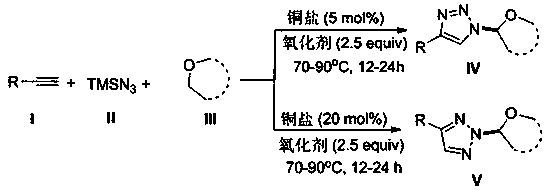
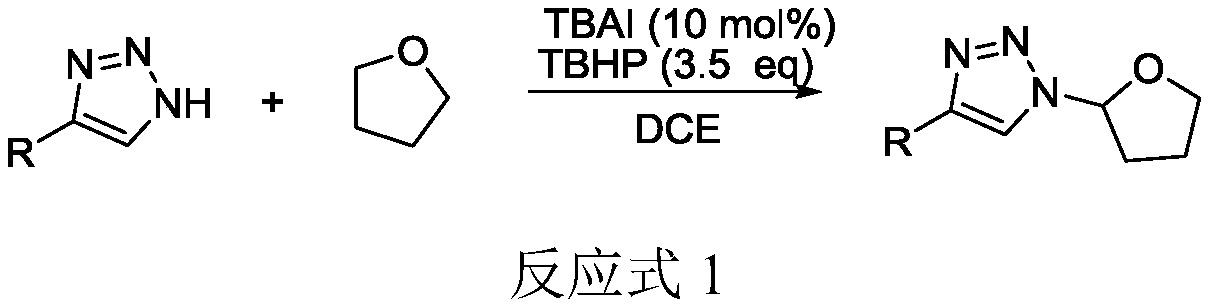
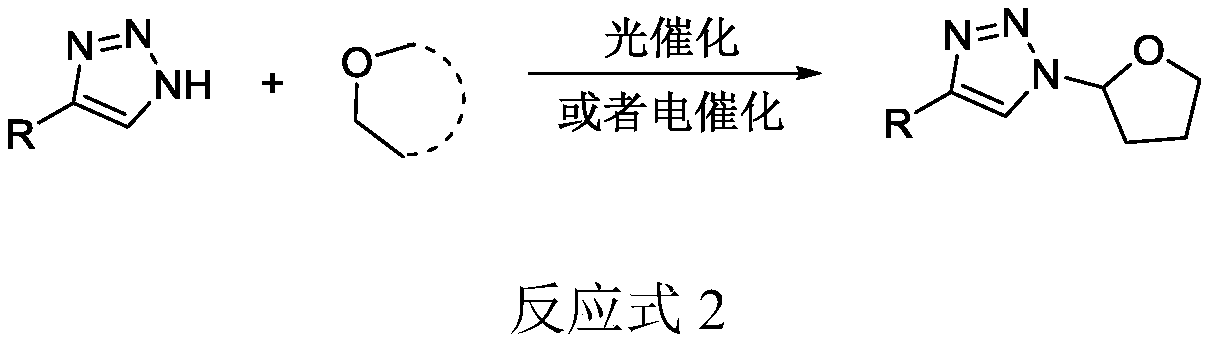
Selective production method of N-1-oxyalkyl-substituted 1,2,3-triazole compound and N2-oxyalkyl-substituted 1,2,3-triazole compound
A compound and selective technology, applied in organic chemistry and other directions, can solve the problems of no commercialization, cannot be selectively obtained at the same time, and single reaction product structure, and achieve the effects of simple operation, excellent selectivity, and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] At room temperature, CuCl (5mol%), phenylacetylene (0.2mmol), TMSN 3 (0.4mmol), tetrahydrofuran (THF, 1mL, 12mmol), and tert-butanol peroxide (TBHP, 0.5mmol), mix well. Close the reaction tube, stir and react at 80°C for 16h. After the reaction is completed, concentrate under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which is then washed with a mixture of petroleum ether and ethyl acetate with a volume ratio of 5:1. After washing with deagent, flash silica gel column chromatography, 36.6 mg of the N-1 oxoalkyl-substituted 1,2,3-triazole product 4a of this example was obtained, with a yield of 85%.
[0030] 1 H NMR (500MHz, DMSO-d 6 ):δ8.70(s,1H),7.93–7.87(m,2H),7.46(t,J=4.7Hz,2H),7.35(t,J=4.3Hz,1H),6.39–6.27(m, 1H),4.11–4.07(m,1H),3.99–3.95(m,1H),2.51–2.44(m,2H),2.25–2.15(m,1H),2.10–2.01(m,1H); 13 C NMR (125MHz, DMSO-d 6 ): δ147.0, 131.1, 129.3, 128.4, 125.7, 120.3, 89.3, 69.4, 32.1, 24.3
Embodiment 2
[0032]
[0033] At room temperature, CuCl (5mol%), p-tolueneacetylene (0.2mmol), TMSN 3 (0.4mmol), tetrahydrofuran (THF, 1mL, 12mmol), and tert-butanol peroxide (TBHP, 0.5mmol), mix well. Close the reaction tube, stir and react at 80°C for 16h. After the reaction is completed, concentrate under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which is then washed with a mixture of petroleum ether and ethyl acetate with a volume ratio of 5:1. Washing with deagent, silica gel column flash column chromatography, obtained 30.2 mg of the N-1 oxoalkyl-substituted 1,2,3-triazole product 4b of this example, with a yield of 66%.
[0034] 1 H NMR (500MHz, DMSO-d 6 ):δ8.61(s,1H),7.76(d,J=8.1Hz,2H),7.24(d,J=7.9Hz,2H),6.38–6.30(m,1H),4.08–4.04(m, 1H),3.97–3.92(m,1H),2.47–2.38(m,2H),2.32(s,3H),2.22–2.13(m,1H),2.07–2.00(m,1H); 13 C NMR (125MHz, DMSO-d 6 ): δ147.0, 137.7, 129.9, 128.4, 125.6, 119.8, 89.2, 69.4, 32.1, 24.3, 21.3
Embodiment 3
[0036]
[0037] At room temperature, CuCl (5mol%), p-chlorophenylacetylene (0.2mmol), TMSN 3 (0.4mmol), tetrahydrofuran (THF, 1mL, 12mmol), and tert-butanol peroxide (TBHP, 0.5mmol), mix well. Close the reaction tube, stir and react at 80°C for 16h. After the reaction is completed, concentrate under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which is then washed with a mixture of petroleum ether and ethyl acetate with a volume ratio of 5:1. After washing with deagent, flash column chromatography on silica gel column, 35.4 mg of the N-1 oxoalkyl-substituted 1,2,3-triazole product 4c of this example was obtained, with a yield of 71%.
[0038] 1 H NMR (500MHz, DMSO-d 6):δ8.75(s,1H),7.94–7.90(m,2H),7.54–7.51(m,2H),6.37(t,J=4.7Hz,1H),4.11–4.07(m,1H), 3.99–3.95(m,1H),2.49–2.43(m,2H),2.23–2.14(m,1H),2.09–2.03(m,1H); 13 C NMR (125MHz, DMSO-d 6 ): δ145.9, 132.8, 130.1, 129.4, 127.4, 120.6, 89.4, 69.5, 32.1, 24.2; HRMS calc.for C12H13ClN3O(M+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com