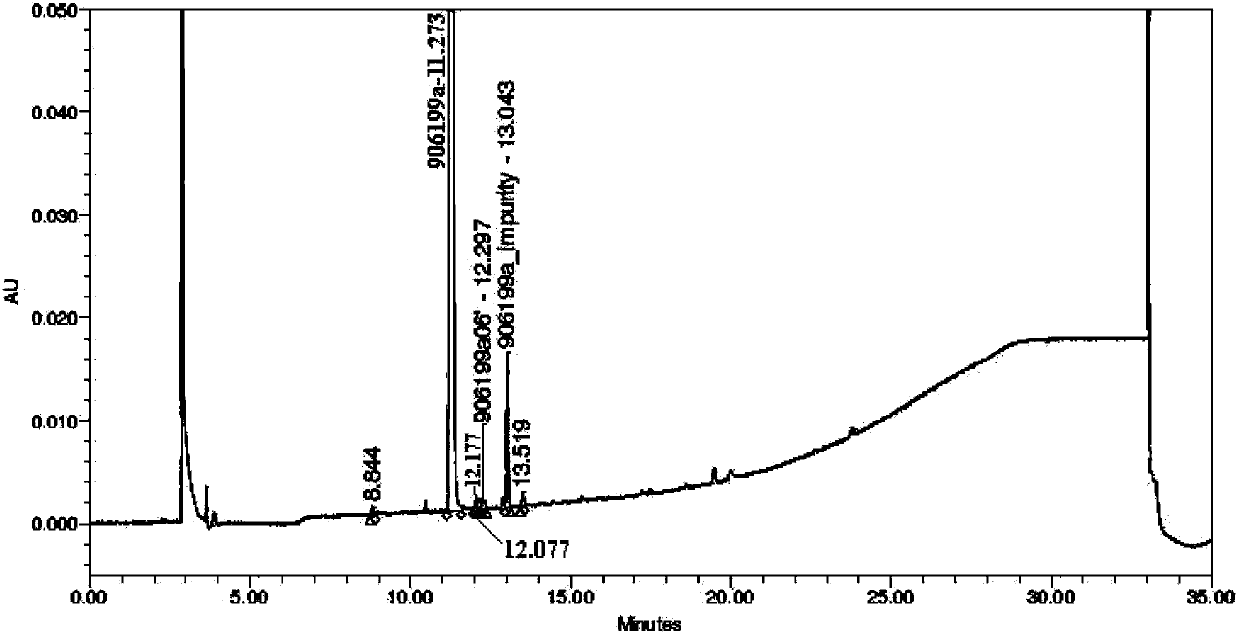
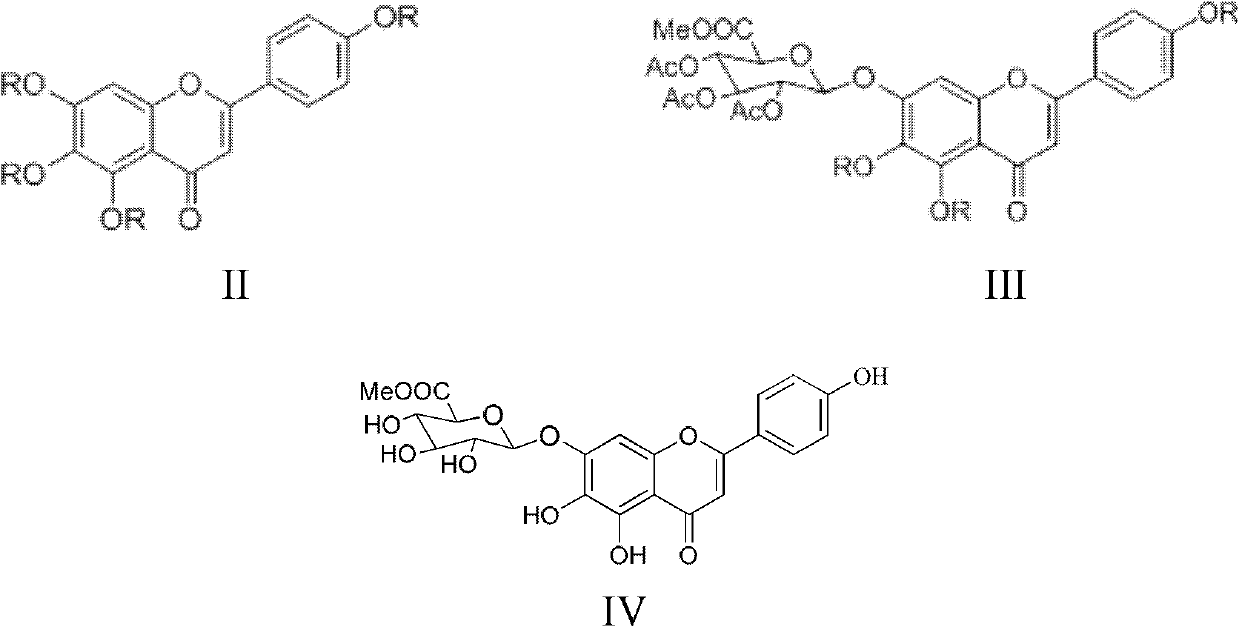
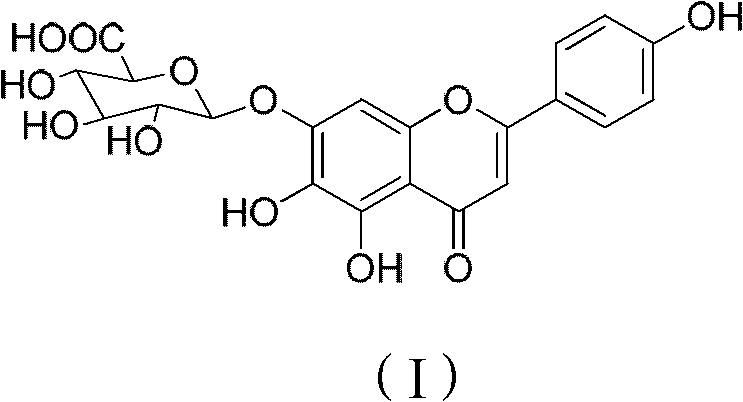
Method for preparing 5,6,4'-trihydroxy flavone-7-0-D-glucuronic acid
A technology of glucuronic acid and trihydroxyflavone is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., and can solve the problems of expensive reaction reagents, cumbersome process operations, harsh reaction conditions, etc. The effect of shortening the reaction route and shortening the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Preparation of 5,6,7,4'-tetra-pivaloyloxyflavone
[0058] 10g (70mmol) of 5,6,7,4'-tetrahydroxyflavone was dissolved in 60mL of pyridine, and 86mL of pivaloyl chloride (d=0.976g / mL, 1.4mol) was slowly added dropwise at room temperature, and heated to 130°C for reflux reaction 6 After ~8 hours, TLC detected that the reaction was complete. When the reaction liquid was cooled to about 70°C, 100 mL of ethyl acetate was added, and a large amount of white solid gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration gave 5,6,7,4'-tetrapivaloyloxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 18 g of pure 5,6,7,4'-tetrapivaloyloxyflavone, a white product with a chromatographic purity of over 98.00% and a yield of 84%. 1H-NMR (400MHz, CDC13), δ (ppm): 7.75 (2H, d, J = 8.6Hz), 7.13 (1H, s), 7.12 (2H, d, J = 8.6Hz), 6.48 (1H, s ), 1.59(9H, s), 1.54(9H...
Embodiment 2
[0068] 1. Preparation of 5,6,7,4'-tetrabenzoyloxyflavone
[0069] Dissolve 10g (35mmol) of 5,6,7,4'-tetrahydroxyflavone in 70mL of pyridine, slowly add 81mL of benzoyl chloride (d=1.212, 700mmol) under stirring at room temperature, heat to 180°C and reflux for about 9 hours, TLC It was detected that the reaction was complete. When the reaction solution was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solids gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,6,7,4'-tetrabenzoyloxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 21.4 g of pure 5,6,7,4'-tetrabenzoyloxyflavone, an off-white product, with a chromatographic purity of over 98.0% and a yield of 87%. 1H-NMR (400MHz, DMSO), δ (ppm): 8.56 (2H, d, J = 8.9Hz), 8.55 (2H, d, J = 8.9Hz), 8.54 (2H, d, J = 8.9Hz), 8.52 (2H, d, J = 8.9Hz), 8.10 (2H, d...
Embodiment 3
[0077] 1. Preparation of 5,6,7,4'-tetraacetoxyflavone
[0078] Dissolve 10g (35mmol) of 5,6,7,4'-tetrahydroxyflavone in 50mL of pyridine, slowly add 30mL of acetic anhydride (d=1.08, 400mmol) dropwise at room temperature, heat to 140°C and reflux for 4-5 hours, TLC The detection reaction is complete. When the reaction solution was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solid gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,6,7,4'-tetraacetoxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 13.7 g of pure off-white 5,6,7,4'-tetraacetoxyflavone, with a chromatographic purity of over 98.00% and a yield of 86%. 1H-NMR (400MHz, CDC13), δ (ppm): 7.64 (2H, d, J = 8.7Hz), 7.03 (1H, s), 7.02 (2H, d, J = 8.6Hz), 6.39 (1H, s ), 2.21(3H, s), 2.14(3H, s), 2.12(3H, s), 2.11(3H, s).
[0079] 2. Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com