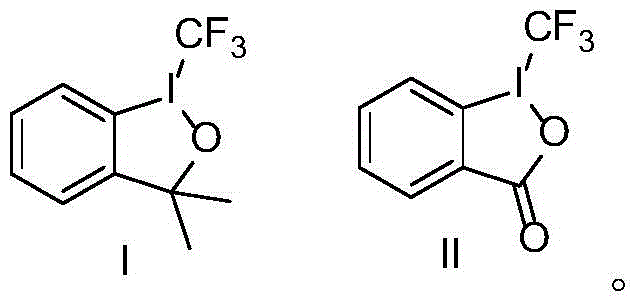
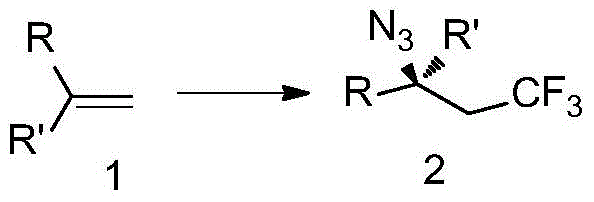
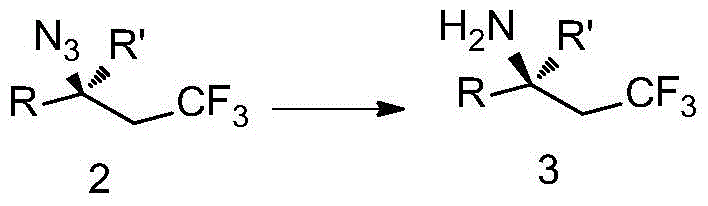
Trifluoromethyl-substituted azide, amine and heterocyclic compounds and preparation method
A technology for azide compounds and amine compounds, applied in the field of amines and heterocyclic compounds, trifluoromethyl substituted azides, can solve the problems of low atom economy, high reaction risk, long reaction steps, etc., to achieve Good atom economy, high selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0098] Add Togni reagent (99.2mg, 0.3mmol) to a 10mL sealed tube, weigh the catalyst Cu(CH 3 CN) 4 PF 6 (3.7mg, 0.01mmol), then transfer out of the glove box, add magnets and 1mL solvent under the protection of nitrogen, and then add TMSN 3 (54μL, 0.4mmol) and styrene (0.2mmol) were added to the sealed tube. After the reaction was stirred at room temperature for 6h, 5mL ethyl acetate was added. The reaction solution was washed twice with water (20mL×2), and then washed once with saturated saline (20mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 37.4 mg of product The total yield is 87%, 1 H NMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0099] 1 H NMR(400MHz, CDCl 3 )δ7.44-7.28(m,5H), 4.77(dd,J=8.4,5.2Hz,1H), 2.65(dqd,J=15.6,10.4,8.4Hz,1H), 2.48(dqd,J=15.6, 10.4, 5.2Hz, 1H). 13 C NMR(100MHz, CDCl 3 )δ137.6,129.2,129.0,126.6,125.2(d,J=276.4Hz), 59.9(q,J=3.2Hz), 40.3(q,J=28.3Hz). 19 F NMR(376MHz, CDCl 3 )δ-64.10(t,J...
example 2
[0102] Add Togni reagent (99.2mg, 0.3mmol) to a 10mL sealed tube, weigh the catalyst Cu(CH 3 CN) 4 PF 6 (3.7mg, 0.01mmol), then transfer out of the glove box, add magnets and 1mL solvent under the protection of nitrogen, and then add TMSN 3 (54μL, 0.4mmol) and (0.2mmol) was added to the sealed tube. After the reaction was stirred at room temperature for 6h, 5mL ethyl acetate was added. The reaction solution was washed twice with water (20mL×2), and then washed once with saturated saline (20mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 48.6mg of product The total yield is 89%, 1 HNMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0103] 1 H NMR(400MHz, CDCl 3 )δ7.34(d,J=8.1Hz,2H), 7.29(d,J=8.1Hz,2H), 6.21(b,1H), 4.77(dd,J=8.6,5.0Hz,1H), 3.66( s, 2H), 2.61 (dqd, J=15.2, 10.4, 8.6 Hz, 1H), 2.47 (dqd, J=15.2, 10.4, 5.0 Hz, 1H). 13 C NMR(100MHz, CDCl 3 )δ172.9, 136.8, 134.2, 130.2, 126.9, 125.1 (q, J=277.4Hz), 59.5 (q, J...
example 3
[0106] Add Togni reagent (99.2mg, 0.3mmol) to a 10mL sealed tube, weigh the catalyst Cu(CH 3 CN) 4 PF 6 (3.7mg, 0.01mmol), then transfer out of the glove box, add magnets and 1mL solvent under the protection of nitrogen, and then add TMSN 3 (54μL, 0.4mmol) and (0.2mmol) was added to the sealed tube. After the reaction was stirred at room temperature for 6h, 5mL ethyl acetate was added. The reaction solution was washed twice with water (20 mL×2), and then washed once with saturated saline (20 mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 42.8 mg of product The total yield is 93%, 1 H NMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0107] 1 H NMR(400MHz, CDCl 3 )δ7.49-7.39(m,4H),7.37-7.32(m,1H), 2.66(dq,J=15.6,10.4Hz,1H), 2.60(dq,J=15.6,10.4Hz,1H),1.88 (q,J=0.9Hz,3H). 13 C NMR(100MHz, CDCl 3 )δ141.9,128.7,128.1,125.3,125.0(d,J=278.3Hz), 62.8(q,J=2.1Hz), 45.2(q,J=27.3Hz), 24.2(q,J=1.7Hz). 19 F NMR(376MHz, CDCl 3 )δ-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com