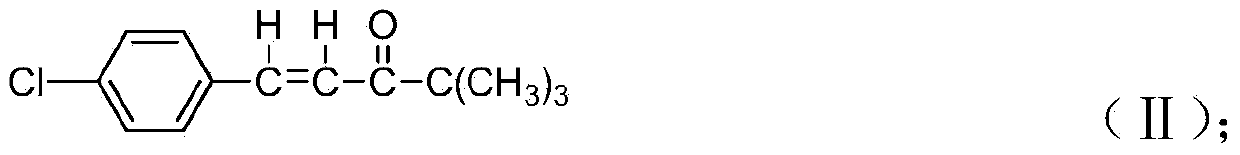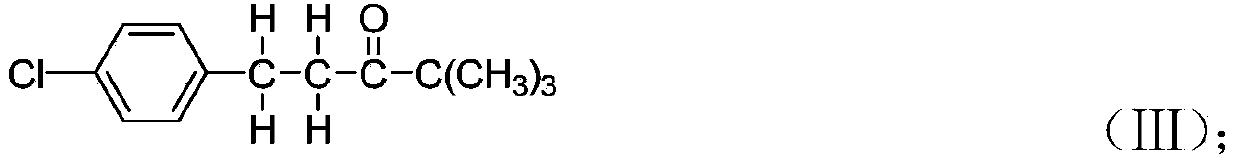Preparation method of tebuconazole
A technology of tebuconazole and pentene, which is applied in the field of preparation of tebuconazole, can solve the problems of low product content, low reaction yield, large amount of sewage and the like, achieves good selectivity and reduces isomer by-products. The effect of generating and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of 4,4-dimethyl-1-(4-chlorophenyl)-1-penten-3-one: put 140ml methanol and 28.0g p-chlorobenzaldehyde (melted) into a 500ml four-neck flask, put 22.0g of pinacolone, 12.0g of sodium hydroxide, start stirring after the injection, slowly raise the temperature to 70°C and reflux, keep it for 5h, after the reaction, slowly cool down to 0±2°C, keep it for 0.5h, filter to get 43.5g of 4,4-di Methyl-1-(4-chlorophenyl)-1-penten-3-one, yield 97.6%.
Embodiment 2
[0054] Synthesis of 4,4-dimethyl-1-(4-chlorophenyl)-3-pentanone: 4,4-dimethyl-1-(4-chlorophenyl)-1 obtained in Example 1 -Penten-3-one 43.5 into the hydrogenation tank, while adding methanol 230ml, red aluminum solution 20.0g (red aluminum solution consists of 70% dihydrobis-(2 methoxyethoxy) sodium aluminate and 30% Composition of toluene, purchased from Nanjing Zhongheng Chemical Materials Co., Ltd., trade name: red aluminum), followed by nitrogen replacement three times, hydrogen replacement three times, slowly heating up, and at the same time starting to pass hydrogen reaction, control the temperature of the kettle at 30-67 °C, and the pressure <1.0Mpa. In the later stage, the temperature is controlled at 62-67°C, and the pressure is 0.7-0.9Mpa to react. When the pressure of the kettle remains basically unchanged, samples are taken for HPLC analysis. After passing the test, cool down to 35°C, let go to remove the methanol, first release the solvent under normal pressure ...
Embodiment 3
[0055] Embodiment 3 (comparative example 1)
[0056] Synthesis of 4,4-dimethyl-1-(4-chlorophenyl)-3-pentanone: 4,4-dimethyl-1-(4-chlorophenyl)-1 obtained in Example 1 - 43.5g of penten-3-one was put into the hydrogenation kettle, and 230ml of methanol was added at the same time, and 8.1g of Raney nickel was added at the same time, followed by three replacements with nitrogen, three replacements with hydrogen, and slowly raising the temperature. Temperature 10-67 ℃, pressure < 1.5Mpa. In the later stage, the temperature is controlled at 62-67°C, and the pressure is 1.2-1.5Mpa to react. When the pressure of the kettle remains basically unchanged, samples are taken for HPLC analysis. After passing the test, cool down to 35°C, vent to remove methanol, first remove methanol at 80°C under normal pressure, keep warm for 0.5h, then change to negative pressure precipitation (-0.095Mpa), heat up to 110°C and keep warm for 1h, until the methanol removal is qualified, 41.3g of yellow is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com