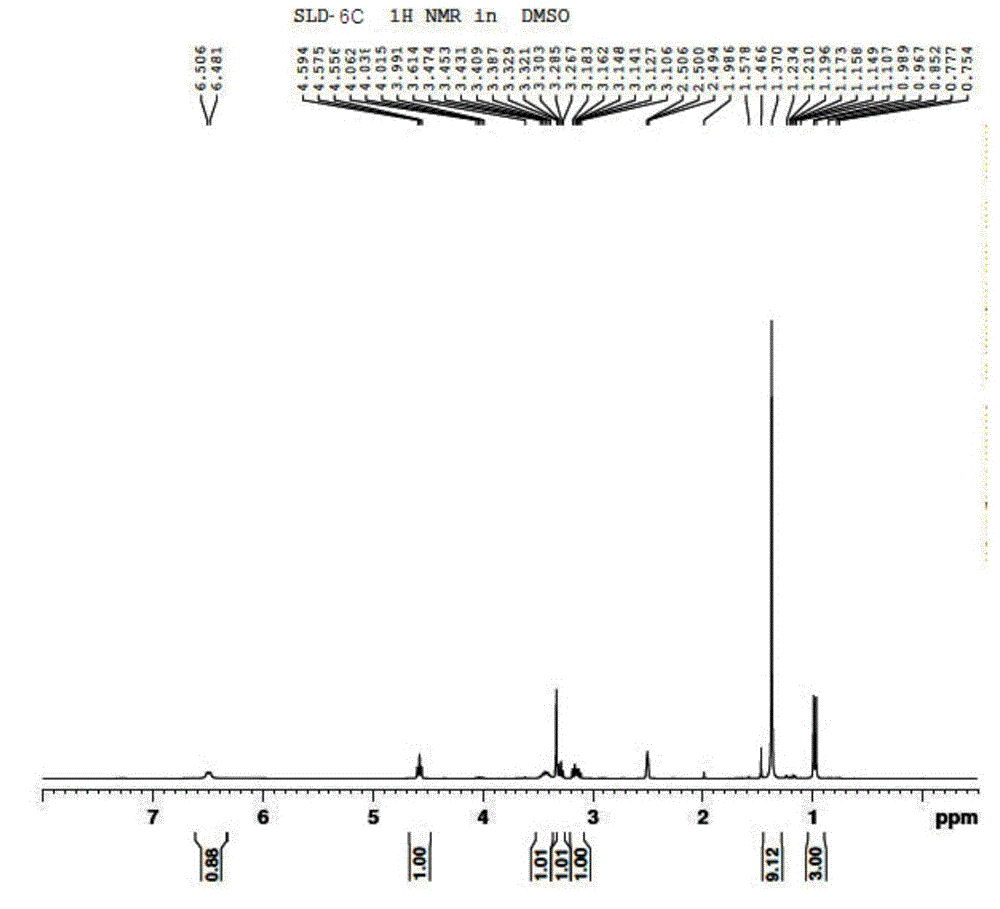
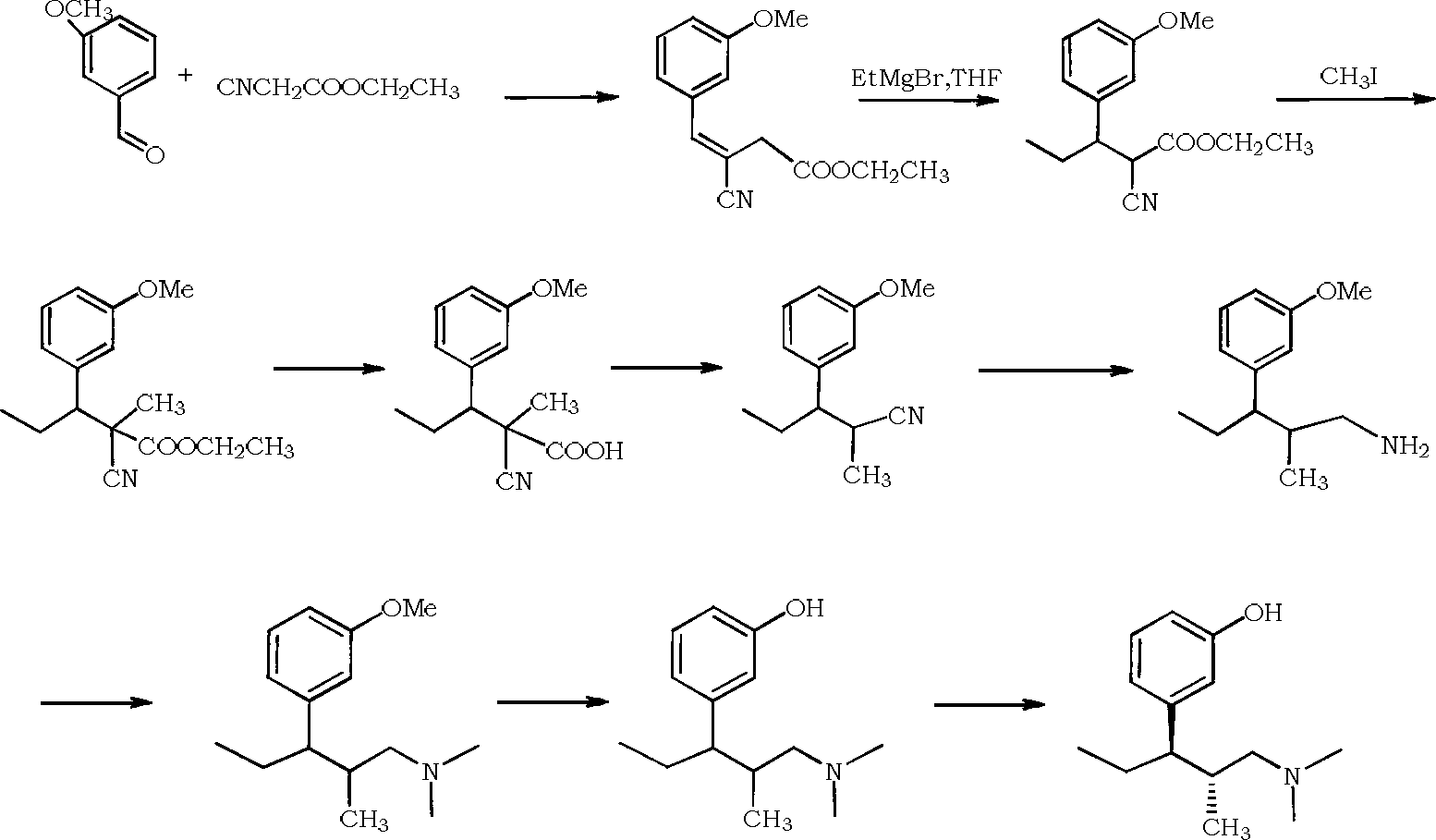
Preparation of 2-bromo-4,5-dimethoxy benzenepropanenitrile
InactiveCN101407474AHigh yieldStarting materials are readily availableCarboxylic acid nitrile preparationOrganic compound preparationFiltrationBenzaldehyde
The invention relates to a method for preparing 2-bromine-4, 5-dimethoxy benzene propane nitrile, which consists of steps: 3-4-dimethoxy benzaldehyde and bromine are dissolved in acetic acid to be reacted; when solid is precipitated, 2-bromine-4, 5-dimethoxy benzaldehyde crystal is obtained through suction filtration; the crystal and acetonitrile are dissolved in organic solvent; after a catalytic reaction, yellow solid is precipitated, cooled, extracted and dried to get 2-bromine-4, 5-dimethoxy cinnamonitrile solid which is then dissolved in methanol with reducing agent and is reduced to obtain 2-bromine-4, 5-dimethoxy benzene propane nitrile after a reflux reaction; or the obtained 2-bromine-4, 5-dimethoxy cinnamonitrile is reduced with hydrogen under the catalysis of palladium-charcoal to get 2-bromine-4, 5-dimethoxy benzene propane nitrile. The 2-bromine-4, 5-dimethoxy benzene propane nitrile prepared by the invention has high yield coefficient; original raw materials of the preparation method are easy to get; the price is low; the reaction operation is simple; the reaction route is short; and the method is easy for industrialization production.
Owner:DONGHUA UNIV
Hydrophobic POSS (Polyhedral Oligomeric Silsesquioxane)-based hybridization fluorinated acrylate resin as well as preparation method and application thereof
InactiveCN103435742AImprove adhesionGood adhesionPolyurea/polyurethane coatingsScale structureSolvent
The invention discloses a hydrophobicPOSS (Polyhedral Oligomeric Silsesquioxane)-based hybridization fluorinated acrylate resin as well as a preparation method and an application thereof. The formula of the hydrophobic POSS-based hybridization fluorinated acrylate resin comprises the following raw materials in percentage by weight: 2-12% of POSS-based monomer, 2-11% of hard monomer, 3-16% of soft monomer, 1.5-7% of fluoroacrylate monomer, 3-11% of crosslinking monomer, 0.4-1.3% of triggering agent and 50-79% of solvent. The application of the hydrophobic POSS-based hybridization fluorinated acrylate resin is characterized in that a crosslinking-type copolymer is mixed with a curing agent, the hydrophobic POSS-based hybridization fluorinated acrylate resin aggregates and is self-assembled in a film-forming solvent so as to form a micellar solution, then a phase isolation technology is used for ensuring that the solvent volatilizes on filter paper or a metal screen to form a film so as to construct a composite multi-scale structure, so that a super-hydrophobic coating is prepared. The preparation method has the advantages that a free radical solution polymerization method is adopted, synthetic conditions are simple, reaction routes are simple, raw materials are simple and easy to get, and the preparation technological processes of organic / inorganic hybrid materials are greatly simplified; the hydrophobic POSS-based hybridization fluorinated acrylate resin has wide application prospect in the field of waterproof and dampproof coating and the like.
Owner:SOUTH CHINA UNIV OF TECH
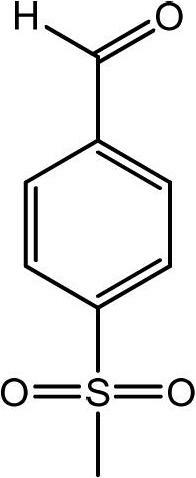
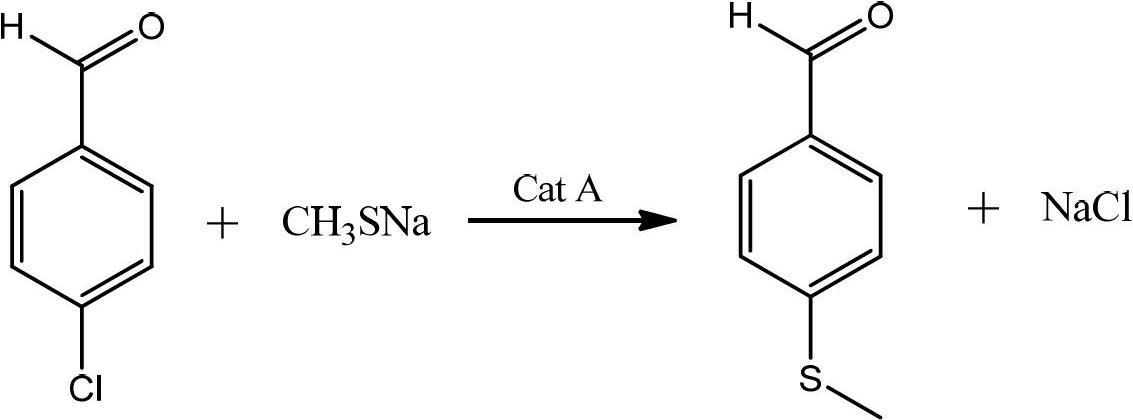
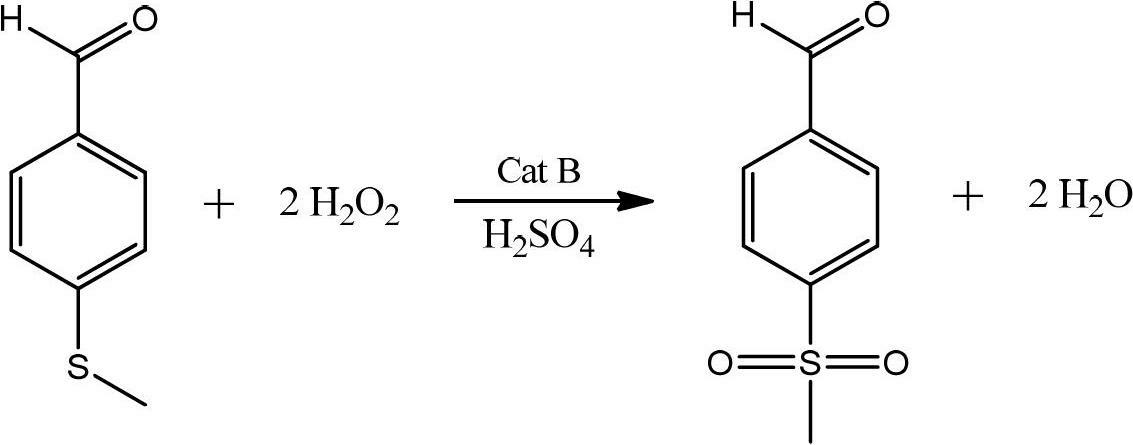
Preparation method of p-methylsulfonyl benzaldehyde
ActiveCN102675167AShort reaction pathShorten the production cycleOrganic chemistryOrganic compound preparationP-chlorobenzaldehydeBenzaldehyde
The invention discloses a preparation method of p-methylsulfonyl benzaldehyde, which takes p-chlorobenzaldehyde as starting material, and comprises the steps of: enabling the starting material to have reaction with sodium methyl mercaptide water solution under the action of phase transfer catalyst to obtain p-methylthio benzaldehyde; and oxidizing the p-methylthio benzaldehyde by hydrogen peroxide under the action of sulfuric acid and oxidation catalyst to obtain the p-methylsulfonyl benzaldehyde. The preparation method is easy in obtaining of raw material of the p-methylsulfonyl benzaldehyde, simple and convenient in operation, low in cost, high in yield and suitable for industrial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
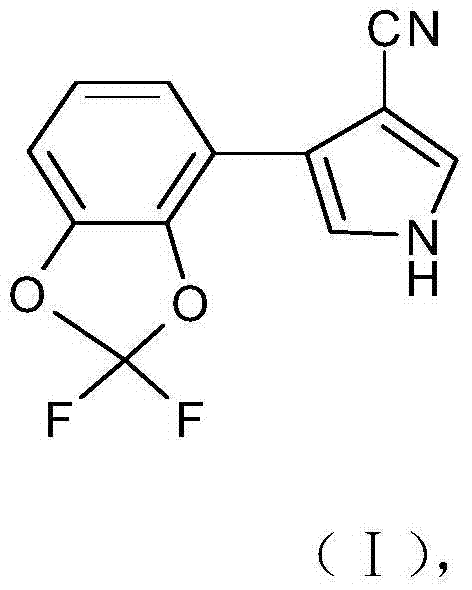
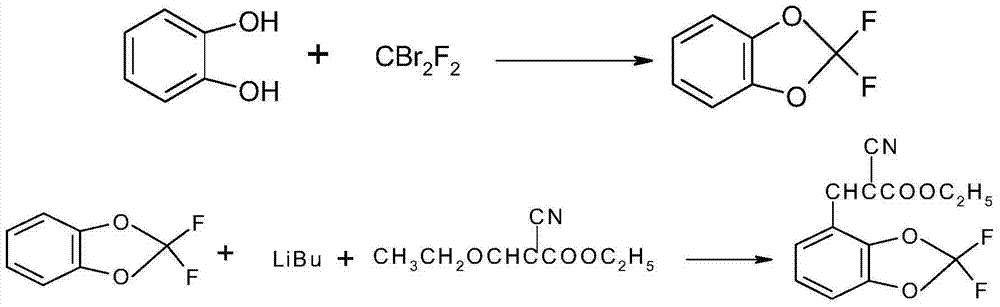
Synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile
The invention discloses a synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile. The method comprises the following steps of: preparing an intermediate 2,2-difluorobenz-1,3-dioxole through reacting catechol with dibromodifluoromethane, and preparing an intermediate 2-cyano-3-(2,2-difluorobenz-1,3-dioxole-4-yl)-2-acrylate. The fludioxonil prepared by the synthetic method provided by the invention has the purity of more than 99.0% and the total yield of more than 45.0%; the synthetic method has the advantages of cheap and easily available raw materials, simple process, high product yield in each step, good purity, low production cost, applicability to batch industrial production and the like.
Owner:XIAN MODERN CHEM RES INST
POSS (Polyhedral Oligomeric Silsesquioxane) based hybridized fluorinated acrylate resin, and preparation method and application thereof
InactiveCN103435741AEfficient separation characteristicsSignificance of great practical applicationPolyurea/polyurethane coatingsCross-linkScale structure
The invention discloses a POSS (Polyhedral Oligomeric Silsesquioxane) based hybridized fluorinated acrylate resin, and a preparation method and an application thereof. The formula of the resin comprises the following components by weight percent: 0.9-14% of POSS based monomer, 1.5-10% of hard monomer, 2.5-14% of soft monomer, 4-10.5% of acrylic higher ester monomer, 1.8-6% of fluoridized acrylate monomer, 1.8-9% of cross-linked monomer, 0.4-1.5% of initiator and 50-80% of solvent. According to the invention, the acrylic higher ester monomer is introduced as an oleophilic monomer into a fluorinated acrylate copolymer and then a hydrophobic and oleophilic compound multi-scale structure is established on filter paper or a metal filter screen by virtue of aggregating self-assembly of the POSS base in the copolymer; therefore, a hydrophobic and oleophilic coating is prepared. The resin coating prepared from the resin provided by the invention is applicable to the oil-water separation field and capable of removing trace moisture in the fuel oil, and has great practical application significance.
Owner:SOUTH CHINA UNIV OF TECH
New preparation method of lapatinib
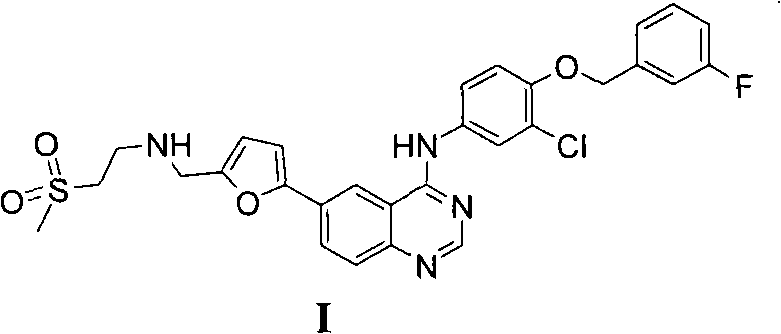
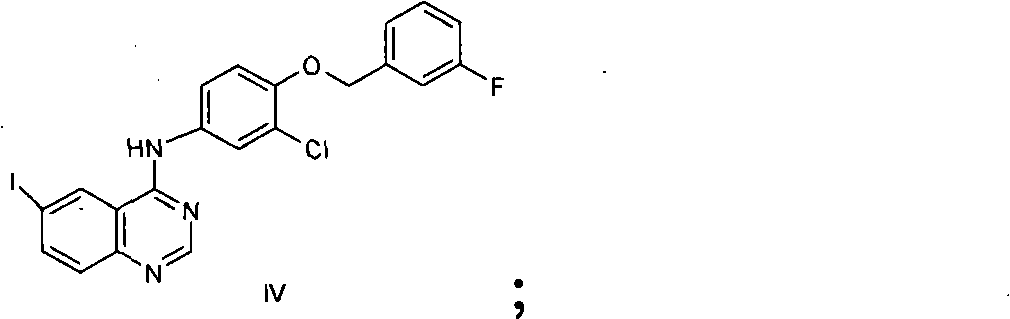
The invention provides a novel method for preparing lapatinib. Specifically, the method for preparing a compound shown in a formula I or medicinal salt thereof comprises the following steps of: (1) reacting a compound shown in a formula II or salt thereof and a compound shown in a formula III at the first temperature; (2) reacting a reaction product obtained in the step (1) and a compound shown in a formula IV in reaction liquid in the step (1) at the second temperature; (3) adding a reducing agent into reaction liquid in the step (2) to reduce the reaction product obtained in the step (2) to obtain the compound shown in the formula I; and optionally (4) reacting the compound shown in the formula I and obtained in the step (3) and acid to obtain medicinal salt of the compound shown in the formula I. The method is high in yield, and can overcome one or more disadvantages of the conventional method and the purity of a product is high.
Owner:QILU PHARMA +1
Method for selective preparation of p-xylene and toluene from p-methylcyclohexene carboxaldehyde
ActiveCN105712817AHigh selectivityImprove responseHydrocarbonsBulk chemical productionMetal catalystFixed bed
The invention relates to a method for selective preparation of p-xylene and toluene from p-methylcyclohexene carboxaldehyde. The method comprises that p-methylcyclohexene carboxaldehyde undergoes dehydroaromatization and in-situ hydrodeoxygenation reactions at a temperature of 200-500 DEG C in the presence of a loaded metal catalyst to produce p-xylene and toluene. The raw material undergoes a reaction in a fixed bed reactor. The raw material goes through a catalyst bed layer through inert gas purging so that p-xylene and toluene are obtained. The method has simple processes and high desired product selectivity. Isoprene and acraldehyde as raw materials from biomass resources undergo a one-step reaction to produce a substrate. The method provides a novel method for preparing a fragrant chemical product directly from biomass.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
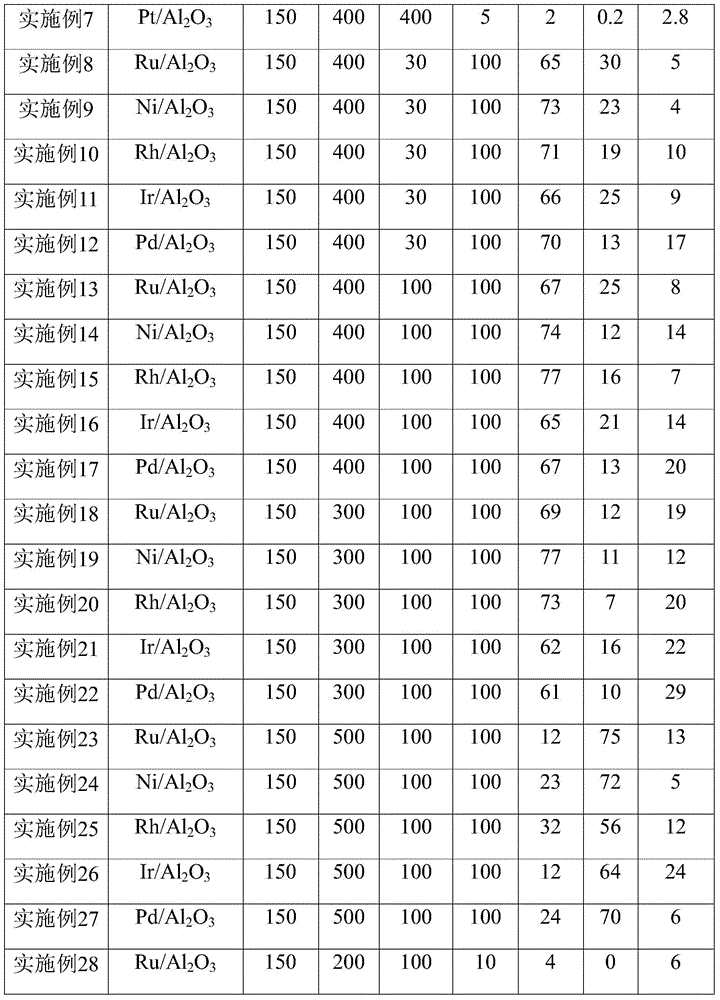
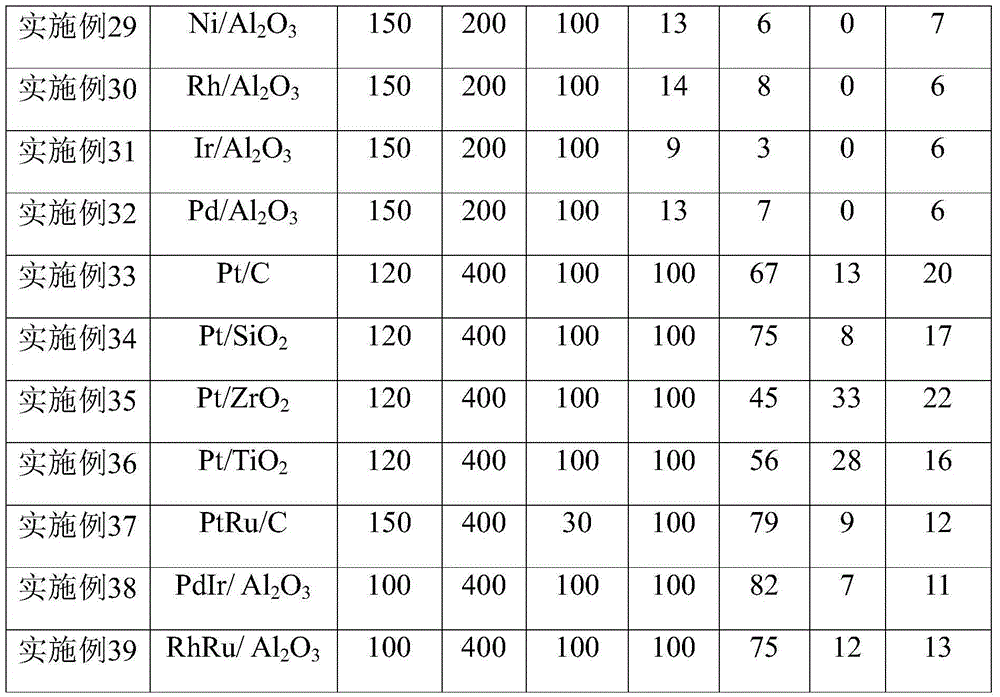
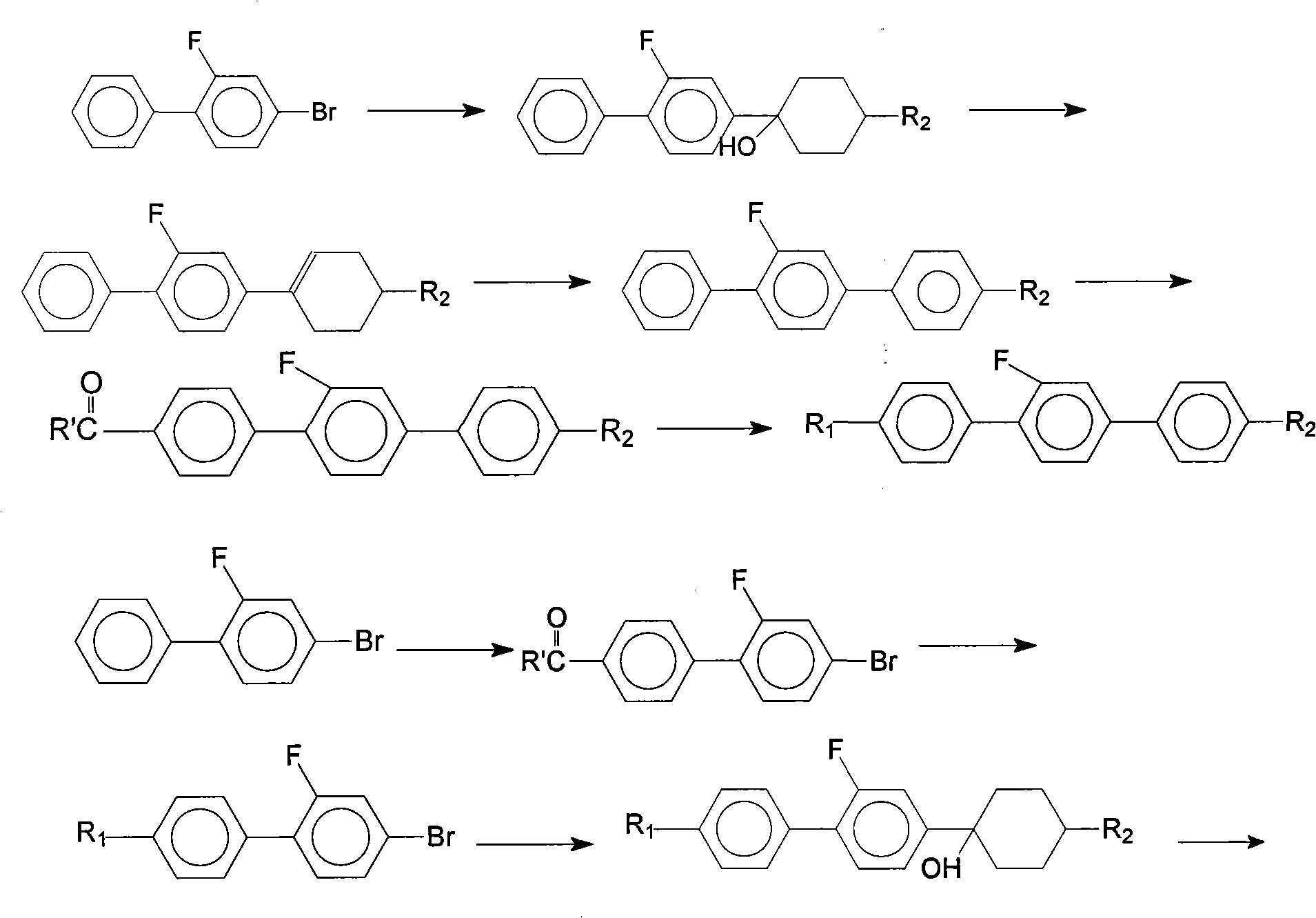
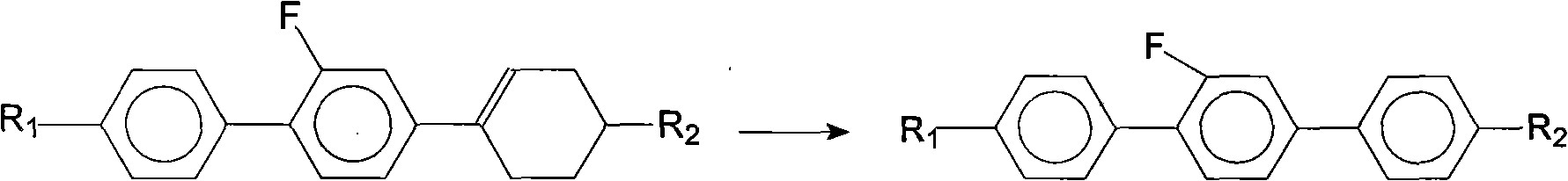
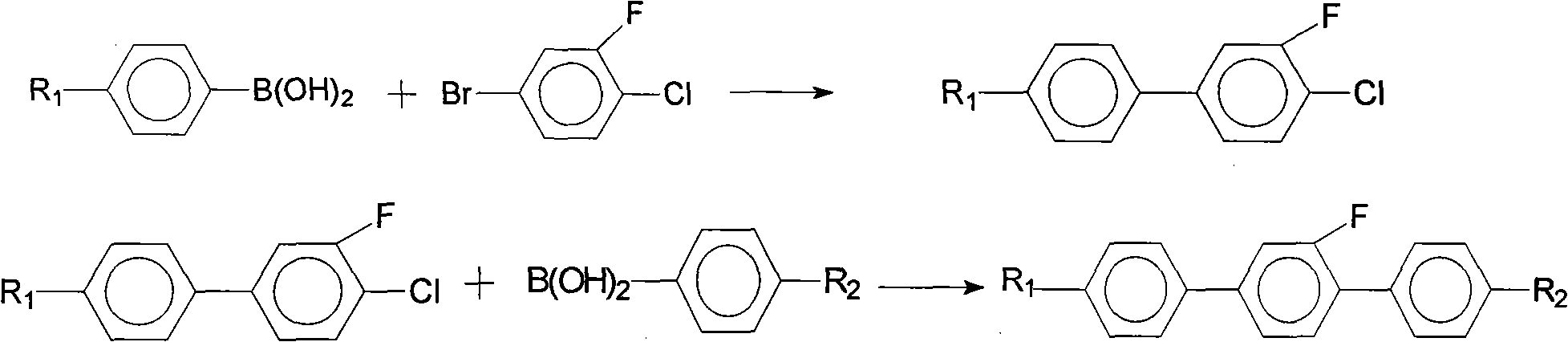
Method for synthesis of 2'-fluoro terphenyl liquid crystal
InactiveCN102399117ALow priceShort reaction pathLiquid crystal compositionsOrganic chemistry methodsBoronic acidReaction temperature
The present invention discloses a method for synthesis of 2'-fluoro terphenyl liquid crystal. With the present invention, problems of more reaction steps, low yield, heavy environmental hazard, expensive catalysts, or hazard on the human body in the prior art are solved. The technical scheme of the present invention is that: a catalyst is added to an organic solvent of the raw material to carry out a suzuki coupling reaction to realize the synthesis of the 2'-fluoro terphenyl liquid crystal. According to the present invention, 4-bromo-3-fluoro iodobenzene, a phenyl boronic acid derivative and a base are adopted as raw materials, and are subjected to two suzuki coupling reactions in the solvent under the effect of the catalyst at the reaction temperature of 70-100 DEG C to obtain the 2'-fluoro terphenyl liquid crystal, wherein the times of the two suzuki coupling reactions are respectively 4-10 hours and 10-20 hours. The method of the present invention has advantages of simple process, mild conditions, low preparation cost, high yield, environmental protection, and strong practicability.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
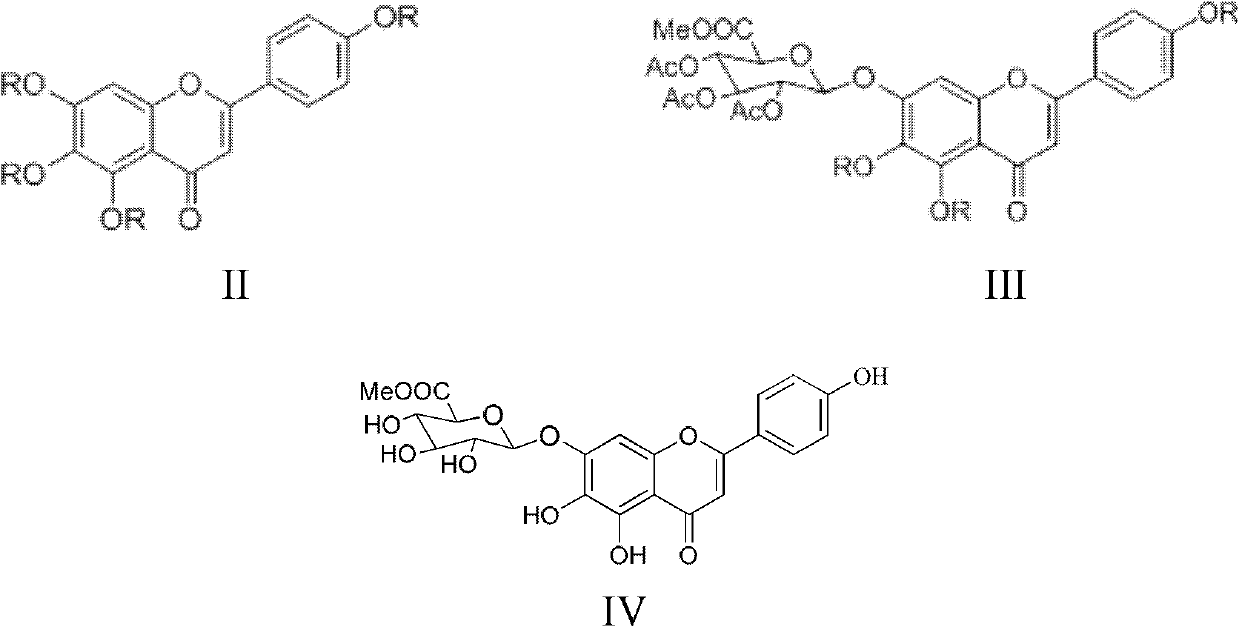
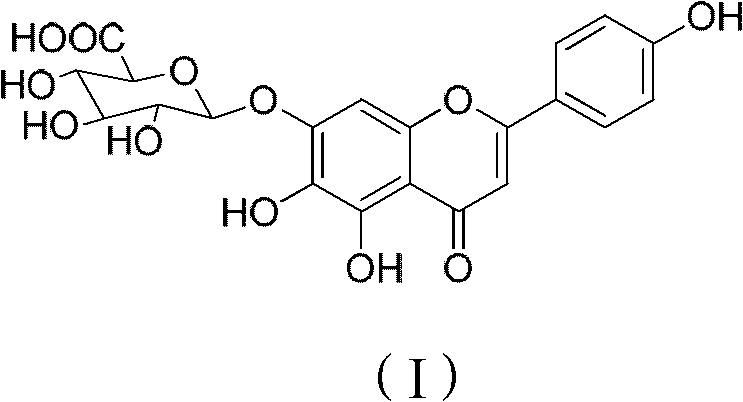
Method for preparing 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid
ActiveCN103374050AShort reaction pathImprove reaction rate and yieldSugar derivativesSugar derivatives preparationD-GLUCURONIC ACIDDrugs synthesis
The invention relates to the field of drug synthesis and discloses a method for preparing 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid. The method is characterized by taking 5,6,7,4'-tetrahydroxy flavone as a raw material, adopting a brand-new synthetic route and carrying out acylation reaction, glycosylation reaction and two-step hydrolysis reactions, thus preparing high-purity 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid. The preparation method has the advantages of short synthetic route, low cost, high reaction yield and easiness in product purification, and is suitable for industrial production of 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid.
Owner:KPC PHARM INC
(Ti,M)C nano solid solution powder and preparation method thereof
ActiveCN107758666AHigh reactivityWell mixedMaterial nanotechnologyOxy/sulfo carbidesCrucibleHigh energy
The invention discloses (Ti,M)C nano solid solution powder and a preparation method thereof. The (Ti,M)C nano solid solution powder is prepared from the following components in percentage by weight: 35 to 79.9 percent of Ti, 0.1 to 35 percent of M and the balance of C, wherein the M is at least one of W, Mo, Cr, Ta, V and Nb. The preparation method comprises the following steps of mixing Ti powder, carbon black powder and a powdery raw material of the component M, carrying out high-energy ball milling, drying a mixture obtained after the ball milling, then putting the dried mixture into a crucible, adding a halogenating agent, afterwards, in the protection condition of an Ar gas, carrying out heat preservation for 2h to 4h at 900 to 1,000 DEG C, so as to obtain halogenating agent mixed (Ti,M)C nano solid solution powder, finally, dissolving the halogenating agent by using distilled water, centrifuging and drying, so that the (Ti,M)C nano solid solution powder is subsequently obtained.The method provided by the invention can be used for completing the carbonization and solid-solution reactions of (Ti,M)C at 900 to 1,000 DEG C; the prepared (Ti,M)C solid solution powder has the characteristic of a single-phase component and has the average particle size which is less than 100nm; the powder is high in purity and the content of the C is easy to precisely regulate and control.
Owner:自贡市泰昶硬质材料有限责任公司
Preparation method of sitagliptin intermediate
ActiveCN103755596AChiral raw materials are cheap and readily availableShort reaction pathCarbamic acid derivatives preparationOrganic compound preparationPhenethylaminesChemistry
The invention discloses a preparation method of a sitagliptin intermediate represented by formula 1. The preparation method comprises the following steps: step A, carrying out a reaction of beta-ketonic ester 2 with a chiral amine to obtain a chiral enamine 3, wherein the chiral amine is R(+)-alpha-phenylethylamine or D-(-)-phenylglycinol; step B, reducing the chiral enamine 3 to obtain a compound 4; step C, carrying out catalytic hydrogenation of the compound 4 to remove a chiral auxiliary group, and thus obtaining a compound 5; step D, protecting amino of the compound 5 by Boc to obtain a compound 6; and step E, hydrolyzing the compound 6 to obtain beta-amino acid 1. The method has the advantages of cheap and easily obtained chiral raw materials, short reaction route, simple operation, mild reaction conditions, no special requirements on equipment, high yield, low cost and small environment protection pressure, and has relatively good industrial application and economic value.
Owner:ZHEJIANG UNIV OF TECH
A synthetic process of a green efficient agricultural fungicide
ActiveCN104262239AShort reaction pathSimple and safe operationOrganic chemistryMeth-Agricultural engineering
A synthetic process of a green efficient agricultural fungicide is disclosed. The synthetic process includes: preparing 4-(methoxymethene)-3-isobenzofuranone by adopting 3-isochromanone as an initial raw material; preparing methyl (E)-2-(2-halomethylphenyl)-3-methoxy acrylate from the 4-(methoxymethene)-3-isobenzofuranone in methanol, a third inert solvent and a halogenating agent; and reacting the methyl (E)-2-(2-halomethylphenyl)-3-methoxy acrylate and 2-hydroxy-6-(trifluoromethyl)pyridine or 6-trifluoromethyl-2-pyridinol salt to obtain picoxystrobin. The synthetic process has characteristics of short reaction steps, mild reaction conditions, simple, convenient and feasible post-treatment, little three-waste, low cost, high yield, and suitability for industrial production. A product is good in appearance, and has external standard purity higher than 98%.
Owner:ZHEJIANG TIDE CROP TECH
Method for preparing DL-threonine chelated copper serving as feed additive
InactiveCN101838214AHigh yieldRaw materials are easy to getOrganic compound preparationAnimal feeding stuffGlycineFood additive
The invention discloses a method for preparing DL-threonine chelated copper serving as a feed additive. The invention adopts a technical scheme of taking aminoacetic acid as raw material, allowing aminoacetic acid to react with basic copper carbonate to form glycine chelated copper and allowing glycine chelated copper to react with acetaldehyde to form DL-threonine chelated copper. Compared with the conventional method for preparing threonine chelated copper, the method of the invention has the following outstanding advantages that: 1) as threonine and threonine chelated copper are prepared from aminoacetic acid, the method is readily available in raw material, needs no threonine and is low in cost; 2) as the feed additive amino-acid chelated copper does not need separation, and DL-threonine copper is directly utilized, so over 50 percent of cost is reduced; and 3) the preparation of the DL-threonine chelated copper is performed at normal temperature under normal pressure and is short in reaction route, little in equipment investment and convenient for industrial production.
Owner:INST OF SUBTROPICAL AGRI CHINESE ACAD OF SCI
Preparation method of high-purity DOPO (9,10-dihydro-9-oxa-10- phosphaphenanthrene-10-oxide) derivative
The invention provides a preparation method of a high-purity DOPO (9,10-dihydro-9-oxa-10- phosphaphenanthrene-10-oxide) derivative, and relates to a flame retardant in the field of chemical materials. According to the preparation method, CDOP (10-chloro-9,10-dihydro-9-oxa-10-phosphaphenanthrene) is used as a raw material and reacts with ethylene glycol under the action of a catalyst, wherein the reaction temperature is 10-200 DEG C, and the reaction time is 5 to 30 hours, so that a product with high optical purity can be directly obtained. The preparation method has the advantages that reaction routes are short, the product does not contain another two isomers, and the melting point of the product reaches up to about 300 DEG C. The high-purity DOPO derivative can be used as the flame retardant.
Owner:JIANGSU YOKE TECH
Preparation method of glufosinate-ammonium and derivatives thereof
InactiveCN103288874AHigh yieldEasy to detectBiocideGroup 5/15 element organic compoundsHydantoin derivativesReaction temperature
The invention discloses a preparation method of glufosinate-ammonium and derivatives thereof. The method comprises the following steps: (1) by using a substituted hydantoin and methylphosphonate compounds as a raw material, adding alkali, carrying out nucleophilic substitution reaction at the reaction temperature of 20-100 DEG C in a reaction solvent to obtain a hydantoin derivative, and tracing product generation condition by high performance liquid chromatography in the reaction process to judge the reaction endpoint, wherein the molar ratio of the methylphosphonate compounds, substituted hydantoin and alkali is 1:(1.0-5.0):(1.0-4.0); (2) carrying out reflux reaction for prepared hydantoin derivative prepared and an inorganic acid in water for 30-60 hours; after reaction, adding ammonia water to adjust the pH of the liquor to 12; spirally distilling to remove the solvent; adding absolute methanol for reflux for 30-60 minutes; filtering; spirally drying the mother liquor; adding methanol for recrystallization to obtain pure glufosinate-ammonium shown in formula (I) or derivatives thereof, wherein the molar ratio of the hydantoin derivative and inorganic acid is 1.0:(4.0-9.0).
Owner:SHANDONG ACADEMY OF PESTICIDE SCI +1
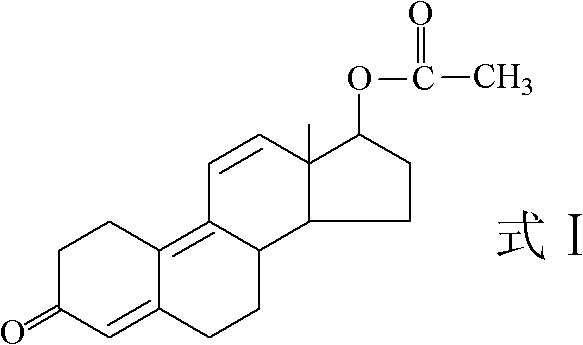
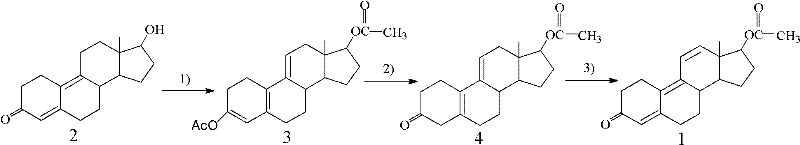
Preparation method for trenbolone acetic ester
The invention provides a preparation method for trenbolone acetic ester. In the preparation method, 17beta-hydroxy-estra-4,9-diene-3-keton as an initiative material (compound 2). The method sequentially comprises the following steps of: 1) esterifying 17-position hydroxyl and 3-position enol of the compound 2 to obtain 3,5(10),9(11)-estratriene-3,17beta-glycol diacetic ester (compound 3); 2) hydrolyzing 3-position enol ester of the compound 3 to obtain 17beta-hydroxy-estra-5(10),9(11)-diene-3-ketone acetic ester (compound 4); and 3) dehydrogenizing 5(10),9(11) conjugated double bond of the compound 4 by using dichloro dicyan quinone (DDQ) to obtain the trenbolone acetic ester (compound 1). The method plays an important role in preparation of medicines (such as finaplix, Revalor, Parabol 25 mg, Forplix, Toreter and the like) by using trenbolone acetic ester as the raw material medicine and has wide application prospect.
Owner:湖南科益新生物医药有限公司
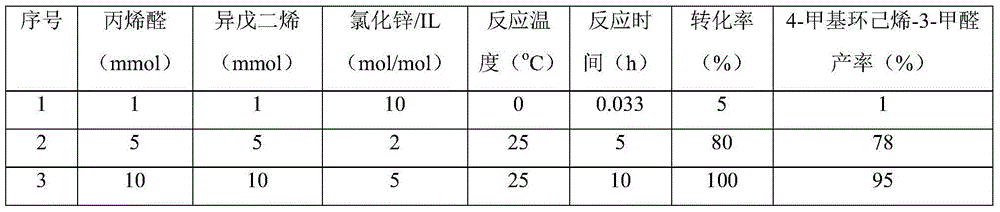
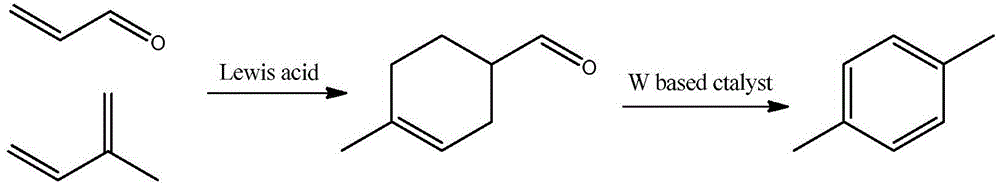
Method for preparing 4-methylbenzaldehyde from isoprene and acrolein
ActiveCN104693016AReduce dependenceExtensive sources of raw materialsOrganic compound preparationCarbonyl compound preparationCyclohexene4-Methylbenzaldehyde
The invention relates to a method for preparing 4-methylbenzaldehyde from isoprene and acrolein. The method specifically comprises the following steps: enabling acrolein and analogues thereof to react with isoprene to carry out a Diels-Alder reaction at a proper temperature under the action of Lewis acidic ionic liquid to generate 4-methyl cyclohexene-3-formaldehyde and analogues thereof; by taking an organic solvent as a reaction medium and graphite oxide as a catalyst, carrying out a dehydrogenation reaction on 4-methyl cyclohexene-3-formaldehyde under proper temperature and pressure, and preparing 4-methylbenzaldehyde (4-methylbenzaldehyde, MBAD for short). According to the method disclosed by the invention, the isoprene and acrolein are taken as raw materials and are subjected to a simple two-step reaction to generate an aromatic target product with high additional value; the raw materials can be prepared from biomass resources, the product is cheap, regenerative, convenient to operate and high in yield, and a novel method for directly preparing chemicals from biomasses is provided.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
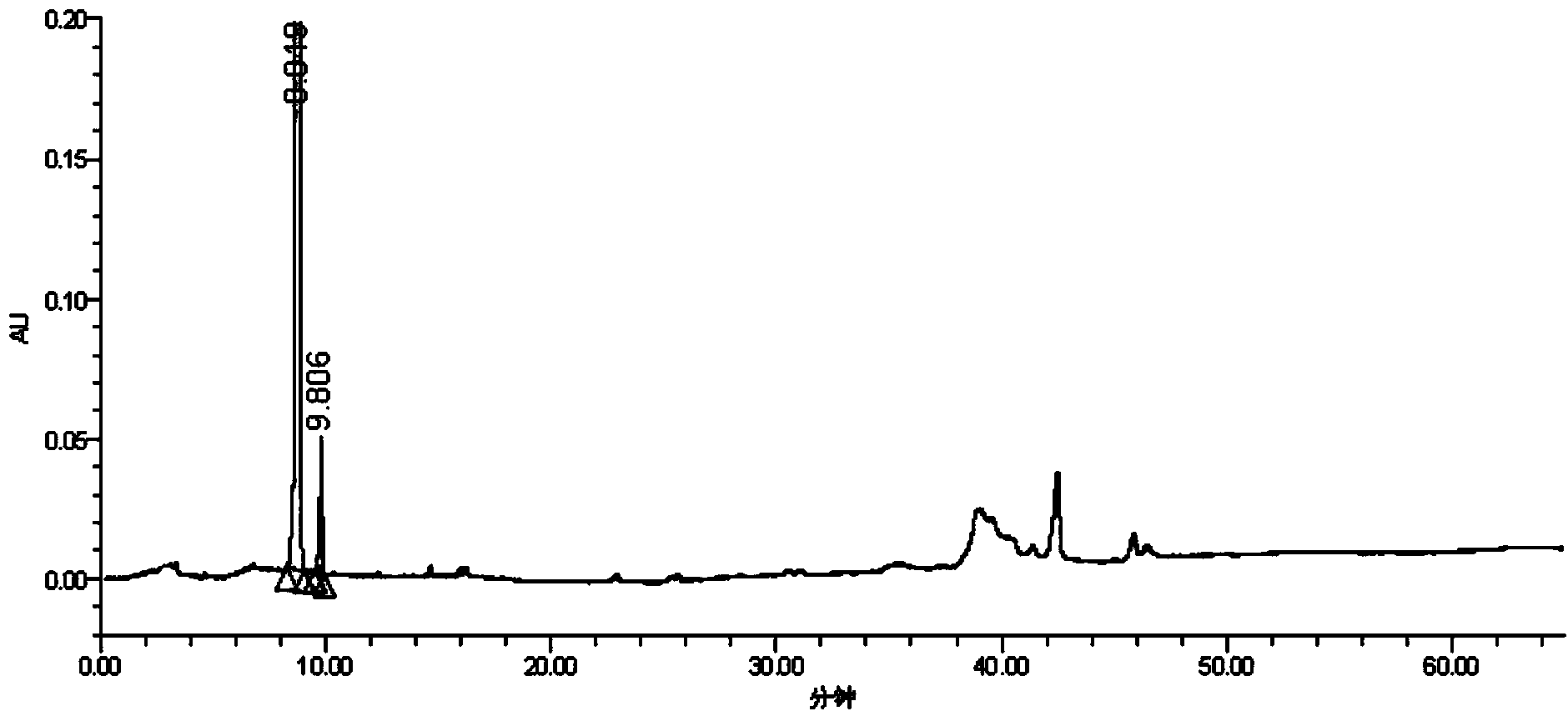
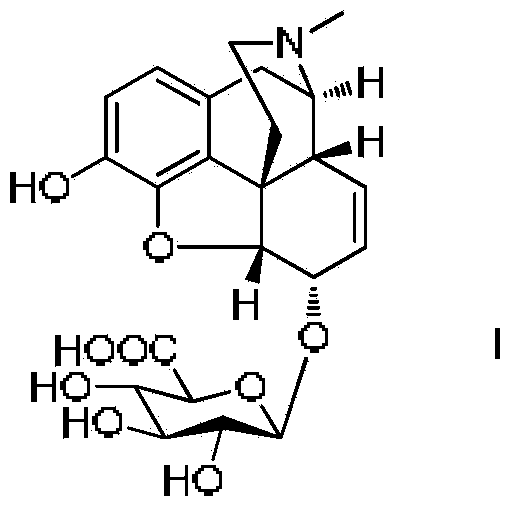
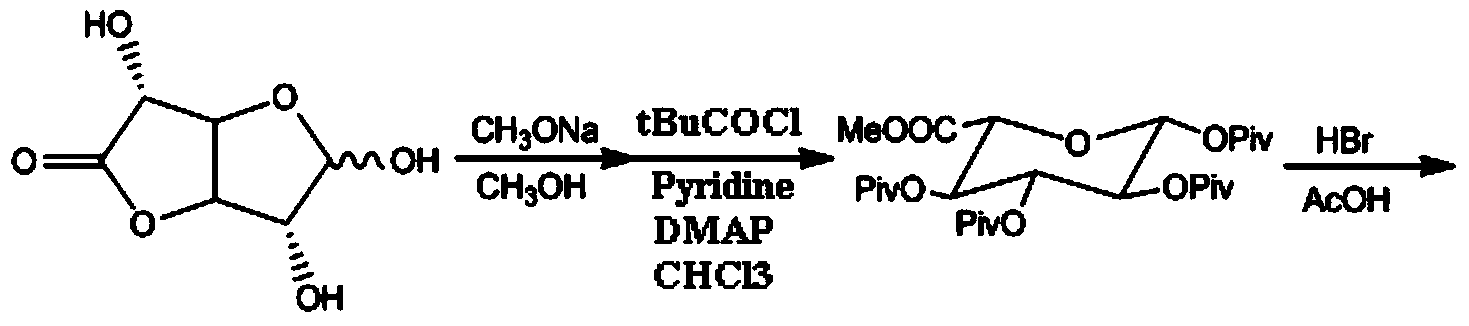
Synthesis method and intermediate compound of morphine-6-Beta-D-glucuronide
ActiveCN103864866AMedication safetyShort reaction pathEsterified saccharide compoundsSugar derivativesOrganic solventLithium hydroxide
The invention discloses a synthesis method and an intermediate compound of morphine-6-Beta-D-glucuronide. The synthesis method comprises the following steps: (1) carrying out a reaction on 3-acetyl morphine and acyl-protected glucuronate in an organic solvent 1 under the catalysis of lewis acid so as to obtain an intermediate shown as a formula (IV); (2) hydrolyzing the intermediate shown as the formula (IV) with lithium hydroxide and neutralizing the intermediate shown as the formula (IV) with hydrobromic acid in a mixture solvent of C1-C4 alkanol and water; and washing the intermediate with the C1-C4 alkanol and drying, thereby obtaining the intermediate compound of the morphine-6-Beta-D-glucuronide, wherein the definitions of substituent groups in the formula (III) and the formula (IV) are shown in the specification. The synthesis method has the advantages of moderate condition and high yield, and is simple to operate and easy to industrialize.
Owner:YICHANG HUMANWELL PHARMA
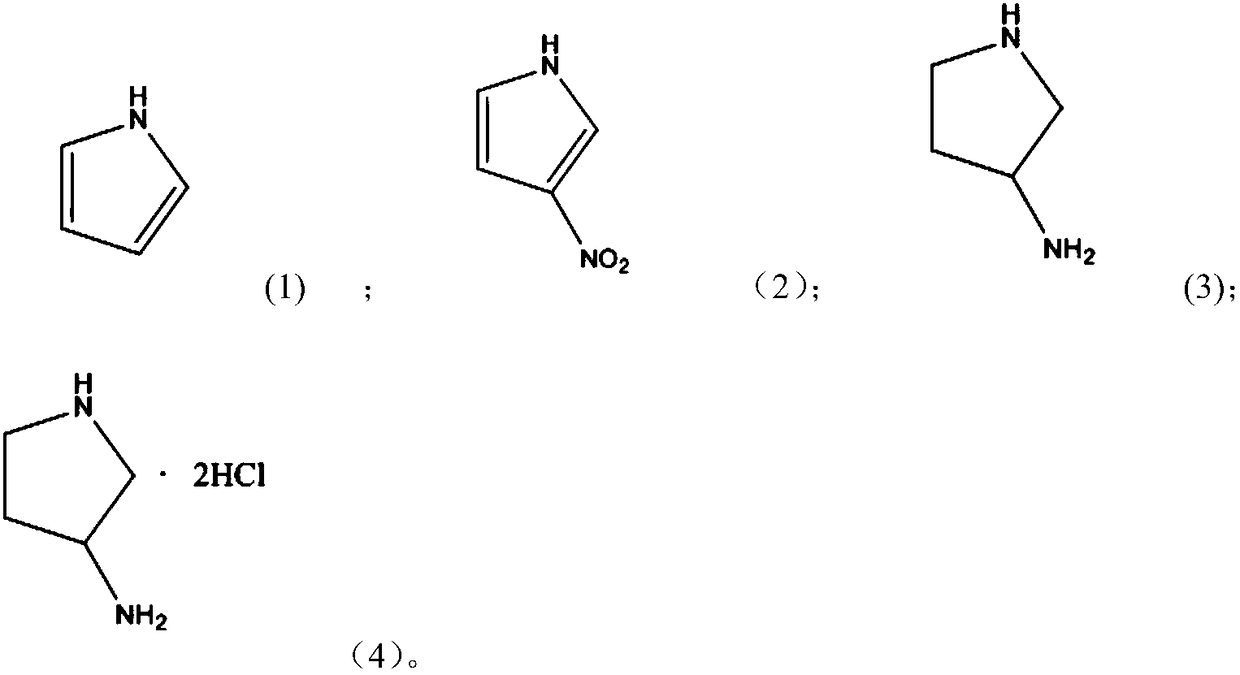
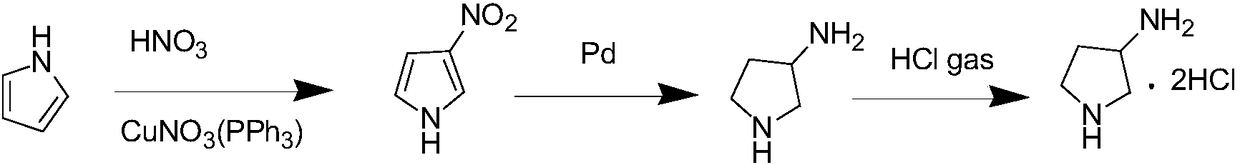
Method for preparing 3-aminopyrrolidine hydrochloride by using one-pot method
InactiveCN108440361AReaction raw materials are cheap and easy to obtainShort reaction pathOrganic chemistryWater bathsIce water
The invention discloses a method for preparing 3-aminopyrrolidine hydrochloride by using a one-pot method. Under an ice-water bathing condition, pyrrole is dissolved in acetonitrile, a catalyst di(triphenyl phosphine) cupric nitrate is added at the same time, nitric acid is added dropwise to conduct a reaction to generate reaction liquid containing 3-nitropyrrole, the reaction liquid is subjectedto extraction by water and ethyl acetate, and an organic phase is obtained after separation is conducted; the obtained organic phase is subjected to reduction by pumping in hydrogen gas under the palladium catalysis condition, and a crude product of 3-aminopyrrolidine is obtained; and a solvent ethyl acetate is added into the crude product of 3-aminopyrrolidine, HCl gas is pumped in to conduct a reaction, and 3-aminopyrrolidine hydrochloride is obtained. The experiment route of the method is simple and short, the raw materials are cheap and easy to get, and the reaction condition is mild.
Owner:NANJING YUANSHU MEDICAL TECH CO LTD
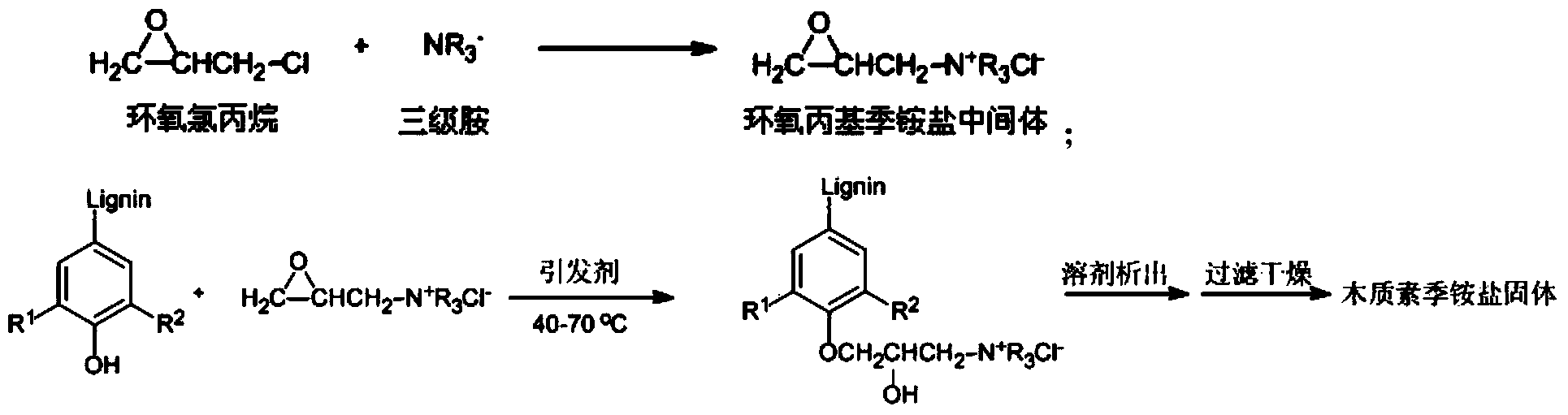
New method for preparing solid lignin quaternary ammonium salt and application of solid lignin quaternary ammonium salt
ActiveCN104059233AShort reaction pathMild reaction conditionsTobacco smoke filtersAmmoniumOrganic solvent
The invention discloses a new method for preparing solid lignin quaternary ammonium salt. Epichlorohydrin is added into tertiary amine, and a glycidyl quaternary ammonium salt intermediate is obtained after a reaction; on the condition that an initiating agent exists, the intermediate reacts with alkali lignin; then a reactant is added into a polarity organic solvent, and a precipitate is the solid lignin quaternary ammonium salt. According to the new method for preparing the solid lignin quaternary ammonium salt, the raw materials and reagents are easy to obtain, the reaction path is short, the reaction condition is moderate, and postprocessing is simple. The invention further discloses the application of the solid lignin quaternary ammonium salt obtained through the method.
Owner:CHINA TOBACCO JIANGSU INDAL
Preparation method of agricultural bactericide
InactiveCN104151233AShort reaction pathSimple and safe operationOrganic chemistryMeth-Agricultural science
Owner:ZHEJIANG TIDE CROP TECH
Preparation method of baricitinib
InactiveCN108129482ALow costAvoid participating in the reactionOrganic chemistryBulk chemical productionDiethyl phosphatePurification methods
The invention discloses a preparation method of baricitinib and belongs to the technical field of drug preparation. The method comprises that 4-chloropyrrolopyrimidine as a starting raw material is subjected to amino protection, the product, hydrazine hydrate and acrolein undergo a one-pot displacement reaction and a cyclization reaction to produce an intermediate 4, a starting raw material 1, 3-dibromoacetone and ethylene glycol undergo a condensation reaction to produce an intermediate 5, the intermediate 5 and ethyl sulfonamide undergo a condensation reaction to produce an intermediate 6, the intermediate 6 and diethyl cyanomethylphosphonate undergo a reaction under action of a strong base to produce an intermediate 7, the intermediate 4 and the intermediate 7 undergo an addition reaction under the action of a catalyst, and the product undergoes a deprotection reaction to produce a desired product 1. The preparation method needs mild reaction conditions. The intermediate purification method is simple and easy, has a total yield of 40-55% and is suitable for industrial production.
Owner:JIANGSU ZHONGBANG PHARMA
Method for selective synthesis of p-xylene from 4-methyl-3-cyclohexene-1-carbaldehyde
ActiveCN106883091AThe reaction process is simpleShort reaction pathHydrocarbonsBulk chemical productionBiomassTungsten
The invention relates to a method for selective preparation of p-xylene from 4-methyl-3-cyclohexene-1-carbaldehyde. Specifically speaking, the method comprises the following steps: dehydrogenation and aromatization as well as in-situ hydrogenation and deoxidation reactions of 4-methyl-3-cyclohexene-1-carbaldehyde are carried out in the effects of a tungsten-based catalyst at 250-450 DEG C, and p-xylene is prepared. The method can be carried out in a fixed bed reactor, a fluidized bed reactor or a moving-bed reactor separately, and reaction raw materials are directly injected into a reactor by an injection pump, or the reaction raw materials pass a catalyst bed layer with purging of carrier gas in order to obtain p-xylene. The process has the advantages of simple reaction process, and high selectivity of target product; the substrates can be obtained by Diels-Alder reaction from isoprene and acrolein which are derived from biomass resources and are used as raw materials, and mole yield of p-xylene reaches 90%. Compared with the prior art, the method has the advantages of cheap raw materials and wide sources, economy of reaction carbon atoms, and the like; the tungsten-based catalyst is cheap, and a new route for preparing p-xylene from biomass is provided.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthetic method for N-phosphonyl methyl imino diacetic acid
InactiveCN1944444AShort reaction pathEasy to getGroup 5/15 element organic compoundsOrganic chemistryGlyphosate toxicity
The present invention provides technological process of preparing PMIDA as the intermediate of glyphosate, and features that imino diacetonitrile is used as material in preparing PMIDA (N-phosphonyl methyl imino diaceteic acid).
Owner:重庆三峡英力化工有限公司
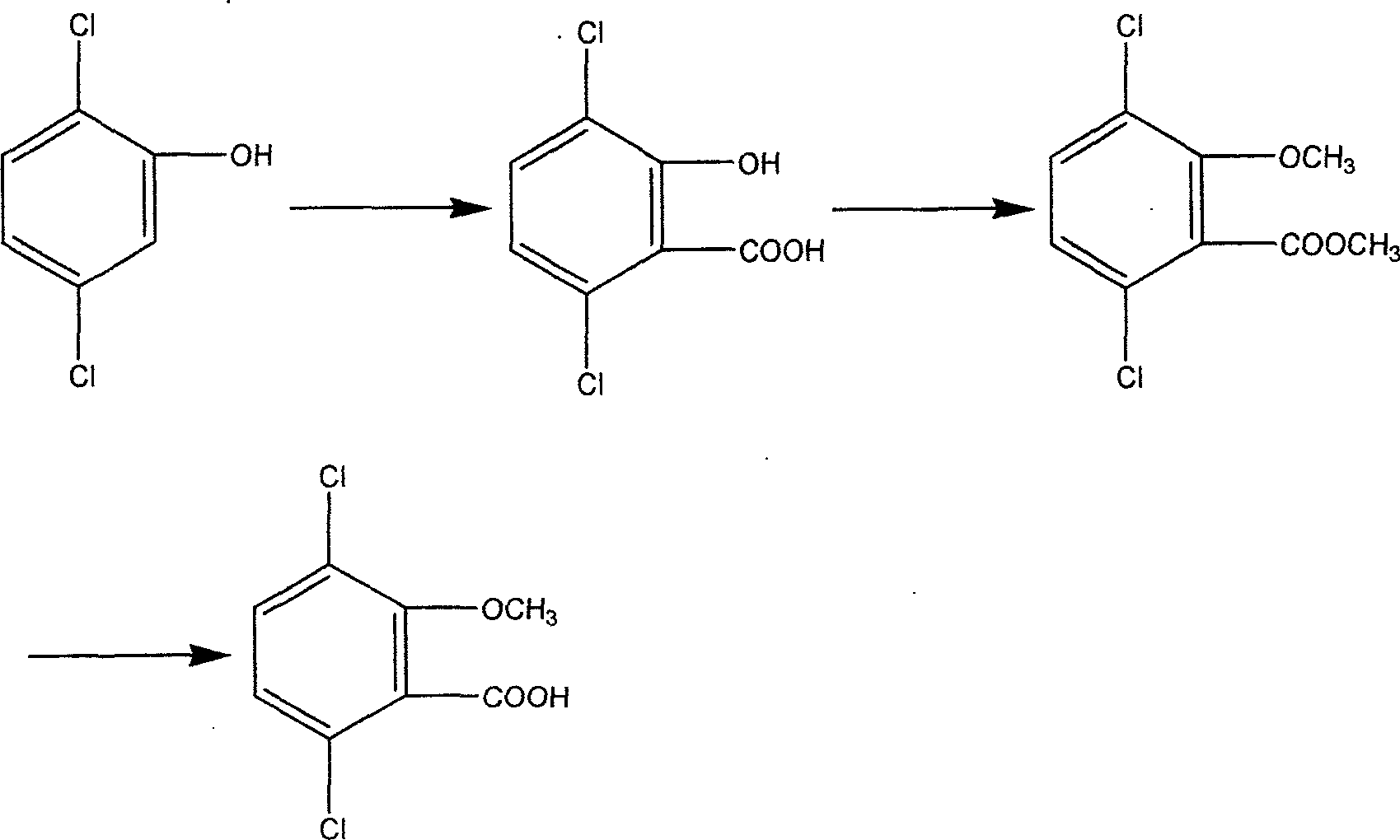
Process for catalyzing oxidating synthesizing 2,5-dichlorophenol
InactiveCN1793096AWill not remainImprove protectionOrganic chemistryOrganic compound preparationCatalytic oxidationDichlorophenol
The invention relates to a method to compound 2, 5-dichlorophenol by catalytic oxidation. The feature is that 1, 4-dichlorobenzene adopts heteropolyacid, heteropolyacid salt or the load of heteropolyacid as catalyst, uses H2O2 as oxidant to take catalytic oxidation reaction solution. After reacting, decreasing pressure and distilling, the 2, 5-dichlorophenol would be gained. The method has short reaction way, simple technology, and is benefit to environment protection.
Owner:SHENYANG RES INST OF CHEM IND +1
Preparation method of isoxazoline insecticide
ActiveCN112457267ACheap and easy to getShort reaction pathOrganic chemistryBulk chemical productionChemical industryPtru catalyst
The invention relates to a preparation method of an isoxazoline insecticide, which relates to the technical field of chemical industry, and comprises the following steps: 1) coupling reaction: mixingan intermediate 1 with nitromethane in a solvent, adding a palladium catalyst and a ligand to carry out catalytic coupling, reacting, and separating and purifying to obtain an intermediate 2; 2) cyclization reaction: mixing the intermediate 2 and an intermediate 3, adding the mixture into a mixed solution of a dehydrating agent and alkali, carrying out full cyclization reaction, and purifying to obtain an intermediate 4; and 3) condensation reaction: mixing the intermediate 4 and an intermediate 5, adding a condensing agent, carrying out full condensation reaction, and carrying out separationand purification to obtain an isoxazoline compound 6. The method has the advantages of short reaction route, accessible raw materials, fewer three wastes and high purity of the purified product, and is suitable for industrial production.
Owner:麦蒂辛生物医药科技成都有限公司
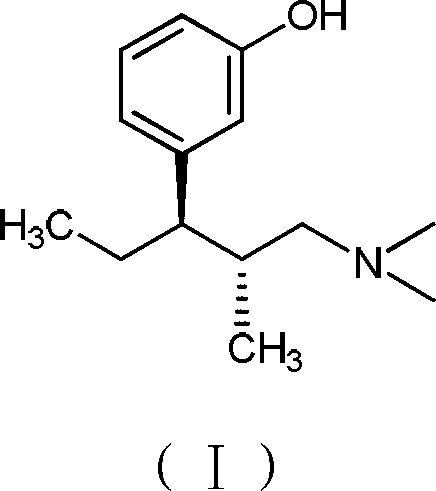
Preparation method of silodosin intermediate
ActiveCN104974073AShort reaction pathReduce manufacturing costOrganic chemistryPhotochemistrySilodosin
A method for preparing silodosin intermediate is disclosed. Said intermediate has the structure of (M), wherein R is benzyl or benzoyl. Said preparation method has the characteristics of short reaction route, simple operation, low cost, high yield, stable process, etc. The method is suitable for industrial production, and has extra-high industrial application value. Intermediate compounds for preparing the intermediate represented by M are also disclosed.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
Synthetic method of cefoxitin acid
The invention discloses a synthetic method of cefoxitin acid. Cephalotin acid used as a raw material reacts with prepared tert-butyl hypochlorite in the presence of sodium methylate to obtain a methoxy substance; the methoxy substance is subjected to a hydrolysis reaction and salt formation is carried out in the presence of benzathine to obtain methoxy cefalotin benzathine; methoxy cefalotin benzathine reacts with CSI, and hydrolysis is carried out to obtain a cefoxitin acid crude product; and the cefoxitin acid crude product undergoes the step of recrystallization to finally prepare cefoxitin acid. The synthetic method is simple to operate and is low-cost. By the synthetic method, quality and total yield reach 95.8%. The produced cefoxitin acid is a white solid powder, and purity of the product reaches more than 99.0%. The product has good quality and is suitable for industrial production.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
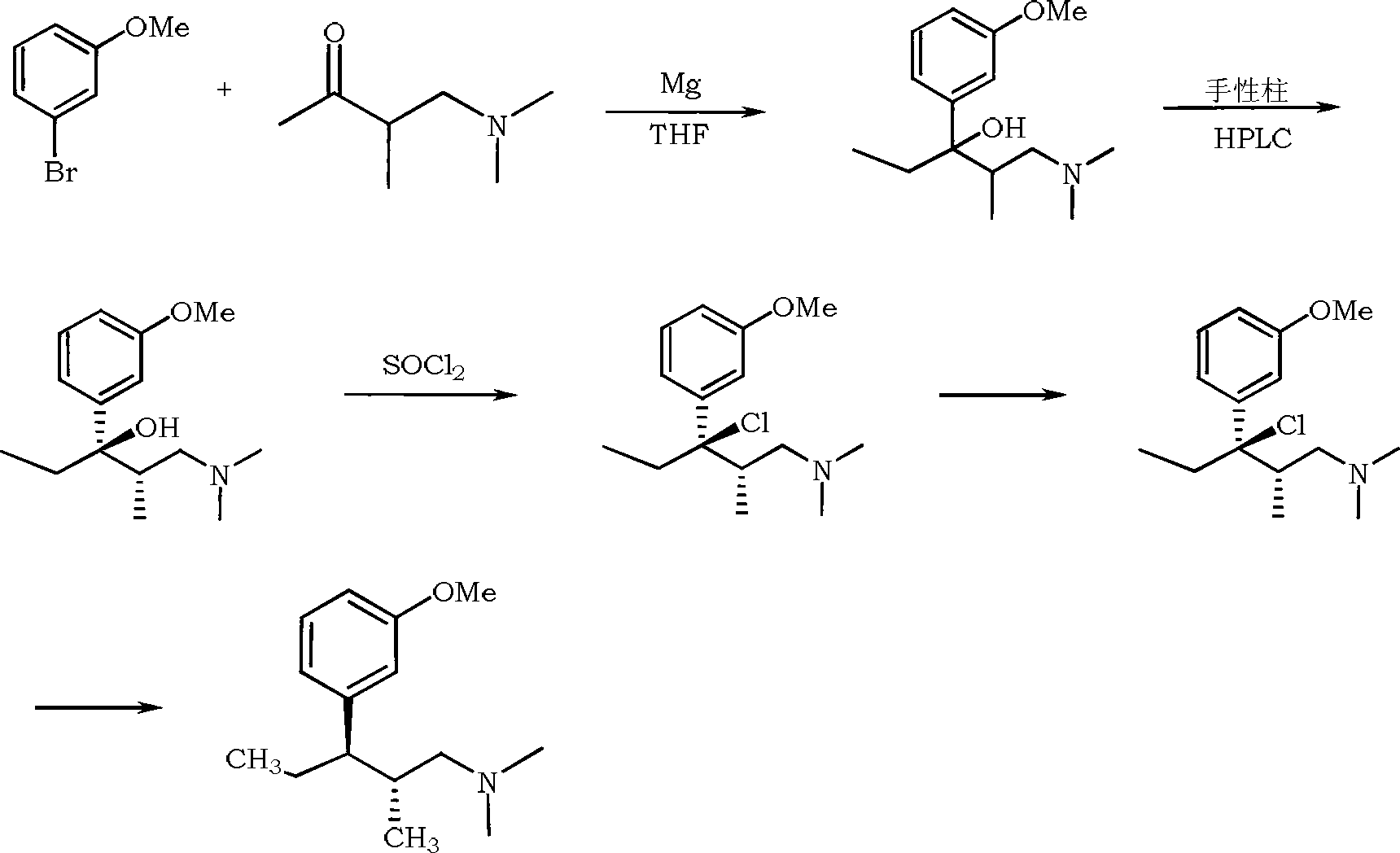
Synthesis method of tapentadol
ActiveCN102936205AEasy to operateOperation diversificationOrganic compound preparationAmino-hyroxy compound preparationTapentadol HydrochlorideMethyl group
The invention discloses a synthesis method of tapentadol. The method includes taking 1-dimethylamino-2-methyl-3-pentanone as a starting material, obtaining 3-bromine-N,N-2-trimethylpentyl-1-amine after reduction and halogenation reactions, then performing a coupling reaction with a metal reagent under the catalysis of transition metal to obtain 3-(3-methoxyphenyl)-N,N-2-trimethylpentyl-1-amine, and obtaining the tapentadol after demethylation and resolution. The synthesis method has the advantages that the raw materials are low in cost and easy to obtain, the steps are few, the operation is simple, and the cost can be effectively reduced; highly purified tapentadol can be obtained through the method, and the liquid phase purity and ee value of hydrochloric acid tapentadol obtained after salification can reach above 99%; and the method can be applied to the medical field.
Owner:HEFEI NEWSTAR PHARMA & CHEM
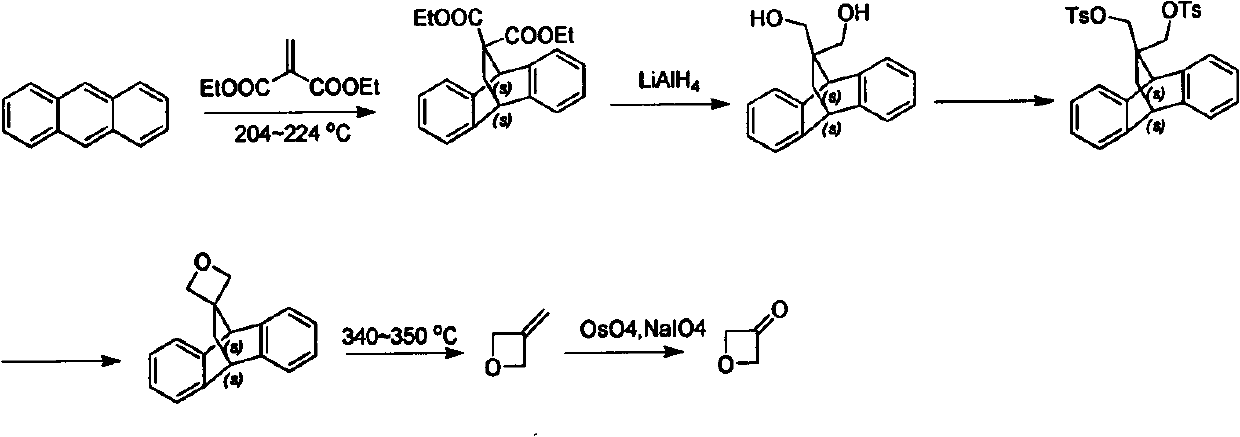
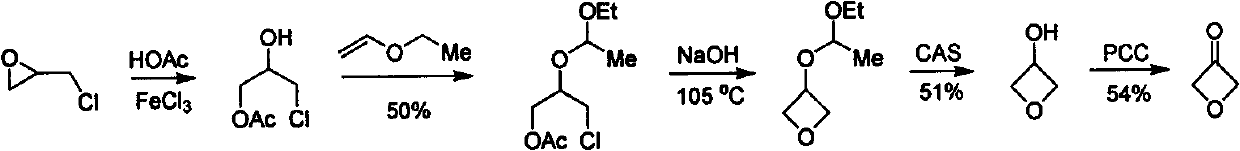
Novel method for synthesizing 3-oxetanone
The invention discloses a novel method for synthesizing 3-oxetanone. 1,3-dichloroacetone is taken as an initial raw material, a key intermediate is obtained through hydrolysis and ring-closing one-pot reaction, the yield is over 80 percent, the 3-oxetanone is obtained through deprotection, the total yield is over 50 percent, and the route is shown in the specification. The method has the advantages that: the use of a dangerous chemical reagent is avoided, the reaction route is shortened, the reaction cost is reduced, the reaction yield is improved, the method is easy to operate, and the industrialized production can be realized.
Owner:TIANJIN QUANHECHENG TECH
Popular searches
Ease of industrial production Simple preparation process Synthesis conditions are simple Raw materials are easy to obtain Simple and fast operation Suitable for industrial production Short synthetic route Reaction conditions are easy to control Suitable for mass industrial production Huge cost savings
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com