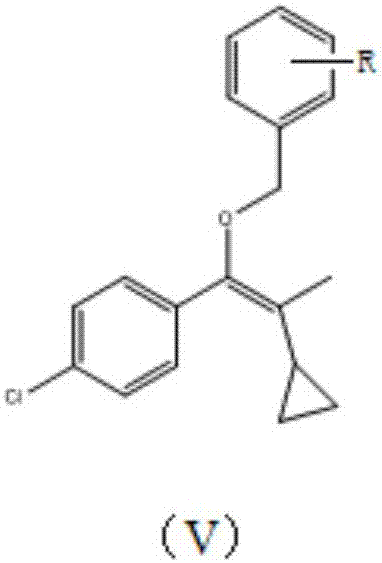
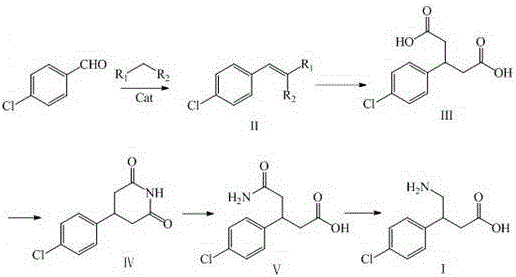
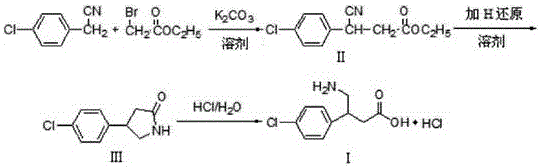
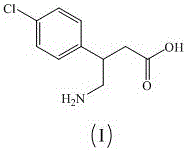
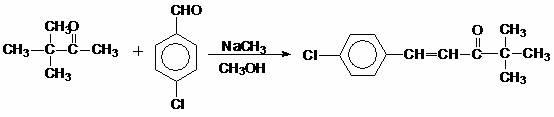Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
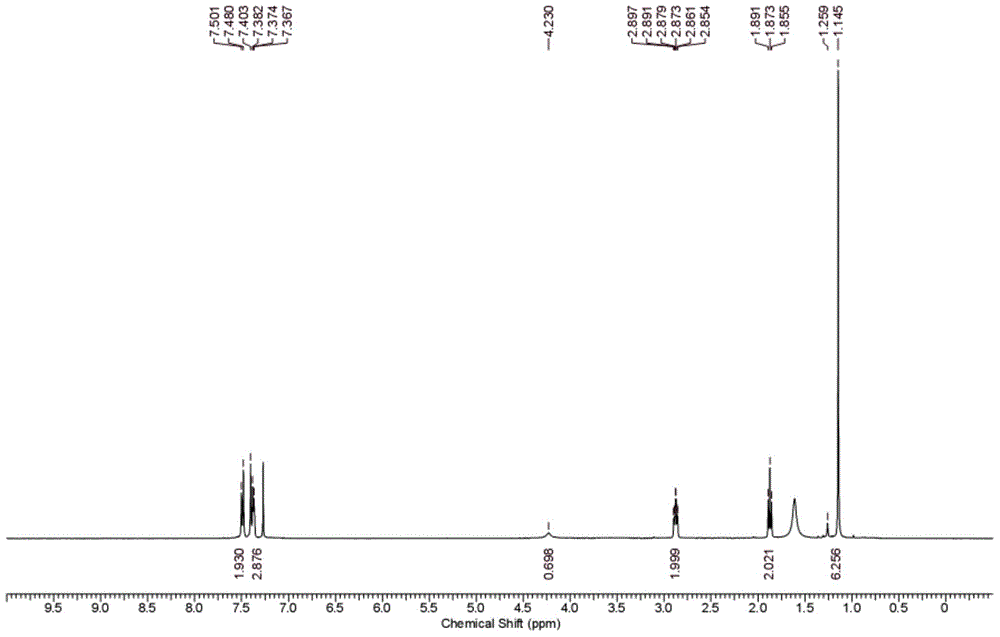
101 results about "P-chlorobenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
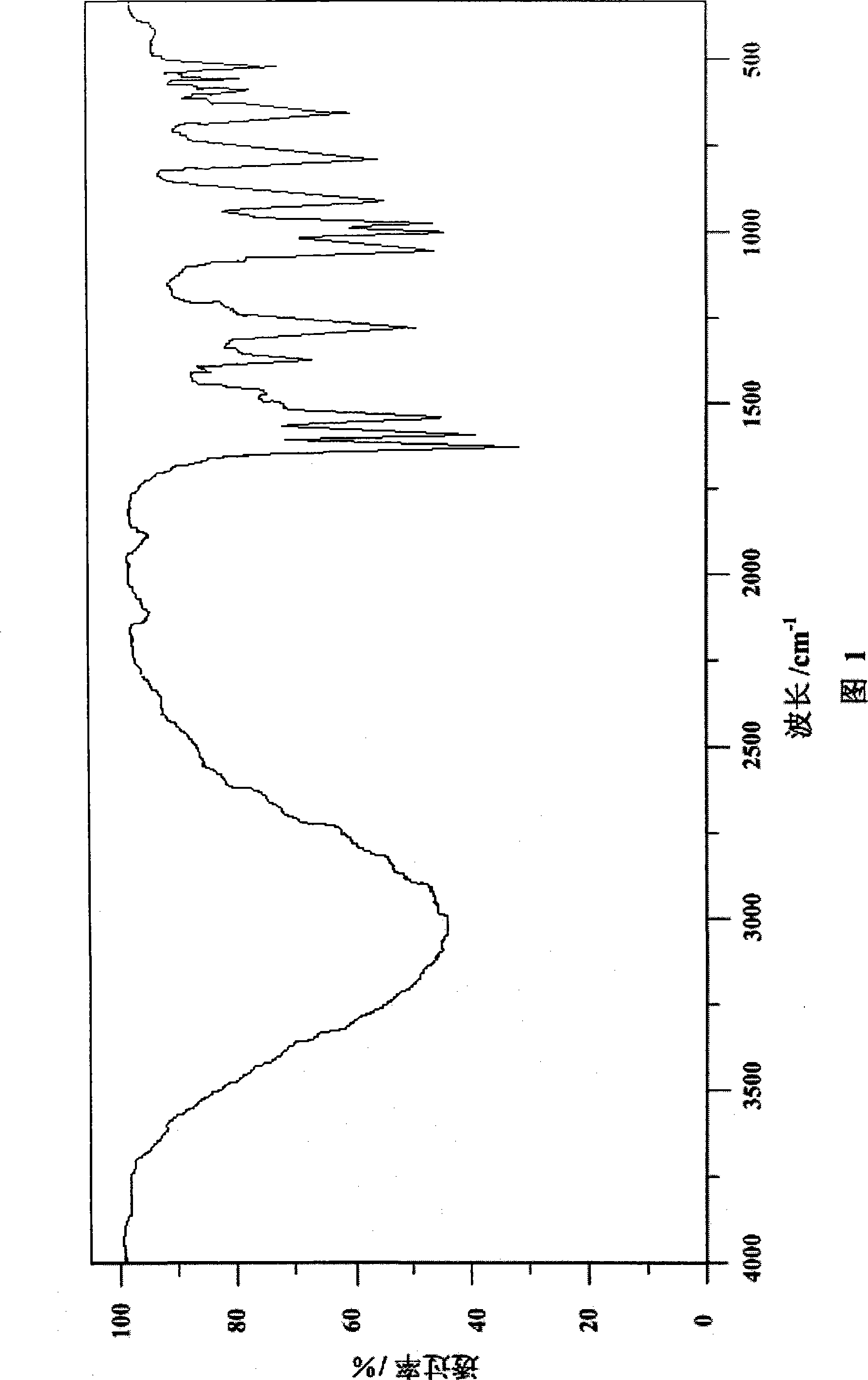
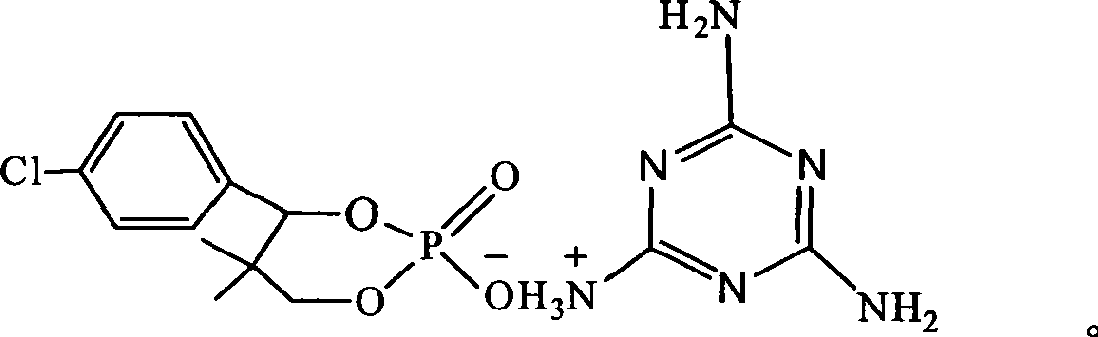
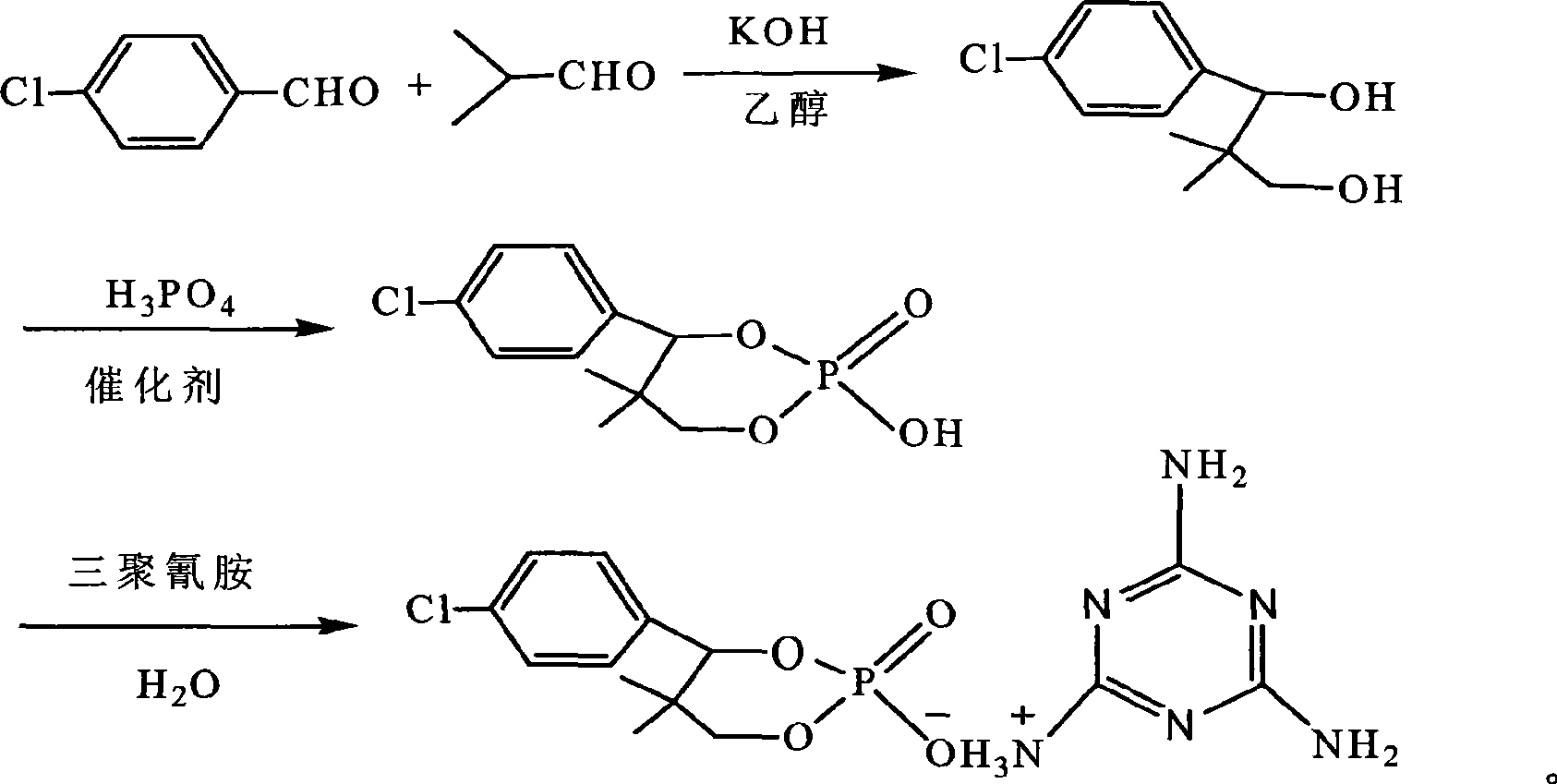
Phosphor-nitrogen expansion type combustion inhibitor and method of producing the same
InactiveCN101429438ALow viscosityReduce pollutionGroup 5/15 element organic compoundsBenzoic acidMelamine phosphate
The invention discloses a phosphorus-nitrogen swelling type fire retardant and a method for preparing the same. The chemical name of the fire retardant is 5, 5-dimethyl-4-p-chlorophenyl-1, 3, 2-dioxaphosphorinane phosphonate melamine salt. The preparation method comprises the following steps: (1) p-chlorobenzaldehyde and iso-butyraldehyde react with alkali ethanol solution, are cooled down and filtered to obtain an intermediate product (I); (2) the intermediate product (I) and phosphoric acid are added to solvent, and added with paratoluenesulfonic acid or benzoic acid or sodium salt of benzoic acid; and the mixing solution is distilled after reaction to reclaim the solvent to obtain an intermediate product(II); and (3) melamine and the intermediate product(II) are added to water for reaction, cooled down and filtered to obtain a filter cake; and the filter cake is dried to obtain phosphorus-nitrogen swelling type fire retardant. The phosphorus-nitrogen swelling type fire retardant has the advantages of high thermal stability, good compatibility, no halogen, less smoke and environmental protection. The preparation method has the advantages of materials available, simple process, mild reaction conditions, small viscosity of reaction liquid, low requirement on equipment and little environmental pollution.
Owner:SHANDONG TIANYI CHEM
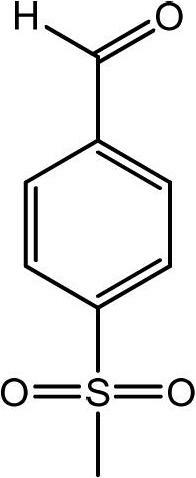
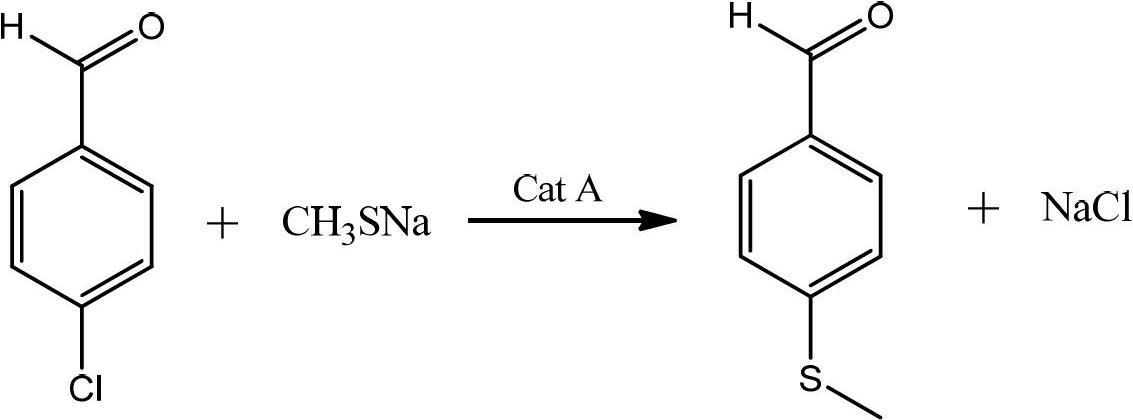
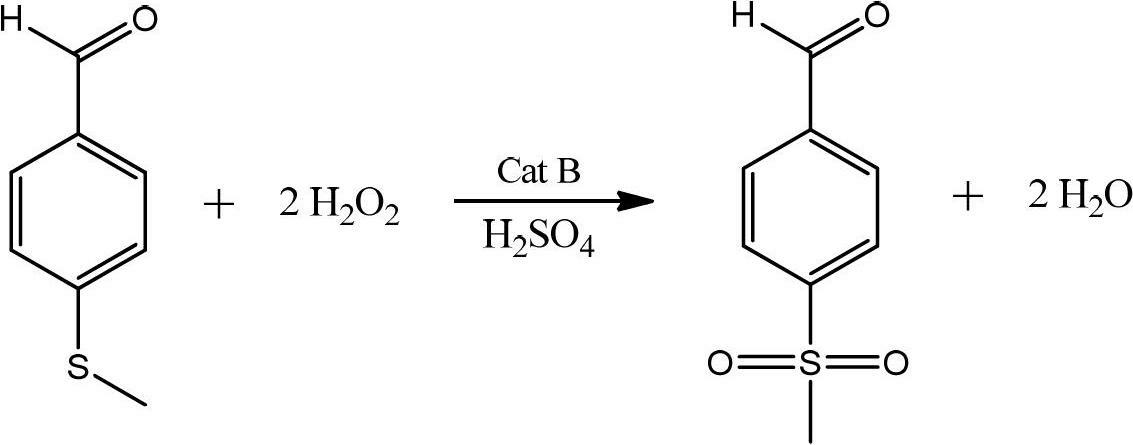
Preparation method of p-methylsulfonyl benzaldehyde
ActiveCN102675167AShort reaction pathShorten the production cycleOrganic chemistryOrganic compound preparationP-chlorobenzaldehydeBenzaldehyde
The invention discloses a preparation method of p-methylsulfonyl benzaldehyde, which takes p-chlorobenzaldehyde as starting material, and comprises the steps of: enabling the starting material to have reaction with sodium methyl mercaptide water solution under the action of phase transfer catalyst to obtain p-methylthio benzaldehyde; and oxidizing the p-methylthio benzaldehyde by hydrogen peroxide under the action of sulfuric acid and oxidation catalyst to obtain the p-methylsulfonyl benzaldehyde. The preparation method is easy in obtaining of raw material of the p-methylsulfonyl benzaldehyde, simple and convenient in operation, low in cost, high in yield and suitable for industrial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Preparation method of tebuconazole
ActiveCN103435564AReduce generationIncrease contentOrganic chemistryEthylene oxideHydrogenation reaction
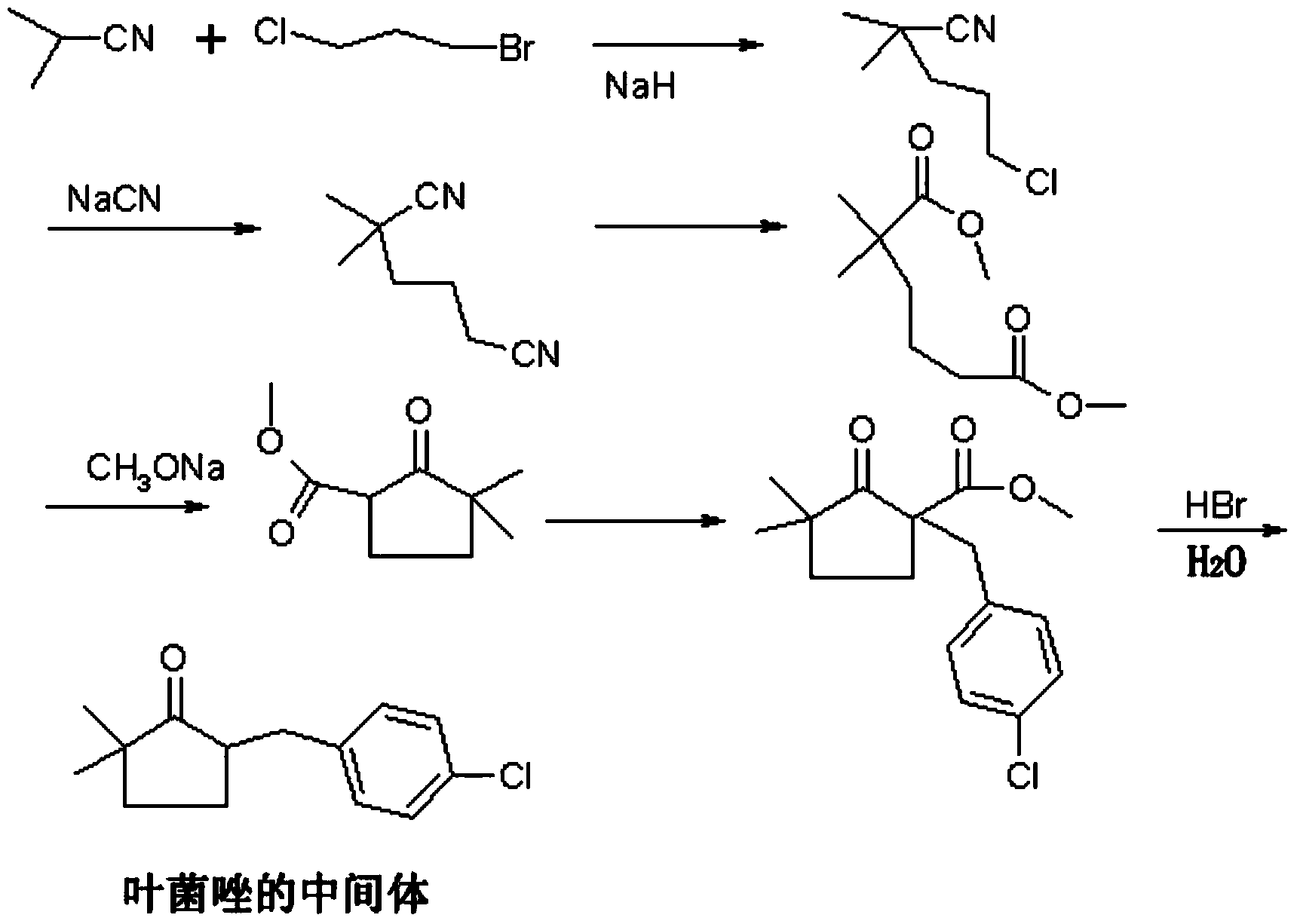
The invention discloses a preparation method of tebuconazole. The preparation method using p-chlorobenzaldehyde and pinacolone as initial raw materials comprises the following steps of: obtaining 2-(4-chlorobenzene ethyl)-2-tertiary butyl ethylene oxide through condensation reaction, hydrogenation reaction and epoxidation reaction in sequence; obtaining tebuconazole through ring-opening reaction of 2-(4-chlorobenzene ethyl)-2-tertiary butyl ethylene oxide and triazole under the co-catalysis of organic amine and crown ether. A catalysis technology of a composite catalyst which is composed of organic amine and crown ether is adopted, so that the isomer byproducts in the ring-opening reaction are greatly reduced, the ring-opening yield is improved to 92.8% from 80% of the conventional process, the product quality is improved to 98.5% or higher from 95%, and the total yield (in terms of p-chlorobenzaldehyde) is improved to 82% or higher from 65% of the conventional process.
Owner:SHANGYU NUTRICHEM +1
Catalyzer for liquid-phase catalytic oxidation producing of chlorobenzaldehyde by using p-chlorotoluene
InactiveCN101138729AHigh activityHigh selectivityOrganic compound preparationOxygen compounds preparation by hydrocarbon oxidationChlorobenzeneP-chlorobenzaldehyde
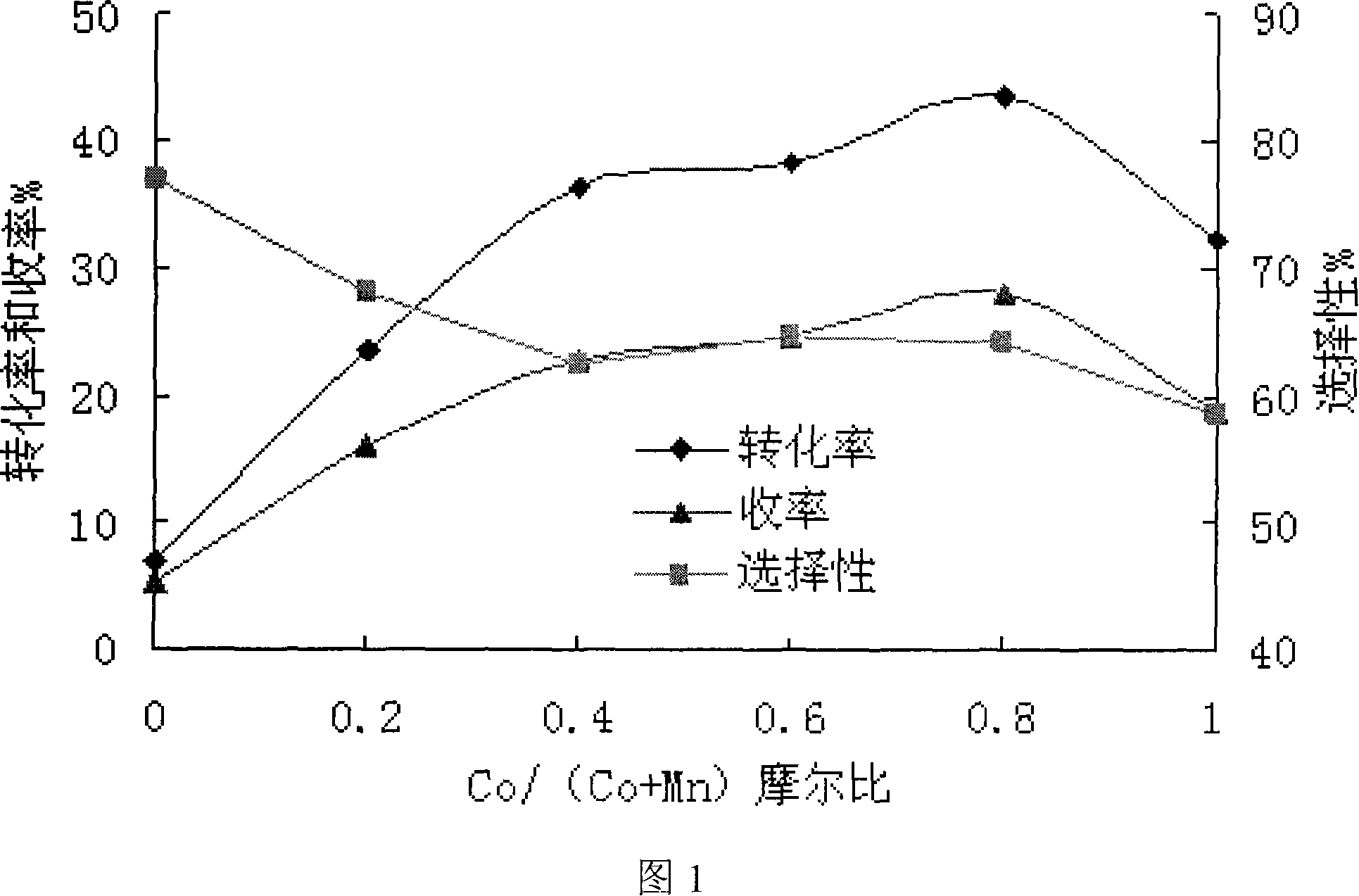
The present invention relates to a catalyst for preparation of p-chlorobenzaldehyde through liquid phase catalytic oxidation. The catalyst is a compound catalyst, which is formed by loading an element provided with catalytic activity on a carrier of aluminum oxide. Active componentS of the catalyst of the present invention are cobalt and manganese, which are loaded on the carrier of the aluminum oxide. The molar ratio of cobalt and manganese is 0.1-5. In addition, the active components of the present invention can also comprise nickel, copper, iron, cerium, lanthanum, magnesium or zirconium and bromine, besides cobalt and manganese. The catalyst of the present invention is applied the reaction course for preparation of p-chlorobenzaldehyde, and is characterized by the simple technique, the high p-chlorobenzaldehyde reaction yield ratio, the low reaction temperature, the simple catalyst separation, and so on.
Owner:天津佰腾生产力促进中心有限公司
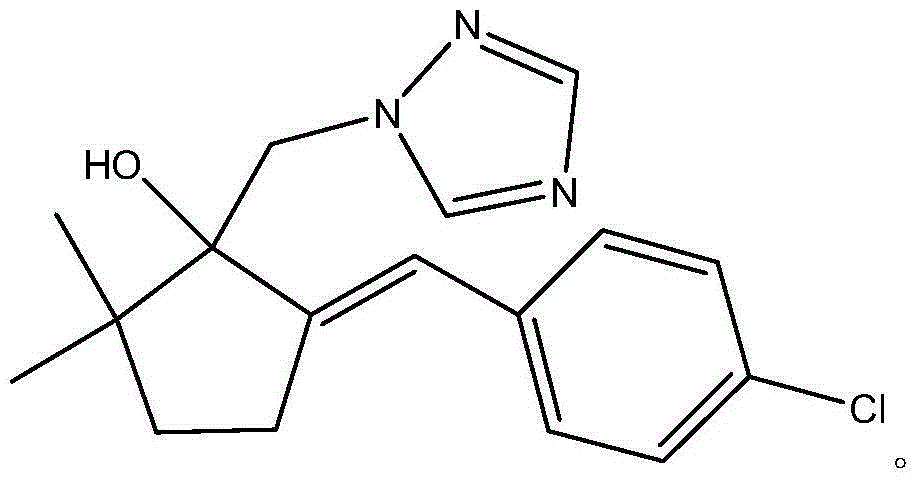
High purity metconazole and preparation method thereof
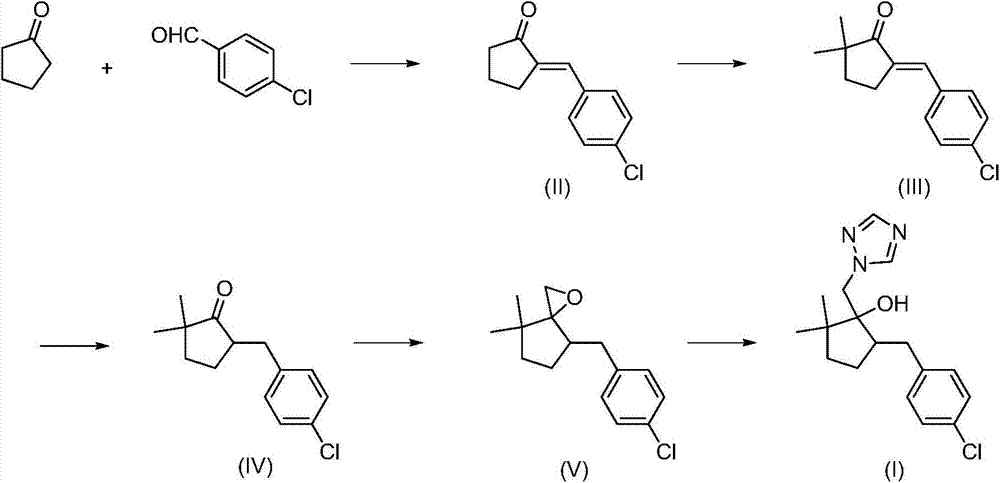
The invention discloses high purity metconazole and a preparation method thereof; the preparation is as follows: exocyclic double bond alpha, beta-unsaturated ketone (II) can be obtained by condensation reaction of cyclopentanone and p-chlorobenzaldehyde; then the exocyclic double bond alpha, beta-unsaturated ketone (II) is reacted with a methylation reagent to obtain alpha', alpha'-dimethyl substituted exocyclic double bond alpha, beta unsaturated ketone (III); then in the presence of a catalyst, the alpha', alpha'-dimethyl substituted exocyclic double bond alpha, beta unsaturated ketone (III) is reacted with hydrogen to obtain 2, 2-dimethyl-5-(4-chloro-benzyl) cyclopentanone (IV) with the double bond being reduced; an epoxypropane compound (V) is obtained by Johnson-Corey-Chaykovsky of the reduced product (IV); finally the epoxypropane compound (V) is reacted with 1, 2, 4 - triazole, and then the high purity metconazole (I) can be obtained by recrystallization. The preparing method has the advantages of chip and easy available raw materials, short route, good selectivity, high overall yield and good atom economy, and is very suitable for industrial production.
Owner:SHANGHAI JIAO TONG UNIV +1
Synthetic method of p-chlorobenzaldehyde
InactiveCN104447251AGood choiceHigh yieldCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationChlorobenzeneP-chlorobenzaldehyde
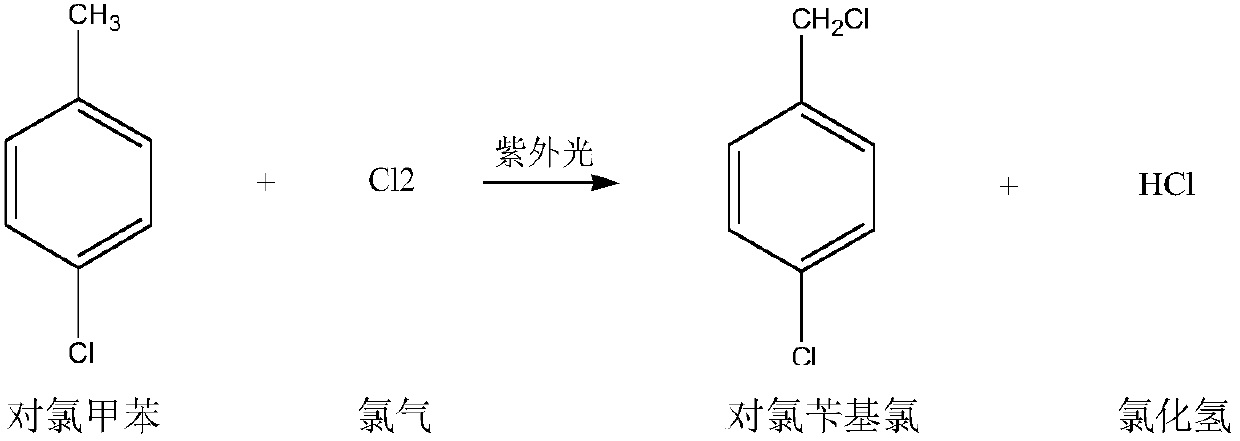
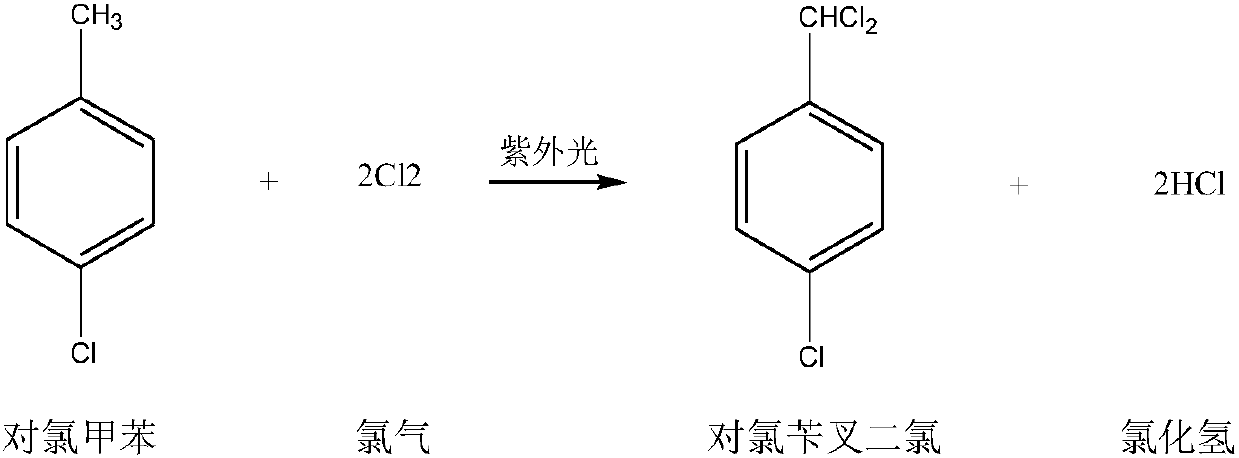
The invention discloses a synthetic method of p-chlorobenzaldehyde. The synthetic method comprises the following steps: performing illumination and chlorine reaction on p-chlorotoluene in the presence of a phosphorus trichloride initiator to generate p-chlorobenzal chloride, refining, and then performing catalytic hydrolysis for 6-12 hours to obtain p-chlorobenzaldehyde. The synthetic method of p-chlorobenzaldehyde, disclosed by the invention, is good in selectivity and high in yield.
Owner:CHANGSHU XINHUA CHEM
Method for preparing chlorophenylglycine
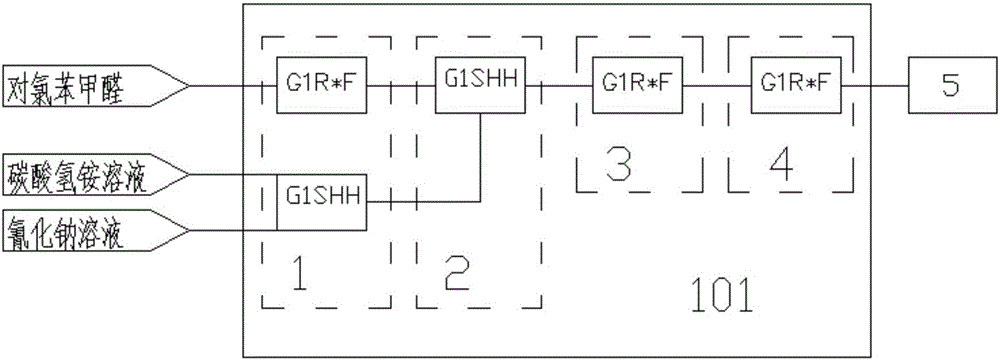
ActiveCN106083628AShort reaction timeReduce production processOrganic compound preparationAmino-carboxyl compound preparationP-chlorobenzaldehydeReaction speed
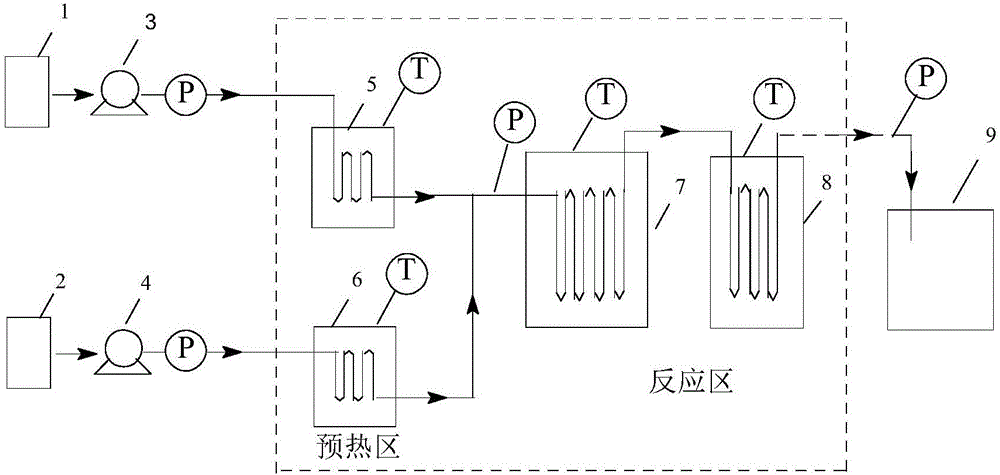
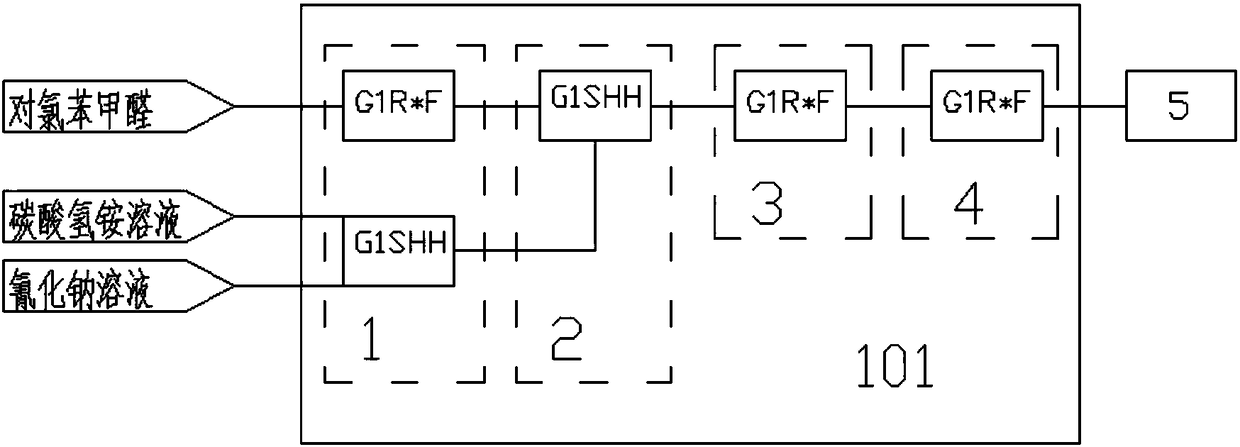
The invention relates to a method for preparing chlorophenylglycine. The method is characterized in that p-chlorobenzaldehyde, ammonium bicarbonate and sodium cyanide are subjected to a complete reaction in a micro-channel reactor, and a tubular-type reactor is used for preparing the chlorophenylglycine. According to the invention, the raw materials are subjected to cyclization in the micro-channel reactor through a flow counter to prepare an intermediate p-chlorophenylhydantoin, alkaline hydrolysis is carried out on the intermediate through the tubular-type reactor by the flow counter to prepare the chlorophenylglycine, the reaction is complete in 8-18 minutes, and the chlorophenylglycine is introduced into a reaction bottle and is subjected to acidifying crystallization. The product purity is 98.0% and above, and the yield is 95% and above. The method has the advantages of concise technical route and fast reaction speed, can increase the work efficiency and production capacity, and can ensure the production and operation security of the processes.
Owner:内蒙古诚信永安化工有限公司
Combined purification method of o/p-chlorobenzaldehyde rectification raffinate and process wastewater
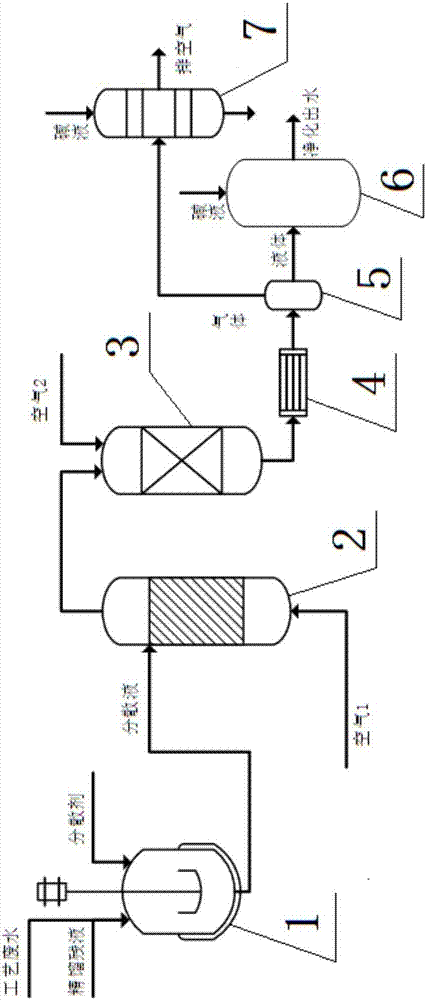
ActiveCN107010761AEasy to handleReduce energy consumptionWater treatment parameter controlGas treatmentPurification methodsFluidized bed
The invention discloses a combined purification method of o / p-chlorobenzaldehyde rectification raffinate and process wastewater. The method concretely comprises the following steps: adding a dispersant to the rectification raffinate and process wastewater to reduce the viscosity in order to form a dispersion; allowing the dispersion to enter a fluidized bed reactor and undergo catalytic cracking oxidation, and allowing the obtained dispersiont o enter a fixed bed reactor and undergo catalytic oxidation purification; carrying out heat transfer condensation and gas-liquid separation on the obtained fixed bed outlet gas, allowing the obtained liquid to enter a neutralization tank, neutralizing HCl and Cl2 dissolved in the liquid by a diluted alkali solution, discharging neutralized liquid to a rain discharging system, allowing the gas to enter an alkali solution absorption tower to absorb HCl and Cl2 in the gas, and directly discharging the absorbed gas to air. The method allows the rectification raffinate and process wastewater to be simultaneously treated at a low temperature through a raffinate emulsification-fluidized bed catalytic cracking oxidation-fixed bed catalytic oxidation purification technology, the content of VOCs in the treated gas is less than 45 mg / m<3>, the content of CODs in the liquid does not exceed 80 mg O2 / L, and the high-efficiency, low-cost and low-energy consumption treatment of the rectification raffinate and the process wastewater is realized in a one-step manner.
Owner:南京资环工程技术研究院有限公司
Preparation method of metconazole and intermediate thereof
ActiveCN103664561AIncrease contentHigh yieldOrganic compound preparationCarboxylic acid esters preparationP-chlorobenzaldehydePhotochemistry
The invention relates to a novel method for preparing metconazole. The method takes p-chlorobenzaldehyde and 2-methoxycarbonyl cyclopentanone represented by the formula (1) as the primary raw materials, then after a series of reactions, obtains an intermediate of metconazole, namely 5-(4-chlorobenzyl)-2,2-dimethyl cyclopentanone represented by the formula (5); then subjects the intermediate to reacts with trimethylsulfonium bromide so as to obtain a reaction product, and finally subjects the reaction product to react with 1,2,4-1H-triazole so as to obtain the metconazole. The preparation method has the advantages of simple technology, available raw materials, low cost, little discharge of waste water, waste gas, and waste solid, and high content and yield of target product metconazole, wherein the metconazole content can reach 95% or more, and the total yield can reach 65% or more, and is suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
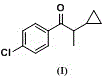
Process for preparing 1-(4-chlorphenyl)-4,4-dimethyl-3-pentanone
ActiveCN103242151AIncrease contentLess side effectsOrganic compound preparationCarbonyl compound preparationHydrogenP-chlorobenzaldehyde
The invention discloses a process for preparing 1-(4-chlorphenyl)-4,4-dimethyl-3-pentanone. The process is characterized by comprising the following steps: 1, preparing ketene, namely condensing p-chlorobenzaldehyde and pinacolin under the temperature of 70 DEG C by taking methanol as a solvent and taking sodium hydroxide as a catalyst to prepare the ketene; and 2, preparing pentanone, namely dissolving the ketene in methanol, adding the catalyst of sodium hydroxide, introducing hydrogen for hydrogenation under the temperature of 50-80 DEG C to prepare pentanone. The process has the beneficial effects that sodium hydroxide serves as the catalyst and methanol serves as the solvent in the ketene preparation process, so that the side reaction is effectively reduced, the content of the ketene is improved, and the reaction time is greatly shortened; and moreover, sodium hydroxide serves as the catalyst in the pentanone preparation process, so that the problems that the precious metal serves as the hydrogenation catalyst and the hydrogenation is incomplete in the prior art are effectively solved, the production cost is greatly reduced, and the hydrogenation conversion rate is over 99.2 percent.
Owner:内蒙古沙洲化学科技有限公司
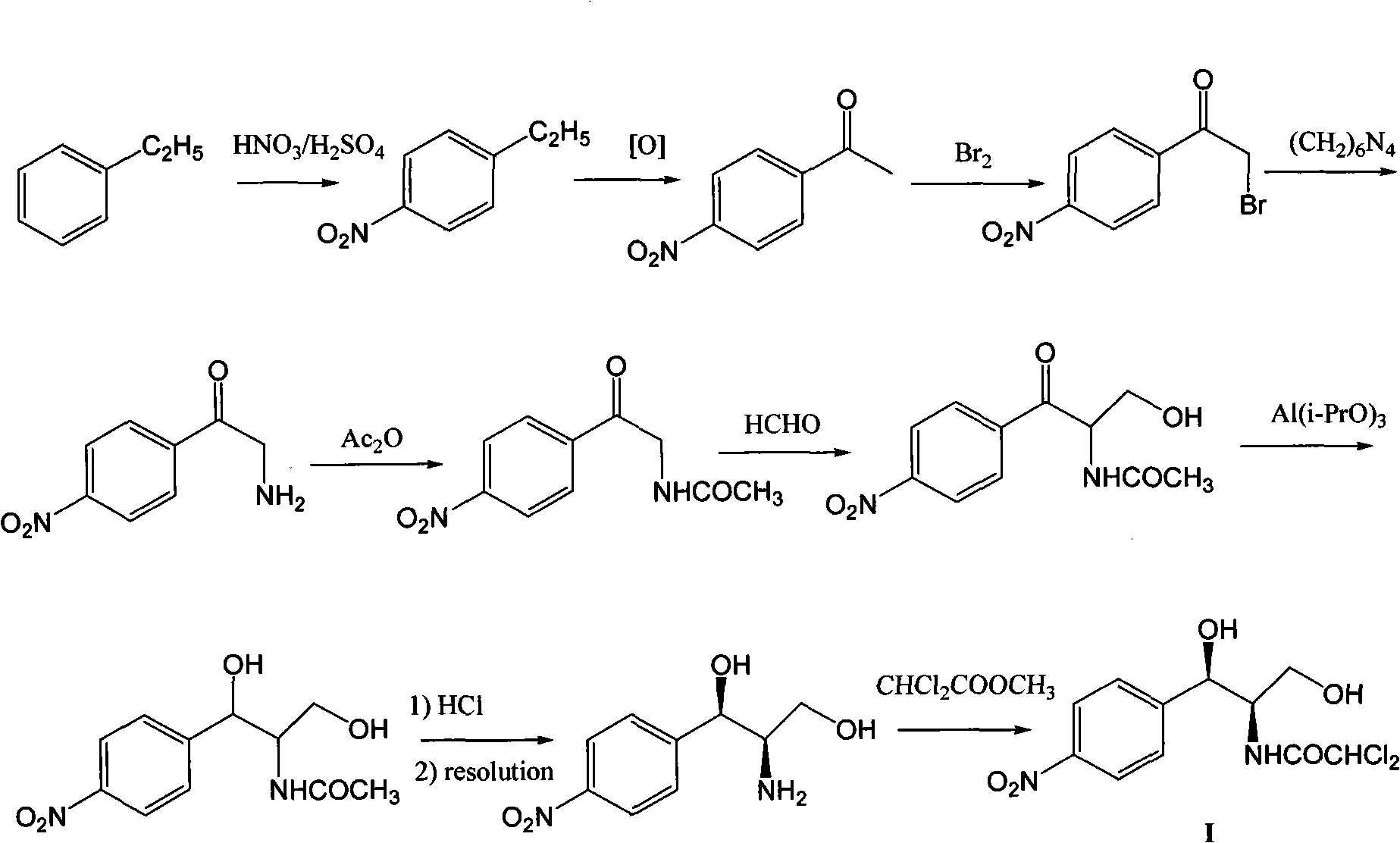
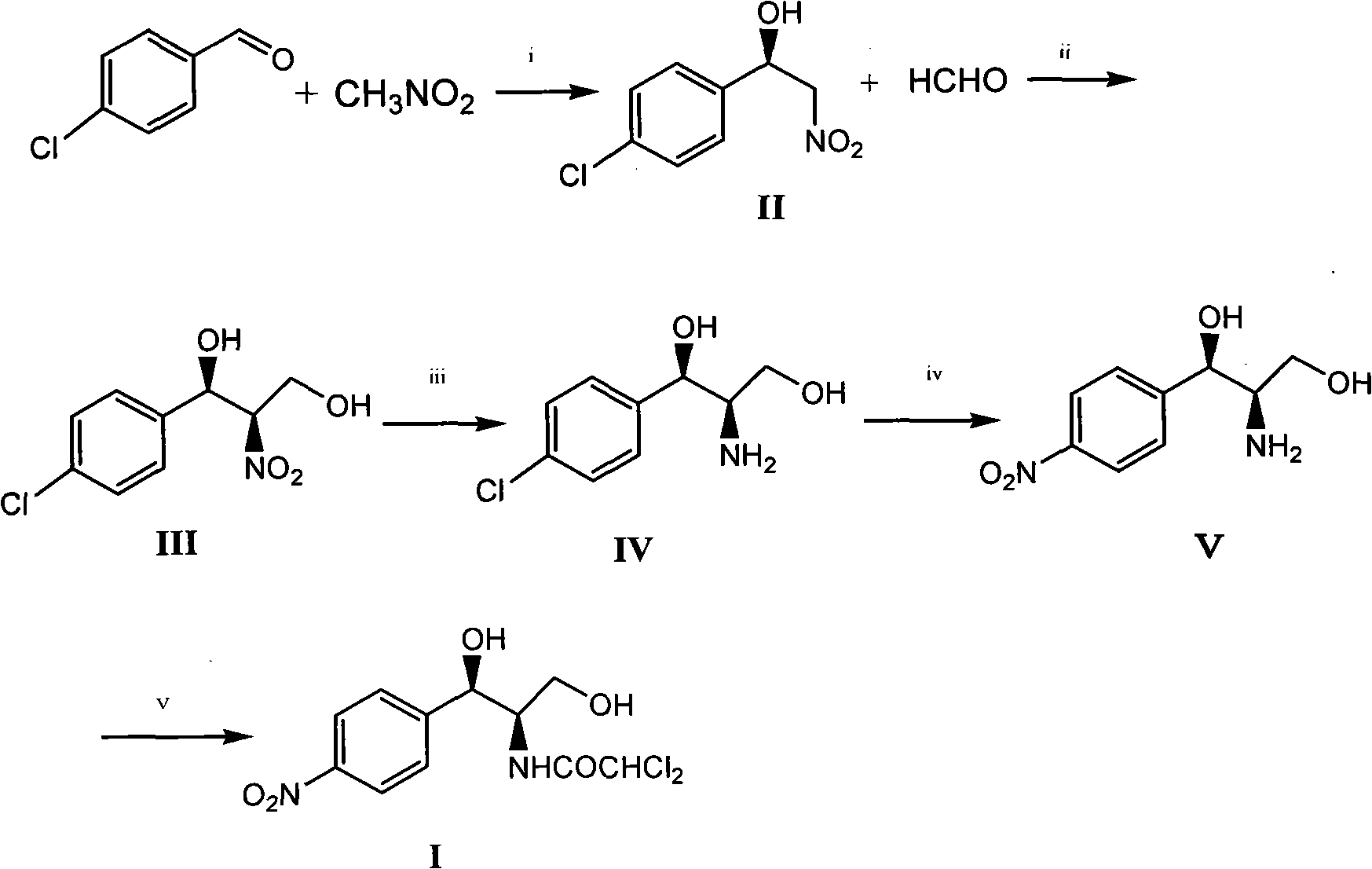
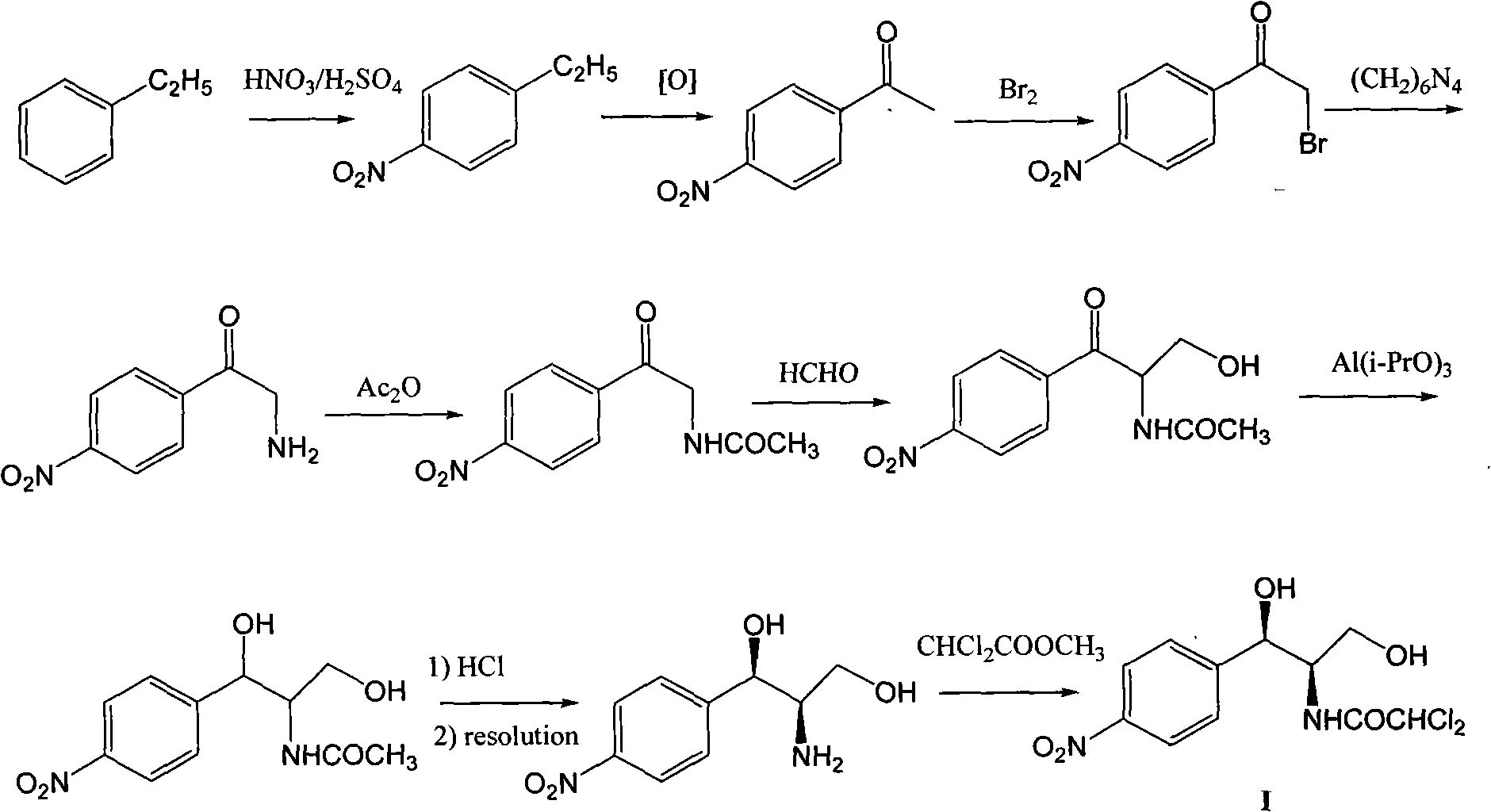
Method for synthesizing chloramphenicol from nitromethane
ActiveCN102399164ARaw materials are cheap and easy to getAvoid splittingOrganic compound preparationCarboxylic acid amides preparationP-chlorobenzaldehydeNitromethane
The invention relates to a method for synthesizing a broad spectrum antibiotic of chloramphenicol. The method comprises the following steps of: synthesizing (R)-2-nitro-1-(4-chlorophenyl)ethanol by using p-chlorobenzaldehyde and nitromethane as raw materials in the presence of a chiral catalyst; reacting with formaldehyde to obtain (1R,2R)-2-nitro-1-(4-chlorophenyl)-1,3-propanediol, and performing catalytic hydrogenation to obtain (1R,2R)-2-amino-1-(4-chlorophenyl)-1,3-propanediol; and performing nitro substitution and dichloro acetylization on the intermediate to obtain the chloramphenicol. By the method, the common chiral resolution and aluminum isopropoxide reduction in the industry at present can be avoided, three wastes are reduced, the raw materials and reagents are cheap and readily available, the method comprises a few synthesizing steps, the yield is high, and the method is more suitable for industrial production.
Owner:WUHAN WUYAO SCI & TECH
Preparation method for p-chlorobenzaldehyde
ActiveCN109651111ALower the temperature of the substitution reactionHigh selectivityCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationBenzeneP-chlorobenzaldehyde
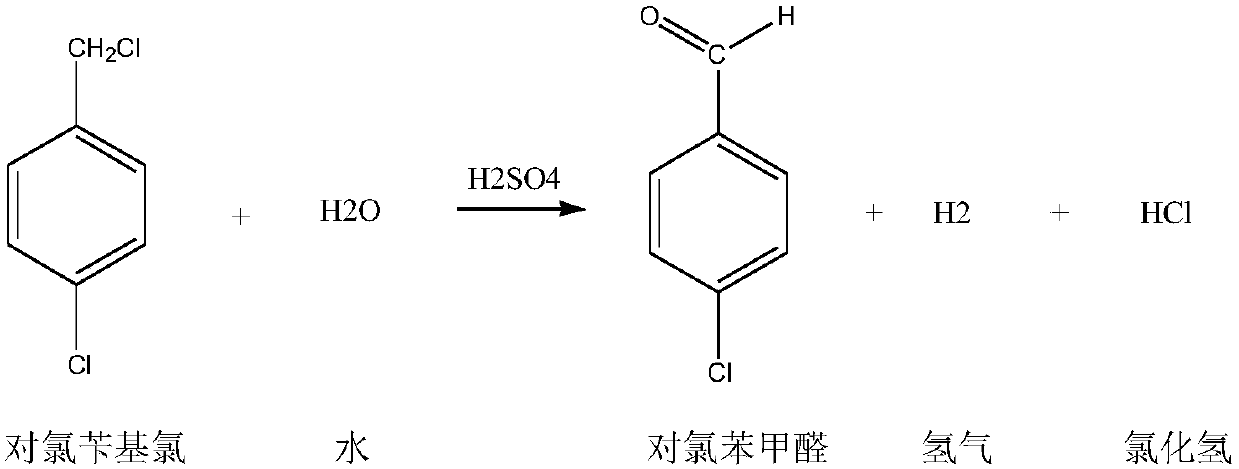
The invention discloses a preparation method for p-chlorobenzaldehyde. P-chlorotoluene can be taken as a raw material to perform substitution reaction with chlorine under the action of a catalyst so that a mixture of 4-chlorobenzyl chloride and 4-chloro-1-(dichloromethyl)benzene; and p-chlorobenzaldehyde can be obtained by the mixture of the 4-chlorobenzyl chloride and the 4-chloro-1-(dichloromethyl)benzene under the action of the catalyst through hydrolysis reaction and air catalytic oxidation reaction. Through the action of the catalyst, the reaction temperature of the substitution reactioncan be reduced, and the selectivity of the substitution reaction can be enhanced; the yield of the p-chlorobenzaldehyde can be enhanced by adopting a mode of combining the hydrolysis reaction and aircatalytic oxidation reaction; and the wastewater quantity of the hydrolysis reaction can be reduced through the mode of applying the catalyst.
Owner:烟台舜康生物科技有限公司
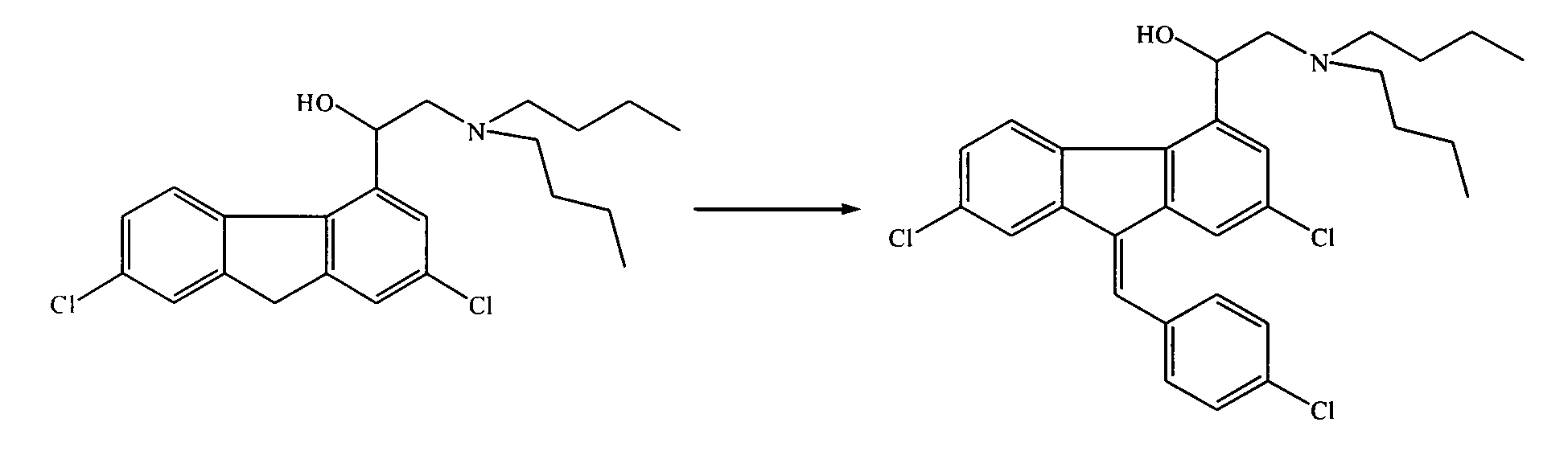
One-step green synthesis process of antimalarial raw material benflumetol
InactiveCN103319356ASimple reaction systemSimple control processOrganic compound preparationAmino-hyroxy compound preparationRefluxP-chlorobenzaldehyde
The invention discloses a semisynthesis process for producing benflumetol by taking alpha (di-n-butylamide)-2,7-dichloro-4-fluorenemethanol as a raw material. The process comprises the following steps: dispersing alpha (di-n-butylamide)-2,7-dichloro-4-fluorenemethanol in a mixed solvent, carrying out alkali catalysis and heating reflux on the obtained mixture, and carrying out condensation reaction on the obtained object and p-chlorobenzaldehyde, reducing the temperature to room temperature, and continuing to stir the obtained product so as to separate out coarse benflumetol grains; carrying out recrystallization on the coarse benflumetol grains by using acetone crystallization so as to obtain a fine benflumetol product with a content and a purity of more than 99.0%.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Method for preparing p-chlorobenzaldehyde
InactiveCN104557492ASimple processEase of industrial productionCarbonyl compound preparation by hydrolysisChlorobenzeneP-chlorobenzaldehyde
The invention discloses a method for preparing p-chlorobenzaldehyde, which comprises the following steps: reacting p-chlorotoluene with chlorine in the presence of an initiator under the irradiation of a mercury lamp at the chlorination temperature of 90-140 DEG C to obtain a p-chlorobenzyl chloride / p-trichlorotoluene mixture; and while controlling the average chlorination degree at 1.4-1.6, carrying out catalytic hydrolysis in a 3-6 wt% nitric acid solution for 6-12 hours to obtain the p-chlorobenzaldehyde. By using the method disclosed by the invention, the next hydrolysis step can be directly carried out without separating the intermediate; and thus, the method is simple in technique and can easily implement industrialized production.
Owner:CHANGSHU XINHUA CHEM
7-(4-chlorphenyl)-5,6-dihydro-7ah-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone and synthetic method thereof
The invention provides 7-(4-chlorphenyl)-5,6-dihydro-7aH-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone and a synthetic method thereof. The method comprises the following steps of: uniformly mixing 3-amino-1,2,4-triazole, p-chlorobenzaldehyde, 2-hydroxyl-1,4-naphthaquinone and p-methylbenzene sulfonic acid with a usage amount of catalyst, heating and stirring, controlling the temperature at 110-125 DEG C and reacting for 2.5-3 hours. The 7-(4-chlorphenyl)5,6-dihydro-7aH-benzo[h]1,2,4-triazolo[3,4-b]quinazoline-5,6-diketone prepared according to the invention introduces chlorine atoms, thus having stronger activity and being more beneficial to absorption. The method provided by the invention is green and environment-friendly, and has the characteristics that the raw materials are available, the operation is simple and the yield is high.
Owner:XINXIANG MEDICAL UNIV
Preparation method of p-chlorophenylglycine
InactiveCN111470994AAvoid poisoningAvoid pollutionOrganic compound preparationCatalystsChlorobenzenePtru catalyst
The invention provides a preparation method of p-chlorophenylglycine, which comprises the following steps: adding a mixed solution of a chloroform solution, a catalyst and p-chlorobenzaldehyde into asodium hydroxide solution, mixing, and dropwisely adding liquid ammonia while stirring for 2-6 hours; after finishing dropwise adding the liquid ammonia, adding an ammonium bicarbonate solution, and reacting for 5-10 hours at room temperature; after the reaction is finished, distilling and concentrating a reaction solution, decolorizing and filtering by using activated carbon, adjusting the pH value of a filtrate to 6.0 by using an inorganic acid, cooling and filtering to obtain a crude product and mother liquor, washing the crude product by using water, ethanol and diethyl ether, and drying to obtain a finished product p-chlorophenylglycine; wherein the catalyst is trioctyl methyl ammonium chloride; wherein the molar ratio of the p-chlorobenzaldehyde to the trioctyl methyl ammonium chloride to the chloroform to the sodium hydroxide to the ammonium bicarbonate is 1.0 : (0.02-0.38) : (1.5-2.5) : (6.0-10.0) : (0.03-0.07). The synthesis process is simple, raw materials are easy to obtain,the reaction is milder, and toxicity and pollution of cyanide are avoided.
Owner:上海开荣化工科技有限公司
Synthetic method of 1-(4-chlorophenyl)-2-cyclopropyl-1-acetone
InactiveCN106187726AReduce usageShort reaction stepsOrganic compound preparationGroup 5/15 element organic compoundsOrganic solventP-chlorobenzaldehyde
The invention relates to a synthetic method of 1-(4-chlorophenyl)-2-cyclopropyl-1-acetone. The synthetic method comprises the following specific steps: (1) dissolving p-chlorobenzaldehyde in an organic solvent, adding dialkyl phosphate, adding base at a certain temperature to carry out a catalytic reaction for a period of time; (2) continuously adding base after the catalytic reaction, dropwise adding cyclopropyl methyl ketone within a certain temperature range, reacting for a period of time, postprocessing, and distilling to obtain high-purity 1-(4-chlorophenyl)-2-cyclopropyl-1-acetone. The reaction equation is as shown in the specification. The method has the following advantages: the reaction steps are short; utilization rate of equipment is high; application amount of a solvent is small; production cost is low; the method is safe and environment friendly and is convenient to operate; the postprocessing mode is simple; purification is easy; and through distillation, 1-(4-chlorophenyl)-2-cyclopropyl-1-acetone with content being 97% and above is obtained.
Owner:YANCHENEG HUIHUANG CHEM
Synthetic method of 4-fluorobenzaldehyde
InactiveCN101353297ALow priceShort synthetic routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSulfolaneHexamethylphosphoramide
The invention relates to a synthetic method of p-flurobenzaldehyde, which belongs to the chemical and pharmaceutical field. In the synthetic method, the p-chlorobenzaldehyde and potassium fluoride react under the condition of a solvent and a catalyst at high temperature, wherein, the solvent is one of sulfolane, dimethyl sulfoxide, dimethylformamide, dimethylacetamide, hexamethyl phosphoramide, nitrobenzene and ortho-nitrotoluene; the catalyst is one of or the mixture of two or more than two of benzyl triethyl amine chloride, tetrabutylammonium bromide, hexadecyltrimethylammonium chloride, methyltrioctylammonium chloride, tetraphenylphosphonium bromide, methyltriphenylphosphonium bromide, benzyl triphenyl phosphonium bromide and polyethylene glycol dimethyl ether. Compared with the conventional preparation method, the synthetic method of the invention has the advantages of cheap raw materials, short synthetic route and little 'three wastes' discharge. As the amount of the catalyst used is reduced and the price of the solvent is low, the production cost of the p-flurobenzaldehyde is decreased.
Owner:王俊华
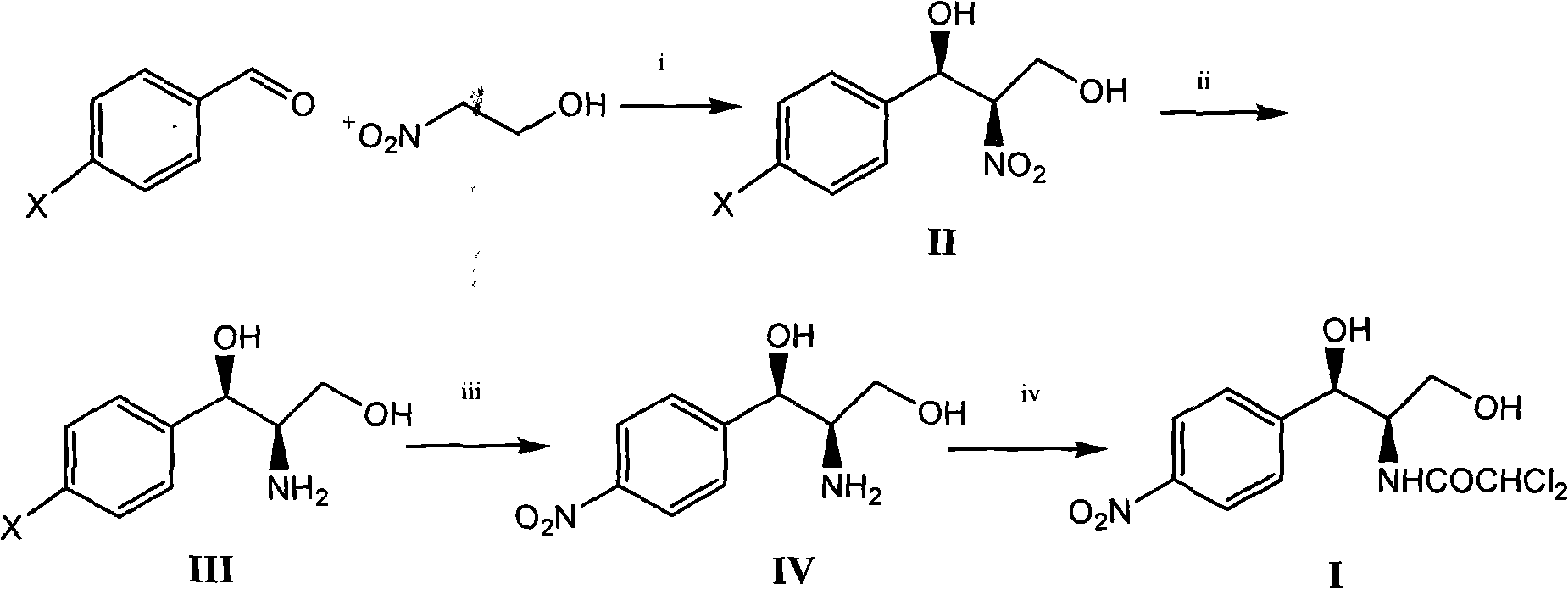
Method for preparing chloramphenicol from 4-chloro-benzaldehyde
ActiveCN102399163AHigh purityHigh yieldOrganic compound preparationCarboxylic acid amides preparationBenzaldehydeP-chlorobenzaldehyde
The invention relates to a method for preparing a broad spectrum antibiotic of chloramphenicol. The method comprises the following steps of: synthesizing (1R,2R)-2-nitro-1-(4-chlorophenyl)-1,3-propanediol by using 4-chlorobenzaldehyde and 2-nitroethylalcohol as raw materials in the presence of a chiral catalyst, and performing catalytic hydrogenation to obtain (1R,2R)-2-amino-1-(4-chlorophenyl)-1,3-propanediol; and performing nitro substitution and dichloro acetylization on the intermediate to obtain the chloramphenicol. By the method, the common chiral resolution and aluminum isopropoxide reduction in the industry at present can be avoided, three wastes are reduced, the raw materials and reagents are cheap and readily available, p-chlorobenzaldehyde is selected as a raw material, the method comprises a few synthesizing steps, the yield is high, and the method is suitable for industrial production.
Owner:GRAND PHARM (CHINA) CO LTD
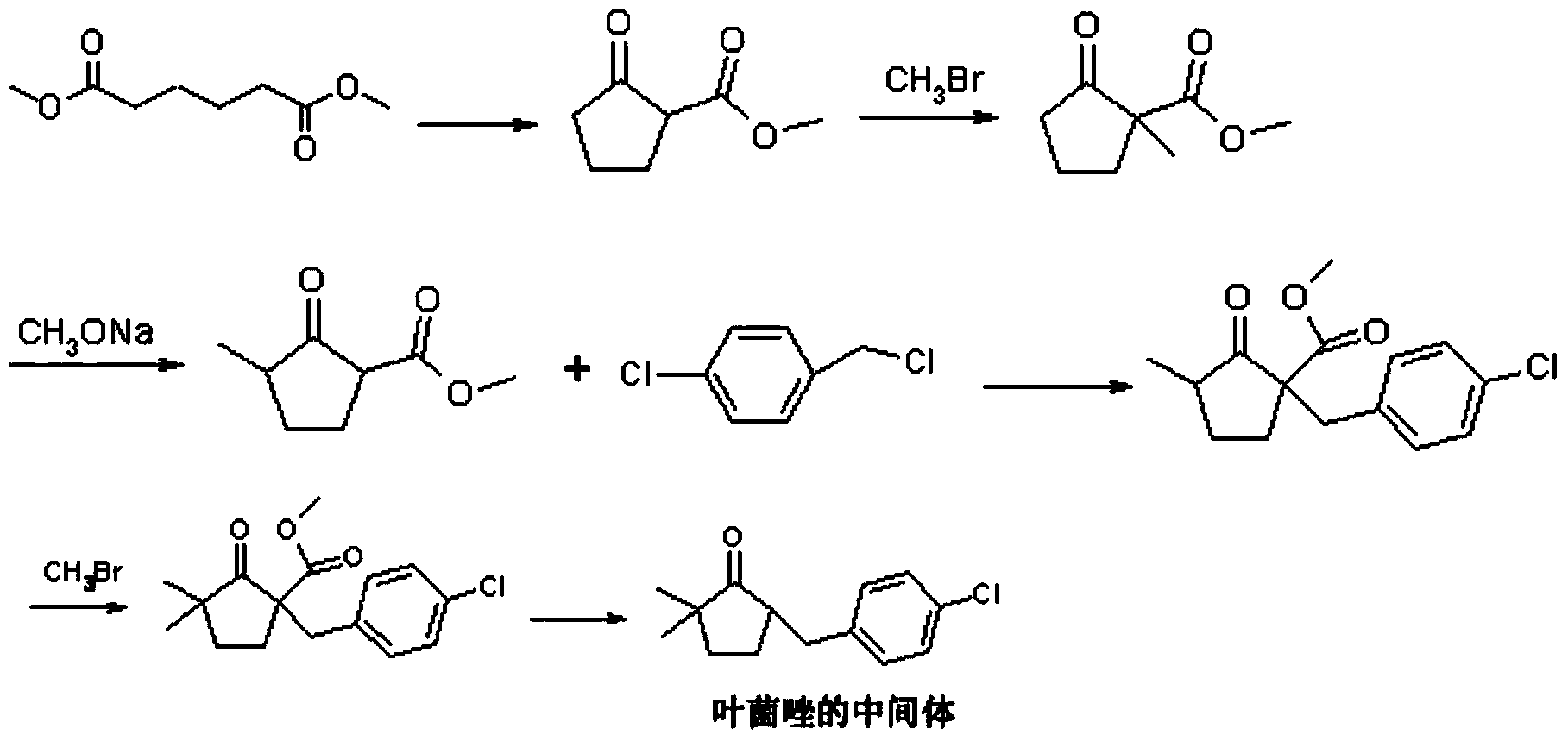
Preparation method of Metconazole
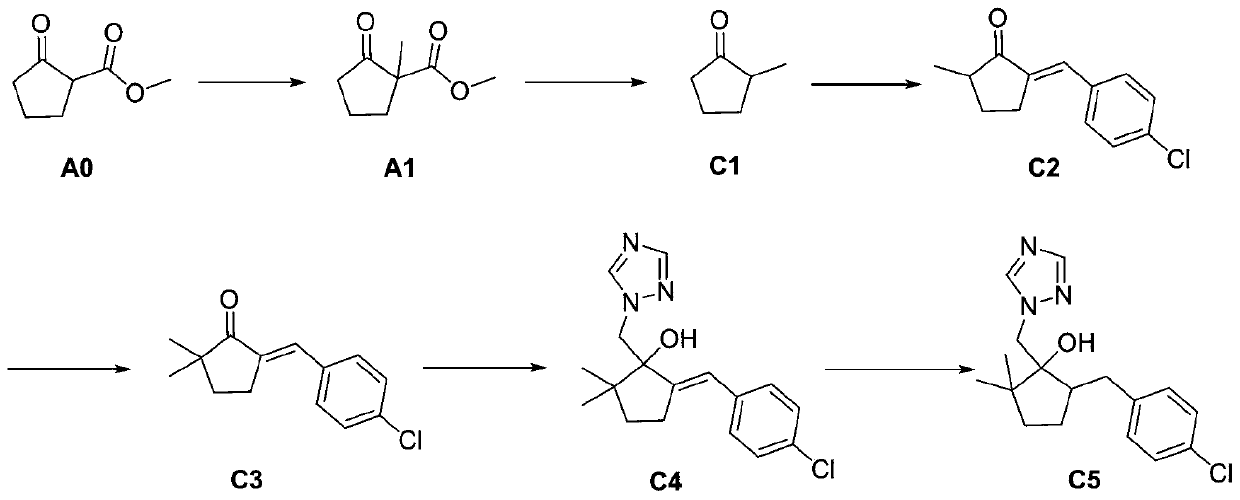
InactiveCN110204500AImprove conversion rateHigh yieldOrganic chemistryP-chlorobenzaldehydeReaction step
The invention discloses a preparation method of Metconazole, and the method comprises the following steps: carrying out water decarboxylation on a methylated product obtained by methylation of 2-methoxy acyl cyclopentanone to obtain 2-methyl cyclopentanone; reacting 2-methyl cyclopentanone with p-chlorobenzaldehyde to obtain 2-(4-chlorobenzylidene)-5-methyl cyclopentanone; carrying out methylationto obtain 5-(4-chlorobenzylidene)-2,2-dimethyl cyclopentanone; 5-(4-Chlorobenzylidene)-2,2-dimethylcyclopentanone undergoes Corey-Chaykovsky reaction to obtain an epoxide; reacts the epoxide with triazole to obtain a ring-opening product; and carrying out catalytic hydrogenation on the ring-opening product to obtain the Metconazole. The raw materials adopted by the preparation method are low in cost and easy to obtain in the market. The reactions in each step are conventional, the reaction steps are simple and easy to implement, and the method is simple process, mild in reaction conditions and easy in operation. The method has the advantages of high conversion rate, short reaction time and easy control, and is beneficial to industrial production.
Owner:九江德思光电材料有限公司
Method for preparing p-chlorobenzaldehyde through continuous oxidization of p-chlorotoluene
InactiveCN106748685AShort reaction timeMild reaction conditionsCarbonyl compound preparation by oxidationChemical/physical/physico-chemical stationary reactorsP-chlorobenzaldehydeOrganic synthesis
The invention relates to a method for preparing p-chlorobenzaldehyde through continuous oxidization of p-chlorotoluene and belongs to the technical field of organic synthesis processes. The method is a process technology for preparing p-chlorobenzaldehyde by continuously oxidizing p-chlorotoluene in a tubular reactor with a p-chlorotoluene compound as a raw material, one or more metal ion complexes of cobalt, molybdenum and bromine as a catalyst, hydrogen peroxide as an oxidizing agent and acetic acid as a solvent. The method has mild conditions and takes short time, the utilization rate of the raw material is high, effective control in the reaction process can be realized, safe, stable and continuous operation is realized, and the production efficiency is high.
Owner:CHANGZHOU UNIV
A kind of method for preparing p-chlorophenylglycine
ActiveCN106083628BShort reaction timeReduce production processOrganic compound preparationAmino-carboxyl compound preparationP-chlorobenzaldehydeSodium cyanide
The invention relates to a method for preparing chlorophenylglycine. The method is characterized in that p-chlorobenzaldehyde, ammonium bicarbonate and sodium cyanide are subjected to a complete reaction in a micro-channel reactor, and a tubular-type reactor is used for preparing the chlorophenylglycine. According to the invention, the raw materials are subjected to cyclization in the micro-channel reactor through a flow counter to prepare an intermediate p-chlorophenylhydantoin, alkaline hydrolysis is carried out on the intermediate through the tubular-type reactor by the flow counter to prepare the chlorophenylglycine, the reaction is complete in 8-18 minutes, and the chlorophenylglycine is introduced into a reaction bottle and is subjected to acidifying crystallization. The product purity is 98.0% and above, and the yield is 95% and above. The method has the advantages of concise technical route and fast reaction speed, can increase the work efficiency and production capacity, and can ensure the production and operation security of the processes.
Owner:内蒙古诚信永安化工有限公司
Synthetic method and applications of modified polyaspartic acid with Schiff base structure
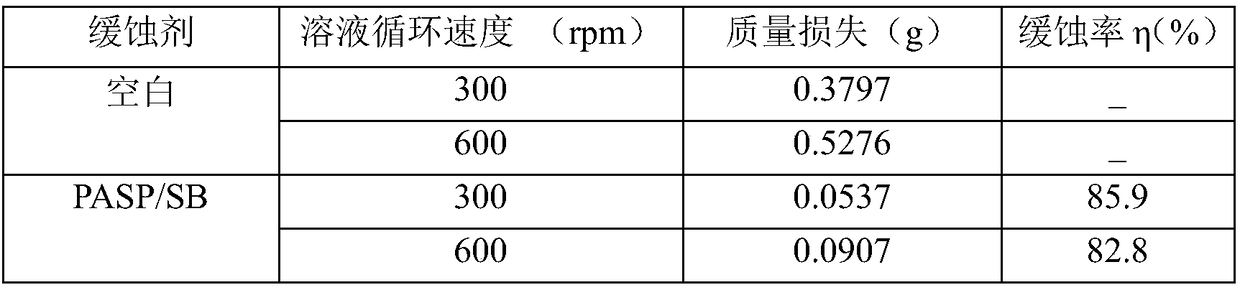
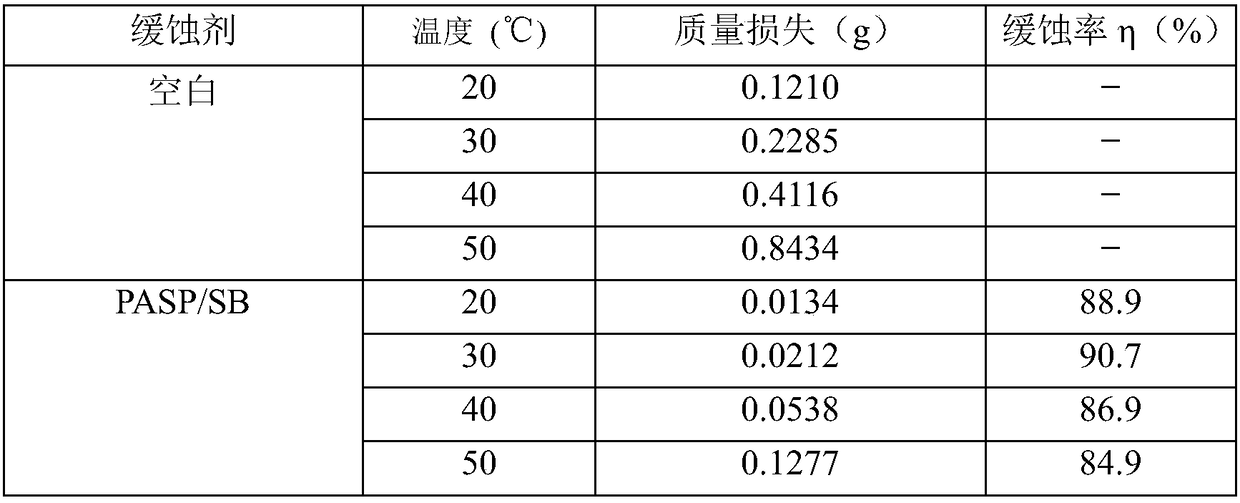
ActiveCN108373536ASignificant corrosion inhibitionProtected from beingWater bathsP-chlorobenzaldehyde
The invention discloses a synthetic method and applications of a modified polyaspartic acid with a Schiff base structure. The synthetic method comprises following steps: cis-butenedioic anhydride is introduced into a reactor containing distilled water, and is subjected to water bath stirring at 30 to 60 DEG C; ammonia water is introduced into the reactor drop by drop, and reaction is carried out for 0.5 to 3h at 60 to 100 DEG C; at argon atmosphere, oil bath treatment is adopted to increase the temperature to 180 to 240 DEG C, reaction is carried out for 20 to 40min, N, N-dimethyl formamide isadded for dissolving of a sticky substance, and product PSI is obtained via reaction; PSI and thiocarbazide are dissolved in a reactor containing distilled water, heating stirring is carried out for6 to 12h so as to obtain product PASP / CD; and at last the obtained PASP / CD aqueous solution and an alcoholic solution of p-chlorobenzaldehyde are subjected to heating backflow so as to obtain the modified polyaspartic acid with a Schiff base structure. The main chain of the modified polyaspartic acid corrosion inhibitor with a Schiff base structure is a biodegradable polymer, and the corrosion inhibition efficiency is 90% or higher.
Owner:HEBEI UNIV OF TECH
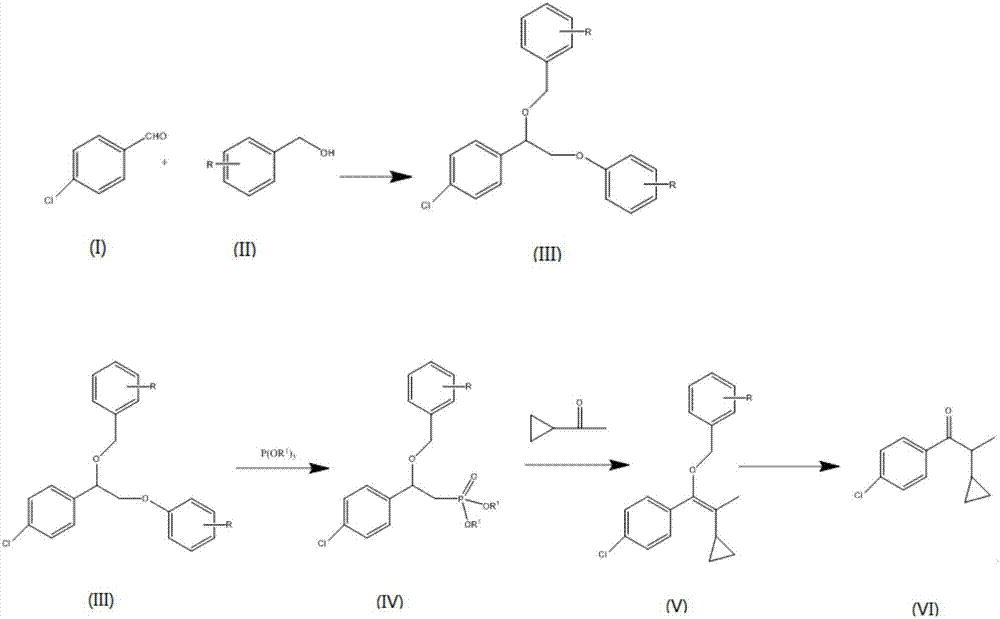
Preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
ActiveCN107285998AOperational securityEasy to controlOrganic compound preparationGroup 5/15 element organic compoundsTriethylphosphiteP-chlorobenzaldehyde
The invention discloses a preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone. The method comprises the steps of preparing acetal by adopting p-chlorobenzaldehyde and phenylcarbinol or a derivative thereof as raw materials under the action of a catalyst, reacting an acetal product with trimethyl phosphite or triethyl phosphite in an environment of a polar solvent under the catalysis of lewis acid to generate alpha-benzyloxy p-chlorobenzyl dimethyl phosphate, further reacting the alpha-benzyloxy p-chlorobenzyl dimethyl phosphate with cyclopropyl methyl ketone under the combined action of a base catalyst, a phase transfer catalyst and a cocatalyst to prepare a benzyloxy-propylene derivative; and then carrying out acidifying hydrolysis on the benzyloxy-propylene derivative to obtain the1-(4-chlorphenyl)-2-cyclopropyl-1-acetone. Compared with the prior art, the method has the advantages of being safe in operation, controllable in process, low in toxicity in the process, mild in reaction condition, suitable for industrial production and high in product purity.
Owner:JIANGSU SWORD AGROCHEM
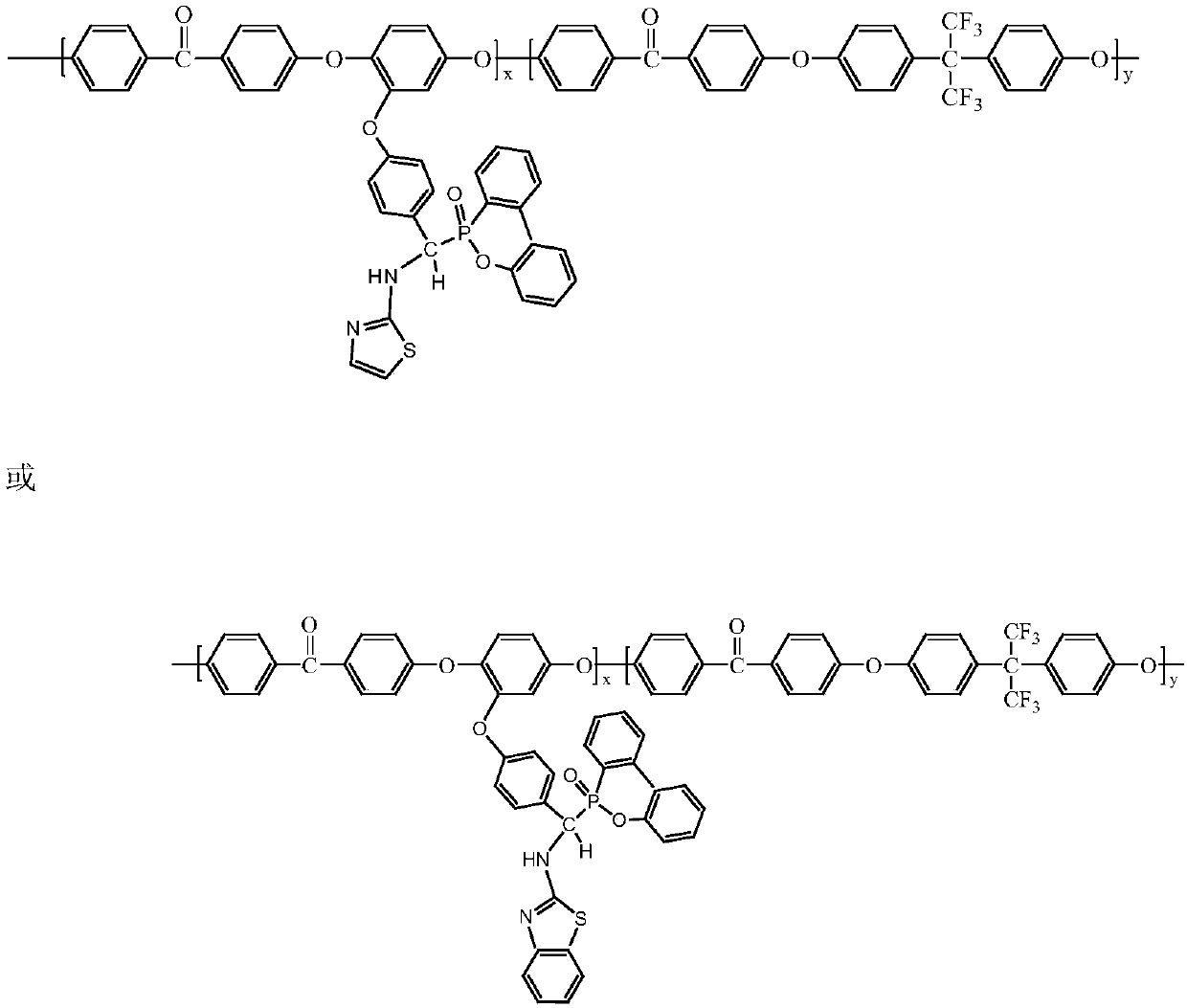
Preparation method of 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO) side group polyaryletherketone containing ternary flame-retardant material
The invention relates to a preparation method of a 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO) side group polyaryletherketone containing a ternary flame-retardant material. The method comprises the following steps: placing multi-component bisphenol, difluorobenzophenone and a catalyst in an organic solvent, carrying out twice temperature-control reactions, then pouring the reaction product into water, carrying out dialysis and vacuum drying on the precipitate to obtain a methoxy substituent group-containing polyaryletherketone base material; dissolving the methoxy substituent group-containing polyaryletherketone in a boron trifluoride solution, filtering the reactant, and carrying out Soxhlet extraction and drying to obtain polyaryletherketone; adding p-fluorobenzaldehyde orp-chlorobenzaldehyde and 2-aminothiazole or 2-aminobenzothiazole into a solvent to carry out a reaction to obtain a 2DOPO group-containing thiazole derivative or DOPO group-containing benzothiazole derivative; and placing hydroxyl substituent group-containing polyaryletherketone, the DOPO side group-containing thiazole derivative and a catalyst in an organic solvent, carrying out a secondary reaction for precipitation, and carrying out dialysis vacuum drying to obtain the DOPO side group-containing polyaryletherketone. The prepared DOPO side group-containing polyaryletherketone with a high molecular weight has excellent comprehensive performance, and requirements of a high-level application can be met.
Owner:FUJIAN NORMAL UNIV
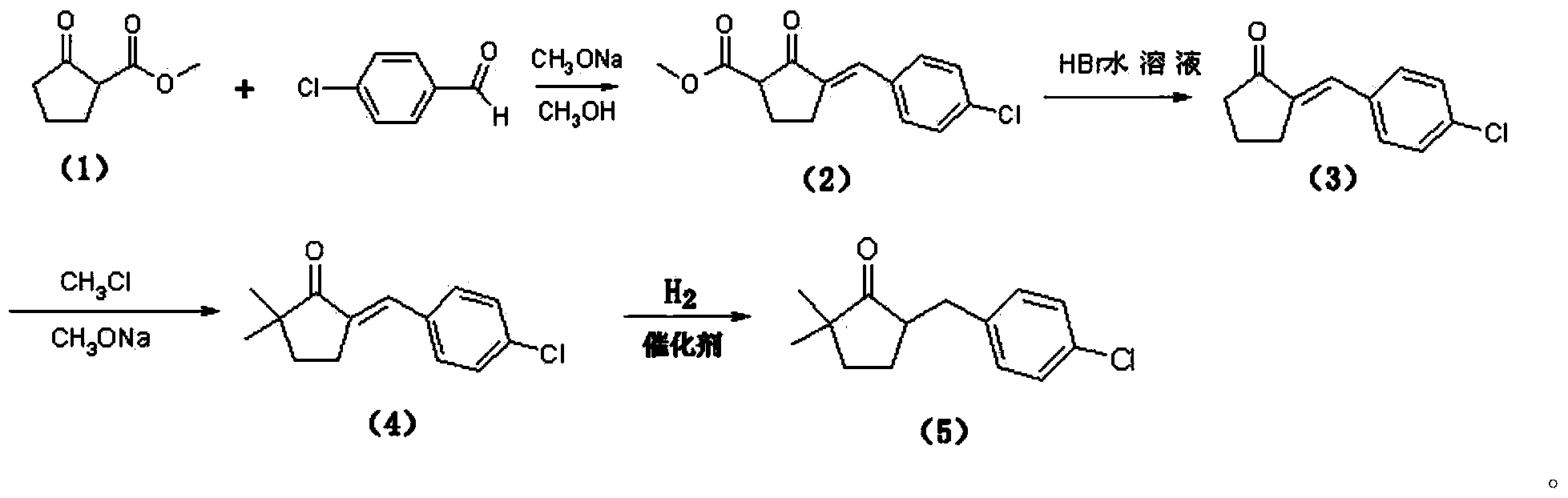
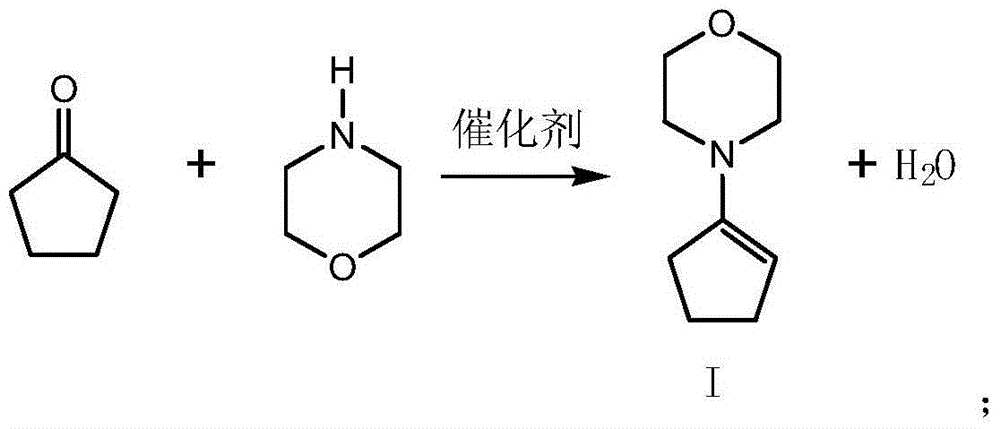
Preparation method for triticonazole intermediate
ActiveCN104130117AControl impurity contentHigh purityCarbonyl compound preparation by condensationAluminium sulfateOrganic solvent
The invention relates to a preparation method for a triticonazole intermediate 5-(4-chlorobenzylidene)-2,2-dimethylcyclopentanone. The preparation method comprises: (1) performing an oximation reaction on cyclopentanone and morpholine in an organic solvent in the presence of p-methylbenzene sulfonic acid at a temperature of 40-80 DEG C, so as to generate an intermediate I; (2) performing a condensation reaction on the intermediate I and p-chlorobenzaldehyde in a solvent at a temperature of 40-80 DEG C, so as to generate an intermediate II; (3) hydrolyzing the intermediate II under an acidic condition to generate an intermediate III; and (4) performing a methylation reaction on the intermediate III and a halogenated methane in a polar solvent in the presence of a strong base at a temperature of 40-80 DEG C, so as to generate 5-(4-chlorobenzylidene)-2,2-dimethylcyclopentanone. The technology is relatively simple, is capable of effectively controlling the impurity content in the product a improving the product impurity, and is low in cost and beneficial for realizing nationalization of triticonazole.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Synthetic method of cyproconazole intermediate
InactiveCN106083542AHigh process fluencyHigh reaction yieldOrganic compound preparationGroup 5/15 element organic compoundsTriethylphosphiteP-chlorobenzaldehyde
Owner:YANCHENEG HUIHUANG CHEM
Green industrial production method of baclofen
InactiveCN106187794AImprove friendlinessHigh reaction yieldCarboxylic acid nitrile preparationOrganic compound preparationDrugs synthesisP-chlorobenzaldehyde
The invention discloses a green industrial production method of baclofen and belongs to the technical field of medicine synthesis. The method is carried out with p-chlorobenzaldehyde as an initial raw material and includes five reactions of Knoevenagel condensation, alkaline hydrolysis, imidation, alkaline hydrolysis, and Hofmann degradation, thus preparing the baclofen satisfying clinical medicinal application. The method employs raw materials being very easy to obtain and being low in cost and is simple in synthesis operations, wherein the reactions are carried out under aqueous conditions. The method is very low in environment pollution and high in yield, and is a novel industrial production method of the baclofen.
Owner:ANHUI YIXINMING PHARMA TECH
Preparation method of p-chlorine methyl cinnamate
InactiveCN102659579AHelp generateNon-volatileOrganic compound preparationCarboxylic acid esters preparationP-chlorobenzaldehydeBenzaldehyde
The invention provides a preparation method of p-chlorine methyl cinnamate, which comprises the following steps: taking basic ionic liquid 1-butyl-3-methylimidazole carbonate as a solvent and a catalyst, tetrabutyl ammonium bromide as a phase-transfer catalyst for stirring and mixing with methyl acetate under the temperature of 5 DGE C, then adding p-chlorine benzaldehyde for a condensation reaction, adding dilute sulfuric acid after the reaction, wherein the pH value of a reaction solution is between 6.5 and 7.2; filtering, wherein the obtained filtrate can be taken as a mixed liquid of ionic liquid and the phase-transfer catalyst; filtering the obtained solid, washing and drying to obtain p-chlorine methyl cinnamate. The method of the invention has the advantages of simple method, mild reaction condition, convenient operation, easy catalyst recovery and multitime repetitive usage; the product has the advantages of reliable and stable quality, high yield and no environmental pollution, and the method of the invention is a green synthetic method.
Owner:湖北远成赛创科技有限公司
Method for preparing 4,4-dimethyl-1-(4-chlorphenyl)-3-pentanone
InactiveCN102351674AReduce unit consumptionImprove reaction speedOrganic compound preparationCarbonyl compound preparationSodium methoxideDistillation
The invention discloses a method for preparing 4,4-dimethyl-1-(4-chlorphenyl)-3-pentanone, which comprises the following steps: reacting 3,3-dimethyl-2-butanone and p-chlorobenzaldehyde under reflux for 5-10 hours by using methanol as a solvent and sodium methoxide as an alkalizer, recycling part of methanol, cooling to 10-20 DEG C, filtering to obtain the 4,4-dimethyl-1-(4-chlorphenyl)-3-pentanone, and drying, wherein the mol ratio of p-chlorobenzaldehyde:3,3-dimethyl-2-butanone:sodium methoxide is 1:(1.0-1.3):(1.2-1.5). Methanol is substituted for potassium hydroxide, so that water generated in reaction is decomposed by sodium methoxide, thereby effectively enhancing the reaction speed and reaction yield; and since the reaction system does not contain water, the solvent can be recycled by simple distillation, thereby greatly lowering the facility request and reducing the raw material unit consumption. The synthesis yield of the method is higher than 97%, the product content is higher than 96%, and the recovery rate of the solvent methanol is up to 85%; and thus, the invention can easily implement industrial production, has good practicality, and can generate high economic benefit and social benefit.
Owner:苏州诚和医药化学有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com