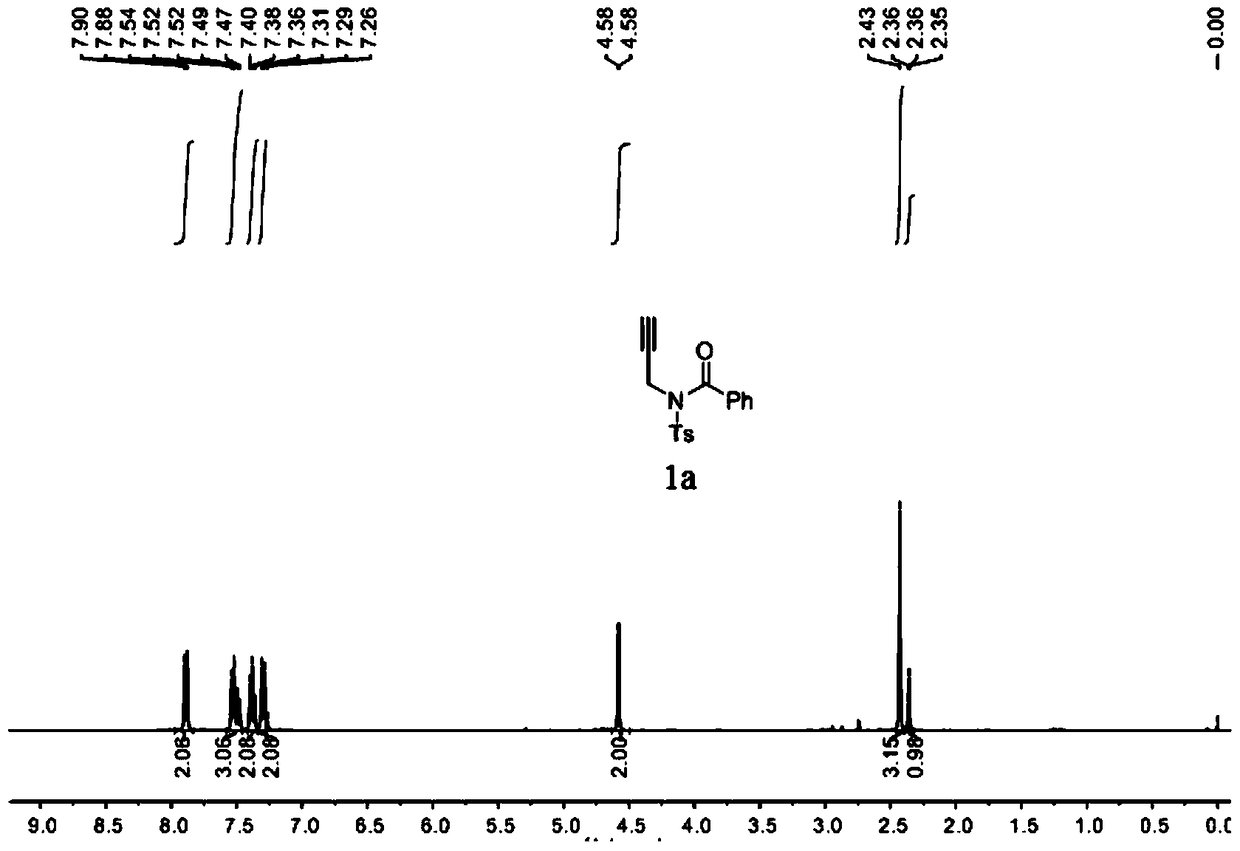
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Azidotrimethylsilane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
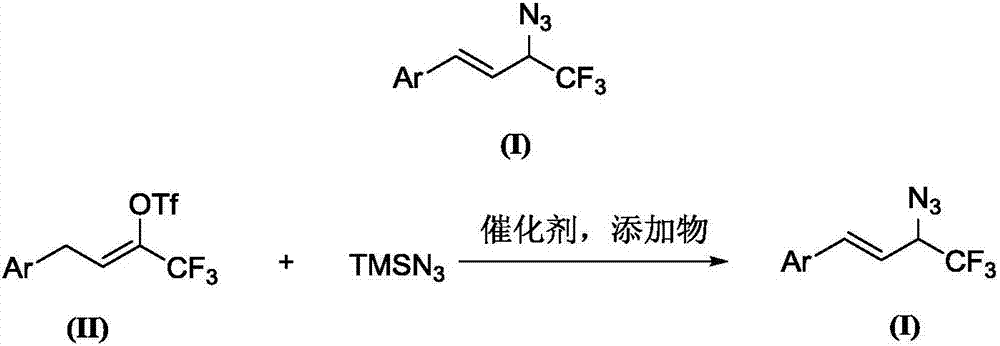
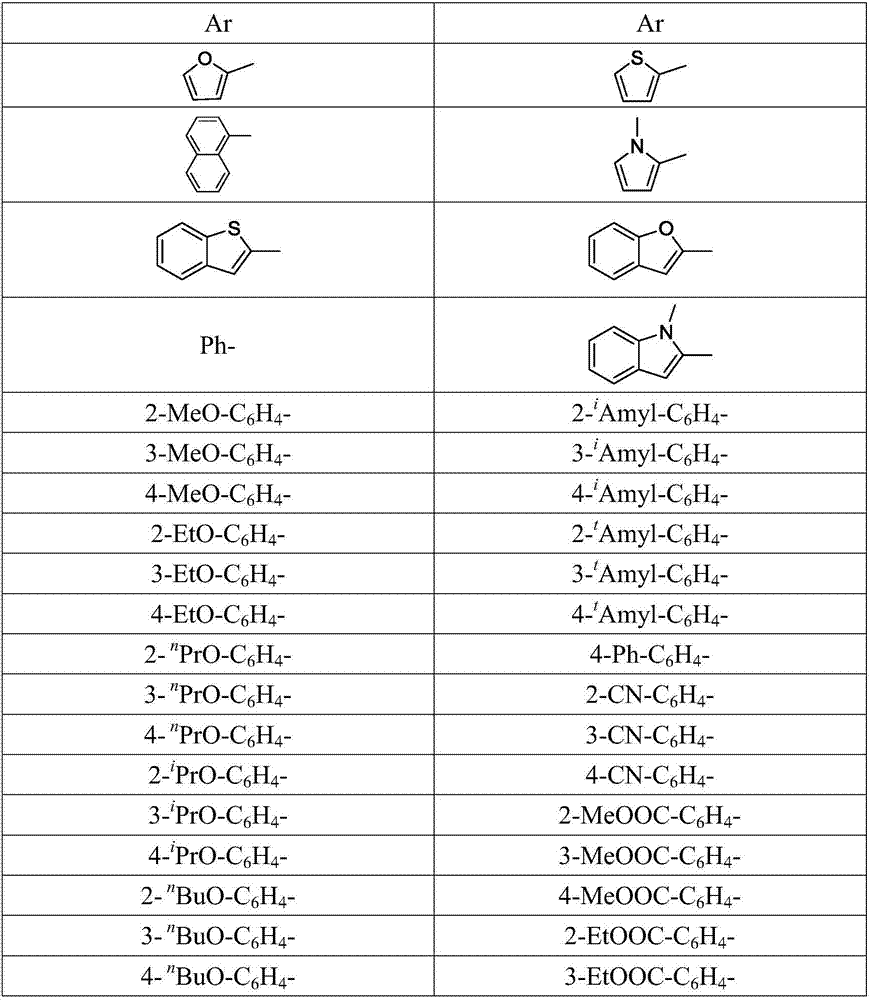
Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
ActiveCN104649857AMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzidotrimethylsilane
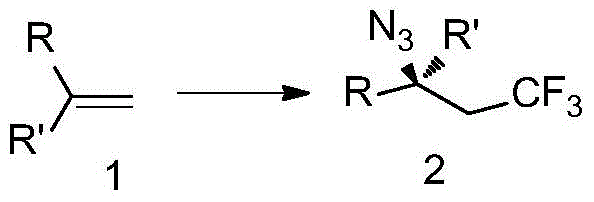
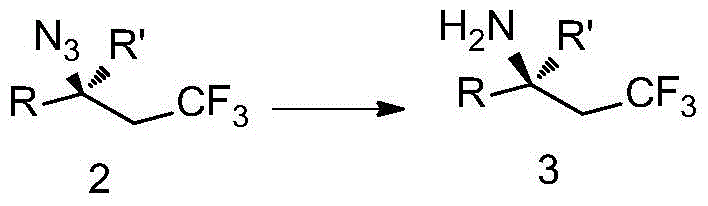
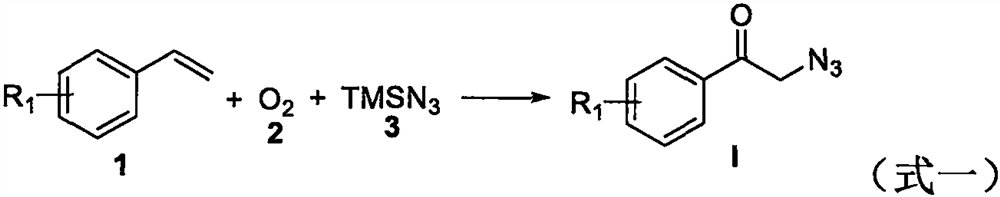
The invention discloses trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof. The preparing method of the trifluoromethyl-substituted azide compounds includes following steps of: subjecting a trifluoromethylation agent, azidotrimethylsilane and a carbon-carbon double bond of an olefin to addition in an organic solvent under the existence of a catalyst to obtain a compound in which one carbon in the carbon-carbon double bond of the olefin has trifluoromethyl and the other carbon has an azide group. The preparing methods utilize the trifluoromethylation agent which is mild relatively, directly form a carbon-nitrogen bond and a carbon-carbon bond by double-functionalization of olefins, and efficiently synthesize the trifluoromethyl-substituted azide, amine and heterocycle compounds with high selectivity. The preparing methods are easily available in raw materials, mild in reaction conditions, good in atom economy, high in selectivity, simple in after-treatment, environmental friendly, high in yields and suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
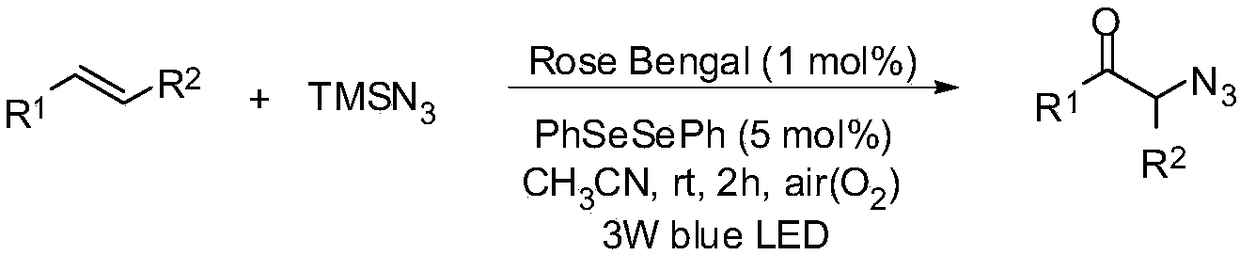
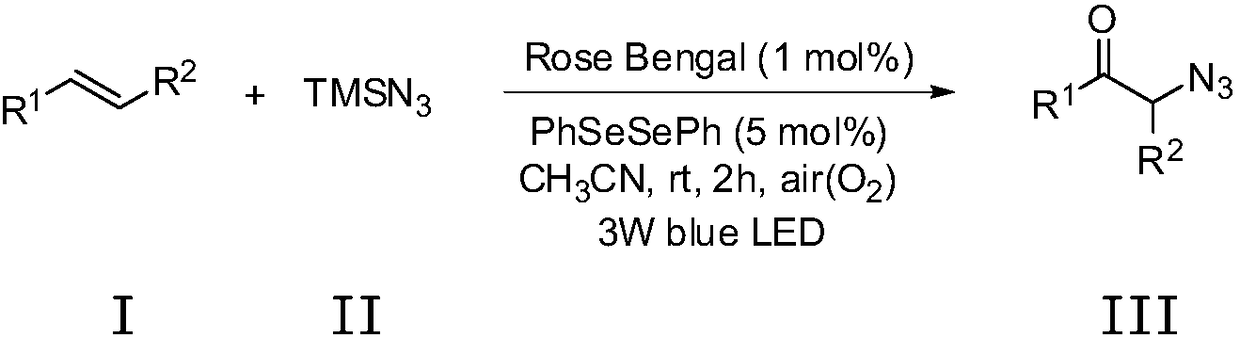
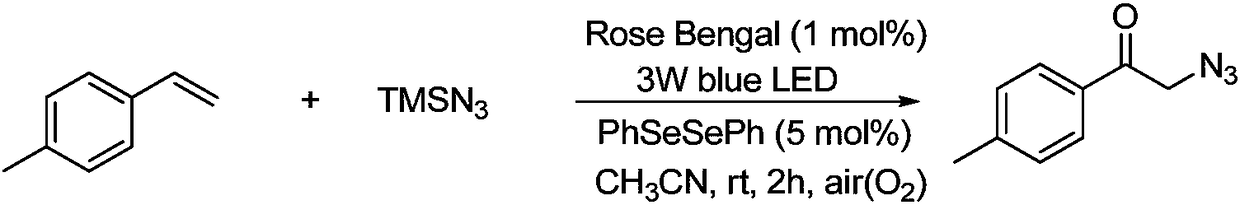
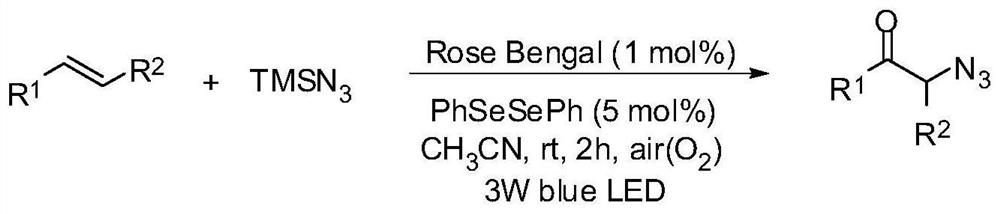
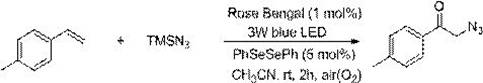
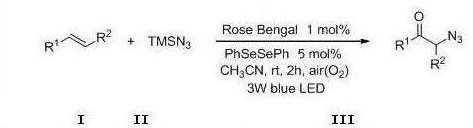
Method for preparing alpha-azidoketone compounds based on photocatalysis
ActiveCN108586283ALower reaction conditionsImprove securityOrganic chemistryArylAzidotrimethylsilane
The invention discloses a method for preparing alpha-azidoketone compounds based on photocatalysis. The method is based on a reaction formula shown in the description, wherein R<1> is optionally-substituted aryl, heteroaryl or 1-12 carbon alkyl; R<2> is optionally-substituted aryl, heteroaryl or 1-12 carbon alkyl, or a hydrogen atom. One substance represented by a general formula I is set as one olefin, a substance represented by a general formula II is set as azidotrimethylsilane (TMSN3), and one substance represented by a general formula III is set as one alpha-azidoketone compound, one alpha-azidoketone compound is synthesized under visible light catalysis. According to the method disclosed by the invention, a photocatalyst is designed, one alpha-azidoketone compound is synthesized by adopting air as an oxidant under the visible light catalysis, an equivalent-weight inorganic oxidant, metal reagent and pre-prepared complex raw material are not used, so that the reaction conditions are reduced, and the reaction safety is improved.
Owner:QUFU NORMAL UNIV
Preparation method of ortho-trifluoromethyl-substituted azide compound
ActiveCN108640808AMild method conditionsEasy to handleSulfonic acid amide preparationFunctional group formation/introductionAzidotrimethylsilaneSolvent
The invention discloses a preparation method of an ortho-trifluoromethyl-substituted azide compound. The preparation method of the ortho-trifluoromethyl-substituted azide compound comprises the following steps of adding manganese salts, olefin derivatives, sodium trifluoromethanesulfinate, azidotrimethylsilane and a peroxy compound into a solvent in the presence of inert gas, and carrying out reaction at 25-75 DEG C for 6-12 hours; and then, carrying out column chromatography separation so as to be subjected to purifying, and obtaining the ortho-trifluoromethyl-substituted azide compound. Themole ratio of the manganese salts to the olefin derivatives to the sodium trifluoromethanesulfinate to the azidotrimethylsilane to the peroxy compound is 0.1-0.2) to 1 to (1.5-2.5) to (2.5-3.5) to (2.5-3.5); and the dosage of the solvent is 6-7 milliliters of the solvent for per millimole of the olefin derivatives. The preparation method of the ortho-trifluoromethyl-substituted azide compound is low in raw material costs, mild in reaction conditions, simple in post-treatment and suitable for industrial production.
Owner:HEBEI UNIV OF TECH
Preparation method of ortho-diazido compound
InactiveCN106467475AMild reaction conditionsSimple post-processingOrganic chemistryAzidotrimethylsilaneSilanes
The invention relates to a preparation method of an ortho-diazido compound. The method comprises the following specific steps: dissolving a manganese salt catalyst, an olefin compound, a peroxide oxidizing agent and azidotrimethyl silane in a solvent under the protection of inert gas according to the molar ratio of 0.01-3.0: 1.0:1.0-2.0, reacting at the temperature of minus 30 to minus 100 DEG C for 6-24 h, and carrying out separation and purification to obtain a diazido compound. According to the method, raw materials used are cheap and easily available, peroxide used as an industrial raw material is used as an oxidizing agent, and cheap and stable manganese is used as a catalyst so as to obtain the diazido compound. The purchased solvent is directly used for the reaction. The solvent used is a commonly-used cheap solvent without any special treatment. The reaction condition is mild, and aftertreatment is simple. The preparation method is suitable for industrial production.
Owner:SHANGHAI UNIV
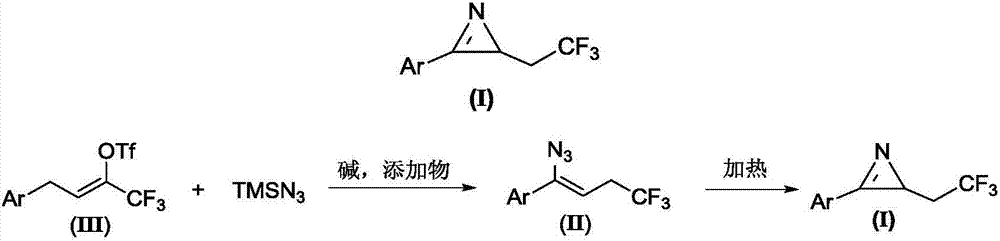
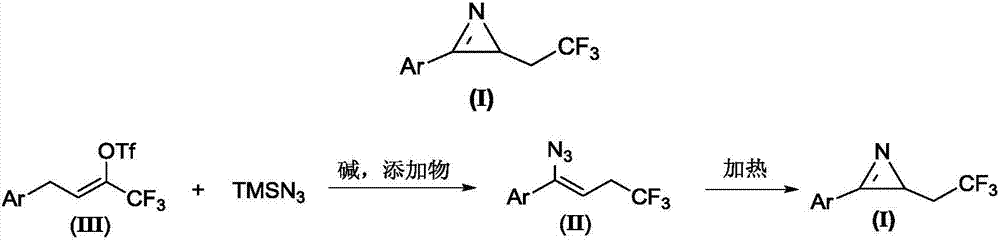
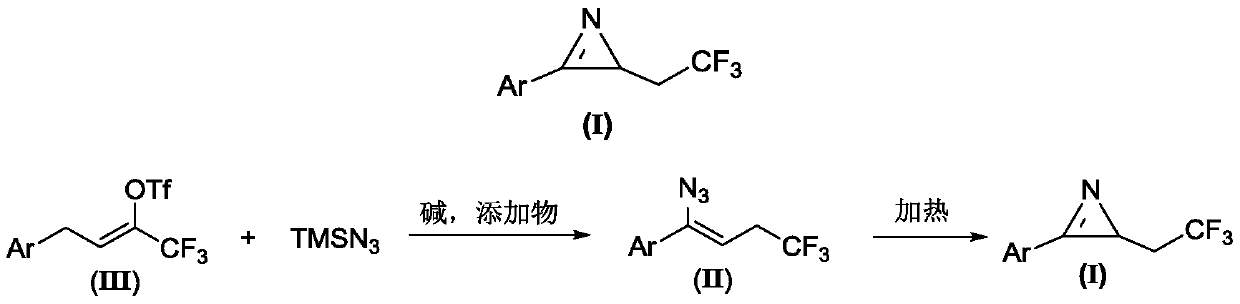
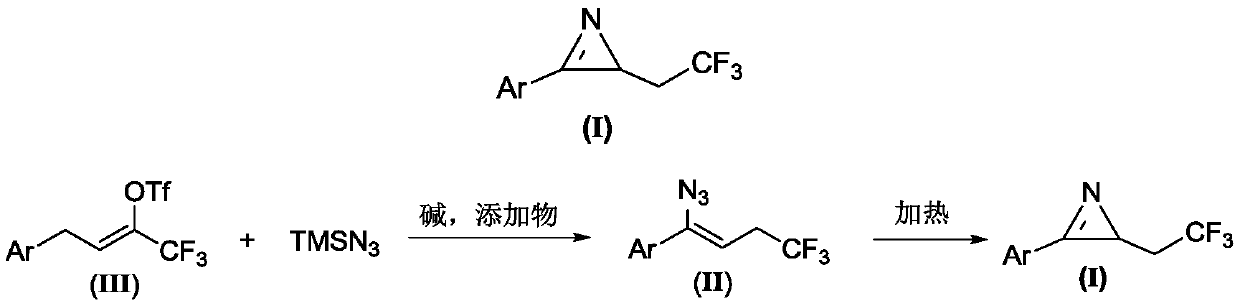
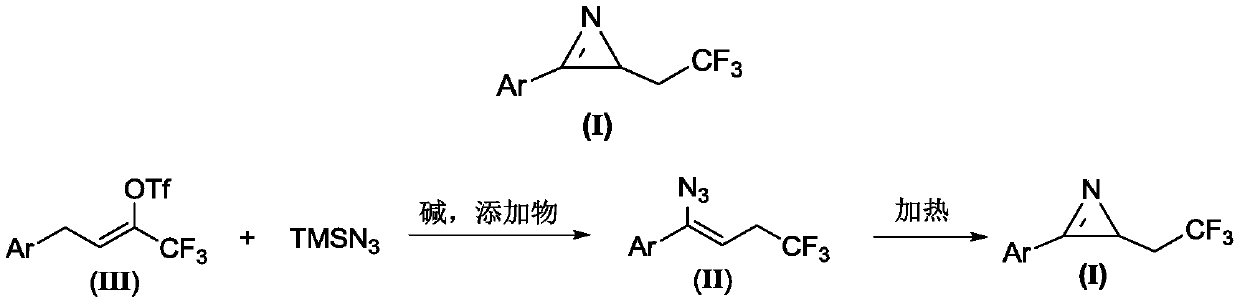
Method for preparing 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound
The invention relates to a method for preparing a 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound. The structure of the 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound is shown in figure 1, and a compound III and azidotrimethylsilane are adopted as raw materials to undergo two steps of reactions: (1) in the presence of alkali and additives, performing an azido reaction to obtain a compound which is shown in the general formula II; (2) preparing the compound which is shown in the general formula I through a denitrification cyclization reaction. The image is shown in the description. The method for synthesis of the 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound is convenient and low in cost, and meanwhile the application of an expensive and unstable trifluoromethylation reagent is avoided.
Owner:DALIAN UNIV OF TECH
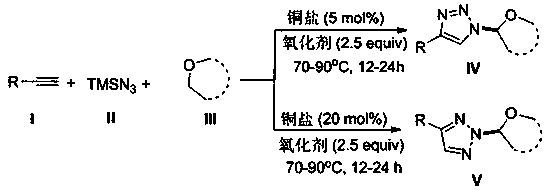
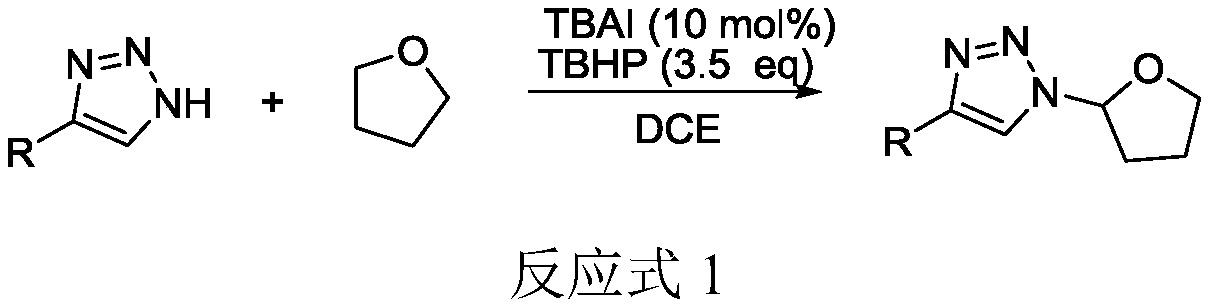
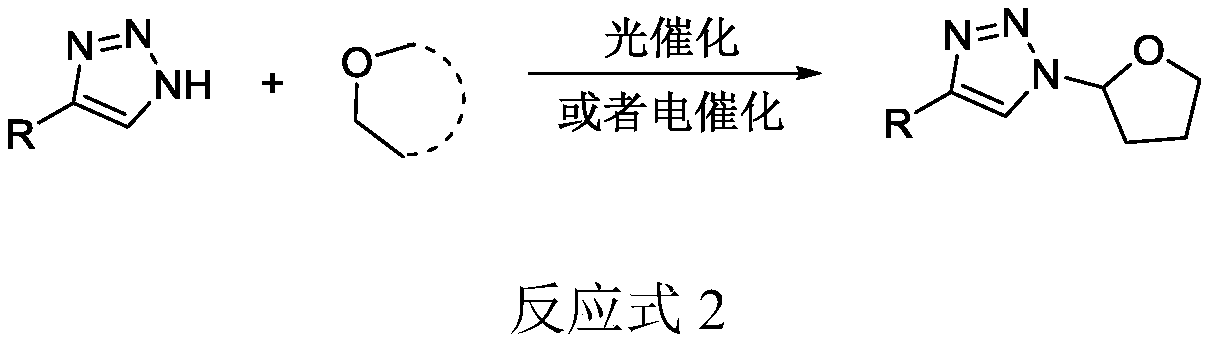
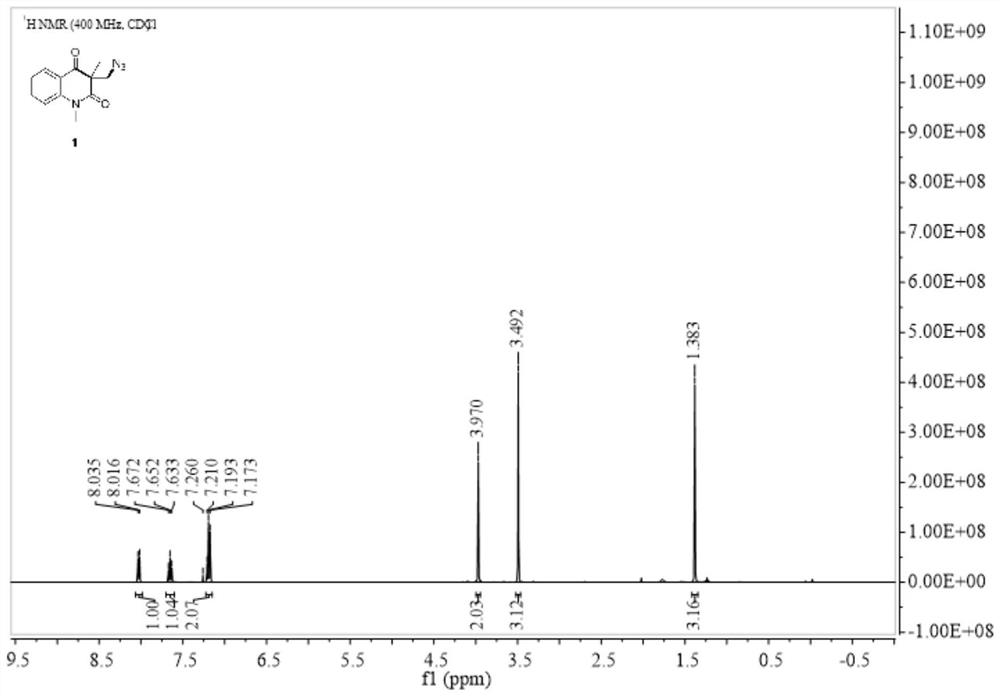
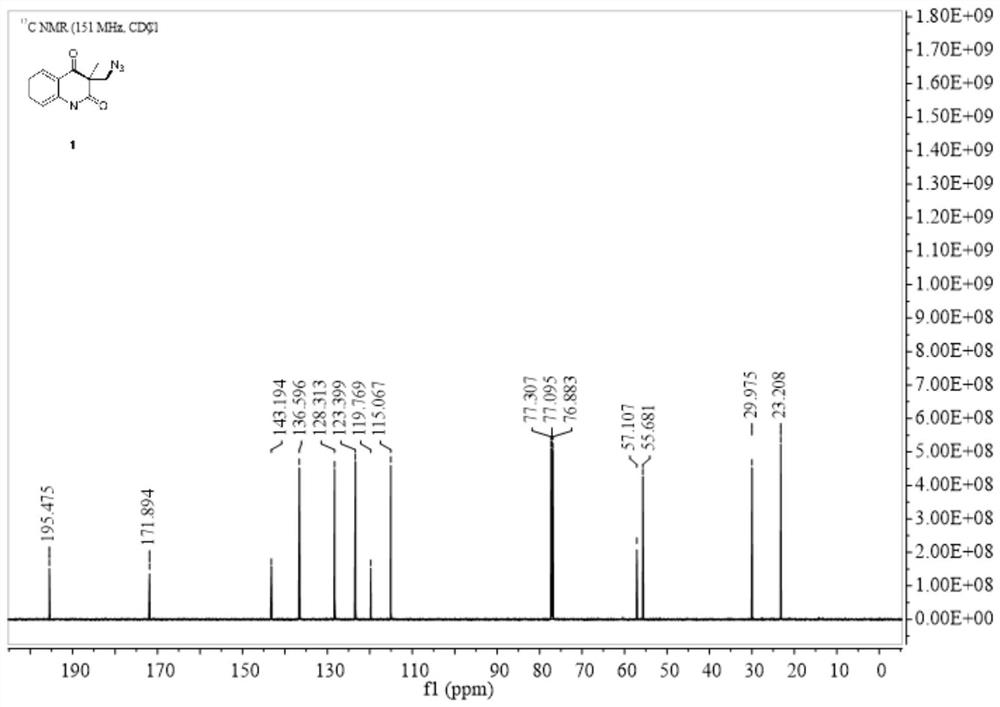
Selective production method of N-1-oxyalkyl-substituted 1,2,3-triazole compound and N2-oxyalkyl-substituted 1,2,3-triazole compound
ActiveCN110372675ASimple and fast operationRaw materials are cheap and easy to getOrganic chemistryAzidotrimethylsilaneAlkyne
The invention discloses a selective production method of a N-1-oxyalkyl-substituted 1,2,3-triazole compound and a N2-oxyalkyl-substituted 1,2,3-triazole compound. The selective production method of the N-1-oxyalkyl-substituted 1,2,3-triazole compound and the N2-oxyalkyl-substituted 1,2,3-triazole compound comprises the steps of mixing alkyne, azidotrimethylsilane and an ether solvent, then addinga copper catalyst and a tert-butyl hydroperoxide oxidizing agent, and carrying out a reaction at 70-90 DEG C; when the dosage of the copper catalyst is 5 mol%, the N-1-oxyalkyl-substituted 1,2,3-triazole compound is obtained from the reaction; and when the dosage of the copper catalyst is 20 mol%, the N2-oxyalkyl-substituted 1,2,3-triazole compound is obtained from the reaction. The selective production method of the N-1-oxyalkyl-substituted 1,2,3-triazole compound and the N2-oxyalkyl-substituted 1,2,3-triazole compound has the advantages that the simple commercial raw materials are adopted, by changing the dosage of the copper catalyst, the N-1-oxyalkyl-substituted 1,2,3-triazole compound and the N2-oxyalkyl-substituted 1,2,3-triazole compound can be selectively synthesized, and the reaction has high application values.
Owner:QUFU NORMAL UNIV
Method for preparing azide-substituted quinoline-2, 4-diketone through free radical tandem carbon cyclization reaction without metal catalysis
InactiveCN113264877AAvoid pollutionSolve technical problems of pollutionOrganic chemistryFungicidesAzidotrimethylsilaneMeth-
The invention discloses a method for preparing azide-substituted quinoline-2, 4-diketone through free radical tandem carbon cyclization reaction without metal catalysis, and relates to the field of chemical preparation, in particular to a preparation method of quinoline-2, 4-diketone. The invention aims to solve the problems of high production cost, increased side reaction occurrence probability and environmental pollution due to adoption of metal catalysis. The method comprises the following steps: reacting methacrylamide, a pre-activated azidotrimethylsilane solution and dimethyl sulfoxide at room temperature, quenching the mixture with H2O, extracting for three times with CH2Cl2, concentrating an organic solvent in vacuum, and purifying residues through rapid column chromatography to obtain the azide-substituted quinoline-2, 4-diketone. The method solves the technical problems of high production cost, side reaction occurrence probability increase and environmental pollution caused by metal catalysis, and the compound prepared by the method has the activity of inhibiting phytopathogen, can be applied to prevention and treatment of phytopathogen, and can be applied to preparation of anti-phytopathogen drugs.
Owner:YANTAI UNIV
Synthesis method for preparing a large amount of alpha-vinyl azide compounds
ActiveCN108178736AReduce dosageEasy to retouchOrganic chemistry methodsSulfonic acid amide preparationAzidotrimethylsilaneOrganic synthesis
The invention relates to a synthesis method for preparing a large amount of alpha-vinyl azide compounds, and belongs to the technical field of organic synthesis chemistry. In the prior art, alpha-vinyl azide compounds have unique reactivity, the synthesis and the applications of the alpha-vinyl azide compounds are important, and the alpha-vinyl azide compounds have good application prospects in the field of organic synthesis. According to the present invention, a large amount of stereospecific alpha-vinyl azide compounds are subjected to one-step efficient synthesis by using the simple and easily-available raw material acetylene-based compound and the azidotrimethylsilane; the synthesis method has advantages of simple and easily-available raw materials, wide application, low catalyst consumption, batch synthesis, simple operation method and efficient reaction, and is suitable for synthesis of a large amount of alpha-vinyl azide compounds; and the product has the stereospecific structure.
Owner:NORTHEAST NORMAL UNIVERSITY
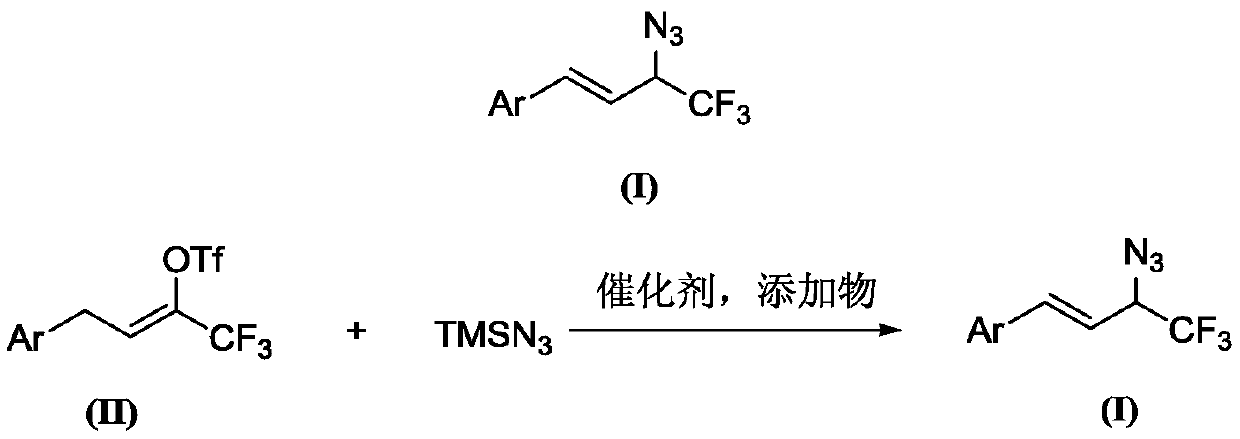
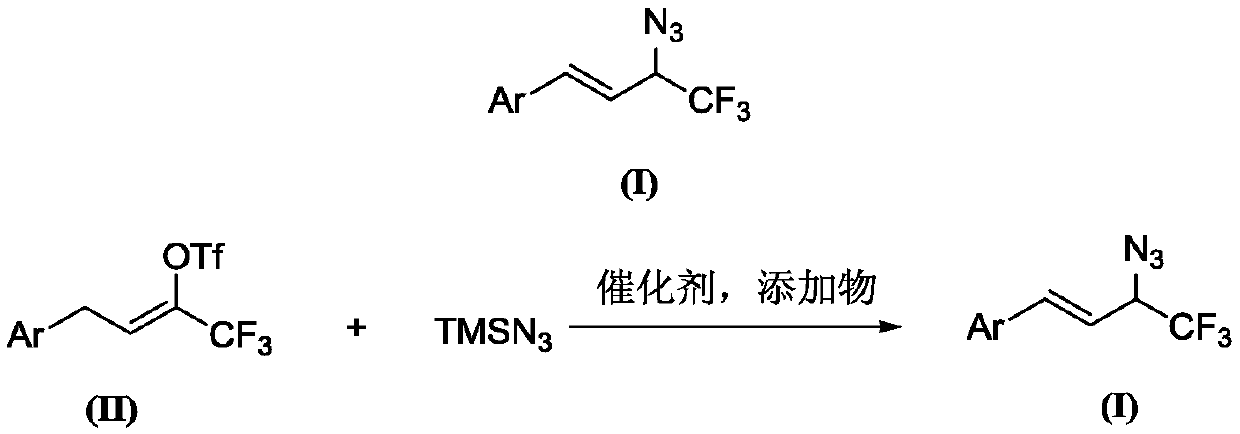
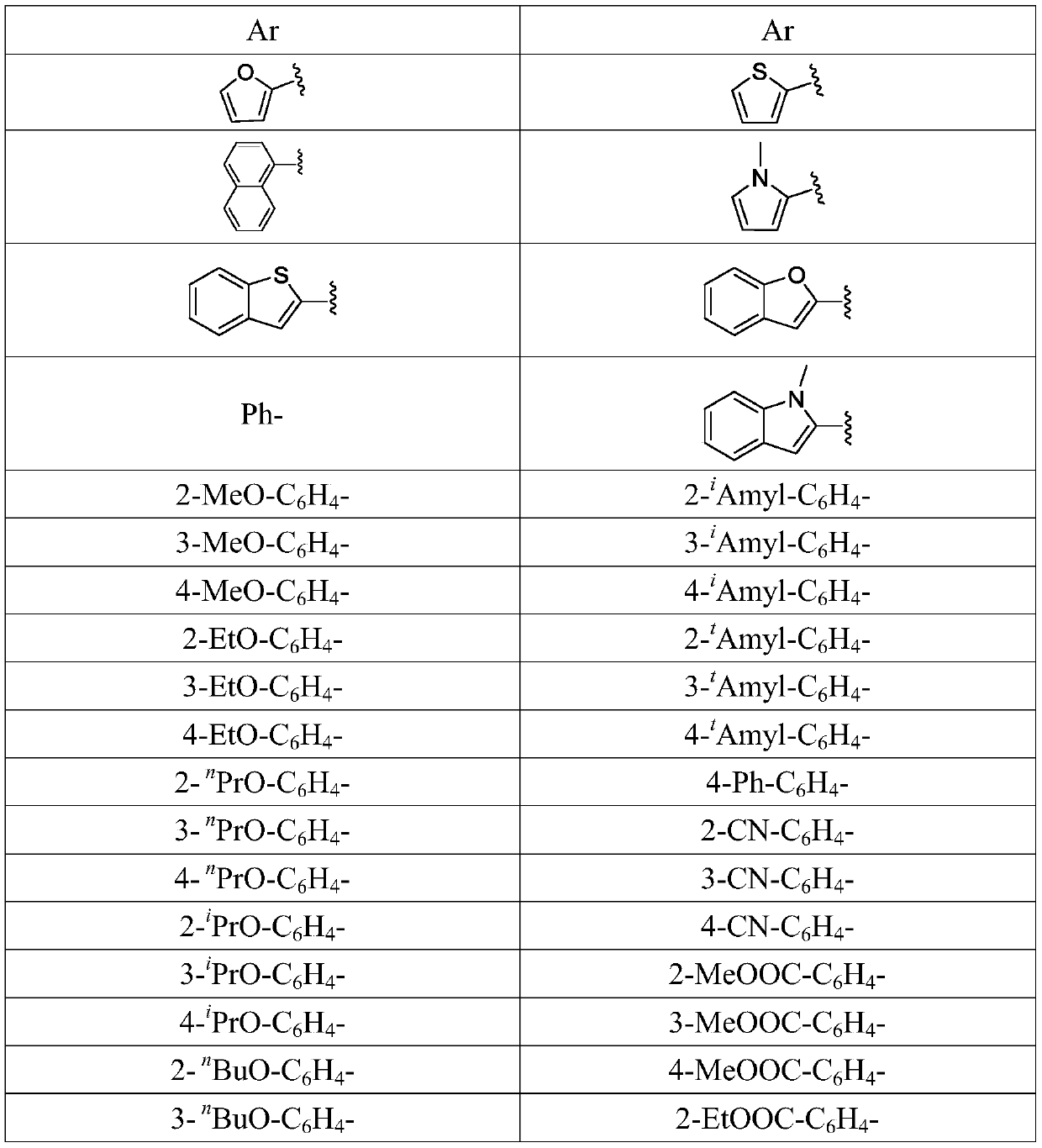
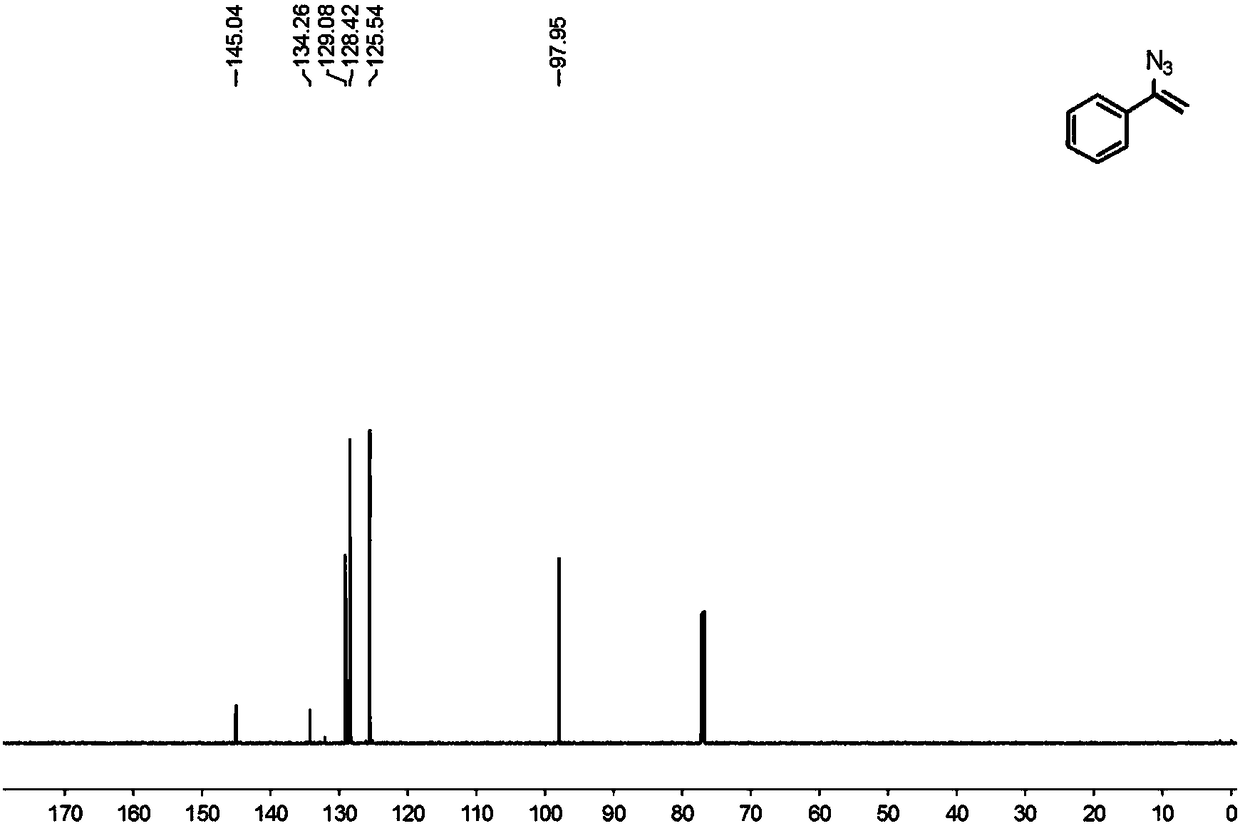
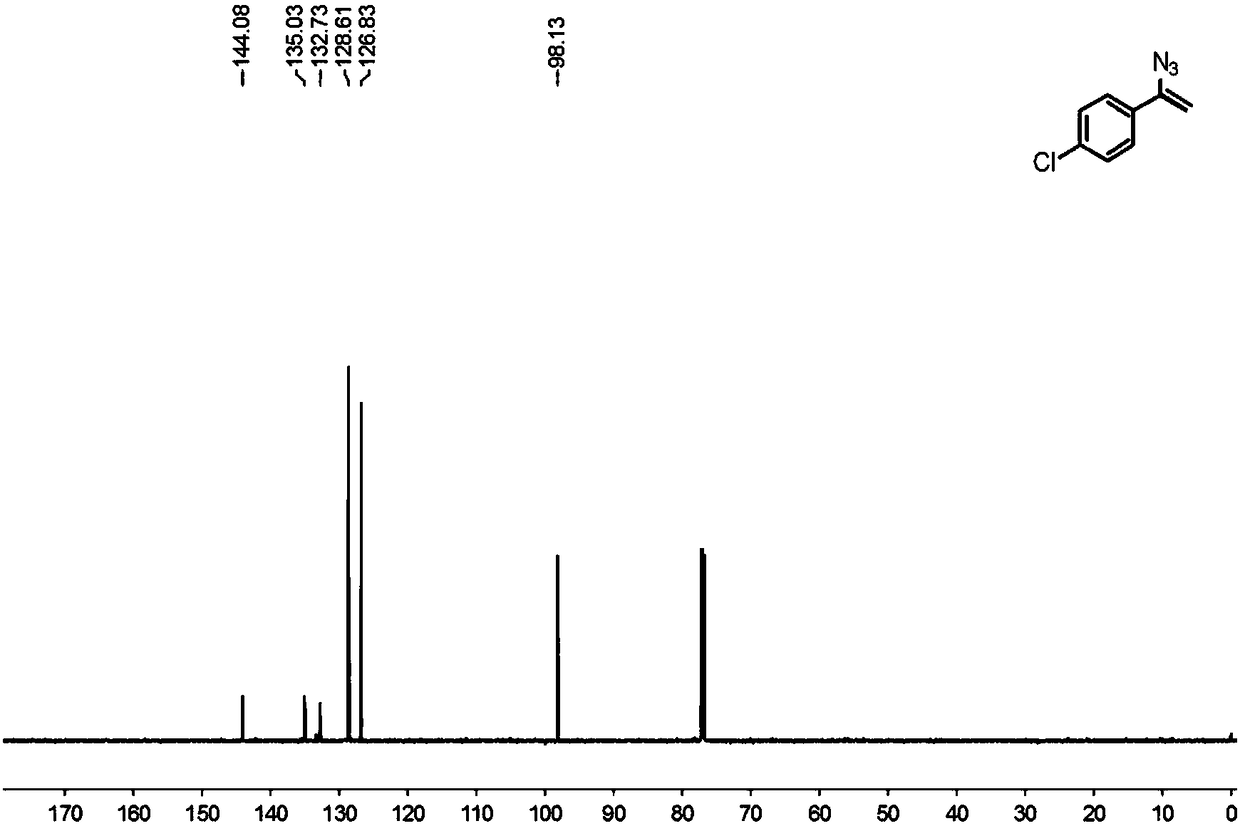
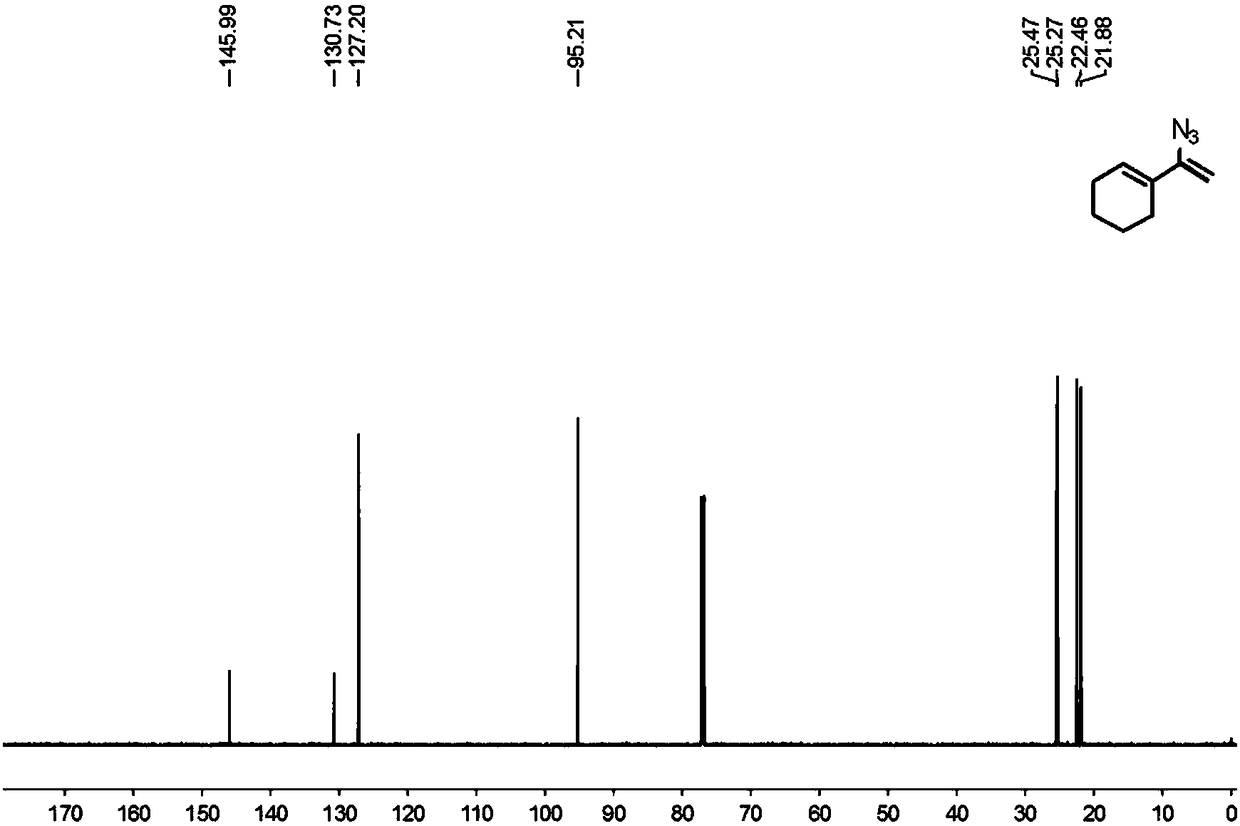
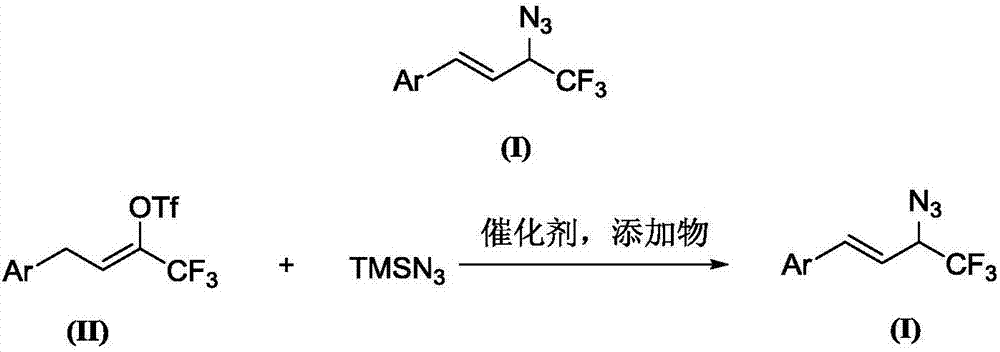
Preparation method of 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound
The invention relates to a preparation method of a 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound, and belongs to the technical field of compound preparation. According to the preparation method of the 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound, the compound shown by a general formula I and azidotrimethylsilane are used as raw materials to take a reaction according to a following reaction formula under the existence of catalysts and additives so as to obtain the compound shown by the general formula I. The reaction formula is shown in the specification. The method provided by the invention has the advantages that the raw materials which have low cost and can be easily obtained in a wide range are used as catalysts; a convenient and low-cost method is provided for the synthesis of the 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound; meanwhile, the use of expensive and instable trifluoromethylation reagents is avoided.
Owner:DALIAN UNIV OF TECH +1
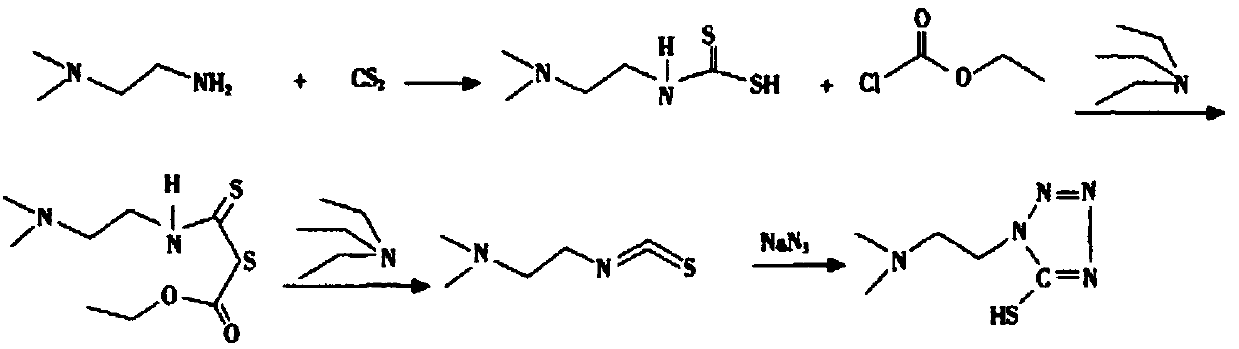
Synthesis method of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole
ActiveCN110950816AThe synthesis process is simpleHigh yieldOrganic chemistryAzidotrimethylsilaneTetrazole
The invention belongs to the technical field of medicines, and particularly relates to a synthesis method of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole. The method comprises the following steps: reacting N, N-dimethylethylenediamine with thiophosgene to obtain isothiocyanate, reacting isothiocyanate with azidotrimethylsilane to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazole hydrochloride, dissolving 1-(2-dimethylaminoethyl)-5-mercaptotetrazole hydrochloride in water, and carrying out decolorizing and crystallizing to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazole. According to themethod, the isothiocyanate is synthesized from the N, N-dimethylethylenediamine and the thiophosgene through a one-step method, so that the synthesis process is simplified and the yield is greatly increased; non-toxic azidotrimethylsilane is used for replacing virulent sodium azide in the cyclization process, so that the safety of the process is improved, and the method is more suitable for industrial amplification.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
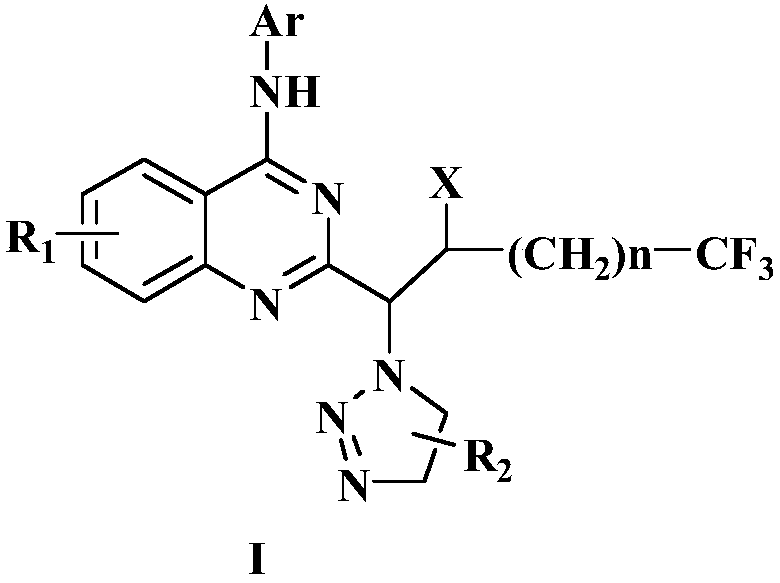
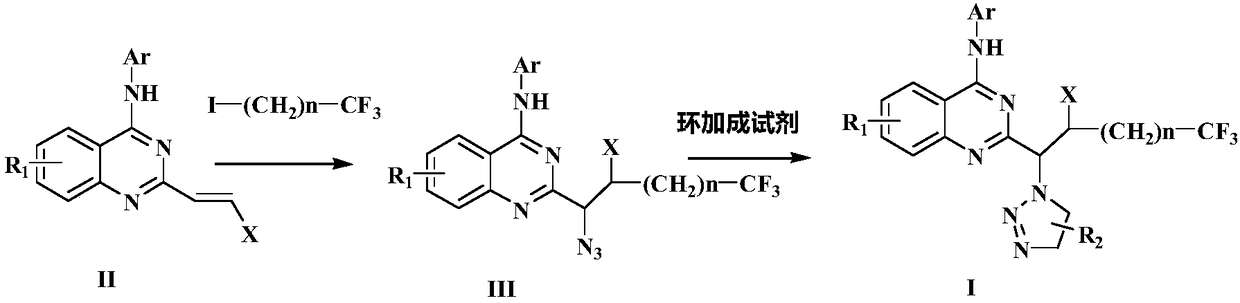
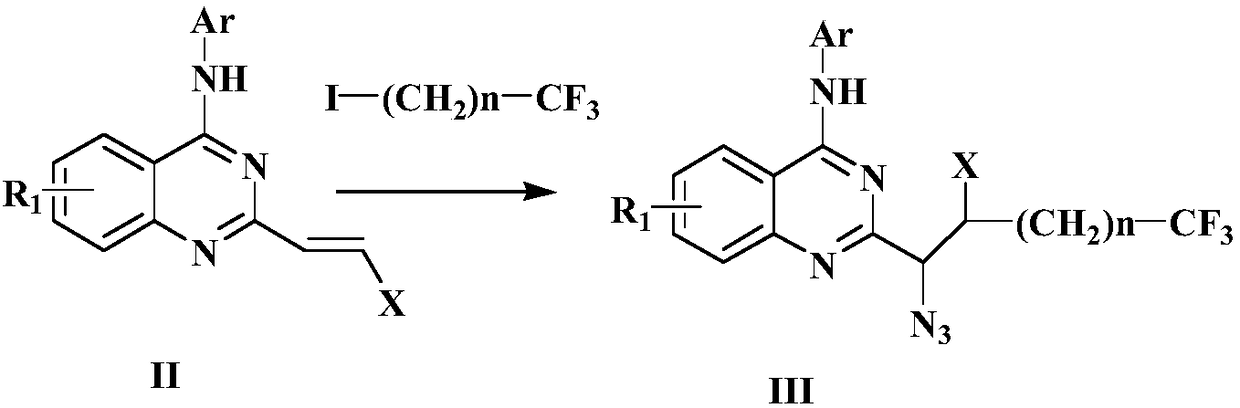
Preparation method of disubstituted quinazoline medicine compounds containing triazole parent nucleus
The invention provides a preparation method of trifluoromethyl substituted and 1,2,3-triazole structure unit containing 2,4-disubstituted quinazoline medicine compounds shown in the following formula(I). The method comprises the steps as follows: an alkenyl quinoline compound reacts with trifluoromethyl substituted alkyl iodide and azidotrimethylsilane in an organic solvent in the presence of a peroxide free radical initiator and an organic metal salt catalyst, and an intermediate compound is obtained; the intermediate compound and a cycloaddition reagent are subjected to a cycloaddition reaction under the protection of nitrogen in an organic solvent in the presence of alkali and bis(2-diphenylphosphinophenyl)ether ligand, and the compound shown in the formula (I) is obtained. The cycloaddition reagent is selected from beta-carbonyl carboxylate or alkyne compounds.
Owner:张海英
Preparation method of ketamine and synthesis method of intermediate compound
ActiveCN110818587AReduce usageReduce manufacturing costOrganic chemistryOrganic compound preparationAzidotrimethylsilaneEthylic acid
The invention discloses an intermediate compound II for synthesizing ketamine, and the chemical structural formula of the intermediate compound II is disclosed in the invention. The compound II is directly synthesized by one-step reaction of a compound under the action of iodobenzene acetate and azidotrimethylsilane; and ketamine is synthesized from the compound II through two steps: 1) reducing azide into amino; and 2) carrying out aminomethylation reaction. Compared with the prior art, the HPLC purity of the product can reach 97% or above, meanwhile, the ketamine prepared through the methodis high in industrialization degree, the quality of the product is greatly improved, intermediate impurities do not exist, the process route is easy to operate, the cost is low, and conditions are mild.
Owner:YICHANG HUMANWELL PHARMA +1
Synthetic method of alpha-hydroxyl alkenyl azide compound
InactiveCN103664686BSpecific reactivityStructural StereospecificityOrganic chemistryPropanolAzidotrimethylsilane
The invention relates to a synthetic method of alpha-hydroxyl alkenyl azide compound, and belongs to the technical field of organic synthetic chemistry. As alpha-hydroxyl alkenyl azide derivatives have particular reactivity, much attention has been paid to the synthesis and application of the alpha-hydroxyl alkenyl azide derivatives. The alpha-hydroxyl alkenyl azide derivatives are synthesis precursors of nitrogenous heterocyclic compounds. By utilizing propargyl alcohol which is simple, stable and easy to get and azide trimethyl silicane, the alpha-hydroxyl alkenyl azide compound can be compounded by one step; the synthetic method of the alpha-hydroxyl alkenyl azide compound has the characteristics that the operation is simple and efficient, the raw materials and reagents are stable and easy to get, and the product structure is stereospecific; the synthetic method is applicable to various alpha-hydroxyl alkenyl azide compounds.
Owner:NORTHEAST NORMAL UNIVERSITY
A kind of preparation method of ketamine and the synthetic method of intermediate compound
ActiveCN110818587BReduce usageReduce manufacturing costOrganic chemistryOrganic compound preparationAzidotrimethylsilaneTrimethylsilane
The invention discloses an intermediate compound II for synthesizing ketamine. Its chemical structural formula is: the compound II is directly synthesized by one-step reaction of the compound under the action of iodobenzene acetate and azidetrimethylsilane. Compound II synthesizes ketamine through two steps: 1) reduction of azide to amino group; 2) aminomethylation reaction. Compared with the prior art, the HPLC purity of the product of the invention can reach more than 97%. At the same time, the ketamine prepared by the method of the invention has a high degree of industrialization, and the quality of the product is greatly improved compared with the prior art, and there is no intermediate impurity; the process route is simple to operate, low in cost and mild in condition.
Owner:YICHANG HUMANWELL PHARMA +1
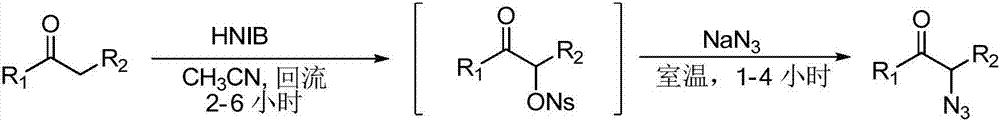
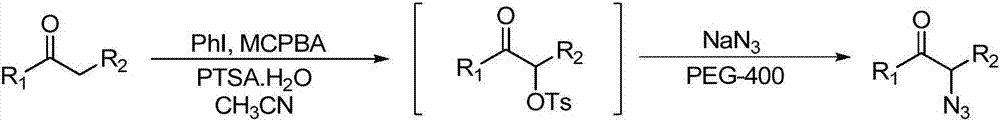
A kind of preparation method of α-azide aryl ketone derivative
ActiveCN109776352BHas a dual roleRealization of azidation/oxidation reactionsOrganic chemistryAzidotrimethylsilaneCombinatorial chemistry
The present invention relates to a kind of preparation method of α-azide aryl ketone derivative, this method is by adding substituted styrene, azidotrimethylsilane and oxidizing agent in Schlenk reaction bottle, then add water as solvent again, at a certain temperature , stirring and reacting under an oxygen atmosphere to obtain the azidation / oxidation product α-azide aryl ketone derivative.
Owner:NINGBO UNIV
A photocatalytic method for the preparation of α-azide ketone compounds
Owner:QUFU NORMAL UNIV
Continuous synthetic method for azidotrimethylsilane
ActiveCN109305984AReduce the risk of accumulatingShort reaction timeGroup 4/14 element organic compoundsAzidotrimethylsilaneTrimethylsilyl
The invention provides a continuous synthetic method for azidotrimethylsilane. The continuous synthetic method comprises the following steps of at a protective atmosphere, continuously conveying trimethyl-chlorosilane and an azide as reaction raw materials to a continuous synthesizer to perform nucleophilic substitution reaction to obtain the azidotrimethylsilane, and continuously discharging theazidotrimethylsilane, wherein the protective atmosphere is selected from nitrogen and / or inert gas. In the continuous synthetic method, by adopting a continuous synthetic technology to synthesize theazidotrimethylsilane; and in the whole reaction process, the required reaction time is short, the yield is large, and the performance of a product is stable. Meanwhile, as the invention adopts the continuous synthetic technology, the reaction heat can be timely brought away from the continuous synthesizer at any time, so that lowering of the risk of heat accumulation when scale reaction is amplified is facilitated, operation is simplified, and the safety of continuous batch production is improved. Therefore, by adopting the continuous synthetic technology provided by the invention to prepare the azidotrimethylsilane, improvement on safety of the whole reaction process and quality of the product is facilitated.
Owner:ASYMCHEM LAB TIANJIN
A kind of preparation method of 2-(2,2,2-trifluoroethyl)-3-aryl-2h-azapropenidine compound
The invention relates to a method for preparing a 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound. The structure of the 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound is shown in figure 1, and a compound III and azidotrimethylsilane are adopted as raw materials to undergo two steps of reactions: (1) in the presence of alkali and additives, performing an azido reaction to obtain a compound which is shown in the general formula II; (2) preparing the compound which is shown in the general formula I through a denitrification cyclization reaction. The image is shown in the description. The method for synthesis of the 2-(2,2,2-trifluoroethyl)-3-aryl-2H-azirines compound is convenient and low in cost, and meanwhile the application of an expensive and unstable trifluoromethylation reagent is avoided.
Owner:DALIAN UNIV OF TECH
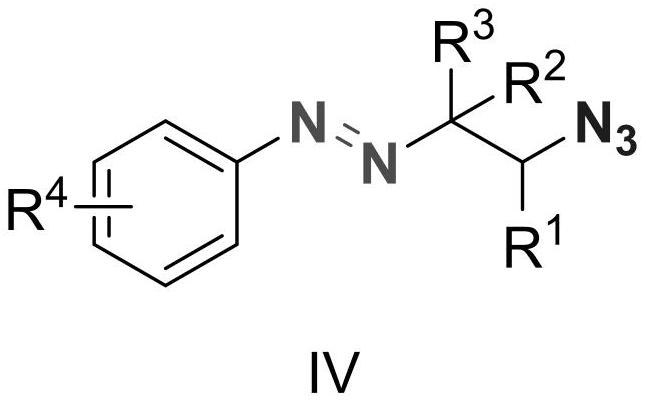
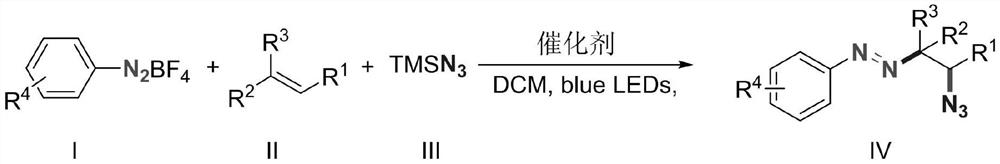
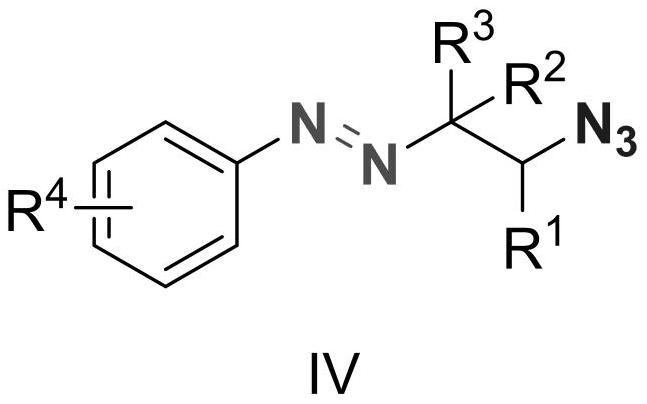
Alkyl aryl asymmetric azo and synthesis method thereof
ActiveCN112939807AConvenient researchHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationTetrafluoroborateAzidotrimethylsilane
The invention discloses alkylaryl asymmetric azo and a synthesis method thereof. The structural formula of the alkylaryl asymmetric azo is shown as a formula (IV). According to the synthesis method of the alkylaryl asymmetric azo, diazonium tetrafluoroborate, olefin and trimethylsilyl azide are used as raw materials, dichloromethane and other organic solvents are used as reaction solvents at normal temperature, a common blue LED lamp is used as a light source, reaction is carried out in the presence of a photocatalyst, and the alkylaryl asymmetric azo is synthesized through a three-component cascade reaction. The synthesis method provided by the invention has the advantages of mild reaction conditions, simple experimental operation, good reaction selectivity, high product yield, easy product separation and the like.
Owner:HANGZHOU NORMAL UNIVERSITY
Synthetic method for preparing large quantities of α-vinyl azides
ActiveCN108178736BReduce dosageEasy to retouchOrganic chemistry methodsSulfonic acid amide preparationAzidotrimethylsilaneOrganic synthesis
Owner:NORTHEAST NORMAL UNIVERSITY
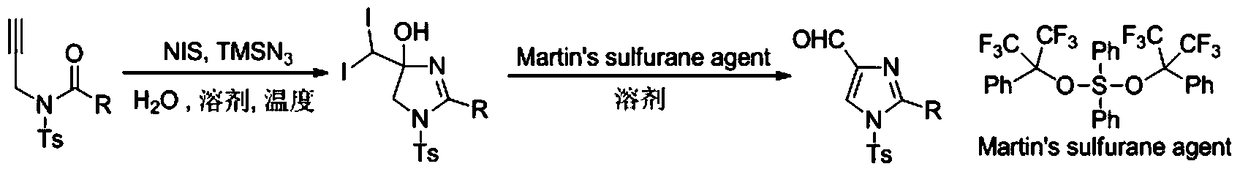
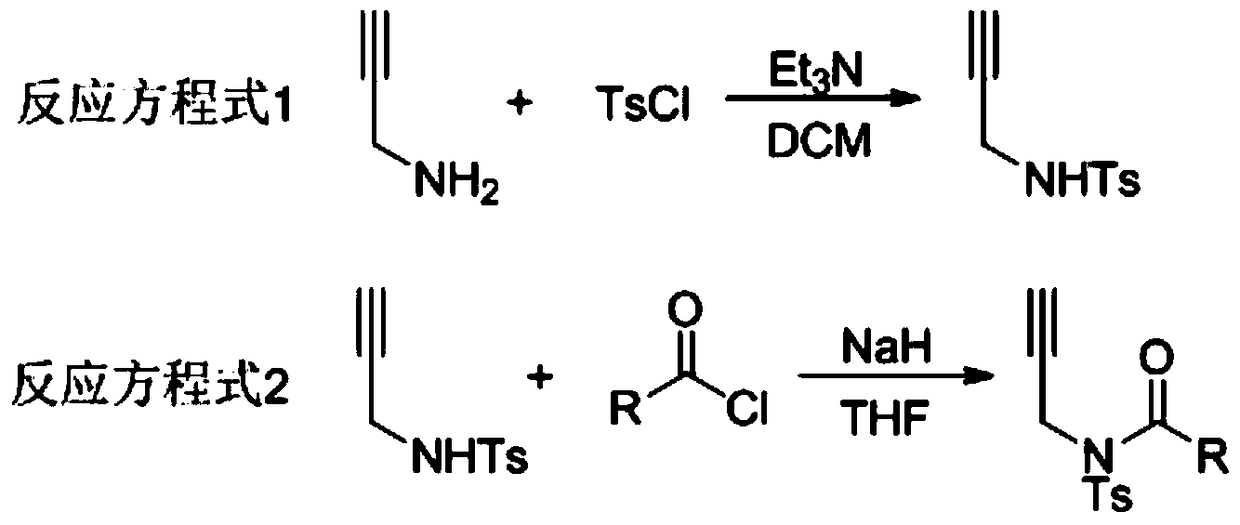
Preparation method of 1,5-disubsituted tetrazole compound
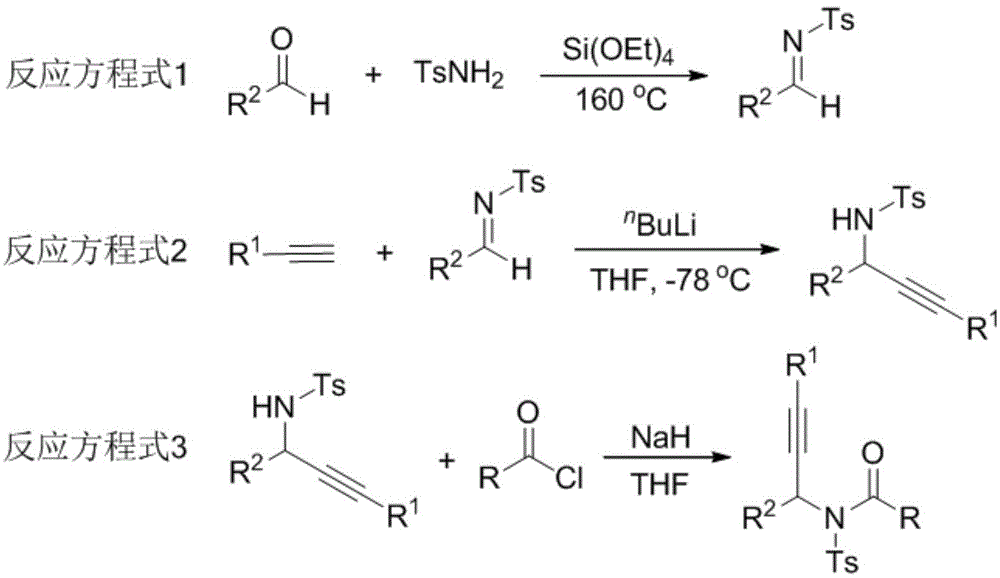
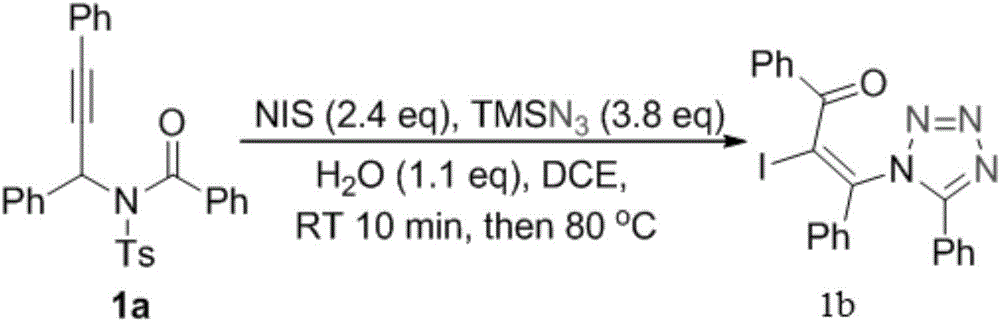
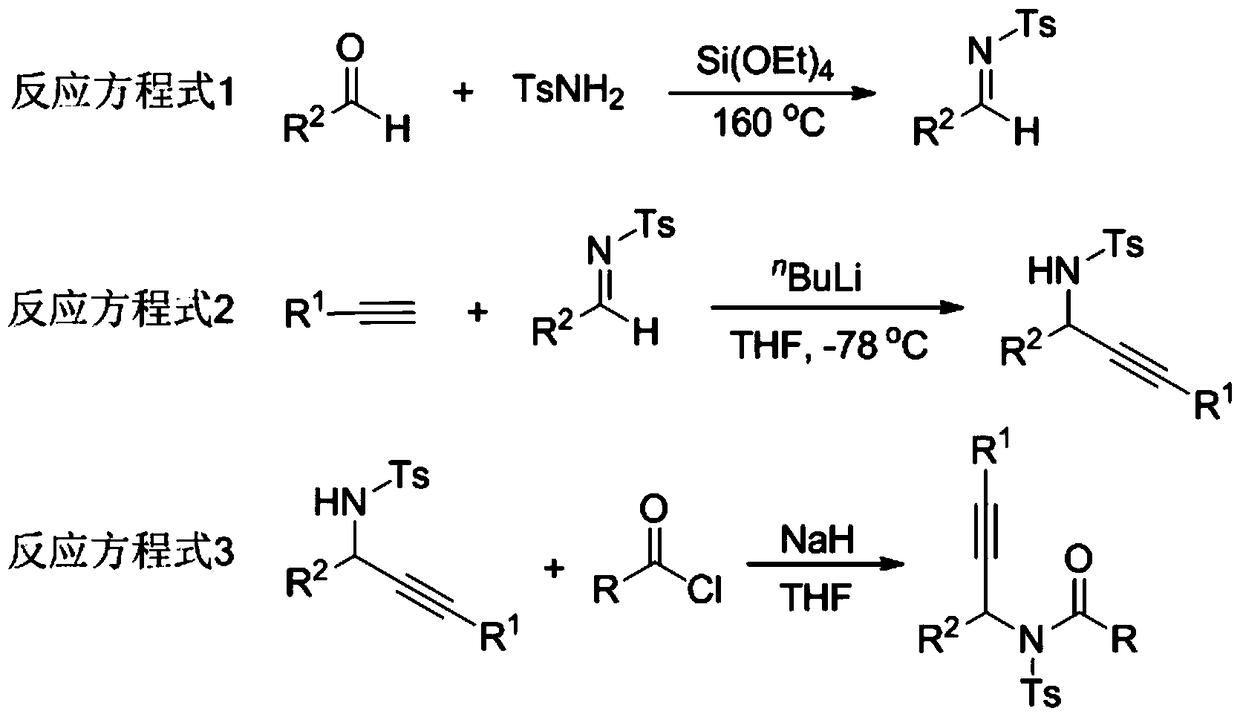
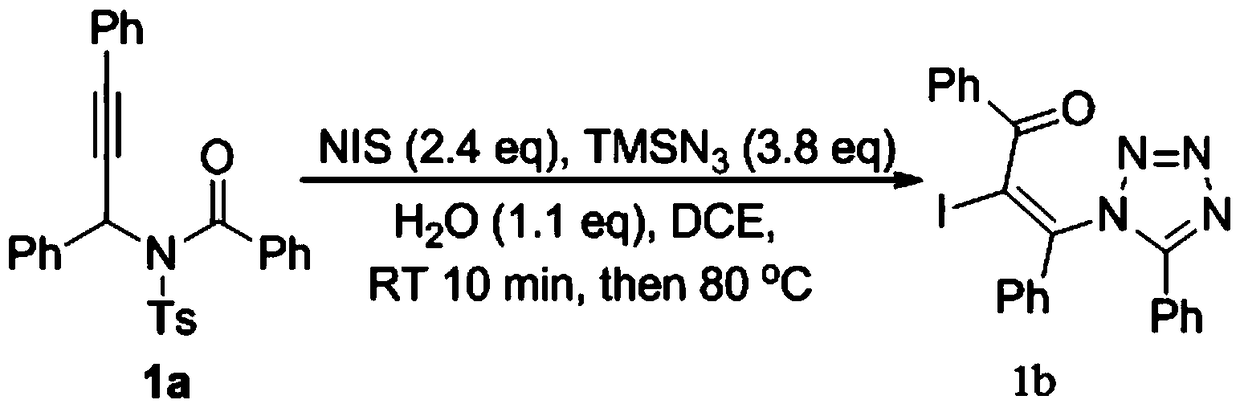
The invention relates to a preparation method of 1,5-disubsituted tetrazole compound. The method concretely comprises the following steps: propynoic acid amide which is obtained by simple preparation is used as a raw material, in a condition with N-Iodosuccinimide (NIS), azidotrimethylsilane (TMSN3) and water, an intramolecular oxygen atom transfer reaction is carried out, and the 1,5-disubsituted tetrazole compound is obtained. Propynoic acid amide is simply prepared from cheap and easily available initial raw materials, TMSN3 is used as a nitrogen source, the one-step intramolecular oxygen atom transfer reaction is carried out, and 1,5-disubsituted tetrazole is obtained. The method has the advantages of simple operation, and mild reaction conditions without metal catalysts.
Owner:中科榆林能源技术运营有限责任公司
A kind of preparation method of 1,5-disubstituted tetrazole compound
The invention relates to a preparation method of a 1,5-disubstituted tetrazole compound. The specific method is to use propynylamide which is simply prepared as a raw material, and under the conditions of N-iodosuccinimide (NIS), azidotrimethylsilane (TMSN3) and water, intramolecular oxygen atom transfer occurs The reaction yields 1,5-disubstituted tetrazole compounds. Propigylamide can be easily prepared from cheap and easy-to-obtain starting materials, and TMSN3 is used as a nitrogen source, and an intramolecular oxygen atom transfer reaction occurs in one step to obtain 1,5-disubstituted tetrazole. A metal catalyst is required.
Owner:中科榆林能源技术运营有限责任公司
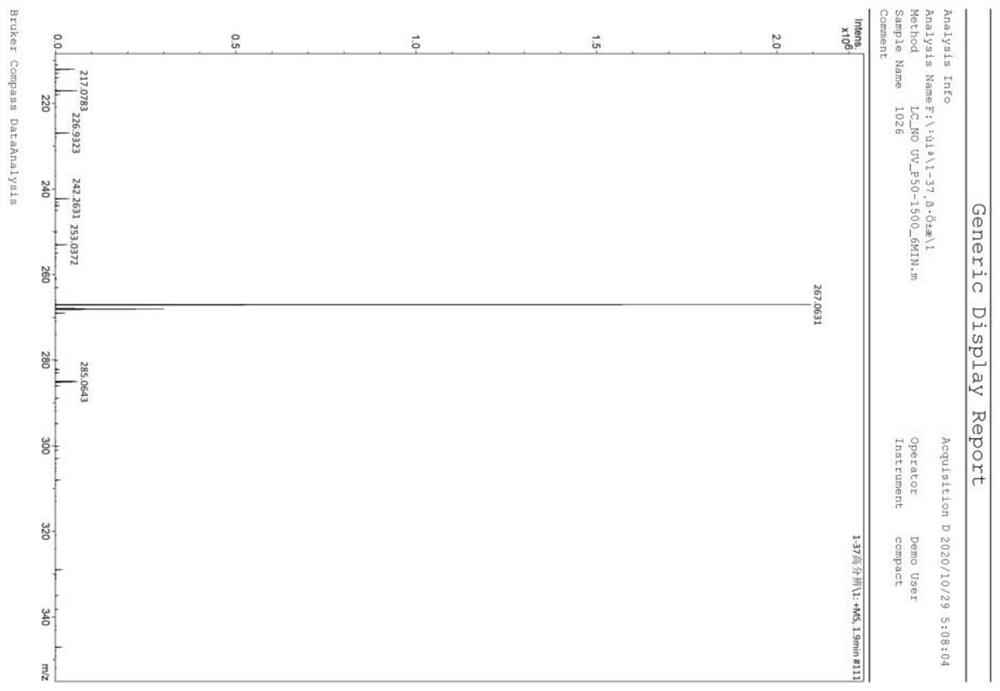
Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds
ActiveCN111057058AReduce dosageEasy to meet quality requirementsOrganic chemistryAzidotrimethylsilanePtru catalyst
The invention discloses a method for preparing 1H-[1,2,4]triazole compounds. The method comprises the following steps: mixing beta-(2-aminoaryl)-alpha,beta-acetylenone (1), azidotrimethylsilane (2), afluorine-containing catalyst and a reaction solvent, reacting at 0-60 DEG C for 3-8 h, monitoring by TLC until the raw materials are completely converted, and carrying out post-treatment on the reacted solution to obtain the product (I). The cheap and easily available fluorine-containing reagent is used as a catalyst, the dosage of the catalyst is small, and no metal reagent is used, so post-treatment is simple, and the quality requirements of medicines and electronic materials are easily met; azidotrimethylsilane is used for replacing sodium azide to serve as an azido source, so toxicity issmall, safety is high, reaction conditions are mild, the reaction can be smoothly carried out at the room temperature, the substrate range is wide, and the yield is high.
Owner:ZHEJIANG UNIV OF TECH
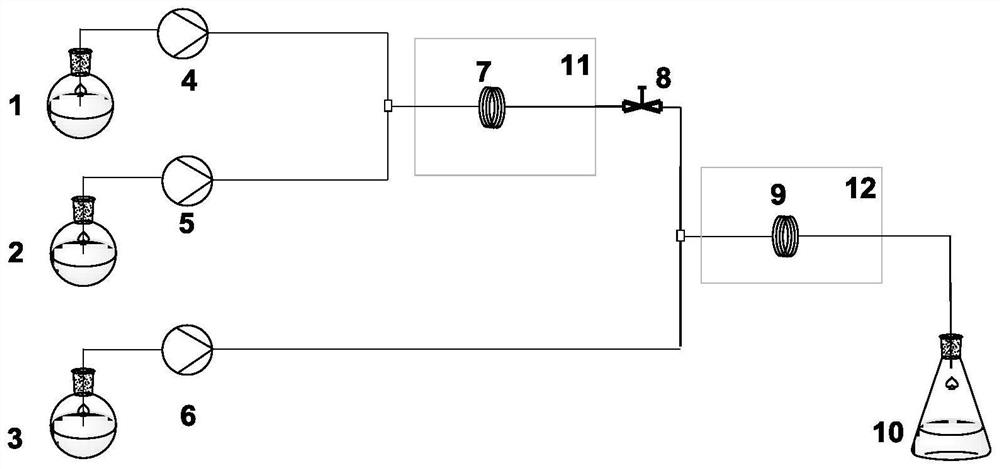
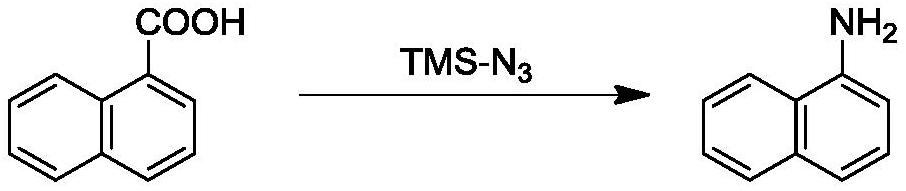
Method for rapidly preparing 1-naphthylamine based on microchannel continuous flow technology
PendingCN114805081AHigh yieldRealize industrial productionOrganic compound preparationChemical/physical/physico-chemical microreactorsSchmidt reactionMicroreactor
The invention discloses a method for rapidly preparing 1-naphthylamine based on a microchannel continuous flow technology. The method comprises the following steps: taking 1-naphthoic acid and azidotrimethylsilane as raw materials, and carrying out Schmidt amination reaction in a microreactor to generate the 1-naphthylamine. The method comprises the following steps: pumping a 1-naphthoic acid solution and an azidotrimethylsilane solution into a first micro-channel reactor, and reacting at a certain temperature for amination; and pumping a quenching agent into the reaction system, mixing the quenching agent with the reaction liquid from the first micro-channel reactor in the second micro-channel reactor, and carrying out reaction at a certain temperature to quench the reaction liquid so as to generate the stable product 1-naphthylamine. By coupling the micro-channel continuous flow technology with intrinsic safety, the safe and continuous operation of the amination process is realized, the danger level of Schmidt reaction in which azide compounds participate is greatly reduced, the production efficiency is remarkably improved, and the production cost is reduced. And a high-yield naphthylamine product is obtained under safe, controllable, environment-friendly, efficient and continuous conditions in the reaction.
Owner:BIRDO (SHANGHAI) PHARM R&D CO LTD
A kind of preparation method of azide compound substituted by ortho trifluoromethyl group
ActiveCN108640808BMild method conditionsEasy to handleSulfonic acid amide preparationFunctional group formation/introductionAzidotrimethylsilaneMeth-
Owner:HEBEI UNIV OF TECH
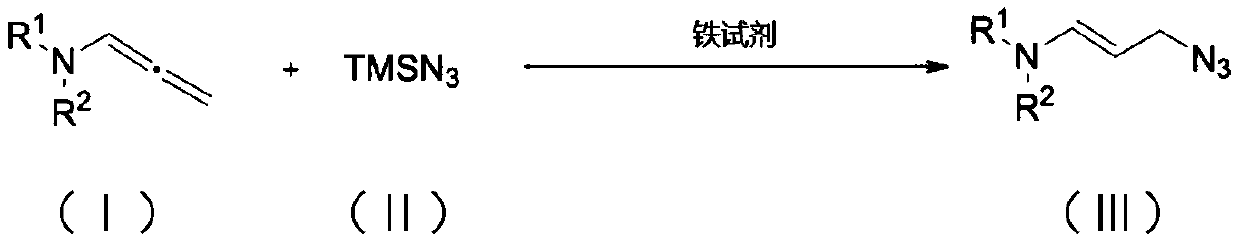
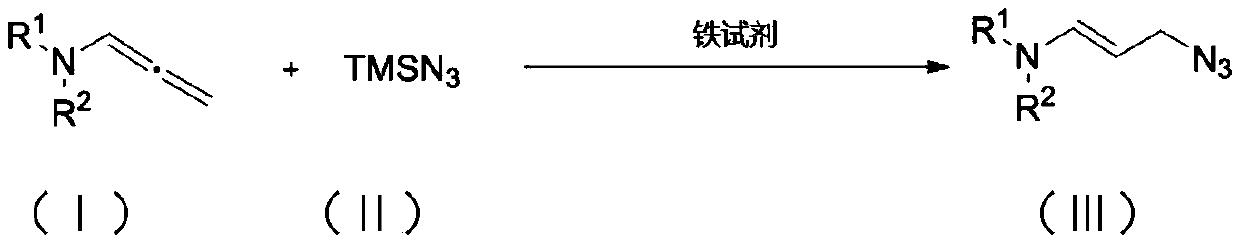
Method for synthesizing allyl azide derivative
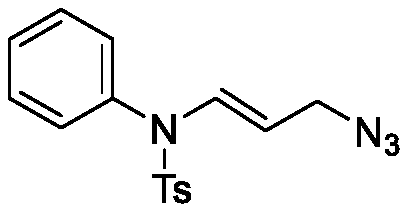
ActiveCN111187185AMild reaction conditionsQuick responseSulfonic acid amide preparationAzidotrimethylsilaneSilanes
The invention discloses a method for synthesizing an allyl azide derivative. The method comprises the step of inducing an allene amine compound with a structure as shown in a formula (I) which is described in the specification to react with azidotrimethylsilane with a structure as shown in a formula (II) which is described in the specification by using an iron reagent in an organic solvent so as to synthesize the allyl azide derivative with a structure as shown in a formula (III) which is described in the specification. In the formulas, the substituent group R<1> is an aryl group, a benzyl group or an alkyl group; and the substituent R<2> is a p-toluenesulfonyl group, an acetyl group or a methanesulfonyl group.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Trifluoromethyl-substituted azide, amine and heterocyclic compounds and preparation method
ActiveCN104649857BMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzirine
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
A kind of preparation method of 1-aryl-3-azido-4,4,4-trifluoro-1-butene compound
The invention relates to a preparation method of a 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound, and belongs to the technical field of compound preparation. According to the preparation method of the 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound, the compound shown by a general formula I and azidotrimethylsilane are used as raw materials to take a reaction according to a following reaction formula under the existence of catalysts and additives so as to obtain the compound shown by the general formula I. The reaction formula is shown in the specification. The method provided by the invention has the advantages that the raw materials which have low cost and can be easily obtained in a wide range are used as catalysts; a convenient and low-cost method is provided for the synthesis of the 1-aryl-3-azide-4,4,4-trifluoro-1-butene compound; meanwhile, the use of expensive and instable trifluoromethylation reagents is avoided.
Owner:DALIAN UNIV OF TECH +1
Alpha-azidocarbonyl compounds and preparation method thereof
InactiveCN107056648AHigh yieldEfficient responseOrganic chemistryAzidotrimethylsilaneOrganic solvent
The invention relates to alpha-azidocarbonyl compounds and a preparation method thereof. The method comprises the step of carrying out alkyne difunctionalization reaction on terminal alkynes and azidotrimethylsilane used as reaction raw materials in an organic solvent under the acceleration actions of a copper catalyst and a persulfate oxidizer, thereby obtaining the alpha-azidocarbonyl compounds. In the azido free radical oxidizing process, air is used as the terminal oxidizer to perform a key function in the reaction process. The method has the advantages of wide substrate range, ambient operation, mild reaction conditions, simple after-treatment, and high product yield and purity, provides a new synthesis route and method for alpha-azidocarbonyl compounds, and thus, has great application potential and research value.
Owner:WENZHOU MEDICAL UNIV
A method for preparing 4-imidazole formaldehyde derivatives by reductive cyclization involving tmsn3
The invention relates to a method for preparing 4-imidazole formaldehyde derivatives through reductive cyclization reaction participated by TMSN3. Concrete method is to be raw material by the propynyl amide that simple preparation obtains, take N-iodosuccinimide (NIS) as iodine source, azidotrimethylsilane (TMSN3) as reductive agent, under the condition of water as additive , a reductive cyclization reaction occurs to obtain dihydroimidazole, and then under the condition of the dehydration reagent bis[Α,Α-bis(trifluoromethyl)benzyl alcohol]diphenylsulfide, a high-yield 4-imidazole formaldehyde derivative is obtained thing. Propigylamide can be easily prepared from cheap and easy-to-obtain starting materials, and TMSN3 is used as a nitrogen source and a reducing agent at the same time, one-step reductive cyclization and intramolecular oxygen atom transfer reaction to obtain dihydroimidazole, and then a simple dehydration reaction is 4-imidazole formaldehyde derivatives can be obtained, the reaction is simple to operate, the reaction conditions are mild, and no metal catalyst is needed.
Owner:中科榆林能源技术运营有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
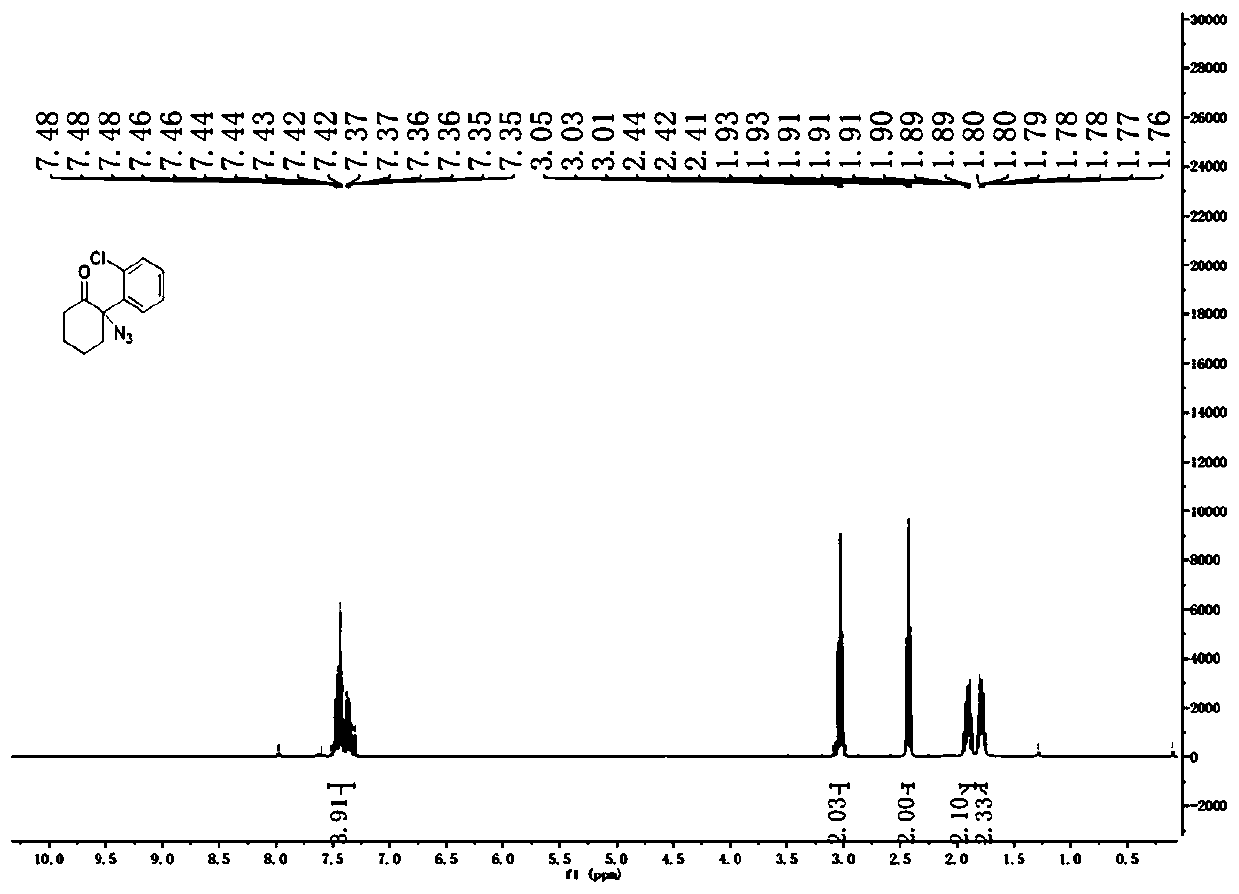


![Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8523479f-99d1-4100-b46f-be10e0a35328/FDA0002354576290000011.png)
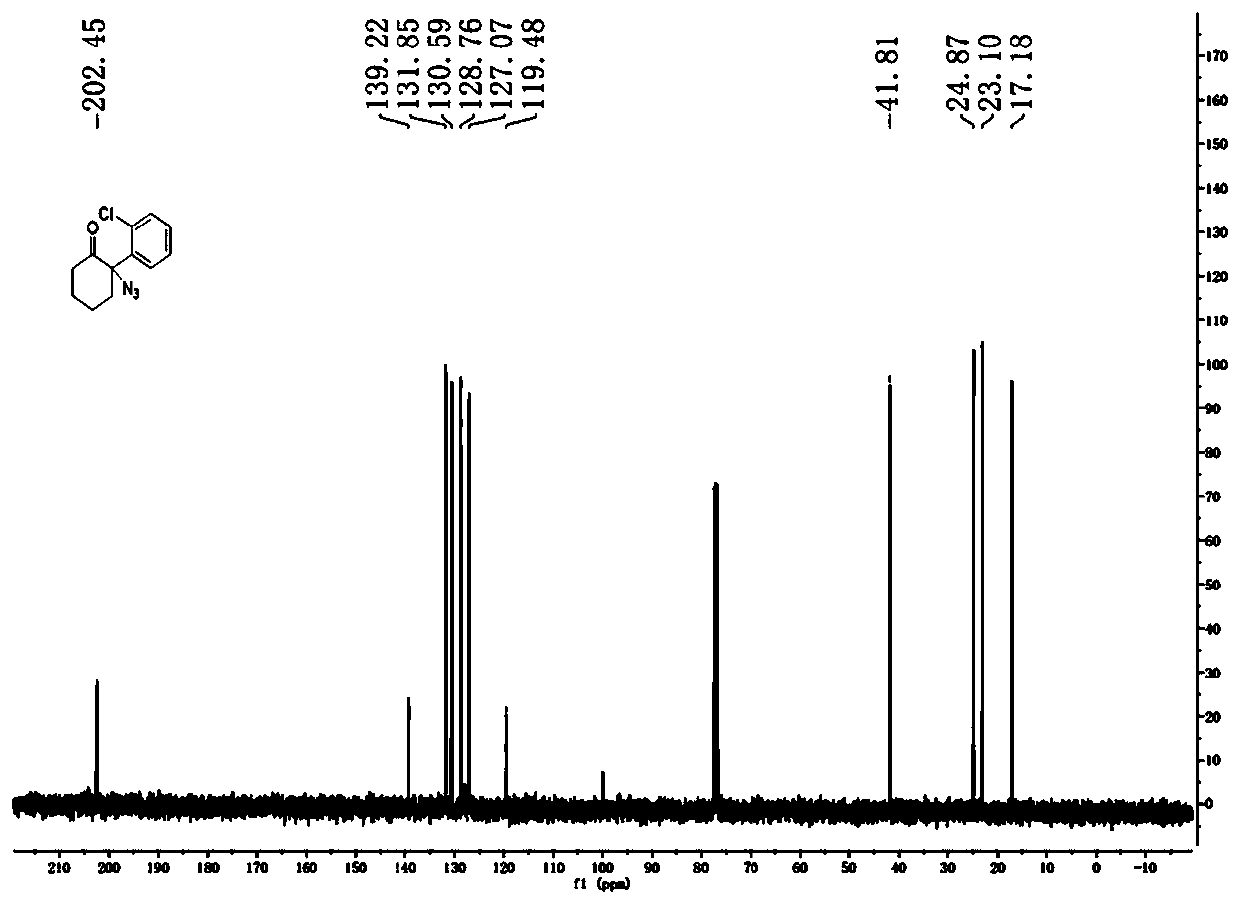
![Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8523479f-99d1-4100-b46f-be10e0a35328/BDA0002354576300000011.png)
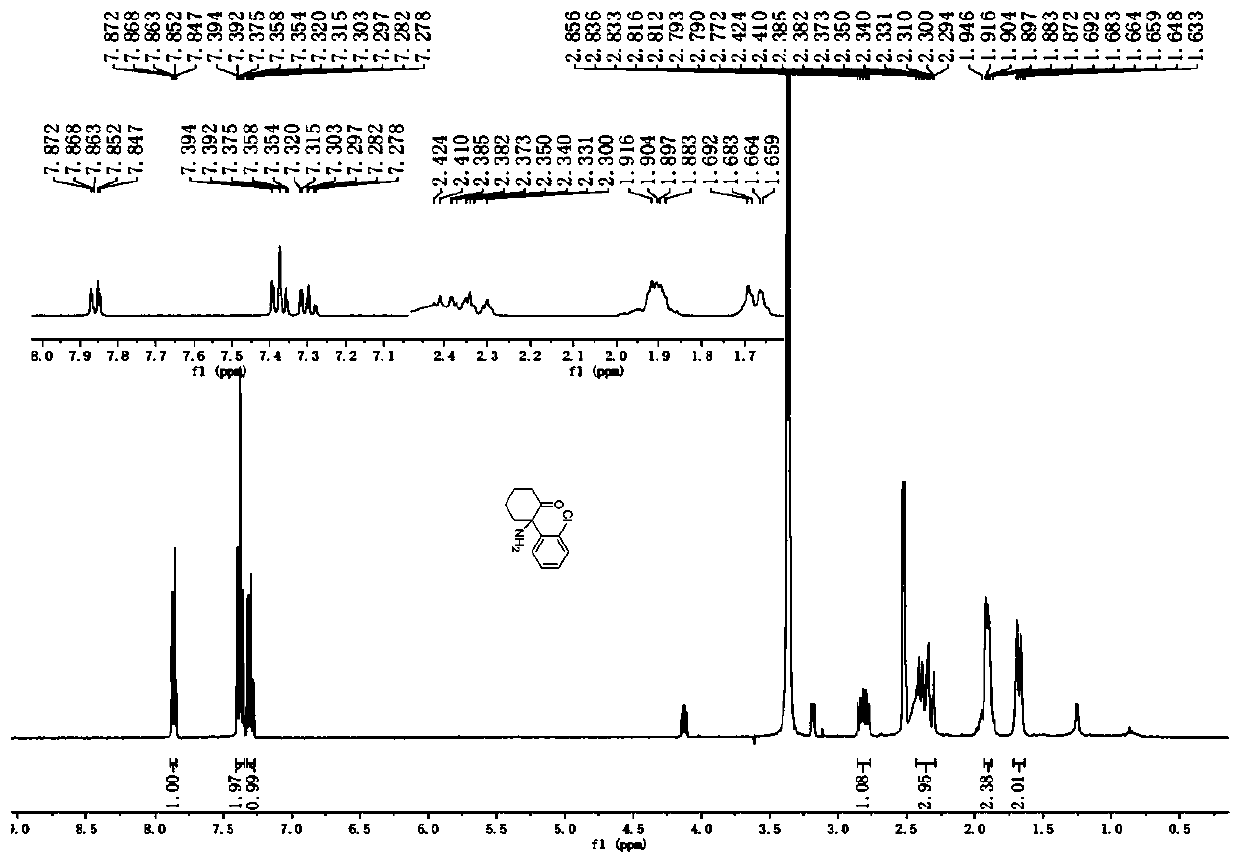
![Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds Method for preparing 1H-[1,2,3]-triazolo[4,5-c]quinoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8523479f-99d1-4100-b46f-be10e0a35328/BDA0002354576300000012.png)