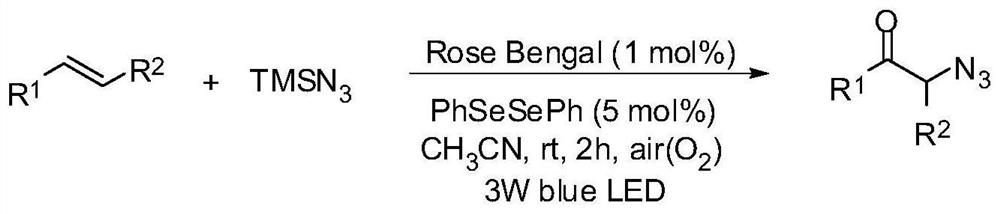
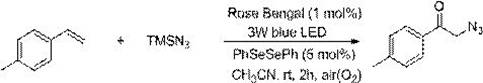
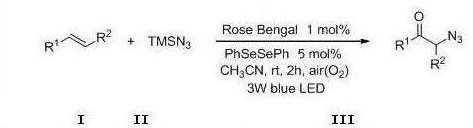
A photocatalytic method for the preparation of α-azide ketone compounds
A technology for azide ketones and compounds, which is applied in the field of photocatalytic preparation of α-azide ketone compounds, can solve the problems of cumbersome operation steps, many by-products, harsh conditions, etc., and achieve safe and reliable process, simplified operation, and mild reaction conditional effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120]
[0121] At room temperature, add styrene 20.8mg, azidotrimethylsilane 46mg, photocatalyst Rose Bengal 2.03mg, diphenyl diselenide 3.14mg and acetonitrile 2ml in sequence in a 15mL reaction tube Under the irradiation of a colored LED lamp, the reaction was stirred in the air for 2 h. After the reaction is detected by TLC, it is concentrated to solvent-free under vacuum (0.08Mpa) to obtain the crude product, which is then washed with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the silica gel column is quickly Through column chromatography, the α-azide ketone product of this example was obtained as 29.6 mg of a yellow oily solid, with a yield of 92%.
[0122] 1 H NMR (CDCl 3 ,500 MHz,ppm):δ7.91(d,J=7.3 Hz,2H),7.63(t,J=7.5 Hz,1H),7.50(t,J=8.0 Hz,2H),4.56(s,2H ); 13 C NMR (CDCl 3 ,125MHz,ppm): δ 193.2,134.4,134.2,129.0,127.9,54.9; HRMS calc.for C 8 h 7 N 3 ONa(M+Na) + , 184.0487; found, 184.0491.
Embodiment 2
[0124]
[0125] At room temperature, add 23.6mg of 4-methylstyrene, 46mg of azidotrimethylsilane, 2.03mg of photocatalyst Rose Bengal, 3.14mg of diphenyldiselenide and 2ml of acetonitrile into a 15mL reaction tube, and mix well , and then under the irradiation of a 3w blue LED lamp, the reaction was stirred in the air for 2h. After the reaction is detected by TLC, it is concentrated to solvent-free under vacuum (0.08Mpa) to obtain the crude product, which is then washed with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the silica gel column is quickly Column chromatography obtained the α-azide ketone product of this embodiment, which was 33.9 mg of white oily solid, with a yield of 97%.
[0126] 1 H NMR (CDCl 3 ,500 MHz,ppm):δ7.80(d,J=8.2 Hz,2H),7.29(d,J=8.0 Hz,2H),4.53(s,2H),2.42(s,3H); 13 C NMR (CDCl 3 ,125MHz,ppm): δ192.8,145.2, 131.9,129.7,128.0,54.8,21.8; HRMS calc.for C 9 h 9 N 3 ONa(M+Na) + ,198.0643; found, 198.0647. ...
Embodiment 3
[0128]
[0129] At room temperature, add 23.6mg of 3-methylstyrene, 46mg of azidotrimethylsilane, 2.03mg of photocatalyst Rose Bengal, 3.14mg of diphenyldiselenide and 2ml of acetonitrile into a 15mL reaction tube, and mix well , and then under the irradiation of a 3w blue LED lamp, the reaction was stirred in the air for 2h. After the completion of the reaction detected by TLC, it was concentrated to solvent-free under vacuum (0.08Mpa) to obtain the crude product, which was then washed with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the silica gel column was quickly Through column chromatography, the α-azide ketone product of this example was obtained as 33.8 mg of a yellow solid with a yield of 96%.
[0130] 1 H NMR (CDCl 3 ,500 MHz,ppm):δ7.71(s,1H),7.67(d,J=7.7 Hz,1H),7.43(d, J=7.6 Hz,1H),7.37(t,J=7.7 Hz,1H ),4.54(s,2H),2.41(s,3H); 13 C NMR (CDCl 3 , 125MHz,ppm): δ193.4,138.9,134.9,134.4,128.8,128.4,125.1,54.9,21.3; HRMS cal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com