Method for preparing p-chlorobenzaldehyde through continuous oxidization of p-chlorotoluene
A technology of p-chlorobenzaldehyde and p-chlorotoluene, which is applied in the field of organic synthesis process technology, can solve the problems of low selectivity of p-chlorobenzaldehyde, target product selectivity, low yield, low conversion rate, etc., and achieve improved reaction Rate and utilization of raw materials, avoiding the use of co-catalysts, and the effect of saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
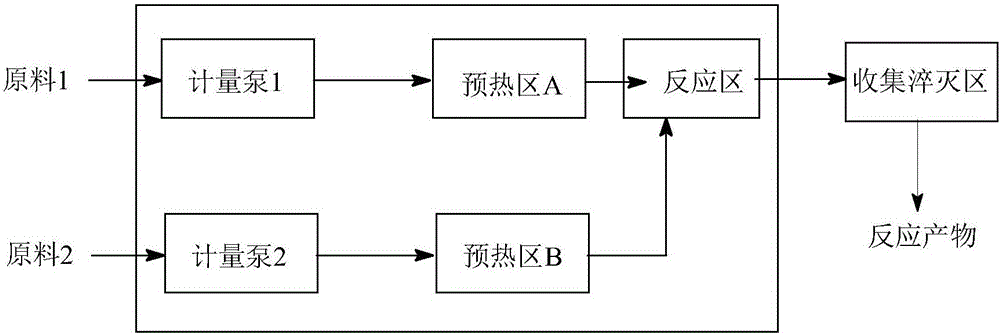
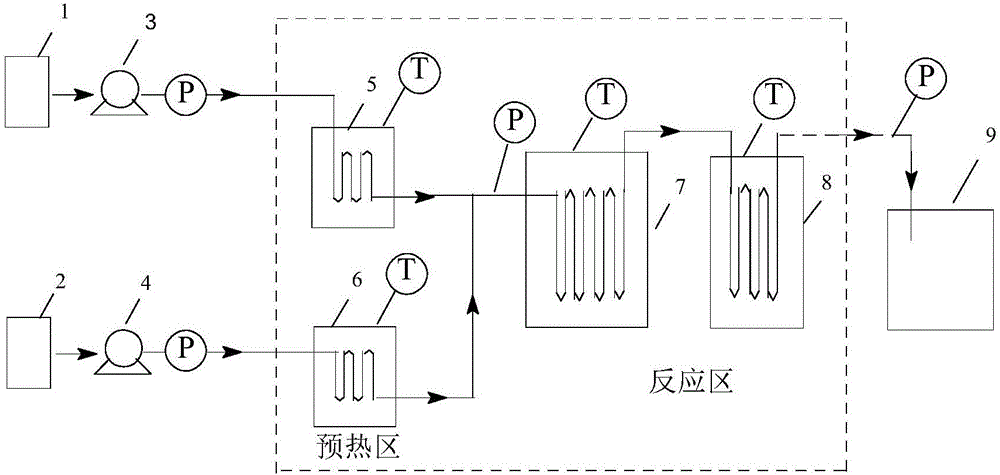
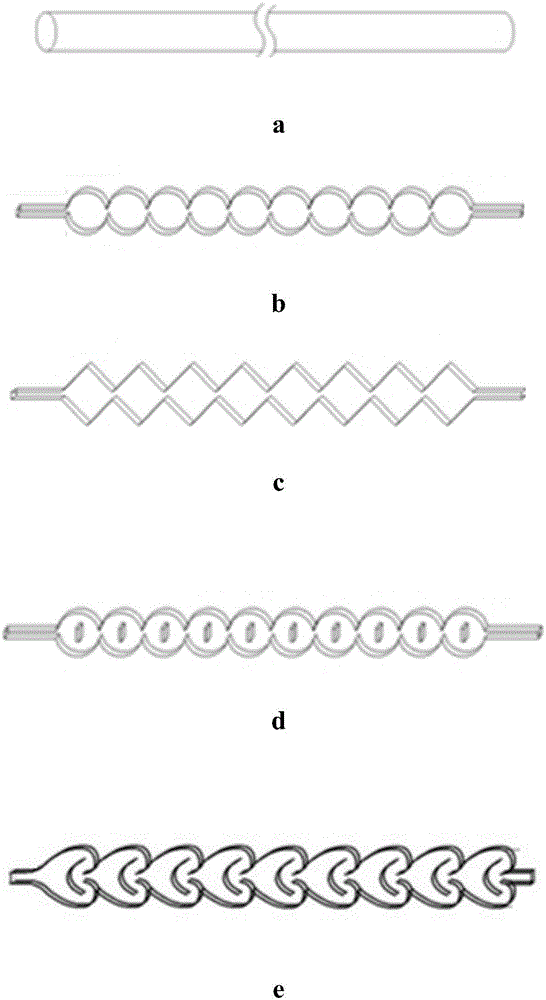
[0032] (1) Device: refer to figure 2 Determine the connection mode of the tubular reactor. The pipeline type is: (3a+3b) direct flow channel + round cake pulse variable diameter rectangular flat pipeline. The inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time. The heat exchange medium is heat conduction Oil.
[0033] (2) Dissolve 3.03g of cobalt acetate and 3.03g of sodium molybdate in 200ml of p-chlorotoluene and 200ml of acetic acid to form a mixed solution. At this time, n (cobalt acetate): n (p-chlorotoluene) = 0.0075:1, and 3.03g Sodium bromide dissolved in 15% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n (sodium bromide):n (p-chlorotoluene)=0.0075:1, p-chlorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by constant flow pump respectively with the flow velocity of 8.33ml / min and 16.67ml / min, at this moment n (H ...
Embodiment 2
[0035] (1) Device: refer to figure 2 Determine the connection mode of the tubular reactor. The pipeline type is: (3a+3c) direct flow channel + oblique square cake pulse variable diameter rectangular flat pipeline. The inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time. The heat exchange medium is heat transfer oil.
[0036] (2) Dissolve 6.06g of cobalt acetate and 6.06g of sodium molybdate in 200ml of p-chlorotoluene and 200ml of acetic acid to form a mixed solution. At this time, n (cobalt acetate): n (p-chlorotoluene) = 0.015:1, and 6.06g Sodium bromide dissolved in 15% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n(sodium bromide):n(p-chlorotoluene)=0.015:1, p-chlorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by constant flow pump respectively with the flow velocity of 8.33ml / min and 16.67ml / min, at this moment n (...
Embodiment 3
[0038] (1) Device: refer to figure 2 Determine the connection mode of the tubular reactor. The pipeline type is: (3a+3d) straight-through channel + enhanced mixing circular cake type rectangular flat pipeline. The inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time, and the heat transfer medium is heat transfer oil. .
[0039] (2) Dissolve 6.06g of cobalt acetate and 6.06g of sodium molybdate in 200ml of p-chlorotoluene and 200ml of acetic acid to form a mixed solution. At this time, n (cobalt acetate): n (p-chlorotoluene) = 0.015:1, and 6.06g Sodium bromide dissolved in 15% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n(sodium bromide):n(p-chlorotoluene)=0.015:1, p-chlorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by constant flow pump respectively with the flow velocity of 8.33ml / min and 16.67ml / min, at this moment n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com