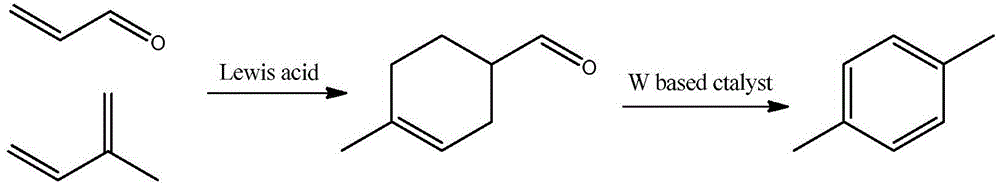
Method for selective synthesis of p-xylene from 4-methyl-3-cyclohexene-1-carbaldehyde
A technology of cyclohexene formaldehyde and p-xylene, which is applied in the direction of chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of high energy consumption and long process route, and achieve high selectivity and simple reaction process , the effect of short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: fill 30wt%W in tubular reactor (inner diameter 10mm) 2 C / AC solid catalyst 1.0g, heated to 400°C, flowing nitrogen as a carrier gas from the upper part of the reaction tube at a flow rate of 20ml / min, using N 2 The carrier gas was purged for 1 hour to remove moisture, and the obtained 4-methyl-3-cyclohexene carboxaldehyde was injected into the vaporization chamber at 1 ml / h, and supplied to the catalyst layer under the guidance of nitrogen gas. After reacting for 1 h, 792 mg of an organic layer was obtained from the collection container at the lower end of the reaction tube. Combining GC-MS to quantitatively calculate the conversion rate and p-xylene yield, the reaction results are listed in Table 1
Embodiment 2-5
[0041] Embodiment 2-5: the same condition as embodiment 1, the catalyst is replaced by 30wt%W 2 C / CB, W 2 C / ACF, W 2 C / SiO 2 , W 2 C / Al 2 o 3 , Other reaction conditions are identical with embodiment 1, calculate the conversion rate and yield of reaction, reaction result is listed in table 1.
[0042] Embodiment 6-11: the catalyst identical with embodiment 1, reaction temperature is respectively controlled as 250 DEG C, 275 DEG C, 300 DEG C, 325 DEG C, 350 DEG C, 375 DEG C, other reaction conditions are identical with embodiment 1, calculate the conversion of reaction Rate and yield, the reaction results are listed in Table 2.
Embodiment 12-17
[0043] Embodiment 12-17: the same condition as embodiment 1, will W 2 The load of C / AC is changed to 1wt%, 5wt%, 10wt%, 15wt%, 45wt%, 60wt%, other conditions are the same as Example 1, calculate the conversion rate and yield of reaction, the reaction result is listed in the table 3 in.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com