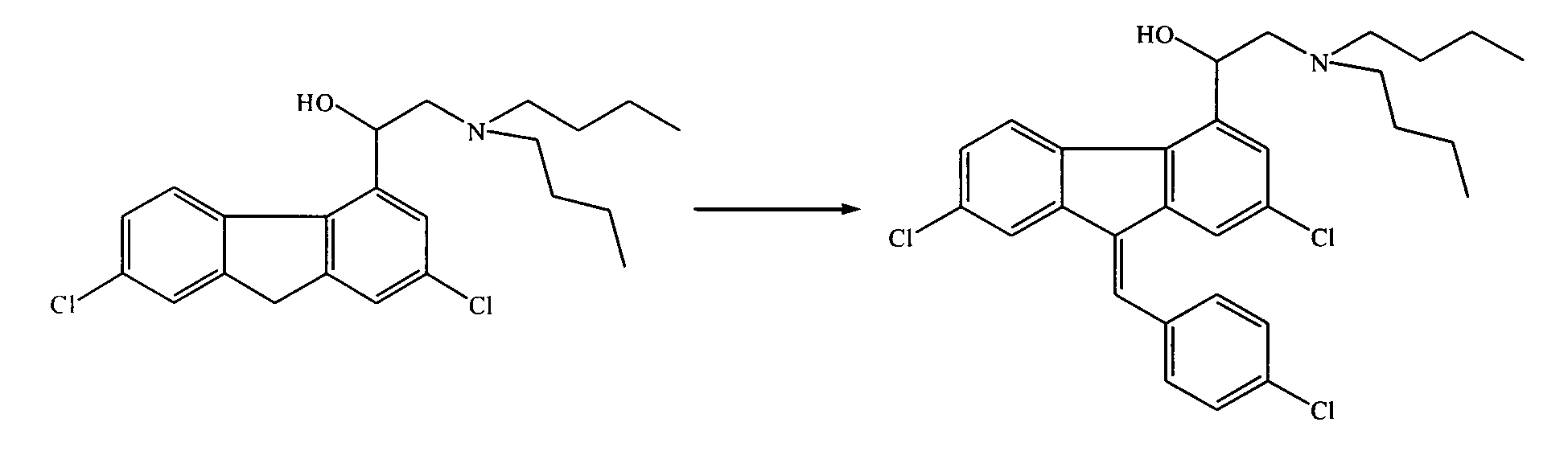
One-step green synthesis process of antimalarial raw material benflumetol
A benfluorenol and anti-malarial technology, applied in the field of high-yield and high-purity benfluorenol, can solve the problems of severe condensation reaction conditions, low atom economy, low yield of high-purity fine benfluorenol, etc. Controllable, simple reaction system, green production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 benzfluorenol
[0022] Put 500.0kg of α-(di-n-butylamino)-2,7-dichloro-4-fluorenemethanol into a 500L stainless steel reaction kettle, add 3000L of methanol-ethylene glycol (V:V=9:1), stir at room temperature to dissolve scattered. Then 10.0 kg of sodium methoxide was added, and the reaction was stirred at room temperature for 1 h. Then add 180.0 kg of p-chlorobenzaldehyde at one time, then raise the temperature to reflux, and control the reaction at 75°C for about 15 hours. Thin-layer chromatography monitors the raw material α-(di-n-butylamino)-2,7-dichloro-4- The fluorenylmethanol spots disappeared. While stirring, the temperature was lowered to room temperature, and the stirring reaction was continued for 2 h, and benzfluorenol crystals were precipitated, filtered under reduced pressure, and washed with a small amount of methanol or ethanol to obtain crude benzfluorenol.
Embodiment 2
[0023] The synthesis of embodiment 2 benzfluorenol
[0024] 500.0kg of α-(di-n-butylamino)-2,7-dichloro-4-fluorenemethanol was placed in a 500L stainless steel reaction kettle, and 2500L of ethanol-ethylene glycol (V:V=95:5) was added, stirred at room temperature to dissolve scattered. Then 11.0 kg of sodium ethoxide was added, and the reaction was stirred at room temperature for 1 h. Then add 180.0kg of p-chlorobenzaldehyde at one time, then raise the temperature to reflux, and control the reaction at 85°C for about 12h. Thin-layer chromatography monitors the raw material α-(di-n-butylamino)-2,7-dichloro-4- The fluorenylmethanol spots disappeared. While stirring, the temperature was lowered to room temperature, and the stirring reaction was continued for 3 h, and benzfluorenol crystals were precipitated, filtered under reduced pressure, and washed with a small amount of methanol or ethanol to obtain crude benzfluorenol.
Embodiment 3
[0025] The synthesis of embodiment 3 benzfluorenol
[0026] 500.0kg of α-(di-n-butylamino)-2,7-dichloro-4-fluorenemethanol was placed in a 500L stainless steel reaction kettle, and 2500L of toluene-ethylene glycol (V:V=85:15) was added, stirred at room temperature to dissolve scattered. Then 5.0 kg of sodium methoxide and 8.0 kg of potassium acetate were added, and the reaction was stirred at room temperature for 2 h. Then add 185.0 kg of p-chlorobenzaldehyde at one time, then raise the temperature to reflux, and control the reaction at 85 ° C for about 16 hours. Thin-layer chromatography monitors the raw material α-(di-n-butylamino)-2,7-dichloro-4- The fluorenylmethanol spots disappeared. While stirring, the temperature was lowered to room temperature, and the stirring reaction was continued for 3 h, and benzfluorenol crystals were precipitated, filtered under reduced pressure, and washed with a small amount of methanol or ethanol to obtain crude benzfluorenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com