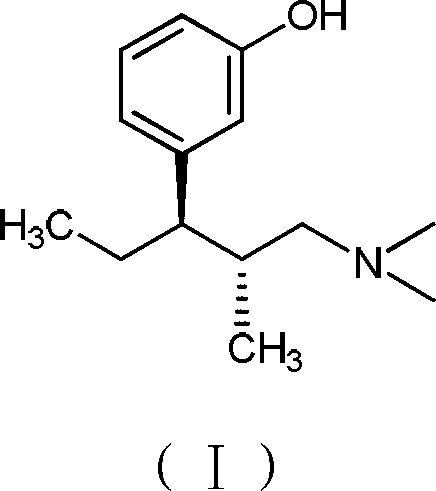
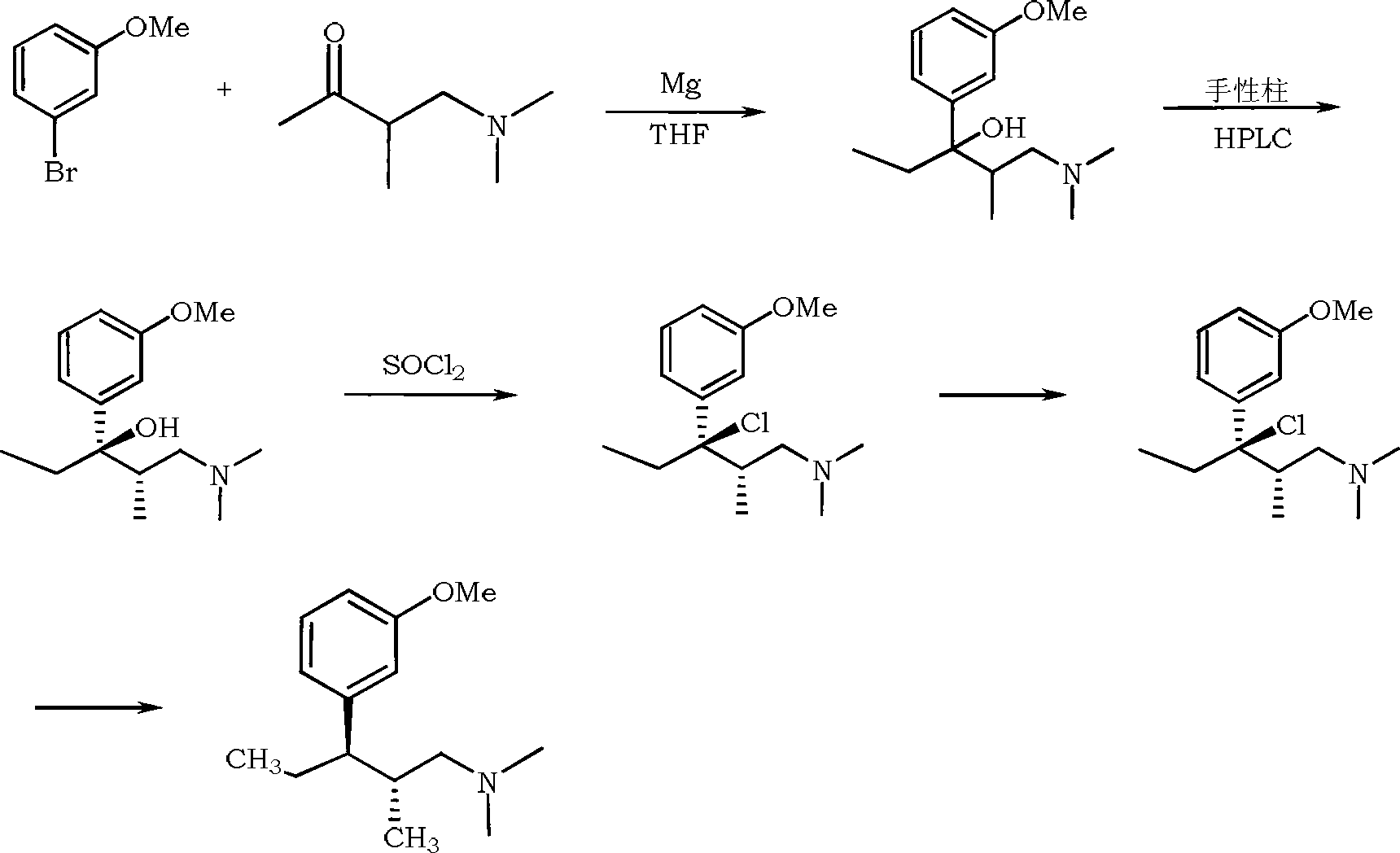
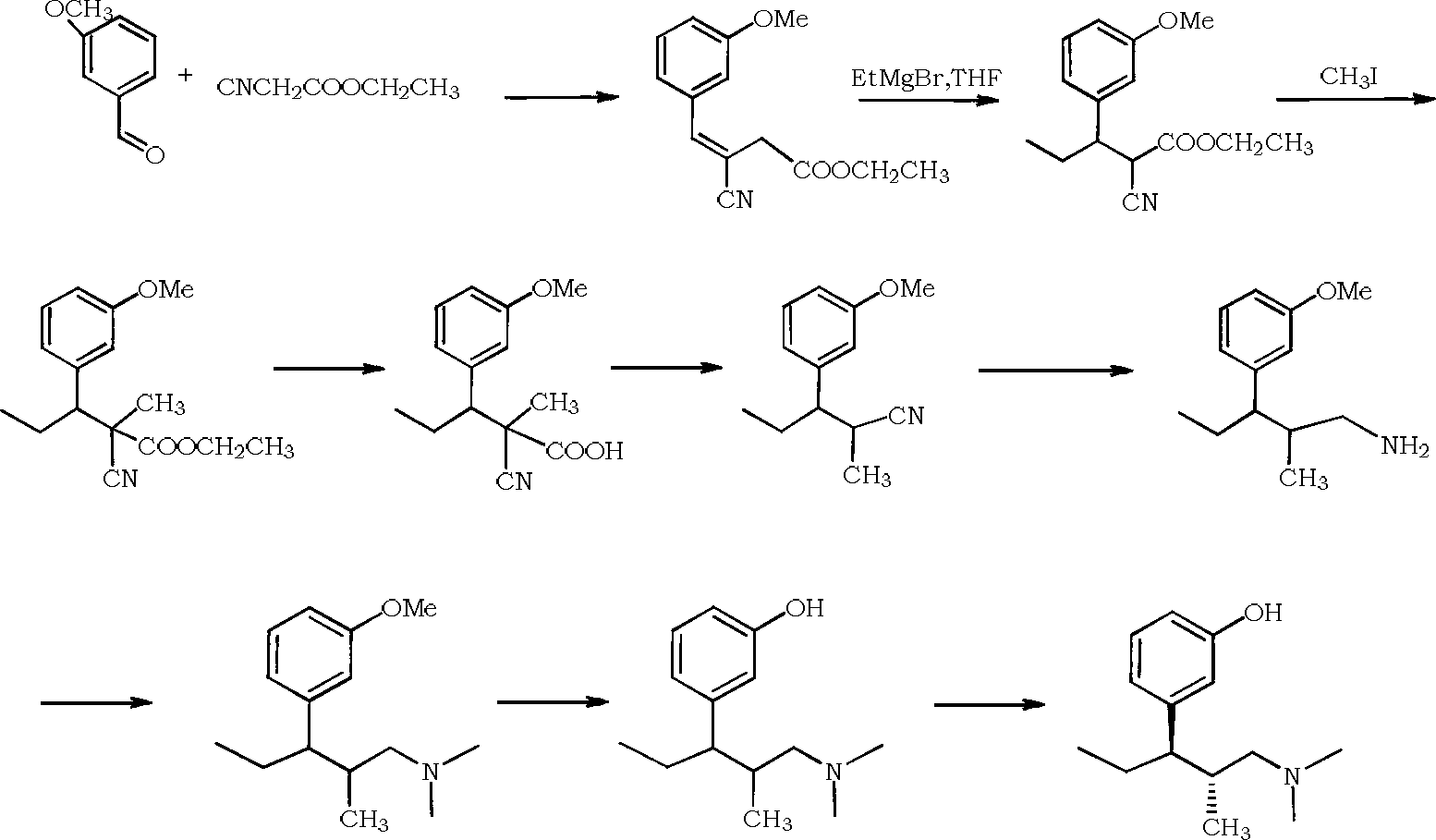
Synthesis method of tapentadol
A synthesis method and tapentadol technology, applied in the field of drug synthesis, can solve the problems of long reaction route steps, high toxicity and high production requirements, and achieve the effects of fewer synthesis steps, easy operation and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of 1-(dimethylamino)-2-methylpentan-3-ol (compound III)
[0062] 1.5L methanol, 1-dimethylamino-2-methyl-3-pentanone (143.23g, 1mol, compound II) were added to the reaction flask, stirred and passed into N 2 , when the system is down to about 0°C, add 60g (1.52mol) 96% sodium borohydride in batches, continue the insulation reaction for 8 hours after the addition (TLC monitoring in the reaction process), distill the solvent under reduced pressure, pour the reactant Pour into water, adjust the pH to neutral, extract with ethyl acetate, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure to obtain 124 g of colorless oil, yield: 85.4%, purity (GC): 96.2%.
[0063] Molecular formula: C 8 h 19 NO; molecular weight: 145.24; MS (m / z): 146.15 (M + +H).
Embodiment 2
[0064] Example 2 Preparation of 3-bromo-N, N, 2-trimethylpentan-1-amine (compound IV-A)
[0065] Add 1-(dimethylamino)-2-methylpentan-3-ol (145.3g, 1mol, compound III) and 2L of dichloromethane into the reaction flask, and when the temperature is cooled to about 0°C in an ice bath, dropwise 270.7(1mol)PBr 3 , the addition was completed in about 2 hours, and the stirring reaction was continued for 1 minute after the addition, and the degree of the reaction was monitored by TLC. After the reaction was completed, the reactant was poured into water, extracted with dichloromethane, and the organic layer was washed with aqueous sodium bicarbonate and saturated salt Washed with water, then washed with water, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to obtain 172g of yellow oil, yield: 82.6%, purity (GC): 97.6%.
[0066] Molecular formula: C 8 h 18BrN; molecular weight: 208.14; MS (m / z): 208.1 (M + ).
Embodiment 3
[0067] Example 3 Preparation of 3-(3-methoxyphenyl)-N,N-2-trimethylpentan-1-amine (compound VI)
[0068] Under the protection of nitrogen, bis(cyclooctyl 1,5-diene)nickel (1.37g, 0.005mol), 4,7-diphenyl-1,10-phenanthroline (CAS: 1945-77-3, 8.44g, 0.01mol), sec-butanol (CAS: 78-92-2, 200mL) were put into the reaction flask, stirred for 15 minutes, anhydrous potassium tert-butoxide (15.7g, 0.14mol), 3-methoxy phenylboronic acid (12.2g, 0.08mol, CAS: 5720-06-9) and 3-bromo-N,N,2-trimethylpentan-1-amine (20.8g, 0.1mol, compound IV-A), Slowly heated to 60°C, kept stirring and reacted overnight, TLC monitored the extent of the reaction, stopped the reaction after the reaction was complete, cooled to room temperature, slowly added the reaction solution into saturated saline, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, The filtrate was concentrated to dryness under reduced pressure and separated on a silica gel column to obtain 12.1g of light yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com