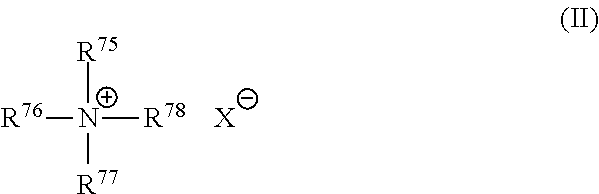Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7092results about "Gaseous substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
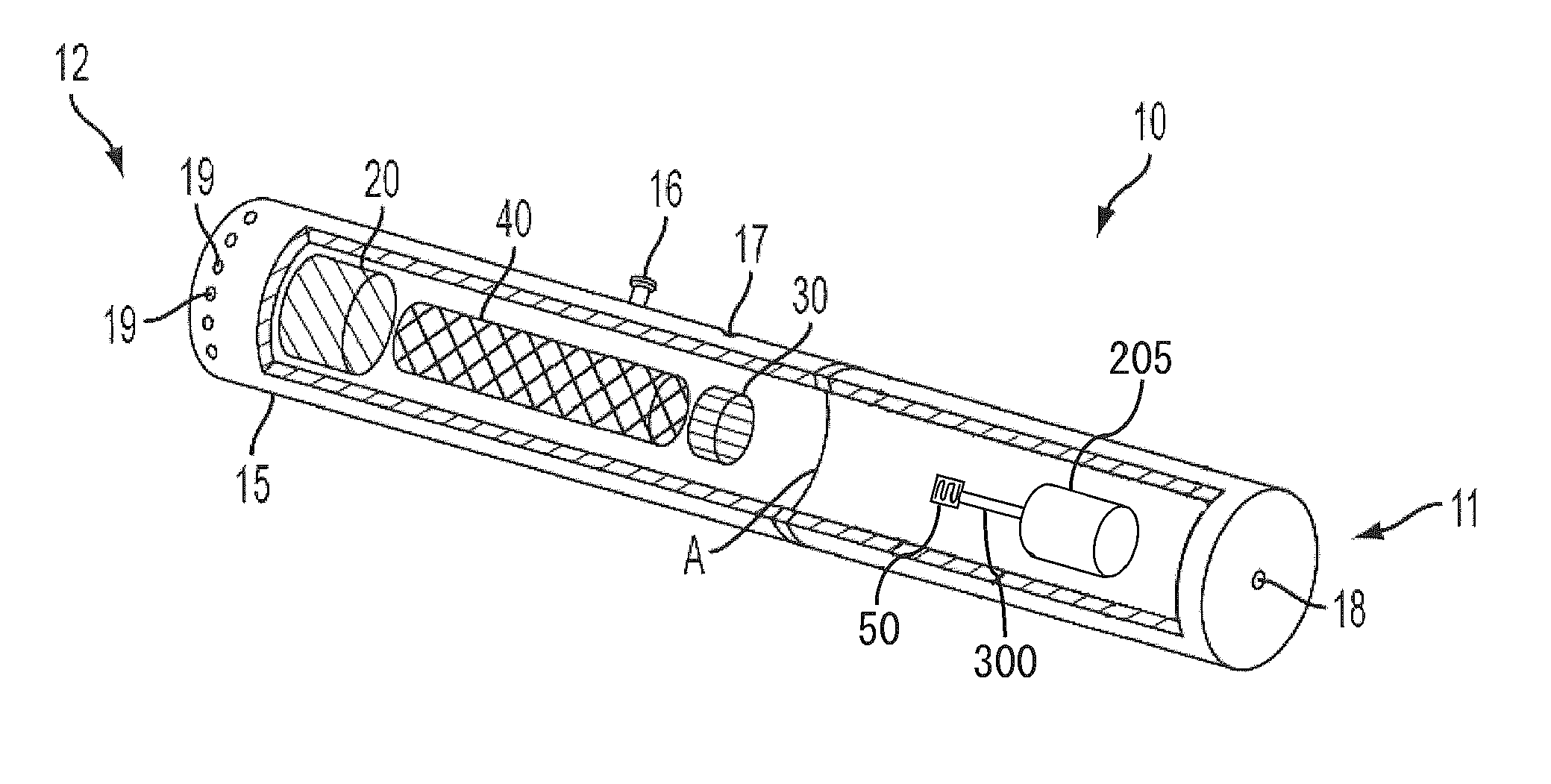
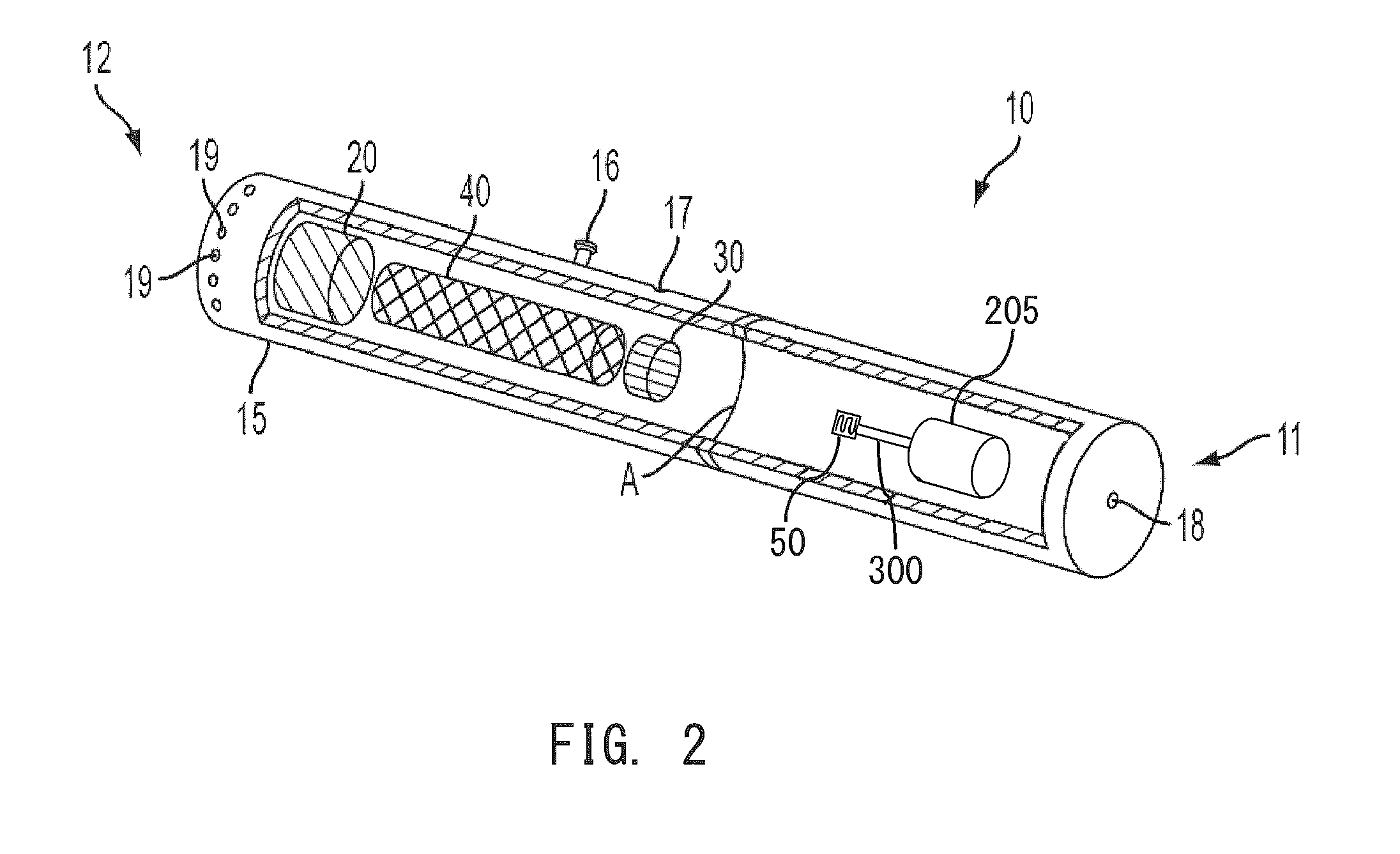
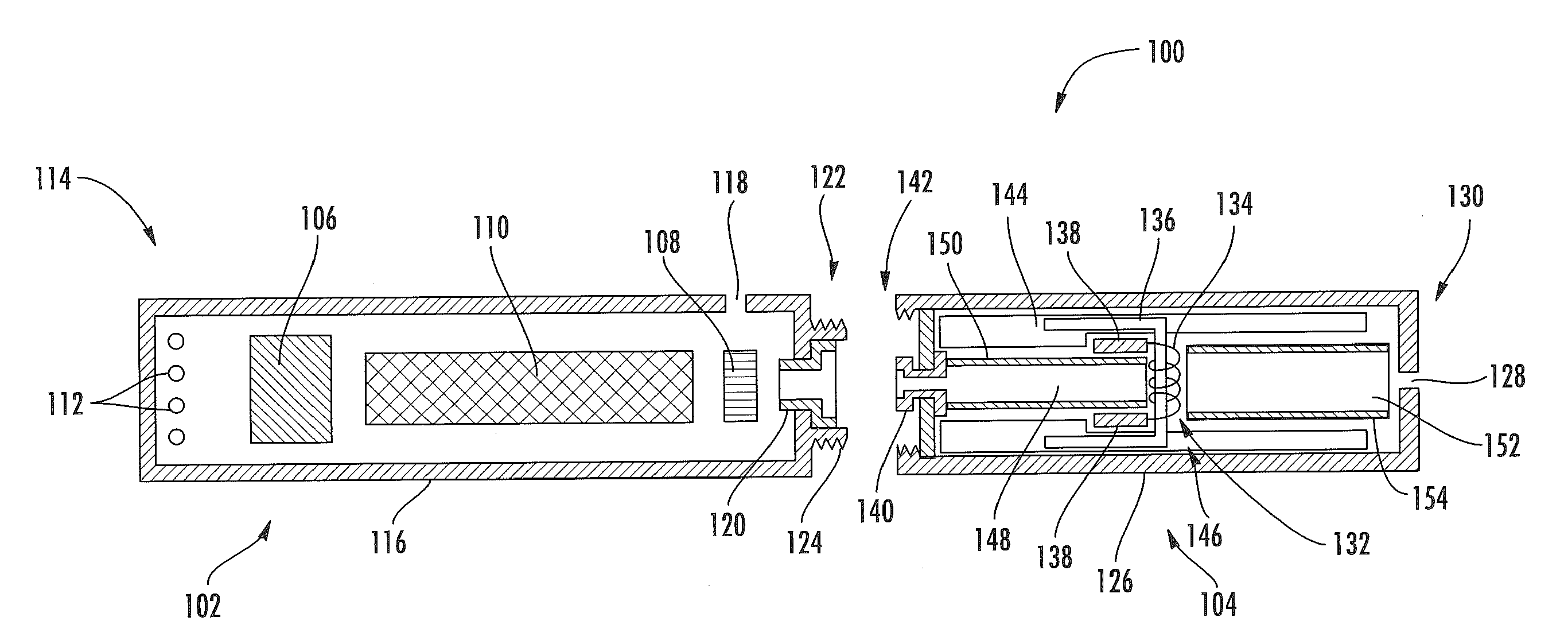
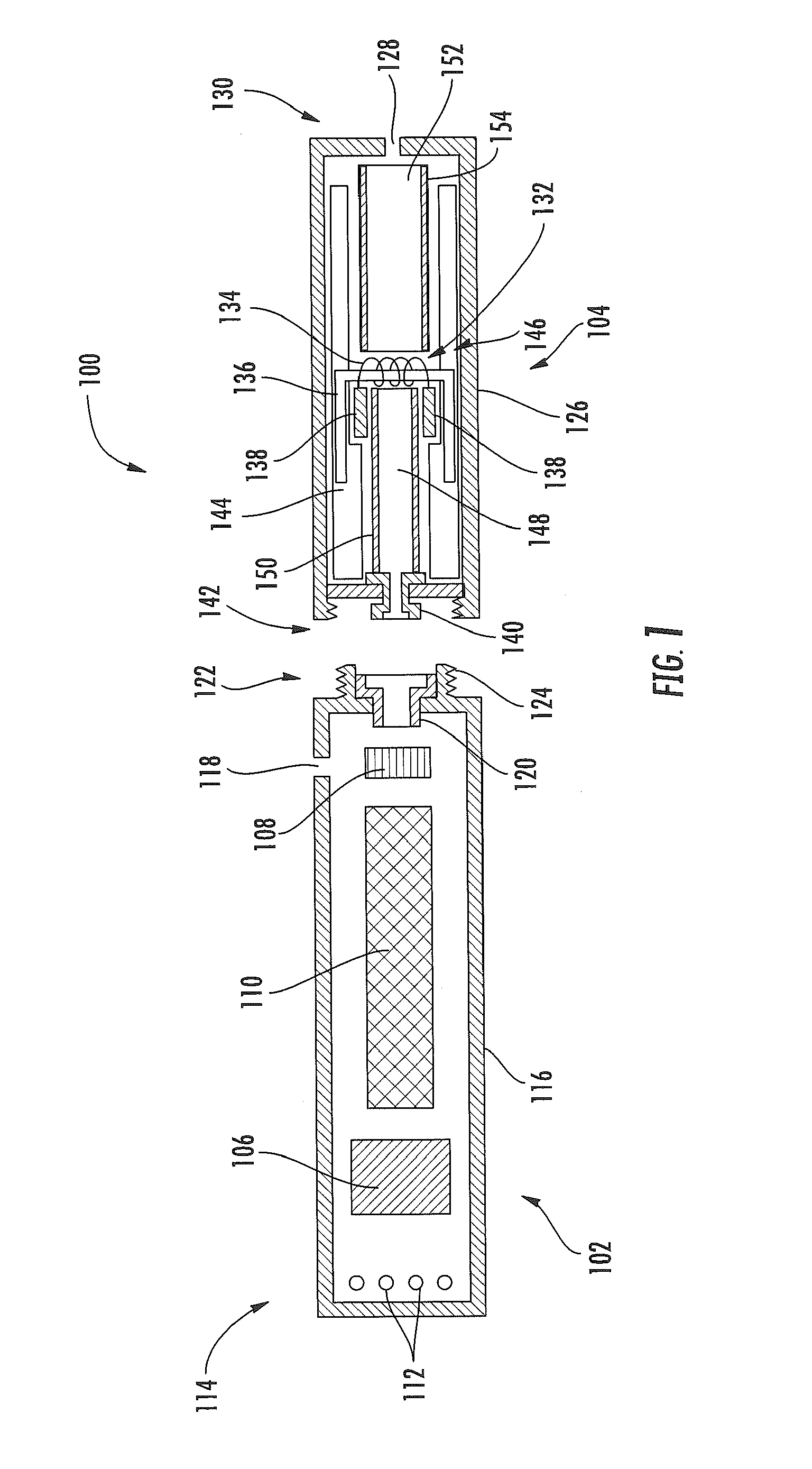
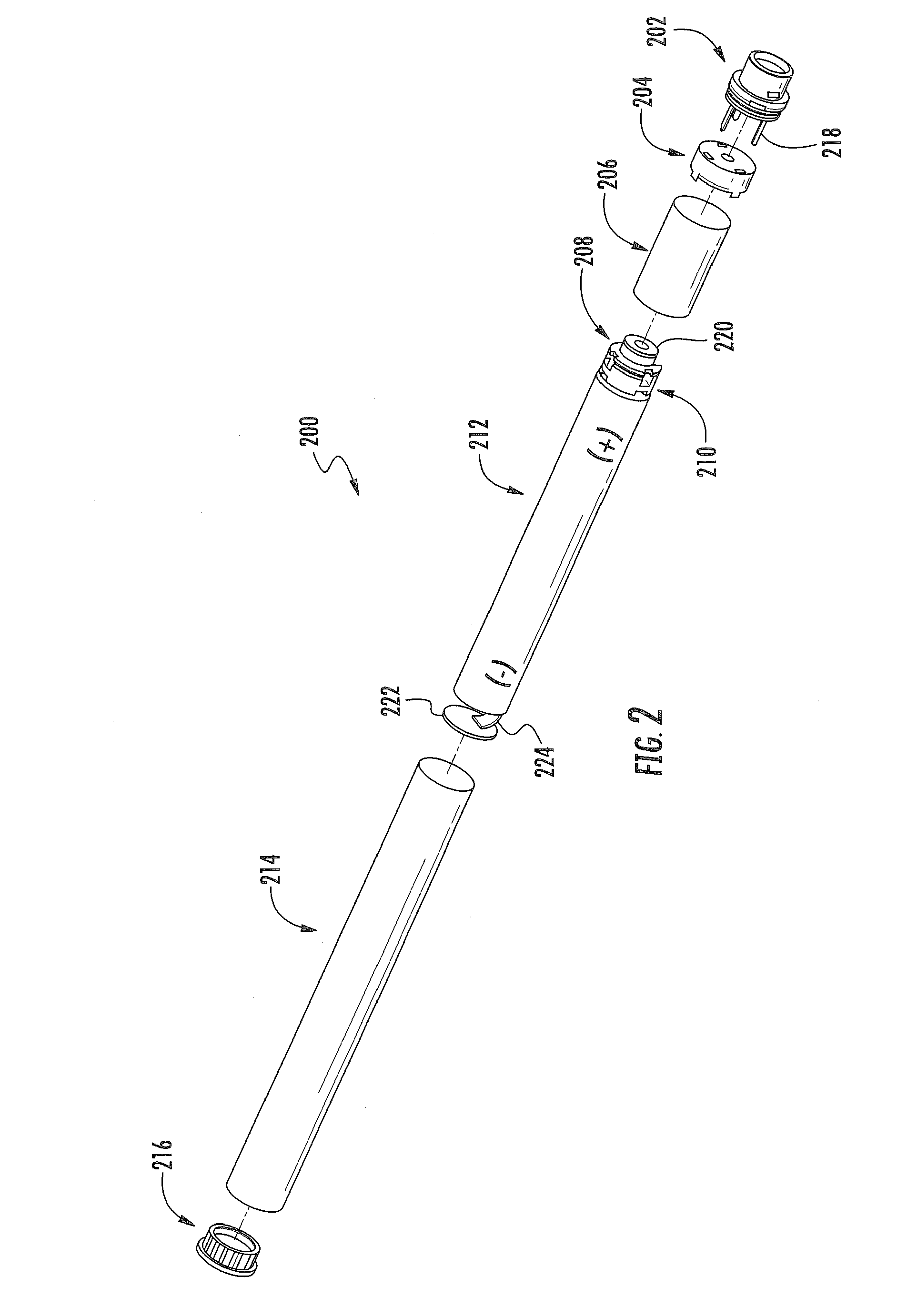
Electronic smoking article comprising one or more microheaters
The present disclosure relates to an electronic smoking article that provides for improved aerosol delivery. Particularly, the article comprises one or more microheaters. In various embodiments, the microheaters provide for improved control of vaporization of an aerosol precursor composition and provide for reduced power requirements to achieve consistent aerosolization. The present disclosure further relates to methods of forming an aerosol in a smoking article.
Owner:RAI STRATEGIC HLDG INC
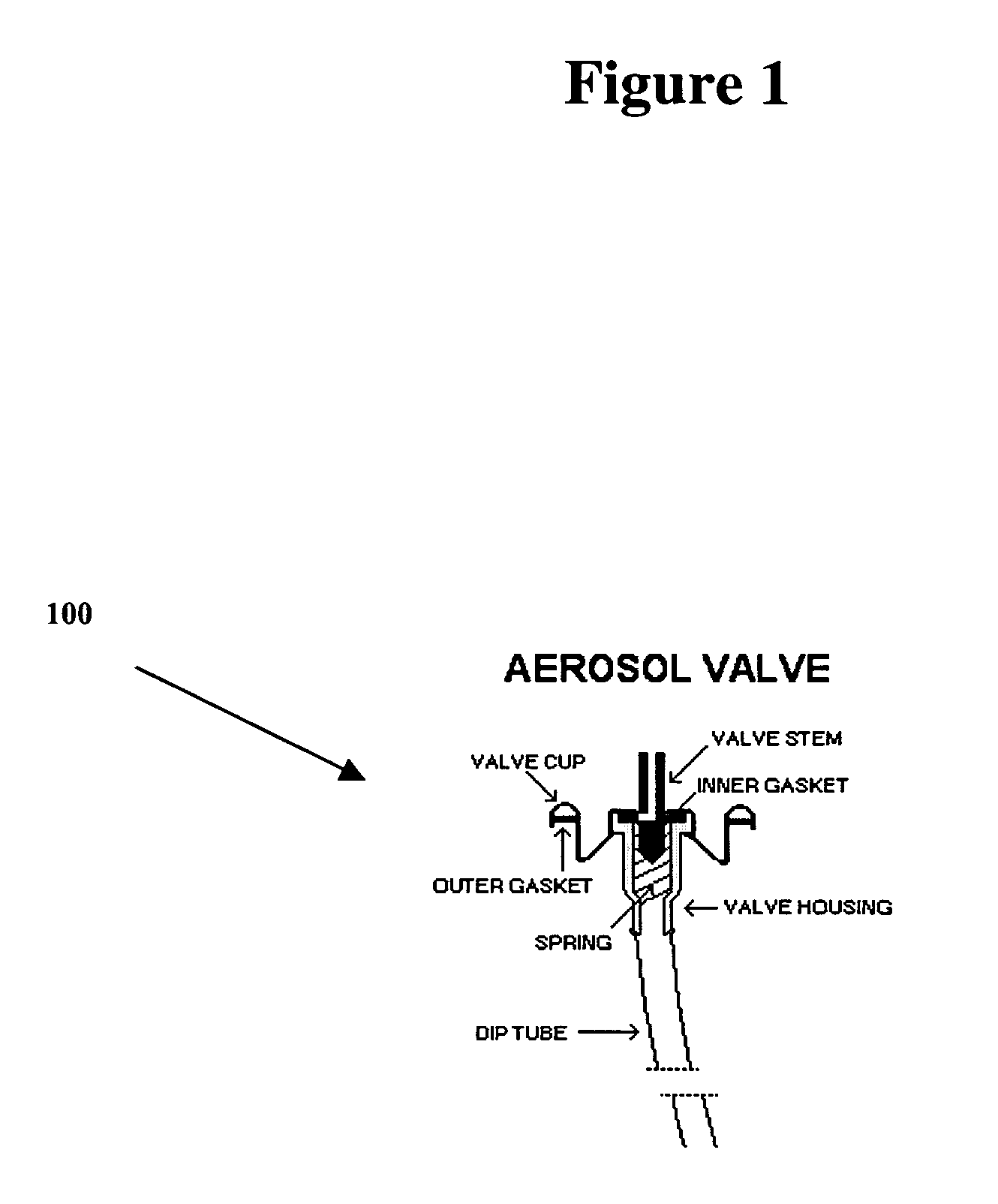
Medicament dispensing device with a display indicative of the state of an internal medicament reservoir
ActiveUS7331340B2Liquid surface applicatorsPowdered material dispensingDisplay deviceBiomedical engineering
A metered dose inhaler for use with a removable pressurized aerosol canister, or reservoir, having a display for indicating to a user the state of the canister. A memory device on the canister or a housing which houses the canister stores information indicative of doses dispensed from, or remaining in, the canister. That information is processed to provide and display information representative of the state of the canister.
Owner:IVAX CORP
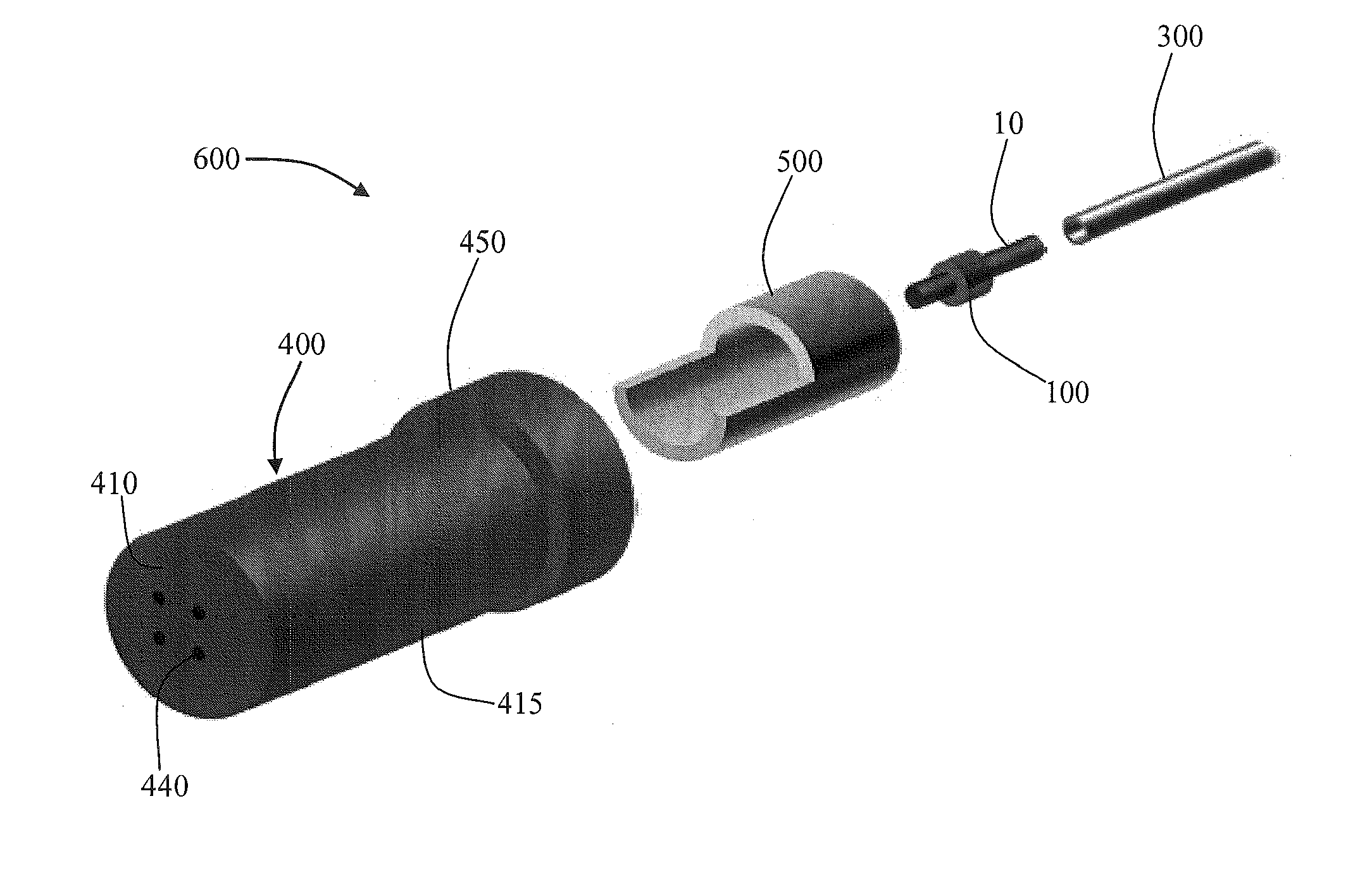
Cartridge and control body of an aerosol delivery device including Anti-rotation mechanism and related method
ActiveUS20140261495A1Prevent rotationWave amplification devicesMedical devicesEngineeringAerosol delivery
The present disclosure relates to aerosol delivery devices including a cartridge and a control body. The control body may include a coupler and the cartridge may include a base. The base may be configured to releasably engage the cartridge. Further the base and the coupler may include anti-rotation mechanisms configured to prevent rotation of the cartridge relative to the base when engaged with one another. The anti-rotation mechanisms may include alternating protrusions and recesses in some embodiments. Related methods are also provided.
Owner:RAI STRATEGIC HLDG INC
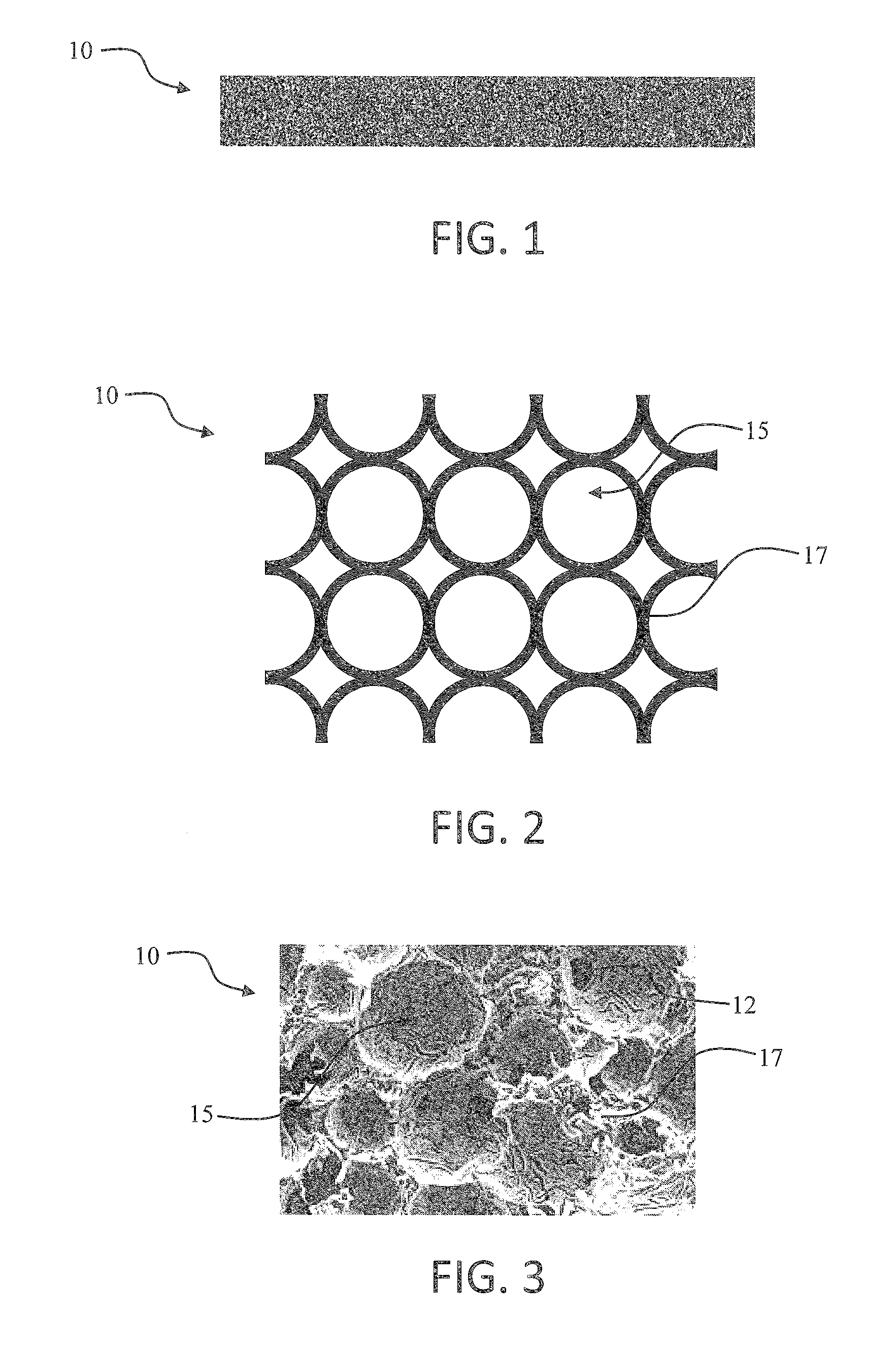
Carbon conductive substrate for electronic smoking article
ActiveUS20150059780A1Improve natureReduce materialSteam generation using steam absorptionTobacco pipesPorous carbonEngineering
The present disclosure provides components useful in heating, particularly heating of an aerosol precursor solution so as to vaporize the solution and form an aerosol. The disclosure particularly provides an electrically conductive, porous carbon heater. The heater may be combined with an aerosol precursor transport element that also is formed of carbon. The heater and transport element may form an atomizer that can be useful in an electronic smoking article, such as in a cartridge that is adapted for attachment to a control body. In some embodiments, the disclosure provides a cartridge of an electronic smoking article, the cartridge being formed substantially completely of carbon.
Owner:RAI STRATEGIC HLDG INC
Deodorizing and sanitizing employing a wicking device
ActiveUS7285255B2Difficult to cleanAvoid accumulationLavatory sanitoryGaseous substancesCapillary actionChemistry
Owner:ECOLAB USA INC
Perfume encapsulates
InactiveUS20040087477A1Improve stabilityImprove retentionGaseous substancesCapsule deliveryFlavorMelamine formaldehyde
A perfume encapsulate comprises an aminoplast capsule, the capsule shell comprising urea-formaldehyde or melamine-formaldehyde polymer and a second polymer comprising a polymer or copolymer of one or more anhydrides, preferably ethylene / maleic anhydride copolymer. The second polymer improves the stability of the capsules with respect to surfactant, thus improving perfume retention properties and enabling use of the capsules in aqueous surfactant-containing products in a way that has not hitherto been possible.
Owner:QUEST INTERNATIONAL
Benefit agent containing delivery particle
Owner:THE PROCTER & GAMBLE COMPANY
Antibiotic kit and composition and uses thereof
The present invention relates to a therapeutic kit to provide a safe and effective dosage of an antibiotic agent, including an aerosol packaging assembly including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam, wherein the pressurized product comprises a foamable composition including: an antibiotic agent; at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, an organic polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight, a surface-active agent, about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent, water; and liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Desiccant entrained polymer
The present invention includes a composition having a co-continuous interconnecting channel morphology. These co-continuous interconnecting channels are predominately occupied with a polymer and particles that control the percolation through the composition. The particles are composed of a material such as an absorbing agent, releasing agent and / or activation agent. The polymer composition may be used to form a desired shaped article such as plug type inserts and liners for closed containers, or it may be formed into a film, sheet, bead or pellet.
Owner:CSP TECH NORTH AMERICA
Steroid kit and foamable composition and uses thereof
InactiveUS20060018937A1Preventing and alleviatingCosmetic preparationsSenses disorderActive agentFilm-forming agent
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a steroid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a steroid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
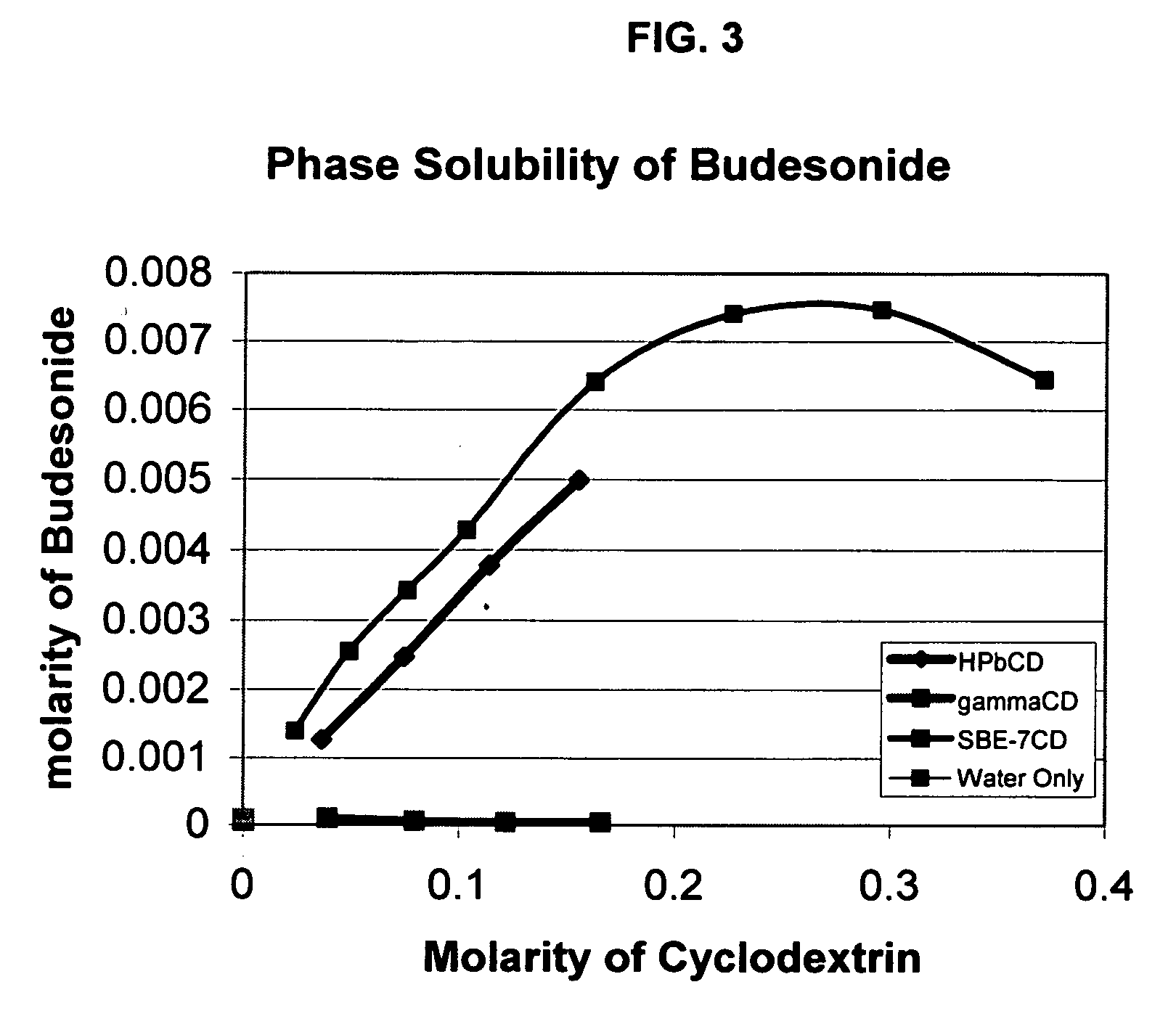
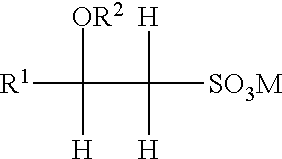
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Delivery particle
Owner:THE PROCTER & GAMBLE COMPANY
Compositions comprising encapsulated material
InactiveUS20060039934A1High weight ratioImprove barrier propertiesAntibacterial agentsCosmetic preparationsWater basedSolvent
A composition such as a water-based consumer product comprises material (e.g. perfume) encapsulated within shell capsules, each capsule comprising an encapsulating wall having an inner surface and an outer surface, with a coating on the inner surface and / or outer surface of the shell wall, the composition further comprising surfactant and / or solvent. The coating can improve the barrier properties of the shell and can enhance retention of the encapsulated materials within the shell.
Owner:QUEST INTERNATIONAL
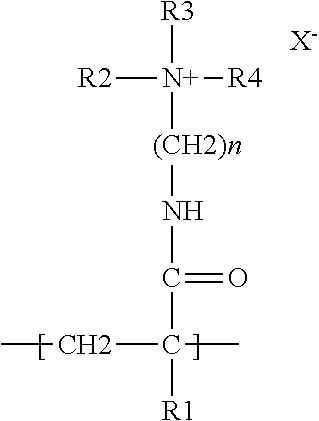
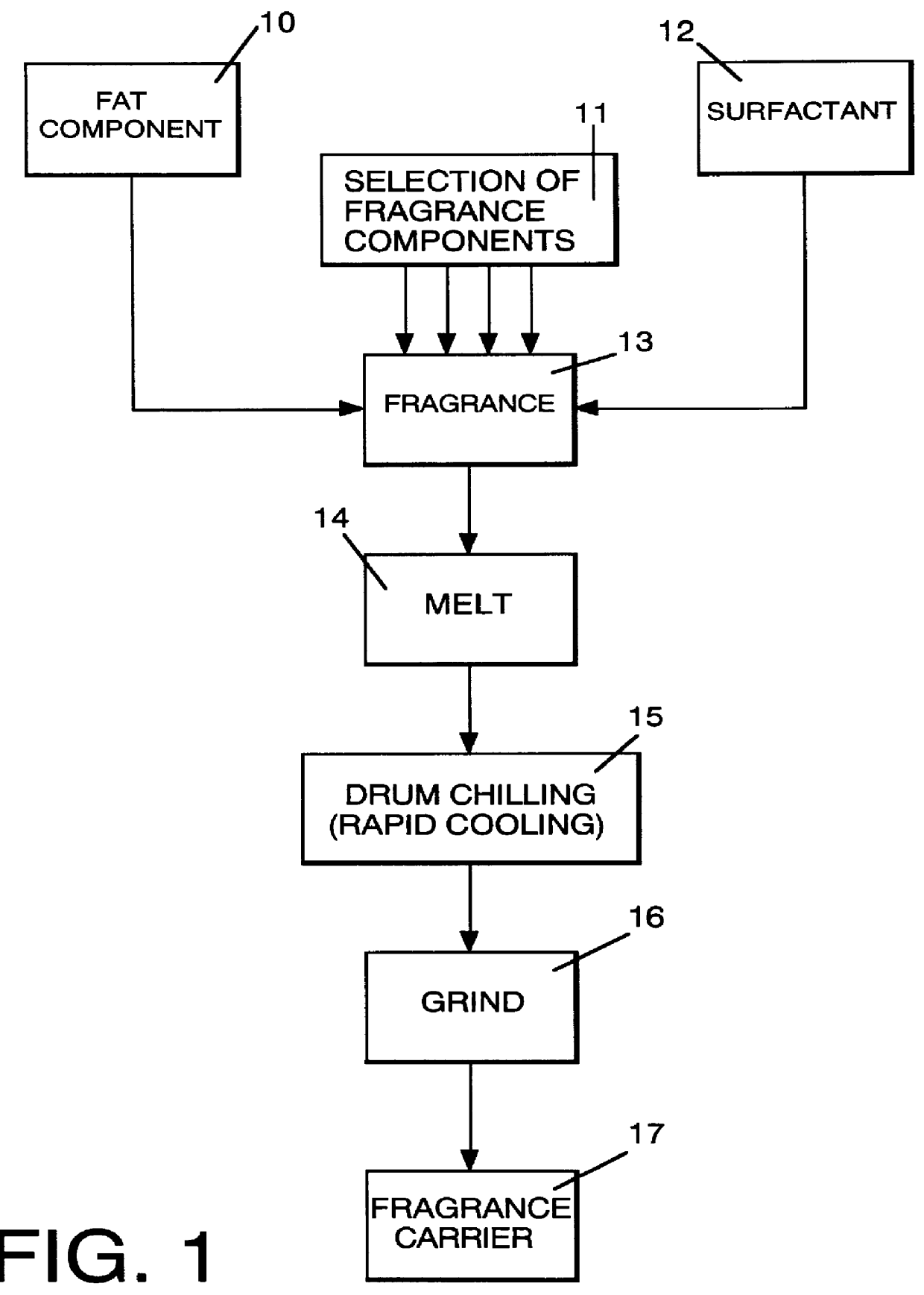
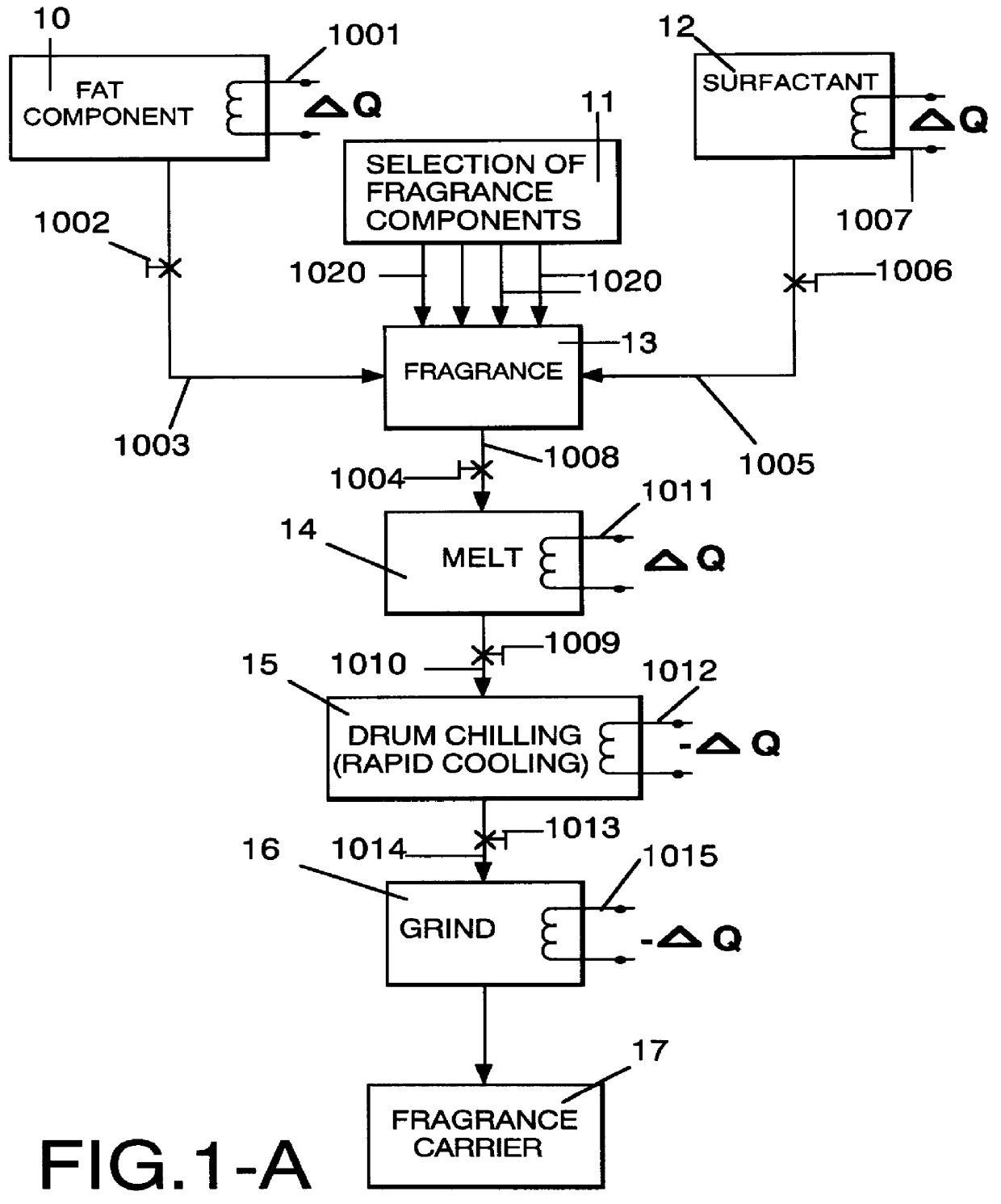
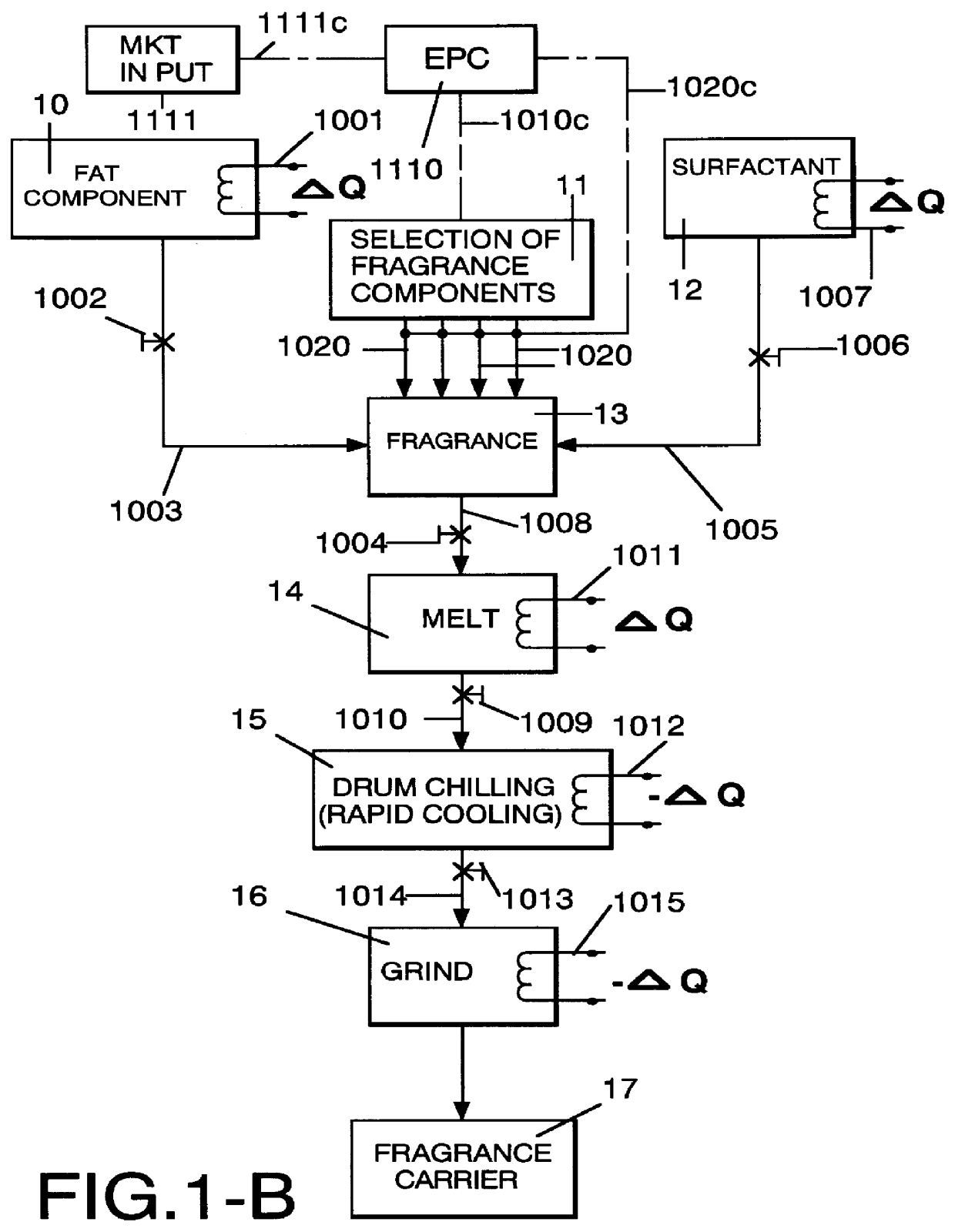
Method employing drum chilling and apparatus therefor for producing fragrance-containing long lasting solid particle
InactiveUS6051540AImproved substantivityMaximum flexibilityNon-ionic surface-active compoundsGaseous substancesDesiccantSolid particle
A method and apparatus is disclosed for producing a fragrance-containing solid particle, capable of controllably releasing the fragrance to the environment in which the particle is contained for incorporation into laundry detergents, fabric softener compositions and drier-added fabric softener articles.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Methods for emitting volatile compositions
InactiveUS7223361B2Maximize perceptibilityLighting and heating apparatusUsing liquid separation agentEngineeringComposition methods
Owner:THE PROCTER & GAMBLE COMPANY
Hypochlorite Technology
This invention generally relates to compositions and method of producing diluted hypohalous acid and hypohalous acid vapor. These compositions can be used to treat allergen containing surfaces, hard surfaces, food contact surfaces, hospital surfaces, food surfaces, kitchen surfaces, bathroom surfaces, human surfaces, animal surfaces, children's items, outdoor surfaces, soft surfaces, and medical instruments. These compositions can be converted to solid particulate or granular compositions. These compositions can be put into a variety of containers which preserve the stability. These compositions can be used to treat allergens and molds and as part of a mold detection system. These compositions can be dispersed into the air to enable microbiological control.
Owner:BROMBERG STEVEN E +29
Receptor specific transepithelial transport of therapeutics
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
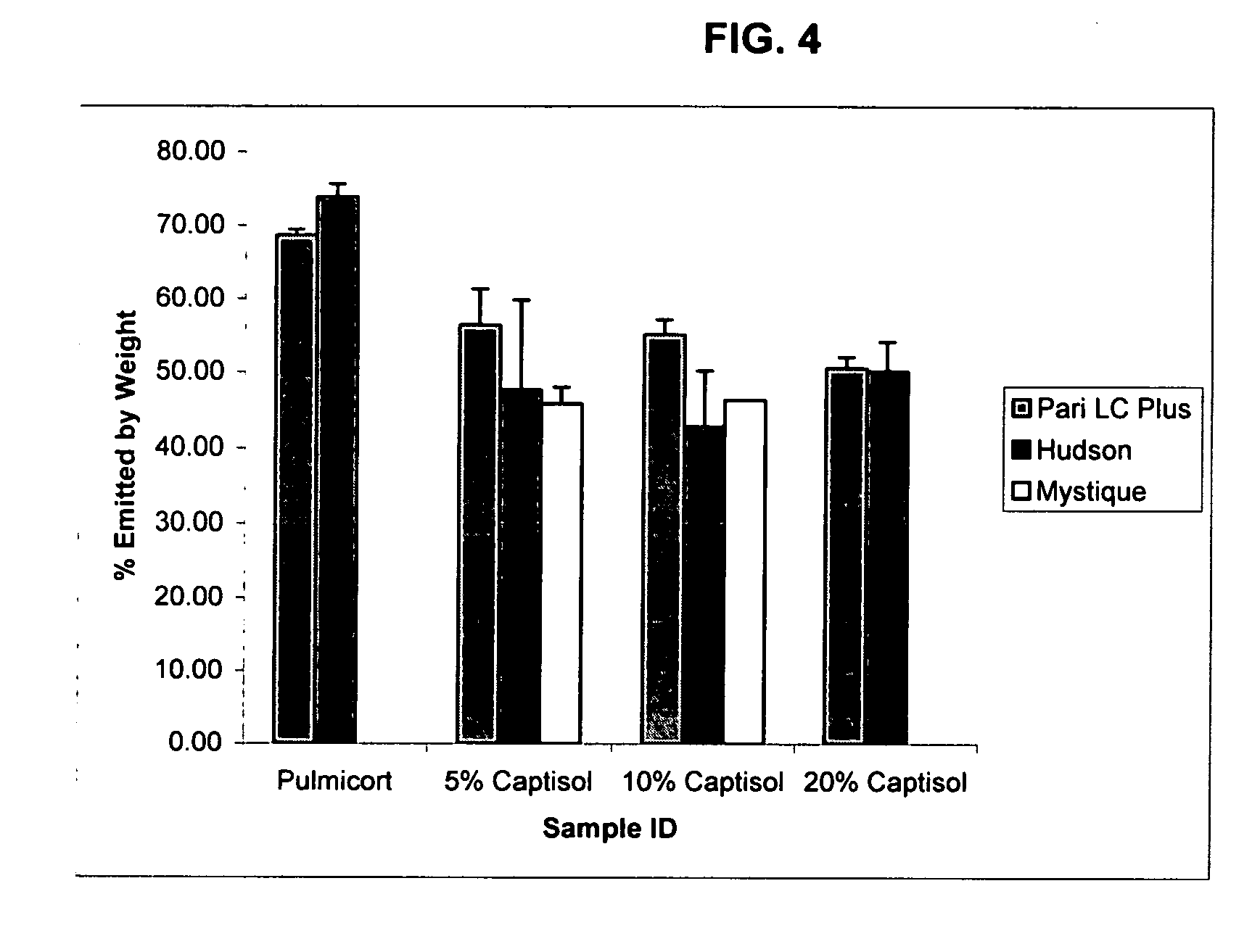
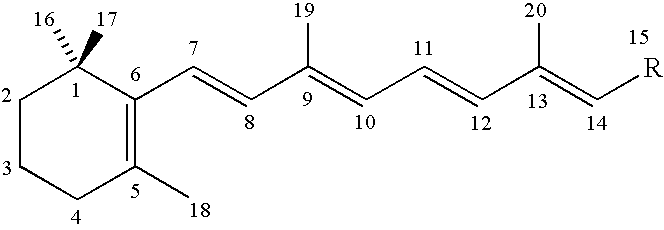
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Pharmaceutical composition
InactiveUS6946120B2Improve solubilityImprove abilitiesCosmetic preparationsOrganic active ingredientsAlcoholPolyol
A pharmaceutical composition for topical administration, including, as the pharmaceutically active component, at least 5% by weight, based on the total weight of the composition of a piperidinopyrimidine derivative or a pharmaceutically acceptable salt thereof; an acid in an amount to completely solubilise the piperidinopyrimidine derivative or a pharmaceutically acceptable salt thereof; a solvent composition including at least two of water, a lower alcohol and a co-solvent selected from one or more of the group consisting of aromatic and polyhydric alcohols; wherein when the co-solvent includes propylene glycol, it is present in an amount of less than approximately 10% by weight.
Owner:STIEFEL RESEARCH AUSTRALIA PTY LTD
Methods for making pharmaceutical formulations comprising microparticles with improved dispersibility, suspendability or wettability
InactiveUS20050079138A1Good dispersibilityImproved suspendabilityPowder deliveryGranulation by liquid drop formationPowder mixtureMicroparticle
Methods are provided for making a dry powder blend pharmaceutical formulation, comprising the steps of: (a) providing microparticles which comprise a pharmaceutical agent; (b) blending the microparticles with at least one excipient in the form of particles to form a powder blend; and (c) jet milling the powder blend to form a dry powder blend pharmaceutical formulation having improved dispersibility, suspendability, or wettability as compared to the microparticles of step (a) or the powder blend of step (b). The method can further include dispersing the dry powder blend pharmaceutical formulation in a liquid pharmaceutically acceptable vehicle to make an formulation suitable for injection. Alternatively, the method can further include processing the dry powder blend pharmaceutical formulation into a solid oral dosage form. In one embodiment, the microparticles of step (a) are formed by a solvent precipitation or crystallization process.
Owner:ACUSPHERE INC
Cosmetic and pharmaceutical foam with solid matter
InactiveUS20050186147A1Good lookingLower yield strengthCosmetic preparationsAerosol deliveryMedicineSolvent
A foamable composition includes about 2 to about 30% by weight solid particles; about 2 to about 75% by weight hydrophobic solvent; about 10 to about 85% by weight water; about 0.1% to about 5% by weight surface-active agent; about 0.1% to about 5 wt % by weight stabilizer / gelling agent; and a liquefied or compressed gas propellant in a container, which upon release provides a breakable foam suitable for topical administration.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Porous plastic media with antiviral or antimicrobial properties and processes for making the same
This invention relates to novel porous materials that possess antiviral and / or antimicrobial properties. The invention encompasses a porous material having antiviral or antimicrobial properties which is comprised of a porous substrate and an antiviral or antimicrobial agent. The invention also encompasses a process for making porous materials that possess antiviral and / or antimicrobial properties and the products of the process.
Owner:POREX CORP
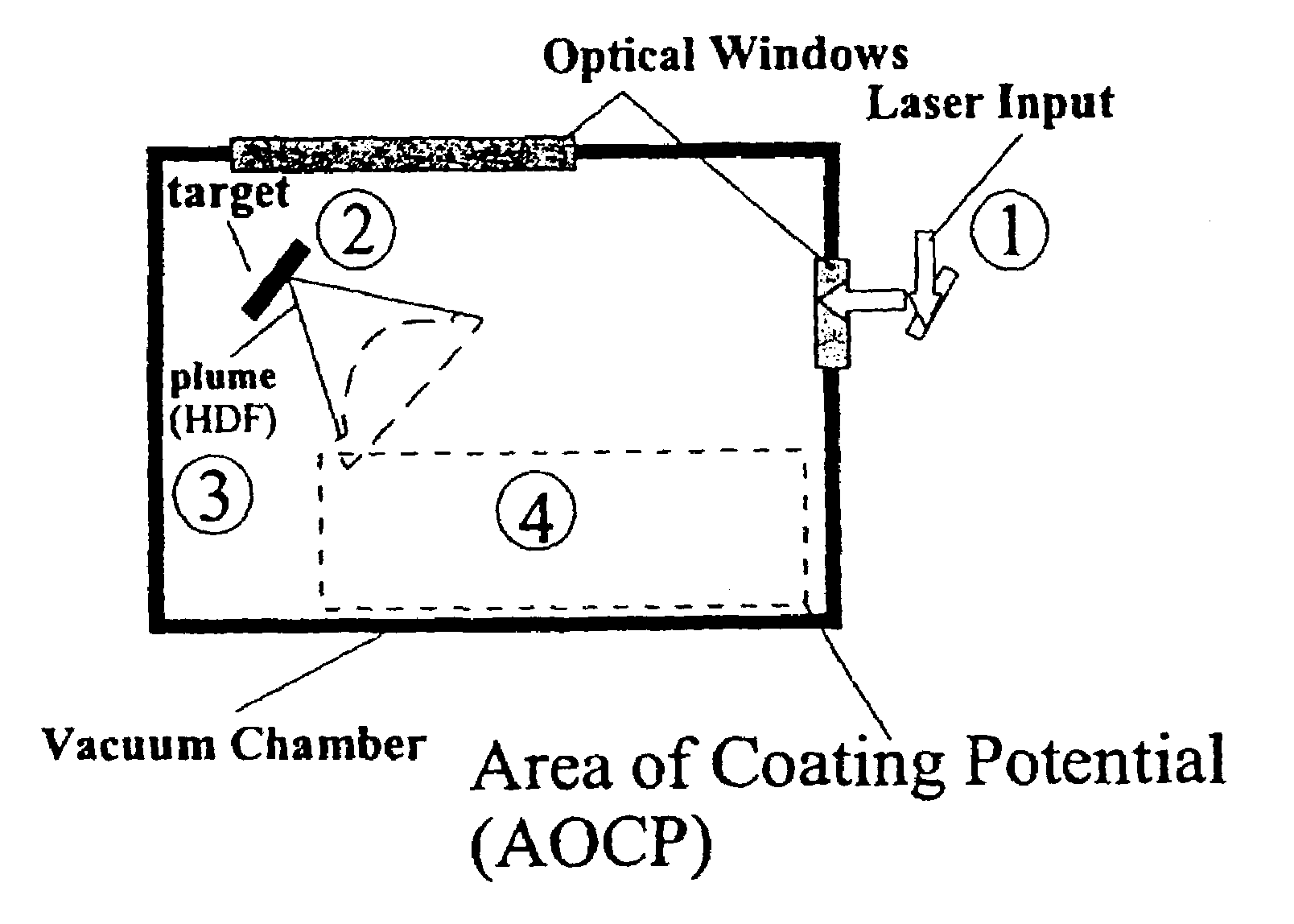
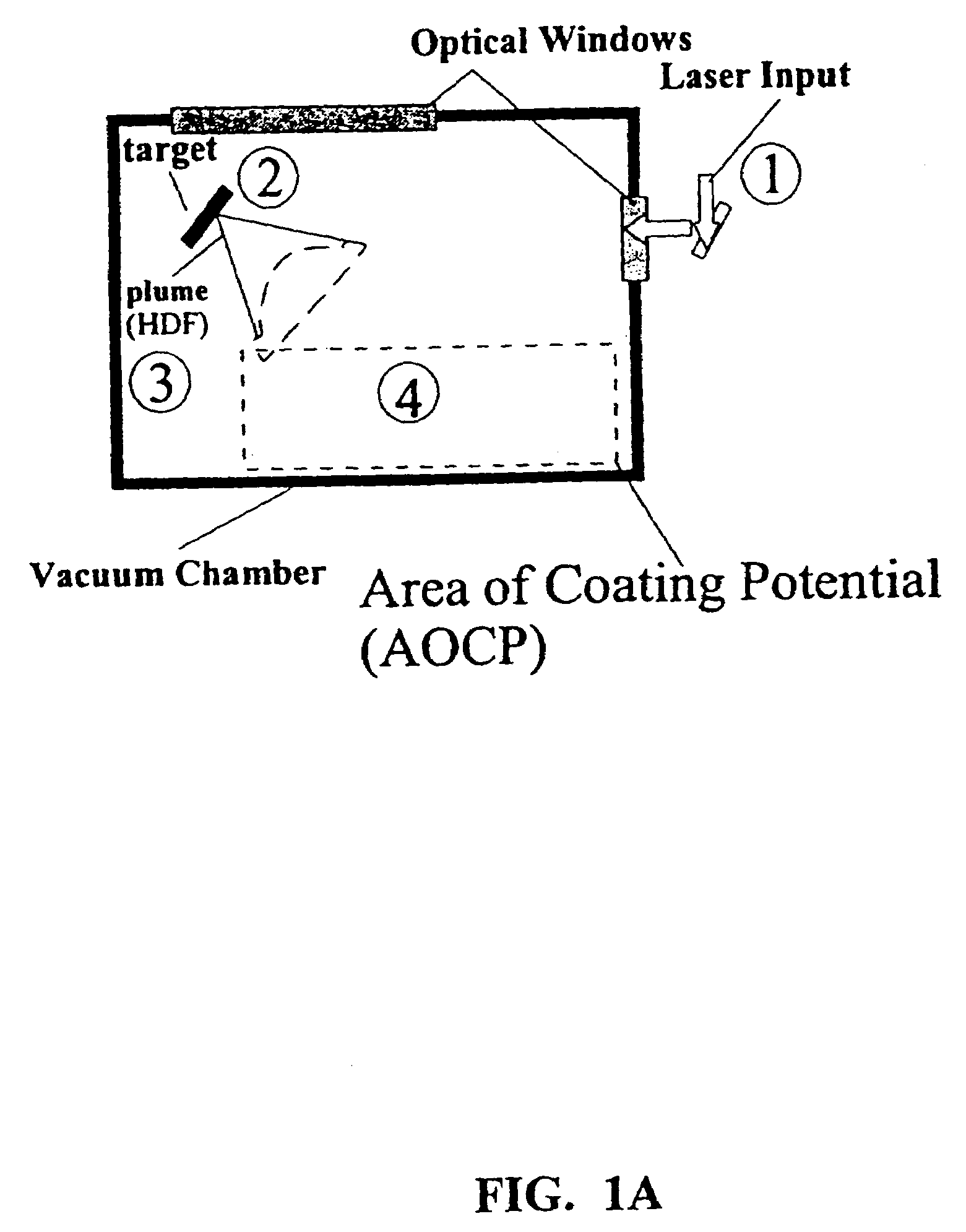
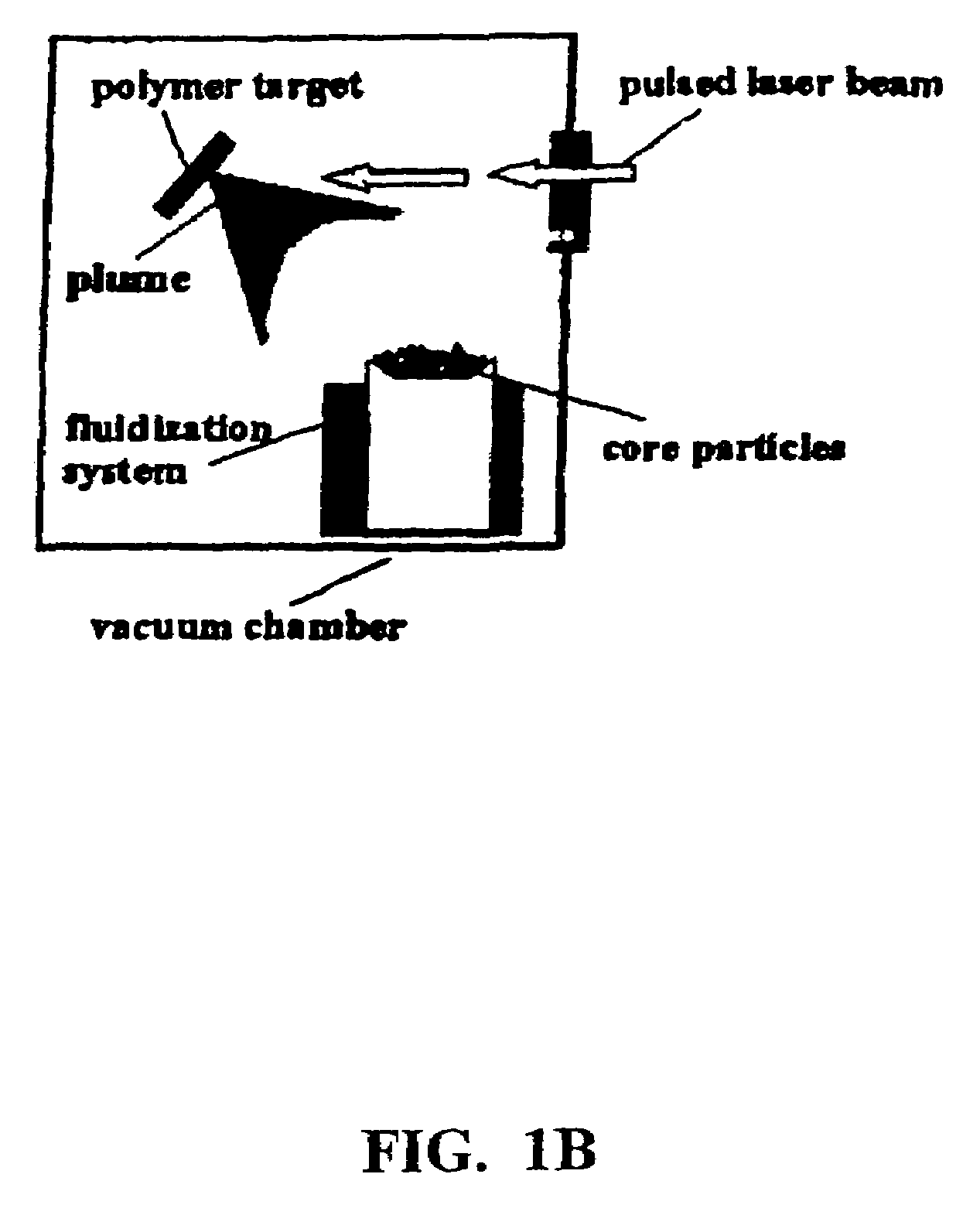
Methods for preparing coated drug particles and pharmaceutical formulations thereof
Disclosed are methods using pulsed laser ablation to prepare coated drug particles of uniform size and thickness. The coated drug particles ranged in size from several nanometers to several millimeters in diameter size, and were coated with organic polymer particle having average diameter sizes from about 1 to 50 nm. In illustrative embodiments, coated drug particles or drug delivery particles are disclosed comprising a biodegradable or biocompatible polymer coating having controlled thickness and controlled coating uniformity, that offer superior pharmaceutical properties for controlled delivery and increased bioavailability.
Owner:FLORIDA UNIV PF +2
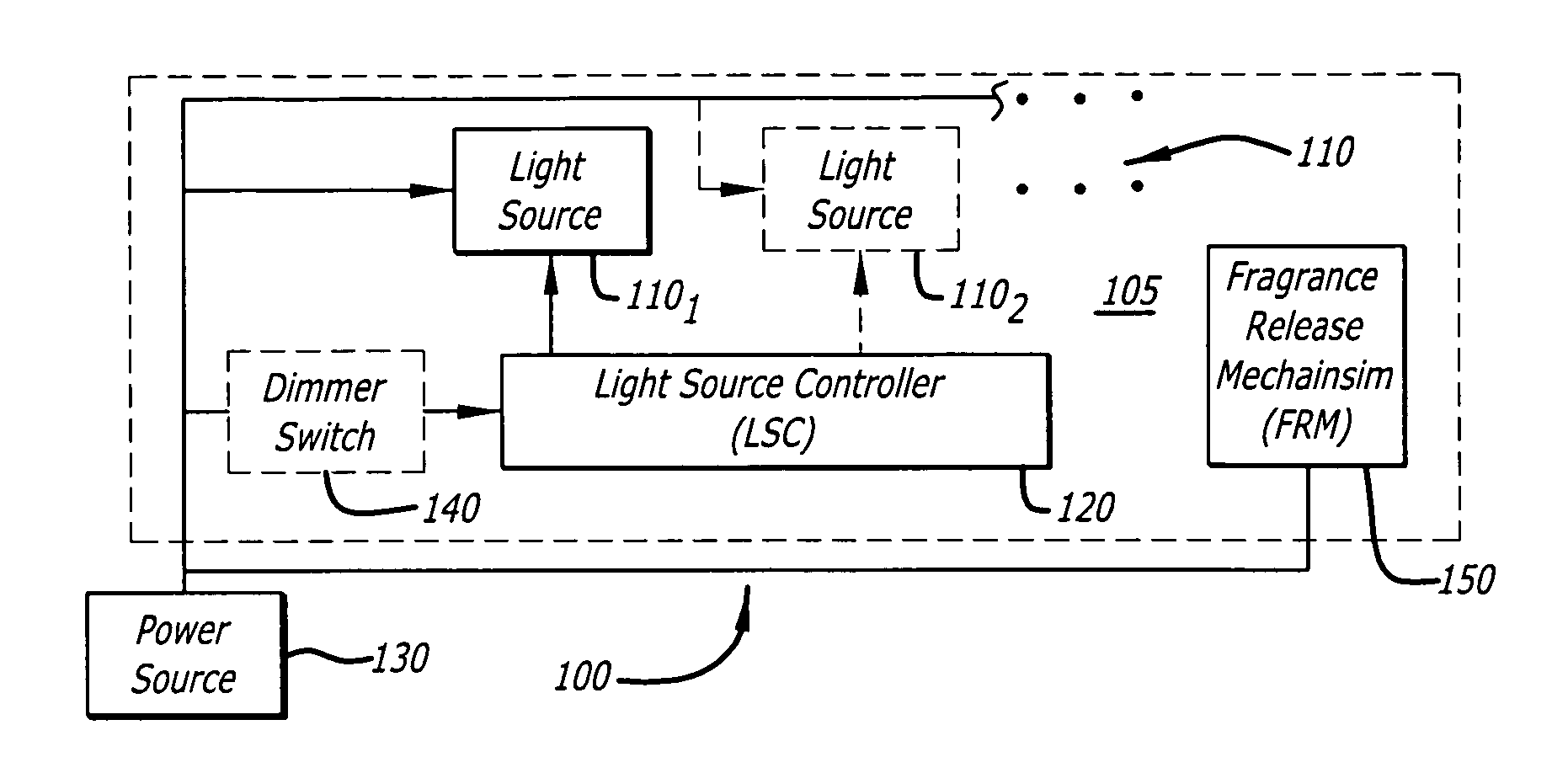
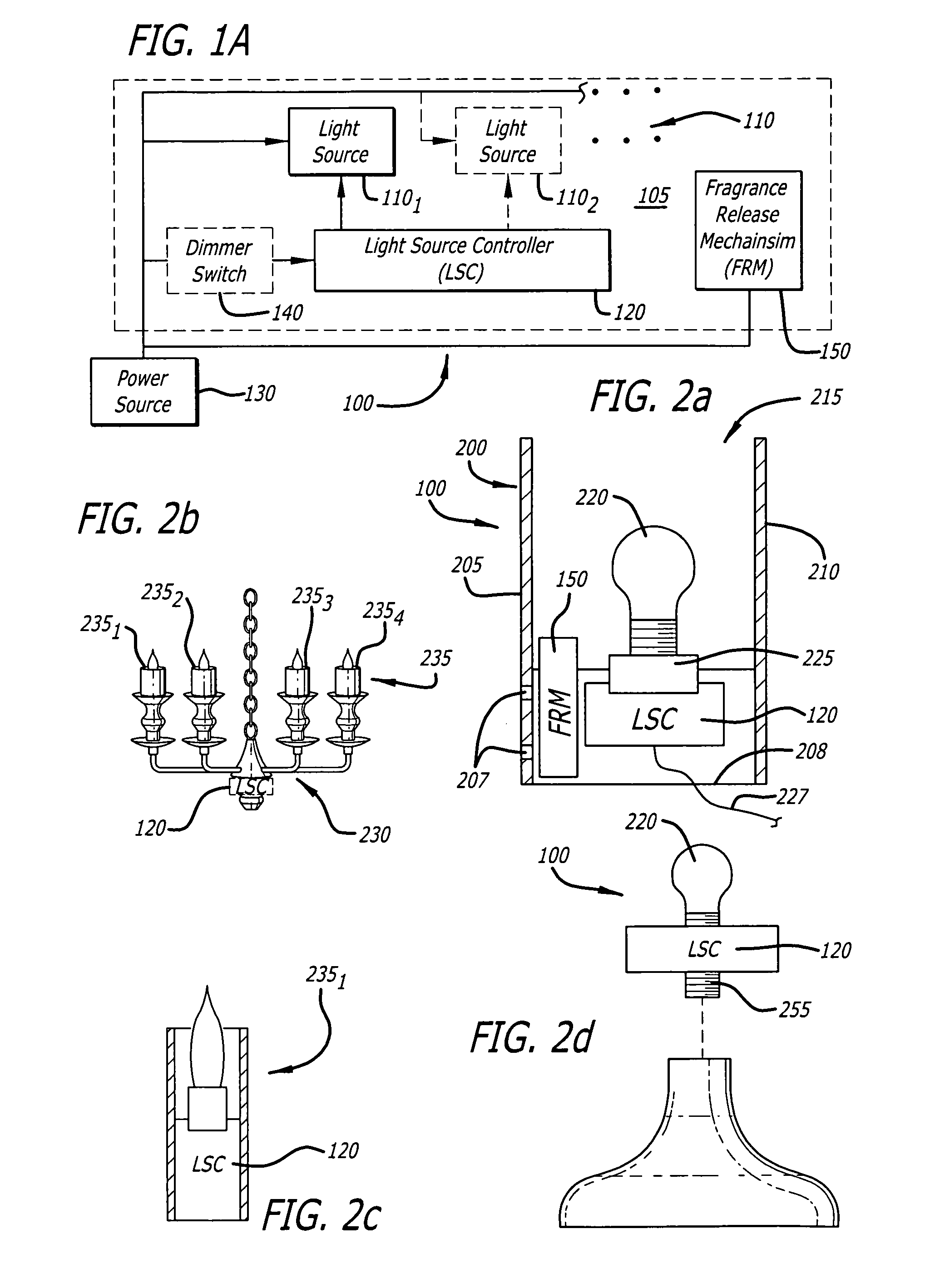
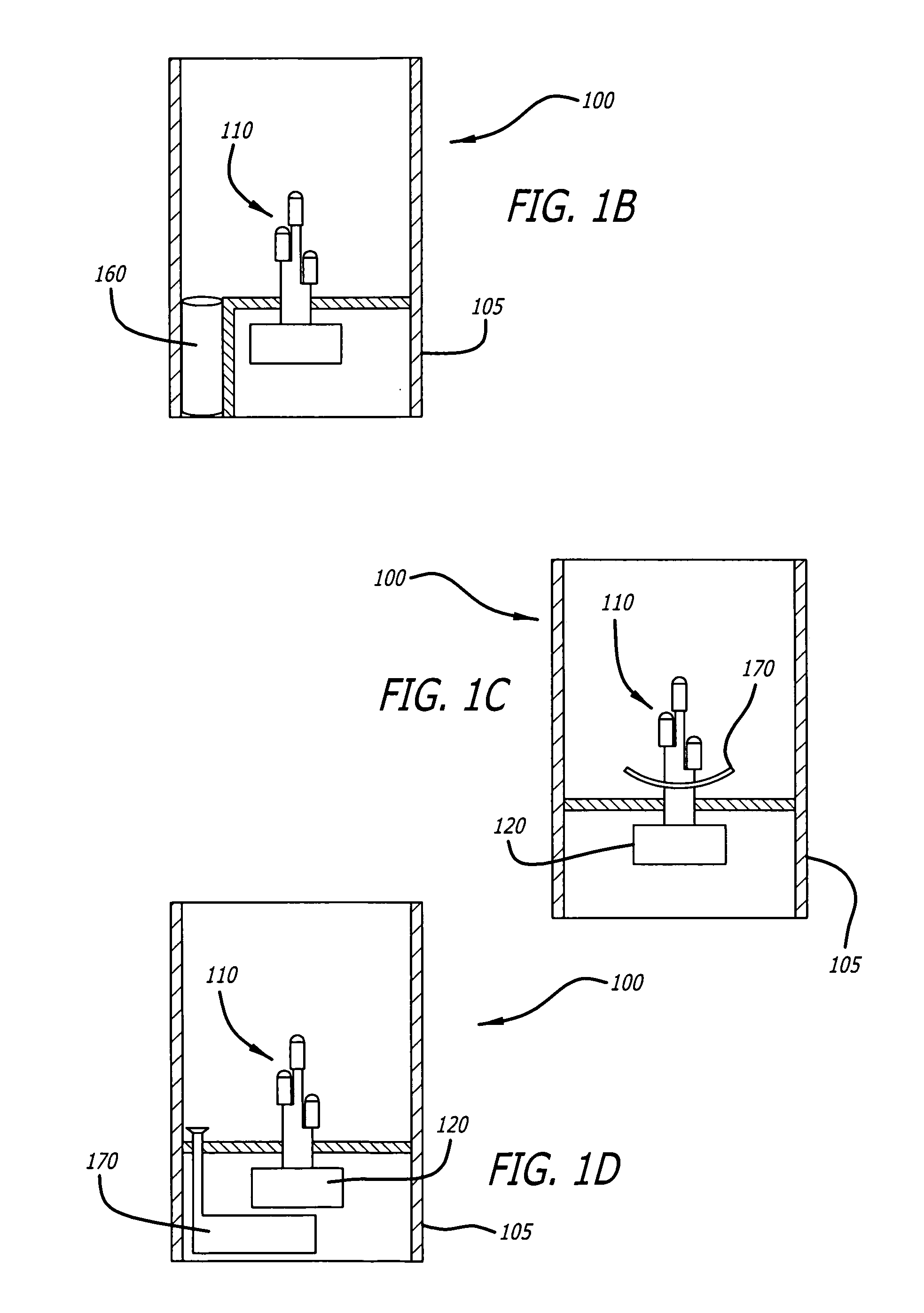
Candle emulation device
According to one embodiment of the invention, a candle emulation device comprises a light source, a light source controller and an optional fragrance-release mechanism. The light source controller is coupled to the light source and is adapted to control the light source in order to produce a lighting effect that emulates lighting from a candle flame. The fragrance-release mechanism is adapted to release a fragrance into air surrounding the candle emulation device.
Owner:IDC ENCHANTED LIGHTING
Retinoid immunomodulating kit and composition and uses thereof
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a retinoid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a retinoid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
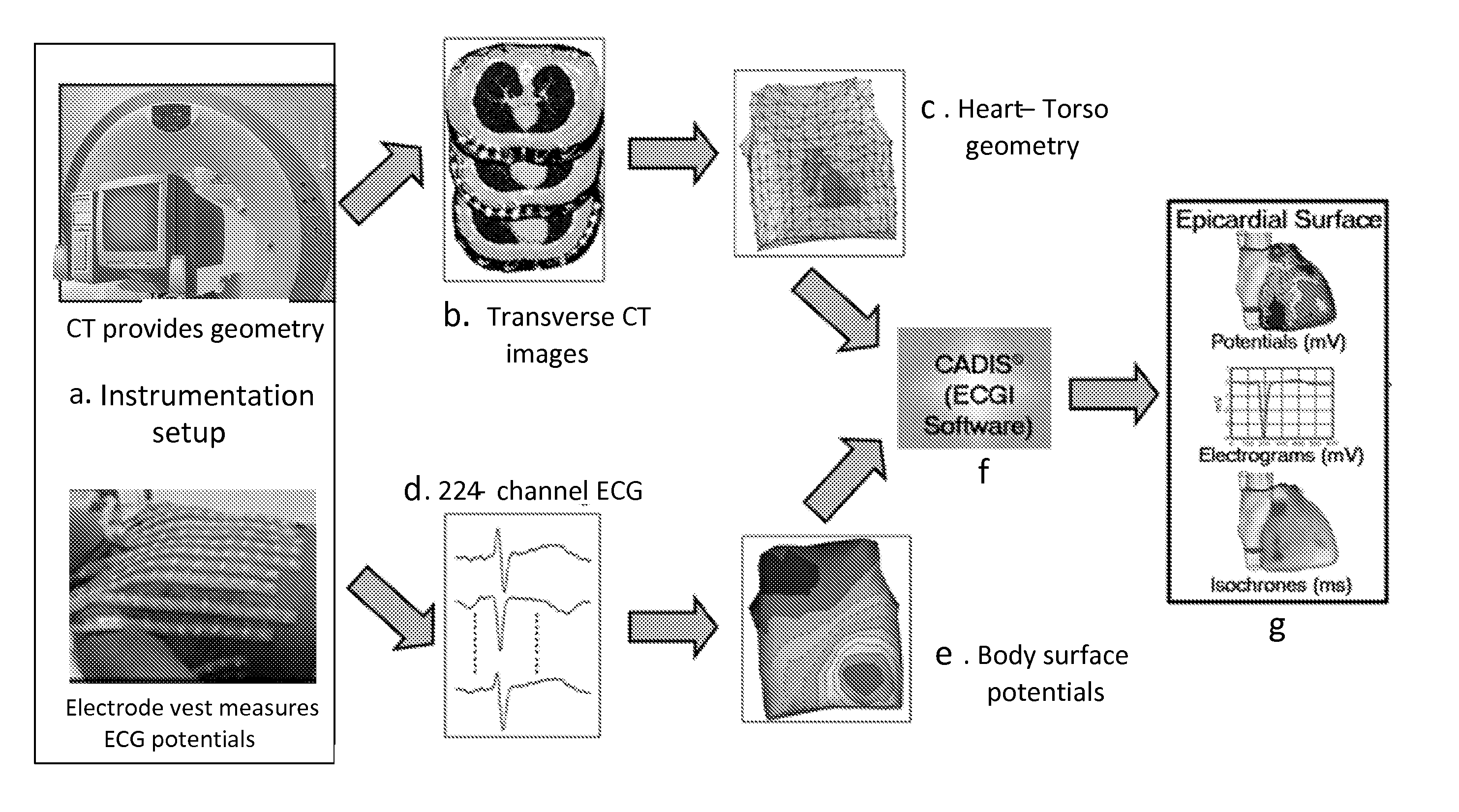
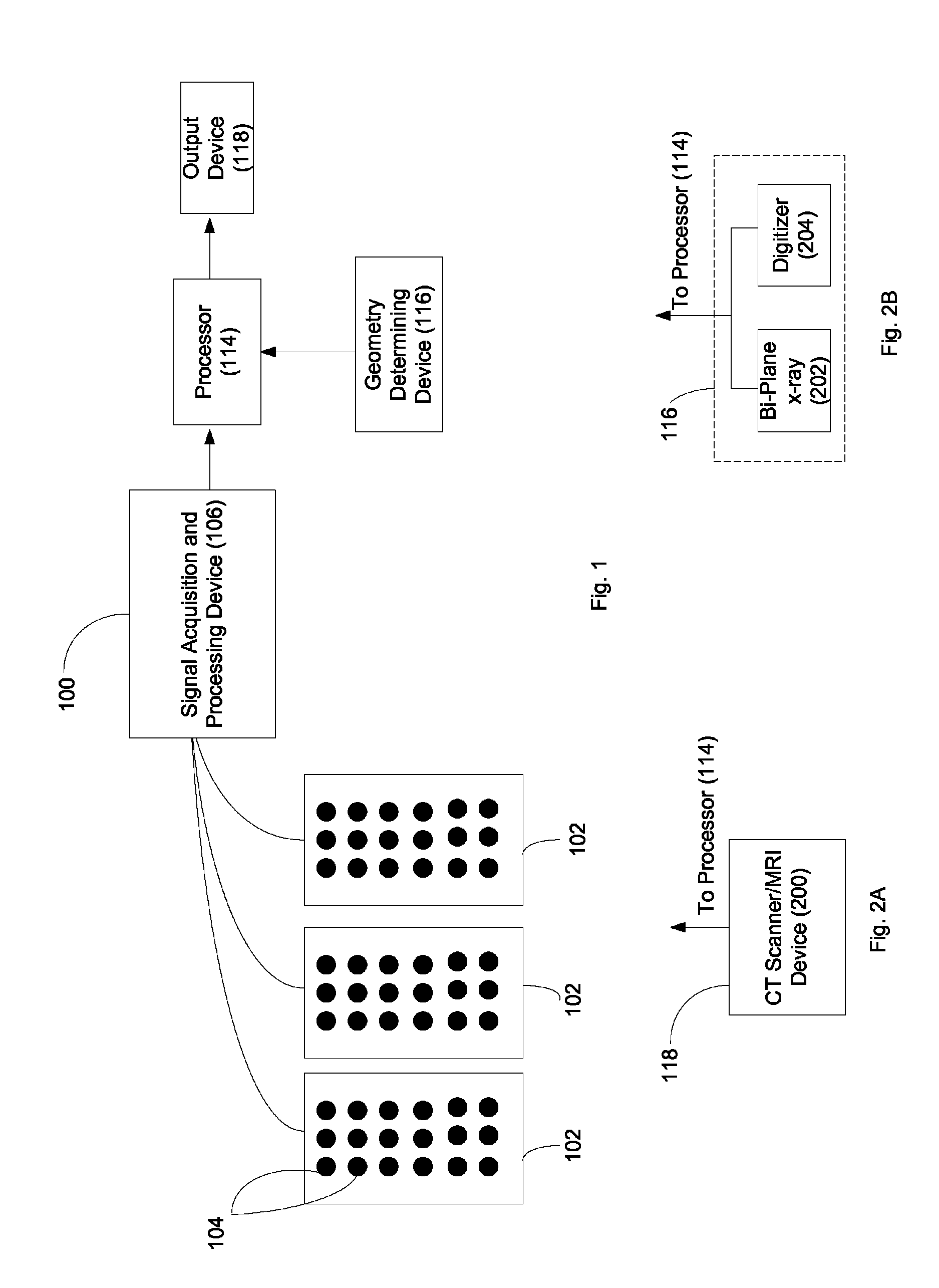
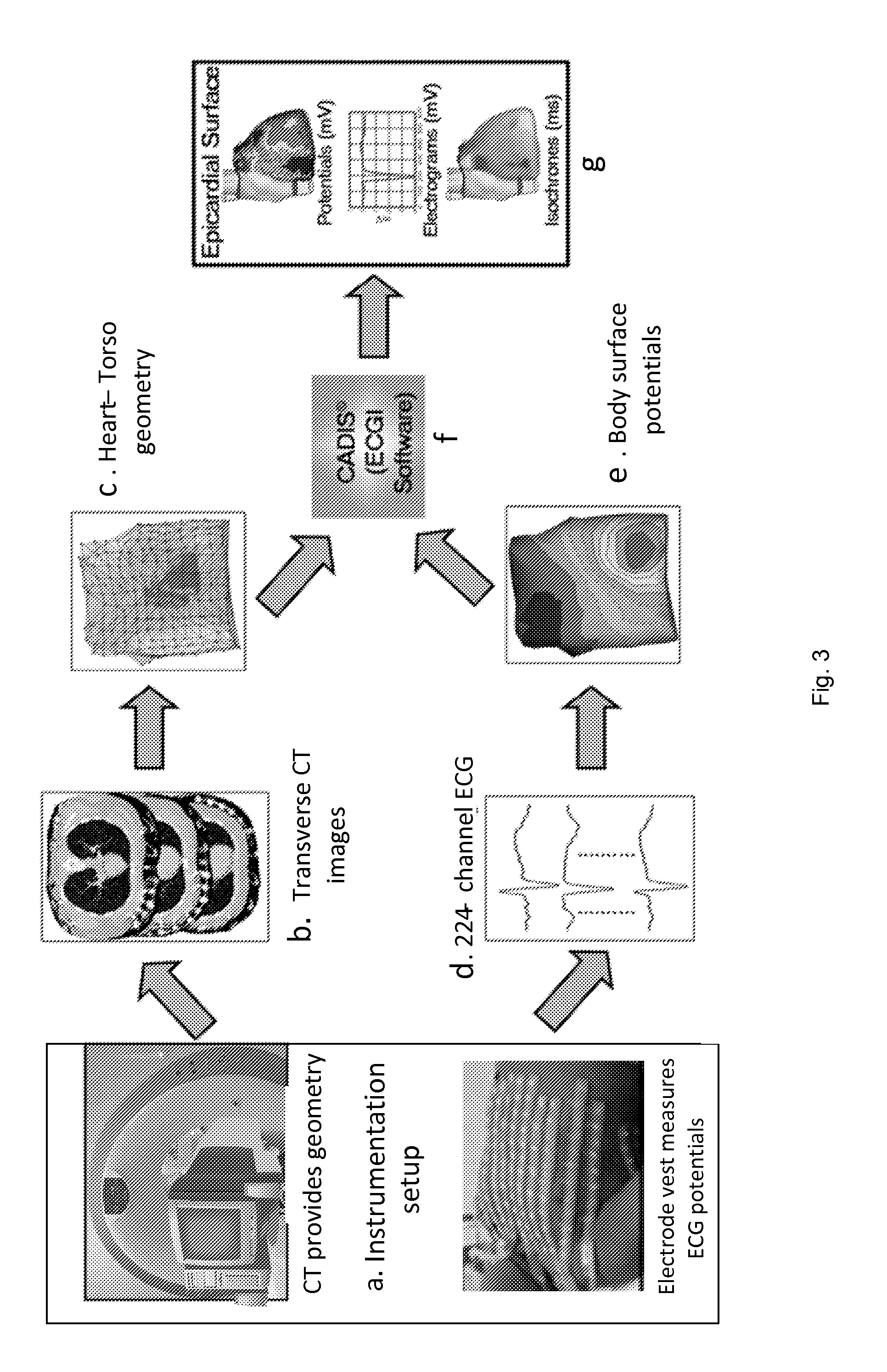
System and method for noninvasive electrocardiographic imaging (ECGI)
ActiveUS7983743B2Easy to rebuildImprove performanceComputerised tomographsGaseous substancesElectricityEngineering
Owner:CASE WESTERN RESERVE UNIV +1
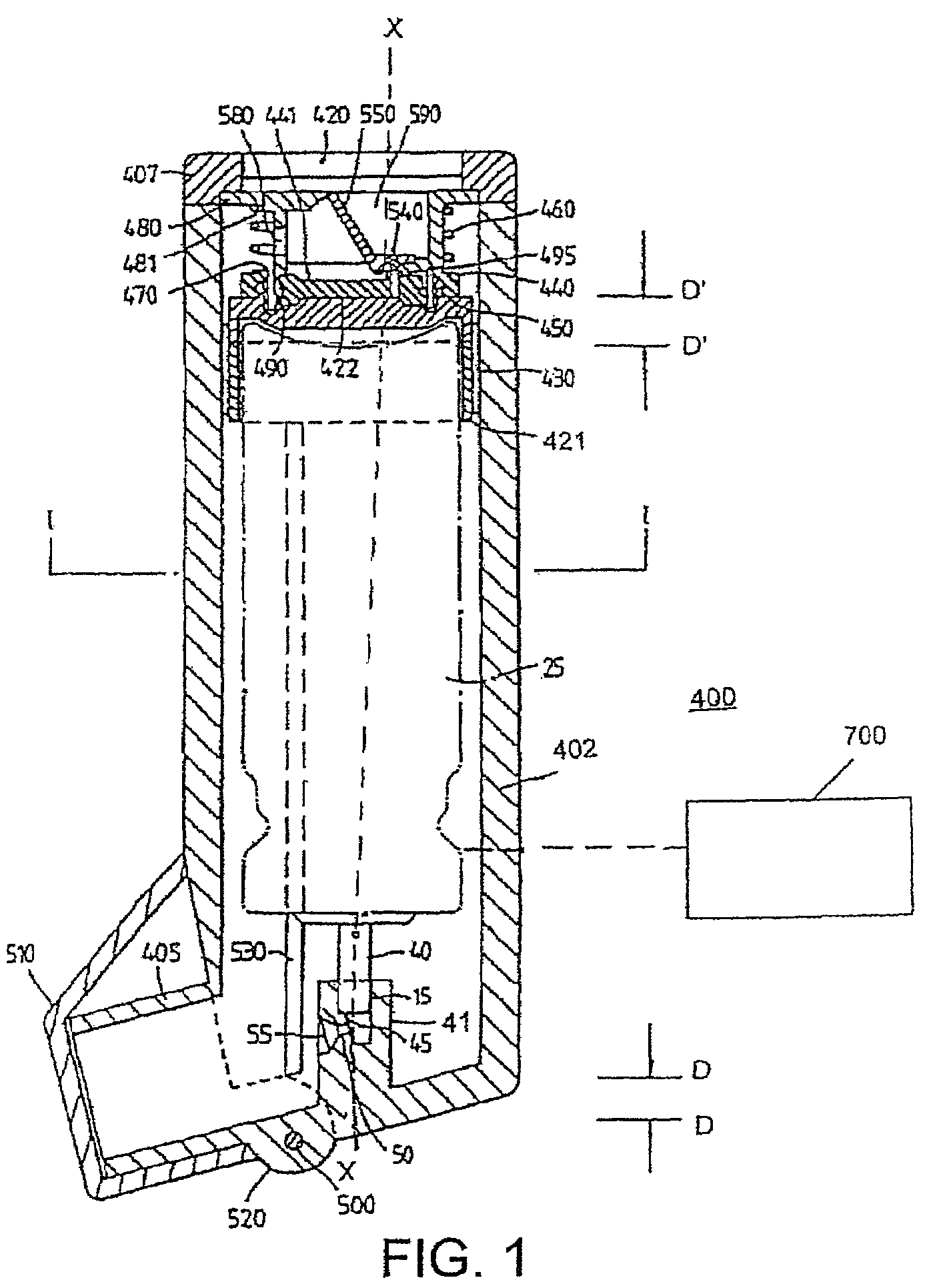
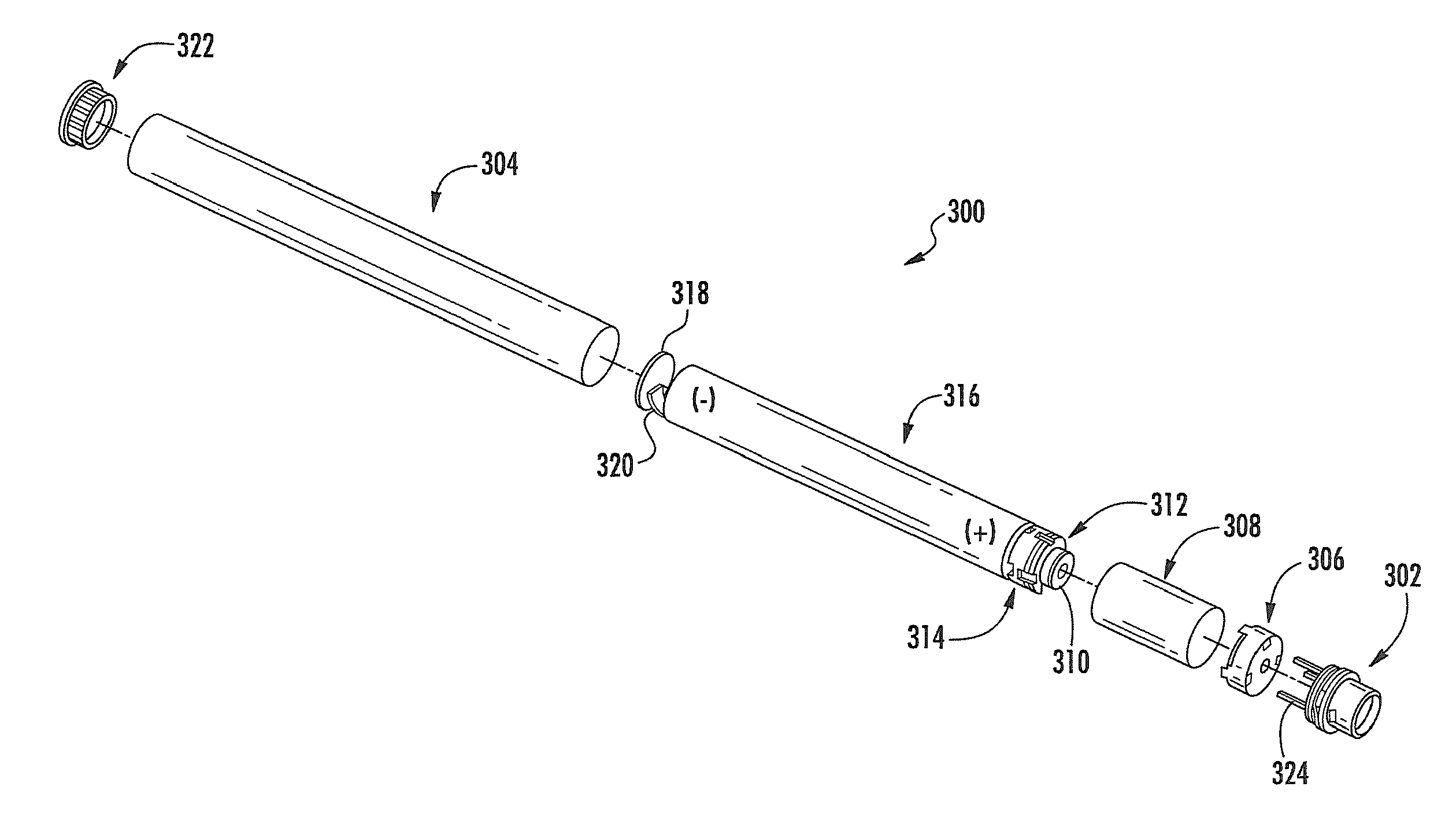
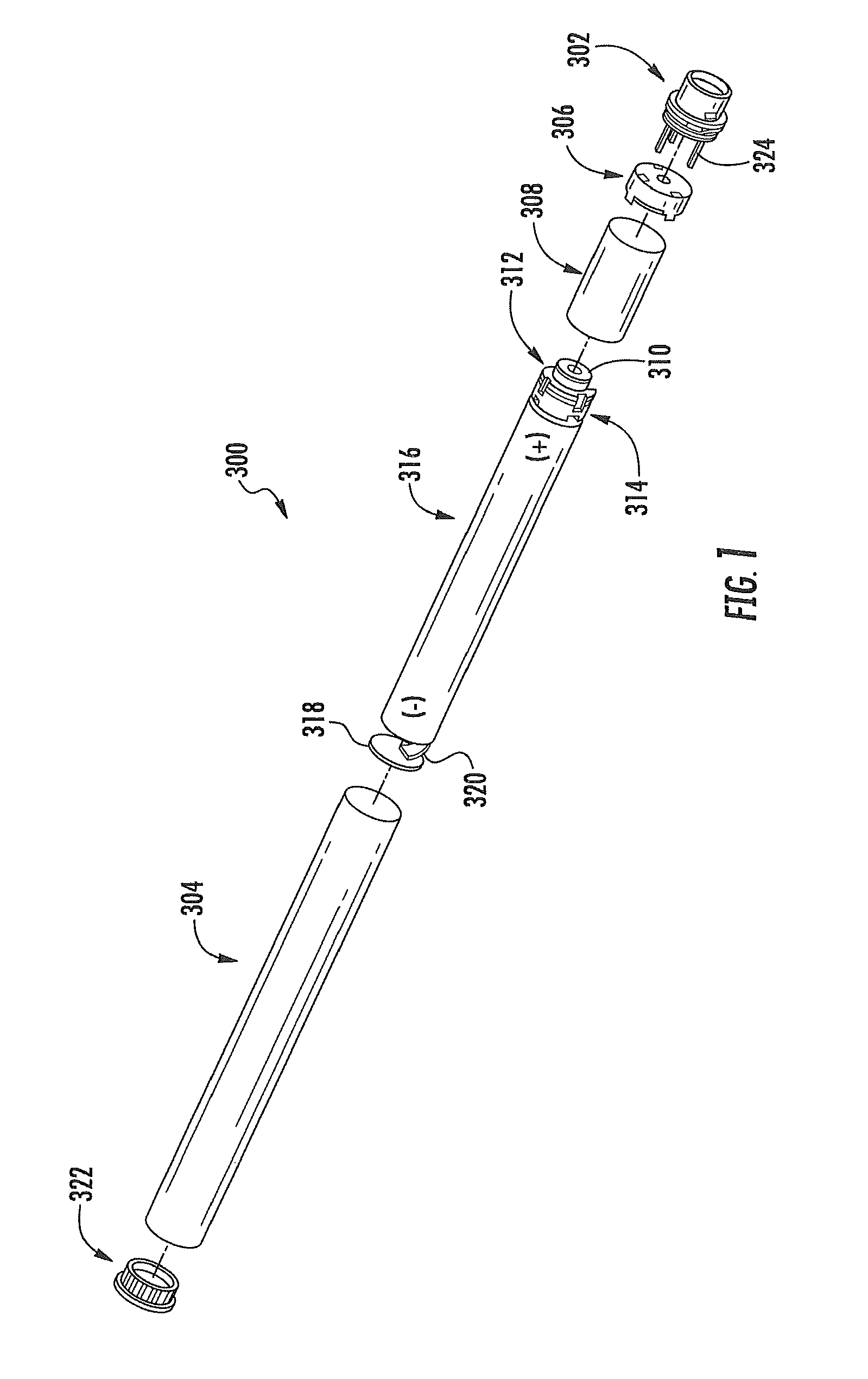
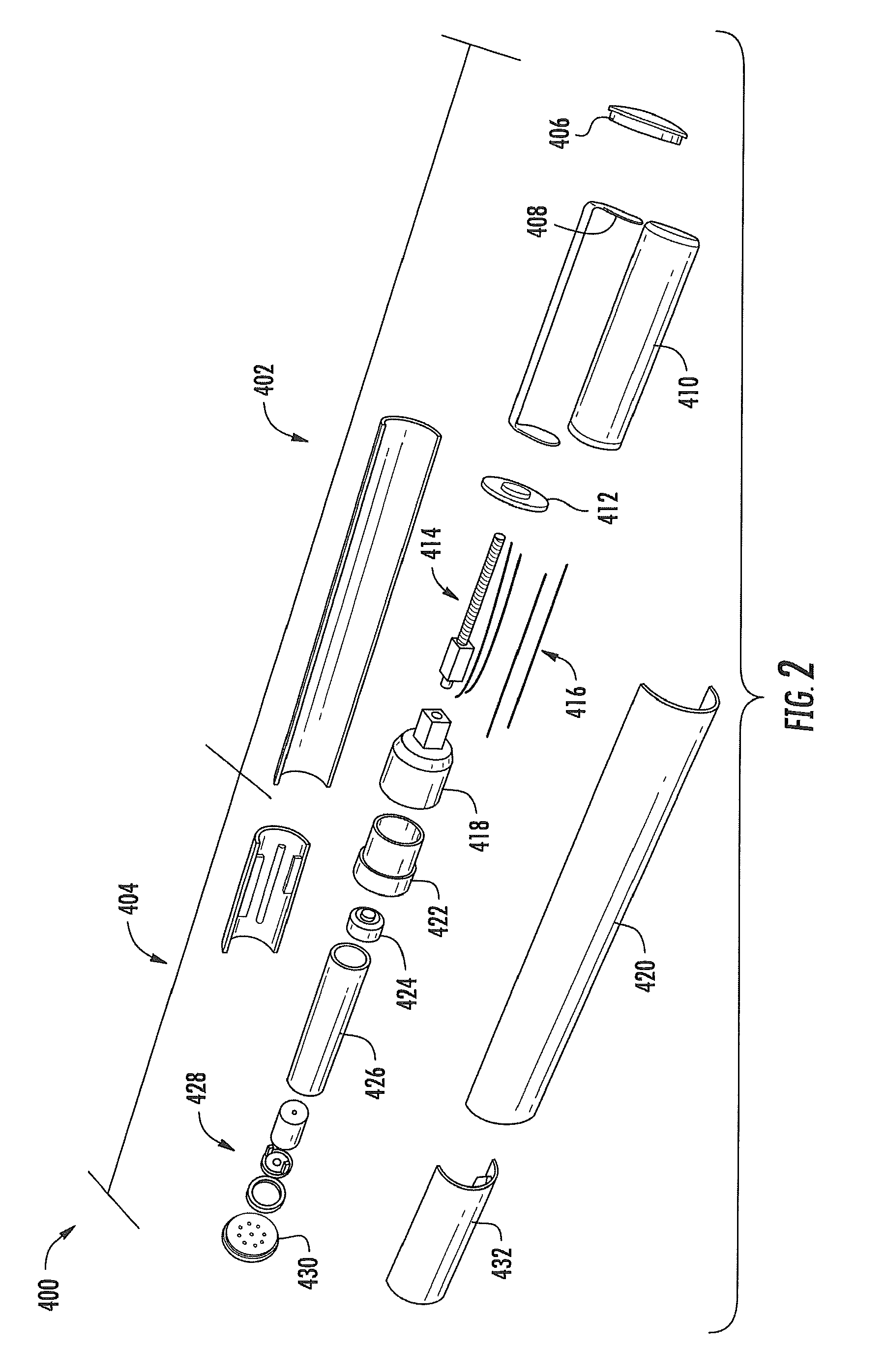
Aerosol Delivery Device Including a Positive Displacement Aerosol Delivery Mechanism
ActiveUS20150117842A1Avoid flowSpace heating and ventilationMedical devicesAerosol deliveryEngineering
The present disclosure relates to aerosol delivery devices. The aerosol delivery devices include mechanisms configured to deliver an aerosol precursor composition from a reservoir to an atomizer including a heating element to produce a vapor. An actuator may displace a rod to dispense the aerosol precursor composition to the atomizer. Thereby, the rod may move a piston in a pump housing to dispense the aerosol precursor composition. The atomizer may define a chamber in which the heating element is positioned and at which the aerosol precursor composition is vaporized.
Owner:RAI STRATEGIC HLDG INC
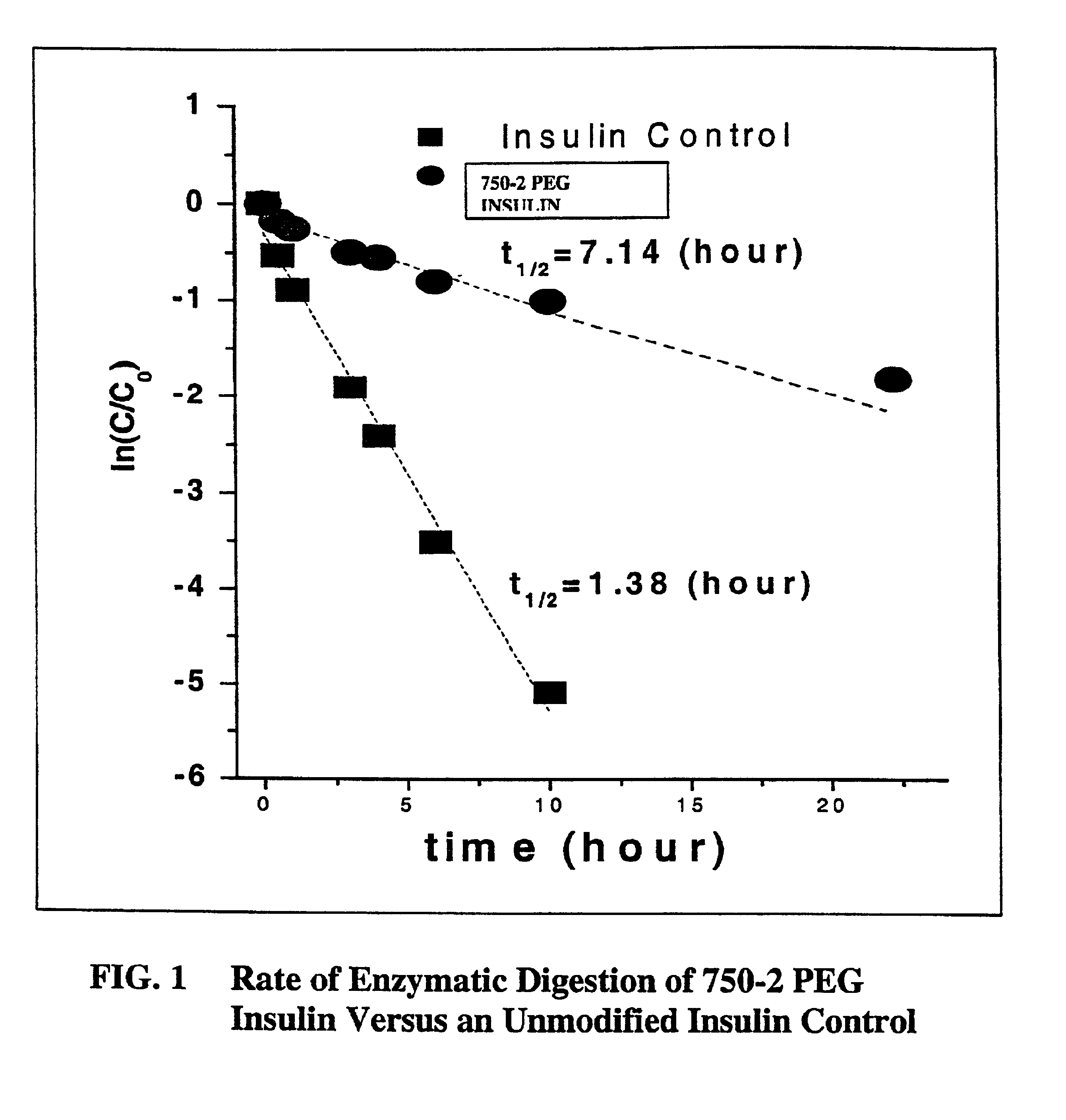
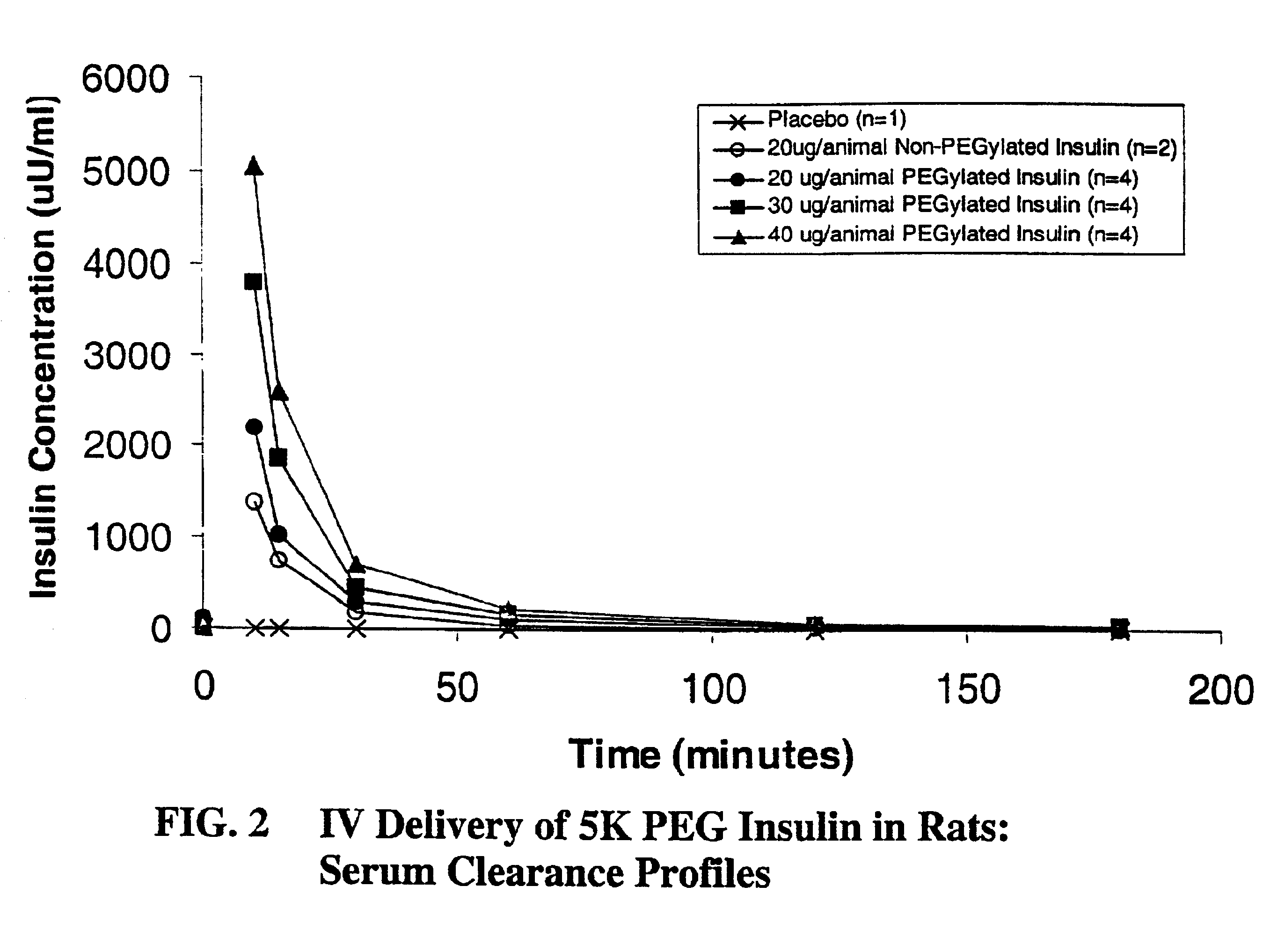
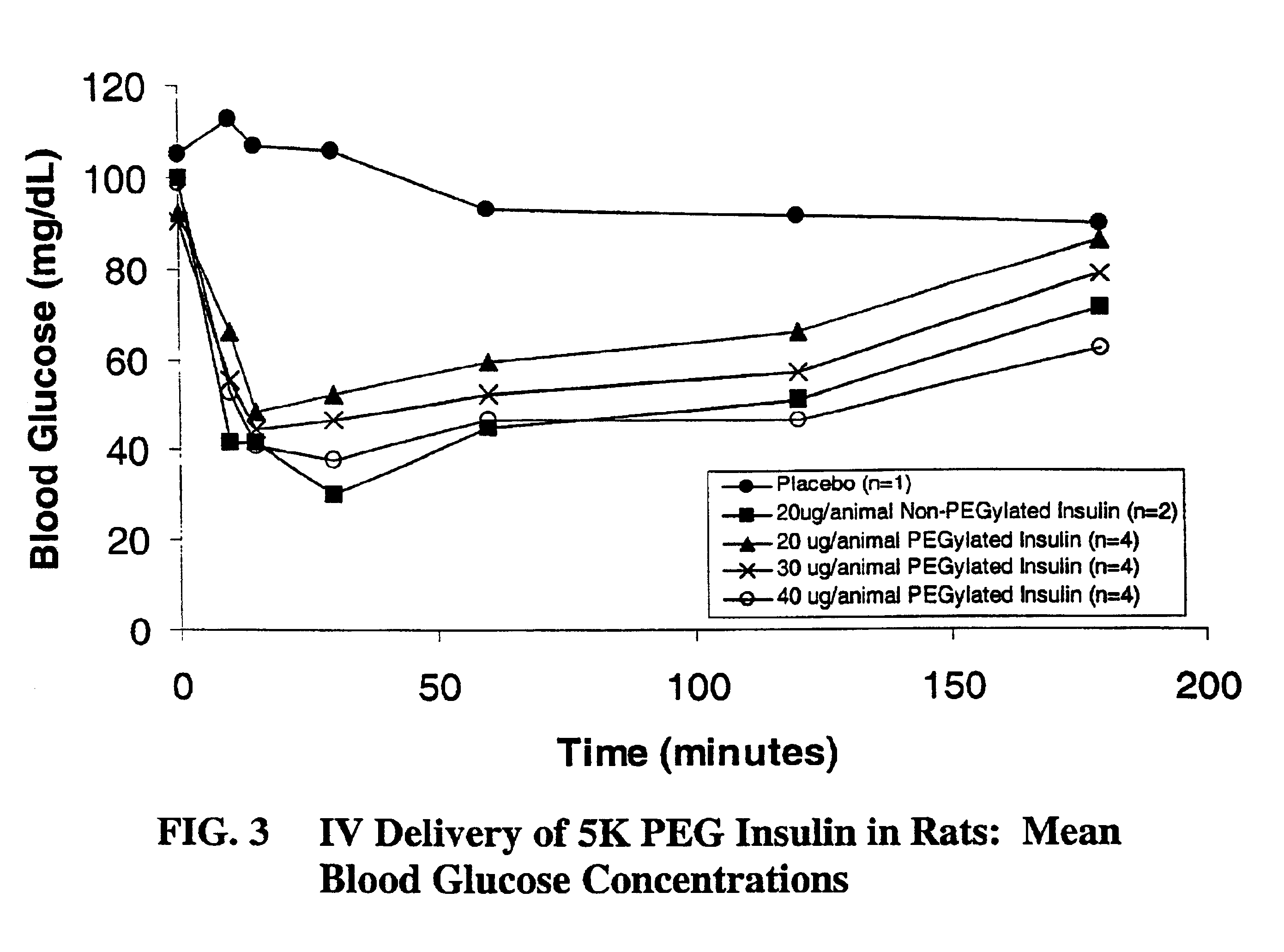
Pulmonary administration of chemically modified insulin
InactiveUS6838076B2Reduce probabilityPowder deliveryPeptide/protein ingredientsPolymer modifiedEndocrinology
The present invention provides active, hydrophilic polymer-modified derivatives of insulin. The insulin derivatives of the invention are, in one aspect, suitable for delivery to the lung and exhibit pharmakokinetic and / or pharmacodynamic properties that are significantly improved over native insulin.
Owner:SHEARWATER CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com