Preparation method of silodosin intermediate
A technology for organic ligands and compounds, applied in the field of preparing silodosin intermediates and new intermediate compounds, can solve problems such as unsuitable industrial production, and achieve the effects of simple operation, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
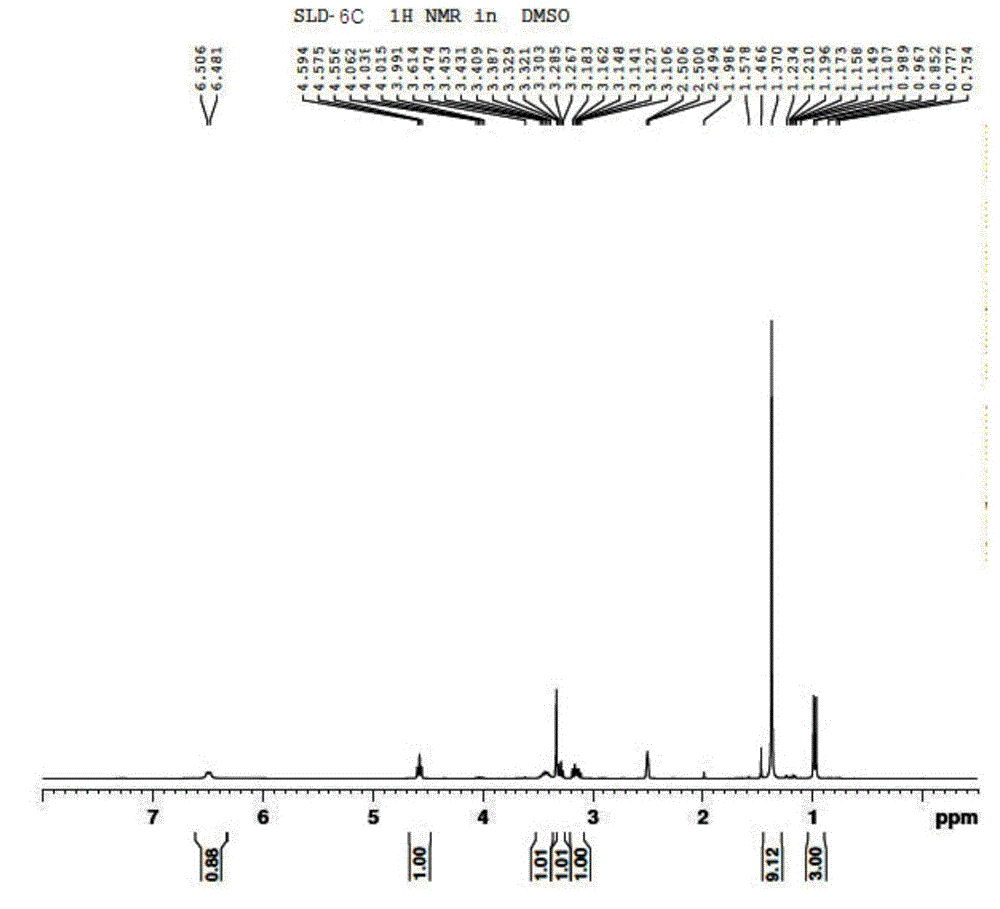
Image
Examples
Embodiment 1
[0081] 1) Preparation of compound SLD-8C
[0082]
[0083] Weigh 107g of SLD-9C (commercially available) into a 2L three-necked flask, add 600mL of methanol, stir mechanically and add 131mL of thionyl chloride dropwise in an ice-water bath, and build an exhaust gas absorption device, and then reflux for 5 to 8 hours after dropping.
[0084] After the completion of the reaction monitored by TLC, methanol was directly concentrated and evaporated, washed with 120mL*3 toluene, concentrated and evaporated to remove residual toluene, and pumped for 24 hours to obtain 167g of white solid SLD-8C, yield: 99.6%.
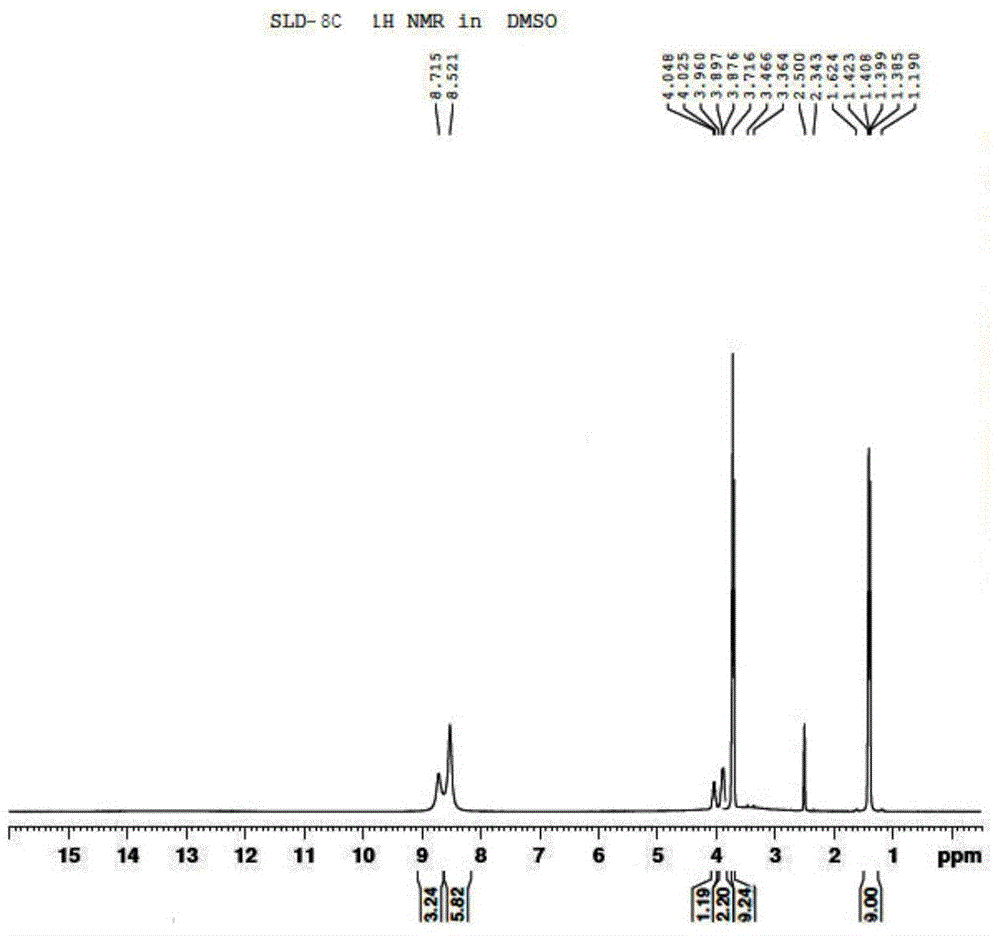
[0085] 1 H-NMR (d 6 -DMSO) δ: 8.71 (s, 1H); 8.52 (s, 2H); 3.71 ~ 4.05 (m, 1H); 3.36 ~ 3.46 (s, 3H); 1.19 ~ 1.62 (d, 3H).
[0086] Compound SLD-8C 1 H NMR chart see figure 1 .
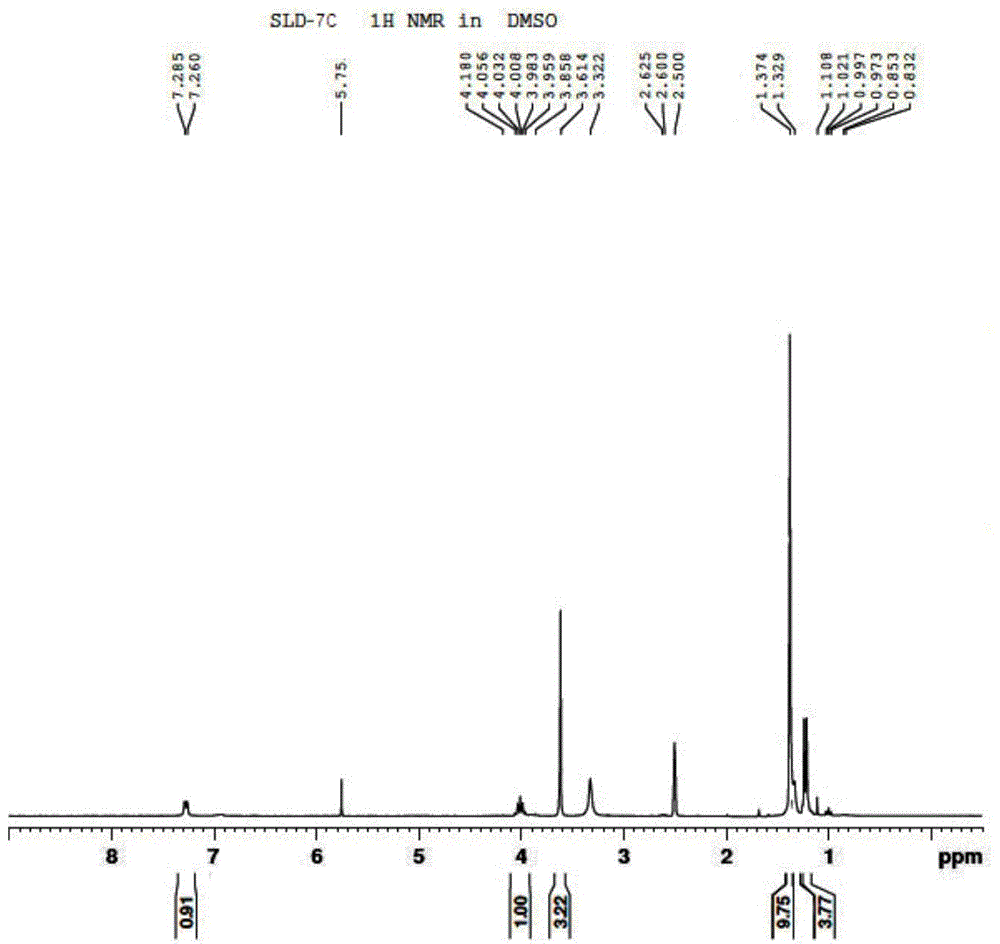
[0087] 3) Preparation of compound SLD-7C
[0088]
[0089] Weigh 167g of SLD-8C, 242g of triethylamine, and 1L of dichloromethane into a 2L three-necked flask, add dropwise 313g of di-tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com