Metal organic framework material used for absorbing and separating CO2 and preparation method thereof
A metal-organic framework and CO2 technology, which is applied in the field of modification of porous metal framework materials, can solve the problems of poor selectivity and achieve the effects of increased adsorption performance, strong adsorption capacity, and enhanced interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Two-step synthetic method prepares Cu-BTC / PEI composite body
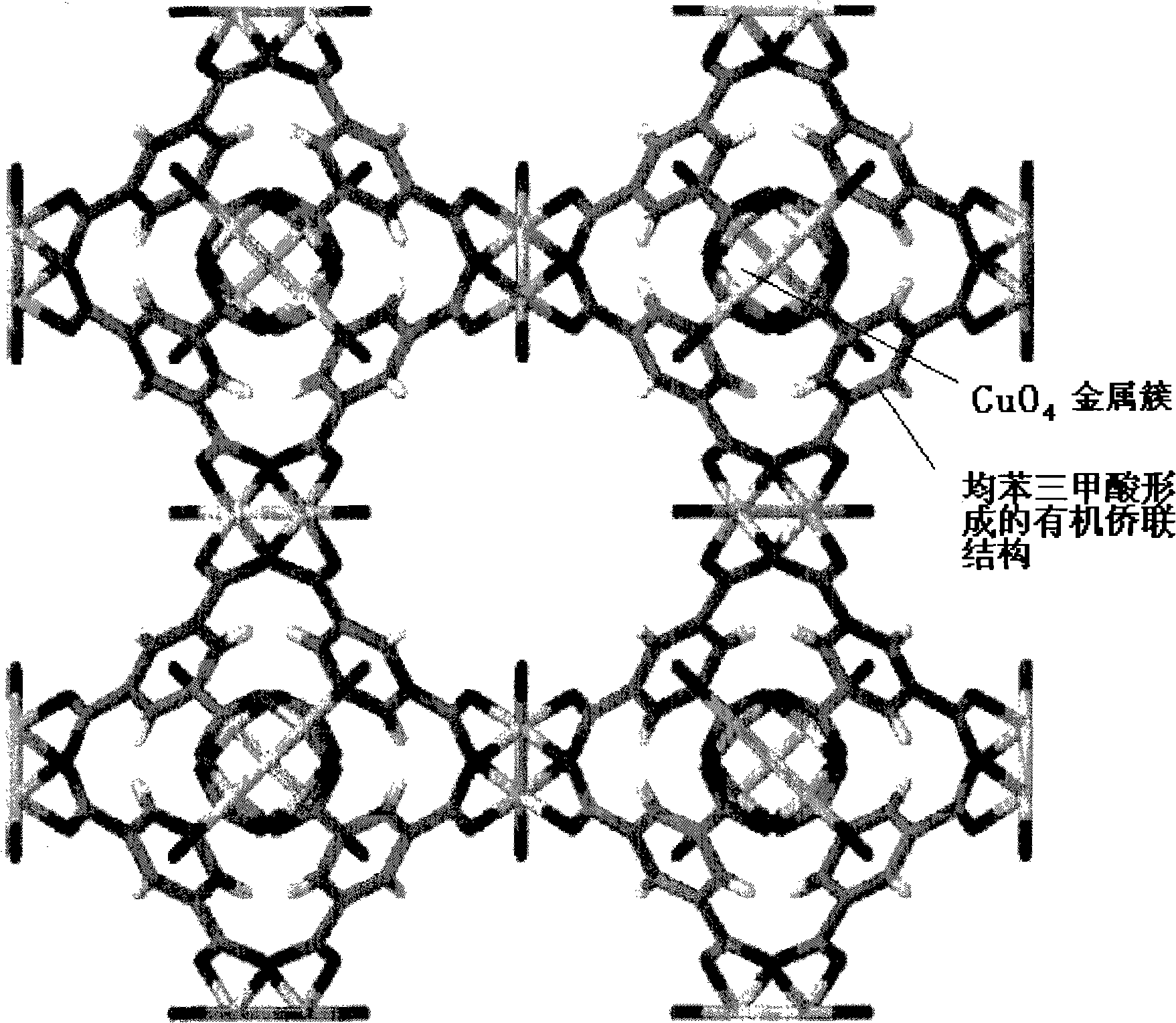
[0026] The first step is to synthesize Cu-BTC crystal
[0027] 1.7922g (7.71mmol) Cu(NO 3 ) 2 2.5H 2 O was dissolved in 24ml of deionized water, 0.8806g (4.19mmol) of trimesic acid was dissolved in 24ml of ethanol, and then the two solutions were mixed in a 125ml polytetrafluoroethylene reactor. The reactor was heated from room temperature to 140° C., then continued to heat for 24 hours, and then cooled to room temperature to obtain a solution.
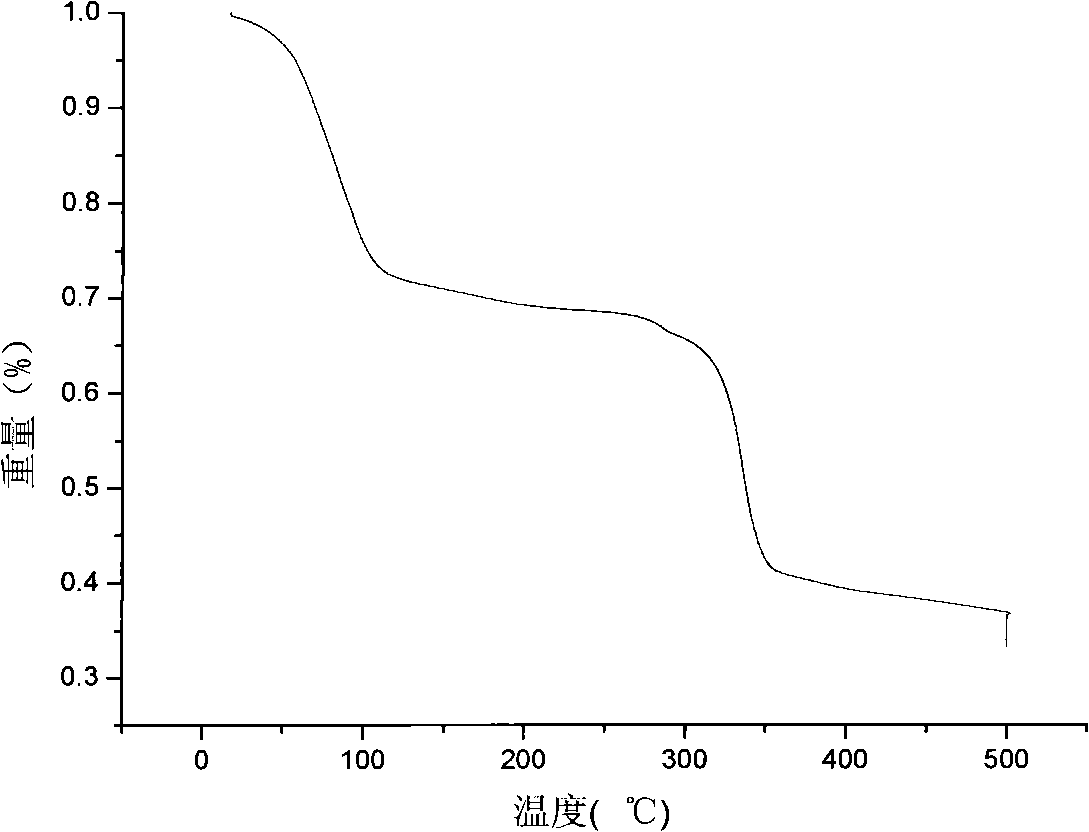
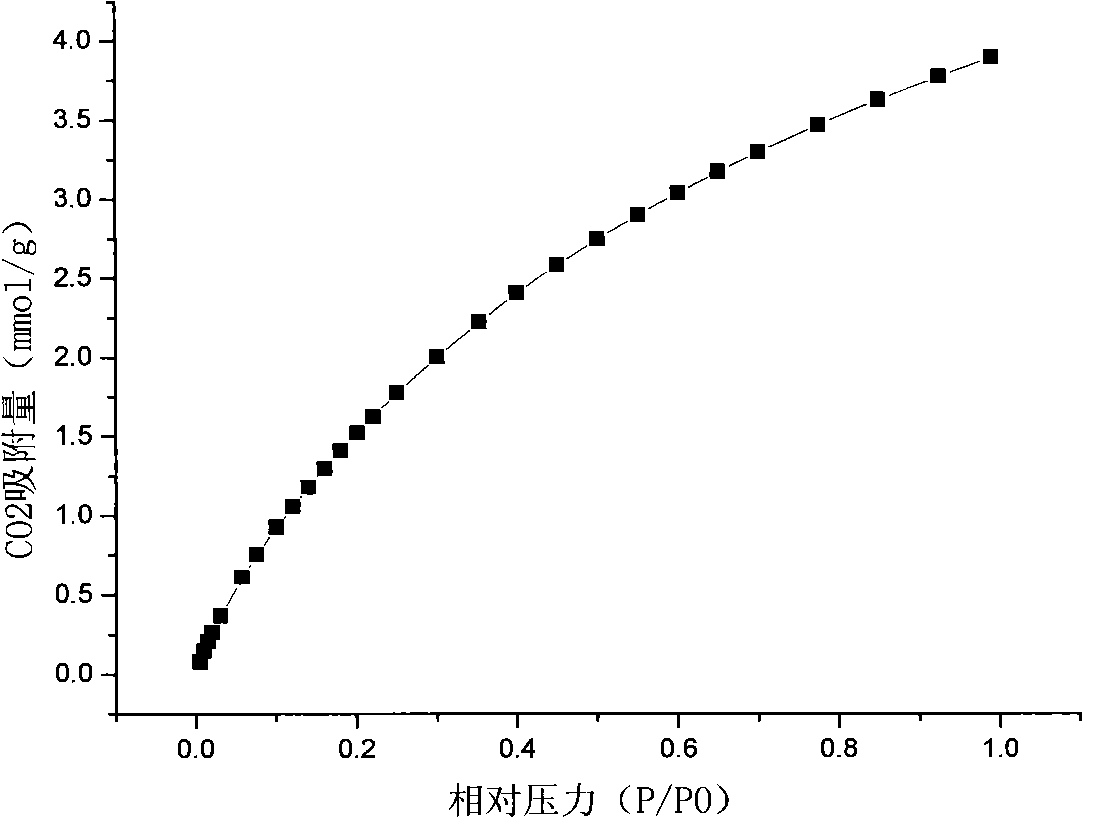
[0028] The above solution was filtered to remove the generated Cu-BTC blue crystals and washed three times with water and methanol respectively. Finally, the product was placed in a vacuum oven at 45°C for 2 days and stored in a desiccator. The specific surface area is 1000m 2 / g, the pore volume is 0.41cm 3 / g.
[0029]
[0030] The above are some possible coordination modes of BTC and metal ions (a) three monodentate; (b) three bidentate; ...
Embodiment 2
[0033] Example 2 Preparation of Cu-BTC / PEI composite by one-step synthesis
[0034] 1.7922g (7.71mmol) Cu(NO 3 ) 2 2.5H 2 O is dissolved in 24ml deionized water, 0.8806g (4.19mmol) 1,3,5-trimellitic acid is dissolved in 24ml ethanol, and a certain amount of polyethyleneimine PEI is dissolved in distilled water to obtain a concentration of 0.05g / ml PEI aqueous solution, then take 6ml of PEI aqueous solution and mix it with the other two solutions in a 125ml polytetrafluoroethylene reactor. The reactor was heated from room temperature to 140° C., then continued to heat for 24 hours, and then cooled to room temperature to obtain a solution. The above solution was filtered to remove the generated crystals, and the crystals were washed with water and methanol three times respectively. Finally, the product was placed in a vacuum oven at 45°C for 2 days and stored in a desiccator.
Embodiment 3
[0035] Example 3 One-step synthesis method to prepare Zn-BTC / PEI complex
[0036] 2.2936g (7.71mmol) Zn(NO 3 ) 2 ·6H 2 O is dissolved in 24ml deionized water, 0.8806g (4.19mmol) 1,3,5-trimellitic acid is dissolved in 24ml ethanol, and a certain amount of polyethyleneimine PEI is dissolved in distilled water to obtain a concentration of 0.05g / ml PEI aqueous solution, then take 6ml of PEI aqueous solution and mix it with the other two solutions in a 125ml polytetrafluoroethylene reactor. The reactor was heated from room temperature to 140° C., then continued to heat for 24 hours, and then cooled to room temperature to obtain a solution. The above solution was filtered to remove the generated crystals, and the crystals were washed with water and methanol three times respectively. Finally, the product was placed in a vacuum oven at 45°C for 2 days and stored in a desiccator.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com