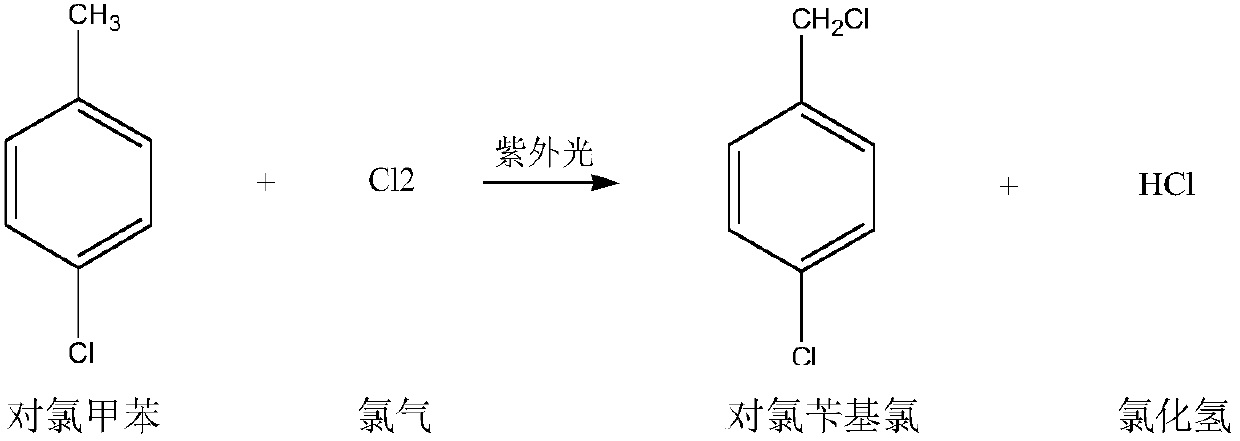
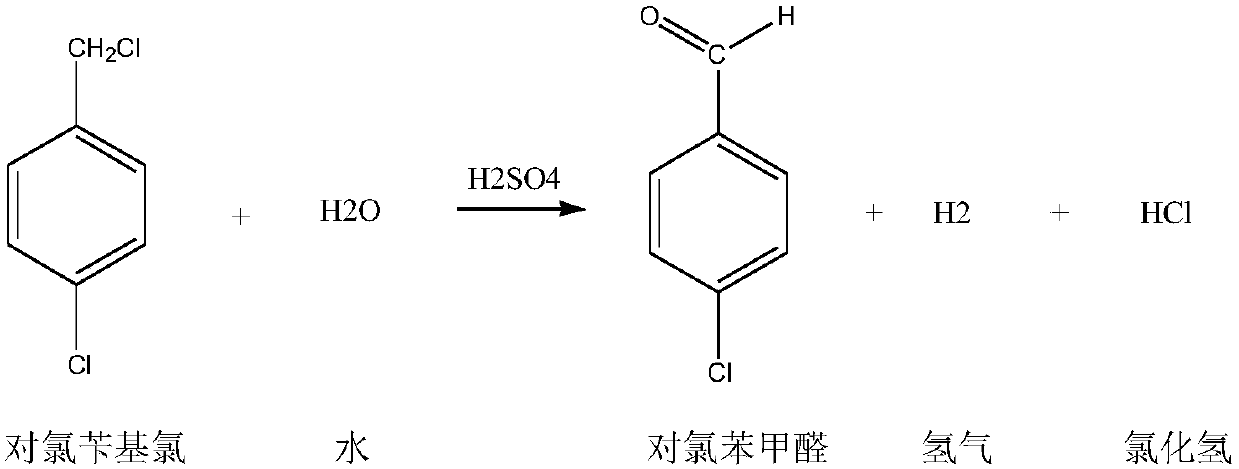
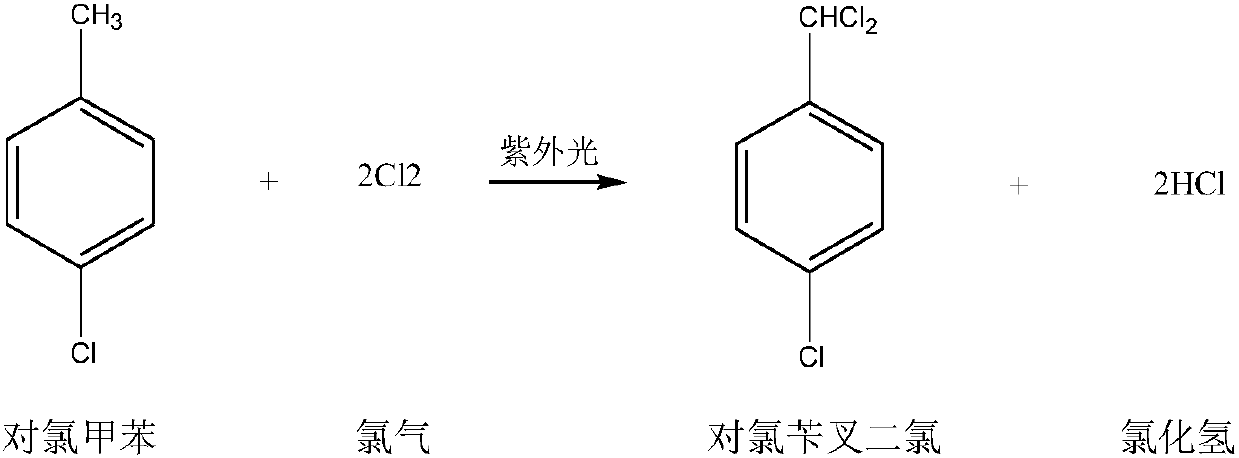
Preparation method for p-chlorobenzaldehyde
A technology of p-chlorobenzaldehyde and p-chlorotoluene, which is applied to the preparation of halogenated hydrocarbons, chemical instruments and methods, and hydrolysis to prepare carbonyl compounds, etc., can solve the problems of large amount of hydrolysis wastewater, low selectivity, and high cost, and reduce waste water. The total amount, high reaction selectivity, and the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Substitution reaction:
[0068] Drop into the p-chlorotoluene of content 99.2% in the dry 500ml four-necked flask that has stirring paddle, chlorine gas pipe, nitrogen gas pipe, reflux condenser (condenser emptying port is connected to tail gas absorption device): 220.0g (1.7252mol ), Bromine: 2.5g. The outer wall of the four-necked flask was heated with heat transfer oil, and the temperature of the heat transfer oil was set at 90°C. Start stirring, turn on nitrogen, and feed 0.5 l / min nitrogen into the four-necked flask for protection. When the temperature of the heat transfer oil reaches 85°C, feed 0.5l / min chlorine gas into the four-necked flask to control the inner temperature of the four-necked flask to 85-90°C (pull out the nitrogen tube, measure the inner temperature with a thermometer, and control the temperature by adjusting the temperature of the heat transfer oil. internal temperature) for a substitution reaction. During the reaction process, the progress ...
Embodiment 2
[0078] Substitution reaction:
[0079] Drop into the p-chlorotoluene of content 99.2% in the dry 500ml four-necked flask that has stirring paddle, chlorine gas pipe, nitrogen gas pipe, reflux condenser (condenser emptying port is connected to tail gas absorption device): 220.2g (1.7268mol ), Bromine: 3.5g. The outer wall of the four-necked flask was heated with heat transfer oil, and the temperature of the heat transfer oil was set at 90°C. Start stirring, turn on nitrogen, and feed 0.5 l / min nitrogen into the four-necked flask for protection. When the temperature of the heat transfer oil reaches 85°C, feed 0.5l / min chlorine gas into the four-necked flask to control the inner temperature of the four-necked flask to 85-90°C (pull out the nitrogen tube, measure the inner temperature with a thermometer, and control the temperature by adjusting the temperature of the heat transfer oil. internal temperature) for a substitution reaction. During the reaction process, the progress ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com