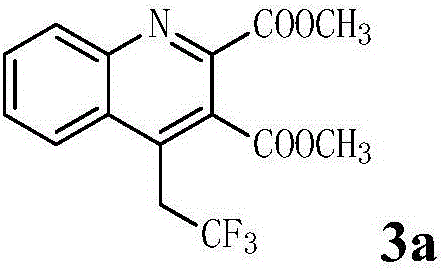
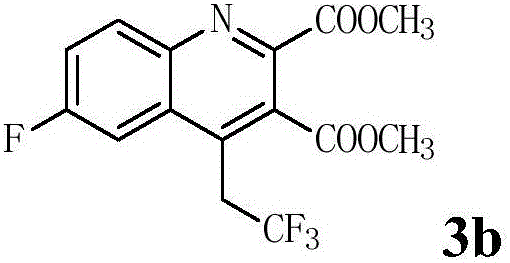
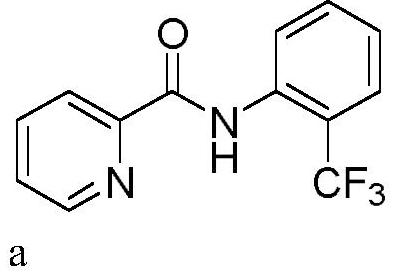
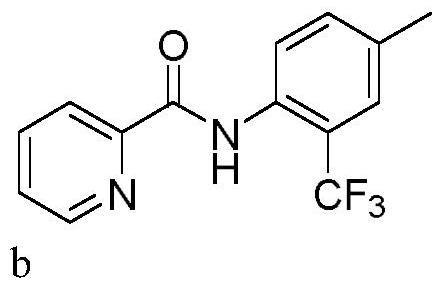
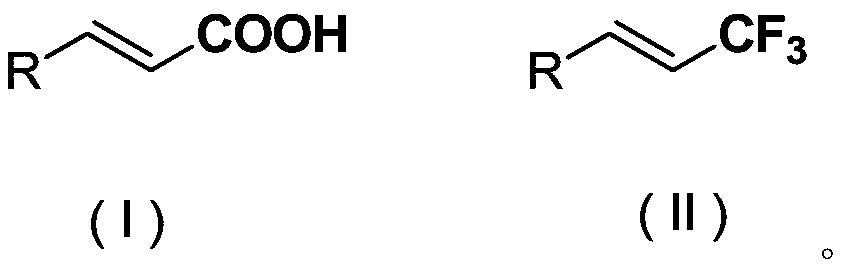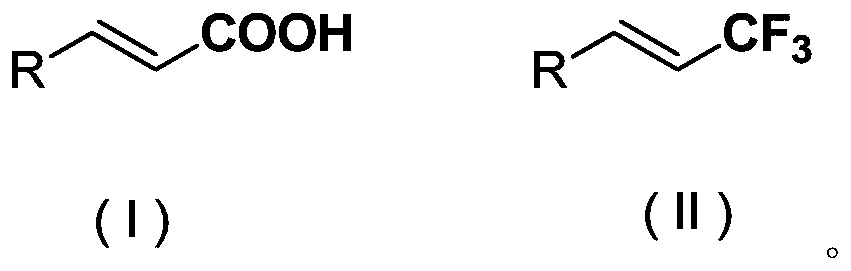Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Trifluoromethylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
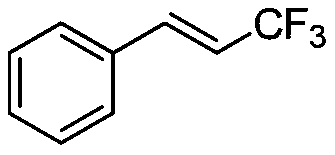
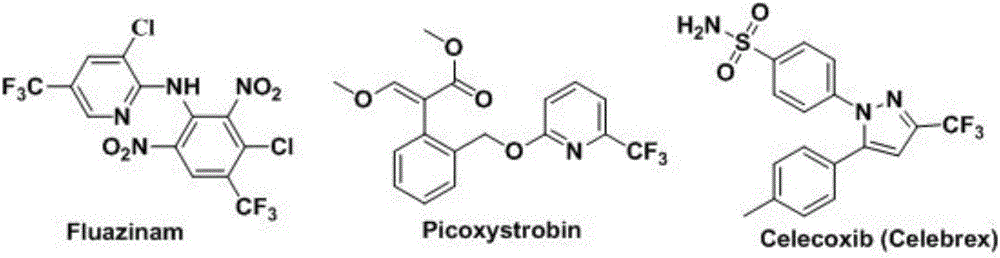
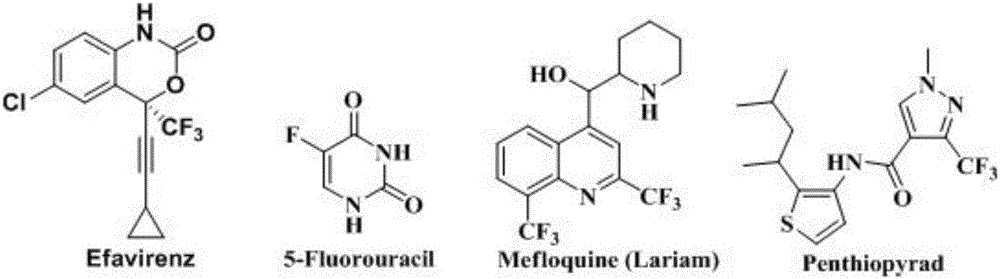
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin. The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.
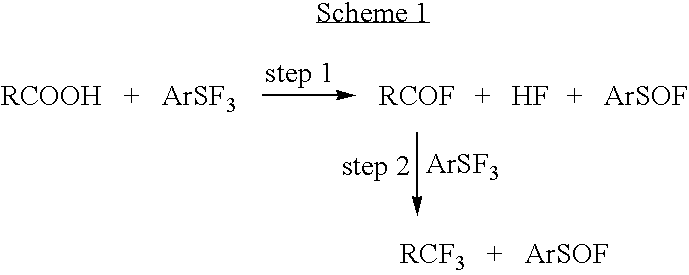
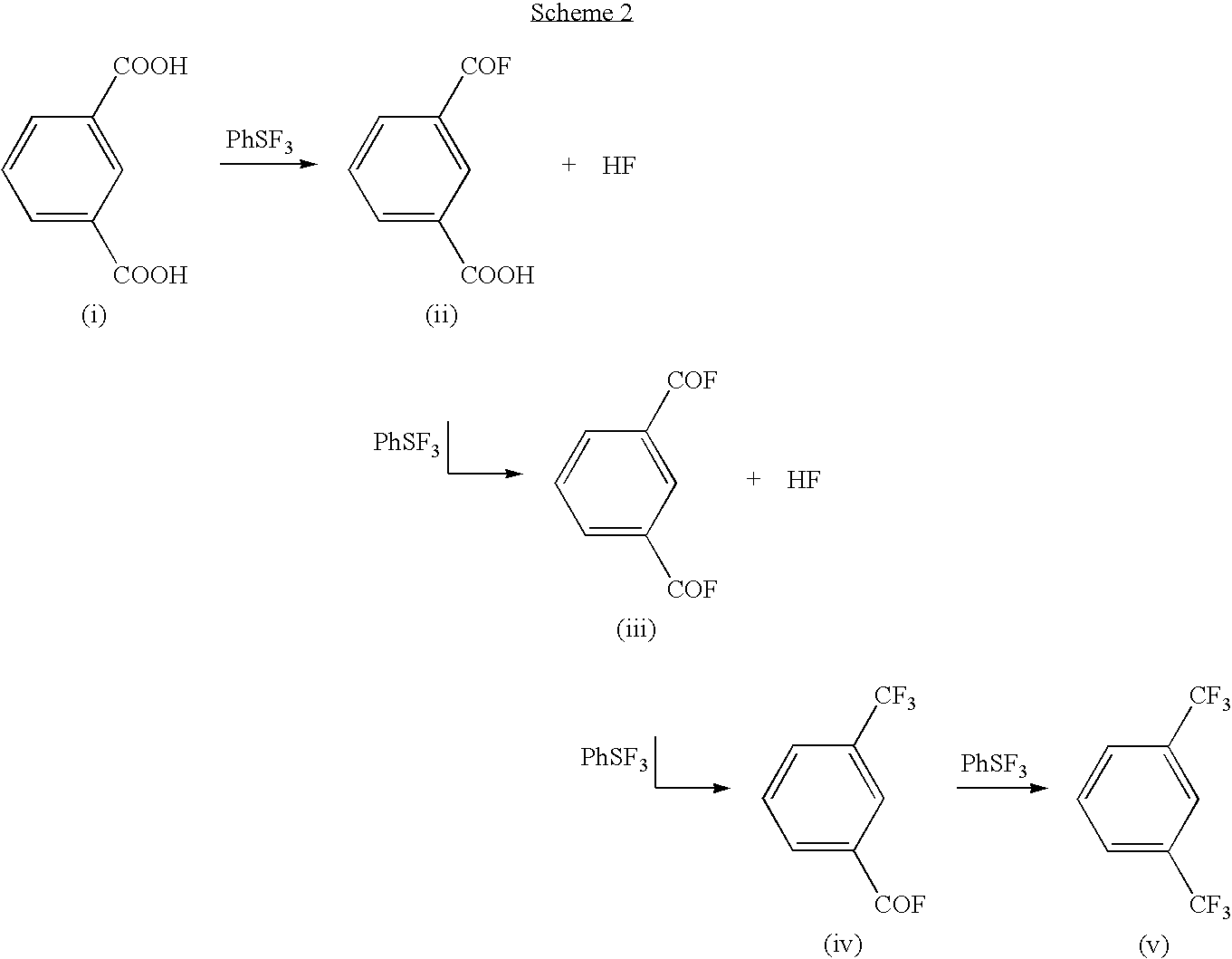
Methods and compositions for producing difluoromethylene-and trifluoromethyl-containing compounds
InactiveUS20100234605A1Tremendous potential in electronicGreat potentialOrganic compound preparationPreparation by halogen replacementTrifluoromethylationTrifluoride
New methods for producing difluoromethylene-containing compounds with phenylsulfur trifluoride or a primary alkyl-substituted phenylsulfur trifluoride are disclosed. Also, new methods for producing trifluoromethyl-containing compounds with phenylsulfur trifluoride or primary alkyl-substituted phenylsulfur trifluoride are also disclosed.
Owner:UBE IND LTD
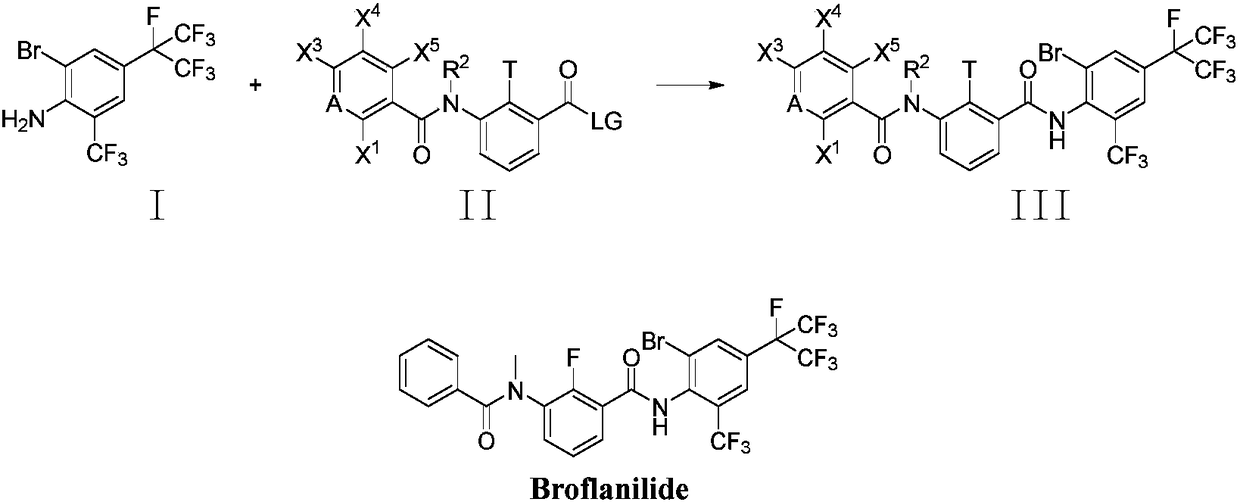
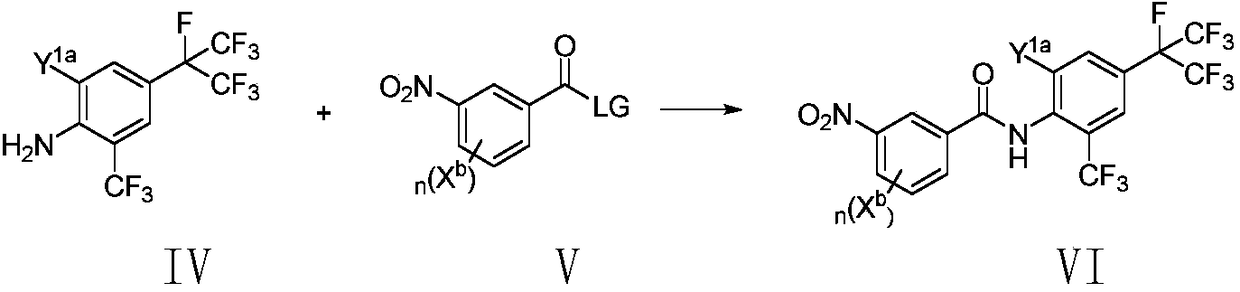
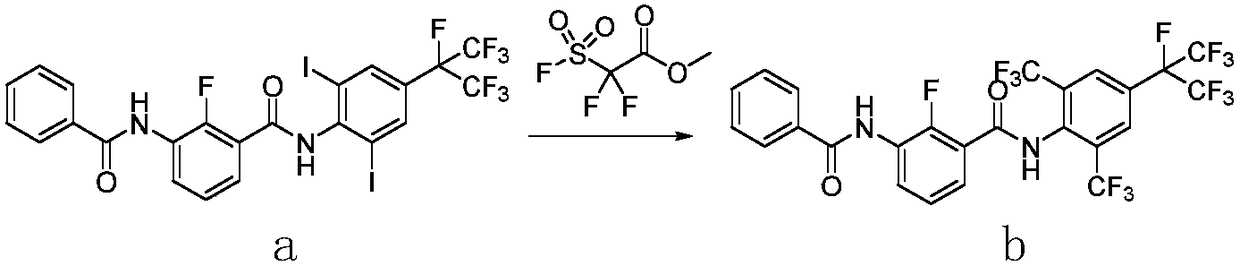
Method for preparing o-trifluoromethylaniline compound and intermediate thereof
ActiveCN109206335AMild reaction conditionsHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationTrifluoromethylationOrganic synthesis
The invention belongs to the field of organic synthesis, and specifically relates to a method for preparing an o-trifluoromethylaniline compound and an intermediate thereof. The reaction formula is asshown in the specification. In the formula, each substituent group is described in the specification. Specifically, a compound VII and a compound VIII react in a suitable reaction solvent under the action of a suitable catalyst to prepare a compound IX, and the compound IX further reacts with a suitable trifluoromethylating reagent under suitable reaction conditions to prepare a compound X. The invention provides an effective preparation method for the industrial development of the insecticidal compounds.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
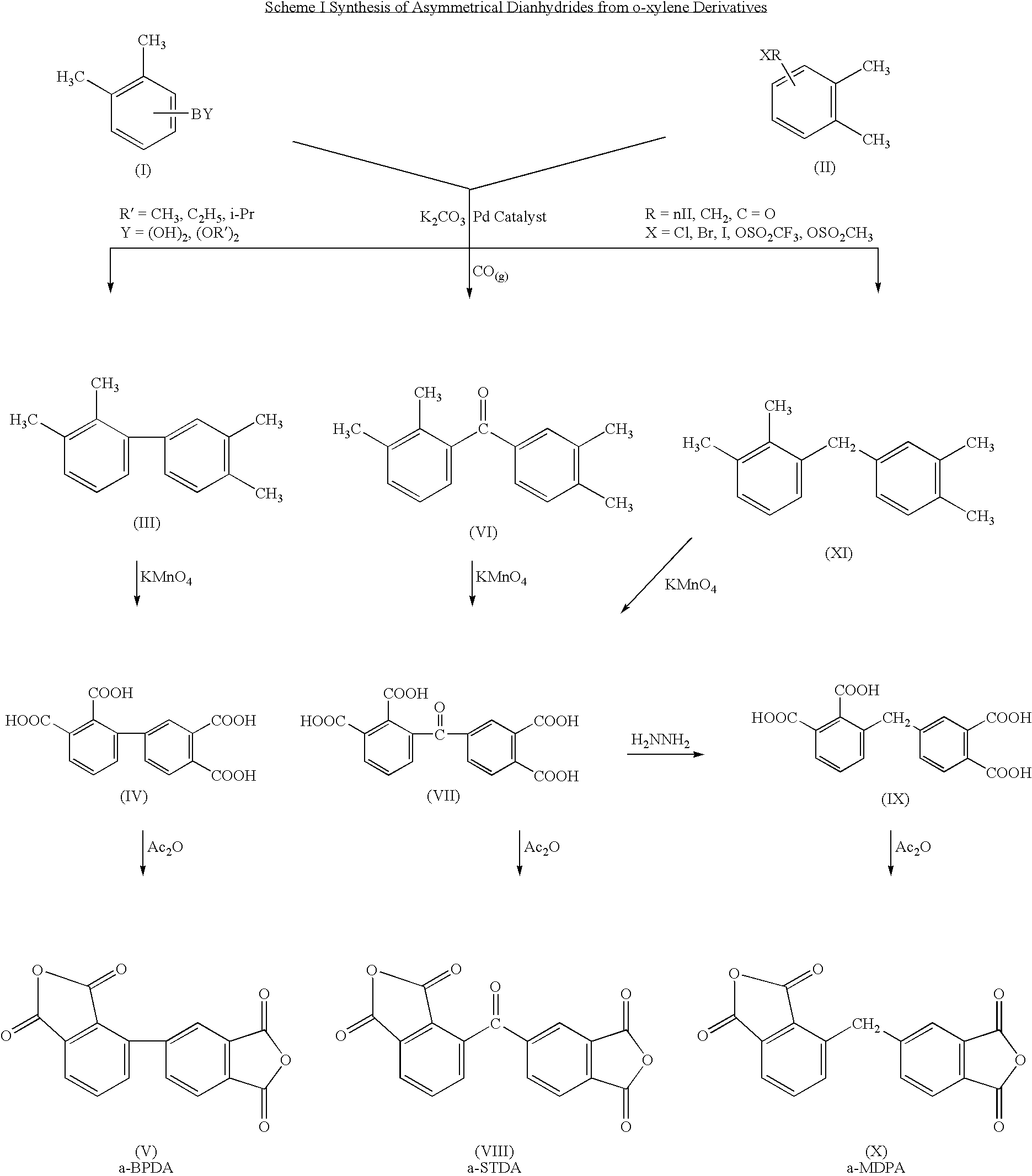
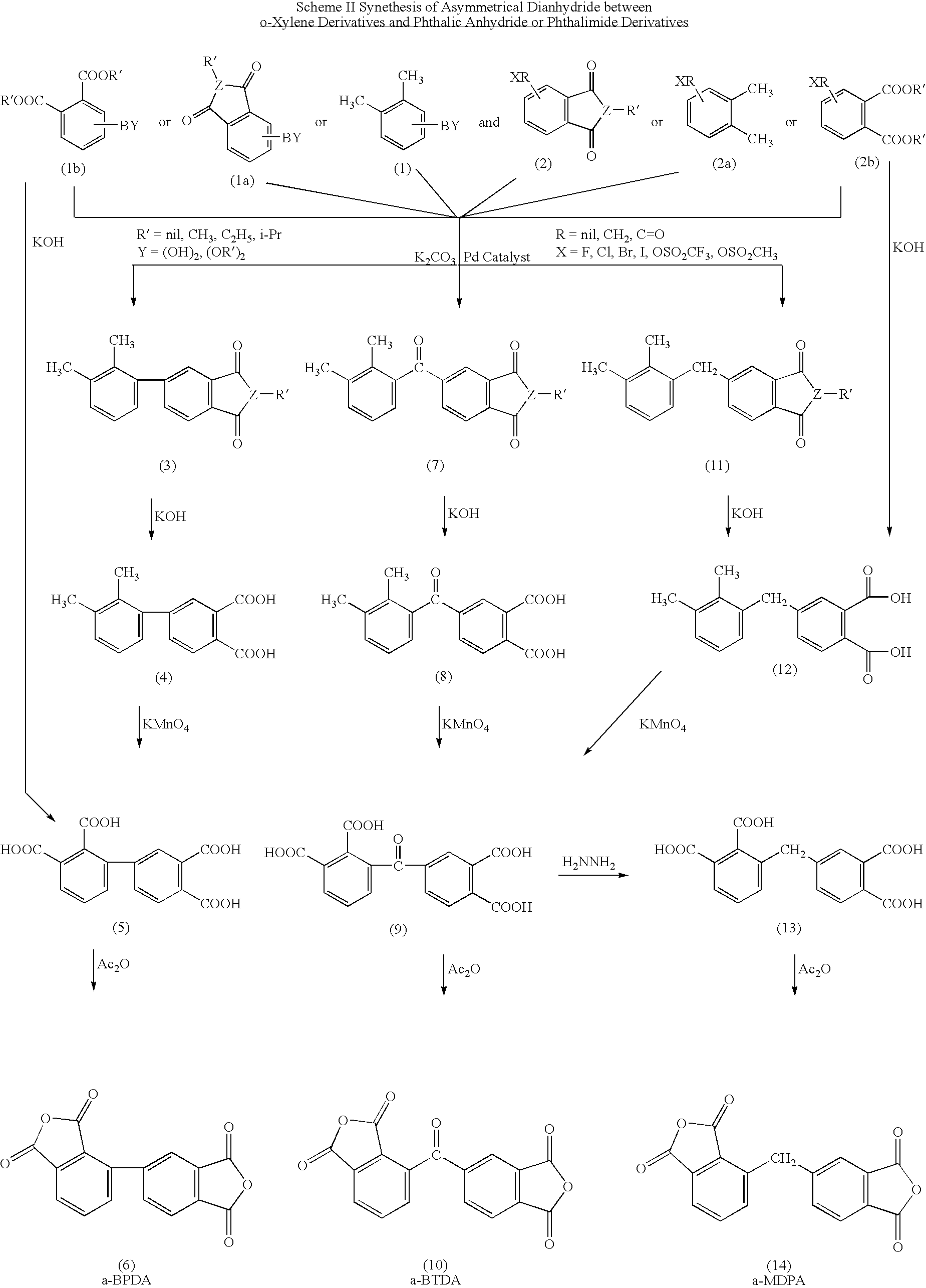
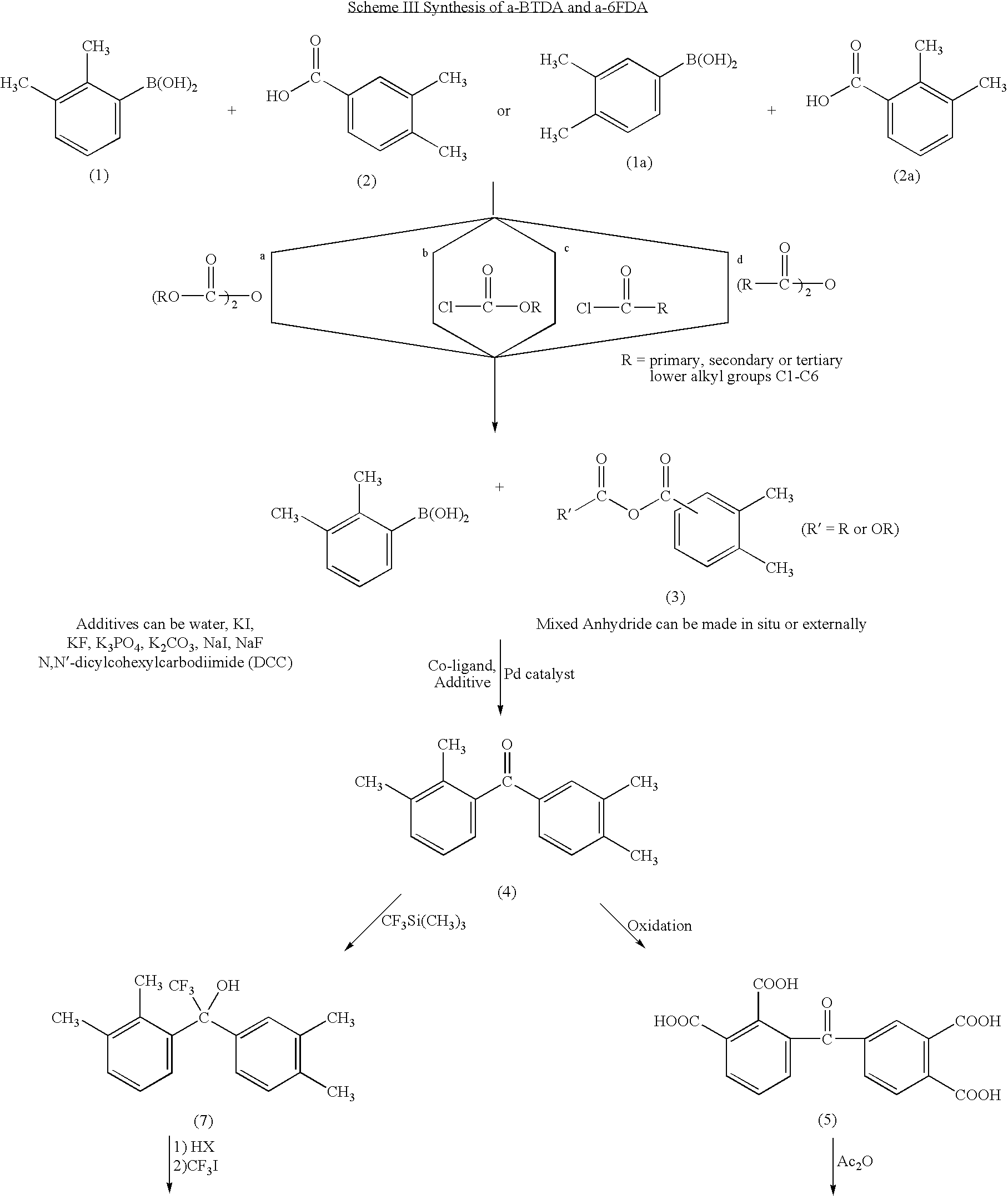
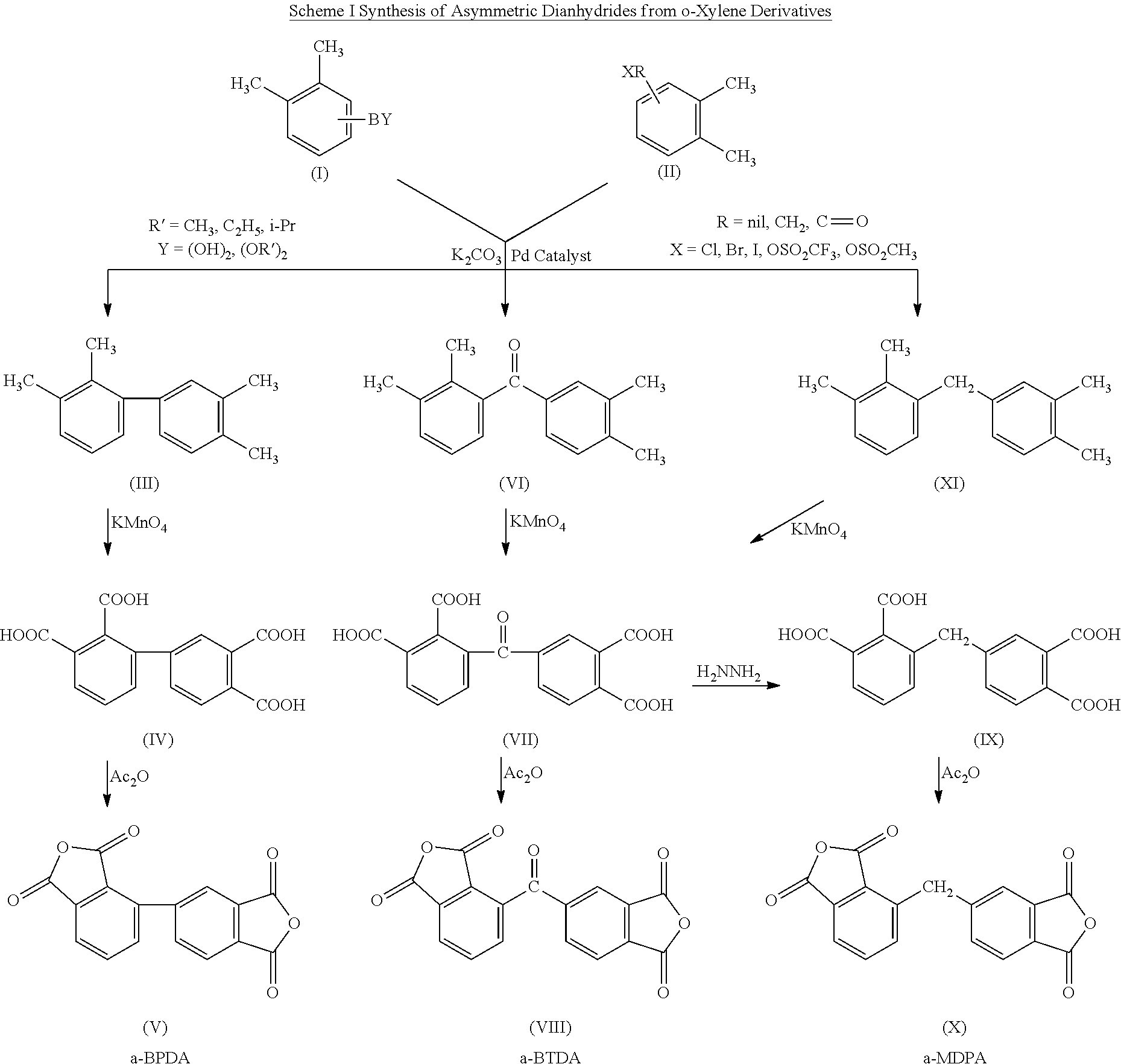
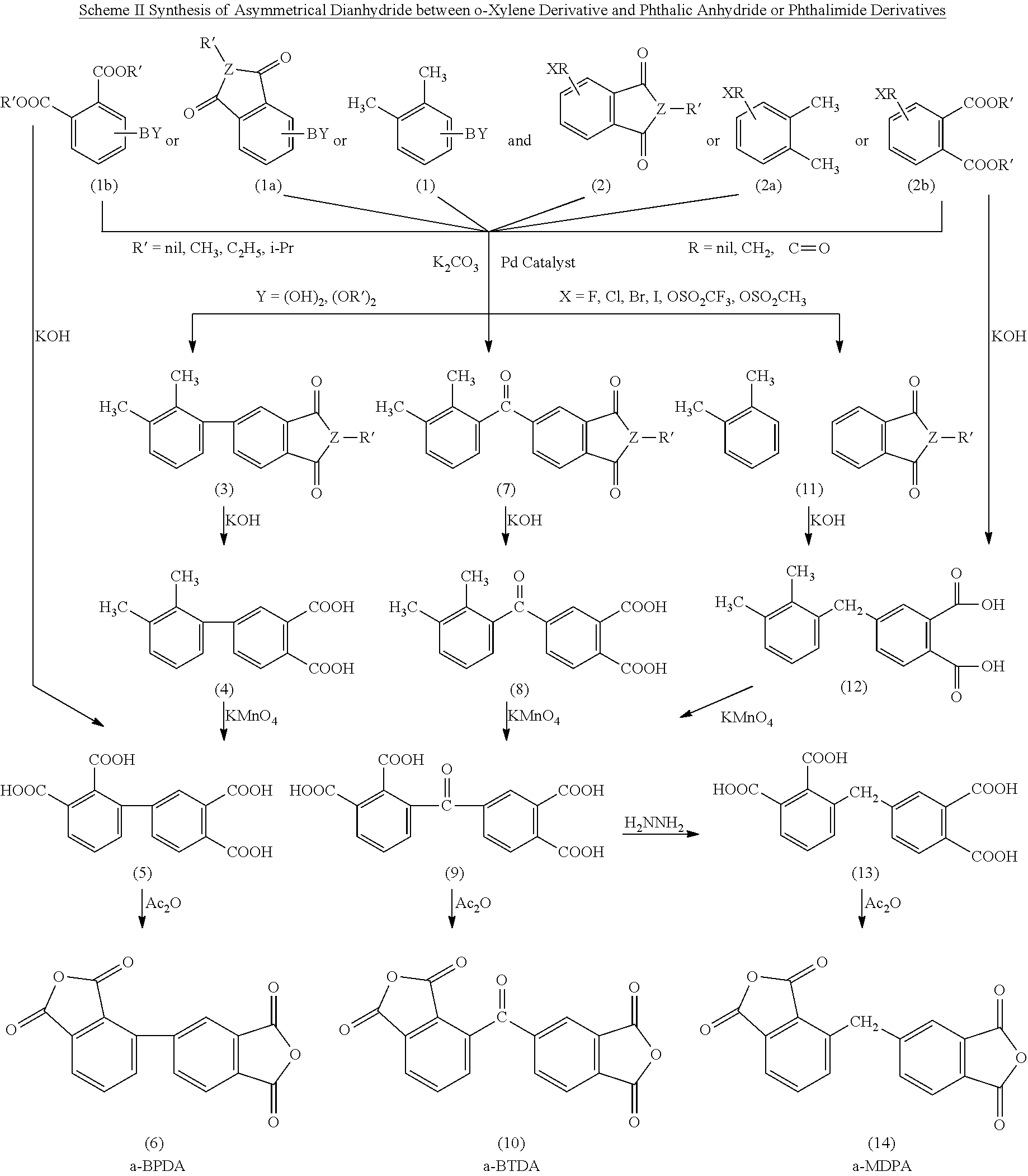
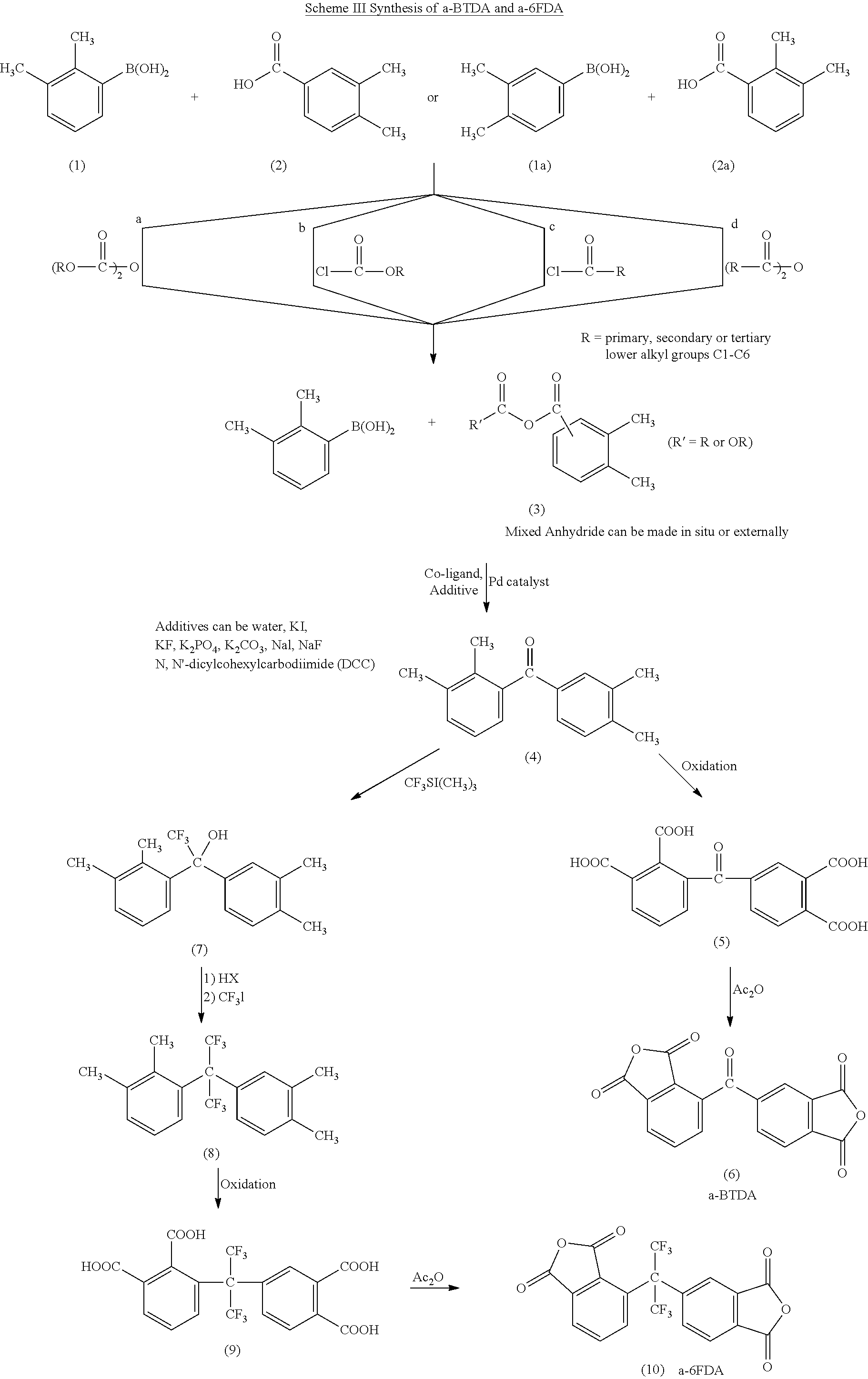
Polyimides derived from novel asymmetric dianhydrides
ActiveUS8093348B1Improve wear resistanceImprove adhesionOrganic chemistryTrifluoromethylationO-Xylene
This invention relates to the compositions and processes for preparing thermoset and thermoplastic polyimides derived from novel asymmetrical dianhydrides: specifically 2,3,3′,4′ benzophenone dianhydride (a-BTDA), and 3,4′-(hexafluoroisopropylidene)diphthalic anhydride (a-6FDA). The a-BTDA anhydride is prepared by Suzuki coupling with catalysts from a mixed anhydride of 3,4-dimethylbenzoic acid or 2,3-dimethylbenzoic acid with 2,3-dimethylphenylboronic acid or 3,4-dimethylphenylboronic acid respectively, to form 2,3,3′,4′-tetramethylbenzophenone which is oxidized to form 2,3,3′,4′-benzophenonetetracarboxylic acid followed by cyclodehydration to obtain a-BTDA. The a-6FDA is prepared by nucleophilic triflouoromethylation of 2,3,3′,4′-tetramethylbenzophenone with trifluoromethyltrimethylsilane to form 3,4′-(trifluoromethylmethanol)-bis(o-xylene) which is converted to 3,4′-(hexafluoroisopropylidene-bis(o-xylene). The 3,4′-(hexafluoroisopropylidene)-bis(o-xylene) is oxidized to the corresponding tetraacid followed by cyclodehydration to yield a-6FDA.
Owner:UNITED STATES GOVERNMENT ADMINSRATOR OF NAT AERONAUTICS & SPACE ADMINISTATION
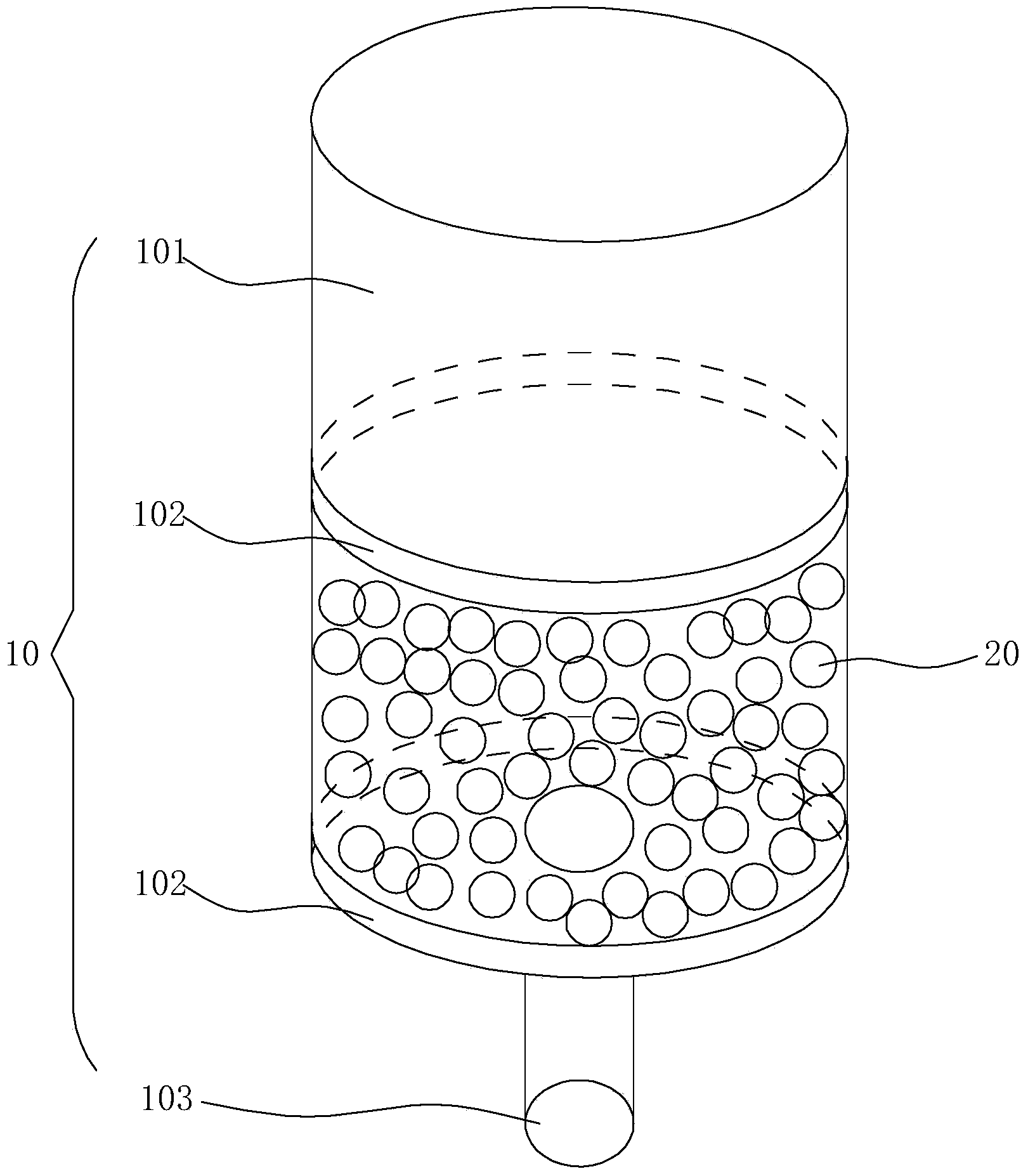
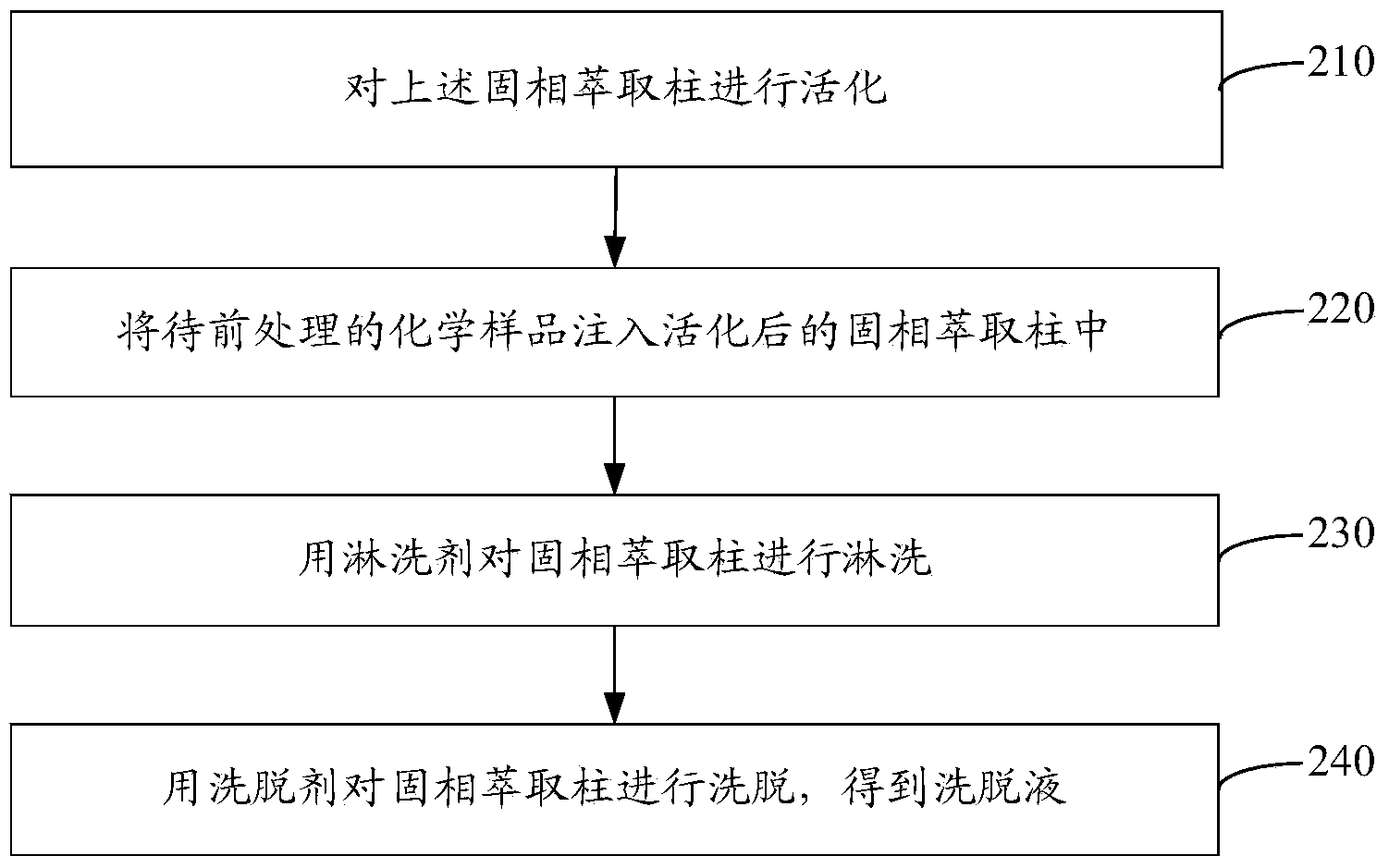
Solid phase extraction column and preparation method thereof as well as chemical sample pretreatment method based on solid phase extraction column
ActiveCN104258598AEffectively fixedAvoid undetectableComponent separationSolid sorbent liquid separationTrifluoromethylationPretreatment method
The invention relates to a solid phase extraction column and a preparation method thereof as well as a chemical sample pretreatment method based on the solid phase extraction column. The solid phase extraction column comprises a separation column and a solid phase extraction agent, wherein the separation column is filled with the solid phase extraction agent; the solid phase extraction agent is graphene or modified graphene; graphene is single-layered graphene or oligo-layered graphene; modified graphene is selected from at least one of aminated graphene, carboxylated graphene, cyano graphene, nitryl graphene, boric acid based graphene, phosphoric acid based graphene, hydroxylated graphene, sulfhydrylated graphene, methylated graphene, allylated graphene, trifluoromethylated graphene, dodecylated graphene, octadecylated graphene, fluorinated graphene, brominated graphene, chlorinated graphene and iodated graphene. The solid phase extraction column is used for pre-treating a chemical sample and can realize efficient separation effect; and the problem that target components cannot be detected in subsequent detection or a real value cannot be detected to cause data distortion is avoided.
Owner:SINOPHENE NOVEL MATERIALS
Fluorine-containing heterocyclic compound and preparation method thereof
ActiveCN106220581AEasy to synthesizeReduce usageOrganic compound preparationCarboxylic acid amides preparationTrifluoromethylationAryl
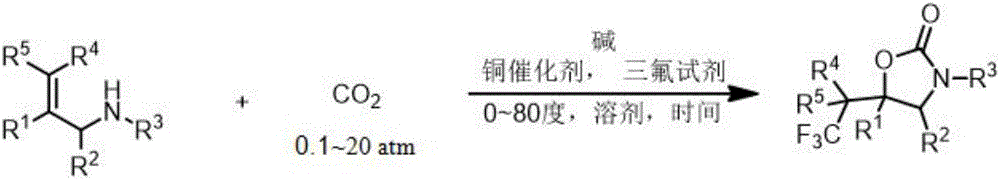
The invention relates to a method for preparing a 2-oxazolinone compound containing a trifluoromethyl group from an allyl amine compound in virtue of carbon dioxide. Specifically, the allyl amine compound as shown in a formula 1 which is described in the specification reacts with CO2 in a solvent in the presence of a copper catalyst, alkali and a trifluoromethylation reagent so as to prepare the 2-oxazolinone compound as shown in a formula 2 which is described in the specification. In the formula 2, R<1>, R<2>, R<4> and R<5> are independently selected from a group consisting of H, halogen, a cyano group, an alkyl group, a carbonyl group, an ester group, an aryl group, a heteroaryl group and the like, R4 or R5 can optionally form a ring with R1, and the alkyl group, the carbonyl group, the ester group, the aryl group and the heteroaryl group can be optionally substituted by halogen, the alkyl group or the like; and R3 is H, halogen, an alkyl group, an aryl group, or the like.
Owner:SICHUAN UNIV
Preparing method by using unactivated olefin hydrogen trifluoride methylation and application thereof
ActiveCN106892800ARich sourcesLow costOrganic compound preparationCarboxylic acid esters preparationTrifluoromethylationTrifluoride
The invention discloses a preparing method by using unactivated olefin hydrogen trifluoride methylation and application thereof. The method comprises the following steps: A, adding unactivated olefin I, sodium trifluoromethanesulfonate and a photocatalystIr[dF(CF3)ppy]2(dtbpy)PF6 into a Schlenk tube; B, vacuumizing, replacing argon, adding methyl alcohol; C, irradiating the Schlenk tube with a fluorescent lamp, and stirring to react; and D, after reaction is finished, adding water into the system to perform a quenching reaction, extracting with ethyl acetate, separating an organic phase, drying, filtering, performing rotary evaporation to remove a solvent, chromatographing the residue through an ethyl acetate / petroleum ether mixed solvent to obtain a target product II, wherein the proportion of the ethyl acetate / petroleum ether mixed solvent is selected according to different polarities of the product, and silica gel is adopted as a solid phase in column chromatography. The method applied to medicinal molecule synthesis is feasible, is simple and convenient to operate, and is used for implementing an unactivated olefin hydrogen trifluoride methylation reaction to prepare a series of trifluoromethyl-containing target compounds by selecting low-price trifluoromethyl reagent under a mild condition.
Owner:HUBEI ENG UNIV
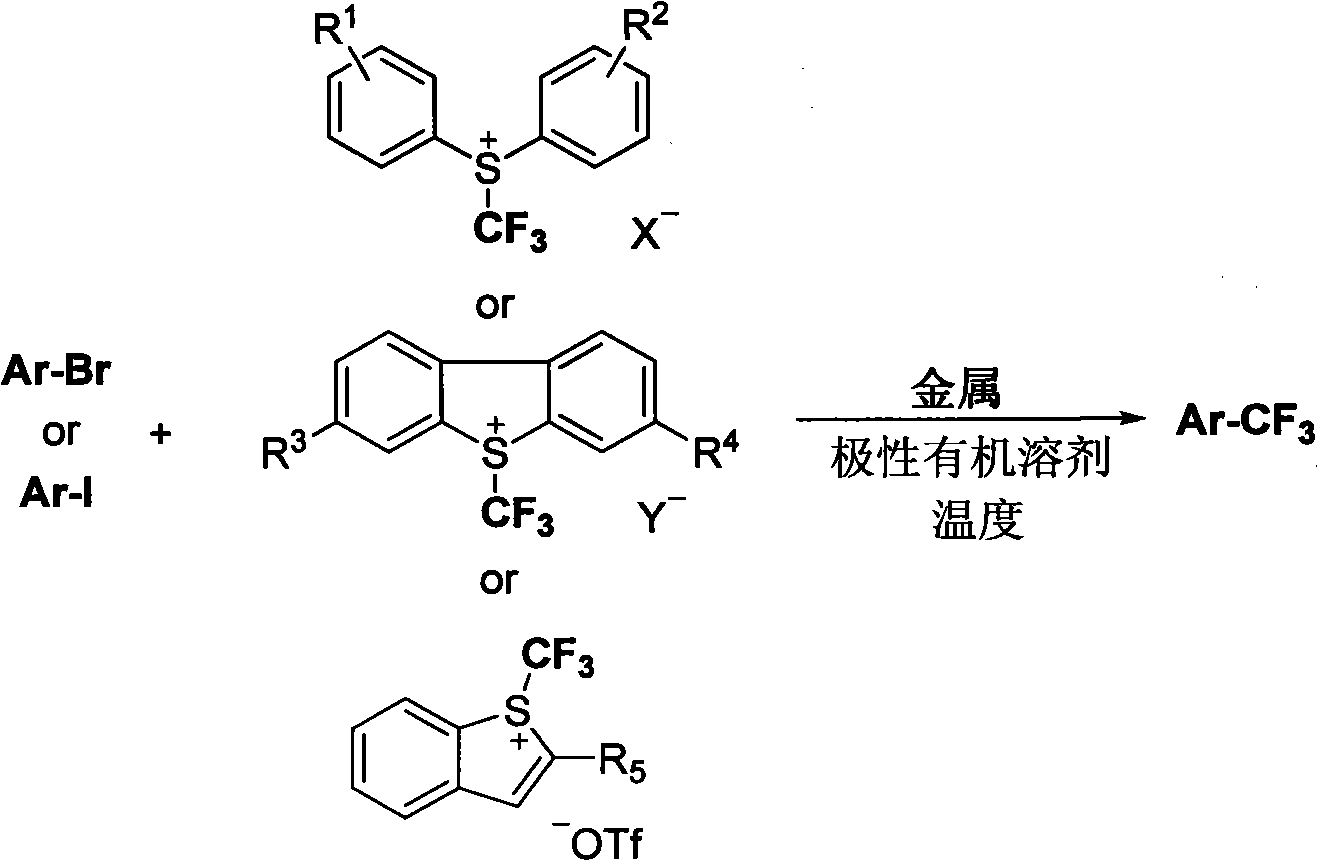
Trifluoromethylation of trifluoromethyl aryl sulfonium salt to heterocyclic compound under metal trigger
InactiveCN101973829AAchieve trifluoromethylationEasy to operateOrganic halogenationTrifluoromethylationAryl
The invention relates to a novel method for trifluoromethylating trifluoromethyl aryl sulfonium salt to a heterocyclic compound. The method comprises the following steps: the trifluoromethyl aryl sulfonium salt is reduced by metal to generate a trifluoromethyl metal compound, and reacts with the heterocyclic compound containing halogen; and a trifluoromethylated heterocyclic product is finally generated. The method is adopted for trifluoromethylating the heterocyclic compound, has simple operation and mild reacting condition, and can obtain a target product with nearly quantitative yield.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
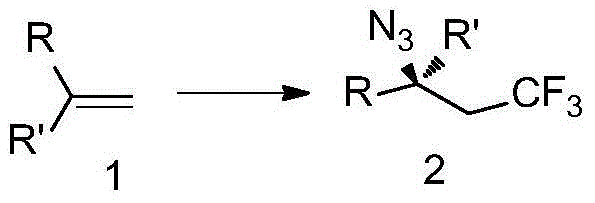
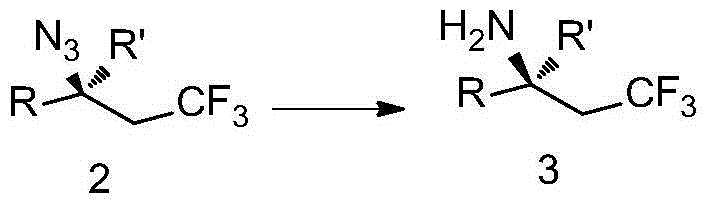
Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
ActiveCN104649857AMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzidotrimethylsilane
The invention discloses trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof. The preparing method of the trifluoromethyl-substituted azide compounds includes following steps of: subjecting a trifluoromethylation agent, azidotrimethylsilane and a carbon-carbon double bond of an olefin to addition in an organic solvent under the existence of a catalyst to obtain a compound in which one carbon in the carbon-carbon double bond of the olefin has trifluoromethyl and the other carbon has an azide group. The preparing methods utilize the trifluoromethylation agent which is mild relatively, directly form a carbon-nitrogen bond and a carbon-carbon bond by double-functionalization of olefins, and efficiently synthesize the trifluoromethyl-substituted azide, amine and heterocycle compounds with high selectivity. The preparing methods are easily available in raw materials, mild in reaction conditions, good in atom economy, high in selectivity, simple in after-treatment, environmental friendly, high in yields and suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
New trifluoro methylation reagent and preparation and application thereof
ActiveCN104045585APreparation by OH and halogen introductionOrganic halogenationTrifluoromethylationReagent
The invention relates to a new trifluoro methylation reagent and preparation and application thereof, and in particular relates to a compound with a structure shown as a formula I, a preparing method thereof and application thereof as a trifluoro methylation reagent. In the formula, the definition of each group is as defined in the specification. The starting materials of the new trifluoro methylation reagent are cheap and easy to obtain, the reaction condition is mild, the post treatment is simple, the product can be used as a variety of potential trifluoro methylation reagents, the yield is high, the equipment requirement is low, and the new trifluoro methylation reagent has wide prospects of industrial application. (FSO2CF2COO) zM I.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
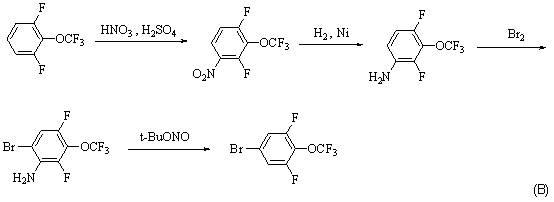
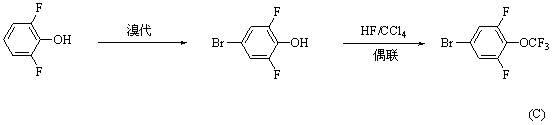
Synthesis method for 4-bromo-2, 6-difluoro-trifluoromethoxybenzene
ActiveCN102531854AReduce usageThere are few types of useChemical recyclingEther preparation by ester reactionsTrifluoromethylationSynthesis methods
A synthesis method for 4-bromo-2, 6-difluoro-trifluoromethoxybenzene adopts 3, 4, 5-trifluorobromobenzene(I) as material and sequentially carries out substitution, dealkylation and trifluoromethylation reactions to prepare 4-bromo-2, 6-difluoro-trifluoromethoxybenzene (IV). The method has the advantages of short synthesis route, high yield, low cost, easy industrialization and the like.
Owner:河北凡克新材料有限公司
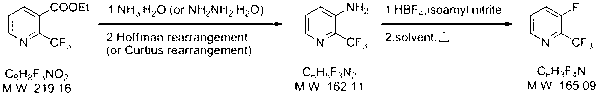
Synthesizing method of 2-trifluoromethyl-3-fluoropyridin
ActiveCN102977009ALow costSimple and fast operationOrganic chemistryTrifluoromethylationChemical industry
The invention relates to the field of medicine and chemical industry. Aims at solving problems of high trifluoromethylation reagent toxicity, or high cost, complicated reaction, and low conversion rate existing in synthesizing reactions of trifluoromethylation similar compounds, the invention provides a synthesizing method of 2-trifluoromethyl-3-fluoropyridin. The method comprises the steps that: (2) with 2-trifluoromethyl ethyl nicotinate as a raw material, 2-trifluoromethyl-3-aminopyridine is produced through a reaction; and (2) the product obtained in the step (1) is subjected to a reaction with fluoboric acid, such that 2-trifluoromethyl-3-fluoropyridin is obtained. According to the invention, the raw materials have low cost, and are easy to obtain. Also, post treatment is simple. The method is suitable for laboratory small-scale preparation, and is suitable for large-scale industrialized production.
Owner:HANGZHOU ALLSINO CHEM
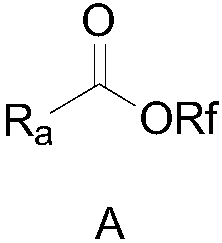
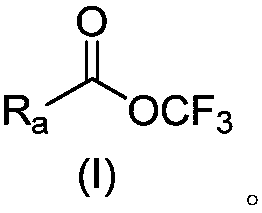
Perfluoro alkoxylation reagent and preparation method and application thereof
ActiveCN108516935AEasy to operateRaw materials are cheap and easy to getPreparation from carboxylic acid halidesSugar derivativesTrifluoromethylationHigh volume manufacturing
The invention provides a perfluoro alkoxylation reagent and a preparation method and application thereof. Specifically, the provided perfluoro alkoxylation reagent shown as a formula A can be used fornormal perfluoro alkoxylation reactions in the field, especially trifluoromethylation. Raw materials related to the perfluoro alkoxylation reagent are low in price and easy to obtain, the reaction condition is mild, the operation is simple, the cost is low, and the reagent is easy to popularize and suitable for large-scale production. The formula A is as shown in the description.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Compositions and processes of preparing and using the same
ActiveUS20140039182A1Organic compound preparationHydroxy compound preparationTrifluoromethylationChemical compound
The present invention relates to compositions, for example, the DBU / Hexafluoroacetone hydrate salt, and processes of preparing and using the same for the modification of chemical compounds via the release of trifluoroacetate. The DBU / Hexafluoroacetone hydrate salt can perform trifluoromethylation reactions on chemical compounds, such as carbonyl group-containing compounds.
Owner:PURDUE RES FOUND INC
Preparation method and application of aryl trifluoromethyl compound
InactiveCN108191612ASimple reaction conditionsLow production costOrganic compound preparationAmino-hyroxy compound preparationTrifluoromethylOrganic solvent
The invention discloses a preparation method and application of an aryl trifluoromethyl compound. The preparation method comprises the step of reacting a compound A with a trifluoromethyl reagent in an organic solvent system containing a catalyst under the illumination and alkaling conditions to obtain the aryl trifluoromethyl compound. The structural formula of the compound A is shown as the follows: A (the formula is shown in the description). The R in the structural formula of the compound A is one or more than two of alkyl, alkoxy, hydroxy, halogen, nitryl and carboxyl. The preparation method disclosed by the invention has the advantages that reaction conditions are simple; the aryl trifluoromethyl compound can be obtained by reaction raw materials under the action of the illumination,the alkaling conditions and the catalyst; no ligand or precursor compound is needed in the preparation process; a product has catalyst residues.
Owner:深圳蓝新科技有限公司
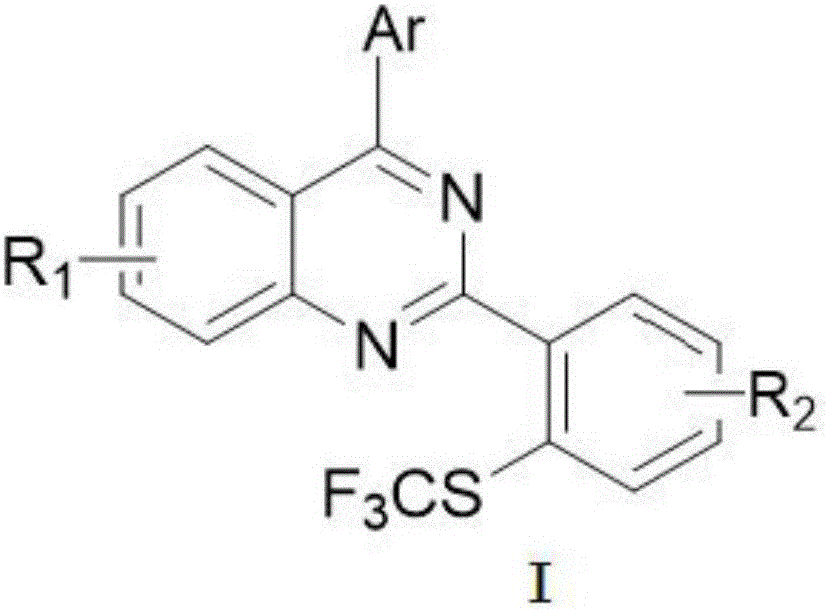
Method for preparing 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline
InactiveCN106632087AHigh yieldHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsPentamethylcyclopentadieneTrifluoromethylation
The invention discloses a 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound and a preparation method thereof. The preparation method comprises the following steps: taking 2,4-diarylquinazoline as a reaction substrate, carrying out a reaction with NIS under the catalytic action of pentamethylcyclopentadienylrhodium (III) chloride dimer / silver hexafluoroantimonate under a condition of 80 DEG C, carrying out a reaction with a sulfur trifluoromethylation reagent, and taking copper iodide as a catalyst, wherein the reaction temperatire is 85 DEG C, and the reaction time is 7-10 hours; performing carbon hydrogen bond activation process, thereby obtaining the 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound. The preparation method disclosed by the invention is mild in reaction conditions, easy and convenient to operate, low in cost, few in side reactions, high in product purity and convenient for separation and purification and can be suitable for large-scale preparation, and the obtained product has high drug activity and excellent potential application prospects.
Owner:JIANGXI NORMAL UNIV
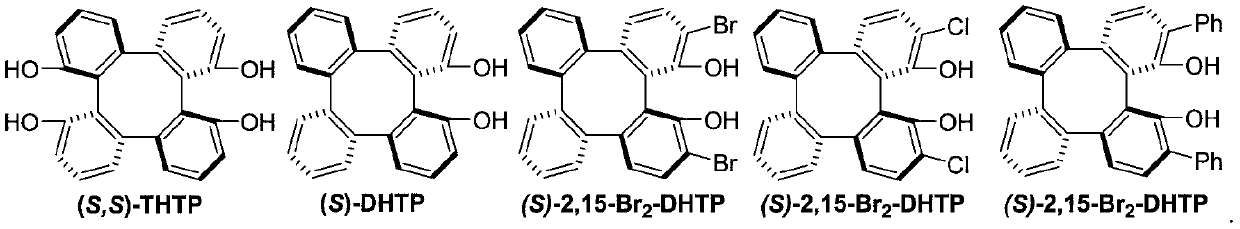
Method for synthesizing optically active trifluoromethyl compound by asymmetric conjugate addition reaction of organic boronic acid and alpha, beta-unsaturated ketone
ActiveCN110078605AHigh yieldHigh enantioselectivityOrganic compound preparationCarbonyl compound preparationTrifluoromethylationBoronic acid
The invention discloses a method for synthesizing an optically active trifluoromethyl compound by asymmetric conjugate addition reaction of an organic boronic acid and alpha, beta-unsaturated ketone,and belongs to the technical field of asymmetric synthesis in the organic chemistry. A reaction equation is as shown in the specification. The method comprises the following specific steps: taking beta-CF3-alpha, beta-unsaturated ketone 1 and organic boric acid 2 as raw materials, and carrying out the asymmetric conjugate addition reaction to obtain the trifluoromethyl compound in the presence ofchiral tetrabenzocyclooctatetraenic or chiral binaphthol catalysts, as well as molecular sieves and magnesium tert-butoxide additives, wherein R<1> is selected from phenyl, substituted phenyl, 2-naphthyl, 1-naphthyl, 2-thienyl, 3-thienyl and cyclohexyl, and R<2> is selected from styryl, 2-furanyl and 2-benzofuranyl. The method has the advantages that reaction raw materials are easy to obtain, reaction conditions are mild, post-treatment is simple, the catalyst can be recycled and reused, the product yield and the enantioselectivity are good to excellent, and the product contains a trifluoromethyl chiral center.
Owner:HENAN NORMAL UNIV
Chromane compound and preparation method thereof
InactiveCN109320489AMild reaction conditionsEasy to operateOrganic chemistryTrifluoromethylationSolvent
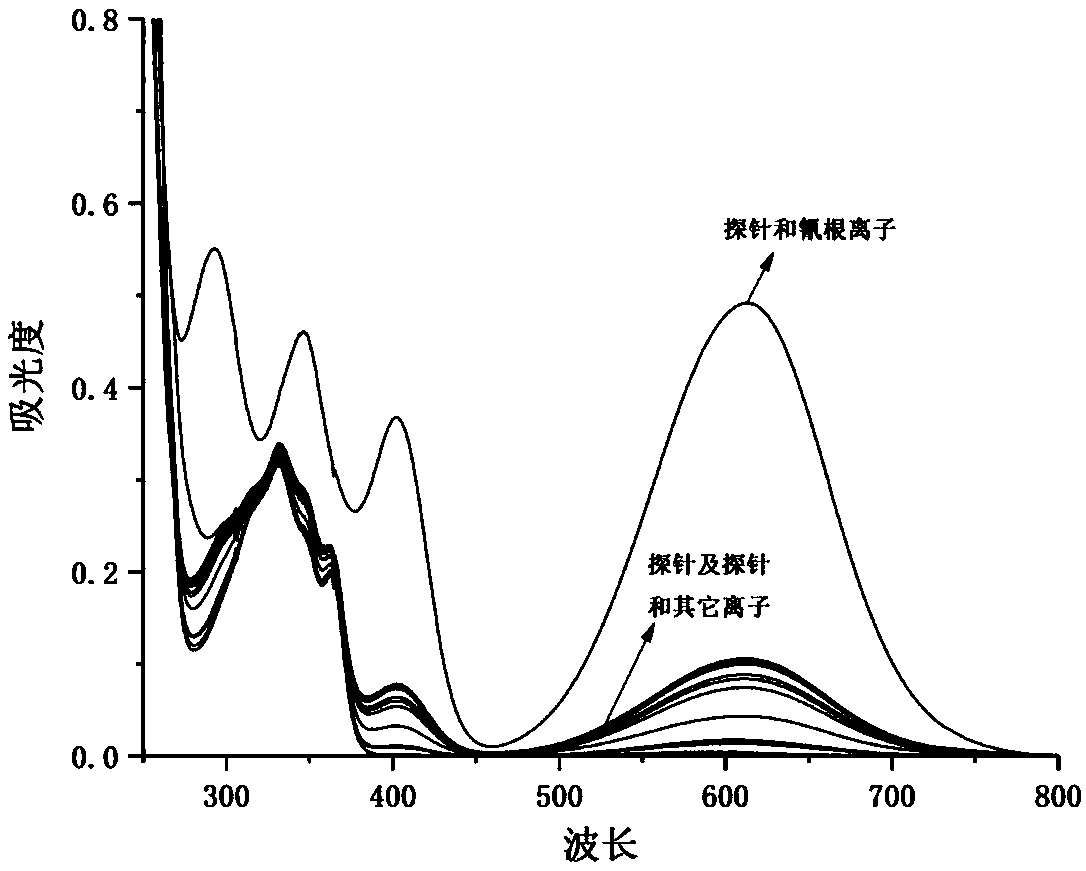
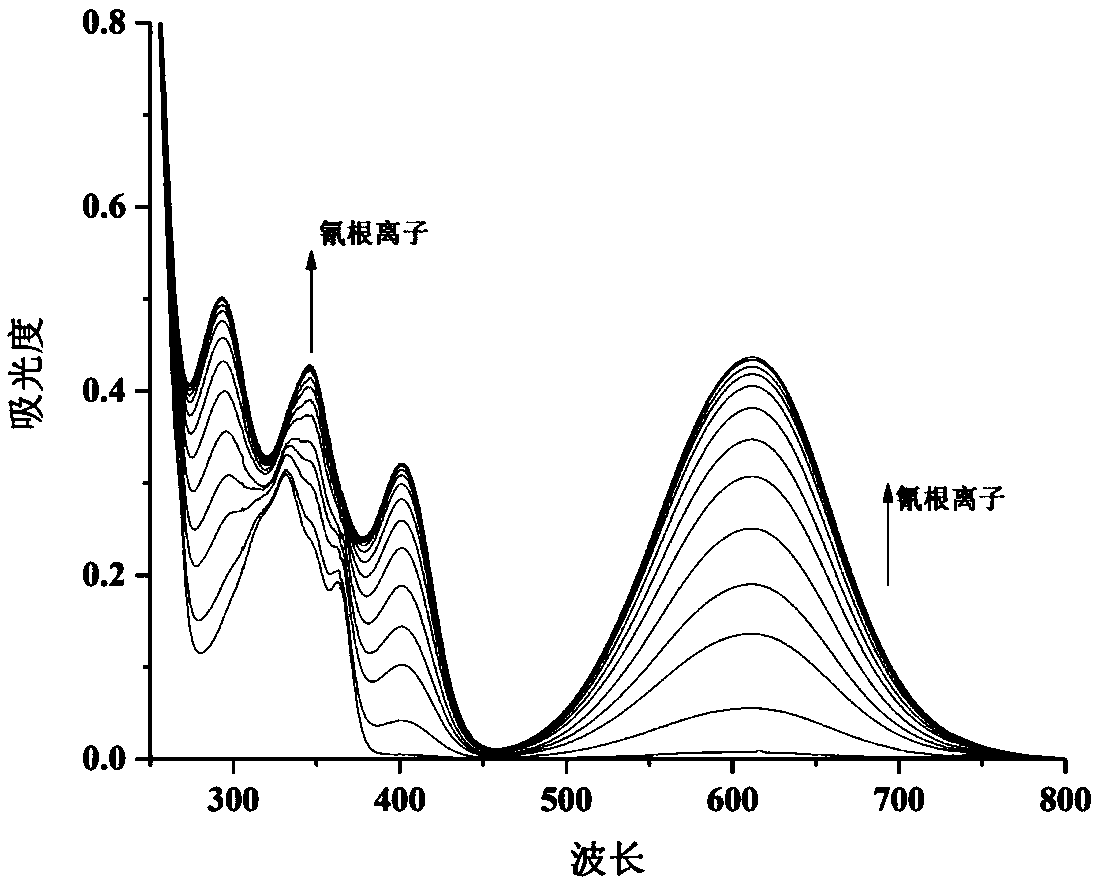
The invention discloses a chromane compound and a preparation method thereof. The preparation method comprises the following specific steps: dispersing olefin shown as a structure (I), a trifluoromethyl reagent shown as a structure (II) and an oxidizing agent in a solvent; heating and stirring the mixture to obtain the chromane compound shown as a structure (III), wherein the structure (III) is shown in the description. The invention further provides a novel method for building a trifluoromethylation chromane compound by taking an olefin compound (I) as a starting raw material of a reaction and sodium trifluoromethanesulfonate (II) as a trifluoromethyl source, and persulfate as an oxidizing agent, and performing free radical addition, free radical arylation cyclization and oxidization on trifluoromethyl and the olefin. The method has the advantages of mild reaction conditions, easiness in operation, diverse products, and the capability of realizing scale production.
Owner:XINYANG NORMAL UNIVERSITY
Preparation method of 3,5-dihalobenzotrifluoride and 3'-chloro-5'-(trifluoromethyl)phenyltrifluoroethanone
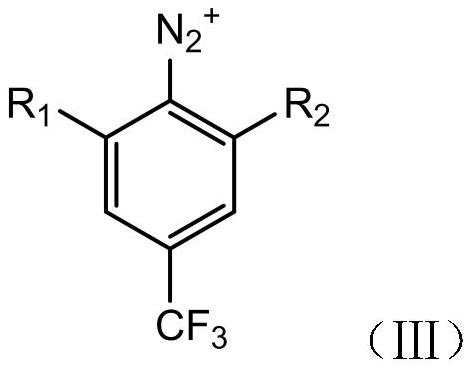
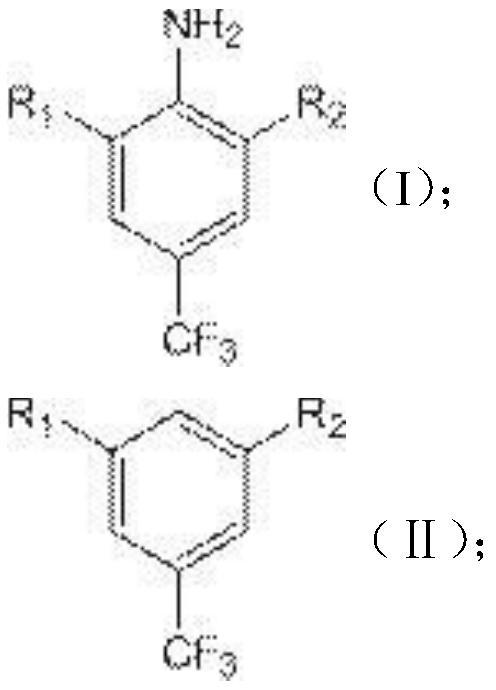
ActiveCN112110790AImprove economyReduce manufacturing costOrganic compound preparationMagnesium organic compoundsTrifluoromethylationMethyl benzene
The invention relates to the technical field of chemical pharmacy, particularly to a preparation method of 3,5-dihalobenzotrifluoride. According to the preparation method, 3,5-dihalogen-4-amino trifluorotoluene is used as a raw material and is subjected to diazotization deamination reaction to obtain the 3,5-dihalobenzotrifluoride, so that the production cost is relatively low, and the economic effect is relatively good. The invention further relates to a preparation method of 3'-chloro-5'-(trifluoromethyl)phenyltrifluoroethanone. According to the preparation method, 3,5-dihalobenzotrifluorideis used as a raw material, and is subjected to a Grignard reagent reaction, then a nucleophilic addition reaction is carried out with and a trifluoromethylation reagent, and a good economic effect isalso achieved.
Owner:TAIZHOU ABSOBIOTEC CO LTD
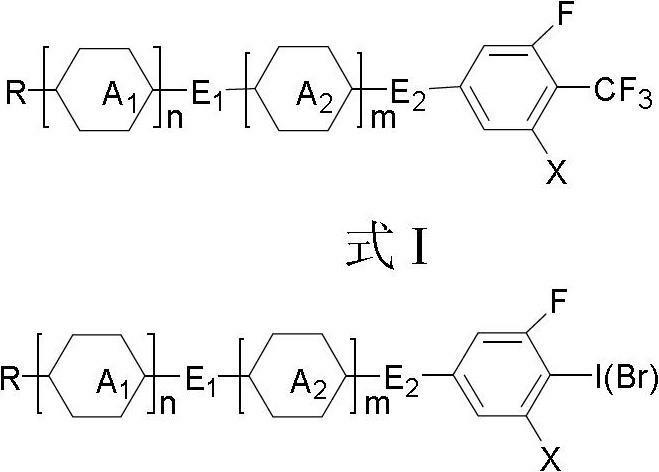
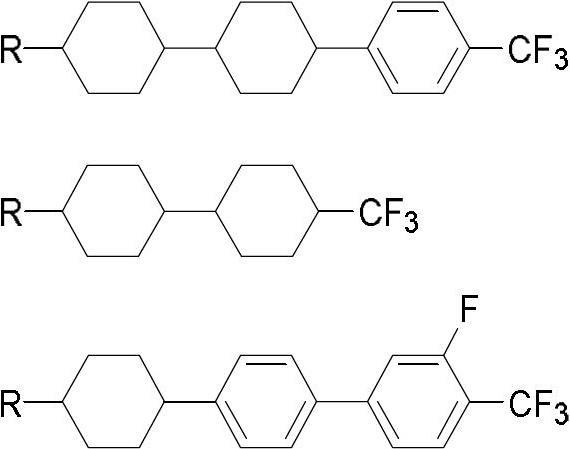
Method for preparing trifluoromethyl-benzene-containing liquid crystals
ActiveCN102675041AHigh yieldQuality improvementLiquid crystal compositionsHalogenated hydrocarbon preparationTrifluoromethylationDielectric anisotropy
The invention discloses a method for preparing trifluoromethyl-benzene-containing liquid crystals, which comprises the following steps: evenly mixing a compound disclosed as Formula II and a trifluoromethylating reagent to carry out catalytic reaction, and obtaining a compound disclosed as Formula I after the reaction finishes. Compared with other methods, the method disclosed by the invention uses potassium trifluoroacetate as the trifluoromethylating reagent instead of sodium trifluoroacetate, thereby greatly enhancing the yield. The method has the advantage of accessible raw materials, is simple, is especially suitable for synthesizing high-dielectric-anisotropy liquid crystal compound monomers, and can effectively enhance the reaction yield and the product quality. Formula I and formula II are shown in the description.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
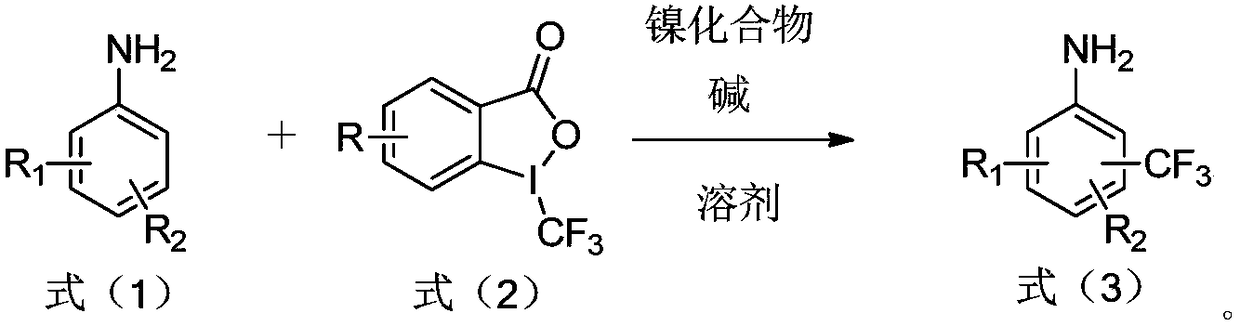
Preparation method of trifluoromethylamine
ActiveCN108503552AReduce manufacturing costLow costCarboxylic acid nitrile preparationAmino preparation from aminesTrifluoromethylationNickel compounds
The invention relates to a preparation method of trifluoromethylamine. The method includes the following steps that aromatic amine shown in the formula (1) and a trifluoromethyl reagent shown in the formula (2) react in a solvent under the condition that an alkali and / or nickel compound exists, and the trifluoromethylamine compound shown in the formula (3) is generated. According to the preparation method of trifluoromethylamine, aromatic amine and 1-trifluoromethyl-1,2-iodobenzoyl-3(H)-ketone serve as raw materials and react under the condition that the alkali and / or nickel compound exists through the amino positioning effect on aromatic nucleus. The synthesis steps of the method are simple, the cost of the raw materials is low, the production cost of trifluoromethylamine can be greatly reduced, and large-scale industrialized production is promoted.
Owner:TETRANOV PHARMA CO LTD
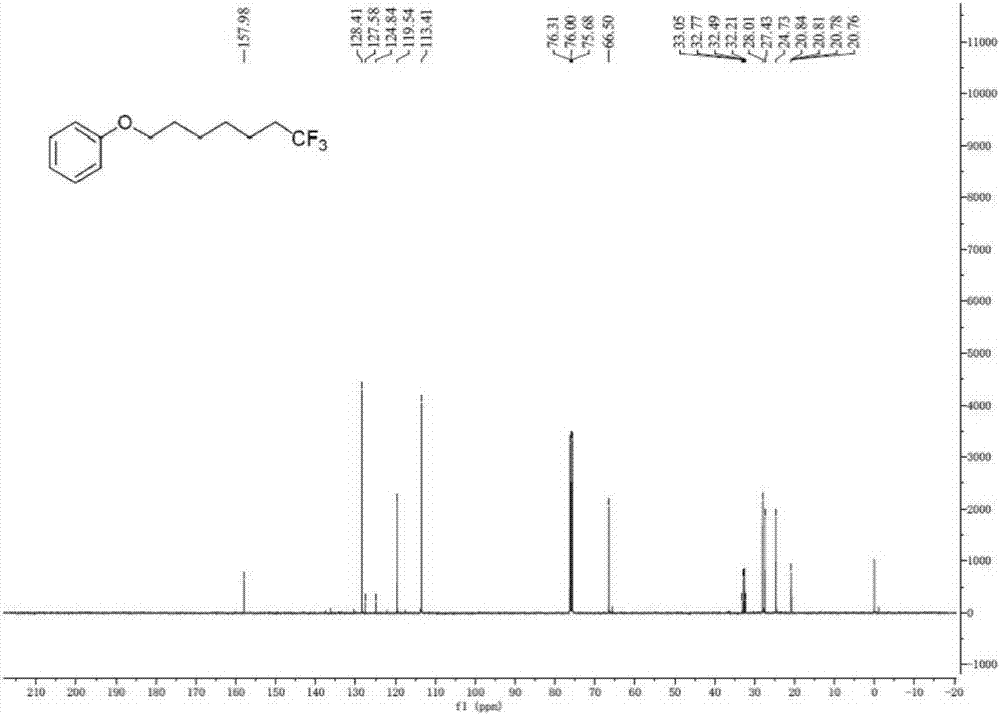
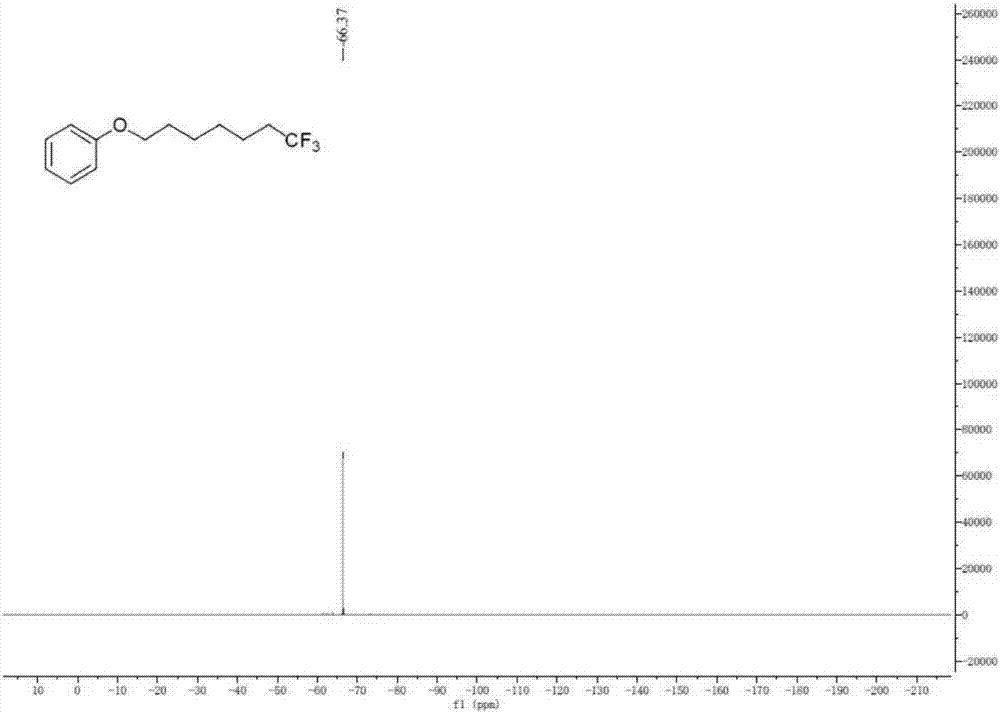
Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device
The invention discloses a method for continuously preparing a dihydrobenzo [j] phenanthridine compound containing a trifluoromethyl functional group by using a micro-channel reaction device, which comprises the following steps: (1) dissolving a 1,7-eneyne compound and alkali in a proper solvent to obtain a material I; (2) dissolving a trifluoromethyl reagent and a photocatalyst in a proper solventto obtain a material II; (3) respectively pumping the material I and the material II into a micro-channel reaction device, fully mixing, and carrying out a photocatalytic trifluoromethylation reaction to obtain a reaction solution; and (4) quenching the reaction liquid, adding a corresponding organic solvent for extraction, collecting an organic phase, drying, concentrating and recrystallizing toobtain a target product. The micro-channel reaction device is used for preparing the 1,7-eneyne trifluoromethylation product, the reaction conditions are milder, the reaction rate can be effectivelycontrolled, the reaction time is shortened, continuous production is achieved, side reactions are reduced, the maximum product yield can reach 99.3%, the amplification effect is basically avoided, andindustrial amplification is facilitated.
Owner:NANJING UNIV OF TECH
Synthesis of asymmetric tetracarboxylic acids and dianhydrides
ActiveUS7381849B1Improve wear resistanceImprove adhesionOrganic compound preparationCarbonyl compound preparationPhenylboronic acidTrifluoromethylation
This invention relates to the compositions and processes for preparing 2,3,3′,4′-tetramethylbenzophenone and asymmetrical dianhydrides such as 2,3,3′,4′ benzophenone dianhydride (a-BTDA), and 3,4′-(hexafluoroisopropylidene)diphthalic anhydride (a-6FDA). a-BTDA is prepared by Suzuki coupling with catalysts from a mixed anhydride of 3,4-dimethylbenzoic acid and 2,3-dimethylbenzoic acid with a respective 2,3-dimethylphenylboronic acid and 3,4-dimethyl phenylboronic acid to form 2,3,3′,4′-tetramethylbenzophenone which is oxidized to 2,3,3′,4′-benzophenonetetracarboxylic acid followed by cyclodehydration to obtain a-BTDA. The a-6FDA was prepared by nucleophilic trifluoromethylation of 2,3,3′,4′-tetramethylbenzophenone with trifluoromethyltrimethylsilane to form 3,4′-(trifluoromethylmethanol) bis(o-xylene) which is converted to 3,4′-(hexafluoroisopropylidene-bis(o-xylene). The 3,4′-(hexafluoroisopropylidene)-bis(o-xylene) is oxidized to the corresponding tetraacid followed by cyclodehydration to yield a-6FDA.
Owner:NASA
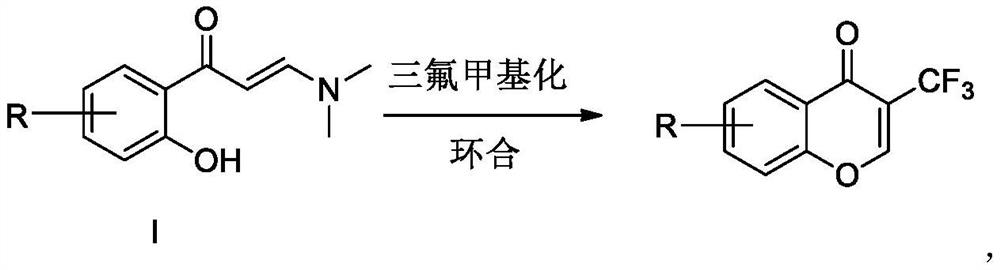
Preparation method of trifluoromethyl chromone compound
The invention discloses a preparation method of a trifluoromethyl chromone compound, which is prepared by carrying out trifluoromethylation and cyclization reaction on a compound as shown in a formulaI in the presence of organic alkali without irradiation of visible light to initiate reaction or action of a photo-catalyst. Compared with the prior art, the method has the advantages of simple operation, safety, reliability, easy amplification, environmental friendliness and the like. R is a mono-substituted or multi-substituted group at any position on a benzene ring, and can be halogen, alkyl,alkoxy and the like in the same or different manner.
Owner:浙江瑞博制药有限公司
Trifluoromethoxylation of arenes via intramolecular trifluoromethoxy group migration
ActiveUS20170298008A1Urea derivatives preparationCarbamic acid derivatives preparationTrifluoromethylationSolvent
The present invention provides a process of producing a trifluoromethoxylated aryl or trifluoromethoxylated heteroaryl having the structure:whereinA is an aryl or heteroaryl, each with or without subsutitution; and R1 is —H, -(alkyl), -(alkenyl), -(alkynyl), -(aryl), -(heteroaryl), -(alkylaryl), -(alkylheteroaryl), —NH-(alkyl), —N(alkyl)7, —NH-(alkenyl), —NH-(alkynyl) —NH-(aryl), —NH-(heteroaryl), —O-(alkyl), —O-(alkenyl), —O-(alkynyl), —O-(aryl), —O-(heteroaryl), —S-(alkyl), —S-(alkenyl), —S-(alkynyl), —S-(aryl), or —S-(heteroaryl), comprising:(a) reacting a compound having the structure:with a trifluoromethylating agent in the presence of a base in a first suitable solvent under conditions to produce a compound having the structure:and(b) maintaining the compound produced in step (a) in a second suitable solvent under conditions sufficient to produce the trifluoromethoxylated aryl or trifluormethoxylated heteroaryl having the structure:
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
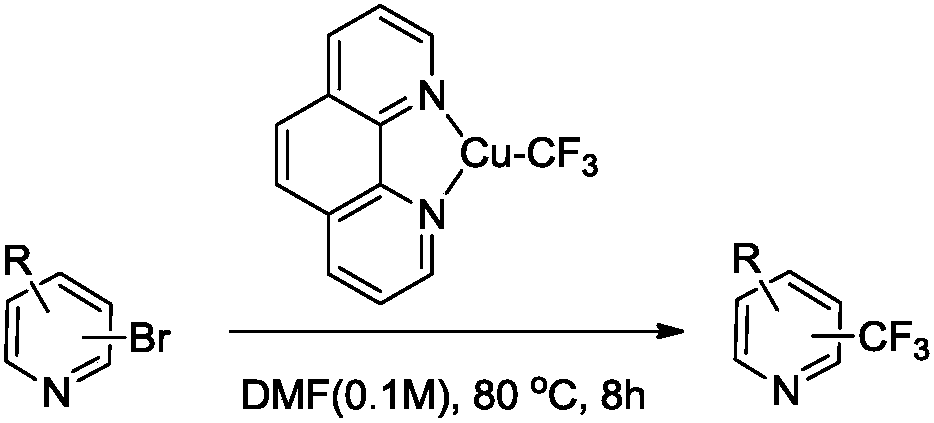
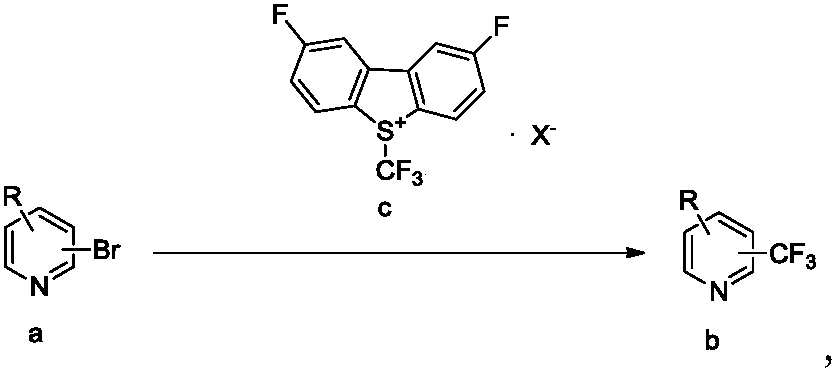
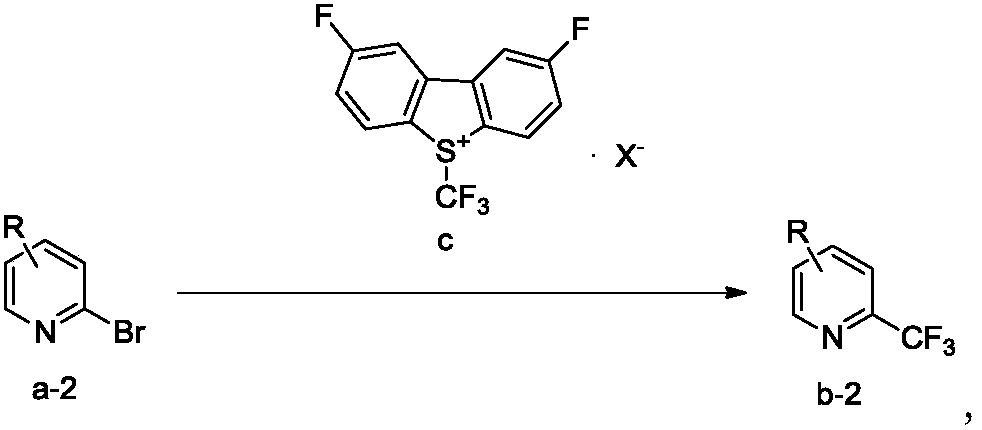
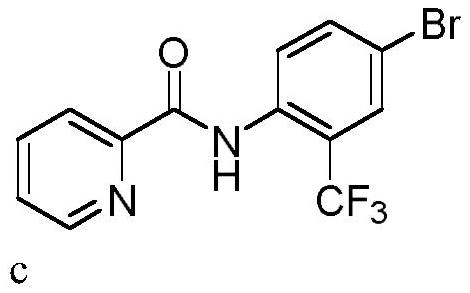
Trifluoromethylation process for bromo-pyridine and derivatives thereof
ActiveCN108239021ASuitable for mass productionEasy to prepareOrganic chemistryTrifluoromethylationPyridine
The invention belongs to the field of organic chemistry and relates to a trifluoromethylation process for bromo-pyridine and derivatives thereof. The process disclosed by the invention comprises the following steps: by taking a bromo-pyridine compound with a formula a structure as a raw material, performing trifluoromethylation under the action of a Maben reagent fluoro-S-( trifluoromethyl)-dibenzothiophene salt having a formula c structure, thereby obtaining the tirfluoromethylpyridine compound with a formula b structure. The structural formula is as shown in the specification. In the formula, X- is Bronst conjugate base, R is H or -CN or halogen or C1-C6 alkyl or C1-C6 alkoxy or -OH or -R1OH or COR2 or -CO2R3 or -CONR4 or -NR5R6; R1 is C1-C6 akyl; R2, R3 and R4 are identically or differently H or C1-C6 alkyl; and the R5 and R6 are identically or differently H or O or C1-C6 alkyl or C1-C6 alkoxy.
Owner:瑞博(杭州)医药科技有限公司
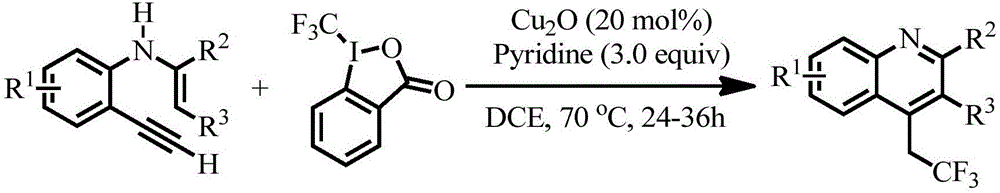
Preparation method of 4-(2',2'2'-trifluoro)ethylquinoline series
The invention discloses a preparation method of 4-(2',2'2'-trifluoro)ethylquinoline series. The preparation method includes adopting N-(2-ethynyl aryl)enamine as a reaction substrate, and enabling the N-(2-ethynyl aryl)enamine and a trifluoromethylation reagent to undergo trifluoromethylation and cyclization reaction for 24-36 hours in an organic solvent under catalysis of transition metal copper under an alkaline condition so as to obtain the 4-(2',2'2'-trifluoro)ethylquinoline series by a cascade cyclization reaction policy. The preparation method has the advantages that the preparation method is high in selectivity, simple and convenient to operate, high in product purity and convenient to separate and purify, and the 4-(2',2'2'-trifluoro)ethylquinoline series are novel in structure (confirmed by characterizations such as 1H, NMR, 13C NMR, 19F NMR and HRMS), have potential biological activity and pharmaceutical activity and can be further synthesized into compounds more complex in structure and widely used in the fields of pesticides and pharmaceuticals as intermediates.
Owner:JIANGXI NORMAL UNIV
Electrochemical synthesis method of trifluoromethylated aryl amide derivative
ActiveCN111690947AHigh yieldMild reaction conditionsElectrolysis componentsOrganic chemistryTrifluoromethylationAryl
The invention discloses an electrochemical synthesis method of a trifluoromethylated aryl amide derivative. The method comprises the steps: adding a substrate aryl amide, a trifluoromethylation reagent, an electrolyte and a solvent into a reaction container equipped with an electrode, carrying out a constant-current stirring reaction on the reaction mixture at the temperature of 25-75 DEG C for 30-180 min at a constant current of 5-20 mA, and carrying out post-treatment on the reaction solution to obtain the product trifluoromethylated aryl amide derivative. The structural formula of the substrate aryl amide is represented by a formula (Ia) or a formula (Ib), and correspondingly, the structural formula of the obtained product trifluoromethylated aryl amide derivative is represented by a formula (IIa) or a formula (IIb); the method is environment-friendly, simple, convenient and efficient; the structural formula of the substrate is described in the specification, the structural formulaof the product is described in the specification.
Owner:ZHEJIANG UNIV OF TECH
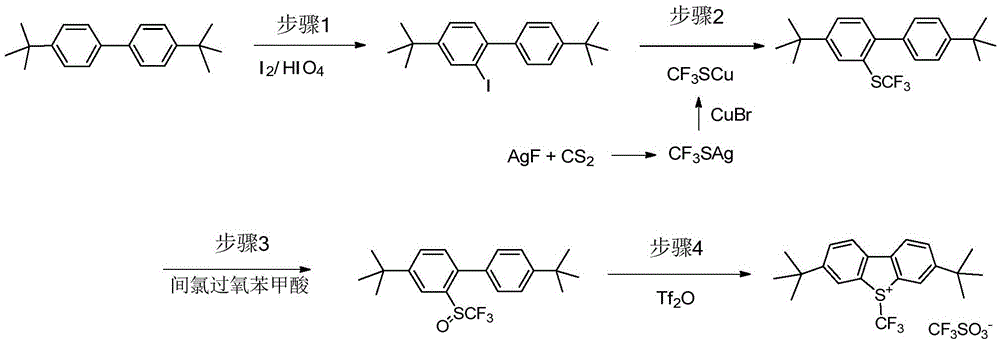
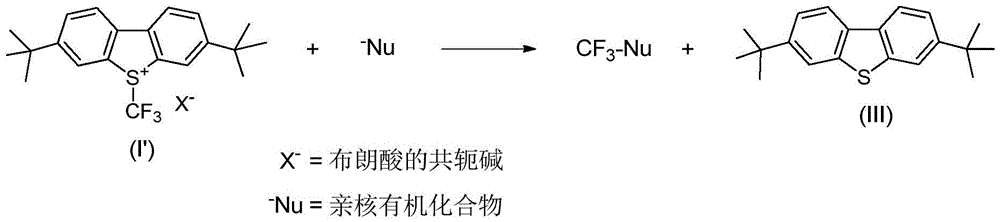
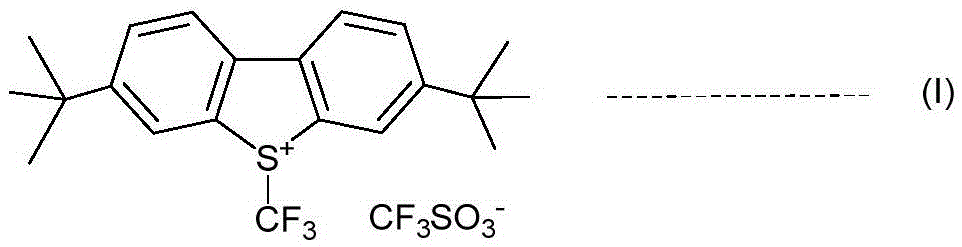
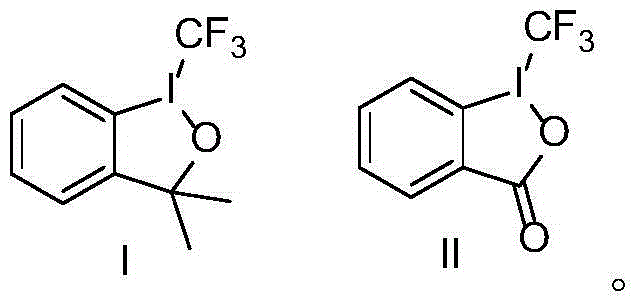
An industrial production method for 3,7-bis(tertiary butyl)-S-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate
The invention relates to an industrial production method for 3,7-bis(tertiary butyl)-S-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate (formula I) that is a useful trifiuoroniethylation agent. The method includes a one-pot reaction process and a simple filtration separation process. The method also comprises a process of recovering raw materials after trifiuoroniethylation of an organic compound.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
4-trifluoromethyl-6-bromo-2-substituted acetonitrile-1,8-naphthalimide compounds as well as preparation method and application thereof
ActiveCN109053572AReduce manufacturing costHigh yieldOrganic chemistryMaterial analysis by observing effect on chemical indicatorTrifluoromethylationBenzene
The invention discloses 4-trifluoromethyl-6-bromo-2-substituted acetonitrile-1,8-naphthalimide compounds as well as a preparation method and an application thereof. The structural formula of the 4-trifluoromethyl-6-bromo-2-substituent acetonitrile-1,8-naphthalimide compounds is shown in the description, wherein R1 is an alkyl group of C1-C10 straight chains or branched chains; R2 is a phenyl group, a naphthyl group, a substituent phenyl group, a five-member or 6-member heteroaryl group or a benzo five-member or 6-member heteroaryl group. N-R1-1,8-naphthalimide compounds are used as initial materials, N-R1-4-trofluoromethyl-6-bromo-1,8-naphthalimide is obtained after trifluoromethylation and bromination and then reacts with R2 substituted acetonitrile under the alkali catalysis action, andthe compounds are obtained. The prepared 4-trifluoromethyl-6-bromo-2-substituted acetonitrile-1,8-naphthalimide compounds can be used as a colorimetric probe and a fluorescent probe for detecting cyanide ions.
Owner:SHANGHAI INST OF TECH
Method for synthesizing compounds containing vinyl trifluoromethyl structure
InactiveCN109972166ALow priceEasy post-processingElectrolysis componentsElectrolytic organic productionTrifluoromethylationElectrochemical anodization
The invention belongs to the field of organic substance synthesis, specifically relates to a method for synthesizing compounds containing a vinyl trifluoromethyl structure, and particularly relates toa method for synthesizing compounds containing a vinyl trifluoromethyl structure through electrochemical decarboxylation trifluoro-methylation. Specifically, in a solvent, alpha, beta-unsaturated carboxylic acids are taken as the raw materials, electrolyte and a trifluoromethyl source are added; through electrochemical anode oxidation reactions, compounds containing a vinyl trifluoromethyl structure are prepared, the anode is a carbon rod, and the cathode is a platinum sheet. Compared with reported methods, the provided method does not use any metal catalyst or external oxidizing agent; the used electrolyte and trifluoromethyl source are cheap, nontoxic, and odorless; the post treatment is simple, the method is suitable for industrial production, the reaction conditions are mild, the operation is simple, and the yield is high.
Owner:WUYI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
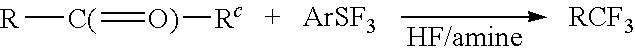

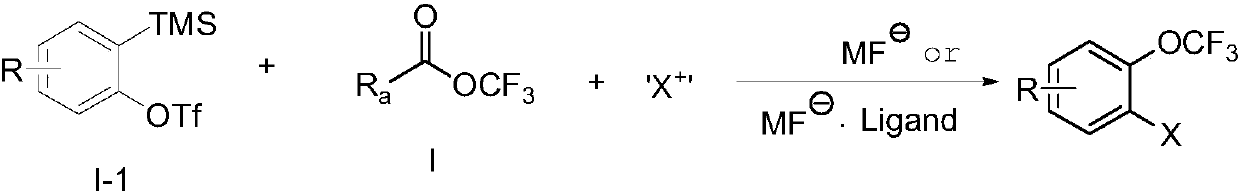
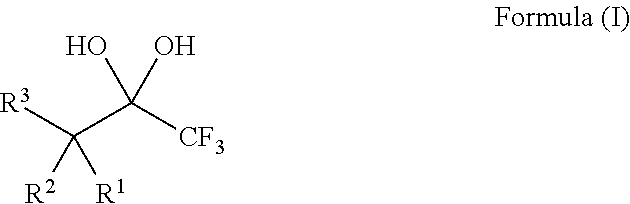
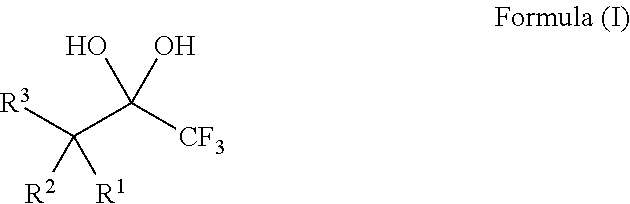
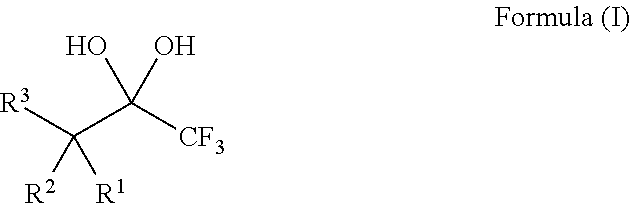

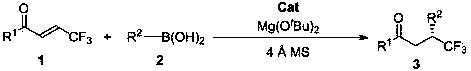
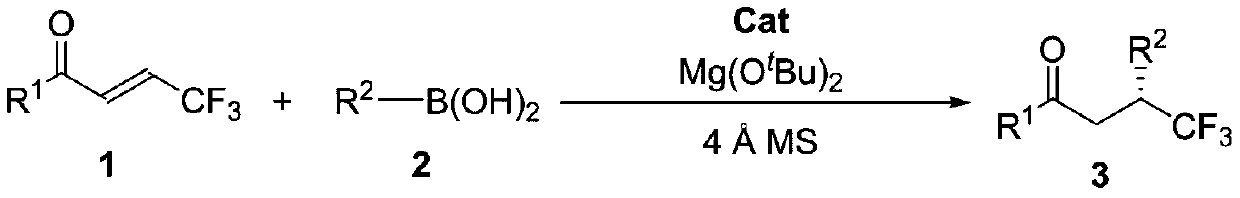
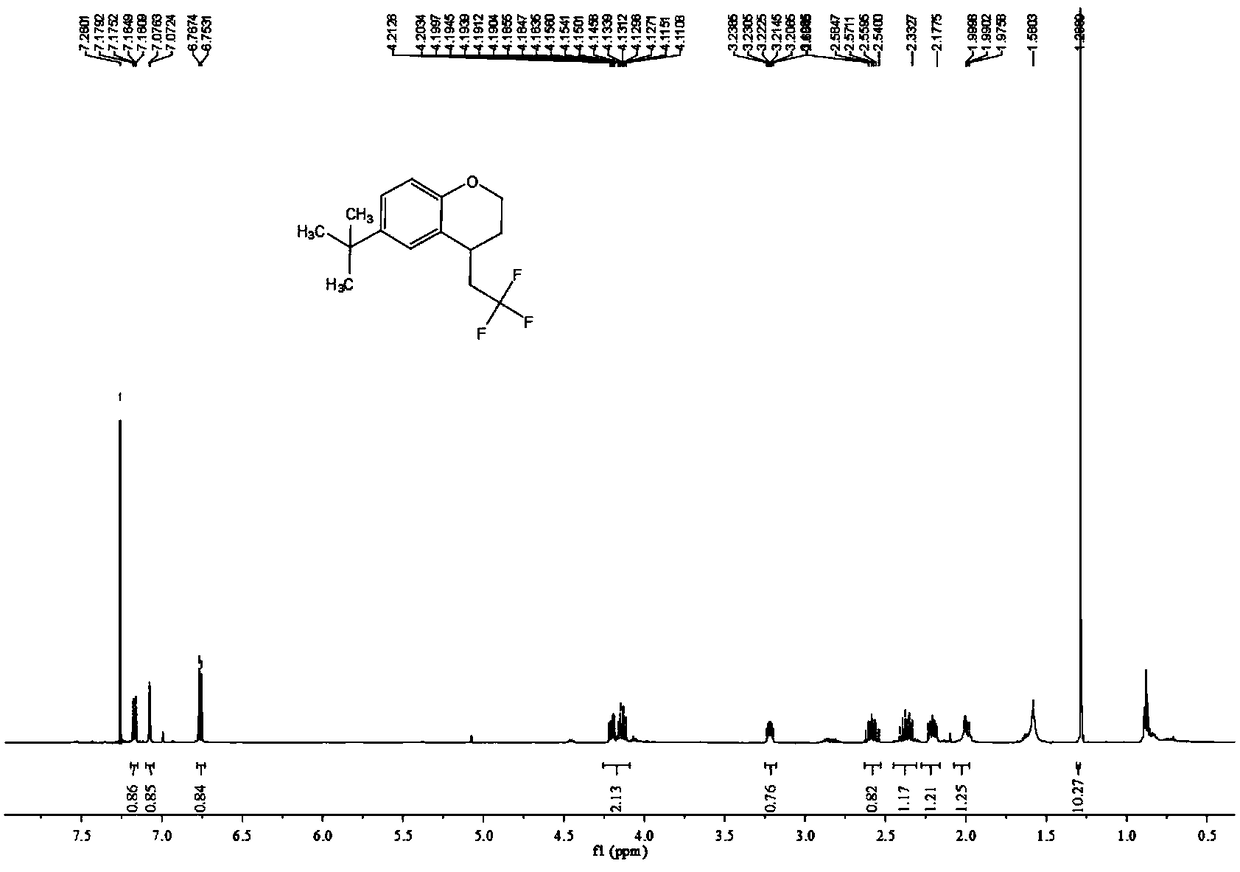
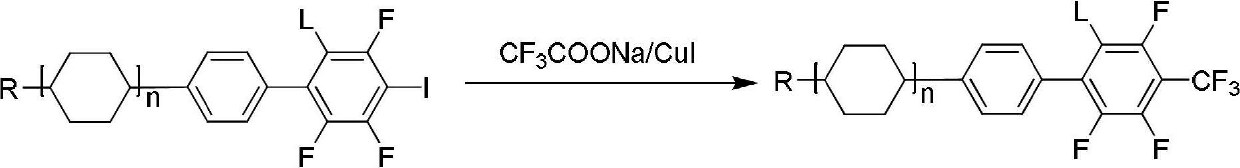
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000011.png)
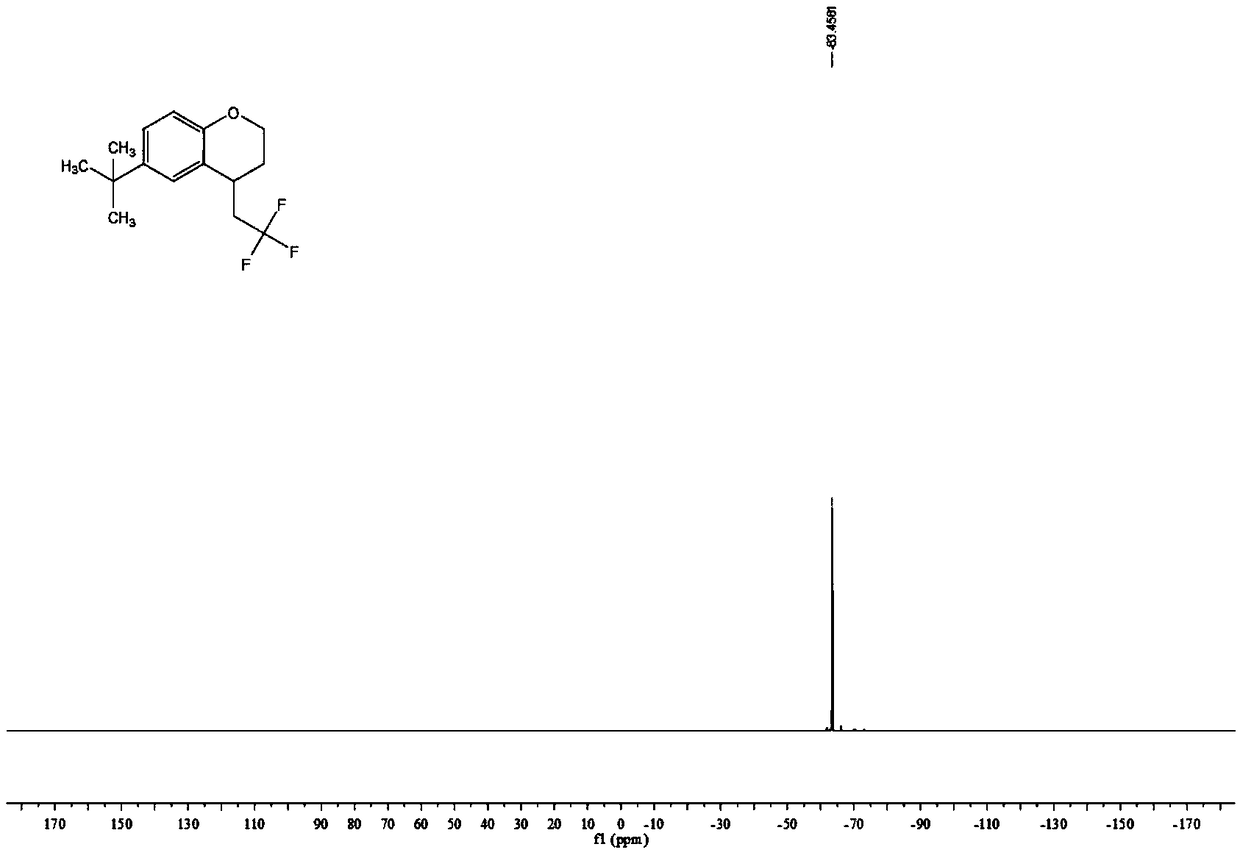
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000012.png)
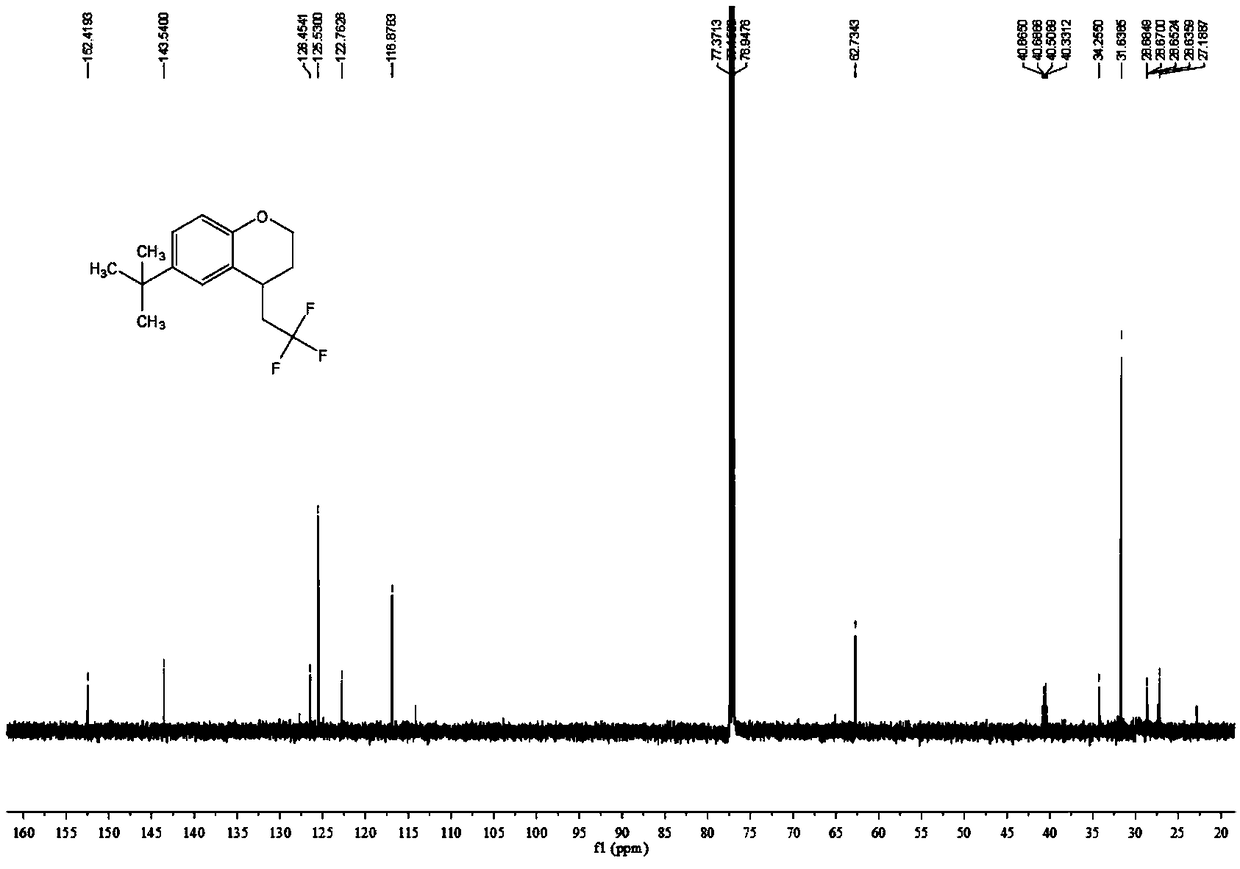
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000021.png)