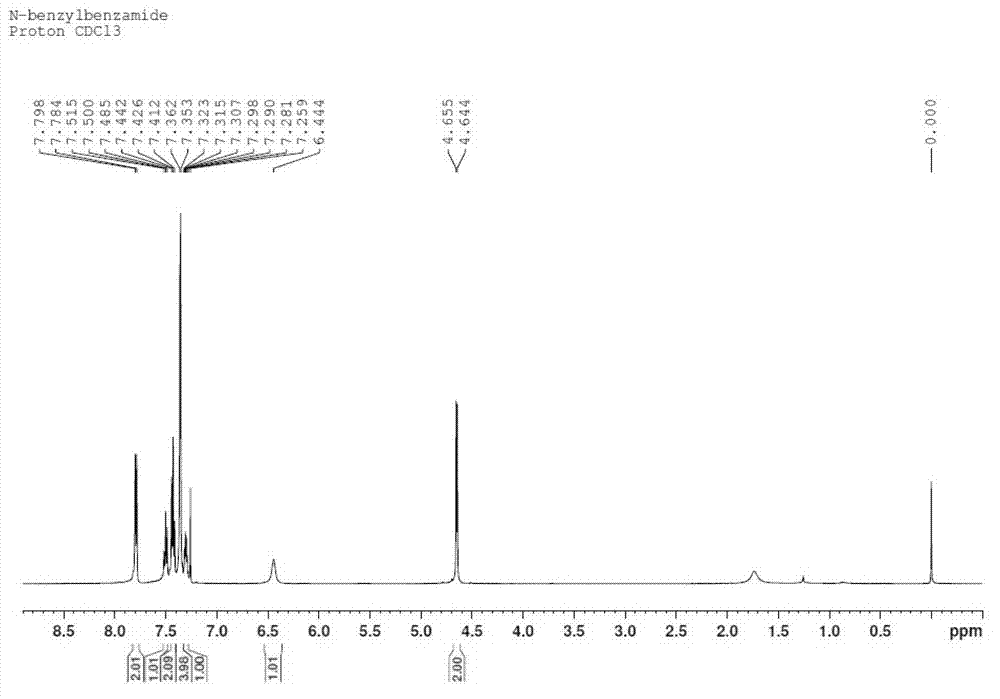
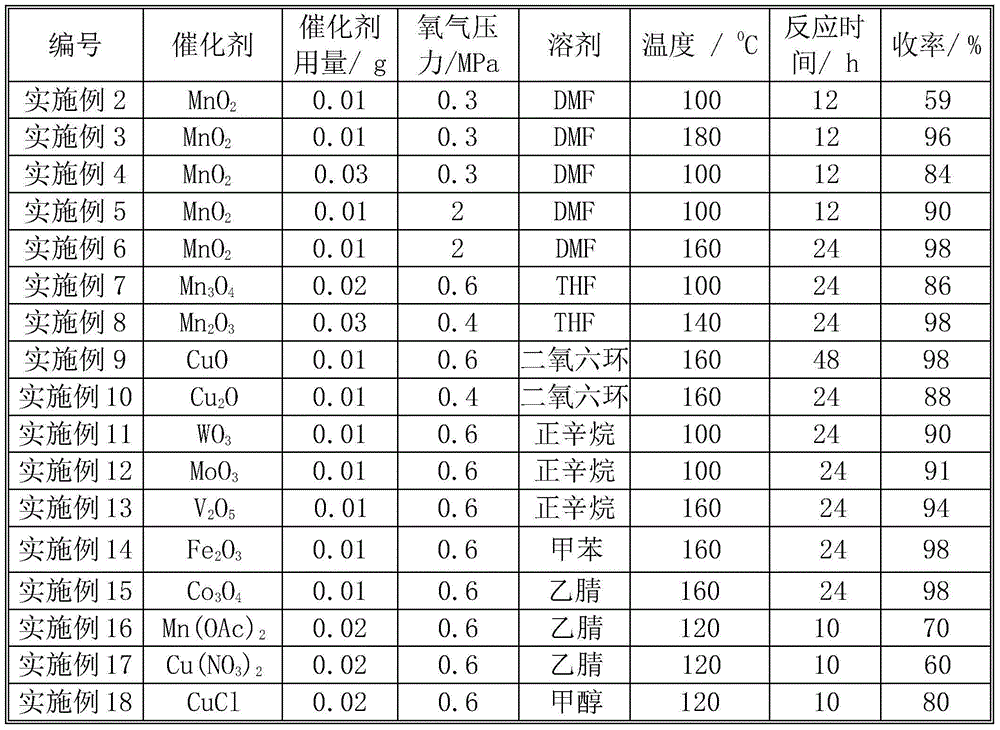
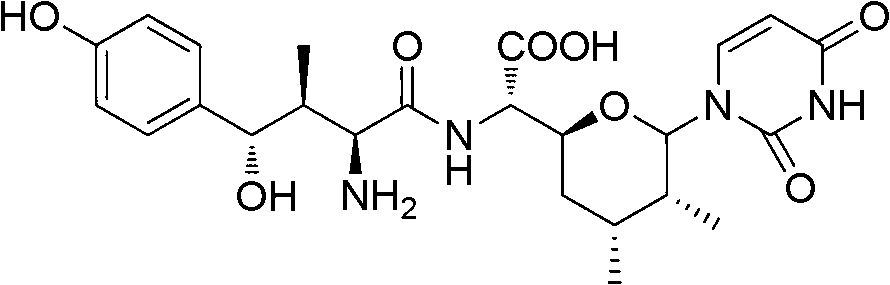
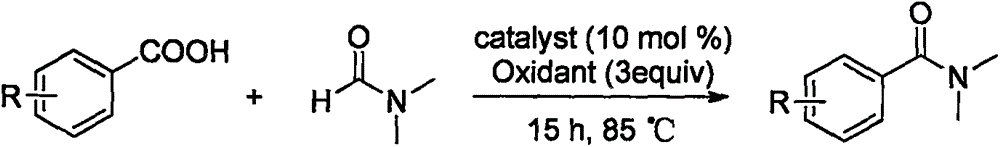
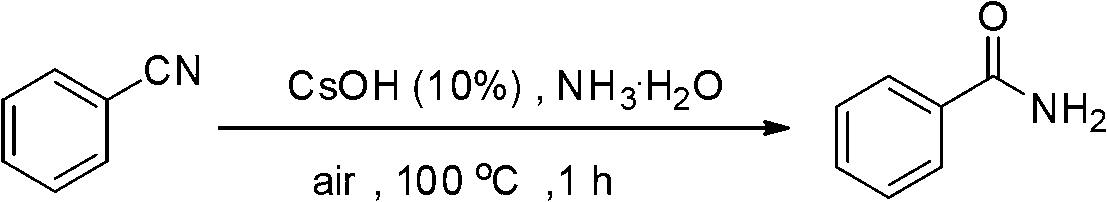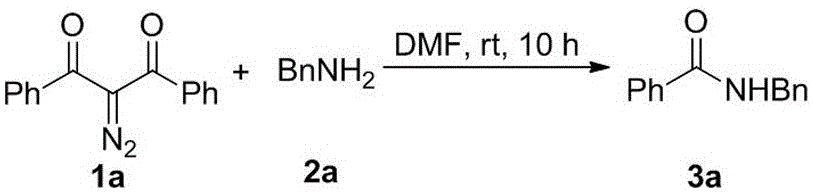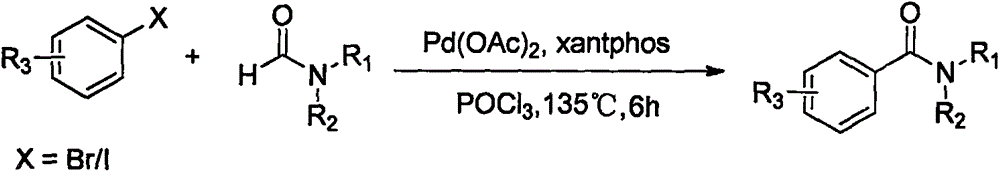Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
190results about "Amide group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macromolecule thiolated modified derivatives and cross-linking material thereof
ActiveCN101200504AAvoid stickingEasy to manufacturePeptide preparation methodsAmide group formation/introductionChemical structureProduction rate
The present invention discloses a macromolecular sulfydryl modified derivative with general formula (I) or (II) as well as corresponding dithio-bond cross-linking material and corresponding cross-linking material of sulfydryl reaction active cross linker. Wherein, R1 and R2 include alkylidene, substituted alkylidene, aromatic base, polyether polyols, etc. and R1 and R2 can have the same or different chemical structure; P refers to macromolecular compound residue including carboxyl in the side chain and the molecular weight of the macromolecular sulfydryl modified derivative is 10 to 5 million. The side-chain chemical structure of the macromolecular sulfydryl modified derivative with general formula (I) or (II) is flexible and changeable with notable advantages of adjustable performance, mild preparation conditions, high production rate, high modification and controllability. The cross-linking material of the macromolecular sulfydryl modified derivative provided by the present invention can be used to inhibit the attachment of cells and used as the matrix for cell adhesion and growth.
Owner:BIOREGEN BIOMEDICAL (CHANGZHOU) CO LTD
Method for the synthesis of amides and related products from esters or ester-like compounds
InactiveUS20050027120A1Improve practicalityUrea derivatives preparationOrganic compound preparationCarbamateOxazolidinediones
A versatile, eco-friendly, and efficient method for the convenient conversion of esters and ester-like compounds into amides, peptides, carbamates, ureas, oxamides, oxamates, hydrazides, oxazolidinones, pyrazolones, oxazolidinediones, barbituric acids, and other molecules containing one or more OCN moieties in the presence of a diol or polyol is disclosed.
Owner:REACTIMEX DE C V
Method of making hydroxyalkyl amide containing reduced level of unreacted alkanolamine
ActiveUS20050107623A1Decrease in levelFatty acid chemical modificationOrganic compound preparationMetalAlkanolamine
A method for reacting alkanolamine with ester in the presence of a metal silicate compound and, optionally, a catalyst, to produce a hydroxyalkyl amide composition with a decreased level of alkanolamine and residual catalyst.
Owner:CHEMTURA CORP
Benzoxazepin oxazolidinone compounds and methods of use
Described herein are benzoxazepin oxazolidinone compounds with phosphoinositide-3 kinase (PI3K) modulation activity or function having the Formula I structure:or stereoisomers, tautomers, or pharmaceutically acceptable salts thereof, and with the substituents and structural features described herein. Also described are pharmaceutical compositions and medicaments that include the Formula I compounds, as well as methods of using such PI3K modulators, alone and in combination with other therapeutic agents, for treating diseases or conditions that are mediated or dependent upon PI3K dysregulation.
Owner:GENENTECH INC
Novel method for synthesizing N-substitute amide derivative
InactiveCN107056567AEasy to separateYield is not affectedOrganic compound preparationCarboxylic acid amides preparationAir atmosphereReaction temperature
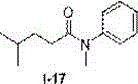
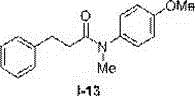
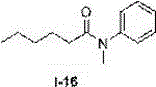
The invention discloses a novel method for synthesizing an N-substitute amide derivative. The novel method is characterized by comprising the steps that at the air atmosphere, no catalyst or alkali or other any additives are added, an organic amine compound shown in a formula (I) is adopted as a reaction substrate, a solvent shown in a formula (II) is adopted as an acylation reagent, an acylation reaction is performed under the reaction temperature of 120-150 DEG C to generate the N-substitute amide derivative shown in a formula (III), and the equation is shown in the description. The novel method has the advantages that environmental protection is achieved, and post-treatment and product separation are easy; the range of the substrate is wide, and the substrate can be primary amine and can also be secondary amine; the solvent can be amide and can also be carboxylic acid, and the solvent can be adopted as the acylation reagent to participate in the reaction; the reaction efficiency is high, and the majority of reactions can reach the quantified yield; water and air have no effect on the reaction, inert gas shielding is not needed, and operation is easy.
Owner:WENZHOU UNIVERSITY
Branched chain-containing aromatic compound
ActiveUS20120059149A1Easy to operatePeptide/protein ingredientsCarboxylic acid amides preparationOperabilityCrystallization
Owner:AJINOMOTO CO INC
Preparation method of amide
InactiveCN103113177AIncrease profitFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryReaction step
The invention discloses a preparation method of amide. The amide is the amide containing the structure shown in the specification. The method comprises the following step: carrying out one-step reaction by adopting a corresponding amide type compound as a starting reactant, a reducing agent and a corresponding acid to prepare a final amide product. According to the preparation method disclosed by the invention, the reaction steps are reduced, the cost can be effectively reduced, and the process flow is simplified.
Owner:JINAN ZHIHE MEDICAL TECH
Benzoxazepin oxazolidinone compounds and methods of use
Described herein are benzoxazepin oxazolidinone compounds with phosphoinositide-3 kinase (PI3K) modulation activity or function having the Formula I structure:or stereoisomers, tautomers, or pharmaceutically acceptable salts thereof, and with the substituents and structural features described herein. Also described are pharmaceutical compositions and medicaments that include the Formula I compounds, as well as methods of using such PI3K modulators, alone and in combination with other therapeutic agents, for treating diseases or conditions that are mediated or dependent upon PI3K dysregulation.
Owner:GENENTECH INC
Method for synthesizing piperlongumine compounds
InactiveCN101774875ASuitable for mass productionLow priceAmide group formation/introductionCarboxylic acidPhotochemistry
The invention provides a method for synthesizing piperlongumine compounds, wherein the compounds are coupled by adopting acromatic carboxylic acid and amido compounds in the presence of coupling reagent. The method has the advantages of mild reaction condition and simple and convenient operation method, and is suitable for batch production of the compounds.
Owner:BEIJING OKEANOS TECH
Method of making hydroxyalkyl amide containing reduced level of unreacted alkanolamine
ActiveUS7244857B2Decrease in levelFatty acid chemical modificationOrganic compound preparationMetalAlkanolamine
A method for reacting alkanolamine with ester in the presence of a metal silicate compound and, optionally, a catalyst, to produce a hydroxyalkyl amide composition with a decreased level of alkanolamine and residual catalyst.
Owner:CHEMTURA CORP
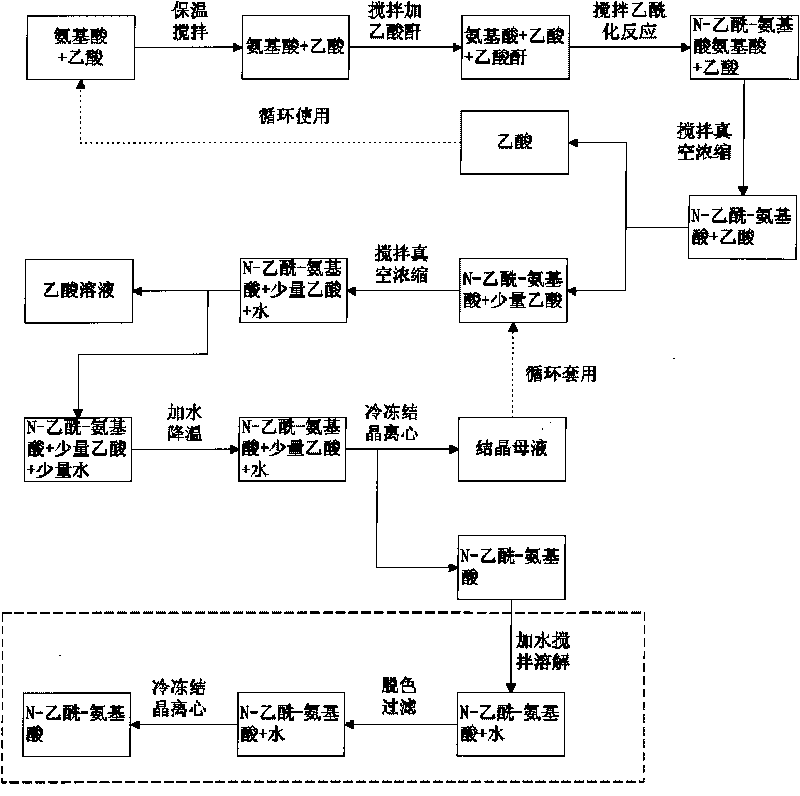
Method for preparing N-acetylamino acid
InactiveCN101723772AHigh purityReduce dosageOrganic compound preparationCarboxylic acid amides preparationAcetic acidAcetic anhydride
The invention discloses a method for preparing N-acetylamino acid, comprising the following steps of: mixing amino acid and acetic acid in the mol ratio of 1:(4-7), raising temperature to 40-70 DEG C and stirring and keeping the temperature for 1-6 hours; evenly feeding acetic anhydride the mole number of which is equivalent to 1.0-1.4 times that of the amino acid within 1-5 hours and stirring and keeping the temperature for 1-4 hours; rapidly distilling under the condition that the vacuum degree is 0.07-0.08 Mpa and the temperature is 60-90 DEG C until the volume of the distilled liquid is 1.0-1.3 times the adding volume of the acetic acid; and then adding water the mass of which is 1-3 times that of the amino acid, stirring and reducing the temperature to separate out crystals, stading for 1-24 hours, separating solid and liquid and drying the solid. The method consumes few reagents and greatly reduces the production cost; and products are easy to separate and the total yield is high up to 92-98 percent. The generated acetylamino acid has high purity and is beneficial to subsequent technological process.
Owner:TIANJIN UNIV
Method for preparing amide compound
InactiveCN102746077AMild reaction conditionsMeet the requirementsOrganic compound preparationSulfonic acid esters preparationSodium iodidePotassium iodine
The invention discloses a method for preparing an amide compound, which comprises the steps of configuration of reaction system. Methyl ketone, amine, catalyst, an oxidizing agent and solvent are use, wherein the general formula of the methyl ketone is RCOCH3, and R is selected from one kind of C6-C14 aryl group, C2-C8 alkenyl group, and five-or six-membered heterocyclic group; the amine is primary amine; the catalyst is selected from one kind of potassium iodide, iodine, tetramethyl ammonium iodide, tetrabutyl ammonium iodide, tetrahexyl ammonium iodide, bismuth iodide, lithium iodide, phenyl trimethyl ammonium iodide, benzyl trimethyl ammonium iodide or sodium iodide; the oxidizing agent is tert-butyl hydroperoxide; the solvent is selected from one kind of water, dichloromethane, ethyl acetate, toluene, 1,2-dichloroethane, 1,1,1-trichloroethane, acetonitrile, and isopropanol. The methyl ketone, the catalyst and the amine are added into the reaction system under the temperature of 0 DEG C and stirred for 5 minutes, and then the oxidizing agent is added to react for 2-48 hours to obtain the amide compound. The method provided by the invention is green, moderate, high in selectivity and wide in application range.
Owner:云梦星联物流有限公司
PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID
InactiveUS20090192313A1Excellent FXa inhibitory effectOrganic halogenationAmino group formation/introductionCarboxylic acidPyridine
5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid of formula (5) or a salt thereof, [F14]is prepared by reacting a compound of formula (6) or a salt thereof,with a trihalogenoacetyl halide in the presence of a base, followed by hydrolysis.
Owner:DAIICHI SANKYO CO LTD
A kind of supported gold catalyzed the method for hydration of nitrile to prepare amide
InactiveCN102285850AMeet product quality requirementsEasy to separateOrganic compound preparationCarboxylic acid amides preparationNitro compoundHydration reaction
The invention belongs to the technical field of preparation of amide, and particularly relates to a method for preparing amide from nitrile by hydration in presence of supported gold as catalyst. In the method, supported gold is used as a catalyst, and nitrile is used as a reaction substrate and is subjected to hydration reaction with water at room temperature or at the temperature of 30-200 DEG C to prepare the amide, wherein the used nitrile is an aliphatic compound of the nitrile or an aromatic nitro compound of the nitrile; the aromatic nitro compound has or does not have one or more substituents; and the one or more substituents are one or more electron-donating groups and / or electron-withdrawing groups. The method has the advantages of easiness, convenience and safety in operation, readily available raw materials, low pollution, high yield and easiness in separation, recovery and recycling of the catalyst.
Owner:FUDAN UNIV
Preparation method for fatty acyl amide
ActiveCN104926578AEfficient preparationThe synthesis process is simpleOrganic compound preparationCarboxylic acid amides preparationChemical synthesisOrganic solvent
The invention discloses a preparation method for fatty acyl amide, belonging to the technical field of organic chemical synthesis. The method comprises the following steps: using substituted terminal olefin, carbon monoxide, and aminal or amine as raw materials, then carrying out catalyzation or co-catalyzation with aldehyde via a transition metal catalyst, and carrying out reaction in an organic solvent at 50 to 120 DEG C for 12 to 24 hours under the participation of a ligand or the co-participation of the ligand with an additive; removing the organic solvent when the reaction is completed, and carrying out column chromatography so as to obtain a fatty acyl amide compound. The preparation method for the fatty acyl amide has the following advantages: the raw materials, the catalyst and the additive for the reaction are cheap and easily available; synthetic process is simple; synthetic cost is greatly reduced; reaction conditions are mild; industrialization is easily realized; the raw materials and the catalyst for the reaction are clean and non-toxic and has small pollution to the environment.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesizing method for amide compound
ActiveCN104710259ADiversity is cheap and readily availableReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationAir atmosphereAlcohol
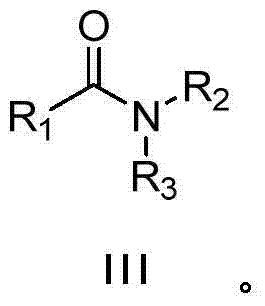
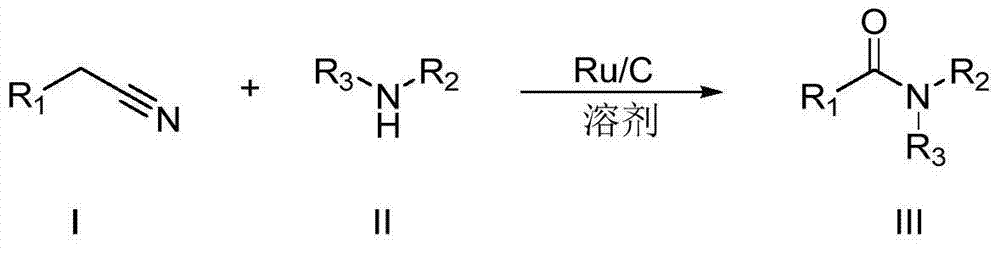
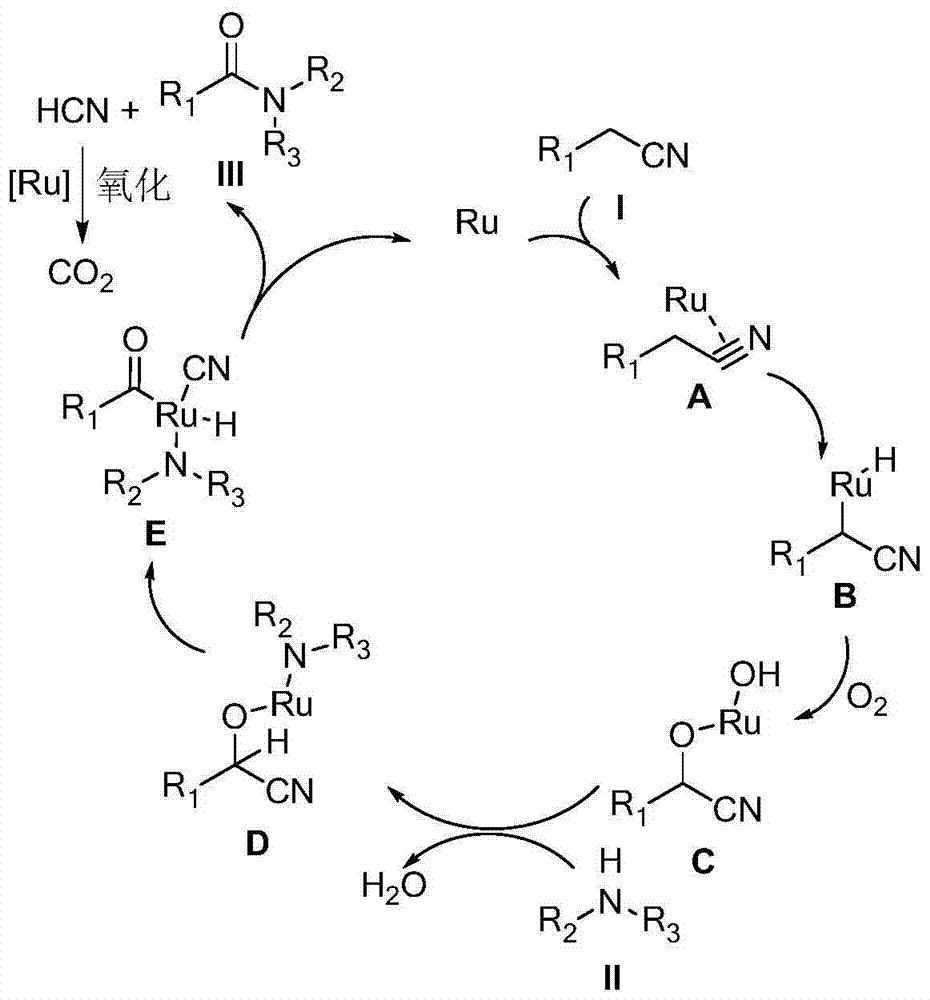
The invention discloses a synthesizing method for an amide compound shown in formula (III). The synthesizing method comprises the following steps: adopting replacing acetonitrile shown in formula (I) and amines compound shown in formula (II) as raw materials in air atmosphere; adopting Ru / C as a catalyst; conducting reaction in solvent to generate the amide compound shown in formula (III); the solvent is one selected from ethyl alcohol, furanidine, methyl sulfoxide and o-dichlorobenzene. The synthesizing method being novel synthesizing route is simple to operate, convenient in post-treatment, high in product yield, and good in product purity; particularly, the catalyst and the solvent can be used repeatedly; air is taken as oxidant; not only production cost is reduced, but also the method is environmental-friendly, and very suitable for large-scale industrialization production.
Owner:ZHEJIANG UNIV OF TECH
Ionic type iron (III) complex with monophenol functionalization tetrahydroglyoxaline cations and preparation method and application thereof
ActiveCN105001031AClear structureEasy to purifyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsImidePtru catalyst
The invention discloses an ionic type iron (III) complex with monophenol functionalization tetrahydroglyoxaline cations and a preparation method and application thereof. The ionic type iron (III) complex with the monophenol functionalization tetrahydroglyoxaline cations is adopted as a catalyst to efficiently catalyze the oxidative coupling reaction of aldehyde and secondary amide to prepare imide for the first time, oxidative coupling of aromatic aldehyde, aliphatic aldehyde and the secondary amide can be efficiently catalyzed, the oxidative coupling reaction with aromatic heterocyclic aldehyde and large-steric-hindrance secondary amide as a substrate can be efficiently catalyzed, and catalytic activity and substrate applicability are superior to those in the prior art.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
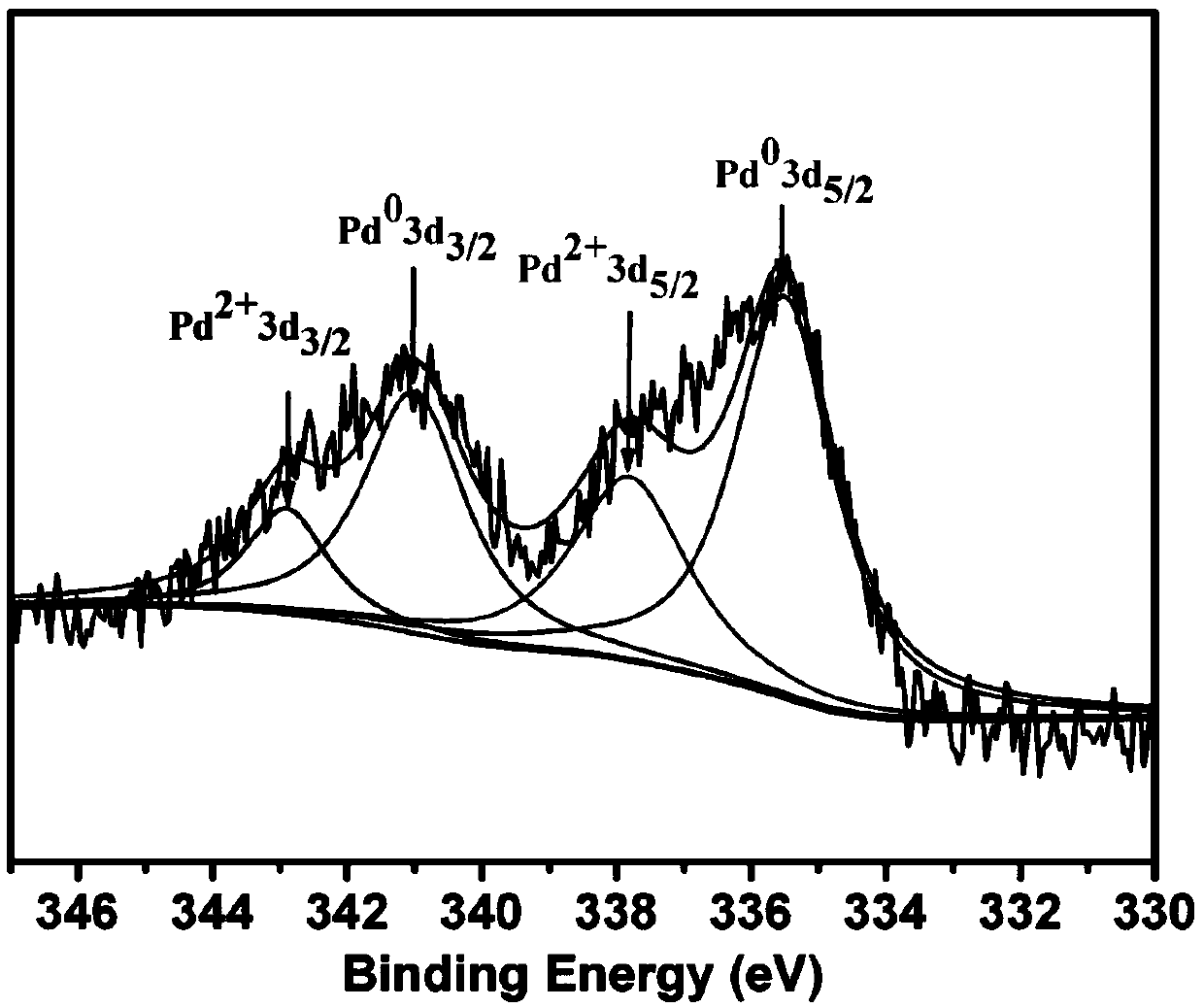
Organic porous polymer palladium-supported catalyst and preparation and application methods thereof
InactiveCN109746045AReduce churnLarge specific surface areaOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBromineCarbonylation
The invention relates to the technical field of preparation of catalysts, particularly to an organic porous polymer palladium-supported catalyst and preparation and application methods thereof. The organic porous polymer palladium-supported catalyst containing dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium functional units is prepared by taking dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium and 1, 3, 5-triphenylbenzene as copolymerizing monomers and through a one-step outer crosslinking method. The organic porous polymer palladium-supported catalyst canachieve efficient catalytic carbonylation of brominated aromatic hydrocarbons to synthesize aromatic carbonyl compounds, and meanwhile, is large in specific surface area, wide in pore size distribution range, mild in reaction conditions, high in yield and good in recycling performance, thereby achieving high application potential.
Owner:LIUPANSHUI NORMAL UNIV
Preparation method of methanamide
ActiveCN104447379AImprove responseMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationSolventControllability
The invention relates to a preparation method of methanamide. The method uses formamide or its derivative and amine (primary or secondary amine) as reactants, which are subjected to amidomethylation in the presence of a catalyst to prepare methanamide. The method is characterized in that solid acidic metal oxide or precious metal loaded acidic metal oxide is used as the catalyst and the reaction is carried out efficiently under mild conditions. The reaction process is as follows: mixing formamide with certain concentration or its derivatives with primary or secondary amine and a certain amount of catalyst, placing the mixture into pressure vessel without adding or additionally adding other solvent, sealing, stirring at a temperature no less than 60 DEG C, and reacting for no less than 0.5 h to obtain the reaction product methanamide. The method has the advantages of simple preparation of the catalyst, easy separation process of the catalyst and product, repeated usage of the catalyst, high controllability of the reaction process, and yield of the product formamide reaching more than 95%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of amide from nitrile
InactiveCN102863305ALow priceLow toxicityOrganic compound preparationCarboxylic acid amides preparationOrganic solventNitrogen
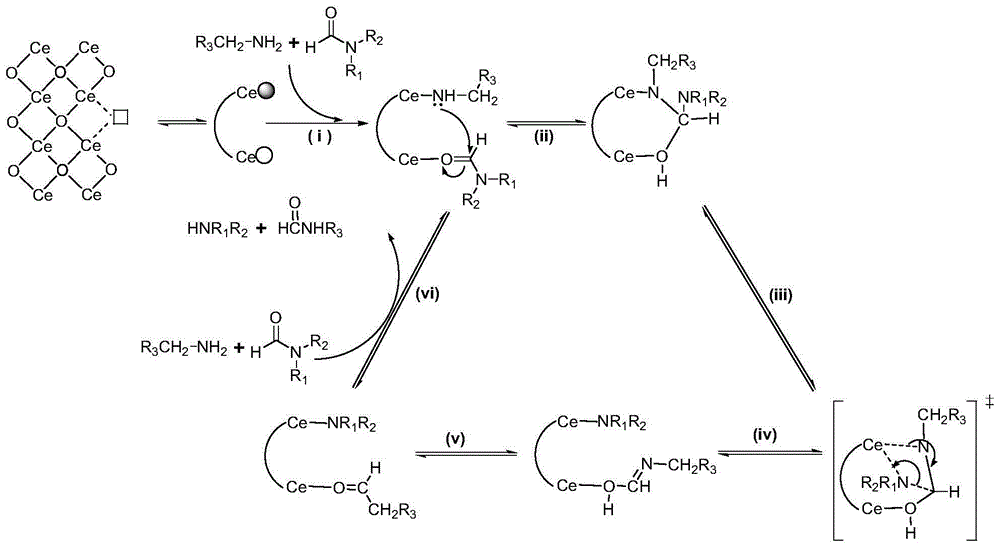
The invention discloses a preparation method of amide from nitrile. The method includes reaction of hydrolysis by heating nitrile under air or nitrogen condition and in ammonia water by alkaline catalyst. The method can implement simple, mild and green hydrolysis and synthesize a series of amide compounds. The catalyst is low in price, small in toxicity, low in dosage, mild in reaction condition and easy to operate in air. Organic solvent is not required in reaction, and ammonia water which is cheap and easy to obtain is used as green solvent. Pollution possibly caused by organic solvent to environment, excess hydrolysis caused by using excess alkali and the like can be reduced greatly.
Owner:WENZHOU UNIVERSITY
Method for preparing N-aryl amide without solvent and catalyst
InactiveCN106674040AOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsSolvent free
The invention discloses a method for preparing N-aryl amide without a solvent and a catalyst. The method is characterized by obtaining the N-aryl amide under solvent-free and catalyst-free action; a molar ratio of substituted meldrum acid and arylamine is (1 to 5) to (5 to 1). According to the method disclosed by the invention, the defects that acyl chloride, anhydride, a dehydration coupling reagent, the solvent, a phase transfer catalyst or a metal catalyst, and the like are required to be adopted in the prior art are overcome; the method has the following advantages that (1) the substituted meldrum acid is used as an acylating agent, so that pre-activation on carboxylic acid or use of the dehydration coupling reagent is avoided; (2) due to easiness in preparation of the substituted meldrum acid, certain difficult-to-obtain or expensive carboxylic acid and activated derivatives are prevented from being used; (3) a solvent-free mode is adopted, so that the use of a toxic organic solvent or emission of wastewater is avoided; (4) no acid, base or metal catalysts exist, the influence of the acid and the base on sensitive groups and equipment and the residue of metal ions in a product are avoided. A synthesis method disclosed by the invention can play an important role in industrial production for preparing the N-aryl amide and particularly for preparing the N-aryl amide with complex carboxylic acid.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
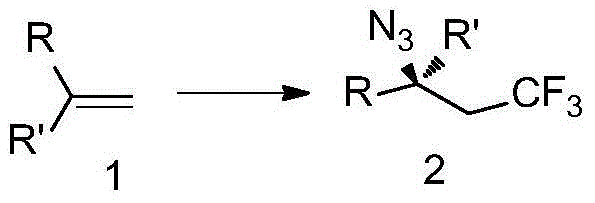
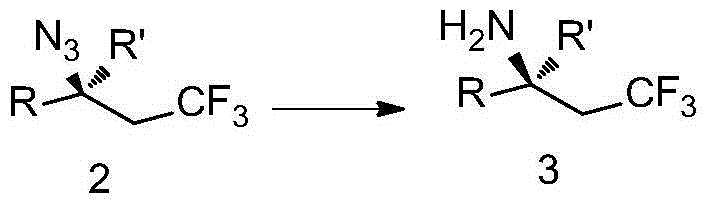
Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
ActiveCN104649857AMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzidotrimethylsilane
The invention discloses trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof. The preparing method of the trifluoromethyl-substituted azide compounds includes following steps of: subjecting a trifluoromethylation agent, azidotrimethylsilane and a carbon-carbon double bond of an olefin to addition in an organic solvent under the existence of a catalyst to obtain a compound in which one carbon in the carbon-carbon double bond of the olefin has trifluoromethyl and the other carbon has an azide group. The preparing methods utilize the trifluoromethylation agent which is mild relatively, directly form a carbon-nitrogen bond and a carbon-carbon bond by double-functionalization of olefins, and efficiently synthesize the trifluoromethyl-substituted azide, amine and heterocycle compounds with high selectivity. The preparing methods are easily available in raw materials, mild in reaction conditions, good in atom economy, high in selectivity, simple in after-treatment, environmental friendly, high in yields and suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
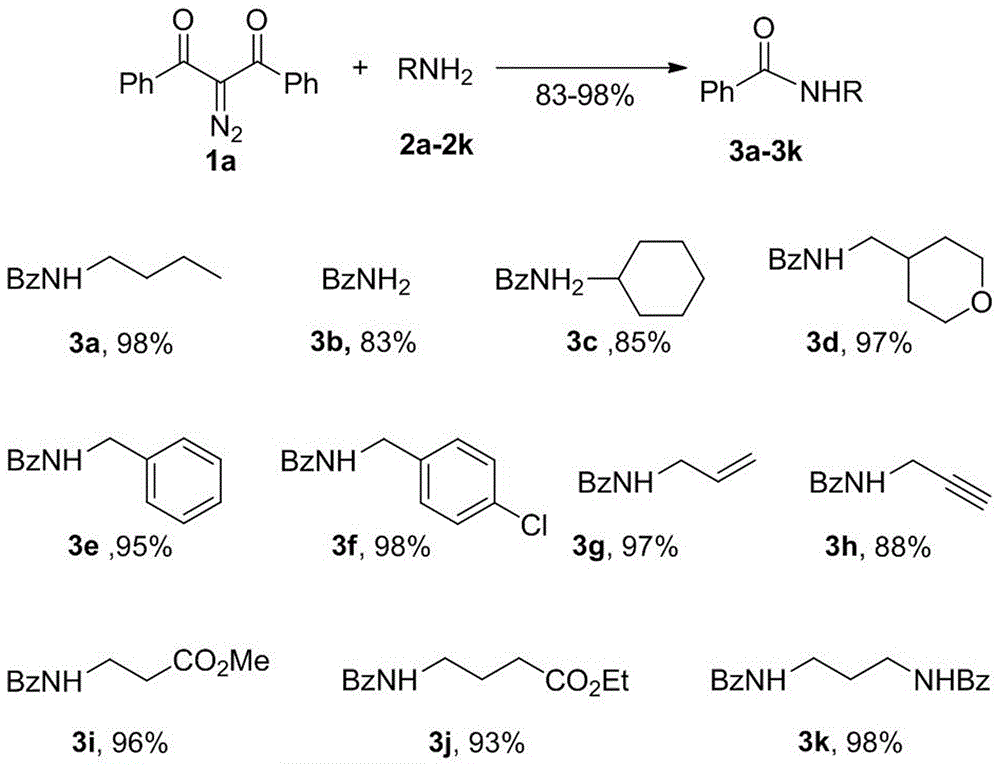
Method for preparing amide compound from 2-diazo-1, 3-dicarbonyl compound as acylating agent
ActiveCN106554290AOvercome limitationsMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationDiazoRaw material
The invention provides a method for preparing an amide compound from a 2-diazo-1, 3-dicarbonyl compound as an acylating agent under non-metallic catalysis and neutral conditions. The method uses 2-diazo-1, 3-dicarbonyl compound as a raw material and carries out different benzoyl protection on different amino compounds so that a series of amide compounds are prepared. The method is carried out under neutral conditions, prevents the limitation of reaction substrates under conventional alkaline conditions, and has mild reaction conditions, high reaction efficiency and a simple operation method. The method provides a new and convenient method for preparation of amide compounds and protection of amino groups and can be used in the fields of chemical medicine, biology and materials.
Owner:山西师范大学
Method for synthesizing N-alkenamide
InactiveCN103113176ANo harmImprove economyOrganic compound preparationCarboxylic acid amides preparationIridiumPtru catalyst
The invention discloses a method for synthesizing N-alkenamide. The method comprises the following preparation processes: adding oxime, transition metal catalyst ruthenium, iridium and / or rhodium complex and an organic solvent into a reaction container; reacting the reaction mixture at the temperature of 90-130 DEG C for hours, and cooling to room temperature; adding compound alcohol and alkali, reacting the reaction mixture at the temperature of 90-130 DEG C for hours, and performing separation on columns to obtain a target compound. Compared with the prior art, the method starts from commercial or easily synthesized oxime, rearrangement is generated in the presence of a transition metal catalyst, an amide intermediate is generated, the intermediate and alcohol are subjected to an alkylation reaction so as to obtain N-alkenamide, and the reaction has three obvious advantages that 1) the commercial or easily synthesized oxime and approximately non-toxic alcohol serve as initial raw materials; 2) only the water is generated to serve as a byproduct, and environmental harm is avoided; and 3) the reaction atom is high in economic efficiency. Thus, the reaction meets the green and chemical requirements and has wide development prospects.
Owner:NANJING UNIV OF SCI & TECH
Method for preparing formamide by catalytic oxidation of tertiary amine
InactiveCN105016937AAtom economy is highMild responseOrganic compound preparationCarboxylic acid amides preparationCatalytic oxidationFormamide
The invention provides a novel method for preparing formamide. In the method, tertiary amine serves as a substrate, air or oxygen serves as an oxygen source, and tertiary amine is oxidized into formamide under the effect of a catalyst. The method is high in oxidation efficiency and product yield; the air or oxygen serves as the oxygen source, and the method is economical and environmentally friendly and has very good application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Thiol-modified macromolecule derivatives and cross-linked materials thereof
ActiveUS20100152423A1Avoid stickingGelation rateConnective tissue peptidesPeptide/protein ingredientsChemical structureCross-link
The disclosed is thiol-modified macromolecule derivatives having the general formula of (I) or (II), as well as the disulfide bond cross-linked materials and the thiol-reactive crosslinker cross-linked materials, wherein R1 and R2 are alkylene groups, substituted alkylene groups, aromatic groups or polyether groups and the like, respectively. R1 and R2 may have same or different chemical structure, and P is a residue of a macromolecule with side carboxyl group. The thiol-modified macromolecule derivative has a molecular weight of from 1000 to 5,000,000. The thiol-modified macromolecule derivative having the general formula of (I) or (II) of the present invention has a side chain with flexible chemical structure and adjustable properties, and has a number of advantages, such as mild reaction condition, high production yield, high degree of modifying, and the controllable modifying degree and so on. The cross-linked material made from the thiol-modified macromolecule derivative of the present invention can be used to inhibit cell attachment and be used as the matrix for cell attachment growth.
Owner:BIOREGEN BIOMEDICAL (CHANGZHOU) CO LTD
Dicyclohexyl trifluoromethanesulfonate ammonium salt and application thereof
ActiveCN101648893ARecyclableLow priceAmino preparation from aminesOrganic compound preparationTriflic acidPhenol
The invention relates to a dicyclohexyl trifluoromethanesulfonate ammonium salt and application thereof. The dicyclohexyl trifluoromethanesulfonate ammonium salt is prepared from trifluoromethanesulfonate and dicyclohexylamine. The dicyclohexyl trifluoromethanesulfonate ammonium salt can be applied to catalyzing the condensation reaction of three components such as aldehydes, amide and phenol, the condensation reaction of the three components such as the aldehydes, the amide and the phenol takes the phenol, the aldehydes and the amine as raw materials and is completely finished in an organic solvent under the action of the dicyclohexyl trifluoromethanesulfonate ammonium salt, and the reaction solution is separated and purified to obtain the corresponding multi-component condensation product. The invention develops a novel trifluoromethanesulfonate ammonium salt, promotes the application of a new generation of acid organic catalyst in the multi-component reaction, and promotes the further development of the multi-component reaction.
Owner:台州小试生物科技有限公司
Mixed acid catalysis system for ketoxime beckmann rearrangement reaction
ActiveCN107051581ALow viscosityReduce contentLactams preparationOrganic compound preparationOrganic acidBeckmann rearrangement
The invention discloses a mixed acid catalysis system for a ketoxime beckmann rearrangement reaction. The mixed acid catalysis system is characterized in that the catalysis system is a homogeneous system consisting of an organic acid and an inorganic acid, and serves as a catalyst in a reaction of preparing amide through ketoxime rearrangement; the reaction temperature is 60-130 DEG C, preferably 80-120 DEG C; the reaction time is 1-240min, preferably 20-90min; the molar ratio of the organic acid to the inorganic acid is 0.5-50, preferably 3-20; the molar ratio of the inorganic acid to the ketoxime is 0.1-10, preferably 1-5. According to the catalysis system, the ketoxime can be efficiently converted into a corresponding amide compound in a relatively mild reaction condition; moreover, the content of byproducts of the reaction can be controlled, and particularly, the content of heavy byproducts is very low. The organic acid part of the catalysis system can be recycled, and the reaction condition in use is mild; the conversion rate of the ketoxime rearrangement reaction is high, and the selectivity is good; the content of byproducts after the rearrangement reaction is low, and particularly, the content of heavy byproducts is very low in an optimized condition; the aftertreatment process is hopefully to be simplified; the catalysis system has a good potential in industrial application.
Owner:TSINGHUA UNIV
Method for synthesizing beta-amidocarbonyl compounds
InactiveCN102816042ARaw materials are easy to getSimple processOrganic compound preparationCarboxylic acid amides preparationArylAcetyl chloride
The invention provides a method for synthesizing beta-amidocarbonyl compounds. The reaction general formula is disclosed in the specification, wherein R1, R2, R3 and R4 are respectively a substituent group; R1 can be H, F, Cl, Br, NO2, CH3, OCH3 or any other single group, and can also be 2,4-2Cl, 2-Cl-5-F or any other double-substituent group, and the quantity and position of the substituent groups are not defined; R2 can be H, and can also be NO2, CH3, OCH2CH3, aromatic group or any other substituent group; and R3 can be H, and can also be CH3, COOC2H5 or any other substituent group, or hexahydric cycloalkyl group formed by R2, R3 and carbon atom connected with R2 and R3. The reaction is carried out at room temperature in the presence of acetyl chloride by using titanium tetrachloride as a catalyst; and the purification adopts recrystallization, column chromatography or any other separation method. The invention has the advantages of accessible raw materials, simple technique, mild reaction conditions and cheap and nontoxic catalyst; and the applicable substrate range for reaction is wide, and thus, different substrates can be utilized to synthesize a series of beta-amidocarbonyl compounds.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Synthesis method of amide aryl compound
InactiveCN106496056AWidely applicable preparation methodStable in natureCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsCarboxylic acid
The invention relates to a synthesis method of an amide aryl compounds. According to the method, Ru-(p-cymene) C12 is taken as a catalyst, K2S2O8 is taken as an oxidizing agent, Xantphos is taken as a ligand, one reactant (N, N-dialkyl formamide) is taken as a solvent, and a substrate aryl carboxylic acid and the N, N-dialkyl formamide are subjected to coupling reaction, so that the amide aryl compound is obtained. The reaction substrate is low in cost and easy to get, stable in performance, small in toxicity and mild in reaction conditions, and has a wide applicability for substrate with different functional groups. The amide aryl compound efficiently established through the synthesis method belongs to an important molecular skeleton of various medicines, bioactive molecules and natural products, and the synthesis method provides a widely applicable preparation method for the synthesis of the compounds.
Owner:中国人民解放军63975部队
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com














![PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7bb1b68e-1669-495e-81d3-cb3f6b0c50d5/US20090192313A1-20090730-C00001.png)
![PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7bb1b68e-1669-495e-81d3-cb3f6b0c50d5/US20090192313A1-20090730-C00002.png)
![PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID PROCESS FOR PRODUCING 5-METHYL-4,5,6,7-TETRAHYDROTHIAZOLO[5,4-c]PYRIDINE-2-CARBOXYLIC ACID](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7bb1b68e-1669-495e-81d3-cb3f6b0c50d5/US20090192313A1-20090730-C00003.png)