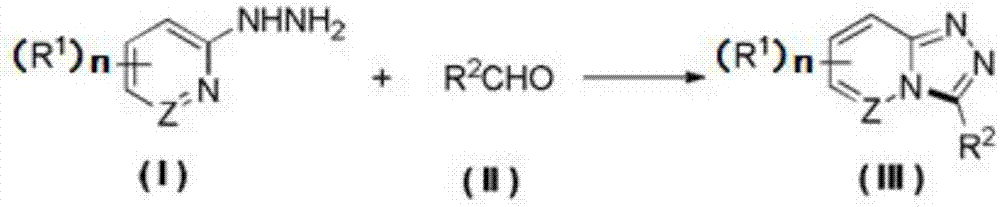
Synthesis method of 1,2,4-triazolohetercyclic compound
A technology for aldehyde compounds and heterocycles, which is applied in the field of synthesizing 1,2,4-triazoloheterocycles, can solve the problems of expensive reagents, toxic and harmful prices, etc., and achieves simple reaction system and high total yield , easy to obtain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 2-hydrazinopyridine (65.5mg, 0.6mmol) and benzaldehyde (51μL, 0.5mmol) in 5mL of acetonitrile solvent, stir and react at 25°C for 1 hour, then add tetrabutylammonium tetrafluoroborate (164.6mg , 0.5mmol), 4mL acetonitrile, 1mL water, and then install a graphite carbon rod electrode as the anode, and a platinum sheet electrode as the cathode, turn on the power, set the current to 10mA, and stop the power after 4 hours. Cool to room temperature, wash with water, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography to obtain the product 3-phenyl-[1,2,4]triazolo[4,3-a]pyridine 83.9 mg, the yield is 86%, and the product is an off-white solid. m.p.:171–173°C; 1 H NMR (500MHz, CDCl 3 )δ8.30(dt,J=7.0,1.2Hz,1H),7.88–7.82(m,3H),7.63–7.55(m,3H),7.31(ddd,J=9.3,6.5,1.1Hz,1H) ,6.89(td,J=6.8,1.1Hz,1H)ppm.
Embodiment 2
[0023] According to the method described in Example 1, the difference is that the amount of 2-hydrazinopyridine is 54.6mg (0.5mmol), to obtain the product 3-phenyl-[1,2,4]triazolo[4,3-a ] Pyridine 58.6mg, the yield is 60%, and the product is off-white solid.
Embodiment 3
[0025] According to the method described in Example 1, the difference is that the amount of 2-hydrazinopyridine is 81.88mg (0.75mmol), to obtain the product 3-phenyl-[1,2,4]triazolo[4,3-a ] Pyridine 78.0 mg, the yield was 80%, and the product was off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com