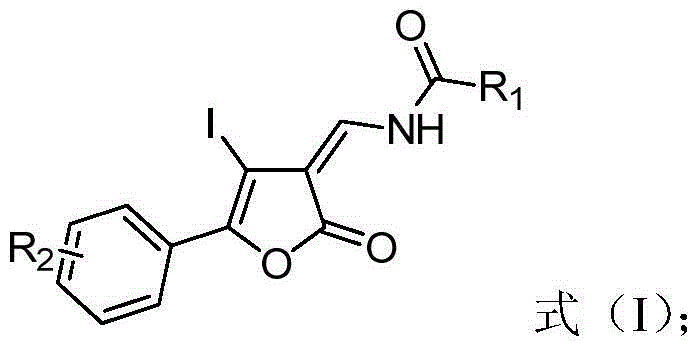
Furanone derivative and synthetic method thereof
A synthetic method and technology of furanone, which is applied in iodine-catalyzed furanone derivatives and its synthesis field, can solve the problems that raw materials are not easy to obtain, and achieve the effects of cheap catalyst, environmental friendliness and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: trans-N-(4-iodo-5-(4 , Synthesis of -methoxyphenyl)-2-oxofuran-3-ylidene)methylbenzamide
[0027]
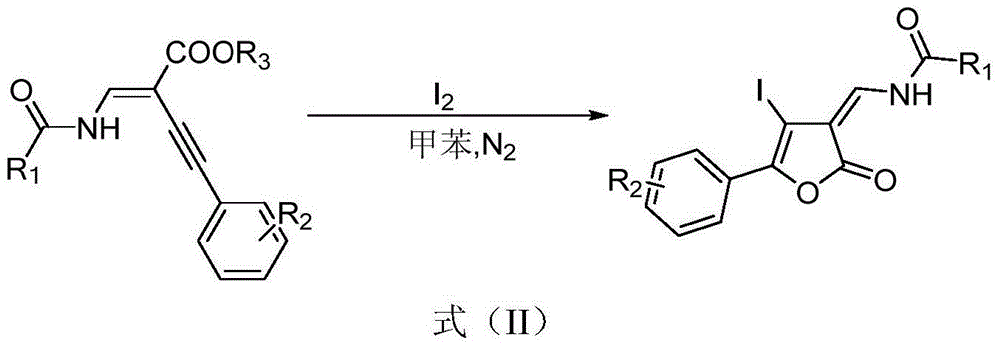
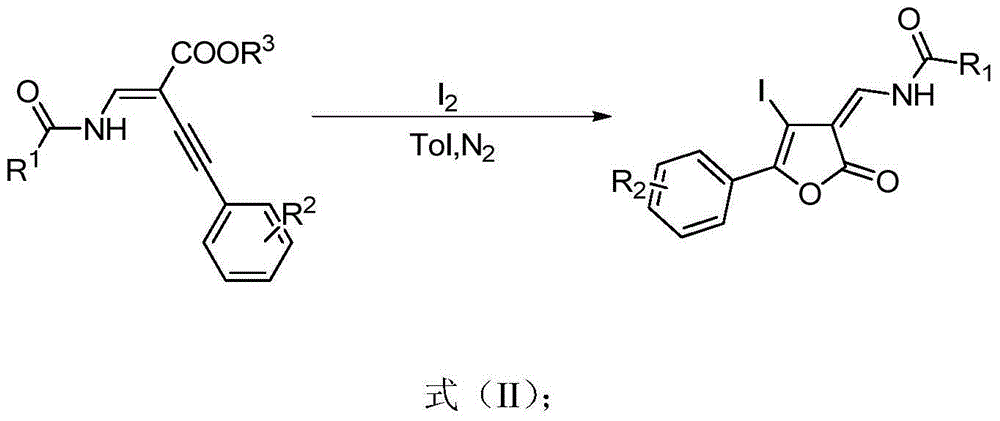
[0028] N-amido-substituted enynyl esters, iodine, and solvents are selected respectively: trans-ethyl-2-(benzoylmethylene)-4-(4,-methoxyphenyl)but-3-ynoic acid Methyl ester, I 2 , toluene, the amount of raw materials is trans-ethyl-2-(benzoylmethylene)-4-(4,-methoxyphenyl) but-3-ynoic acid methyl ester 0.2mmol, iodine simple substance 0.4mmol, Toluene 2ml. Reacted at 100°C for 6 hours to obtain the target product, a yellow solid, with an isolated yield of 65%, mp: 215-216°C
[0029] NMR data: 1 H NMR (500MHz, CD 2 Cl 2 , Me 4 Si)δ3.85(s,3H),6.99(d,J=9.0Hz,2H),7.57(t,J=8.0Hz,2H),7.66(t,J=7.5Hz,1H),7.93-8.01 (m,5H),11.16(d,J=11.0Hz,1H); 13 C NMR (125MHz, CD 2 Cl 2 , Me 4 Si)d 55.99, 58.39, 112.17, 114.53, 121.42, 128.41, 129.31, 129.71, 132.13, 134.02, 136.53, 149.68, 161.65, 164.33, 168.58;
[0030] High resolution variable rate mass spectrometry...
Embodiment 3
[0035] Embodiment 3: Synthesis of trans-N-(4-iodo-2-oxo-5-phenylfuran-3-ylidene)methylbenzamide
[0036]
[0037] N-amido-substituted enynyl esters, iodine, and solvents are selected respectively: trans-ethyl-2-(benzoylmethylene)-4-phenylbut-3-ynoic acid methyl ester, I 2 , Toluene, the amount of raw materials is trans-ethyl-2-(benzoylmethylene)-4-phenylbut-3-ynoic acid methyl ester 0.2mmol, iodine simple substance 0.4mmol, toluene 2ml. React at 100°C for 6 hours to obtain the target product, a yellow solid, with an isolated yield of 64%, mp: 148-150°C.
[0038] NMR data: 1 H NMR (400MHz, CDCl 3 , Me 4 Si) δ7.41-7.46(m, 3H), 7.55(t, J=7.6Hz, 2H), 7.64(d, J=7.6Hz, 1H), 7.99-8.05(m, 5H), 11.23(d, J=11.2Hz,1H); 13 C NMR (100.6MHz, CDCl 3 , Me 4 Si)d 59.85, 111.59, 127.24, 128.15, 128.44, 128.67, 129.35, 130.24, 131.42, 133.81, 137.24, 149.30, 164.26, 168.39;
[0039] High-resolution mass spectrometry data: HRMS(EI)calcd for C 18 h 12 INO 3 :416.9862, found 416.9864. ...
Embodiment 4
[0040] Example 4: Synthesis of trans-N-(4-iodo-2-oxo-5-phenylfuran-3-ylidene)methyl pivalamide
[0041]
[0042] N-amido-substituted enynyl esters, iodine, and solvents are selected respectively: trans-ethyl-4-phenyl-2-(pivalylaminomethylene) but-3-ynoic acid methyl ester, I 2 , Toluene, the amount of raw materials is trans-ethyl-4-phenyl-2-(pivaloylaminomethylene) but-3-ynoic acid methyl ester 0.2mmol, iodine simple substance 0.4mmol, toluene 2ml. Reacted at 100°C for 6 hours to obtain the target product, a yellow liquid, with an isolated yield of 63%
[0043] NMR data: 1 H NMR (400MHz, CDCl 3 , Me 4 Si)δ1.35(s,9H),7.42-7.48(m,3H),7.82(d,J=11.2Hz,1H),8.02-8.04(m,2H),10.67(d,J=10.8Hz, 1H); 13 C NMR (100.6MHz, CDCl 3 , Me 4 Si)d 26.86, 39.43, 59.87, 110.95, 127.28, 128.60, 128.69, 130.19, 137.51, 149.14, 136.36, 177.00;
[0044] High-resolution mass spectrometry data: HRMS(EI)calcd for C 16 h 16 INO 3 :397.0175, found 397.0177.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com