Preparation method of isoxazoline insecticide
An isoxazoline and pesticide technology, which is applied in the chemical industry, can solve the problems of high cost of commercialized large-scale production products, long routes, long routes, etc., and achieves easy realization of reaction conditions, reduction of three waste discharges, and easy availability of raw material costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
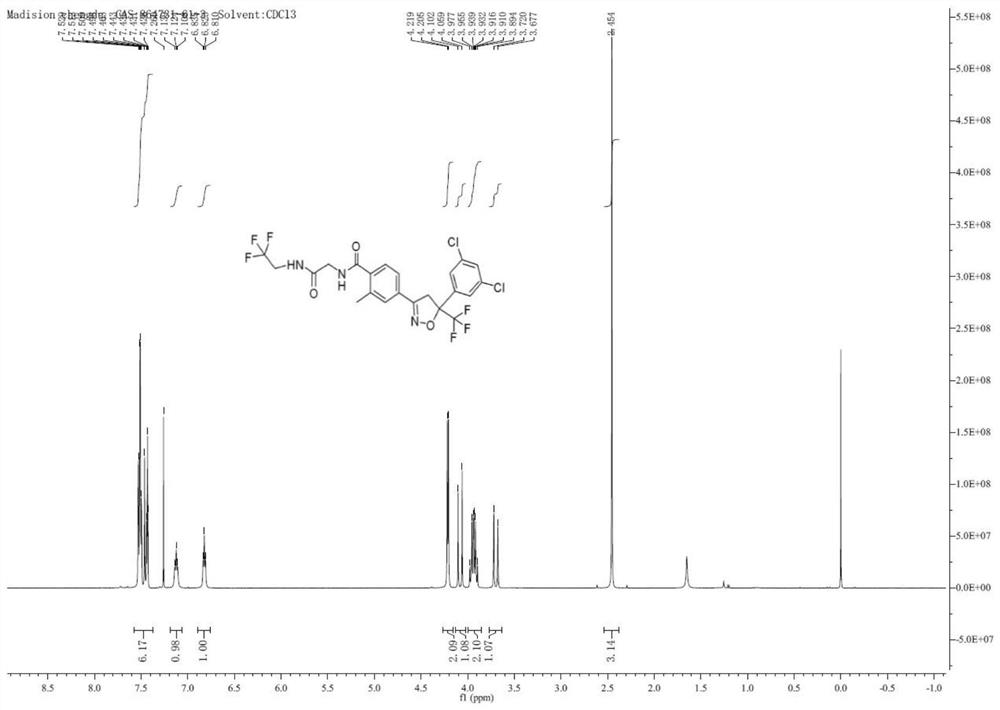
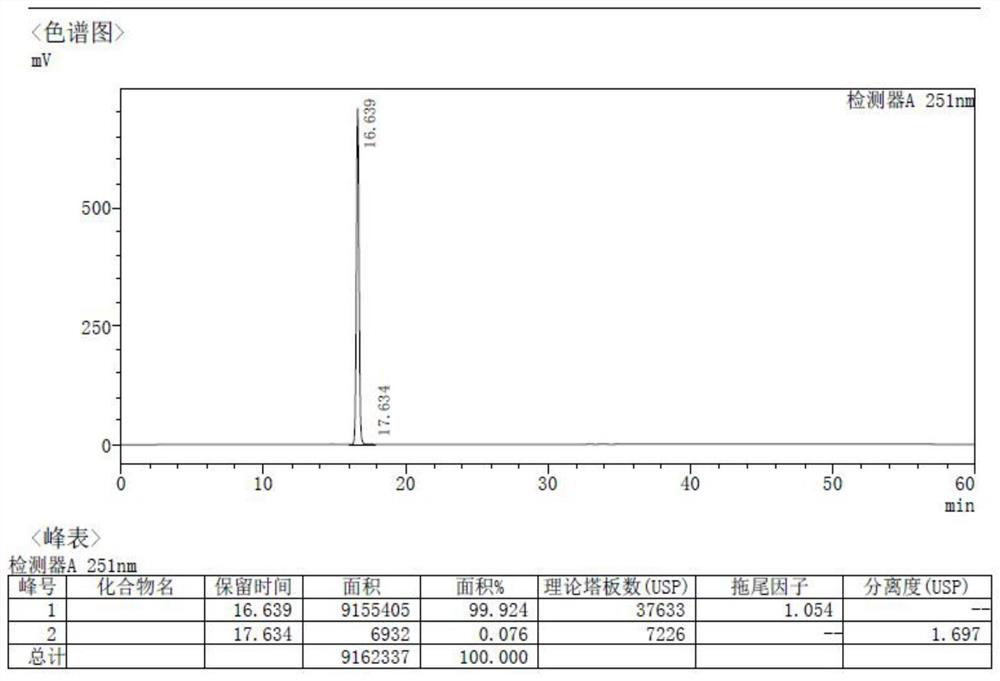
[0051] Embodiment 1 (preparation of Freilaner 1-1)
[0052] 1.1 Coupling reaction:
[0053]
[0054] Add Cs sequentially to the 100 ml reaction vial 2 CO 3 (37.88g, 0.12mol), 3A molecular sieves (10g), Pd 2 (dba) 3 (212.0mg, 0.5%eq), Xphos (1.33g, 6%eq), nitromethane (10mL), nitrogen replacement 3 times, and then dissolve 10g of intermediate 1 (4-bromo-2-methylbenzoic acid) In 10ml of ethylene glycol dimethyl ether, the temperature was raised to 50°C, and the reaction was detected by TLC. After the reaction was complete, it was cooled to room temperature, filtered, and the filter cake was washed with dichloromethane (10mLx2), added to 20ml of ammonium chloride solution, and separated phase, the organic phase was extracted with 20ml of dichloromethane, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was spin-dried under reduced pressure to obtain Intermediate 2 (6.36g, yield 70%).
[0055] 1.2 Ring closi...
Embodiment 2
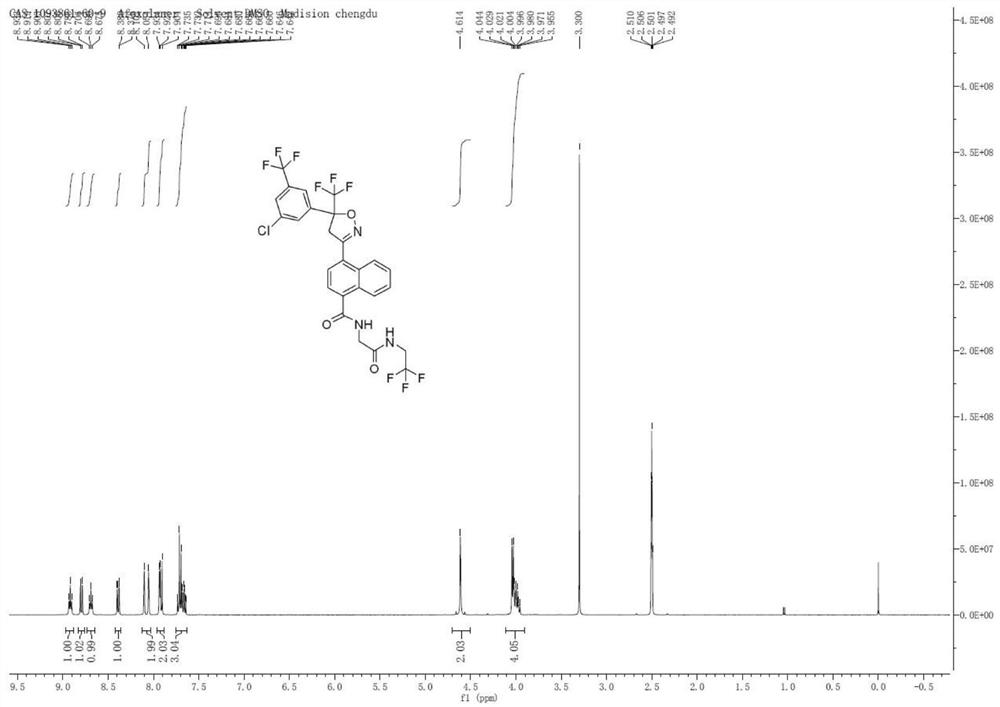
[0065] Embodiment 2 (preparation of Afulaner 1-2)
[0066] 2.1 Coupling reaction:
[0067]
[0068] Add Cs sequentially to the 100 ml reaction vial 2 CO 3 (25.15g, 79.67mmol), 3A molecular sieves (8g), Pd 2 (dba) 3 (182.4mg, 0.5%eq), Xphos (1.14g, 6%eq), nitromethane (10mL), nitrogen replacement 3 times, and then 10g of intermediate 1 (4-bromo-1-naphthoic acid) was dissolved in 10ml In ethylene glycol dimethyl ether, the temperature was raised to 50°C, and the reaction was detected by TLC. After the reaction was complete, it was cooled to room temperature, filtered, and the filter cake was washed with dichloromethane (10mLx2), added to 20ml of ammonium chloride solution, and the phases were separated. The organic phase was extracted with 20ml of dichloromethane, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was spin-dried under reduced pressure to obtain Intermediate 2 (5.80g, yield 63%).
[0069] 2.2...
Embodiment 3
[0078] Embodiment 3 (preparation of lotirana 1-3)
[0079] 3.1 Coupling reaction:
[0080]
[0081] Add Cs sequentially to the 100 ml reaction vial 2 CO 3 (28.55g, 90.46mmol), 3A molecular sieves (9g), Pd 2 (dba) 3 (206.0mg, 0.5%eq), Xphos (1.29g, 6%eq), nitromethane (9mL), nitrogen replacement 3 times, and then 10g of intermediate 1 (5-bromo-3-methylthiophene-2- Formic acid) was dissolved in 10ml of ethylene glycol dimethyl ether, heated to 50°C, and the reaction was detected by TLC. After sufficient reaction, cooled to room temperature, filtered, and the filter cake was washed with dichloromethane (10mLx2), and added to 20ml of ammonium chloride Solution, phase separation, the organic phase was extracted with 20ml of dichloromethane, the combined organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was spin-dried under reduced pressure to obtain intermediate 2 (6.73g, yield 74%) .
[0082] 3.2 Ring closing reaction:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com