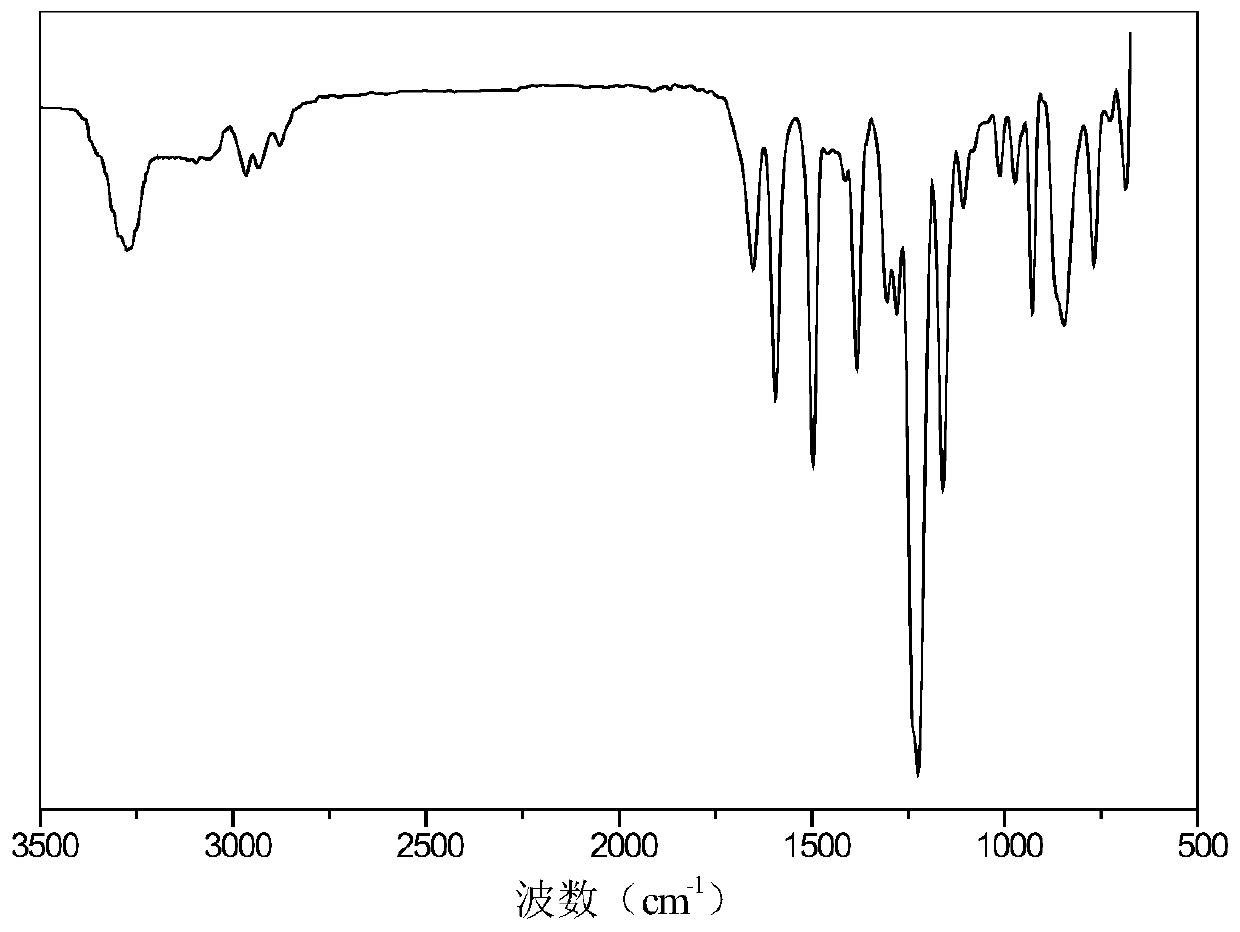
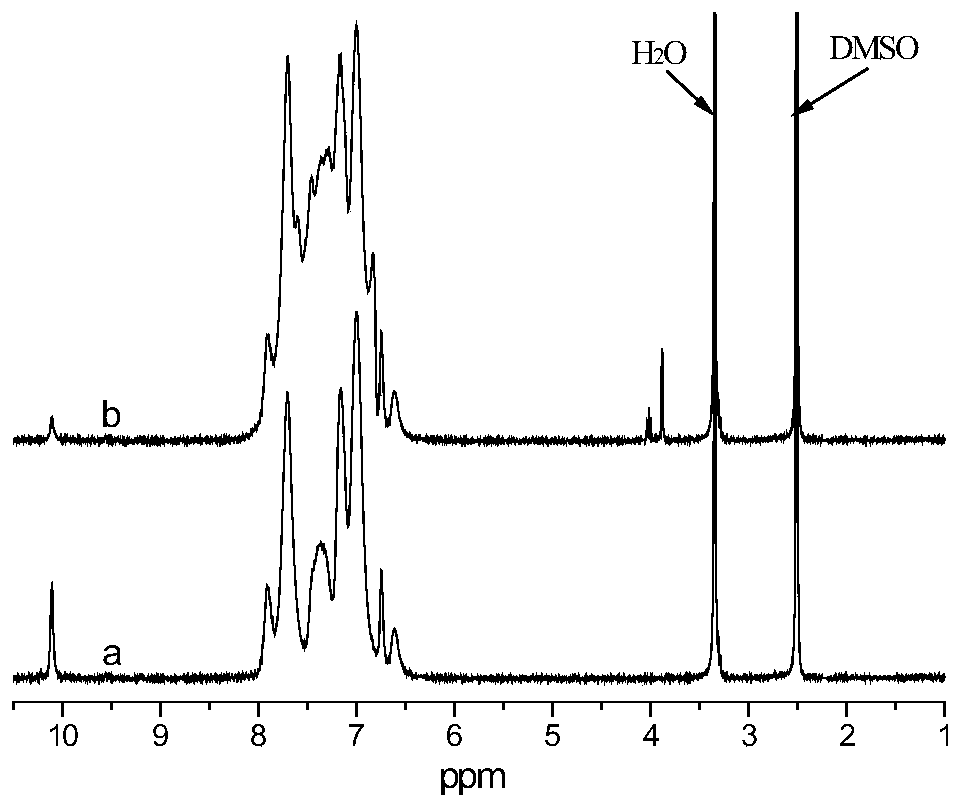
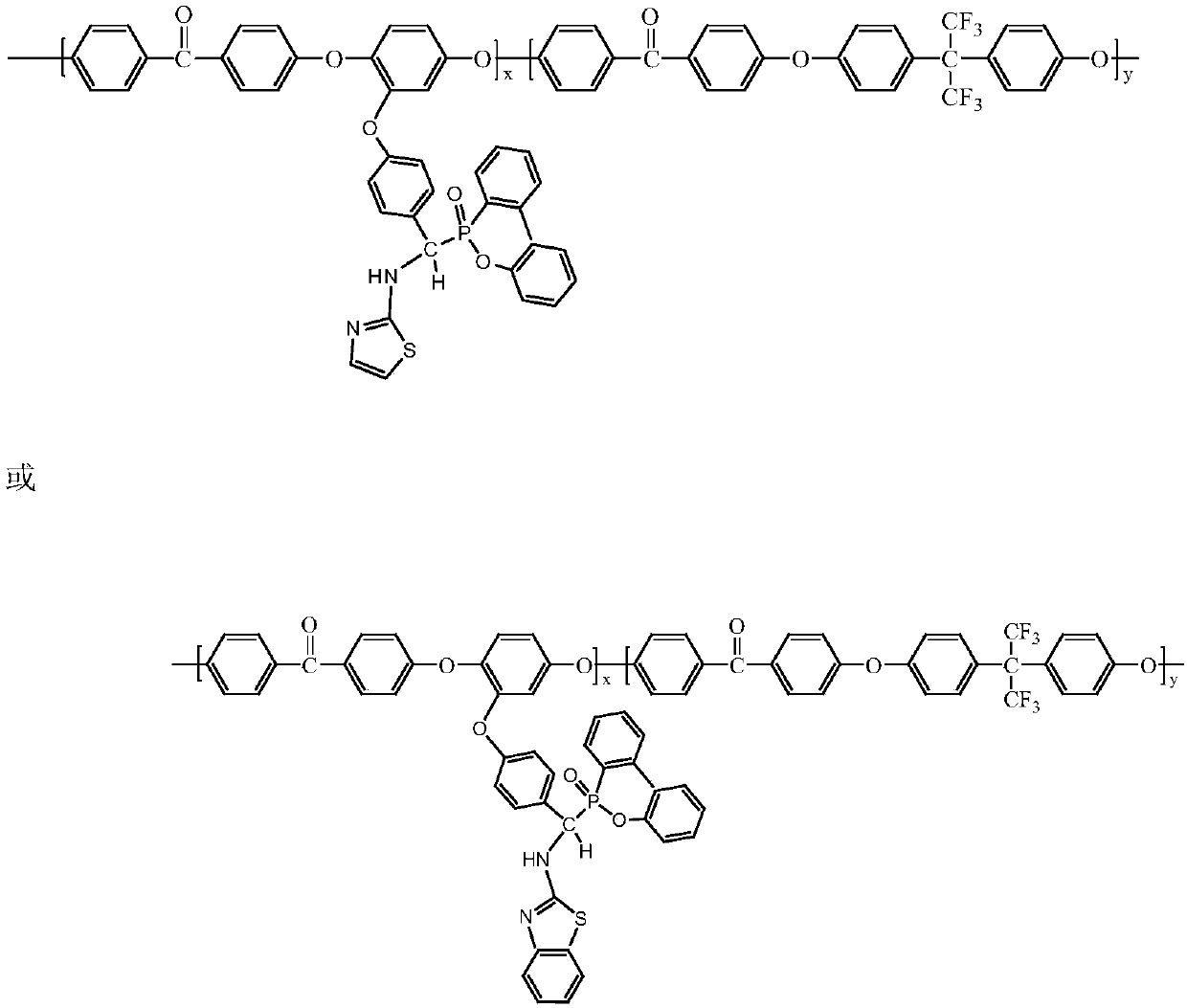
Preparation method of 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO) side group polyaryletherketone containing ternary flame-retardant material
A technology of polyaryletherketone and flame-retardant materials, which is applied in the field of preparation of DOPO side-group polyaryletherketone containing ternary flame-retardant materials, can solve the problems of material mechanical properties decline and affect flame-retardant performance, and achieve good maintenance, The effect of good electrical insulation, good economic benefits and promotional value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] One of embodiment 1 p-fluorobenzaldehyde or p-chlorobenzaldehyde and reactant B
[0032] (1) Add p-fluorobenzaldehyde and 2-aminothiazole or p-fluorobenzaldehyde and 2-aminobenzothiazole to absolute ethanol at a molar ratio of 1:1, react at 50°C for 2 hours in a nitrogen atmosphere, and then add Continue to react with DOPO in an equimolar amount of 2-aminothiazole or 2-aminobenzothiazole for 24 hours, terminate the reaction, and obtain DOPO derivatives respectively. The synthetic route is shown in the figure.
[0033]
[0034] (2) Synthesis of polyaryletherketone containing DOPO side groups: In a three-necked flask, add 1.4014g (10mmol) of o-methoxyhydroquinone, 3.3623g (10mmol) of hexafluorobisphenol A, and difluorobiphenyl Methanone 4.3640g (20mmol), anhydrous potassium carbonate 3.3166g (24mmol), toluene 80mL, DMAc100mL, in N 2 React at 140°C for 3h under airflow, then raise the temperature to 170°C for 6h, immediately pour the reaction solution into deionized wa...
Embodiment 2
[0042] In a three-necked flask, add 0.707g (5mmol) of o-methoxyhydroquinone, 5.0434g (15mmol) of hexafluorobisphenol A, 4.3640g (20mmol) of difluorobenzophenone, and 3.3166g of anhydrous potassium carbonate. (24mmol), toluene 80mL, DMAc100mL, in N 2 React at 140°C for 3h under airflow, then raise the temperature to 170°C for 6h, immediately pour the reaction solution into deionized water for precipitation under stirring, and filter. The filtered precipitate was soaked in deionized water, soaked in running water for 24 hours, filtered, and dried in a vacuum oven at 80° C. for 10 hours to obtain methoxy-containing polyaryletherketone. GPC analysis, Mn = 20200, PDI = 1.84.
[0043] Weigh 5.0 g of methoxy-containing polyaryletherketone and dissolve it in 100 mL of dried dichloromethane. N 2 Under gas protection, in an ice-salt bath, 1.5 mL of boron tribromide solution diluted with 15 mL of dichloromethane was added dropwise. After the dropwise addition was completed within 2 ho...
Embodiment 3
[0047] In a three-necked flask, add 0.2803g (2mmol) of o-methoxyhydroquinone, 6.0521g (18mmol) of hexafluorobisphenol A, 4.3640g (20mmol) of difluorobenzophenone, and 3.3166g of anhydrous potassium carbonate. (24mmol), toluene 80mL, DMAc100mL, in N 2 React at 140°C for 3h under airflow, then raise the temperature to 170°C for 6h, immediately pour the reaction solution into deionized water for precipitation under stirring, and filter. The filtered precipitate was soaked in deionized water, soaked in running water for 24 hours, filtered, and dried in a vacuum oven at 80° C. for 10 hours to obtain methoxy-containing polyaryletherketone.
[0048] Weigh 5.0 g of methoxy-containing polyaryletherketone and dissolve it in 100 mL of dried dichloromethane. N 2 Under gas protection, in an ice-salt bath, 0.6 mL of boron tribromide solution diluted with 6 mL of dichloromethane was added dropwise. After the addition was completed within 2 hours, the temperature was naturally raised to roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com